Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impact of coating type on structure and magnetic properties of biocompatible iron oxide nanoparticles: insights into cluster organization and oxidation stability†
Amal
Nasser
 *ab,
Asma
Qdemat
*ab,
Asma
Qdemat
 c,
Harald
Unterweger
c,
Harald
Unterweger
 d,
Rainer
Tietze
d,
Rainer
Tietze
 d,
Xiao
Sun
d,
Xiao
Sun
 e,
Joachim
Landers
e,
Joachim
Landers
 f,
Juri
Kopp
f,
Juri
Kopp
 f,
Baohu
Wu
f,
Baohu
Wu
 b,
Marie-Sousai
Appavou
b,
Marie-Sousai
Appavou
 b,
Anastasiia
Murmiliuk
b,
Anastasiia
Murmiliuk
 b,
Elliot Paul
Gilbert
b,
Elliot Paul
Gilbert
 g,
Oleg
Petracic
g,
Oleg
Petracic
 *c and
Artem
Feoktystov
*c and
Artem
Feoktystov
 *b
*b
aDepartment of Physics, Technical University Munich (TUM), Garching, Germany
bForschungszentrum Jülich GmbH, Jülich Centre for Neutron Science JCNS at MLZ, Garching, Germany. E-mail: a.nasser@fz-juelich.de; a.feoktystov@fz-juelich.de
cForschungszentrum Jülich GmbH, Jülich Centre for Neutron Science JCNS-2, Jülich, Germany. E-mail: o.petracic@fz-juelich.de
dENT Department, Section of Experimental Oncology and Nanomedicine (SEON), Else Kroener-Fresenius-Stiftung-Professorship, University Hospital Erlangen, Germany
eDeutsches Elektronen-Synchrotron DESY, 22607 Hamburg, Germany
fFaculty of Physics and Center for Nanointegration Duisburg-Essen (CENIDE), University of Duisburg-Essen, D-47057 Duisburg, Germany
gAustralian Centre for Neutron Scattering, Australian Nuclear Science and Technology Organisation, NSW 2234, Australia
First published on 14th September 2024
Abstract
Superparamagnetic iron oxide nanoparticles (SPIONs) are a promising tool for biomedical applications, including drug delivery, imaging, and magnetic hyperthermia. However, their tendency to agglomerate limits their performance efficiency. To overcome this limitation, a coating can be applied during or after synthesis. This work investigates the effect of three biocompatible coatings, namely sodium citrate, (3-aminopropyl)triethoxysilane (APTES), and dextran, on controlling the agglomeration of iron oxide nanoparticles. Various experimental techniques were used to characterize the structural and magnetic properties of the coated nanoparticles, including cryogenic transmission electron microscopy (cryo-TEM), magnetometry, Mössbauer spectroscopy, and small-angle X-ray and neutron scattering. The results indicate that the coatings effectively stabilize the nanoparticles, leading to clusters of different sizes that modify their magnetic behaviour due to magnetic inter-particle interactions. The oxidation kinetics of the nanoparticles prepared with the various coating materials were investigated to characterize their oxidation behaviour and stability over time. This research provides valuable insights into the design of an optimized nanoparticle functionalization strategy for biomedical applications.
1. Introduction
Magnetic nanoparticles can be manipulated by external magnetic fields and concentrated close to the targeted tissue, making them attractive for use in vivo. Superparamagnetic iron oxide nanoparticles (SPIONs) are a type of magnetic nanoparticles that show promise in theranostics, which combines therapy and diagnostics,1–4 and find applications in various medical fields such as imaging, targeted drug delivery, and hyperthermia.5–7 However, the development of these processes depends on a detailed understanding of the magnetic properties of the particles. These properties are influenced by various parameters, including particle size,8 morphology,9 crystalline defects (such as antiphase boundaries),10 composition of iron oxide phases,11 and interparticle interactions.12Successful and reliable medical applications require the long-term stability of SPIONs. To achieve this goal, the particles are typically stabilized using electrostatic or steric coatings, which provide sufficient repulsion to avoid uncontrolled agglomeration.13,14 textcolororange In addition it is important to control the oxidation state.15 Recently, controlled SPIONs agglomeration has gained substantial attention in research due to the improved magnetic response as a consequence of their larger size. Despite their larger size, these particles have a large degree of long-term stability.16–18 Such systems are often termed ‘clustered nanoparticles’ or ‘nanoflowers’ and consist of smaller SPION constituents. Due to the single magnetic cores being closer together or even in contact, magnetic dipolar interactions are increased, or exchange interactions may be found.19
The most commonly used iron oxide phases for magnetic core nanoparticles in biomedical applications are magnetite Fe3O4 and maghemite γ-Fe2O3.20 Both phases have a similar crystallographic structure (cubic inverse spinel) but exhibit different magnetic and electronic properties.21,22 The presence of vacancies in maghemite and the variations in electronic configurations are responsible for the differences in magnetic properties. For instance, maghemite has a lower saturation magnetization of 76 A m2 kg−1 compared to magnetite, which demonstrates a saturation magnetization of 87 A m2 kg−1 at room temperature.23
Controlling specifically the oxidation state of the iron oxide cores is crucial for medical applications, as it significantly impacts their functionality. For example, the oxidation of magnetite to maghemite results in a reduction of the SPION's saturation magnetization. Also the magnetocrystalline anisotropy is affected. Consequently, both the efficiency of the particles and the reproducibility of results are altered.24 Deliberate control of the oxidation state can hence lead e.g. to an increase of the heat delivery in magnetic hyperthermia applications.25
In our study, we have selected several coating agents, such as negatively charged citrate, positively charged APTES, and neutral hydrophilic polymer dextran, to stabilize the magnetic nanoparticles and control the magnetite oxidation. While numerous studies have explored the impacts of these coatings on cluster formation and the resulting physicochemical properties of magnetic nanoparticles,26–32 the specifics of their magnetic properties remain debated.33,34 To investigate the influence of particle size, structural organization, and aging on the magnetic properties of these clusters in detail, we employed magnetometry combined with small-angle X-ray scattering (SAXS) and cryogenic transmission electron microscopy (cryo-TEM). Additionally, we utilized small-angle neutron scattering (SANS) to examine the magnetic structure of the clustered particles for the citrate-coated system. In addition, we employ Mössbauer spectroscopy combined with magnetometry to investigate the time-dependent oxidation behavior of the particle-coating species. This approach hence provides information on the aging process, from storage to the final product. By using both Mössbauer spectroscopy and magnetometry, we were able to track the changes in Fe2+ and Fe3+ composition as a function of time for various coating types, determining the net magnetic properties and examining how fast this oxidation takes place and the proportion of the oxidized form.
2. Materials and methods
2.1. Synthesis of iron oxide nanoparticles
The iron oxide particles used in this study were prepared via the co-precipitation method.35 This synthesis route was chosen for its simplicity, higher yield, and relatively low cost.36–38 Initially, a mixture of FeCl2 and FeCl3 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio was dissolved in distilled water and stirred in an argon atmosphere to prevent oxidation. Subsequently, an ammonia solution (25%) was added for the precipitation reaction of the iron oxide. In the following stage, the corresponding coating materials were added to the solution to optimize the surface coating and control the cluster size, including citrate molecules, (3-aminopropyl)triethoxysilan (APTES), and dextran. The coated particles were dispersed in water and then sealed in containers under N2 gas for the aging study, while others were filled under atmospheric conditions. Below is a summary of the materials' details.
2 molar ratio was dissolved in distilled water and stirred in an argon atmosphere to prevent oxidation. Subsequently, an ammonia solution (25%) was added for the precipitation reaction of the iron oxide. In the following stage, the corresponding coating materials were added to the solution to optimize the surface coating and control the cluster size, including citrate molecules, (3-aminopropyl)triethoxysilan (APTES), and dextran. The coated particles were dispersed in water and then sealed in containers under N2 gas for the aging study, while others were filled under atmospheric conditions. Below is a summary of the materials' details.
2.2. Characterization methods
| Component | FexOy | Citrate | H2O | D2O |
|---|---|---|---|---|
| The magnetic scattering length density (ρmag) of a single iron oxide core is taken from ref. 10. It is assumed that the value of ρmag for the cluster particles is comparable to that of the single cores. | ||||
| Density g cm−3 | 5.74 | 1.70 | 1.00 | 1.10 |
| ρ | 6.91 | 1.50 | −0.56 | 6.34 |
| ρ mag | 0.94 | — | — | — |
The SANS scattering intensity of the magnetic particle is a combination of the nuclear, IN(Q), and the magnetic, IM(Q), scattering intensity. The scattering intensity is proportional to the scattering contrast, the difference between the scattering length densities of the solvent and the particle. The dispersion of particles in D2O leads to a low nuclear contrast Δρ (see Table 1). This leads to a reduction in the intensity of nuclear scattering from the cluster particles, allowing the magnetic scattering contribution to be highlighted.43 The scattering intensity in a zero magnetic field is then written as44
 | (1) |
For a magnetic field applied perpendicular to the direction of the neutron beam, the magnetic moments of clusters are aligned by the field, which results in the magnetic scattering contribution being anisotropic in the detector plane. The scattering intensity is then expressed as45
I(Q,θ)H≠0 = IN(Q)|Δρ≈0 + IM(Q)sin2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (2) |
 | (3) |
The quantities V, bH, ![[M with combining tilde]](https://www.rsc.org/images/entities/i_char_004d_0303.gif) z and θ refer to the scattering volume, a constant parameter, the Fourier transform of the z-components of the magnetization vector field, the azimuthal angle between the applied magnetic field H and the scattering vector Q, respectively. The purely nuclear scattering contribution was obtained from the 2D scattering pattern in the sector of ±10° for Q‖H (sin2
z and θ refer to the scattering volume, a constant parameter, the Fourier transform of the z-components of the magnetization vector field, the azimuthal angle between the applied magnetic field H and the scattering vector Q, respectively. The purely nuclear scattering contribution was obtained from the 2D scattering pattern in the sector of ±10° for Q‖H (sin2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ ≈ 0). The field-dependent magnetic scattering amplitude is accessible from the 2D scattering pattern in a sector of ±10° for Q⊥H (sin2
θ ≈ 0). The field-dependent magnetic scattering amplitude is accessible from the 2D scattering pattern in a sector of ±10° for Q⊥H (sin2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ ≈ 1). The contrast variation experiment in a zero magnetic field was also performed by dispersing the C71 particles in mixtures of H2O and D2O to vary the scattering length density of the solvent. This allowed for obtaining additional information about the structural organization of the cluster particles.
θ ≈ 1). The contrast variation experiment in a zero magnetic field was also performed by dispersing the C71 particles in mixtures of H2O and D2O to vary the scattering length density of the solvent. This allowed for obtaining additional information about the structural organization of the cluster particles.
3. Results and discussion
3.1. Morphology and structure
Fig. 1(a)–(c) shows transmission electron microscopy images of samples C71, B47 and D40. Samples C71 (Fig. 1) and B47 (Fig. 1b) display raspberry-like structures consisting of polydisperse small nanoparticle cores in larger clusters, which form network-like structures. The C71 system exhibits less extended structures. On the other hand, the D40 sample (Fig. 1c) shows a different morphology with the presence of smaller particles (1–2 nm). In this case, the dextran coating serves as a polymer matrix that embeds the NPs and forms randomly shaped aggregates. Table 2 summarizes the r0 and σ parameters of the log-normal distribution of the single core nanoparticles. These parameters were determined from the fit according to the following expression: | (4) |
| Coating material | Code | TEM | SAXS | |
|---|---|---|---|---|
| r 0 (nm) | σ | D max (nm) | ||
| Citrate molecule | C71 | 9.5(1) | 0.30(6) | 56 |
| APTES | B47 | 10.7(4) | 0.30(5) | >120 |
| Dextran | D40 | 2.0(8) | 0.20(1) | 36 |
To further investigate the size and structure of the clusters, SAXS intensity curves (see Fig. 2a) were recorded for samples C71, B47 and D40. In SAXS, any effect of the coating molecules on the scattering will be negligible due to the much higher electron density of iron oxide. The presence of a Guinier-like region at low Q in the scattering curve of samples C71 and D40 indicates that the dimension of the scattering particles is less than 1/Qmini.e. the inverse of the minimum Q. On the contrary, the scattering curve of sample B47 at Q < 0.03 Å−1 follows a power-law intensity of I ≃ Q−2.2. The power law behaviour is consistent with the presence of larger scattering objects arising from cluster aggregation generating fractal like-structures.47,48
The pair distance distribution function, P(r), was obtained by applying the indirect Fourier transform (IFT) to the scattering intensity using the GNOM software.49Fig. 2b displays the P(r) profile for both the C71 and D40 samples. The B47 sample had stronger aggregation, so it was not possible to calculate the corresponding IFT. The profiles show a difference in overall particle size, as indicated by zero values occurring at different positions of r = Dmax. The P(r) of the C71 exhibits a shape that is more reminiscent of spherical particles, while the P(r) of the D40 sample is characteristic of elongated particles.
In summary, the SAXS and TEM results show variations in the cluster sizes and the single core nanoparticles, which can be attributed to the coating materials used and the synthesis route. The SAXS result of the polymer dextran shows smaller clusters compared to the citrate and APTES molecules. However, the SAXS result of the APTES coating shows fractal aggregate clusters, which makes it less stable than C71. This information is valuable for improving the design and synthesis of nanoparticles for medical use.
3.2 Aging study
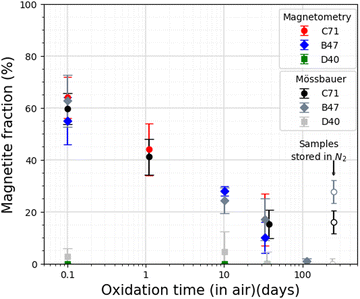
To study nanoparticle aging behaviour as a function of time, Mössbauer spectroscopy and magnetometry measurements were performed on particle dispersions in water repeatedly over a timespan of several weeks. Exemplary Mössbauer spectra of sample C71 recorded at 5 K in a magnetic field of 8 T are shown in Fig. 3, comparing the particle state after a certain time since the synthesis (up to 37 days), assuming a maximum time the particles were under ambient atmosphere between the synthesis and the start of the measurement of ca. 0.1 d. The spectra were reproduced via three sextet subspectra, based on their hyperfine parameters being assigned to Fe3+ in tetrahedral coordination (A sites, green), Fe3+ in octahedral coordination (B sites, blue) and Fe2+ in octahedral coordination (B site, violet). The ferrimagnetic structure of the particles is apparent from the resolution of the A- and B-site sublattice contributions after the application of the magnetic field. Based on the low intensity of absorption lines 2 and 5,50 the particles display a moderate average spin canting angle of ca. 14°. The spin canting angle is defined as the angle between the spin and incidence directions of the γ-ray, with the latter here being identical to the magnetic field direction. Upon aging, the intensity of the B-site Fe2+ subspectrum decreases while the corresponding Fe3+ component increases, leading to more symmetrical B-site absorption lines over aging time. This is due to the shoulder formed by the B-site Fe2+ subspectrum becoming less pronounced, making the oxidation from magnetite to maghemite visible to the naked eye. The magnetite fraction is determined for each spectrum by comparing the Fe2+ fraction in pure magnetite (33.3%) and maghemite (0%). The magnetite fractions in samples C71, B47 and D40 are displayed in Table 3. | ||
| Fig. 3 Mössbauer spectra for sample C71 after various aging times of exposure to air, recorded at 5 K and an applied magnetic field of 8 T. In the C71 1 day spectrum, the vertical arrows mark Mössbauer lines 2 and 5, whose relative intensity indicates the degree of spin canting.51 | ||
| C71 | B47 | D40 | |||
|---|---|---|---|---|---|
| Aging time (day) (in air) | Magnetite fraction (%) | Aging time (day) (in air) | Magnetite fraction (%) | Aging time (day) (in air) | Magnetite fraction (%) |
| 0.1 | 59.7 ± 6 | 0.1 | 62.7 ± 9.3 | 0.1 | 2.7 ± 3.3 |
| 1.1 | 41.1 ± 6.9 | 1.1 | — | 1.1 | — |
| 10.1 | 24.1 ± 7.2 | 9.1 | 24.3 ± 5.4 | 9.1 | 4.5 ± 7.8 |
| 37.1 | 15.3 ± 5.4 | 33.1 | 17.1 ± 7.5 | 35.1 | 0 ± 2.1 |
| 114.1 | — | 114.1 | 0.9 ± 1.2 | 114.1 | — |
| 252 (in N2) | 16 ± 4.4 | 242 (in N2) | 27.7 ± 4.5 | 249 (in N2) | 0 ± 1.9 |
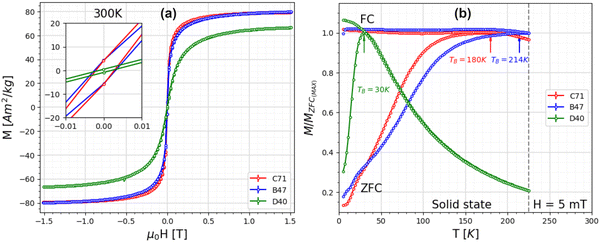
A complementary approach was used to determine the net magnetic properties and quantify the oxidation rate. This involved analyzing the time dependent change in saturation magnetization Msat. The determination of Msat at room temperature is presented in Table 4. To obtain this value, Msat was extrapolated from high-field magnetization data using the law of approach to saturation as described by M(H) = Msat(1 − a/H − b/H2). The magnetite fraction then is estimated by normalizing the net magnetization to the total Fe mass and comparing it to Msat for bulk magnetite, 121 A m2 kgFe−1, and maghemite, 108 A m2 kgFe−1.52 The obtained magnetite and maghemite fractions are used for normalizing saturation magnetization. As shown in Fig. 4, magnetite fractions obtained from Mössbauer spectroscopy and magnetometry are in good agreement. For samples C71 and B47, the initial measurements already indicate ca. 40% of the particle mass was oxidized after a limited exposure time of less than ca. 2 h, which corresponds to a maghemite shell thickness of ca. 0.8–1 nm in single core nanoparticles. This would match the expectation of fast formation of a maghemite surface layer, followed by decelerated further oxidation, resulting in a remaining magnetite fraction of ca. 10–20% in both samples after one month of storage under ambient conditions. After 114 days for sample B47 only minimum Fe2+ is detected in Mössbauer spectroscopy, indicating complete conversion to maghemite within the error margin. In contrast, sample D40 oxidizes faster than the other samples, showing no considerable Fe2+ component already in the initial spectrum, which is also indicated by magnetometry data. For comparison, a second batch of samples C71 and B47 was also stored for six months after preparation under N2. This batch exhibited higher stability against oxidation by preserving a magnetite fraction of around 20–30%, which is comparable to the fraction observed after 10–30 days of exposure to air.
| Aging time (day) (in air) | C71 | B47 | D40 | |||
|---|---|---|---|---|---|---|
| Magnetite fraction (%) | M s (A m2 kg−1) | Magnetite fraction (%) | M s (A m2 kg−1) | Magnetite fraction (%) | M s (A m2 kg−1) | |
| 0.1 | 64 ± 8 | 81.4 ± 0.8 | 54 ± 9 | 80.6 ± 1.6 | 0 | 69.9 ± 1 |
| 1.1 | 44 ± 9 | 79.2 ± 0.9 | 33 ± 1.8 | 78.3 ± 1.3 | 0 | 69.7 ± 0.6 |
| 10.1 | — | — | 28 ± 1.8 | 77.8 ± 1.3 | 0 | 69.9 ± 0.3 |
| 33.1 | 17 ± 10 | 76.3 ± 1.1 | 10 ± 6 | 75.7 ± 0.6 | 0 | 69.9 ± 1 |
| 180 | 3.8 ± 5.3 | 75 ± 0.5 | 0 | 73.5 ± 0.6 | 0 | 69.9 ± 1 |
The saturation magnetization determined for samples C71 and B47 is comparable to the bulk value. However, for sample D40, the saturation magnetization value is 6.6 ± 0.9% lower than for maghemite. This decrease in magnetization may have several causes, including minor fitting artifacts in the extrapolation of Ms due to the non-saturating tendency of the M(H) loops, or crystal defects such as the presence of antiphase boundaries, modified atomic coordination, and an increased number of Fe vacancies.10,53–55 The relatively large error in the saturation magnetization is due to an inaccurate determination of Fe-content using ICP-OES. Treating the entire sample along with the holder might result in significant errors when determining the Fe content.
3.3. Magnetic properties
The hysteresis loops at room temperature (Fig. 5a) for all samples indicate a negligible coercive field, confirming that the nanoparticle clusters exhibit superparamagnetic behaviour. A detailed assessment of the magnetic properties of the clusters was conducted using ZFC/FC curves (Fig. 5b). The blocking temperature, TB, is the temperature at which the transition occurs from the unblocked superparamagnetic to the blocked state. This transition is affected by several factors, such as particle size distribution and magnetic interparticle interactions.56–58 Here, samples C71 and B47 exhibit similar single-core particle sizes but differ in the organization formed by these particles. The two samples have different TB values, with C71 having a TB of 180 K and B47 having a TB of 214 K. The determination of TB is based on the highest value found in the ZFC curve. The broadening of peaks in the ZFC curves can be attributed to either a variation in the particle volume or strong magnetic interactions.59 The SAXS results confirm that magnetic interaction is the main reason for the broadening featured in strongly aggregated single-core nanoparticles. Also, the flattened shape of the FC curve provides evidence for the existence of magnetic inter-particle interactions. Sample D40 displays a TB of about 30 K, which corresponds to the small particle sizes confirmed in TEM measurements.A qualitative assessment of the particle size distribution can be found from the difference in temperatures between the peak position of the ZFC curve and the splitting temperature between ZFC and FC curves.60,61 Sample C71 with a TB of 180 K would correspond to a particle size of 10–15 nm, with a surprisingly narrow size distribution considering the shape of the ZFC/FC curves and the qualitative difference in temperature. However, such an interpretation would be in direct contradiction to the TEM analysis, which reveals a large polydispersity of the single nanoparticle cores. In addition, the blocking temperature from the ZFC curve would correspond to much smaller sizes of particles than obtained in SAXS. Therefore, further studies with small-angle neutron scattering are necessary to obtain the coherent magnetic size of the clusters and clarify the ZFC/FC results.
3.4. Magnetic size determination
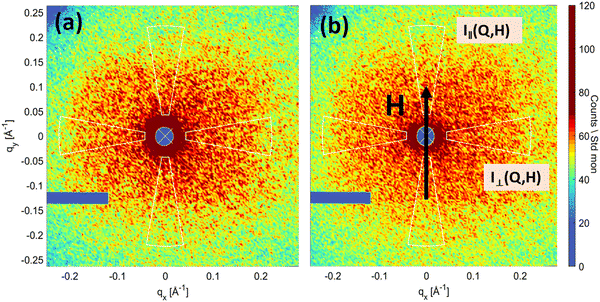
The scattering curves of the sample with high D2O content between 0% and 80% indicate the presence of large structures, which prevents a simple Guinier analysis. In comparison to SAXS data (see Fig. 2a), the SANS curves point to an advanced aggregation in the sample, which occurred during the period between the preparation of contrast variation samples and the measurement (approximately 6 months). We believe that this developed aggregation does not influence the characterization of initial clusters of nanoparticles in the studied Q-range. A clearly observable feature in the curves for large D2O content (above 95%) is present in the mid-Q-range 0.02 < Q < 0.06 Å−1 (Fig. 6a). Although it was not possible to fully compensate for the nuclear signal, this feature is primarily a result of the magnetic scattering associated with single particles (or magnetic correlations between them) constituting the large clusters. It is important to note that the magnetic scattering contribution is independent of the nuclear contrast between the particle and solvent. Using pure H2O as a solvent results in a significant scattering length density contrast between the iron oxide nanoparticles and the water. Thus, the scattering intensity in this case results mainly from the nuclear signal, while the magnetic contribution is negligible.63 To separate the magnetic scattering from the residual nuclear scattering in D2O, the H2O curve is divided by a factor of 131 and subtracted from the 100% D2O curve. The scaling factor is determined manually and approximately corresponds to the ratio of particle contrasts, Δρ2, in H2O and D2O solvents. Its exact determination is difficult due to uncertainties in the determination of the average SLD of the particles, as we could not observe the zero-angle scattering intensity I(0). A Guinier region in the difference curve appears as shown in Fig. 6b and can be attributed to magnetic spherical objects with a  (Fig. 6b (inset)). This size is in agreement with the obtained TB from the ZFC data, which corresponds to a contribution of single nanoparticles (or their magnetic correlations) in the clusters.
(Fig. 6b (inset)). This size is in agreement with the obtained TB from the ZFC data, which corresponds to a contribution of single nanoparticles (or their magnetic correlations) in the clusters.
4. Conclusion
The study investigated the impact of three types of coating materials on the structure, magnetic properties, and long-term stability of biocompatible iron oxide nanoparticles. The coating types are citrate, (3-aminopropyl) triethoxysilane (APTES), and dextran. The study's findings indicate that the type of coating significantly influences the organization of nanoparticles into distinct structures. The SAXS results showed different cluster sizes associated with each type of coating: 36 nm for dextran, 56 nm for citrate, and fractal cluster aggregates for APTES. The underlying particle core sizes are hereby: 2 nm for dextran, 9.5 nm for citrate, and 9.8 nm for APTES. In addition, the oxidation rates of the iron oxide cores were also studied for all three types of coatings. All systems exhibited rapid oxidation after less than 0.1 days (the time between the end of the synthesis and the sealing of the samples under N2 atmosphere). This led to the complete oxidation of the cores to maghemite for the dextran coating, while the citrate and APTES coating showed slower oxidation with 10–20% magnetite fraction after one month. After 3 months, the magnetite fraction is not detectable neither in Mössbauer spectroscopy nor in magnetometry. The varying oxidation behaviours can be attributed to differences in particle size resulting from the used coating agent and the synthesis route. Magnetic SANS at zero field on the citrate-coated nanoparticles revealed the magnetic size, which agrees with the ZFC data. The zero-field magnetic size is slightly larger than the size of a single nanoparticle obtained from TEM. At saturation field, the magnetic size of the clusters is increased, leading to approximately half the cluster size, corroborating the presence of magnetic domains inside the clusters. Overall, it is of great importance to be able to control the desired material properties through the choice of coating and the route of synthesis. In addition, the detailed knowledge of the aging processes of the particles is of equally large importance for officially approved quality standards. Such knowledge can guide the development of more stable nanoparticles that retain their magnetic properties for extended periods of time. Magnetic susceptibility, which, among other properties, depends mainly on the cluster size and core spacing, has a direct impact e.g. on the T2 relaxation time in Magnetic Resonance Imaging (MRI) and on the specific absorption rate (SAR) for hyperthermia applications. It was, for instance, found that in chains of particles, the SAR might increase compared to single particles.65 Consequently, a detailed knowledge of the particle arrangement and its impact on the magnetic properties can help in tuning the synthesis route to obtain the desired characteristics of the nanoparticle systems.Author contributions
Amal Nasser: planning the research study, sample preparation, performing SAXS and SANS experiments, data collection and analysis, writing the manuscript. Asmaa Qdemat & Baohu Wu: performing SAXS experiment and reducting the data. Harald Unterweger & Rainer Tietze: synthesis of samples, discussing the project motivations and the obtained results. Xiao Sun: helping in designing the SAXS experiments and data reduction. Joachim Landers: Mössbauer data analysis, performing mössbauer spectroscopy experiments with Juri Kopp. Marie-Sousai Appavou: performing cryo-TEM experiment. Elliot Paul Gilbert: performing SANS experiment and helping in data reduction assisted with Anastasiia Murmiliuk & Asmaa Qdemat. Artem Feoktystov & Oleg Petracic: supervision, project administration, planning the research work, assisting in data reduction and analysis, discussion, helping in manuscript preparation. All authors read, edited, and approved the final manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is funded by the German Federal Ministry of Education and Research in the framework of the Palestinian-German Science Bridge (PGSB) and by DFG grant LA 5175/1-1. The authors acknowledge the support of the Australian Centre for Neutron Scattering, ANSTO, and the Australian Government through the National Collaborative Research Infrastructure Strategy, supporting the QUOKKA neutron research infrastructure used in this work via ACNS proposal P14278.References
- H. Ittrich, K. Peldschus, N. Raabe, M. Kaul and G. Adam, Superparamagnetic iron oxide nanoparticles in biomedicine: applications and developments in diagnostics and therapy, Fortschr. Röntgenstr., 2013, 185(12), 1149–1166 CrossRef CAS PubMed.
- S. M. Dadfar, K. Roemhild, N. I. Drude, S. von Stillfried, R. Knüchel and F. Kiessling, et al., Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications, Adv. Drug Delivery Rev., 2019, 138, 302–325 CrossRef CAS PubMed.
- P. B. Santhosh and N. P. Ulrih, Multifunctional superparamagnetic iron oxide nanoparticles: promising tools in cancer theranostics, Cancer Lett., 2013, 336(1), 8–17 CrossRef CAS PubMed.
- S. Mornet, S. Vasseur, F. Grasset and E. Duguet, Magnetic nanoparticle design for medical diagnosis and therapy, J. Mater. Chem., 2004, 14(14), 2161–2175 RSC.
- A. Lindemann, K. Lüdtke-Buzug, B. M. Fräderich, K. Gräfe, R. Pries and B. Wollenberg, Biological impact of superparamagnetic iron oxide nanoparticles for magnetic particle imaging of head and neck cancer cells, Int. J. Nanomed., 2014, 5025–5040 CrossRef PubMed.
- R. Tietze, J. Zaloga, H. Unterweger, S. Lyer, R. P. Friedrich and C. Janko, et al., Magnetic nanoparticle-based drug delivery for cancer therapy, Biochem. Biophys. Res. Commun., 2015, 468(3), 463–470 CrossRef CAS PubMed.
- S. Laurent, S. Dutz, U. O. Häfeli and M. Mahmoudi, Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles, Adv. Colloid Interface Sci., 2011, 166(1–2), 8–23 CrossRef CAS PubMed.
- V. Patsula, M. Moskvin, S. Dutz and D. Horák, Size-dependent magnetic properties of iron oxide nanoparticles, J. Phys. Chem. Solids, 2016, 88, 24–30 CrossRef CAS.
- A. G. Roca, L. Gutiérrez, H. Gavilán, M. E. F. Brollo, S. Veintemillas-Verdaguer and M. del Puerto Morales, Design strategies for shape-controlled magnetic iron oxide nanoparticles, Adv. Drug Delivery Rev., 2019, 138, 68–104 CrossRef CAS PubMed.
- T. Köhler, A. Feoktystov, O. Petracic, E. Kentzinger, T. Bhatnagar-Schöffmann and M. Feygenson, et al., Mechanism of magnetization reduction in iron oxide nanoparticles, Nanoscale, 2021, 13(14), 6965–6976 RSC.
- M. Benitez, D. Mishra, P. Szary, G. B. Confalonieri, M. Feyen and A. Lu, et al., Structural and magnetic characterization of self-assembled iron oxide nanoparticle arrays, J. Phys.: Condens. Matter, 2011, 23(12), 126003 CrossRef CAS PubMed.
- O. Petracic, X. Chen, S. Bedanta, W. Kleemann, S. Sahoo and S. Cardoso, et al., Collective states of interacting ferromagnetic nanoparticles, J. Magn. Magn. Mater., 2006, 300(1), 192–197 CrossRef CAS.
- C. Vasilescu, M. Latikka, K. D. Knudsen, V. M. Garamus, V. Socoliuc and R. Turcu, et al., High concentration aqueous magnetic fluids: structure, colloidal stability, magnetic and flow properties, Soft Matter, 2018, 14(32), 6648–6666 RSC.
- M. Mühlberger, C. Janko, H. Unterweger, R. P. Friedrich, B. Friedrich and J. Band, et al., Functionalization of T lymphocytes with citrate-coated superparamagnetic iron oxide nanoparticles for magnetically controlled immune therapy, Int. J. Nanomed., 2019, 8421–8432 CrossRef PubMed.
- M. Escoda-Torroella, C. Moya, A. F. Rodrguez, X. Batlle and A. Labarta, Selective control over the morphology and the oxidation state of iron oxide nanoparticles, Langmuir, 2020, 37(1), 35–45 CrossRef PubMed.
- T. Krasia-Christoforou, V. Socoliuc, K. D. Knudsen, E. Tombácz, R. Turcu and L. Vékás, From single-core nanoparticles in ferrofluids to multi-core magnetic nanocomposites: Assembly strategies, structure, and magnetic behavior, Nanomaterials, 2020, 10(11), 2178 CrossRef CAS PubMed.
- L. Gutiérrez, R. Costo, C. Grüttner, F. Westphal, N. Gehrke and D. Heinke, et al., Synthesis methods to prepare single-and multi-core iron oxide nanoparticles for biomedical applications, Dalton Trans., 2015, 44(7), 2943–2952 RSC.
- A. Gallo-Cordova, J. G. Ovejero, A. M. Pablo-Sainz-Ezquerra, J. Cuya, B. Jeyadevan and S. Veintemillas-Verdaguer, et al., Unravelling an amine-regulated crystallization crossover to prove single/multicore effects on the biomedical and environmental catalytic activity of magnetic iron oxide colloids, J. Colloid Interface Sci., 2022, 608, 1585–1597 CrossRef CAS PubMed.
- L. Gutiérrez, L. De la Cueva, M. Moros, E. Mazaro, S. De Bernardo and J. M. De la Fuente, et al., Aggregation effects on the magnetic properties of iron oxide colloids, Nanotechnology, 2019, 30(11), 112001 CrossRef PubMed.
- H. Shokrollahi, A review of the magnetic properties, synthesis methods and applications of maghemite, J. Magn. Magn. Mater., 2017, 426, 74–81 CrossRef CAS.
- D. Maity and D. Agrawal, Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media, J. Magn. Magn. Mater., 2007, 308(1), 46–55 CrossRef CAS.
- R. Grau-Crespo, A. Y. Al-Baitai, I. Saadoune and N. H. De Leeuw, Vacancy ordering and electronic structure of γ -Fe2O3 (maghemite): a theoretical investigation, J. Phys.: Condens. Matter, 2010, 22(25), 255401 CrossRef PubMed.
- B. D. Cullity and C. D. Graham, Introduction to Magnetic Materials, John Wiley & Sons, 2011, pp. 115–150 Search PubMed.
- G. C. Lavorato, A. A. de Almeida, C. Vericat and M. H. Fonticelli, Redox phase transformations in magnetite nanoparticles: impact on their composition, structure and biomedical applications, Nanotechnology, 2023, 34(19), 192001 CrossRef PubMed.
- K. Jiang, Q. Zhang, D. T. Hinojosa, L. Zhang, Z. Xiao and Y. Yin, et al., Controlled oxidation and surface modification increase heating capacity of magnetic iron oxide nanoparticles, Appl. Phys. Rev., 2021, 8, 031407 CAS.
- C. Dennis, A. Jackson, J. Borchers, P. Hoopes, R. Strawbridge and A. Foreman, et al., Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia, Nanotechnology, 2009, 20(39), 395103 CrossRef CAS PubMed.
- L. Li, K. Y. Mak, C. W. Leung, K. Y. Chan, W. K. Chan and W. Zhong, et al., Effect of synthesis conditions on the properties of citric-acid coated iron oxide nanoparticles, Microelectron. Eng., 2013, 110, 329–334 CrossRef CAS.
- H. Unterweger, R. Tietze, C. Janko, J. Zaloga, S. Lyer and S. Dürr, et al., Development and characterization of magnetic iron oxide nanoparticles with a cisplatin-bearing polymer coating for targeted drug delivery, Int. J. Nanomed., 2014, 3659–3676 CrossRef CAS PubMed.
- B. Friedrich, S. Lyer, C. Janko, H. Unterweger, R. Brox and S. Cunningham, et al., Scavenging of bacteria or bacterial products by magnetic particles functionalized with a broad-spectrum pathogen recognition receptor motif offers diagnostic and therapeutic applications, Acta Biomater., 2022, 141, 418–428 CrossRef CAS PubMed.
- H. Unterweger, L. Dézsi, J. Matuszak, C. Janko, M. Poettler and J. Jordan, et al., Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: Evaluation of size-dependent imaging properties, storage stability and safety, Int. J. Nanomed., 2018, 13, 1899 CrossRef CAS PubMed.
- W. Mekseriwattana, P. Guardia, B. T. Herrero, J. M. de la Fuente, C. Kuhakarn and A. Roig, et al., Riboflavin-citrate conjugate multicore SPIONs with enhanced magnetic responses and cellular uptake in breast cancer cells, Nanoscale Adv., 2022, 4(8), 1988–1998 RSC.
- M. S. Khan, B. J. Gowda, N. Nasir, S. Wahab, M. R. Pichika and A. Sahebkar, et al., Advancements in dextran-based nanocarriers for treatment and imaging of breast cancer, Int. J. Pharm., 2023, 123276 CrossRef CAS PubMed.
- G. B. Confalonieri, V. Vega, A. Ebbing, D. Mishra, P. Szary and V. M. Prida, et al., Template-assisted self-assembly of individual and clusters of magnetic nanoparticles, Nanotechnology, 2011, 22(28), 285608 CrossRef PubMed.
- L. Lartigue, P. Hugounenq, D. Alloyeau, S. P. Clarke, M. Lévy and J. C. Bacri, et al., Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents, ACS Nano, 2012, 6(12), 10935–10949 CrossRef CAS PubMed.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic and L. Vander Elst, et al., Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications, Chem. Rev., 2008, 108(6), 2064–2110 CrossRef CAS PubMed.
- R. Massart, Preparation of aqueous magnetic liquids in alkaline and acidic media, IEEE Trans. Magn., 1981, 17(2), 1247–1248 CrossRef.
- N. Saxena, H. Agraval, K. C. Barick, D. Ray, V. K. Aswal and A. Singh, et al., Thermal and microwave synthesized SPIONs: Energy effects on the efficiency of nano drug carriers, Mater. Sci. Eng., C, 2020, 111, 110792 CrossRef CAS PubMed.
- H. C. Roth, S. P. Schwaminger, M. Schindler, F. E. Wagner and S. Berensmeier, Influencing factors in the CO-precipitation process of superparamagnetic iron oxide nano particles: A model based study, J. Magn. Magn. Mater., 2015, 377, 81–89 CrossRef CAS.
- S. Webers, M. Hess, J. Landers, A. M. Schmidt and H. Wende, Effect of phase transitions in polymer solutions on the magnetic response of embedded nanoparticles, ACS Appl. Polym. Mater., 2020, 2(7), 2676–2685 CrossRef CAS.
- E. P. Gilbert, J. C. Schulz and T. J. Noakes, ‘Quokka’-the small-angle neutron scattering instrument at OPAL, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 385, 1180–1182 CrossRef.
- K. Wood, J. P. Mata, C. J. Garvey, C. M. Wu, W. A. Hamilton and P. Abbeywick, et al., QUOKKA, the pinhole small-angle neutron scattering instrument at the OPAL Research Reactor, Australia: design, performance, operation and scientific highlights, J. Appl. Crystallogr., 2018, 51(2), 294–314 CrossRef CAS.
- S. R. Kline, Reduction and analysis of SANS and USANS data using IGOR Pro, J. Appl. Crystallogr., 2006, 39(6), 895–900 CrossRef CAS.
- D. Eberbeck, C. L. Dennis, N. F. Huls, K. L. Krycka, C. Gruttner and F. Westphal, Multicore magnetic nanoparticles for magnetic particle imaging, IEEE Trans. Magn., 2012, 49(1), 269–274 Search PubMed.
- M. V. Avdeev, E. Dubois, G. Mériguet, E. Wandersman, V. M. Garamus and A. V. Feoktystov, et al., Small-angle neutron scattering analysis of a water-based magnetic fluid with charge stabilization: contrast variation and scattering of polarized neutrons, J. Appl. Crystallogr., 2009, 42(6), 1009–1019 CrossRef CAS.
- S. Mühlbauer, D. Honecker, É. A. Périgo, F. Bergner, S. Disch and A. Heinemann, et al., Magnetic small-angle neutron scattering, Rev. Mod. Phys., 2019, 91(1), 015004 CrossRef.
- U. von Hörsten, 2023, https://udue.de/pi.
- G. Beaucage, Small-angle scattering from polymeric mass fractals of arbitrary mass-fractal dimension, J. Appl. Crystallogr., 1996, 29(2), 134–146 CrossRef CAS.
- A. Y. Cherny, E. M. Anitas, V. A. Osipov and A. I. Kuklin, The structure of deterministic mass and surface fractals: Theory and methods of analyzing small-angle scattering data, Phys. Chem. Chem. Phys., 2019, 21(24), 12748–12762 RSC.
- D. Svergun, Determination of the regularization parameter in indirect-transform methods using perceptual criteria, J. Appl. Crystallogr., 1992, 25(4), 495–503 CrossRef CAS.
- D. Peddis, N. Yaacoub, M. Ferretti, A. Martinelli, G. Piccaluga and A. Musinu, et al., Cationic distribution and spin canting in CoFe2O4 nanoparticles, J. Phys.: Condens. Matter, 2011, 23(42), 426004 CrossRef CAS PubMed.
- J. Landers, F. Stromberg, M. Darbandi, C. Schöppner, W. Keune and H. Wende, Correlation of superparamagnetic relaxation with magnetic dipole interaction in capped iron-oxide nanoparticles, J. Phys.: Condens. Matter, 2014, 27(2), 026002 CrossRef PubMed.
- X. Batlle, N. Pérez, P. Guardia, O. Iglesias, A. Labarta and F. Bartolomé, et al., Magnetic nanoparticles with bulklike properties, J. Appl. Phys., 2011, 109(7), 07B524 CrossRef.
- A. Lak, S. Disch and P. Bender, Embracing defects and disorder in magnetic nanoparticles, Adv. Sci., 2021, 8(7), 2002682 CrossRef CAS PubMed.
- M. Coduri, P. Masala, L. Del Bianco, F. Spizzo, D. Ceresoli and C. Castellano, et al., Local structure and magnetism of Fe2O3 maghemite nanocrystals: The role of crystal dimension, Nanomaterials, 2020, 10(5), 867 CrossRef CAS PubMed.
- M. P. Morales, S. Veintemillas-Verdaguer, M. Montero, C. Serna, A. Roig and L. Casas, et al., Surface and internal spin canting in γ-Fe2O3 nanoparticles, Chem. Mater., 1999, 11(11), 3058–3064 CrossRef CAS.
- O. Petracic, Superparamagnetic nanoparticle ensembles, Superlattices Microstruct., 2010, 47(5), 569–578 CrossRef CAS.
- B. D. Plouffe, D. K. Nagesha, R. S. DiPietro, S. Sridhar, D. Heiman and S. K. Murthy, et al., Thermomagnetic determination of Fe3O4 magnetic nanoparticle diameters for biomedical applications, J. Magnetism Magnetic Mater., 2011, 323(17), 2310–2317 CrossRef CAS.
- N. Nandakumaran, L. Barnsley, A. Feoktystov, S. A. Ivanov, D. L. Huber and L. S. Fruhner, et al., Unravelling magnetic nanochain formation in dispersion for in vivo applications, Adv. Mater., 2021, 33(24), 2008683 CrossRef CAS PubMed.
- R. Frison, G. Cernuto, A. Cervellino, O. Zaharko, G. M. Colonna and A. Guagliardi, et al., Magnetite– ite nanoparticles in the 5–15 nm range: correlating the core–shell composition and the surface structure to the magnetic properties. A total scattering study, Chem. Mater., 2013, 25(23), 4820–4827 CrossRef CAS.
- S. Bedanta, O. Petracic and W. Kleemann, Handbook of magnetic materials, Elsevier, 2015, vol. 23, pp. 1–83 Search PubMed.
- K. Mandel, F. Hutter, C. Gellermann and G. Sextl, Stabilisation effects of superparamagnetic nanoparticles on clustering in nanocomposite microparticles and on magnetic behaviour, J. Magn. Magn. Mater., 2013, 331, 269–275 CrossRef CAS.
- H. Stuhrmann, Neutron small-angle scattering of biological macromolecules in solution, J. Appl. Crystallogr., 1974, 7(2), 173–178 CrossRef CAS.
- M. V. Avdeev and V. L. Aksenov, Small-angle neutron scattering in structure research of magnetic fluids, Phys.-Usp., 2010, 53(10), 971 CrossRef CAS.
- C. Moya, M. Escoda-Torroella, J. Rodríguez-Álvarez, A. I. Figueroa, Í. García and I. B. Ferrer-Vidal, et al., Unveiling the crystal and magnetic texture of iron oxide nanoflowers, Nanoscale, 2024, 16(4), 1942–1951 RSC.
- C. Martinez-Boubeta, K. Simeonidis, A. Makridis, M. Angelakeris, O. Iglesias and P. Guardia, et al., Learning from nature to improve the heat generation of iron-oxide nanoparticles for magnetic hyperthermia applications, Sci. Rep., 2013, 3(1), 1652 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Histogram and lognormal fit for the distribution of individual particles as determined by TEM, FTIR spectra, the Mössbauer spectra for the B47 and D40 samples after different aging times of exposure to air, table of hydrodynamic size, polydispersity index (PDI) and zeta potential (mV), FTIR spectra, as well as ZFC/FC curves and SAXS curves for the fresh sample and after 6 months following preparation for the C71 sample. See DOI: https://doi.org/10.1039/d4cp01735h |
| This journal is © the Owner Societies 2024 |