Open Access Article
Open Access ArticleThe SCAN+U method in the investigation of complex transition metal oxides: a case study on YSr2Cu2FeO7+δ (δ = 0, 1)
Marianela
Gómez-Toledo
 and
Elena M.
Arroyo-de Dompablo
and
Elena M.
Arroyo-de Dompablo
 *
*
Departamento de Química Inorgánica, Universidad Complutense de Madrid, 28040 Madrid, Spain. E-mail: e.arroyo@quim.ucm.es
First published on 10th June 2024
Abstract
Assessment of DFT methods is essential to sustain reliability in the computational investigation of complex transition metal oxides. This work evaluates the performance of the strongly constrained and appropriately normed (SCAN) functional and its extended Hubbard-U methodology (SCAN+U) to model the YSr2Cu2FeO7+δ (0 < δ < 1) perovskite-based system. The influence of the individual UCu and UFe Hubbard parameters (0 < U < 4 eV) on the calculated electronic, magnetic and crystal structures of the end members δ = 1 (metallic) and δ = 0 (insulating) is analyzed. The introduction of the U-correction terms enhances the reproduction of the crystal structures, with a UCu value of 1 eV improving the band gap accuracy for the YSr2Cu2FeO7 phase, while maintaining the metallic characteristics of YSr2Cu2FeO8. At a fixed UCu value, the results are almost insensitive to the UFe value used in the calculations. The findings emphasize that for oxides containing several TM ions, the optimal UTM values may differ from those of the simple TM oxides.
Introduction
Transition metal (TM) oxides are one of the most interesting classes of solids, exhibiting excellent properties and applications in a wide variety of fields. Methods based on density functional theory (DFT) play a central role in designing advanced functional oxides. They enable the identification of promising materials through property prediction and facilitate the understanding of the underlying structure–composition–property relationships. A current challenge is selecting a DFT methodology capable of modelling complex TM oxides with strongly interacting d-electrons. The GGA + U method has been extensively used in the last few decades; to the GGA exchange–correlation functional, a TM-specific Hubbard U parameter is added describing the on-site Coulomb interaction between localized electrons. However, progress in the modelling of correlated TM oxides requires testing and evaluating advanced exchange–correlation functionals.The recently developed strongly constrained and appropriately normed (SCAN) meta-GGA functional1 has demonstrated a superior performance in reproducing the fundamental physical properties and phenomena associated with correlated oxides.2–8 Furthermore, studies have shown that incorporating the SCAN functional into the DFT+U framework (SCAN+U) can enhance the accuracy in reproducing certain physical properties of transition metal oxides.9–13 For example, Carter and coworkers demonstrated that the introduction of the Hubbard term improves the reproduction of the ground-state properties of binary 3d TM oxides, being critical for the prediction of the most stable polymorphs of Mn2O3 and Fe3O4.9,10 The SCAN+U method has also been used to evaluate the formation energy of anion vacancies, which are highly involved in properties related to energy applications, of ABO3 perovskites with various A and B (TM) elements and diverse electronic structures.11 For BiFeO3 polymorphs, the effect of Fe-site doping on the phase stability, as well as on the structural and ferroelectric properties, has been assessed.12 For oxides containing two TM ions, double Nb-perovskites (Sr2SmNbO6, Sr2CoNbO6 and Ba2CoNbO6) have been investigated,14 although the authors dropped the treatment of correlation in Nb to simplify the analysis.
Assessing the performance of the SCAN+U method in modelling complex transition metal oxides represents a crucial step forward in the predictive design of functional oxides, especially defying for oxides containing different TM ions. This study focuses on the SCAN+U method in the investigation of complex oxides YSr2Cu2FeO7+δ, for which the SCAN functional correctly captures the metal-to-insulating transition as a function of the oxygen content.8 The perovskite-related crystal structure of the YSr2Cu2FeO7+δ system (Fig. 1) can be referred to the YBaCuO-type structure with the replacement of one layer of Cu atoms with Fe.15,16 The YSr2Cu2FeO8 (δ = 1) idealized compound, with [FeO6] octahedral units in the FeO2 layers (Fig. 1a), is a metallic oxide with a superconducting transition at a Tc of 70 K.17 Lowering the oxygen content creates vacancies in the O3 positions of the FeO1+δ layer, which leads to a tetrahedral coordination for all Fe atoms in the idealized YSr2Cu2FeO7 (δ = 0) compound (Fig. 1b). The synthetized compounds with δ ∼ 0 are insulators with mainly Fe3+ and Cu2+ ions.8,16,18,19
The combination of Cu and Fe ions in different oxidations states, and the evolution of the electronic properties of the YSr2Cu2FeO7+δ series, provides an excellent framework to examine the applicability of the SCAN+U approach in the DFT investigation of complex oxides. This study delves into the effects of UCu and UFe correction terms on the crystallographic, magnetic and electronic structures of the idealized end-members of YSr2Cu2FeO7+δ (δ = 0 and 1). By conducting calculations with individual U terms ranging from 0 to 4 eV, we determine and discuss the appropriate set of U values for accurately describing the system. A relevant question arises regarding the transferability of UTM parameters from simple oxides to their combinations in complex TM oxides. We will demonstrate that such transferability cannot be guaranteed.
Methodology
Calculations for the ideal stoichiometric end-members of the YSr2Cu2FeO7+δ family, YSr2Cu2FeO8 and YSr2Cu2FeO7, have been performed using the ab initio total energy and molecular dynamics program VASP (Vienna Ab initio Simulation Package) developed at the Universität Wien.20,21 The interaction of core electrons with the nuclei is described using the projector augmented wave (PAW) method22 with 2s22p4 of O, 3s23p63d74s1 of Fe, 3p63d104p1 of Cu, 4s24p65s2 of Sr, and 4s24p64d15s2 of Y being treated as valence electrons. The energy cut-off for the plane wave basis set was fixed at a constant value of 600 eV throughout the calculations. The integration in the Brillouin zone was done on appropriate k-point sets determined by the Monkhorst–Pack scheme. The k-point meshes were set at 2 × 8 × 8 for YSr2Cu2FeO7 and 10 × 10 × 4 for YSr2Cu2FeO8, using a Gaussian smearing parameter of 0.05 eV. For the density of states (DOS) calculations, the tetrahedron method with Bloch corrections23 was used. Self-consistency was achieved with a tolerance in total energy of 1 × 10−4 eV for geometry optimization and 1 × 10−6 eV for DOS calculations.A local Hubbard-U (SCAN+U) was added to Fe and Cu atoms, following the simplified rotationally invariant framework developed by Dudarev et al.24 Typically, U is formulated as Ueff = U − J, where U is the onsite Coulomb term and J is the exchange term. In this work, this effective parameter is simply referred as U. The J value was fixed to 1 eV. Effective U values between 0 and 4 eV were used for the 3d orbitals of Cu and Fe. It should be noted that a value of 4 eV for Fe and Cu was found appropriate in GGA + U investigations.25–27 The local magnetic moments were taken from the difference between the projected electron density of up and down spins onto a 1 Å ionic radius sphere. Bader charge analysis28 was performed on the charge density files29 using the pymatgen package.30
The crystallographic models of YSr2Cu2FeO8 and YSr2Cu2FeO7 were, respectively, constructed taking the initial positions from YSr2Cu2FeO7.86 (ICSD file 11514)17 and YSr2Cu2GaO7 (ICSD file 71263).31 For the metallic YSr2Cu2FeO7.86, López et al.17 found a magnetic structure consisting of in-plane FM coupling within the FeO1+δ layers and a weak AFM coupling between layers along the c-axis (A-type AFM ordering). Due to the soft character of the inter-plane magnetic interactions (TN ∼ 20 K32), subsequent DFT calculations utilized a ferromagnetic (FM) structure to model the idealized YSr2Cu2FeO8,8 which is also employed in the present study. Investigation of the idealized YSr2Cu2FeO7 is carried out in the antiferromagnetic ground state (C-type AFM) and the metastable ferromagnetic structure (FM). Additional SCAN+U calculations have been conducted with the maximum U values tested (UCu = UFe = 4 eV) using the A-type AFM magnetic ordering for YSr2Cu2FeO7+δ with δ = 0 and 1. More details of magnetic structures are given in ref. 8.
Results
The experimental lattice parameters and bond distances of YSr2Cu2FeO7+δ (δ = 0,1) crystal structures are reproduced well using both SCAN and SCAN+U methods, with errors below 3%. For conciseness, Table 1 lists only the values for SCAN+U with the highest U values tested, 4 eV for Fe and Cu. Noteworthily, introducing the U correction term reduces the discrepancies between the theory and experimental structural data. For instance, within the SCAN+U approach, the calculated unit cell volumes (errors of 0.9% and 0.7% for YSr2Cu2FeO8 and YSr2Cu2FeO7, respectively) are closer to the experimental values than those within the SCAN approach (errors of 2% and 1.2% for YSr2Cu2FeO8 and YSr2Cu2FeO7, respectively). Since the Cu-3d states play a critical role in the metal-to-insulating transition in high-Tc cuprate superconductors,33 the Cu–O distances are analyzed in detail. Fig. 2 shows the calculated Cu–O1 (apical oxygen) and Cu–O2 (equatorial oxygen) distances as a function of the UCu and UFe values. The results show that, for both YSr2Cu2FeO8 and YSr2Cu2FeO7, increasing UCu results in a better agreement with the experimental available values. It can be seen in Fig. 2a that, for the metallic phase YSr2Cu2FeO8, at a fixed UCu, applying UFe produces a decrease in d Cu-O1 (a maximum variation of 4% for UCu = 0) and a subtle increase in d Cu–O2 (a maximum variation of 0.7% at UCu = 0). On the other hand, for the C-type AFM-YSr2Cu2FeO7, the Cu–O distance depends mostly on UCu. Hence, at a fixed UCu, changing UFe has little to no effect on the Cu–O bonding distances.| YSr2FeCu2O8 | Experimental | SCAN | SCAN+U | YSr2FeCu2O7 | Experimental | SCAN AFM-C | SCAN+U AFM-C | SCAN FM | SCAN+U FM |
|---|---|---|---|---|---|---|---|---|---|
| a | 3.8145(3) | 3.7861 | 3.7989 | a | 22.9241(8) | 22.8529 | 22.8568 | 22.9157 | 22.8640 |
| c | 11.327(7) | 11.2755 | 11.3116 | b | 5.4584(2) | 5.4411 | 5.4536 | 5.4365 | 5.4646 |
| V | 164.81(1) | 161.62 | 163.26 | c | 5.4080(2) | 5.3744 | 5.3893 | 5.3608 | 5.3956 |
| V per atom | 11.77 | 11.54 | 11.66 | V | 676.70 | 668.22 | 671.79 | 675.96 | 674.14 |
| d Fe–O3 | 1.9072(2) | 1.8930 | 1.8994 | V per atom | 12.89 | 12.85 | 12.91 | 13.00 | 12.96 |
| d Fe–O1 | 1.8434(4) | 1.8417 | 1.8591 | Mean d Fe–O | 1.8757 | 1.8732 | 1.8888 | 1.8880 | 1.8987 |
| d Fe–O1/d Fe–O3 | 0.9663 | 0.9729 | 0.9788 | Fe–O3–Fe | 125.5° | 124.5° | 123.2° | 120.1° | 121.0° |
| d Cu–O2 | 1.9244(4) | 1.9147 | 1.9232 | Mean d Cu–O2 | 1.9335 | 1.9237 | 1.9368 | 1.9207 | 1.9366 |
| d Cu–O1 | 2.117(4) | 2.0812 | 2.0738 | d Cu–O1 | 2.312(4) | 2.3449 | 2.3210 | 2.3600 | 2.3071 |
| d Cu–O1/d Cu–O2 | 1.1000 | 1.0870 | 1.0783 | d Cu–O1/d Cu–O2 | 1.1958 | 1.2148 | 1.2019 | 1.2288 | 1.1913 |
| d Cu–Cu | 3.407(4) | 3.4297 | 3.4459 | d Cu–Cu | 3.338(0) | 3.3095 | 3.3379 | 3.3119 | 3.3676 |
| d Cu–Fe | 3.960(3) | 3.9229 | 3.9329 | d Cu–Fe | 4.085(8) | 4.0815 | 4.0681 | 4.0946 | 4.0543 |
 | ||
| Fig. 2 Calculated Cu–O bond lengths (Å) within the SCAN+U approach as a function of UCu and UFe values for (a) YSr2Cu2FeO8 and (b) YSr2Cu2FeO7 idealized oxides. The experimental bond lengths are depicted as dashed blue lines. The PBE (UFe and UCu = 0 eV) and PBE+U (UFe and UCu = 4 eV) results from a previous investigation8 are denoted by blue and yellow asterisks, respectively. | ||
For the sake of completeness, Fig. 2 incorporates data from a preceding PBE and PBE+U (UCu = UFe = 4 eV) investigation.8 As previously discussed, the PBE functional tends to yield larger bonding lengths than the SCAN functional. Specifically, the Cu–O1 distance in the YSr2Cu2FeO7 oxide exhibits the most significant deviation from experimental values (5.7%). Introducing the U parameter to the PBE method results in changes in the Cu–O bond distances, with d Cu–O1 for δ = 0 decreasing and the others increasing, following the same trends observed with SCAN+U.
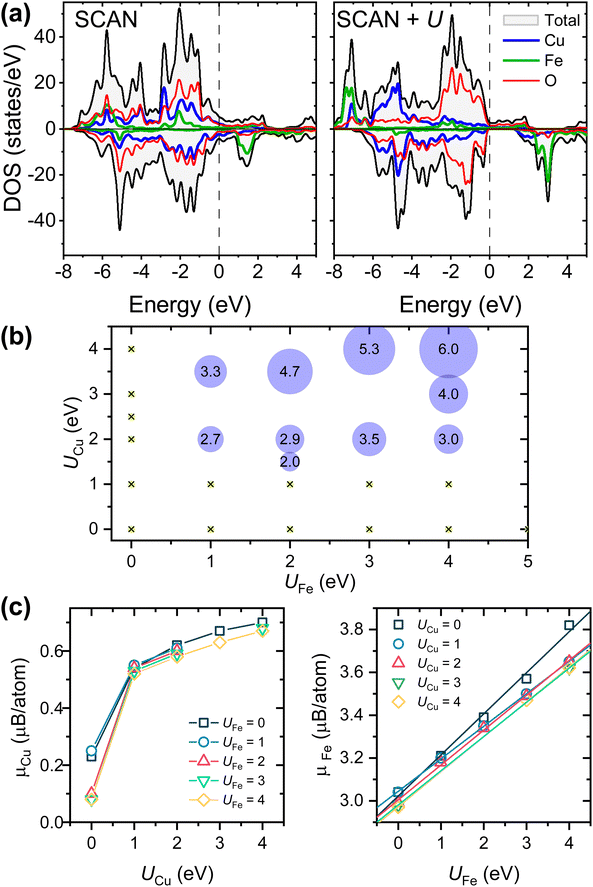
Regarding the electronic properties of YSr2Cu2FeO8, the SCAN functional correctly reproduces the itinerant electron character and metallic behavior of the oxide (calculated DOS in Fig. 3a). Applying the Hubbard correction (SCAN+U) produces a downshift of the occupied Fe/Cu-3d states, keeping the 3d orbitals atomic-like and diminishing their hybridization with the 2p-oxygen (less covalent TM–O bonding). This is clearly observed in Fig. 3a for the case with UCu and UFe = 4 eV. Moreover, since U penalizes the fractional occupation on metal ions, the electrons tend to localize in the majority spin in Cu-3d and Fe-3d orbitals and a band gap opens in the minority spin channel, resulting in a half-metal compound. Fig. 3b shows the dependence of the down-spin band gap with the UCu and UFe values employed in the SCAN+U calculations. Half-metallicity is largely influenced by the U value used for the Cu-3d states. When UCu < 1.0 eV, independent of the UFe tested (maximum 5 eV), the DOS corresponds to a metallic compound. For UCu > 1.0 eV and UFe > 0, an energy gap opens in the down spin channel, whose energy increases with larger UFe (see the band gap values in the circles). In addition to half-metallicity, the introduction of the U parameter, and associated electron localization, produces increasing local magnetic moments (Fig. 3c) that deviate from the experimental observations, that is, μ0(Fe) = 1.7–2 μB per ion and μ0(Cu) = 0 μB.17 In this regard, the introduction of the U term does not benefit property prediction.
Interestingly, as depicted in Fig. 3b and c, maintaining metallic characteristics in YSr2Cu2FeO8 is possible with combinations such as UCu = 0 eV and UFe = 5 eV, as well as UFe = 0 and UCu = 4 eV. This is to say, electron localization does not occur when either UCu or UFe is set to zero. Fig. 4 reveals the impact of increasing only UFe or UCu, while keeping the other U-value equal to zero. It can be inferred that since both Cu-3d and Fe-3d states contribute to the Fermi level, electron localization only occurs when both U-terms are greater than zero. Furthermore, the predominant contribution of Cu 3d-states at the Fermi level, characterized by a higher number of states compared to Fe 3d-states, results in UCu exerting a greater influence on the magnetic and electronic properties as well as on the Cu–O distances.
Fig. 5a shows the calculated SCAN-DOS for the idealized model of C-type AFM-YSr2Cu2FeO7. For this insulating oxide, the band gap extracted from diffuse reflectance spectroscopy (DRS) is in the range of 0.73–1.2 eV.8 As previously reported, the PBE+U (UCu and UFe = 4 eV) predicts a band gap of 0.85 eV, while the bare PBE fails to reproduce the insulating character of YSr2Cu2FeO7. The SCAN functional yields a band gap of 0.63 eV, which widens when the U term is applied (SCAN+U). With U = 4 eV for Fe and Cu (Fig. 5a), the band gap is as wide as 2.3 eV. Fig. 5b unveils how the band gap can be tuned by the choice of the U values. Noteworthily, the band gap depends linearly on UCu, but it is almost insensitive to the choice of UFe, similarly with the afore-mentioned trend in the calculated Cu–O distances in this oxide. The experimental band gap is reproduced for 0 < UCu < 1 eV. For UCu = 0 eV, appropriate values of UFe range from 1 to the maximum value tested (4 eV), whereas for UCu = 1 eV, any UFe value (including UFe = 0) yields the same band gap. Fig. 6 depicts the specific effects of UCu and UFe in the calculated DOS. While both, Cu and Fe-3d states, are significant parts of the top of the valence band, the bottom of the conduction band is formed exclusively by Cu-3d states. As UCu increases, these empty states shift up in energy, thereby opening the band gap.
 | ||
| Fig. 5 (a) Calculated total and atom-projected density of states for YSr2Cu2FeO7 in the C-type AFM configuration within the SCAN and SCAN+U approximation (Ueff = 4 eV for Cu and Fe). The Fermi level is set as the zero of energy. DOS units refer to the calculated cell. Up spin (or majority) and down spin (or minority) contributions are shown. Color code: total black, Cu blue, Fe green, and O red. (b) Dependence of the band gap for C-type AFM-YSr2Cu2FeO7 with the U values on Fe and Cu utilized in the SCAN+U calculations. The experimental band gap range is denoted by a gray region. The PBE (UFe and UCu = 0 eV) and PBE+U values (UFe and UCu = 4 eV) from a previous investigation8 are denoted by blue and yellow asterisks, respectively. | ||
Investigation of the hypothetical FM-YSr2Cu2FeO7 supports the minor impact of UFe in property prediction. The calculated DOSs for the FM configuration (shown in Fig. 7a), indicate that within the SCAN+U approach, the U term drives the oxide from a metallic state (as described by the SCAN) to an insulating state. Fig. 7b shows the band gap opening as a function of the UCu and UFe values used in the calculations. In the same way as in the C-type AFM-YSr2Cu2FeO7, the UCu dominates the band gap opening, being the critical value U = 2 eV. In the insulating state, the magnitude of the band gap is nearly insensitive to UFe; for instance, a band gap of 0.8 eV is obtained when UCu = 4 eV and UFe ranging from 0 to 4 eV. It should be noted that due to the lack of experimental data for FM-YSr2Cu2FeO7, it is not conceivable to extract an optimal U value to model its properties. FM-YSr2Cu2FeO7 is metastable relative to C-type AFM under any DFT approximation, with energy differences per formula unit of 0.248 eV (PBE),8 0.414 eV (SCAN),8 0.424 eV (PBE+U, UFe = UCu = 4 eV),8 and 0.330 eV (SCAN+U, UFe = UCu = 4 eV). It is therefore unlikely that such a phase could ever be prepared.
The stabilization of the C-type AFM magnetic structure in insulating YSr2Cu2FeO7 is attributed to local antiferromagnetic ordering within the Cu/Fe plane layer arising from superexchange interactions via oxygen bridges.8,34 In contrast, in-plane AFM ordering is not observed in the metallic YSr2Cu2FeO8, for which an A-type AFM magnetic structure is reported.17 Noteworthily, the inter-plane magnetic interactions are weak throughout the YSr2Cu2FeO7+δ system.8,17,32,34 This is consistent with the small energy differences found between the inter-plane ferromagnetic and antiferromagnetic couplings in YSr2Cu2FeO7 within the PBE, PBE+U and SCAN approximations.8 For this oxide, the SCAN+U (UFe = UCu = 4 eV) yields a negligible energy (ETotalFM – ETotalFM) difference of 0.004 eV per formula unit. Similarly, for the metallic YSr2Cu2FeO8, the A-type AFM configuration is only 0.018 eV per formula unit more stable than the FM configuration within the SCAN +U (UFe = UCu = 4 eV).
Discussion
The principal drawback of the DFT+U schemes lies in the selection of the adjustable parameter, U. While U values can be derived from self-consistent calculations,35 a common approach involves choosing these in order to match the experimental data, such as equilibrium volumes, formation energies, band gap values, transition pressures, intercalation voltages, and so on.10,25,26,36–38 It is impossible to pinpoint an exact and universally applicable value for the U parameter to accurately reproduce all material properties.37 In this study, suitable ranges of U values are determined through comparison with experimental data on crystalline, magnetic and electronic structures.The appropriate Hubbard U parameter varies depending on the nature and oxidation state of the transition metal (TM) as well as the crystal structure.25 YSr2Cu2FeO7+δ (δ = 0,1) shares the same structural type, and for both compounds incorporating a U correction improves the reproduction of lattice parameters and Cu–O bond lengths. In the YSr2Cu2FeO7+δ system, the oxygen content determines the oxidation states of Cu and Fe as well as the magnetic interactions. For the magnetic structures, the SCAN+U method performs equally well compared to the SCAN, PBE and PBE+U methods: they all capture the occurrence of in-plane AFM ordering for δ ∼ 0, and the soft character of the inter-plane interactions throughout the YSr2Cu2FeO7+δ system. These DFT-trends, even when extracted from the idealized oxides with δ = 0 and 1, are in good agreement with the observed magnetic properties of YSr2Cu2FeO7+δ.17,32,34
The primary impact of U is observed in the electronic properties. In the metallic phase YSr2Cu2FeO8, as expected, the U parameter penalizes electron delocalization, with UCu > 1 eV being detrimental for property prediction. On the other hand, for the insulating phase YSr2Cu2FeO7, the electron correlation is better captured with 0 < UCu < 1 eV and UFe =1–4 eV. Considering these findings, the YSr2Cu2FeO7+δ system is better described with UCu = 1 eV, which improves the accuracy of the band gap for the insulating C-type AFM-YSr2Cu2FeO7, while maintaining the metallic behavior of YSr2Cu2FeO8. Importantly, once UCu is set to 1 eV, varying UFe between 0 and 4 eV has a minor impact on the calculated metal/insulating behavior of YSr2Cu2FeO8/YSr2Cu2FeO7 (Fig. 3b and 5b). The predominant role of UCu is consistent with the specific contributions of Cu-3d and Fe-3d states on the valence and conduction bands of YSr2Cu2FeO7+δ.
In the DFT+U framework, there exists dependence of the appropriate U values on the XC-functional used. Despite the limited number of studies addressing the estimation of suitable U values within the SCAN+U approach, a widespread observation is that the optimal U values for electronic property prediction are notably lower than those within the PBE+U method.9,12,27 This remark is supported by comparing the current SCAN+U results for YSr2Cu2FeO7 with a previous investigation using PBE+U (Fig. 5b).8 In the PBE+U approach, applying Ueff values of ∼4 eV for Cu and Fe ions, a calculated band gap of 0.85 eV is achieved, which falls in the experimental range. However, using the same U values in the SCAN+U method significantly overestimates the band gap, widening it to 2.3 eV. Fig. 2 and 5b suggest that the calculated bonding distances and band gaps exhibit a linear trend with the U value, displaying similar slopes for both SCAN+U and PBE+U methods. Since the bare PBE and SCAN produce different values (either for band gaps or bond-lengths), the optimal U values matching the experimental data definitely depend on the chosen XC functional.
Finally, within the SCAN+U method, the optimal U values for modelling the YSr2Cu2FeO7+δ compounds can be compared to those previously reported for the simple Fe and Cu oxides. Previous studies have suggested that the optimal UFe value is around ∼3 eV for FeO/Fe2O3/Fe3O49 and BiFeO3.12 Carter et al. demonstrated that, while SCAN is sufficient to reproduce the crystal structure and formation energies of CuO and Cu2O, the proper simulation of the electronic structure requires the introduction of UCu at around ∼2 eV.10 However, applying the combination extracted from simple oxides (UCu = 2 eV and UFe = 3 eV), a poor description of the electronic properties of the YSr2Cu2FeO7+δ system is given. This combination leads to a half-metallic behavior (and electron localization) for YSr2Cu2FeO8, and band gaps that deviate from the experimental values for YSr2Cu2FeO7. In short, the appropriate U parameters found for the simple Cu/Fe oxides are not suitable for reproducing the properties of the complex oxides YSr2Cu2FeO7+δ. The differences between the optimal U-values for simple and complex TM oxides are linked to the specific contributions of the TM-d states in the electronic structure.
Conclusions
The SCAN functional effectively models the crystal structure and basic electronic and magnetic properties of complex oxides YSr2Cu2FeO7+δ (0 < δ < 1). Although adding a U correction on top is not necessary, it is found that applying a UCu value of 1 eV leads to a more accurate description of properties. In this case study, the Cu-3d states dominate, respectively, the Fermi level of YSr2Cu2FeO8 and the bottom of the conduction band of YSr2Cu2FeO7. Consequently, UCu has a greater impact than UFe in the calculated electronic properties and magnetic moments. Indeed, for a fixed UCu, the results are almost insensitive to UFe.This case study highlights the importance of analyzing the role of the individual U parameters for the reliable SCAN+U investigations of complex oxides containing several TM ions. The optimal U-values certainly depend on the material under investigation (chemical composition and crystal structure), and U-transferability among materials is particularly risky for such complex oxides. The appropriate combination of UTM terms may differ from the optimal values in the respective simple oxides. Therefore, the convenience of using the SCAN+U approach in the study of oxides containing several TM ions should be assessed for each material, considering whether the benefits in property prediction (compared to the SCAN functional) outweigh the efforts made in determining the optimal combination of individual UTM values.
Author contributions
Marianela Gómez-Toledo: investigation, methodology, software, data curation, validation, formal analysis, visualization, and writing – original draft. Elena M. Arroyo-de Dompablo: investigation, conceptualization, supervision, funding acquisition, project administration, and writing – original draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is part of the project ECSAWE-PID2022-139501OB-C22 funded by the MCIN/AEI/10.13039/501100011033 and by the “ERDF A way of making Europe”. The authors thank funding from the MCIN/AEI/10.13039/501100011033-“NextGenerationEU”/PRTR (project TED2021-130452B-C21) and from the Universidad Complutense de Madrid (FEI-EU-22-01-4129585). Computational resources from the MALTA–cluster (Universidad de Oviedo) and I2Basque are acknowledged. The authors are grateful to S.A. López-Paz and S.García-Martín for their valuable discussions and helpful insights into the experimental aspects of the YSr2Cu2FeO7+δ system.References
- J. Sun, A. Ruzsinszky and J. P. Perdew, Phys. Rev. Lett., 2015, 115, 036402 CrossRef PubMed.
- Y. Hinuma, H. Hayashi, Y. Kumagai, I. Tanaka and F. Oba, Phys. Rev. B, 2017, 96 Search PubMed.
- D. A. Kitchaev, H. Peng, Y. Liu, J. Sun, J. P. Perdew and G. Ceder, Phys. Rev. B, 2016, 93, 045132 CrossRef.
- J. W. Furness, Y. Zhang, C. Lane, I. G. Buda, B. Barbiellini, R. S. Markiewicz, A. Bansil and J. Sun, Commun. Phys., 2018, 1 CAS.
- J. Varignon, M. Bibes and A. Zunger, Phys. Rev. B, 2019, 100 Search PubMed.
- L. Iglesias, M. Bibes and J. Varignon, Phys. Rev. B, 2021, 104, 035123 CrossRef CAS.
- J. Kaczkowski, M. Pugaczowa-Michalska and I. Płowaś-Korus, J. Magn. Magn. Mater., 2022, 548, 168984 CrossRef CAS.
- M. Gómez-Toledo, S. A. López-Paz, S. García-Martín and M. E. Arroyo-de Dompablo, Inorg. Chem., 2023, 62, 3445–3456 CrossRef PubMed.
- G. S. Gautam and E. A. Carter, Phys. Rev. Mater., 2018, 2, 095401 CrossRef.
- O. Y. Long, G. S. Gautam and E. A. Carter, Phys. Rev. Mater., 2020, 4, 045401 CrossRef CAS.
- R. B. Wexler, G. S. Gautam, E. B. Stechel and E. A. Carter, J. Am. Chem. Soc., 2021, 143, 13212–13227 CrossRef CAS PubMed.
- J. Kaczkowski, M. Pugaczowa-Michalska and I. Plowas-Korus, Phys. Chem. Chem. Phys., 2021, 23, 8571–8584 RSC.
- M. Verma and R. Pentcheva, Phys. Rev. Res., 2022, 4 Search PubMed.
- Y. Zeng, Q. Hu, M. Pan, K. Zhang, S. Grasso, C. Hu and Q. Feng, Adv. Powder Mater., 2022, 1, 100019 CrossRef.
- J. Shimoyama, K. Otzschi, T. Hinouchi and K. Kishio, Physica C, 2000, 341, 563–564 CrossRef.
- T. Mochiku, Y. Nakano, A. Hoshikawa, S. Sato, K. Oikawa, T. Ishigaki, T. Kamiyama, K. Kadowaki and K. Hirata, Phys. C, 2004, 412, 115–119 CrossRef.
- S. A. Lopez-Paz, X. Martinez de Irujo-Labalde, J. Sanchez-Marcos, C. Ritter, E. Moran and M. A. Alario-Franco, Inorg. Chem., 2019, 58, 12809–12814 CrossRef CAS PubMed.
- J. E. H. Sansom, E. Kendrick, H. A. Rudge-Pickard, M. S. Islam, A. J. Wright and P. R. Slater, J. Mater. Chem., 2005, 15, 2321–2327 RSC.
- T. Mochiku, Y. Nakano, K. Oikawa, T. Kamiyama, H. Fujii, Y. Hata, I. Suzuki, I. Kakeya, K. Kadowaki and K. Hirata, Phys. C, 2003, 400, 43–52 CrossRef CAS.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- P. E. Bloch, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef PubMed.
- P. E. Blochl, O. Jepsen and O. K. Andersen, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 16223–16233 CrossRef CAS PubMed.
- S. L. Dudarev, G. A. Botton, S. Y. Savrasov, Z. Szotek, W. M. Temmerman and A. P. Sutton, Phys. Status Solidi A, 1998, 166, 429–443 CrossRef CAS.
- L. Wang, T. Maxisch and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 195107 CrossRef.
- M. E. Arroyo y de Dompablo, Y.-L. Lee and D. Morgan, Chem. Mater., 2010, 22, 906–913 CrossRef CAS.
- A. M. Ritzmann, J. M. Dieterich and E. A. Carter, Phys. Chem. Chem. Phys., 2016, 18, 12260–12269 RSC.
- R. F. W. Bader, Atoms in Molecules: A Quantum Theory, Oxford University Press, Oxford, UK, 1990 Search PubMed.
- G. Henkelman, A. Arnaldsson and H. Jónsson, Comput. Mater. Sci., 2006, 36, 354–360 CrossRef.
- S. P. Ong, W. D. Richards, A. Jain, G. Hautier, M. Kocher, S. Cholia, D. Gunter, V. L. Chevrier, K. A. Persson and G. Ceder, Comput. Mater. Sci., 2013, 68, 314–319 CrossRef CAS.
- G. Roth, P. Adelmann, G. Heger, R. Knitter and T. Wolf, J. Phys. I, 1991, 1, 721–741 CrossRef CAS.
- Y. Hata, Y. Mihara, T. Mochiku, J.-I. Suzuki, I. Kakeya, K. Kadowaki, E. Kita and H. Yasuoka, Phys. C, 2004, 417, 17–24 CrossRef CAS.
- M. Imada, A. Fujimori and Y. Tokura, Rev. Mod. Phys., 1998, 70, 1039 CrossRef CAS.
- M. Pissas, G. Kallias, A. Simopoulos, D. Niarchos and R. Sonntag, Physica B, 1998, 253, 1–9 CrossRef CAS.
- F. Zhou, M. Cococcioni, C. A. Marianetti, D. Morgan and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 15, 235121 CrossRef.
- K. W. Lee and W. E. Pickett, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 174428 CrossRef.
- M. E. Arroyo-de Dompablo, A. Morales-Garcia and M. Taravillo, J. Chem. Phys., 2011, 135, 054503 CrossRef CAS PubMed.
- M. E. Arroyo-de Dompablo, P. Rozier, M. Morcrette and J. M. Tarascon, Chem. Mater., 2007, 19, 2411–2422 CrossRef CAS.
| This journal is © the Owner Societies 2024 |