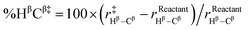Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solvent-induced dual nucleophiles and the α-effect in the SN2 versus E2 competition†
Xiangyu
Wu
 a,
F. Matthias
Bickelhaupt
a,
F. Matthias
Bickelhaupt
 *bcd and
Jing
Xie
*bcd and
Jing
Xie
 *a
*a
aKey Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, 100081, China. E-mail: jingxie@bit.edu.cn
bDepartment of Chemistry and Pharmaceutical Sciences, AIMMS, Vrije Universiteit Amsterdam, De Boelelaan 1108, 1081 HZ Amsterdam, The Netherlands. E-mail: f.m.bickelhaupt@vu.nl
cInstitute for Molecules and Materials (IMM), Radboud University Nijmegen, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands
dDepartment of Chemical Sciences, University of Johannesburg, Auckland Park, Johannesburg 2006, South Africa
First published on 15th March 2024
Abstract
We have quantum chemically investigated how microsolvation affects the various E2 and SN2 pathways, their mutual competition, and the α-effect of the model reaction system HOO−(H2O)n + CH3CH2Cl, at the CCSD(T) level. Interestingly, we identify the dual nature of the α-nucleophile HOO− which, upon solvation, is in equilibrium with HO−. This solvent-induced dual appearance gives rise to a rich network of competing reaction channels. Among both nucleophiles, SN2 is always favored over E2, and this preference increases upon increasing microsolvation. Furthermore, we found a pronounced α-effect, not only for SN2 substitution but also for E2 elimination, i.e., HOO− is more reactive than HO− in both cases. Our activation strain and quantitative molecular orbital analyses reveal the physical mechanisms behind the various computed trends. In particular, we demonstrate that two recently proposed criteria, required for solvent-free nucleophiles to display the α-effect, must also be satisfied by microsolvated HOO−(H2O)n nucleophiles.
Introduction
Bimolecular nucleophilic substitution (SN2) and elimination (E2) are ubiquitous, often mutually competing, reactions. For nearly 50 years, the mechanism of the SN2 reaction has been extensively studied experimentally and theoretically, including its temperature dependence,1–3 benchmark studies,4–11 steric effects,12–21 and solvent effects.14,15,22–29 Solvation can have a tremendous effect on chemical reactions.14,15,22,30–32 Rate constants, for example, can be reduced by up to 16 orders of magnitude from the gas phase to aqueous solution.33 Microsolvation bridges the gap between the gas phase and bulk solution and constitutes a powerful approach for obtaining a detailed understanding of how exactly solvent molecules affect reactions.33–39 Most research in this direction has been focused on microsolvated SN2 reactions of Y−(H2O)n + CH3X.1,23,26,30,32,34,40–50 Significantly less attention has been paid to microsolvated E2 reactions51–53 or solvent effects on the SN2 versus E2 competition.25,27,37,54–56 Previous theoretical studies have shown that the stepwise introduction of solvent molecules (e.g., HF,25 CH3OH27 and H2O37,55) as well as the increase in their solvation power favors SN2 relative to E2 pathways, and can lead to a switch from overall E2 to SN2 reactivity. The mechanism behind this solvation favoring the SN2 pathway is that SN2 reactions have a significantly lower characteristic distortivity and thus lower activation strain than E2 reactions and therefore suffer less from the reduced interaction between the nucleophile and the substrate when the nucleophile's basicity is attenuated upon solvation, as shown by Bickelhaupt, Hamlin et al.55The peroxide anion HOO− is an interesting nucleophile, because the monohydrated peroxide anion displays dual nucleophile character, where both HOO−(H2O) and (HOOH)(OH−) species are similarly stable.57 Moreover, as HOO−(H2O) reacts with CH3Cl,58 both HOO− and HO− anions are possible attacking nucleophiles, and this was observed in our recent direct dynamic simulation work.36 The introduction of water into the HOO− nucleophile enriches the reaction dynamics for it adds the proton-transfer induced HO−-SN2 pathway; one can expect that if the substrate was ethyl halides, the E2 pathway will emerge and make the dynamics more complicated and interesting.
Besides, HOO− is a typical α-nucleophile, possessing a lone pair of electrons adjacent to the nucleophilic atom. The term α-effect59 has been used to describe the enhanced reactivity of α-nucleophiles compared to that of normal nucleophiles by deviating from the Brønsted-type correlations found for normal nucleophiles.60 There has been extensive discussion on the origin of the α-effect, as well as a controversy about whether the α-effect is controlled by the intrinsic properties of the α-nucleophile or by external solvent effects.61–63 In terms of the intrinsic properties, mechanisms such as ground state destabilization, transition state stabilization, and thermodynamic product stability were proposed to be the origin of the α-effect.63–69 The α-effect has been observed in a variety of SN2 reactions,58,61,70–74 yet fewer studies have addressed its relevance to E2 reactions.75,76 A recent study by Hamlin et al.77 proposed two intrinsic criteria for the α-nucleophile to display the α-effect: (1) a higher energy HOMO and (2) a smaller HOMO lobe and overall amplitude of occupied orbitals on the nucleophilic center compared to the normal nucleophile. These criteria were proposed for solvent-free nucleophiles, and it is intriguing to examine whether they suit microsolvated nucleophiles.
In this study, we report a quantum chemical study on the HOO−(H2O)n + CH3CH2Cl reaction (Scheme 1), where n = 0 to 4 is the number of water molecules. We explore the full reaction pathways that, after formation of the initial E2 or SN2 product complexes, lead to the separated products, as shown in Scheme 1. This is in agreement with earlier experimental and simulation studies on closely related microsolvated ion–molecule SN2 reactions, which have shown that the formation of the unsolvated ionic products strongly dominates the formation of the solvated ionic products because of dynamic bottlenecks which make solvent transfer from nucleophiles to leaving groups less likely.45,47,78 The purpose of this study is three-fold, namely, to investigate the effect of solvation on (1) the competing SN2 and E2 reaction pathways, (2) the competing normal HOO−-pathways and the solvent-induced HO−-pathways, and (3) the α-effect on both, the SN2 and E2 reactions, in terms of the nucleophiles' intrinsic properties.
 | ||
| Scheme 1 SN2 and E2 reaction pathways of HOO−(H2O)n=1–4 + CH3CH2Cl reactions. In pathway c′, one water is the conjugate acid of the nucleophile HO−. | ||
Results and discussion
Potential energy surfaces
Fig. 1 depicts the potential energy surfaces (PESs) of the various SN2 and E2 mechanistic pathways of HOO− reacting with CH3CH2Cl, all of which display the typical “double-well” shape in which reactants (R) combine to a reactant complex (RC) that is connected via a transition state (TS) to product complexes (PC) and eventually separate products (P). The SN2 reaction is significantly more exothermic than the E2 reaction, with respective reaction energies of −46.0 and −24.0 kcal mol−1. Depending on the site and spatial direction under which HOO− attacks, the SN2 reaction proceeds either via backside substitution (inv-SN2, a) or via front side substitution that retains the stereostructure (ret-SN2, b),79 whereas the E2 reaction may take place either via anti-elimination (c) or via syn-elimination (d).80 For the inv-SN2 pathway, the nucleophile HOO− attacks Cα from the back side of the leaving group and causes the CH3-moiety to undergo Walden inversion toward the product CH3CH2OOH. For the ret-SN2 pathway, HOO− attacks Cα from the front side of the leaving group and the CH3-moiety retains its geometry in product CH3CH2OOH. For anti-E2 or syn-E2 pathways, HOO− attacks a β proton from the opposite or same side of the leaving group, respectively, leading to the abstraction of this proton and the formation of the E2 products CH2 = CH2 + Cl− + HOOH. The energy barriers decrease along ret-SN2 (16.5 kcal mol−1) > syn-E2 (−3.1) > anti-E2 (−9.7) > inv-SN2 (−14.1). The enthalpy and free energy values show the same trend (Table 1). The inv-SN2 and anti-E2 pathways have the lowest barriers and therefore outperform other reaction channels. Therefore, we now focus on these two reaction mechanisms and label them, for simplicity, as SN2 and E2.| n | HOO−W-path | HO−PT-path | |||||
|---|---|---|---|---|---|---|---|
| ΔE‡ | ΔH‡ | ΔG‡ | ΔE‡ | ΔH‡ | ΔG‡ | ||
| a Computed at CCSD(T)/aug-cc-pVTZ//MP2/6-311++G(d,p). | |||||||
| inv-SN2 | 0 | −14.1 | −13.8 | −3.9 | −14.0 | −14.0 | −5.4 |
| 1 | −4.6 | −3.8 | 5.2 | −1.4 | −0.4 | 9.5 | |
| 2 | 0.6 | 1.7 | 13.2 | 2.7 | 3.8 | 16.3 | |
| 3 | 3.1 | 4.1 | 14.8 | 8.0 | 9.3 | 19.4 | |
| 4 | 8.0 | 8.3 | 18.2 | 10.0 | 11.0 | 22.4 | |
| anti-E2 | 0 | −9.7 | −12.6 | −3.5 | −12.7 | −15.8 | −8.0 |
| 1 | 2.4 | 0.1 | 7.9 | 3.8 | 1.6 | 9.6 | |
| 2 | 7.9 | 6.9 | 19.2 | 8.8 | 6.9 | 17.4 | |
| 3 | 14.0 | 12.5 | 21.6 | 15.2 | 13.7 | 22.0 | |
| 4 | 17.9 | 16.4 | 25.1 | 18.2 | 16.2 | 26.9 | |
| ret-SN2 | 0 | 16.5 | 16.3 | 27.5 | 20.9 | 20.5 | 29.4 |
| syn-E2 | 0 | −3.1 | −6.0 | 4.1 | −5.2 | −8.6 | −0.5 |
It has been shown that hydrated peroxide anions, HOO−(H2O)n, tend to abstract a proton from H2O and form more stable species HO−(HOOH)(H2O)n−1 (Fig. S1, ESI†).36,38,57,58 Consequently, the solvent-induced proton-transfer HO−-moiety is a potential nucleophile to compete with the original nucleophile HOO−. Hence, when HOO−(H2O)n reacts with CH3CH2Cl, four pathways are possible: (a) HOO−W-SN2, “W” indicates that the nucleophile is bound with water molecules; (a′) HO−PT-SN2, “PT” indicates that the HO− nucleophile is induced by proton transfer from water molecules; (c) HOO−W-E2; and (c′) HO−PT-E2 (Scheme 1). Note that in the hydrated system, the HOO−W-E2 and HO−PT-E2 pathways generate the same products.
To show the effect of individual solvent molecules, we plotted the potential energy profiles of the HOO−(H2O)n=0,1,2 + CH3CH2Cl reactions in Fig. 2 for both HOO−W-paths (right panel) and HO−PT-paths (left panel). We used 0, 1 and 2 as prefixes to denote the number of solvent molecules when naming the species. The corresponding transition state structures are shown in Fig. 3. The involvement of multiple H2O molecules complicates the structures, so the most stable structures of each species and corresponding energetics were used in the discussion. Information on higher-energy conformational isomers is provided in Fig. S2–S6 (ESI†) for interested readers. Using HO−(HOOH) + CH3CH2Cl as the reference point, as observed, the HOO−W-SN2 reactions (−18.8 kcal mol−1) are more exothermic than HO−PT-SN2 reactions (−11.5 kcal mol−1), where both are more exothermic than E2 reactions (3.1 kcal mol−1). The addition of water molecules to the ion–molecule system stabilizes each species. For the singly- and doubly-hydrated systems, the potential energy profiles of both SN2 and E2 reactions remain double-well shaped. However, due to the differential stabilization effect of water molecules on the reactants and transition states, the barrier heights changed differently for the SN2 and E2 reactions. The energetic values are presented in Table 1 and details will be discussed in the next section.
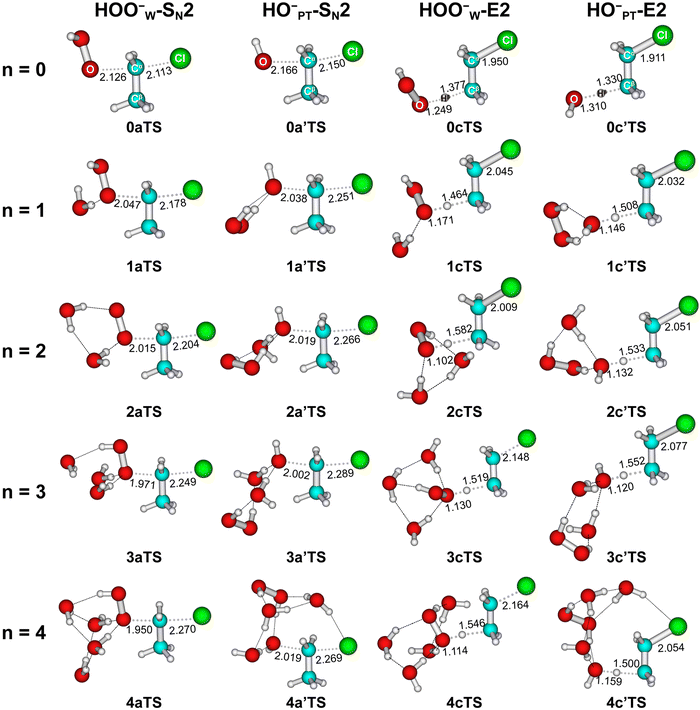 | ||
| Fig. 3 Structures of transition states of HOO−(H2O)n=0–4 + CH3CH2Cl optimized at MP2/6-311++G(d,p). Bond lengths are in angstrom. | ||
Competition SN2 versus E2 and HOO−versus HO−
The reaction barrier, ΔE‡ = E(TS) − E(R), of all four paths increases systematically as the extent of microsolvation (i.e., the number of solvent molecules) increases, as shown in Fig. 4. This trend of an increasing barrier agrees well with the decreasing reaction rate observed previously in experimental studies of microsolvated ion–molecule reactions.1,30,33,34,40,52,54,81,82 Focusing on the competition between SN2 and E2 pathways (Fig. 4a and b), the barrier of E2 pathways increases slightly faster upon microsolvation than that of SN2 pathways. Thus, the extent to which the E2 barrier exceeds that of the SN2 barrier, i.e. ΔΔE‡1 = ΔE‡(E2) − ΔE‡(SN2), increases upon microsolvation. For instance, as n increases from 0 to 4, the ΔΔE‡1 value increases from 4.3 to 7.0, 7.4, 10.8, and 9.9 kcal mol−1 for the HOO−W-path. Consequently, the SN2 paths always dominate and they do so even more at higher degrees of solvation. This enlargement of the E2–SN2 barrier difference (ΔΔE‡1) with a stepwise increase of microsolvation is also computed for the HO−PT-pathways, with corresponding values of 1.3, 5.2, 6.2, 7.2 and 8.3 kcal mol−1, respectively. Our finding consolidates earlier work that focused on the E2 and SN2 reactions of microsolvated model systems HO−(H2O)n + CH3CH2X (X = Cl, Br, I) and F− + CH3CH2F + nHF.25,37,80Regarding the competition between the α-nucleophile HOO−W and the normal nucleophile HO−PT (Fig. 4c and d), the barrier height difference, defined as ΔΔE‡2 = ΔE‡(HO−PT) − ΔE‡(HOO−W), is greater than zero for almost all cases, that is, the α-nucleophile reacts faster in almost all cases. The main trend is that introducing microsolvation, i.e., going from n = 0 to n ≥ 1, enhances the α-effect, i.e., ΔΔE‡2. However, the dependence of the α-effect as a function of introducing more solvent molecules (i.e., along n = 1, 2, 3, 4) is less uniform. For the SN2 paths, the barrier difference ΔΔE‡2(SN2) ranges from 2.0 to 4.9 kcal mol−1, whereas there is a smaller difference between the E2 pathways (Fig. 4d), and ΔΔE‡2(E2) ranges from 0.4 to 1.4 kcal mol−1, indicating that the HO−-E2 path can be strongly competitive to the HOO−-E2 pathway, provided sufficient energy is available to pass the E2 barriers.
In brief, among the four competing pathways, the HOO−W-SN2 path dominates with incremental solvation. In what follows, we seek the reason for barrier height difference upon solvation by analyzing the properties of nucleophiles and transition states.
In line with our previous studies,25,38,55,80 we found herein that microsolvation lowers the energy of the HOMO of both HOO− and HO− systematically upon adding an additional solvent molecule, either H2O or HOOH, by the HOMO–LUMO interaction with the σO–H* (solvent) LUMO (Table S4, ESI†). The HOMO of the microsolvated peroxide anion in HOO−(H2O)2,3,4 (−5.6, −6.0, and −6.6 eV) always remains higher in energy than that of the equivalently microsolvated hydroxide anion in HO−(HOOH)(H2O)1,2,3 (−6.5, −6.9, and −7.3 eV) and HO−(H2O)2,3,4 clusters (−5.7, −6.6, and −7.2 eV; see Table S4, ESI†). As shown previously by Bickelhaupt et al.,25,55 this situation gives rise to a smaller HOMO–LUMO energy gap and a more stabilizing HOMO–LUMO interaction with the LUMO (substrate) (in this work, the substrate is CH3CH2Cl) and, therefore, to a lower barrier for the reactions of microsolvated HOO− than for the corresponding reactions of microsolvated HO−. Indeed, we found a strong correlation of the barrier heights of the SN2 and E2 reactions with the HOMO energy level of the microsolvated peroxide and hydroxide nucleophiles (Fig. 5 and Fig. S8, ESI†). The same holds true for the related quantities of proton affinity (PA) and ethyl cation affinity (ECA), which are measures of the nucleophiles’ ability to bind with a proton and an ethyl cation. The PA and ECA values are defined as the enthalpy change of NuH → Nu− + H+ and CH3CH2Nu → Nu− + CH3CH2+, respectively (see Table S5 for all computed PA and ECA values, ESI†).
With the introduction of solvent molecules, the PA or ECA values of nucleophiles decrease continuously, that is, both types of affinities become weaker. Gratifyingly, the overall barrier heights (ΔE‡) of the SN2 and E2 reactions have, again, a strong linear correlation with the PA or ECA of the various microsolvated peroxide and hydroxide nucleophiles (R2 ≈ 1.00; see Fig. 5). In line with this, there is also a good to reasonable correlation between the height of the barrier and the amount of charge transferred from the nucleophile to the substrate, as computed with natural population analysis (NPA) atomic charges (R2 = 0.85–0.97; see Fig. 5 and Table S7, ESI†).37,83 Thus, a stronger charge transfer goes with a lower barrier.
| %D‡ = %CαCl‡ + %HβCβ‡ |
Indeed, we found a reasonably strong linear correlation of the SN2 and E2 reaction barriers with %D‡ (R2 = 0.81 to 0.98; see Fig. 5). The trend is that the more geometrically distorted the TS, the higher the barrier.
Furthermore, we found that incremental microsolvation makes the transition structures in general more product-like, as elaborated upon in the following. The SN2 pathway and the transition state involve the breaking of the Cα–Cl bond and the formation of the O–Cα bond, whereas the E2 pathway and transition state involve the breaking of both the Cα–Cl bond and the Hβ–Cβ bond and the formation of the peroxide or hydroxide O–Hβ bond (Fig. 3). Table 2 shows that, as the degree of microsolvation increases from 0 to 3, for transition structures of inv-SN2 reactions, the O–Cα bond shortens and the Cα–Cl bond lengthens systematically. In the case of the transition structures of anti-E2 reactions, there are a few irregularities but, by and large, the O–Hβ bond shortens, and both the Cα–Cl bond and the Hβ–Cβ bond lengthen upon going from the unsolvated to the microsolvated situation. In line with these structural characteristics, the Cl leaving group becomes increasingly negatively charged in the transition states of both SN2 and E2 reactions when the degree of microsolvation increases, as reflected by the computed NPA charges (see Table S7, ESI†). For example, the negative charge of the leaving group q(Cl) of the HOO−W SN2 path increases from −0.539 to −0.675 a.u., and the value of the HOO−W E2 path increases from −0.355 to −0.580 a.u. These findings are all consistent with the fact that the transition states become more product-like. Thus, as-stated above, microsolvation shifts the TS to a later, more product-like point along the reaction coordinate.
| HOO−W-path | HO−PT-path | |||||
|---|---|---|---|---|---|---|
| inv-SN2-TS | inv-SN2-TS | |||||
| n | r(O–Cα) | r(Cα–Cl) | r(Hβ–Cβ) | r(O–Cα) | r(Cα–Cl) | r(Hβ–Cβ) |
| 0 | 2.126 | 2.113 | 1.094 | 2.166 | 2.150 | 1.091 |
| 1 | 2.047 | 2.178 | 1.094 | 2.038 | 2.251 | 1.091 |
| 2 | 2.015 | 2.204 | 1.094 | 2.019 | 2.266 | 1.092 |
| 3 | 1.971 | 2.249 | 1.094 | 2.002 | 2.289 | 1.092 |
| 4 | 1.950 | 2.270 | 1.094 | 2.019 | 2.269 | 1.092 |
| anti-E2-TS | anti-E2-TS | |||||||
|---|---|---|---|---|---|---|---|---|
| n | r(O–Hβ) | r(Cα–Cl) | r(Hβ–Cβ) | r(Cα–Cβ) | r(O–Hβ) | r(Cα–Cl) | r(Hβ–Cβ) | r(Cα–Cβ) |
| 0 | 1.249 | 1.950 | 1.377 | 1.458 | 1.310 | 1.911 | 1.330 | 1.470 |
| 1 | 1.171 | 2.045 | 1.464 | 1.433 | 1.146 | 2.032 | 1.508 | 1.435 |
| 2 | 1.102 | 2.009 | 1.582 | 1.442 | 1.132 | 2.051 | 1.533 | 1.430 |
| 3 | 1.130 | 2.148 | 1.519 | 1.412 | 1.120 | 2.077 | 1.552 | 1.425 |
| 4 | 1.114 | 2.164 | 1.546 | 1.411 | 1.159 | 2.054 | 1.500 | 1.428 |
| ΔE‡ = ΔEstrain + ΔEint |
As shown in Fig. S9a and S9b (ESI†), the destabilizing strain energy of the E2 path is significantly larger than that of the SN2 path. The reason is the aforementioned larger characteristic distortion associated with the E2 path in which two bonds are breaking (Cα–Cl and Cβ–H) in the substrate as compared to the lesser characteristic distortion associated with the SN2 path along which only one bond (Cα–Cl) is breaking in the substrate.86 The higher activation strain is what makes the E2 barrier higher than the SN2 barrier, and this can only be inverted if the stabilizing nucleophile–substrate interaction is strong enough. As pointed out by Bickelhaupt et al.,86 the E2 pathway goes with a higher TS acidity, i.e., the lower LUMO in the TS, than the SN2 pathway. However, neither HOO− nor HO− are strong enough bases to cause an inversion of barrier heights as determined by the unfavorably high activation strain for E2 reactions involving the CH3CH2Cl substrate. In view of the fact that introducing solvent molecules makes the nucleophile an even poorer electron donor, the E2 barrier becomes higher relative to the SN2 barrier as the degree of solvation increases.
α-Effect
The HOO− anion is an α-nucleophile, featuring a lone-pair bearing heteroatom adjacent to the nucleophilic center. Its parent normal nucleophile is HO−. The α-effect refers to the dramatically enhanced reactivity of α-nucleophiles compared to their parent normal nucleophiles by deviating downward from the Brønsted-type correlation (reaction barrier versus proton affinity) found for normal nucleophiles.87 To evaluate whether microhydrated HOO− anions display an α-effect, two different Brønsted-type correlations between the barrier height and the basicity can be plotted by choosing different normal nucleophiles.In the Brønsted-type I correlation, one plots reaction barriers (ΔH‡) against the proton affinity (PA) of HOO−(H2O)n and HO−(H2O)n for n = 0 to 4. We found that there is a good correlation between ΔH‡ and the PA of the normal nucleophiles HO−(H2O)n for both SN2 (Fig. 6a) and E2 (Fig. 6b) reactions. Thus, nucleophiles with larger PA values have lower barriers and, therefore, a higher reactivity. All the points of HOO−(H2O)n deviate downward from this correlation line, with deviation values ΔΔH‡ = ΔH‡(HO− Brønsted path PA) − ΔΔH‡(HOO−) of more than 5 kcal mol−1, revealing the existence of the α-effect for microhydrated HOO− anions. The barriers of E2 reactions are more sensitive to the PA, as reflected by the larger slope of the correlation lines.
In the Brønsted-type II correlation, at each degree of hydration n, one plots the ΔH‡ of a set of other reference normal nucleophiles, including HO−, H2N− and HS−, against the PA, and compares this with the barrier for the HOO− anion and its PA. As shown in Fig. 7, when n = 0 to 3, the barriers of HOO−(H2O)n show a downward shift from the Brønsted-type II ΔH‡versus PA correlation line of the normal nucleophiles for both SN2 and E2 reactions. This downward shift becomes less obvious for E2 when n = 1–3. This is consistent with the report of Hamlin et al.,77 where the unsolvated nucleophiles were considered reacting with ethyl halides. To our knowledge, no such correlation has been plotted for microsolvated nucleophiles reacting with ethyl halides, nevertheless, examples with methyl halides exist.58,74,88–90 Experimental studies by Bierbaum's group58,74 and computational investigations by Ren's group88,90 suggested that the α-effect exists for HOO−(H2O) and HOO−(CH3OH) reacting with methyl chloride. Herein, we expand the exploration of this phenomenon to ethyl halides covering both SN2 and E2 reactions. In brief, the Brønsted-type II analyses also suggest that HOO−(H2O)n nucleophiles display the α-effect as compared with their normal nucleophile counterparts.
The mechanism behind the α-effect in SN2 reactions of unsolvated nucleophiles has been recently studied by Hamlin et al.77 who identified two criteria an α-nucleophile needs to fulfill in order to show the α-effect: (1) a small HOMO lobe and overall reduction of occupied amplitude on the nucleophilic center, in order to reduce the repulsive occupied–occupied orbital overlap between the nucleophile and the substrate and (2) a sufficiently high HOMO energy level, in order to still engage in a strong HOMOnucleophile–LUMOsubstrate orbital interaction with the substrate. Herein, we examine whether the microsolvated HOO−(H2O)n nucleophiles satisfy the two criteria. First, as shown in Fig. S10 (ESI†), the key occupied orbitals of nucleophiles, the HOMO lobes of HOO−(H2O)n, are smaller than those of HO−(H2O)n and HO−(HOOH)(H2O)n−1 for nearly all degrees of microsolvation. This is further confirmed by the fact that the total negative charge on the nucleophilic center is significantly lower in the α-nucleophiles than in the corresponding normal nucleophile (see Fig. S1, ESI†). Second, if a DFT method is used, the HOMO levels of HOO−(H2O)n are consistently at higher energy than those of HO−(H2O)n and HO−(HOOH)(H2O)n−1. So, both criteria77 are indeed satisfied also in the case of microsolvated α-nucleophiles HOO−(H2O)n. Accordingly, the microsolvated HOO−(H2O)n anion shall display an α-effect, consistent with the result given by Brønsted-type correlations.
Our above analyses show that the rate-accelerating α-effect in microsolvated E2 and microsolvated SN2 reactions goes hand-in-hand with a rise in orbital energy of the π-antibonding HOMO and the reduced amplitude of density on the nucleophilic center. The mechanism behind this is that the α-nucleophile has, due to its reduced density on the nucleophilic center, less steric (Pauli) repulsion with the substrate than the normal nucleophile and, therefore, a more stabilizing overall interaction;77 this difference in Pauli repulsion does not occur for the proton affinity because the proton has no occupied orbitals. However, if the ethyl cation affinity (ECA) instead of the PA is used as the Brønsted-correlation parameter, the α-effect is diminished; that is, the downward deviation of the barriers for the α-nucleophiles from the barrier versus the ECA Brønsted-type correlation is significantly reduced because the Pauli-reduction lowering in the case of α-nucleophiles relative to normal nucleophiles now happens not only in the interaction with the substrate in the TS of the reaction but also in the interaction with the carbon acid CH3CH2+ which defines the ECA. We had found this previously for SN2 reactions of unsolvated α-nucleophiles.77
Herein, we have been able to extend this finding to microsolvated nucleophiles and to E2 reactions. Thus, the barriers versus ECA correlations were constructed (Fig. S12d, ESI†), and the degree of downward deviation is indeed greatly reduced. In fact, as shown in Fig. 6c and d, the ΔH‡ values of both the HOO−W-path (α-nucleophile) and the HO−W-path (normal nucleophile) have a good linear relationship with the ECA. This phenomenon of reduced deviation is also observed when the HY−(H2O)0–3 is used as the reference, where Y = O, S, and HN (Fig. S15, ESI†), i.e. type II correlation. Altogether, our computed Brønsted-type correlations reveal that the microhydrated HOO−(H2O)0–4 nucleophiles exhibit the α-effect in both SN2 and E2 reactions.
Conclusions
We have computed highly accurate potential energy profiles for various E2 and SN2 pathways involved in the HOO−(H2O)0–4 + CH3CH2Cl reaction system, involving both HOO− and HO− as attacking nucleophiles, based on a correlated CCSD(T)/aug-cc-pVTZ//MP2/6-311++G(d,p) approach. Our work provides both a benchmark description and a unified conceptual framework for a collection of interesting kinetic and structural phenomena that occur in our chemically rich series of model reactions in which microsolvated HOO− has a dual appearance due to the facile solvent-induced formation of microsolvated HO−.The SN2 path dominates the E2 path in our model systems. Adding water molecules further enhances the dominance of the SN2 reaction. This is so for both nucleophiles, HOO−(H2O)0–4 and HO−(HOOH)(H2O)0–3. Thus, the E2 barrier rises further above the SN2 barrier with each additional water molecule. This trend emerges from the combination of two factors: (i) the SN2 mechanism is associated with a smaller characteristic distortion and thus less activation strain ΔEstrain than the E2 mechanism; (ii) therefore, as the nucleophile–substrate interaction ΔEint is weakened due to microsolvation, the barrier for the E2 path rises faster than that for SN2 and the latter pathway becomes more dominant.
In the SN2 substitution, the initial HOO− nucleophile is clearly more reactive than the associated solvent-induced HO− nucleophile. But, in the E2 elimination, the difference in reactivity is significantly smaller, with HOO− still being somewhat more reactive. Thus, we found that the HOO−(H2O)0–4 nucleophiles display the α-effect in both the SN2 and, to a lesser extent, also in the E2 reaction. We show that the microsolvated α-nucleophiles satisfy the earlier proposed criteria for the occurrence of the α-effect, namely, a higher-energy HOMO and less occupied amplitude on the nucleophilic center, as compared to the corresponding normal nucleophile.
Our present work provides a unified description and rationalization of the reaction potential energy surface (PES) and kinetic and structural phenomena determined by this PES. A next leap forward that we envisage is the exploration of the complex dynamics taking place on this mechanistically rich multi-mechanistic PES of the HOO−(H2O)0–4 + CH3CH2Cl reactions.
Computational methods
All calculations were performed using the Gaussian 16 program.91 To find an accurate method, the MP2,92 B97-1,93 B3LYP94 and CAM-B3LYP95 methods were tested on the reaction enthalpies of HOO− + CH3CH2Cl to form CH3CH2OOH + Cl− and CH2 = CH2 + Cl− + HOOH. It turns out that the MP2/6-311++G(d,p) and MP2/aug-cc-pVTZ level of theories gave the best agreement with experimental values (Table S1, ESI†). We selected the MP2/6-311++G(d,p) method to perform the geometry optimization and frequency calculations throughout this work, for it is less time-consuming than the aug-cc-pVTZ basis set, and to be consistent with our previous work.38 The nature of stationary points was confirmed by the frequencies under harmonic oscillator approximation, where energy minimum structures have no imaginary frequency and transition state structures have one imaginary frequency. The intrinsic reaction coordinate (IRC) calculations were performed for all transition states to ensure accuracy. On top of the geometries optimized with MP2/6-311++G(d,p) level of theory, single point calculations were performed using coupled cluster theory CCSD(T)96 with the aug-cc-pVTZ basis set.97,98 If not specified, the energies reported in this work are at the CCSD(T)/aug-cc-pVTZ//MP2/6-311++G(d,p) level of theory.Data availability statement
The data that support the findings of this study are available in the ESI† of this article.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work is supported by the National Natural Science Foundation of China (no. 22273004), the Beijing Natural Science Foundation (no. 2222028), the Teli Fellowship, and the Innovation Foundation (no. 2021CX01026) from the Beijing Institute of Technology, China. F. M. B. is grateful for continued support from the Netherlands Organization of Scientific Research (NWO).References
- A. A. Viggiano, S. T. Arnold, R. A. Morris, A. F. Ahrens and P. M. Hierl, J. Phys. Chem., 1996, 100, 14397–14402 CrossRef CAS.
- J. V. Seeley, R. A. Morris, A. A. Viggiano, H. Wang and W. L. Hase, J. Am. Chem. Soc., 1997, 119, 577–584 CrossRef CAS.
- G. Caldwell, T. F. Magnera and P. Kebarle, J. Am. Chem. Soc., 1984, 106, 959–966 CrossRef CAS.
- A. P. Bento, M. Solà and F. M. Bickelhaupt, J. Chem. Theory Comput., 2008, 4, 929–940 CrossRef CAS PubMed.
- M. Swart, M. Solà and F. M. Bickelhaupt, J. Chem. Theory Comput., 2010, 6, 3145–3152 CrossRef CAS PubMed.
- J. Liang, Q. Su, Y. Wang and Z. Geng, Bull. Chem. Soc. Jpn., 2015, 88, 110–116 CrossRef.
- I. Szabó and G. Czakó, J. Phys. Chem. A, 2017, 121, 5748–5757 CrossRef PubMed.
- V. Tajti and G. Czakó, J. Phys. Chem. A, 2017, 121, 2847–2854 CrossRef CAS PubMed.
- D. A. Tasi, Z. Fábián and G. Czakó, J. Phys. Chem. A, 2018, 122, 5773–5780 CrossRef CAS PubMed.
- D. A. Tasi, C. Tokaji and G. Czakó, Phys. Chem. Chem. Phys., 2021, 23, 13526–13534 RSC.
- Z. Kerekes, D. A. Tasi and G. Czakó, J. Phys. Chem. A, 2022, 126, 889–900 CrossRef CAS PubMed.
- A. A. Mohamed and F. Jensen, J. Phys. Chem. A, 2001, 105, 3259–3268 CrossRef CAS.
- C. K. Regan, S. L. Craig and J. I. Brauman, Science, 2002, 295, 2245–2247 CrossRef CAS PubMed.
- G. Vayner, K. N. Houk, W. L. Jorgensen and J. I. Brauman, J. Am. Chem. Soc., 2004, 126, 9054–9058 CrossRef CAS PubMed.
- X. Chen, C. K. Regan, S. L. Craig, E. H. Krenske, K. N. Houk, W. L. Jorgensen and J. I. Brauman, J. Am. Chem. Soc., 2009, 131, 16162–16170 CrossRef CAS PubMed.
- J. M. Garver, N. Eyet, S. M. Villano, Z. Yang and V. M. Bierbaum, Int. J. Mass Spectrom., 2011, 301, 151–158 CrossRef CAS.
- S. Nettey, C. A. Swift, R. Joviliano, D. O. Noin and S. Gronert, J. Am. Chem. Soc., 2012, 134, 9303–9310 CrossRef CAS PubMed.
- E. Carrascosa, J. Meyer, T. Michaelsen, M. Stei and R. Wester, Chem. Sci., 2018, 9, 693–701 RSC.
- M. Gallegos, A. Costales and Á. Martín Pendás, J. Comput. Chem., 2022, 43, 785–795 CrossRef CAS PubMed.
- M. Gallegos, A. Costales and Á. Martín Pendás, J. Phys. Chem. A, 2022, 126, 1871–1880 CrossRef CAS PubMed.
- X. Lu, C. Shang, L. Li, R. Chen, B. Fu, X. Xu and D. H. Zhang, Nat. Commun., 2022, 13, 4427 CrossRef CAS PubMed.
- J. Chandrasekhar, S. F. Smith and W. L. Jorgensen, J. Am. Chem. Soc., 1984, 106, 3049–3050 CrossRef CAS.
- P. M. Hierl, A. F. Ahrens, M. Henchman, A. A. Viggiano, J. F. Paulson and D. C. Clary, J. Am. Chem. Soc., 1986, 108, 3142–3143 CrossRef CAS.
- J. P. Bergsma, B. J. Gertner, K. R. Wilson and J. T. Hynes, J. Chem. Phys., 1987, 86, 1356–1376 CrossRef CAS.
- F. M. Bickelhaupt, E. J. Baerends and N. M. M. Nibbering, Chem. – Eur. J., 1996, 2, 196–207 CrossRef CAS.
- S. Kato, J. Hacaloglu, G. E. Davico, C. H. DePuy and V. M. Bierbaum, J. Phys. Chem. A, 2004, 108, 9887–9891 CrossRef CAS.
- X. Liu, J. Zhang, L. Yang and W. L. Hase, J. Am. Chem. Soc., 2018, 140, 10995–11005 CrossRef CAS PubMed.
- F. Yu, J. Phys. Chem. A, 2022, 126, 4342–4348 CrossRef CAS PubMed.
- Y. Li, C. Li, D. Gao and D. Wang, J. Phys. Chem. A, 2022, 126, 5527–5533 CrossRef CAS PubMed.
- D. K. Bohme and A. B. Raksit, Can. J. Chem., 1985, 63, 3007–3011 CrossRef CAS.
- J. M. Garver, Y. Fang, N. Eyet, S. M. Villano, V. M. Bierbaum and K. C. Westaway, J. Am. Chem. Soc., 2010, 132, 3808–3814 CrossRef CAS PubMed.
- R. Otto, J. Brox, S. Trippel, M. Stei, T. Best and R. Wester, Nat. Chem., 2012, 4, 534–538 CrossRef CAS PubMed.
- D. K. Bohme and G. I. Mackay, J. Am. Chem. Soc., 1981, 103, 978–979 CrossRef CAS.
- J. Xie, X. Ma, J. Zhang, P. M. Hierl, A. A. Viggiano and W. L. Hase, Int. J. Mass Spectrom., 2017, 418, 122–129 CrossRef CAS.
- X. Ji, C. Zhao and J. Xie, Phys. Chem. Chem. Phys., 2021, 23, 6349–6360 RSC.
- C. Zhao, X. Ma, X. Wu, D. L. Thomsen, V. M. Bierbaum and J. Xie, J. Phys. Chem. Lett., 2021, 12, 7134–7139 CrossRef CAS PubMed.
- X. Wu, S. Zhang and J. Xie, Phys. Chem. Chem. Phys., 2022, 24, 12993–13005 RSC.
- X. Wu, C. Zhao and J. Xie, ChemPhysChem, 2022, 23, e202200285 CrossRef CAS PubMed.
- Y. Hu, X. Wu and J. Xie, Phys. Chem. Chem. Phys., 2023, 25, 1947–1956 RSC.
- D. K. Bohme and A. B. Raksit, J. Am. Chem. Soc., 1984, 106, 3447–3452 CrossRef CAS.
- X. G. Zhao, S. C. Tucker and D. G. Truhlar, J. Am. Chem. Soc., 1991, 113, 826–832 CrossRef CAS.
- R. A. J. O'Hair, G. E. Davico, J. Hacaloglu, T. T. Dang, C. H. DePuy and V. M. Bierbaum, J. Am. Chem. Soc., 1994, 116, 3609–3610 CrossRef.
- S. Re and K. Morokuma, J. Phys. Chem. A, 2001, 105, 7185–7197 CrossRef CAS.
- J. Xie, R. Otto, R. Wester and W. L. Hase, J. Chem. Phys., 2015, 142, 244308 CrossRef PubMed.
- R. Otto, J. Brox, S. Trippel, M. Stei, T. Best and R. Wester, J. Phys. Chem. A, 2013, 117, 8139–8144 CrossRef CAS PubMed.
- J. Xie, M. J. Scott, W. L. Hase, P. M. Hierl and A. A. Viggiano, Z. Phys. Chem., 2015, 229, 1747–1763 CrossRef CAS.
- J. Zhang, L. Yang, J. Xie and W. L. Hase, J. Phys. Chem. Lett., 2016, 7, 660–665 CrossRef CAS PubMed.
- B. Bastian, T. Michaelsen, L. Li, M. Ončák, J. Meyer, D. H. Zhang and R. Wester, J. Phys. Chem. A, 2020, 124, 1929–1939 CrossRef CAS PubMed.
- X. Lu, L. Li, X. Zhang, B. Fu, X. Xu and D. H. Zhang, J. Phys. Chem. Lett., 2022, 13, 5253–5259 CrossRef CAS PubMed.
- R. Otto, J. Xie, J. Brox, S. Trippel, M. Stei, T. Best, M. R. Siebert, W. L. Hase and R. Wester, Faraday Discuss., 2012, 157, 41–57 RSC.
- Y.-R. Wu and W.-P. Hu, J. Am. Chem. Soc., 1999, 121, 10168–10177 CrossRef CAS.
- N. Eyet, S. M. Villano, S. Kato and V. M. Bierbaum, J. Am. Soc. Mass Spectrom., 2007, 18, 1046–1051 CrossRef CAS PubMed.
- N. Eyet, S. M. Villano and V. M. Bierbaum, J. Am. Soc. Mass Spectrom., 2008, 19, 1296–1302 CrossRef CAS.
- N. Eyet, J. J. Melko, S. G. Ard and A. A. Viggiano, Int. J. Mass Spectrom., 2015, 378, 54–58 CrossRef CAS.
- T. Hansen, J. C. Roozee, F. M. Bickelhaupt and T. A. Hamlin, J. Org. Chem., 2022, 87, 1805–1813 CrossRef CAS PubMed.
- F. M. Lisboa and J. R. Pliego, J. Mol. Model., 2022, 28, 159 CrossRef CAS PubMed.
- D. J. Anick, J. Phys. Chem. A, 2011, 115, 6327–6338 CrossRef CAS PubMed.
- D. L. Thomsen, J. N. Reece, C. M. Nichols, S. Hammerum and V. M. Bierbaum, J. Am. Chem. Soc., 2013, 135, 15508–15514 CrossRef CAS PubMed.
- J. O. Edwards and R. G. Pearson, J. Am. Chem. Soc., 1962, 84, 16–24 CrossRef CAS.
- W. P. Jencks and J. Carriuolo, J. Am. Chem. Soc., 1960, 82, 1778–1786 CrossRef CAS.
- E. Buncel, H. Wilson and C. Chuaqui, J. Am. Chem. Soc., 1982, 104, 4896–4900 CrossRef CAS.
- E. Buncel and I.-H. Um, J. Chem. Soc., Chem. Commun., 1986, 595, 10.1039/C39860000595.
- I.-H. Um and E. Buncel, J. Org. Chem., 2000, 65, 577–582 CrossRef CAS PubMed.
- M. Laloi-Diard, J.-F. Verchere, P. Gosselin and F. Terrier, Tetrahedron Lett., 1984, 25, 1267–1268 CrossRef CAS.
- I.-H. Um, E.-J. Lee and E. Buncel, J. Org. Chem., 2001, 66, 4859–4864 CrossRef CAS PubMed.
- J. F. Liebman and R. M. Pollack, J. Org. Chem., 1973, 38, 3444–3445 CrossRef CAS.
- A. Pross and S. S. Shaik, J. Am. Chem. Soc., 1981, 103, 3702–3709 CrossRef CAS.
- S. Hoz, J. Org. Chem., 1982, 47, 3545–3547 CrossRef CAS.
- M. M. Heaton, J. Am. Chem. Soc., 1978, 100, 2004–2008 CrossRef CAS.
- Y. Ren and H. Yamataka, J. Org. Chem., 2007, 72, 5660–5667 CrossRef CAS PubMed.
- Y. Ren and H. Yamataka, Chem. – Eur. J., 2007, 13, 677–682 CrossRef CAS PubMed.
- J. M. Garver, S. Gronert and V. M. Bierbaum, J. Am. Chem. Soc., 2011, 133, 13894–13897 CrossRef CAS PubMed.
- Y. Ren, X.-G. Wei, S.-J. Ren, K.-C. Lau, N.-B. Wong and W.-K. Li, J. Comput. Chem., 2013, 34, 1997–2005 CrossRef CAS PubMed.
- D. L. Thomsen, J. N. Reece, C. M. Nichols, S. Hammerum and V. M. Bierbaum, J. Phys. Chem. A, 2014, 118, 8060–8066 CrossRef CAS PubMed.
- Y. Ren and H. Yamataka, J. Comput. Chem., 2009, 30, 358–365 CrossRef CAS PubMed.
- X.-G. Wei, X.-M. Sun, X.-P. Wu, Y. Ren, N.-B. Wong and W.-K. Li, J. Org. Chem., 2010, 75, 4212–4217 CrossRef CAS PubMed.
- T. Hansen, P. Vermeeren, F. M. Bickelhaupt and T. A. Hamlin, Angew. Chem., Int. Ed., 2021, 60, 20840–20848 CrossRef CAS PubMed.
- X. Liu, J. Xie, J. Zhang, L. Yang and W. L. Hase, J. Phys. Chem. Lett., 2017, 8, 1885–1892 CrossRef CAS PubMed.
- A. P. Bento and F. M. Bickelhaupt, J. Org. Chem., 2008, 73, 7290–7299 CrossRef CAS PubMed.
- F. M. Bickelhaupt, J. Comput. Chem., 1999, 20, 114–128 CrossRef CAS.
- J. V. Seeley, R. A. Morris and A. A. Viggiano, J. Phys. Chem. A, 1997, 101, 4598–4601 CrossRef CAS.
- C. H. DePuy, S. Gronert, A. Mullin and V. M. Bierbaum, J. Am. Chem. Soc., 1990, 112, 8650–8655 CrossRef CAS.
- M. N. Glukhovtsev, A. Pross and L. Radom, J. Am. Chem. Soc., 1996, 118, 6273–6284 CrossRef CAS.
- F. M. Bickelhaupt and K. N. Houk, Angew. Chem., Int. Ed., 2017, 56, 10070–10086 CrossRef CAS PubMed.
- P. Vermeeren, S. C. C. van der Lubbe, C. Fonseca Guerra, F. M. Bickelhaupt and T. A. Hamlin, Nat. Protoc., 2020, 15, 649–667 CrossRef CAS PubMed.
- P. Vermeeren, T. Hansen, P. Jansen, M. Swart, T. A. Hamlin and F. M. Bickelhaupt, Chem. – Eur. J., 2020, 26, 15538–15548 CrossRef CAS PubMed.
- S. Hoz and E. Buncel, Isr. J. Chem., 1985, 26, 313–319 CrossRef CAS.
- W.-Y. Zhao, J. Yu, S.-J. Ren, X.-G. Wei, F.-Z. Qiu, P.-H. Li, H. Li, Y.-P. Zhou, C.-Z. Yin, A.-P. Chen, H. Li, L. Zhang, J. Zhu, Y. Ren and K.-C. Lau, J. Comput. Chem., 2015, 36, 844–852 CrossRef CAS PubMed.
- D. L. Thomsen, C. M. Nichols, J. N. Reece, S. Hammerum and V. M. Bierbaum, J. Am. Soc. Mass Spectrom., 2014, 25, 159–168 CrossRef CAS PubMed.
- L. Yun-Yun, Q. Fang-Zhou, Z. Jun, R. Yi and L. Kai-Chung, J. Mol. Model., 2017, 23, 192 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Revision A.03, Gaussian Inc., Wallingford CT, 2016.
- M. J. Frisch, M. Head-Gordon and J. A. Pople, Chem. Phys. Lett., 1990, 166, 275–280 CrossRef CAS.
- F. A. Hamprecht, A. J. Cohen, D. J. Tozer and N. C. Handy, J. Chem. Phys., 1998, 109, 6264–6271 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- G. D. Purvis and R. J. Bartlett, J. Chem. Phys., 1982, 76, 1910–1918 CrossRef CAS.
- T. H. Dunning, J. Chem. Phys., 1989, 90, 1007–1023 CrossRef CAS.
- D. E. Woon and T. H. Dunning, J. Chem. Phys., 1993, 98, 1358–1371 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp00671b |
| This journal is © the Owner Societies 2024 |