Pressure and temperature dependent kinetics and the reaction mechanism of Criegee intermediates with vinyl alcohol: a theoretical study†
Cuihong
Sun
 a,
Baoen
Xu
a,
Baoen
Xu
 a and
Yanli
Zeng
a and
Yanli
Zeng
 *b
*b
aShijiazhuang Key Laboratory of Low Carbon Energy Materials, Technology Innovation Center of HeBei for Heterocyclic Compound, College of Chemical Engineering, Shijiazhuang University, Shijiazhuang 050035, P. R. China
bCollege of Chemistry and Materials Science, Hebei Normal University, Shijiazhuang 050024, P.R. China. E-mail: yanlizeng@hebtu.edu.cn
First published on 27th February 2024
Abstract
Criegee intermediates (CIs), the key intermediates in the ozonolysis of olefins in atmosphere, have received much attention due to their high activity. The reaction mechanism of the most simple Criegee intermediate CH2OO with vinyl alcohol (VA) was investigated by using the HL//M06-2X/def2TZVP method. The temperature and pressure dependent rate constant and product branching ratio were calculated using the master equation method. For CH2OO + syn-VA, 1,4-insertion is the main reaction channel while for the CH2OO + anti-VA, cycloaddition and 1,2-insertion into the O–H bond are more favorable than the 1,4-insertion reaction. The 1,4-insertion or cycloaddition intermediates are stabilized collisionally at 300 K and 760 torr, and the dissociation products involving OH are formed at higher temperature and lower pressure. The rate constants of the CH2OO reaction with syn-VA and anti-VA both show negative temperature effects, and they are 2.95 × 10−11 and 2.07 × 10−13 cm3 molecule−1 s−1 at 300 K, respectively, and the former is agreement with the prediction in the literature.
1. Introduction
Criegee intermediates (CIs) are also known as carbonyl oxides, the atmospheric intermediates produced in the oxidation of unsaturated hydrocarbons with ozone. In the ozonolysis reactions, the CI is formed vibrationally excited, and the energized CI will undergo rapid unimolecular decomposition or be stabilized collisionally and eventually be lost through bimolecular reactions or UV photolysis.1,2Criegee intermediates may affect the oxidative capacity of the atmosphere by producing OH3–7 and influencing the tropospheric HOx budget, and they also play an important role in the formation of atmospheric aerosols8,9 by reaction with SO2,10,11 which is an important gas-phase source of low-volatility sulfuric acid. As a 1,3-bipole radical with zwitterionic character, a Criegee intermediate can react with H2O,12–14 (H2O)214,15 NH3,16,17 alkane,18–20 atmospheric organic and inorganic acids acids21–26 and alcohols27–31via an insertion reaction, or react with SO2,32,33 carbonyl compounds,34–37 and alkenes38via a cycloaddition reaction, or be associated with other 1,3-bipoles, such as ozone or carbonyl oxides.39–42
The major loss paths for Criegee intermediates are assumed to be the reactions with water vapor, which is ubiquitous throughout the troposphere while the CI reaction with H2O is slow, and the rate constant is about 10−15–10−16 cm3 molecule−1 s−1.43,44 The reaction of CIs + alcohols has a similar 1,2-insertion mechanism to that of CIs + H2O, and the measured rate constants31 by experiment for the simplest Criegee intermediate (CH2OO) with methanol, ethanol, and 2-propanol are (1.4 ± 0.4) × 10−13, (2.3 ± 0.6) × 10−13, and (1.9 ± 0.5) × 10−13 cm3 molecule−1 s−1. Vereecken22 investigated the reaction of CH2OO with HNO3 and HCOOH, and found that a 1,4-insertion mechanism allows for barrierless reactions with high rate constants up to ∼10−10 cm3 molecule−1 s−1. He determined that the 1,4-insertion mechanism relies on the presence of a double bond in the α-position to the acidic OH group. The reactions of CI with enols were predicated to likewise react through the 1,4-insertion mechanism, with the rate constant exceeding 10−11 cm3 molecule−1 s−1. Thus, the CH2OO reaction with enols by 1,4-insertion will be much faster than with H2O, methanol, ethanol, etc. using the 1,2-insertion mechanism.
Vinyl alcohol (VA) is the simplest enol, with high reactivity in atmospheric and combustion chemistry;45 it has been implicated as a precursor to atmospheric carboxylic acids.46–49 The main atmospheric decay channel of vinyl alcohol is the reaction with the OH radical, which has been incorporated into the atmospheric chemistry box model estimating the contribution of photo-tautomerization of acetaldehyde to the worldwide formic acid production.49 Since organic acids are key species in the formation of secondary organic aerosol, the investigation on the bimolecular reaction of vinyl alcohol is of potential importance. Furthermore, the rate constant of VA + CH2OO may be comparable or even faster than that of VA + OH (1.48 × 10−11).50 Under natural conditions, vinyl alcohol has two conformers, syn-VA and anti-VA, and the calculated syn/anti ratio is 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 at 300 K.45 As shown in Scheme 1, both syn-VA and anti-VA display Cs symmetry. Theoretical study shows that the favorable OH-addition site for syn-VA is β-C, while for the anti-VA, the favorable OH-addition site is α-C.50 Likewise, are the main reaction channels for CH2OO with syn-VA and anti-VA both 1,4-insertion, or are there different mechanisms?
15 at 300 K.45 As shown in Scheme 1, both syn-VA and anti-VA display Cs symmetry. Theoretical study shows that the favorable OH-addition site for syn-VA is β-C, while for the anti-VA, the favorable OH-addition site is α-C.50 Likewise, are the main reaction channels for CH2OO with syn-VA and anti-VA both 1,4-insertion, or are there different mechanisms?
In this work, we studied the detailed reaction mechanism of CH2OO with vinyl alcohol (syn and anti), including 1,4-insertion over the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH moiety, 1,2-insertion into the C–H or O–H bond, and cycloaddition across the C
C–OH moiety, 1,2-insertion into the C–H or O–H bond, and cycloaddition across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. The rate constants and products’ branching ratios were calculated using the master equation (ME) method, to determine the product distributions at different temperatures and pressures.
C double bond. The rate constants and products’ branching ratios were calculated using the master equation (ME) method, to determine the product distributions at different temperatures and pressures.
2. Computational method
2.1 Electronic structure calculations
The geometries of the reactants, pre-reactive complexes, transition states, and products of the title reaction were optimized at the M06-2X/def2TZVP level of theory. The harmonic vibration frequency was calculated at the same level of theory to obtain the zero-point energy (ZPE) and to verify the nature (minima or saddle points) of the stationary points. All transition states discussed in this work were verified by intrinsic reaction coordinate (IRC) calculations to identify the reactants and products. The single-point energies of all stationary points were then refined by a higher-level (denoted as HL) single-point energy calculation method. The HL energies were obtained via a combination of coupled cluster calculations with perturbative inclusion of the triplet contribution, CCSD(T),51 and second order Moller–Plesset perturbation theory (MP2).52,53 The complete basis set limit energy was estimated by the extrapolation of results obtained for sequences of the correlation-consistent polarized-valence basis sets. The expression for this method can be as follows:| EHL = E[CCSD(T)/cc-pVTZ] + (E[CCSD(T)/cc-pVTZ] − E[CCSD(T)/cc-pVDZ]) × 0.46286 + E[MP2(FC)/cc-pVQZ] + (E[MP2(FC)/cc-pVQZ] − E[MP2(FC)/cc-pVTZ]) × 0.69377 − E[MP2(FC)/cc-pVTZ] − (E[MP2(FC)/cc-pVTZ] − E[MP2(FC)/cc-pVDZ]) × 0.46286 |
The HL method has been verified to be accurate for both closed shell and open shell species’ calculations.54,55 The rate constants of Criegee intermediates with acrolein and C2H4 reactions35,56 based on the HL potential energy surface have been proved to be consistent with experimental results. All the electronic structure and energetic calculations were carried out using the Gaussian 09 program package.57
2.2 Kinetic calculations
The rate constant and products’ branching ratios were calculated using the master equation method,58–62 which is a powerful tool for calculating the temperature and pressure dependent kinetics of a multiple-channel and multiple-well chemical reaction system. Energy transfer is treated with the single exponential down model with ΔEdown = 200 cm−1. The Lennard-Jones (L-J) parameters of the intermediates C3O3H6 (σ = 6.0 Å and ε/kb = 450 K) are taken from ref. 63 and the L-J parameters of N2 are taken as σ = 3.9 Å and ε/kb = 48 K.58 In order to evaluate the sensitivity of the results for the L-J parameters of the intermediates, σ = 4.5 Å and ε/kb = 350 K22 are used to recalculated the rate constant for comparison, and the results show no obvious change. In this study, the master equation was solved by an ordinary differential equation (ODE) solver to develop directly the number population of the species with time.64–66 The populations of species as functions of time can be obtained directly and the first-order rate constants can be extracted from the reactant population using the “exponential decay” approach. More details and test cases can be found in ref. 55 and 66–68. All the kinetic calculations were carried out by using TheRate program.693. Results and discussion
3.1. Reaction mechanism of CH2OO + syn-VA
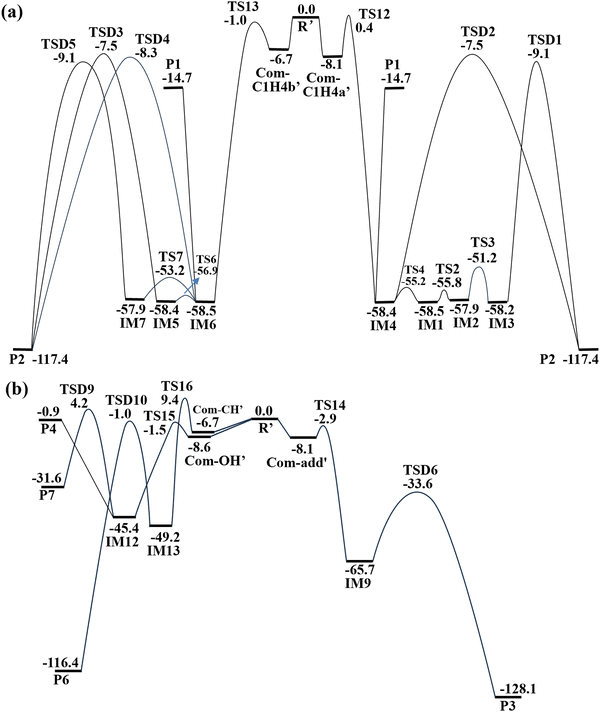
The optimized geometries of pre-reactive complexes, transition states, intermediates, and products are shown in Fig. 1 and 2. The PES for the CH2OO + syn-VA reaction is portrayed in Fig. 3. All the reaction channels initiate from the pre-reactive complex, and they are named Com-C1H4a (Com-C1H4b) for the 1,4-insertion reaction, Com-add for the cycloaddition reaction, Com-OH and Com-CH for the O–H and C–H 1,2-insertion reaction, respectively. | ||
| Fig. 1 The optimized geometries of stationary points in 1,4-insertion reactions for the CH2OO + syn-VA reaction. (a) Channel initiates from Com-C1H4a; (b) Channel initiates from Com-C1H4b. | ||
 | ||
| Fig. 2 The optimized geometries of stationary points for the CH2OO + syn-VA reaction. (a) Cycloaddition reaction; (b) 1,2-insertion reaction in O–H and C–H bonds. | ||
For the 1,4-insertion reaction over the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH moiety, the CH2OO carbon atom and the terminal oxygen atom approaches the β-C and the hydroxyl H atom of syn-VA respectively, forming the pre-reactive complexes Com-C1H4a and Com-C1H4b, which are 7.5 and 9.0 kcal mol−1 more stable than the free reactants. In Com-C1H4a, the central oxygen atom (O9) of CH2OO lies on the same side with hydroxyl O atom (O3) of syn-VA, while in Com-C1H4b, they lie on the opposite side.
C–OH moiety, the CH2OO carbon atom and the terminal oxygen atom approaches the β-C and the hydroxyl H atom of syn-VA respectively, forming the pre-reactive complexes Com-C1H4a and Com-C1H4b, which are 7.5 and 9.0 kcal mol−1 more stable than the free reactants. In Com-C1H4a, the central oxygen atom (O9) of CH2OO lies on the same side with hydroxyl O atom (O3) of syn-VA, while in Com-C1H4b, they lie on the opposite side.
As shown in Fig. 1(a) and 3(a), starting from Com-C1H4a, the CH2OO carbon atom attacks β-C and the hydroxyl H atom shifts to the terminal oxygen of CH2OO forming the intermediate IM1 (3-hydroperoxy-propanal), the relative energies of TS1 and IM1 are −5.7 and −57.5 kcal mol−1. The energized 3-hydroperoxy-propanal can dissociate directly to CHOCH2CH2O + OH (P1, −13.7 kcal mol−1), or it will proceed via two different isomerization and decomposition pathways to form the same product P2 (H2O + malondialdehyde). Firstly, IM1 isomerizes to IM3 by the rotation of –CHO (TS2, −54.8 kcal mol−1) and –OH groups (TS3, −50.2 kcal mol−1), and then decomposes to P2 by the dissociation of the O9–O10 bond accompanied by H11 shifting from C8 to O10 (TSD1, −8.2 kcal mol−1). Secondly, IM1 isomerizes to IM4 by the molecular skeleton rotation (TS4, −54.3 kcal mol−1) to facilitate the subsequent H1 atom shift from C8 to O10, and then decomposes to P2 by H shift and O–O bond fission (TSD2, −6.5 kcal mol−1). The relative energies of the local minima IM2, IM3, IM4, and P2 are −57.0, −57.2, −57.4, and −116.4 kcal mol−1, respectively. The dissociation to CHOCH2CH2O + OH may be energetically more favorable due to the high-energy TSD1 and TSD2 in the formation of H2O + malondialdehyde.
As shown in Fig. 1(b) and 3(a), starting from Com-C1H4b, the 1,4-insertion of CH2OO over C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH moiety forms the intermediate IM5 via TS5, the respective relative energy of TS5 and IM5 are −5.6 and −57.4 kcal mol−1. IM5 can isomerize to IM6 (−57.5 kcal mol−1) by rotation of –CHO and –OH (TS6, −55.9 kcal mol−1), and IM6 then isomerizes to IM7 (−57.0 kcal mol−1) by molecular skeleton rotation (TS7, −52.2 kcal mol−1). IM5, IM6, and IM7 can all decompose to H2O and malondialdehyde, and the relative energies of the corresponding transition state TSD3, TSD4, and TSD5 are −6.5, −7.3 and −8.2 kcal mol−1, respectively. Likewise, IM5 can also form CHOCH2CH2O + OH by direct dissociation.
C–OH moiety forms the intermediate IM5 via TS5, the respective relative energy of TS5 and IM5 are −5.6 and −57.4 kcal mol−1. IM5 can isomerize to IM6 (−57.5 kcal mol−1) by rotation of –CHO and –OH (TS6, −55.9 kcal mol−1), and IM6 then isomerizes to IM7 (−57.0 kcal mol−1) by molecular skeleton rotation (TS7, −52.2 kcal mol−1). IM5, IM6, and IM7 can all decompose to H2O and malondialdehyde, and the relative energies of the corresponding transition state TSD3, TSD4, and TSD5 are −6.5, −7.3 and −8.2 kcal mol−1, respectively. Likewise, IM5 can also form CHOCH2CH2O + OH by direct dissociation.
For the cycloaddition reaction on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, the formation of a five-membered adduct IM8 (−62.8 kcal mol−1) needs to overcome an energy barrier of 0.7 kcal mol−1. As shown in Fig. 2(a), IM8 isomerizes to IM9 (−64.7 kcal mol−1) by the –OH group rotation around C–O bond (TS9), and then IM9 decomposes to products aldehyde and formic acid (P3, −127.1 kcal mol−1) by C1–C2 bond breakage and H12 migration from C8 to C1. It is worth mentioning that the electronic energy of TS9 is only 0.02 kcal mol−1 higher than that of IM8, and the PES from IM8 to TS9 is very flat, which leads to the sum of electronic and zero-point energies of TS9 being slightly lower than IM8. Therefore, only the electronic portion is used to compute the relative height of TS9 to IM8 in this work. For clarity, the relative energy values of IM8 and TS9 in Fig. 3 are kept to two decimal places. The vector of the imaginary frequency in TS9 is the OH rotation around the C–O bond (Fig. S1, ESI†), and the dihedral angle C1C2O3H4 is 73.0, 80.8, and 164.2° in IM8, TS9, and IM9, respectively. The Cartesian coordinates of IM8 and TS9 are shown in the ESI† (Table S1).
C double bond, the formation of a five-membered adduct IM8 (−62.8 kcal mol−1) needs to overcome an energy barrier of 0.7 kcal mol−1. As shown in Fig. 2(a), IM8 isomerizes to IM9 (−64.7 kcal mol−1) by the –OH group rotation around C–O bond (TS9), and then IM9 decomposes to products aldehyde and formic acid (P3, −127.1 kcal mol−1) by C1–C2 bond breakage and H12 migration from C8 to C1. It is worth mentioning that the electronic energy of TS9 is only 0.02 kcal mol−1 higher than that of IM8, and the PES from IM8 to TS9 is very flat, which leads to the sum of electronic and zero-point energies of TS9 being slightly lower than IM8. Therefore, only the electronic portion is used to compute the relative height of TS9 to IM8 in this work. For clarity, the relative energy values of IM8 and TS9 in Fig. 3 are kept to two decimal places. The vector of the imaginary frequency in TS9 is the OH rotation around the C–O bond (Fig. S1, ESI†), and the dihedral angle C1C2O3H4 is 73.0, 80.8, and 164.2° in IM8, TS9, and IM9, respectively. The Cartesian coordinates of IM8 and TS9 are shown in the ESI† (Table S1).
The 1,2-insertion in the O–H bond is a barrierless pathway leading to IM10 with 42.9 kcal mol−1 exoergicity, IM10 may decompose directly to CH2CHOCH2O + OH (P4, 0.1 kcal mol−1), or form CH2OHCHO + HCHO (P5, −99.3 kcal mol−1) via the O3–C8 bond breakage and O10H4 shift from O9 to C1. The 1,2-insertion in the C–H bond needs to overcome the 9.6 kcal mol−1 energy barrier, so it has a negligible contribution compared to other channels.
Comparing the PESs of all the channels in the CH2OO + syn-VA reactions (Fig. 3), we found that all the stationary points in the 1,4-insertion reaction lie below the reactants, and the entrance is barrierless for the 1,4-insertion reaction and 1,2-insertion in the O–H bond. So the 1,4-insertion reaction is more competitive than other channels.
3.2. Reaction mechanism of CH2OO + anti-VA
The optimized geometries of the stationary points in CH2OO + anti-VA reaction are shown in Fig. 4, and the PES are shown in Fig. 5. Similar to the CH2OO + syn-VA reaction, all the reaction channels initiate from the pre-reactive complex, and the corresponding complexes are named Com-C1H4a′ (Com-C1H4b′), Com-add′, Com-OH′ and Com-CH′, respectively.Starting from Com-C1H4a′ and Com-C1H4b′, the 1,4-insertion of CH2OO into anti-VA forms the intermediates IM4 and IM6, respectively. Both IM4 and IM6 can dissociate directly to CHOCH2CH2O + OH. IM4 can isomerize to IM1, IM2, and IM3 successively, IM6 can isomerize to IM5 or IM7, and then IM3, IM4, IM5, IM6, and IM7 decomposes to the product P2 (H2O + malondialdehyde). The cycloaddition reaction of CH2OO + anti-VA forms the adducts IM9 firstly, and IM9 can decompose to the product P3 via TSD6. The optimized structures of IM1–IM9, and their isomerization and dissociation processes have been described in detail in the CH2OO + syn-VA reaction. The O–H and C–H 1,2-insertion reaction results in the formation of respective intermediates IM12 and IM13 and decomposition to the corresponding products P4 (or P7) and P6.
From Fig. 5 we can see that the energies of the stationary points in the cycloaddition and 1,4-insertion reaction are obviously lower than those in other channels. In addition, TS14 (−2.9 kcal mol−1) in the entrance channel of cycloaddition has lower energy than TS12 and TS13 (0.4 and −1.0 kcal mol−1) in the 1,4-insertion reaction. So, the cycloaddition may be favorable in the CH2OO + anti-VA reaction, which is different from the CH2OO + syn-VA reaction.
3.3. Kinetic calculations
The master equation calculations were carried out at the pressure range of 1–760 torr and the temperature range of 200–500 K. According to the reaction PESs, the 1,4-insertion reaction, cycloaddition reaction, and 1,2-insertion reaction in O–H are involved in the master equation calculation, while the 1,2-insertion reaction in C–H bond are excluded because of its higher reaction energy barrier. The calculated TS from the pre-reactive complex is approximately taken as the bottleneck for the present reaction system. The reactants are assumed to be in fast equilibrium with the pre-reactive complex (C), and the rate constant of C → P is much smaller than that of C → CH2OO + vinyl alcohol, like the treatment in a barrierless entrance reaction system.70–72 | ||
| Fig. 6 The rate constants of the CH2OO + syn-VA and CH2OO + anti-VA reactions at 200–500 K and 760 torr. | ||
| Temperature | 200 | 250 | 300 | 350 | 400 | 450 | 500 |
|---|---|---|---|---|---|---|---|
| k (CH2OO + syn-VA) | 3.44 × 10−9 | 1.92 × 10−10 | 2.95 × 10−11 | 8.05 × 10−12 | 3.14 × 10−12 | 3.14 × 10−12 | 3.14 × 10−12 |
| k (CH2OO + anti-VA) | 2.15 × 10−12 | 5.01 × 10−13 | 2.07 × 10−13 | 1.17 × 10−13 | 8.04 × 10−14 | 6.22 × 10−14 | 5.23 × 10−14 |
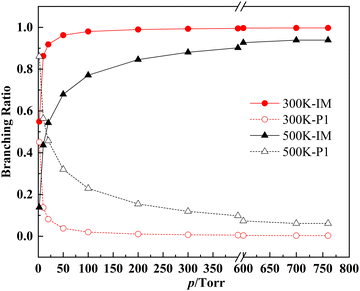
For the CH2OO + syn-VA reaction, the calculation results show that the both the 1,2-insertion and the cycloaddition reaction are negligible compared to the 1,4-insertion reaction. As shown in Fig. 7 and 8, the domain product is the 1,4-insertion intermediate (3-hydroperoxy-propanal) thermalized in collisions with the bath gas at 300 K and 760 torr. The pressure dependence of the products’ branching ratios is obvious at lower pressure ranges. The intermediates IM1–IM7 are shown in Fig. 7 as a whole for clarity, and the individual branching ratios for IM1–IM7 at 300 K and 1 torr, 100 torr and 760 torr are shown in Fig. 8. The ratios of IM1–IM7 are in the order IM6 > IM1 > IM4 > IM5 > IM3 > IM2 > IM7, which are determined by their relative energies (−57.5, −57.5, −57.4, −57.4, −57.2, −57.0, and −57.0 kcal mol−1). The 1,4-insertion intermediate decomposes to CHOCH2CH2O + OH at higher temperature and lower pressure, and the products malondialdehyde + H2O are negligible.
 | ||
| Fig. 8 The branching ratios of the IM1–IM7 in the CH2OO + syn-VA reaction at 300 K and 1 torr, 100 torr and 760 torr. | ||
The products’ branching ratios of the CH2OO + anti-VA reaction are shown in Fig. 9. The dominant product is the cycloaddition intermediate IM9, and it dissociates to P3 (aldehyde and formic acid) gradually with the increase of temperature and the decrease of pressure, and, in the meanwhile, the direct dissociation product P4 (CH2CHOCH2O + OH) of the O–H 1,2-insertion intermediate IM12 has a significant contribution.
Combining the PESs and the kinetic characters of the two reactions, we find that the 1,4-insertion dominated the CH2OO + syn-VA reaction, while the cycloaddition and O–H 1,2-insertion are more favorable in the CH2OO + anti-VA reaction. At 300 K and 760 torr, the 1,4-insertion or cycloaddition intermediates are the main products. At higher temperature and lower pressure, the 1,4-insertion intermediate tends to decompose to CHOCH2CH2O + OH, so the reaction can be regarded as the OH reservoir, and affect the HOx budget, thereby having a potential impact on the oxidative capacity of the atmosphere. In addition, the reaction of CH2OO with vinyl alcohol is significantly faster than CH2OO + saturated alcohol (∼10−13 cm3 molecule−1 s−1),31 and is comparable to the OH + vinyl alcohol reaction (1.48 × 10−11),50 due to the zwitterionic character of CH2OO and special structure of vinyl alcohol, with a double bond in the α-position of the OH group. So this reaction may be an important loss process for both Criegee intermediate and vinyl alcohol.
4. Conclusions
The reaction mechanism of CH2OO with vinyl alcohol has been investigated at the HL//M06-2X/def2TZVP level of theory. The calculation results of the master equation method reveal the temperature and pressure dependent kinetic characters. The following conclusions can be drawn. (1) The 1,4-insertion reaction is the main channel for the CH2OO + syn-VA reaction, while the cycloaddition is more competitive in the CH2OO + anti-VA reaction. (2) The rate constants are 2.95 × 10−11 and 2.07 × 10−13 cm3 molecule−1 s−1 for CH2OO + syn-VA and CH2OO + anti-VA reactions at 300 K and 760 torr, and both show negative temperature effects at 200–500 K. (3) The product distribution is temperature and pressure dependent. At 300 K and 760 torr, the 3-hydroperoxy-propanal formed by 1,4-insertion in the CH2OO + syn-VA reaction and the cycloaddition intermediate in the CH2OO + anti-VA reaction are main products. At higher temperature and lower pressure, 3-hydroperoxy-propanal dissociates to CHOCH2CH2O + OH, and cycloaddition intermediate decomposes to aldehyde and formic acid; in the meanwhile, CH2CHOCH2O + OH are formed by the dissociation of 1,2-insertion (in O-H bond) intermediate in CH2OO + anti-VA reaction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 21603152), the Natural Science Foundation of Hebei Province (B2022205022), and the Science Research Project of Hebei Education Department (ZD2022157). We would also like to thank professor S. W. Zhang for providing technical support on the kinetic calculation of the CH2OO + vinyl alcohol reaction.References
- P. Neeb, O. Horie and G. K. Moortgat, J. Phys. Chem. A, 1998, 102, 6778–6785 CrossRef CAS.
- D. Johnson and G. Marston, Chem. Soc. Rev., 2008, 37, 699–716 RSC.
- K. Samanta, J. M. Beames, M. I. Lester and J. E. Subotnik, J. Chem. Phys., 2014, 141, 134303 CrossRef PubMed.
- F. Liu, J. M. Beames, A. S. Petit, A. B. McCoy and M. I. Lester, Science, 2014, 345, 1596–1598 CrossRef CAS PubMed.
- A. Novelli, L. Vereecken, J. Lelieveld and H. Harder, Phys. Chem. Chem. Phys., 2014, 16, 19941–19951 RSC.
- M. I. Lester and S. J. Klippenstein, Acc. Chem. Res., 2018, 51, 978–985 CrossRef CAS PubMed.
- V. P. Barber, S. Pandit, A. M. Green, N. Trongsiriwat, P. J. Walsh, S. J. Klippenstein and M. I. Lester, J. Am. Chem. Soc., 2018, 140, 10866–10880 CrossRef CAS PubMed.
- Y. Gong and Z. Chen, Atmos. Chem. Phys., 2021, 21, 813–829 CrossRef CAS.
- F. Bianchi, J. Tröstl, H. Junninen, C. Frege, S. Henne, C. R. Hoyle, U. Molteni, E. Herrmann, A. Adamov, N. Bukowiecki, X. Chen, J. Duplissy, M. Gysel, M. Hutterli, J. Kangasluoma, J. Kontkanen, A. Kürten, H. E. Manninen, S. Münch, O. Peräkylä, T. Petäjä, L. Rondo, C. Williamson, E. Weingartner, J. Curtius, D. R. Worsnop, M. Kulmala, J. Dommen and U. Baltensperger, Science, 2016, 352, 1109–1112 CrossRef CAS PubMed.
- H.-L. Huang, W. Chao and J. J.-M. Lin, Proc. Natl. Acad. Sci., 2015, 112, 10857–10862 CrossRef CAS PubMed.
- L. Vereecken, Science, 2013, 340, 154–155 CrossRef CAS PubMed.
- Y.-J. Wu, K. Takahashi and J. J.-M. Lin, J. Phys. Chem. A, 2023, 127, 8059–8072 CrossRef CAS PubMed.
- B. Long, J. L. Bao and D. G. Truhlar, J. Am. Chem. Soc., 2016, 138, 14409–14422 CrossRef CAS PubMed.
- L.-C. Lin, H.-T. Chang, C.-H. Chang, W. Chao, M. C. Smith, C.-H. Chang, J. J.-M. Lin and K. Takahashi, Phys. Chem. Chem. Phys., 2016, 18, 4557–4568 RSC.
- W. Chao, J.-T. Hsieh, C.-H. Chang and J. J.-M. Lin, Science, 2015, 347, 751–754 CrossRef CAS PubMed.
- A. Nazari and V. Saheb, Theor. Chem. Acc., 2022, 141, 66 Search PubMed.
- B. Long, Y. Wang, Y. Xia, X. He, J. L. Bao and D. G. Truhlar, J. Am. Chem. Soc., 2021, 143, 8402–8413 CrossRef CAS PubMed.
- K. Xu, W. Wang, W. Wei, W. Feng, Q. Sun and P. Li, J. Phys. Chem. A, 2017, 121, 7236–7245 CrossRef CAS PubMed.
- T. V. T. Mai, M. v Duong, H. T. Nguyen, K. C. Lin and L. K. Huynh, Chem. Phys. Lett., 2018, 706, 280–284 CrossRef CAS.
- L. Franzon, J. Peltola, R. Valiev, N. Vuorio, T. Kurtén and A. Eskola, J. Phys. Chem. A, 2023, 127, 477–488 CrossRef CAS PubMed.
- O. Welz, A. J. Eskola, L. Sheps, B. Rotavera, J. D. Savee, A. M. Scheer, D. L. Osborn, D. Lowe, A. Murray Booth, P. Xiao, H. K. M. Anwar, C. J. Percival, D. E. Shallcross and C. A. Taatjes, Angew. Chem., Int. Ed., 2014, 53, 4547–4750 CrossRef CAS PubMed.
- L. Vereecken, Phys. Chem. Chem. Phys., 2017, 19, 28630–28640 RSC.
- E. S. Foreman, K. M. Kapnas and C. Murray, Angew. Chem., Int. Ed., 2016, 55, 10419–10422 Search PubMed.
- R. Chhantyal-Pun, M. R. McGillen, J. M. Beames, M. A. H. Khan, C. J. Percival, D. E. Shallcross and A. J. Orr-Ewing, Angew. Chem., Int. Ed., 2017, 56, 9044–9047 CrossRef CAS PubMed.
- P. Raghunath, Y. P. Lee and M. C. Lin, J. Phys. Chem. A, 2017, 121, 3871–3878 CrossRef CAS PubMed.
- C. A. Chung, J. W. Su and Y. P. Lee, Phys. Chem. Chem. Phys., 2019, 21, 21445–21455 RSC.
- W. Chao, Y.-H. Lin, C. Yin, W.-H. Lin, K. Takahashi and J. J.-M. Lin, Phys. Chem. Chem. Phys., 2019, 21, 13633–13640 RSC.
- S. Enami and A. J. Colussi, J. Phys. Chem. A, 2017, 121, 5175–5182 CrossRef CAS PubMed.
- M. R. McGillen, B. F. E. Curchod, R. Chhantyal-Pun, J. M. Beames, N. Watson, M. A. H. Khan, L. McMahon, D. E. Shallcross and A. J. Orr-Ewing, ACS Earth Space Chem., 2017, 1, 664–672 CrossRef CAS.
- Y.-H. Lin, C. Yin, W.-H. Lin, Y.-L. Li, K. Takahashi and J. J.-M. Lin, J. Phys. Chem. Lett., 2018, 9, 7040–7044 CrossRef CAS PubMed.
- S. V. Tadayon, E. S. Foreman and C. Murray, J. Phys. Chem. A, 2018, 122, 258–268 CrossRef CAS PubMed.
- L. Vereecken, H. Harder and A. Novelli, Phys. Chem. Chem. Phys., 2012, 14, 14682–14695 RSC.
- N. U. M. Howes, Z. S. Mir, M. A. Blitz, S. Hardman, T. R. Lewis, D. Stone and P. W. Seakins, Phys. Chem. Chem. Phys., 2018, 20, 22218–22227 RSC.
- A. J. Eskola, M. Dontgen, B. Rotavera, R. L. Caravan, O. Welz, J. D. Savee, D. L. Osborn, D. E. Shallcross, C. J. Percival and C. A. Taatjes, Phys. Chem. Chem. Phys., 2018, 20, 19373–19381 RSC.
- C. Sun, S. Zhang, J. Yue and S. Zhang, J. Phys. Chem. A, 2018, 122, 8729–8737 CrossRef CAS PubMed.
- P.-B. Wang, D. G. Truhlar, Y. Xia and B. Long, Phys. Chem. Chem. Phys., 2022, 24, 13066–13073 RSC.
- Y. Wei, Q. Zhang, X. Huo, W. Wang and Q. Wang, Chemosphere, 2022, 296, 133717 CrossRef CAS PubMed.
- Z. J. Buras, R. M. Elsamra, A. Jalan, J. E. Middaugh and W. H. Green, J. Phys. Chem. A, 2014, 118, 1997–2006 CrossRef CAS PubMed.
- L. Vereecken, H. Harder and A. Novelli, Phys. Chem. Chem. Phys., 2014, 16, 4039–4049 RSC.
- Z. J. Buras, R. M. Elsamra and W. H. Green, J. Phys. Chem. Lett., 2014, 5, 2224–2228 CrossRef CAS PubMed.
- L. Vereecken, A. R. Rickard, M. J. Newland and W. J. Bloss, Phys. Chem. Chem. Phys., 2015, 17, 23847–23858 RSC.
- R. Chhantyal-Pun, A. Davey, D. E. Shallcross, C. J. Percival and A. J. Orr-Ewing, Phys. Chem. Chem. Phys., 2015, 17, 3617–3626 RSC.
- D. Stone, M. Blitz, L. Daubney, N. U. Howes and P. Seakins, Phys. Chem. Chem. Phys., 2014, 16, 1139–1149 RSC.
- T. Berndt, R. Kaethner, J. Voigtlander, F. Stratmann, M. Pfeifle, P. Reichle, M. Sipila, M. Kulmala and M. Olzmann, Phys. Chem. Chem. Phys., 2015, 17, 19862–19873 RSC.
- M. F. Shaw, D. L. Osborn, M. J. T. Jordan and S. H. Kable, J. Phys. Chem. A, 2017, 121, 3679–3688 CrossRef CAS PubMed.
- D. U. Andrews, B. R. Heazlewood, A. T. Maccarone, T. Conroy, R. J. Payne, M. J. Jordan and S. H. Kable, Science, 2012, 337, 1203–1206 CrossRef CAS PubMed.
- A. T. Archibald, M. R. McGillen, C. A. Taatjes, C. J. Percival and D. E. Shallcross, Geophys. Res. Lett., 2007, 34, L21801 CrossRef.
- S. So, U. Wille and G. da Silva, Environ. Sci. Technol., 2014, 48, 6694–6701 CrossRef CAS PubMed.
- M. F. Shaw, B. Sztaray, L. K. Whalley, D. E. Heard, D. B. Millet, M. J. T. Jordan, D. L. Osborn and S. H. Kable, Nat. Commun., 2018, 9, 2584 CrossRef PubMed.
- X. Lei, W. Wang, J. Cai, C. Wang, F. Liu and W. Wang, J. Phys. Chem. A, 2019, 123, 3205–3213 CrossRef CAS PubMed.
- G. E. Scuseria, C. L. Janssen and H. F. Schaefer, III, J. Chem. Phys., 1988, 89, 7382–7387 CrossRef CAS.
- C. Møller and M. S. Plesset, Phys. Rev., 1934, 46, 618–622 CrossRef.
- M. Head-Gordon, J. A. Pople and M. J. Frisch, Chem. Phys. Lett., 1988, 153, 503–506 CrossRef CAS.
- J. A. Miller and S. J. Klippenstein, J. Phys. Chem. A, 2003, 107, 2680–2692 CrossRef CAS.
- C. H. Sun, B. E. Xu and S. W. Zhang, J. Phys. Chem. A, 2014, 118, 3541–3551 CrossRef CAS PubMed.
- C. Sun, B. Xu, L. Lv and S. Zhang, Phys. Chem. Chem. Phys., 2019, 21, 16583–16590 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013 Search PubMed.
- R. G. Gilbert and S. C. Smith, Theory of Unimolecular and Recombination Reactions, Blackwell Scientific, Carlton, Australia, 1990 Search PubMed.
- K. A. Holbrook, Michael J. Pilling and S. H. Robertson, Unimolecular Reactions, Wiley, New York, 1996 Search PubMed.
- S. J. Klippenstein and L. B. Harding, J. Phys. Chem. A, 2000, 104, 2351–2354 CrossRef CAS.
- L. K. Huynh, H. R. Zhang, S. Zhang, E. Eddings, A. Sarofim, M. E. Law, P. R. Westmoreland and T. N. Truong, J. Phys. Chem. A, 2009, 113, 3177–3185 CrossRef CAS PubMed.
- D. R. Glowacki and M. J. Pilling, Chem. Phys. Chem., 2010, 11, 3836–3843 CrossRef CAS PubMed.
- G. da Silva, J. Phys. Chem. A, 2012, 116, 5317–5324 CrossRef CAS PubMed.
- D. W. Chandler and J. A. Miller, J. Chem. Phys., 1984, 81, 455–464 CrossRef CAS.
- J. A. Miller and D. W. Chandler, J. Chem. Phys., 1986, 85, 4502–4508 CrossRef CAS.
- J. A. Miller and S. J. Klippenstein, J. Phys. Chem. A, 2006, 110, 10528–10544 CrossRef CAS PubMed.
- J. A. Miller, S. J. Klippenstein and S. H. Robertson, J. Phys. Chem. A, 2000, 104, 7525–7536 CrossRef CAS.
- L. K. Huynh, H. R. Zhang, S. Zhang, E. Eddings, A. Sarofim, M. E. Law, P. R. Westmoreland and T. N. Truong, J. Phys. Chem. A, 2009, 113, 3177–3185 CrossRef CAS PubMed.
- W. T. Duncan, R. L. Bell and T. N. Truong, J. Comput. Chem., 1998, 19, 1039–1052 CrossRef CAS.
- G. J. R. Aroeira, A. S. Abbott, S. N. Elliott, J. M. Turney and H. F. Schaefer, Phys. Chem. Chem. Phys., 2019, 21, 17760–17771 RSC.
- L. Chen, Y. Huang, Y. Xue, Z. Shen, J. Cao and W. Wang, Atmos. Chem. Phys., 2019, 19, 4075–4091 CrossRef CAS.
- N. A. I. Watson, J. A. Black, T. M. Stonelake, P. J. Knowles and J. M. Beames, J. Phys. Chem. A, 2019, 123, 218–229 CrossRef CAS PubMed.
- W.-m Wei, S. Hong, W.-j Fang, R.-h Zheng and Y.-d Qin, Theor. Chem. Acc., 2019, 138, 13 Search PubMed.
- R. S. Zhu and M. C. Lin, J. Phys. Chem. A, 2000, 104, 10807–10811 CrossRef CAS.
- C. Sun, Y. Liu, B. Xu, Y. Zeng, L. Meng and S. Zhang, J. Chem. Phys., 2013, 139, 154307 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp06115a |
| This journal is © the Owner Societies 2024 |