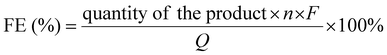The plasmonic effect of Cu on tuning CO2 reduction activity and selectivity†
Jing
Xue
ab,
Zhenlin
Chen
ab,
Kun
Dang
ab,
Lei
Wu
ab,
Hongwei
Ji
ab,
Chuncheng
Chen
 ab,
Yuchao
Zhang
ab,
Yuchao
Zhang
 *ab and
Jincai
Zhao
*ab and
Jincai
Zhao
 ab
ab
aKey Laboratory of Photochemistry, Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: yczhang@iccas.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 20th December 2023
Abstract
Copper (Cu) has been widely used for catalyzing the CO2 reduction reaction (CO2RR), but the plasmonic effect of Cu has rarely been explored for tuning the activity and selectivity of the CO2RR. Herein, we conducted a quantitative analysis on the plasmon-generated photopotential (Ehv) of a Cu nanowire array (NA) photocathode and found that Ehv exclusively reduced the apparent activation energy (Ea) of reducing CO2 to CO without affecting the competitive hydrogen evolution reaction (HER). As a result, the CO production rate was enhanced by 52.6% under plasmon excitation when compared with that under dark conditions. On further incorporation with a polycrystalline Si photovoltaic device, the Cu NA photocathode exhibits good stability in terms of photocurrent and syngas production (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 2
H2 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) within 10 h. This work validates the crucial role of the plasmonic effect of Cu on modulating the activity and selectivity of the CO2RR.
1) within 10 h. This work validates the crucial role of the plasmonic effect of Cu on modulating the activity and selectivity of the CO2RR.
1. Introduction
Plasmonic catalysis has emerged as a promising field where solar energy is efficiently harnessed to drive chemical reactions.1,2 Specifically, the localized surface plasmon resonance (LSPR) phenomenon occurs when a beam of light interacts with metal nanostructures smaller than the wavelength of the incident light, inducing collective oscillation of free electrons on metal surfaces.3,4 After light irradiation, plasmons decay through non-radiation pathways, enhancing surface electromagnetic field, forming hot carriers (electron–hole pairs), and inducing photothermal effects.5–8 Plasmonic metals, such as noble metal gold (Au) and silver (Ag), are recognized as efficient mediators for utilizing solar energy due to their exceptional capability in harvesting a broad spectrum ranging from the ultraviolet-visible (UV-vis) to near-infrared (NIR) region, and are thereby widely researched in water splitting,9–12 ethylene epoxidation,13,14 NH3 decomposition,15 and so on.16–19 However, the scarcity of Au and Ag metals hinders their practical applications. Non-noble metals, particularly copper (Cu), that also exhibit an excellent LSPR effect from the UV-vis to NIR region, present promising opportunities for replacing noble metals in plasmonic catalysis.20,21The concept of carbon neutrality has been proposed to mitigate the escalating atmospheric CO2 levels and convert it into value-added products, thereby achieving the goals of environmental protection and energy storage.22,23 The plasmon-assisted CO2 reduction reaction (CO2RR) provide a sustainable and environmentally friendly approach by harnessing solar energy.24,25 Plasmon-assisted CO2RR systems, such as Au@Ru core–shell nanoflowers and Ag nanopyramids,26–28 have shown great potential not only in improving the activity but also in enhancing the selectivity, as described in several reports. The increase in activity (including current density and product yield) is commonly attributed to the presence of hot carriers or photothermal effects. The selective transfer of hot carriers into antibonding orbitals of the specific surface adsorbates may regulate the reaction path and accelerate chemical reactions by weakening certain chemical bonds.29,30 Plasmon-induced local heating excites the vibrational transitions of all the reactants and accelerates the overall chemical reactions.31,32 It is important to distinguish between hot carrier effects and photothermal effects, as it is crucial for design guidelines for the further optimization of plasmonic nanomaterials.29,33 By analyzing the behavior of the plasmon-induced photocurrent, Tian's group ascribed the rapid response (in the time scale of milliseconds) of photocurrent to the hot carrier effect and the slow response of photocurrent (in the time scale of seconds) to the photothermal effect.32 The utilization of the plasmon-assisted CO2RR can further modulate the product selectivity, facilitating the formation of single/multi-carbon products while suppressing the hydrogen evolution reaction (HER). Cu has been widely studied as an electrocatalyst in the CO2RR,34–36 while there is little research on the plasmonic effect of Cu on the CO2RR. The quantitative analysis of the contribution of light and unraveling the mechanism of selectivity changes induced by plasmon excitation remain elusive.
The Cu nanowire array (NA) electrode synthesized in situ is anisotropic, displaying two LSPR absorption peaks corresponding to plasmonic resonance in the longitudinal and transverse directions. Besides, many previous studies show that light absorption can be tuned by varying the nanowire diameter and the angle of light incidence.21,37 These advantages of the Cu NAs allow for more flexibility in the optimization of its photoelectrochemical (PEC) activity, endowing it with great potential to be an ideal plasmonic catalyst for the CO2RR. In this work, Cu NAs were employed as a plasmonic catalyst for the CO2RR, where Cu NAs simultaneously acted as electrocatalysts and light absorbers. We observed that visible light excitation of the Cu NA photocathode enhanced the activity and selectivity of the CO2RR and inhibited the competitive HER. By combining PEC techniques, including current differential curve, step potential, and electrochemical impedance experiments, we resolved the contribution of hot carrier effects from photothermal effects. The accumulation of hot electrons resulted in the generation of cathodic photopotential (Ehv), about 34.6 mV at −0.8 VRHE under 3.0 W cm−2 of visible light. The Ehv caused lower energy barriers for CO generation (reduced from 26.61 to 18.95 kJ mol−1). Furthermore, when combined with a Si-based solar cell and an Au/TiO2 photoanode, the Cu NA photocathode exhibited a stable photocurrent density of 8.8 mA cm−2 and generated syngas with a CO/H2 molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 within 10 h. These findings provide insights into the mechanistic role of plasmon excitation of Cu in catalyzing the CO2RR.
1 within 10 h. These findings provide insights into the mechanistic role of plasmon excitation of Cu in catalyzing the CO2RR.
2. Materials and methods
2.1. Preparation of Cu nanowire array catalysts
The Cu NAs were prepared by a template-assisted electrodeposition method. The Cu foil was covered with an anodic aluminum oxide (AAO) template, then another Cu foil was added on the AAO template to form a sandwich-like structure. The electrodeposition process was carried out in a two-electrode system. One Cu foil substrate was covered with an AAO template as a counter electrode and another Cu foil as a working electrode. Cu was filled into the channels of the AAO template by applying a constant voltage of 0.35 V for 1200 s in 0.5 M CuSO4·5H2O and 0.1 M H3BO3 electrolyte. After deposition, the sample was placed in 1 M NaOH for 2 h to dissolve the AAO template. The sample was rinsed in deionized water and dried using Ar. As a result, vertically aligned Cu NAs were obtained on the Cu foil. Finally, Cu NAs were further annealed at 473 K in an air atmosphere for 2 h to obtain a stable photocathode.2.2. PEC reduction process
The CO2RR experiment was performed in a custom-made gastight H-type cell, which was composed of two compartments that were separated by a proton exchange membrane (Nafion-117). Each H-cell chamber was equipped with 20 mL of 0.1 M KHCO3. The reference electrode was Ag/AgCl (3 M KCl), and the counter electrode was a graphite rod electrode. The geometric area of the working electrode (Cu NAs) exposed to electrolytes was controlled at 0.5 cm2. Before the CO2RR measurements, CO2 gas was continuously purged into two compartments for at least 30 min to reach saturation (pH = 6.8). The stirring magnet rotated at a speed of 2000 rpm in the cathode chamber during the CO2RR measurement, and a mass flow controller was used to maintain a constant flow rate of 10 sccm for CO2. All PEC measurements were carried out at an electrochemical workstation (CHI 760E). The electrode potential was converted into the potential of reversible hydrogen electrode (RHE) following the conversion relationship: ERHE = EAg/AgCl + 0.059 × pH + 0.197 V − iR drop (R is the solution resistance of 0.1 M KHCO3, which was obtained by electrochemical impedance spectroscopy (EIS) measurement). Linear sweep voltammetry (LSV) curves were obtained at a scan rate of 20 mV s−1 to qualitatively evaluate the catalytic activity under both dark and light conditions. The potential-step experiment was carried out by multi-potential steps using an appropriate step potential (5 mV) for the various potentials with a pulse width of the step potential of 5 s. The frequency range of EIS was from 5000 to 0.1 Hz with an amplitude of 5 mV at different potentials. The double layer capacitance (Cdl) was obtained by the cyclic voltammetry (CV) method. The potential window of the CV curves ranged from 0.15 to 0.25 VRHE, with scan rates varying from 5 to 30 mV s−1. The effect of light irradiation on the photocurrent was investigated at a regular interval of 20 s for light on/off. A 300 W Xenon lamp (Beijing China Education AU-LIGHT Technology Co., Ltd) with an AM 1.5G filter was used as the light source. All Cu NA electrodes were pre-electrochemically reduced at −1.0 VRHE until the current stabilized before conducting PEC measurements.2.3. Solar-driven PEC device for the CO2RR
The CO2RR was carried out in a typical H-type solar cell, in which the Cu NA electrode was placed in the cathode cell filled with 0.1 M KHCO3, while an Au/TiO2 photoanode was placed in a 0.5 M potassium borate buffer electrolyte. The light source was simulated sunlight with a 300 W Xenon lamp coupled with an AM 1.5G filter. A 53 × 30 mm polycrystalline Si solar cell, with a maximum open circuit voltage of 3 V, was exposed to visible light irradiation to generate the required voltage.2.4. CO2RR product analysis
The exhaust trachea was connected to the gas chromatography instrument (GC, Fuli GC9790 Plus) for quantitative analysis of gas products. CO, C2H4 and C2H6 were detected using a flame ionization detector (FID) and H2 was detected using a thermal conductivity detector (TCD). The liquid product (HCOOH) was analyzed quantitatively by ion chromatography (IC). The faradaic efficiency (FE) of the gas product was calculated as follows:where n is the number of electrons transferred, F is the Faraday constant (96
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), and Q represents the total coulomb of electrons passing through the working electrode.
485 C mol−1), and Q represents the total coulomb of electrons passing through the working electrode.
3. Results and discussion
3.1. Morphology and structure analyses
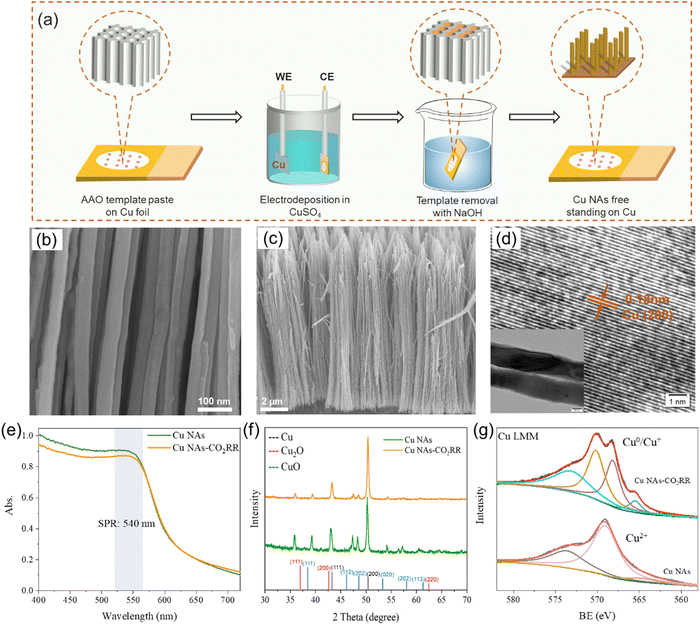
As shown in Fig. 1a, Cu NAs were synthesized by a template-assisted electrodeposition method.38 Scanning electron microscopy (SEM) images in Fig. 1b and c show that the diameter of Cu NA is about 70 nm and the length is 10 μm. Furthermore, high-resolution transmission electron microscopy (HRTEM) revealed that Cu NAs had a lattice fringe with an interplane spacing of 0.18 nm, corresponding to the (200) facet of metallic Cu (Fig. 1d). The UV-vis diffuse reflectance spectrum was tested as the evidence of the absorbance characteristics of plasmonic metals. As shown in Fig. 1e, Cu NAs exhibited wide absorption in the wavelength range of 350–800 nm. The small absorption peak from 500 to 570 nm with maximum absorption at 540 nm was ascribed to the transverse resonance absorption of Cu NAs. As Duan et al. proved,37 plasmonic Cu NAs exhibit two LSPR absorption peaks corresponding to plasmonic resonance in the longitudinal and transverse directions. When the wave vector of the incident light is parallel to the Cu NAs, only transverse modes can be excited. After the PEC reduction process, the LSPR absorption peak position of Cu NAs did not shift. However, the absorption intensity slightly decreased, which is related to the change of the electrode surface color from black (Cu2+) to reddish brown (Cu0/1+). The Cu NAs were also monitored with X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) techniques to study the crystal structure and valence states of the obtained samples (Fig. 1f and g). The synthesized Cu NAs had a polycrystalline structure with Cu+ and Cu2+ presented on the surfaces. After the PEC reduction process, the Cu NAs were predominantly composed of the (200) facet and showed metallic valence state. Meanwhile, rough surfaces were formed due to the deoxidation of Cu1+/2+ during the PEC reduction process (Fig. S1, ESI†). This phenomenon of surface unevenness is consistent with the findings reported by Huang et al.39 The uneven surface can provide more active sites, which is beneficial for the CO2RR performance.403.2. PEC tests for quantitative analysis of plasmonic effects
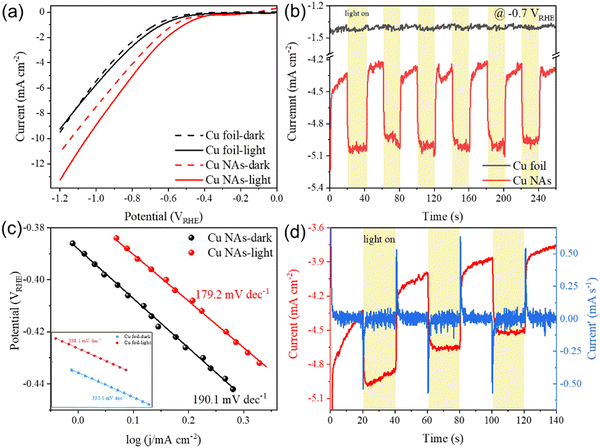
The photo-response induced by the LSPR effect was first compared between the Cu NA photocathode and a commercial Cu foil using linear sweep voltammogram (LSV) measurements in a CO2-saturated 0.1 M KHCO3 electrolyte (Fig. 2a). The Cu NAs exhibited a remarkable photo-response, showing an increase in current density upon LSPR excitation (3.0 W cm−2) compared to the dark conditions, while the current density of the Cu foil remained relatively unchanged under light irradiation. These results were consistent with the measured chronoamperometry (CA) curves under chopped light irradiation at −0.7 VRHE (Fig. 2b), in which ΔjCu NAs = 0.7 mA cm−2, and ΔjCu foil = 0 mA cm−2 (Δj = jphoto − jdark). Tafel analysis showed that the Cu NA photocathode exhibited a slope of 190.1 mV dec−1 in the dark (Fig. 2c). Upon LSPR excitation, the Tafel slope decreased by about 10.9 mV dec−1, indicating that light excitation promotes a faster reaction kinetics.41 In contrast, the Tafel slope of the Cu foil remained unchanged under light irradiation, confirming the lack of photo-response on the Cu foil.The contribution of the hot carrier effect and the photothermal effect was investigated. As shown in Fig. 2d, the photocurrent of the Cu NA photocathode responded immediately upon LSPR excitation, reaching its maximum value within 5 ms, and the current differential curve showed a sharp change. However, the photothermal-induced photocurrent responded much slower (seconds) due to the slow thermal diffusion in surroundings.29,32 Therefore, the measured photocurrent is mainly the contribution of hot carriers. To further eliminate the contribution of photothermal effects, we conducted CA measurements at various temperatures under chopped light irradiation (Fig. S2, ESI†). The control experiment showed that an increase in temperature led to a corresponding current increase in the dark. However, it was worth noting that when the temperature increased from 298 to 313 K, Δj remained around 0.53 mA cm−2 at −0.6 VRHE. This observation suggests that there is no direct correlation between the increase in temperature and the enhancement of Δj, implying a minimal contribution of the photothermal effect to the generation of photocurrent.
The value of jphoto is determined by the applied light intensity (I), and a simple quantitative model has been reported as shown in the Formula derivation section of the ESI† (eqn (S1)–(S5)). We obtained CA curves for the Cu NA photocathode with different light intensities at −0.7 VRHE under chopped light irradiation (Fig. S3, ESI†), and drew a curve of jphoto as a function of light intensity (Fig. 3a). The results showed super-linear relationship between light intensity and jphoto, which served as a distinctive feature of the hot-carrier-driven redox reaction of plasmonic nanomaterials. Accordingly, the photochemical conversion coefficient Φ (s cm2 mol−1) was determined by plotting RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(jphoto) against light intensity.16,42 The slope of the plot gave a Φ of approximately 258.7 s cm2 mol−1, indicating that LSPR excitation of an incident intensity of 1.0 W cm−2 contributed about 0.26 kJ mol−1 to the free energy of the reaction (Fig. S4, ESI†).
ln(jphoto) against light intensity.16,42 The slope of the plot gave a Φ of approximately 258.7 s cm2 mol−1, indicating that LSPR excitation of an incident intensity of 1.0 W cm−2 contributed about 0.26 kJ mol−1 to the free energy of the reaction (Fig. S4, ESI†).
Plasmonic Cu generates hot electrons and holes under light excitation. Hot holes are transferred to the counter electrode through an external circuit assisted by the applied bias, while hot electrons accumulate on the surface of Cu NAs. After that, a stable electron distribution with Fermi levels higher than those under the dark conditions was obtained, generating a cathode photopotential (Ehv).32 The potential step method enables the simulation of photocurrent behaviour and determination of the value of Ehv, in which changes in current (ΔI) was obtained by adjusting the step potential (ΔP) in the dark (Fig. S5a, ESI†). Accordingly, Ehv could be calculated based on the observed changes of photocurrent under light conditions. The linear relationship between ΔI and ΔP in the dark is shown in Fig. S5b (ESI†), and the increase in current caused by illumination is shown in Fig. S5c (ESI†). As summarized in Fig. 3b, the Ehv increased from 25.5 mV at −0.6 VRHE to 34.6 mV at −0.8 VRHE, indicating that the applied potential caused the increase of Ehv. Furthermore, a large difference was observed between the values of Ehv under Ar (12.2 mV) and CO2 (34.6 mV) atmospheres at −0.8 VRHE, suggesting that the presence of a CO2 atmosphere plays a crucial role in enhancing the separation of hot carriers.
To further illustrate the effect of CO2 atmosphere on the separation of hot carriers, transient photovoltage (TPV) and transient photocurrent experiments were conducted in Ar and CO2 atmospheres (Fig. S6, ESI†).43 The fitted time attenuation constant was enhanced to 362.7 ns compared to that for the CO2-free condition (312.2 ns), and the calculated value of Δj was greater in CO2 atmosphere (ΔjCO2 = 0.83 mA cm−2) than in Ar atmosphere (ΔjAr = 0.53 mA cm−2) at −0.7 VRHE. These results confirmed that the CO2 atmosphere promoted hot carrier separation.
The generation of Ehv is reported to supply electrons either for the charging of the electrode/electrolyte double layer or driving faradaic reactions.9 To determine whether the hot electrons were involved in the charging process of the double layer, we tested the CV curves of the non-faradaic region at different scan rates with and without light irradiation (Fig. S7, ESI†). The double layer capacitance (Cdl) was obtained from the slope of current density corresponding to different scan rates.44 The Cdl values of Cu NAs under both dark and light conditions were both 11.2 mF cm−2, as shown in Fig. 3c. The result suggested that the Ehv generated by light irradiation did not contribute to the Cdl component, but was solely utilized for faradaic reactions.
The interfacial hot-electron transfer kinetics was analyzed by using EIS,9 and the Nyquist plots were obtained in the dark and under light conditions at various potentials. The arc radius of the Nyquist plot in the presence of light was smaller than that under the dark conditions (Fig. 3d), suggesting that the charge-transfer process was facilitated by the participation of hot electrons. Further equivalent circuit fitting also proved that the charge-transfer resistance (Rct) could be reduced under light irradiation (Fig. 3e and Table S1, ESI†). The capacitance value is another critical parameter that closely correlates with variations in the amount of surface adsorbed species (such as *CO, Fig. S8, ESI†).45,46 It is reported that the effective capacitance (Ceff) value is related to “surface cleanliness”, in other words, an increase in the number of adsorbed species on the surface will lead to a decrease in the Ceff value.26 Due to the larger aspect ratio of Cu NAs, the electrochemical active surface area is significantly enlarged, resulting in a higher Ceff (on the order of mF) compared to spherical nanomaterials reported in the literature (on the order of μF).47 The Ceff values increased significantly with the increase of potentials after −0.65 VRHE under both dark and light conditions, as depicted in Fig. 3f, indicating a surface with reduced adsorbed species. More importantly, Ceff decreased from 11.3 to 8.4 mF at −0.7 VRHE under light irradiation, indicating an increase in the amount of surface adsorption species of *CO.
3.3. Plasmon-assisted CO2RR performance
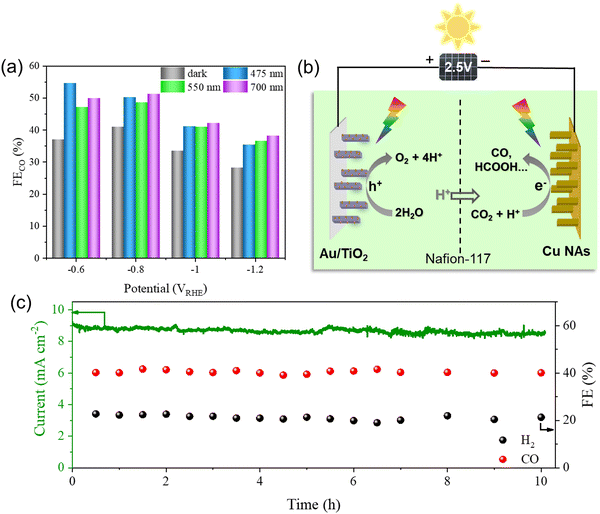
The CO2 reduction products on the Cu NA photocathode were analyzed by using online gas chromatography (GC) and ion chromatography (IC) at various potentials ranging from −0.6 to −1.4 VRHE (Fig. S9–S11, ESI†). Carbon monoxide (CO) and formate (HCOO−) were the main reduction products, with a small amount of C2 (C2H4, C2H6) yield. It is worth noting that light excitation of the plasmonic Cu NAs with 420 nm ≤ λ ≤ 800 nm (3.0 W cm−2) induced a marked change in the FE of products (especially for H2 and CO) compared to that observed during dark electrocatalysis (Fig. S12 and Table S2, ESI†). Specifically, we observed a decrease in the FE for H2 evolution concomitantly with an increase in the FE for CO at all applied potentials. The lowest FE of H2 was observed at −0.8 VRHE, and the FE of H2 decreased from 24.2% in the dark to 20.6% under light irradiation (Fig. 4a). The decrease in HER activity was accompanied by an enhancement in the selectivity for CO2RR. The FE of CO increased by 11.4% compared to that observed without light and accounted for 54.7% of the total products (Fig. 4b). The partial current densities associated with H2 (jH2) and CO (jCO) are shown in Fig. 4c and d. We only observed very little change in jH2 between dark and light conditions, but there was a noticeable increase in jCO. At −1.4 VRHE, the jCO was increased by 53.1%, from 3.2 mA cm−2 in the dark to 4.9 mA cm−2 under light irradiation. Correspondingly, the production rate of CO was increased by 52.6%, from 59.8 μmol cm−2 h−1 in the dark to 91.2 μmol cm−2 h−1 under light irradiation (Fig. 4e), while the production rate of H2 did not change significantly (Fig. 4f and Fig. S13, ESI†). These findings demonstrated that the light irradiation of the Cu NA photocathode preferentially enhanced the catalytic activity towards the CO2RR without affecting the HER across all applied potentials. The CO2 reduction products on the Cu foil were analyzed under different applied potentials in the dark and under light irradiation. The FE of all products (including H2, CO, HCOOH, CH4, and C2) under light irradiation was almost unchanged compared with that dark conditions (Fig. S14, ESI†), which also proved that Cu foil has no photo-response. | ||
| Fig. 4 The FE (a) and (b), partial current density (c) and (d), and production rate (e) and (f) of H2 and CO in the dark and under light irradiation at different applied potentials. | ||
Syngas composed of CO/H2 with different molar ratios can be precisely tailored to match downstream chemical processes to get value-added chemicals.48–50 For example, a molar ratio of CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1
H2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 can be used to synthesize olefins and alcohols, while a molar ratio of CO
2 can be used to synthesize olefins and alcohols, while a molar ratio of CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 2
H2 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can produce short-chain hydrocarbon. The CO/H2 molar ratio in our study could be adjusted from 0.40 to 1.70 in the dark and from 0.67 to 2.60 under light irradiation (Fig. S15, ESI†). Therefore, the strategy of plasmon excitation offers a novel approach for both promoting CO2RR and regulating product composition.
1 can produce short-chain hydrocarbon. The CO/H2 molar ratio in our study could be adjusted from 0.40 to 1.70 in the dark and from 0.67 to 2.60 under light irradiation (Fig. S15, ESI†). Therefore, the strategy of plasmon excitation offers a novel approach for both promoting CO2RR and regulating product composition.
3.4. Effect of plasmon excitation on the reaction mechanism
To gain insights into the energetics involved in the plasmon-assisted hot electron transfer reaction, we measured the apparent activation energy (Ea) for the reaction in the dark and under light irradiation. The reaction rate was measured as a function of temperature ranging from 288 to 313 K with an interval of 5 K (Fig. S16, ESI†). The slope obtained by linear fitting of each Arrhenius plot was used to determine Ea.42,51 It was noted that the Ea was reduced from 26.61 to 18.95 kJ mol−1 upon LSPR excitation for the generation of CO (Fig. 5a), confirming that the LSPR excitation on the Cu NA photocathode efficiently reduces the energy barrier for CO2RR. However, as shown in Fig. 5b, the change of Ea for the HER was minimal (varies from 26.42 to 25.66 kJ mol−1 under light irradiation).To better illustrate the mechanism of the plasmon-assisted CO2RR, reaction free energy profiles are depicted in Fig. 5c. CO is the main CO2RR product via a two-electron pathway, and the process of reducing CO2 to CO goes along CO2 → *CO2 → *COOH → *CO → CO.43,52 First, the adsorption of CO2 on the surface of the Cu NA photocathode begins with an electron transfer, forming *CO2δ−, which is also considered as the rate determining step (RDS). The transfer of a proton leads to the formation of *COOH, followed by a second proton-coupled electron transfer that results in the formation of *CO. Finally, *CO desorbs to form CO gas. As obtained from Fig. 5a, the plasmon-generated Ehv exclusively reduced the Ea in the first step under light irradiation. Cu is widely acknowledged to possess moderate adsorption energy for CO, thereby facilitating the formation of multi-carbon products through C–C coupling. However, the FE of the C2 product did not increase under light irradiation in this work (Fig. S17, ESI†), implying that the plasmon effect on Cu did not promote the coupling between *CO species. This phenomenon reminds us of the transient negative ion (TNI) theory in the study of plasmonic catalysis.53,54 Specifically, the hot electrons generated by LSPR excitation can transfer to an unpopulated adsorbate state to form a TNI, and then the adsorbate shifts to the excited state. If the energy deposited in the adsorbate is insufficient to overcome the activation barrier, the electron will be released back to the metal and the adsorbate will return to the ground state with additional vibration energy. During this process, hot electrons do not participate in the reduction process and only provide additional energy to promote the desorption of the adsorbate. In the process of plasmon-assisted CO2RR, the adsorbed CO was not further reduced by hot electrons to multi electron products, and an increase in the CO production rate was observed. Therefore, we hold that hot electrons only provide the energy to overcome the adsorption energy barrier of *CO through the TNI process.
Considering that light irradiation may cause an increase in the electrolyte temperature,55 we investigated whether the alterations in temperature could cause changes in the FE of CO without light. The temperature-dependent variations of FE are shown in Fig. 5d. The FE of H2 tended to increase with rising temperature, while the FE of CO remained unchanged across different temperatures. The results disclosed that an increase in temperature promoted faster kinetics of HER rather than CO2RR.56 Conversely, it was observed that light irradiation promoted the CO2RR while suppressing the HER, which also ruled out the contribution of the photothermal effect to the CO2RR.
3.5. Solar-driven PEC device for CO2RR
The plasmonic Cu NAs exhibited broad light absorption characteristics, and we investigated the CO2RR performance of the Cu NA photocathode at various wavelengths (475 nm, 550 nm, and 700 nm). Compared to the dark condition, the irradiation of all tested wavelengths led to an enhancement in photocurrents (Fig. S18, ESI†). Notably, excitation at different wavelengths also significantly improved the selectivity of CO (Fig. 6a). Inspired by this, we conducted a solar-driven PEC device that employed the Au/TiO2 photoanode for OER and the Cu NA photocathode for CO2RR (Fig. 6b). The cell was powered by a commercial Si solar cell with an output photovoltage of 2.5 V. The plasmonic Au/TiO2 photoanode exhibited higher photocurrent stability than TiO2 during the OER process due to the generation of electron-rich surface states at the interfaces between Au and TiO2, which was investigated in our previous work (Fig. S19, ESI†). We tested the stability of the Cu NA photocathode during the CO2RR process. As shown in Fig. 6c, the FEs of CO (40%) and H2 (20%), as well as the photocurrent density (8.8 mA cm−2) remained stable during the 10 h of the test. Consequently, the syngas with a stable CO/H2 molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was obtained (Fig. S20, ESI†).
1 was obtained (Fig. S20, ESI†).
4. Conclusions
In summary, taking the Cu NA photocathode for the CO2RR as a model system, the plasmonic effect on tuning CO2 reduction activity and selectivity was investigated. Under plasmon excitation, the photocurrent density of the Cu NA photocathode was enhanced and the Tafel slope was reduced, which was attributed to the contribution of hot carriers. The accumulation of hot electrons resulted in the generation of Ehv, which selectively reduced the Ea of CO2 to CO without affecting the HER. The designed solar-driven PEC device, composed of the plasmonic Cu NA photocathode and an Au/TiO2 photoanode, exhibited good photostability and stable syngas composition (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 2
H2 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). These findings provide insight into the role of LSPR excitation in enhancing the electrocatalytic activity of nanostructured plasmonic metals and tuning the selectivity.
1). These findings provide insight into the role of LSPR excitation in enhancing the electrocatalytic activity of nanostructured plasmonic metals and tuning the selectivity.
Author contributions
Y. Zhang directed the project. J. Xue carried out most of the experiments. Y. Zhang and J. Xue wrote the manuscript, with input from others. C. Zhen and K. Dang conducted the electron microscopy characterization and XRD experiments. L. Wu assisted in the EIS experiments. K. Dang assisted in the Raman experiments. All the authors analyzed the results and reviewed the paper.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2022YFA1505000), the National Natural Science Foundation of China (No. 22072158), the Strategic Priority Research Program of Chinese Academy of Sciences, Grant No. XDB36000000 and CAS Project for Young Scientists in Basic Research YSBR-004.References
- S. Linic, U. Aslam, C. Boerigter and M. Morabito, Photochemical Transformations on Plasmonic Metal Nanoparticles, Nat. Mater., 2015, 14, 567–576 CrossRef CAS PubMed.
- R. Nixon, E. Contreras and P. K. Jain, Electrochemistry with plasmons, Trends Chem., 2023, 5, 605–619 CrossRef CAS.
- P. Lyu, R. Espinoza and S. C. Nguyen, Photocatalysis of Metallic Nanoparticles: Interband vs. Intraband Induced Mechanisms, J. Phys. Chem. C, 2023, 127, 15685–15698 CrossRef CAS PubMed.
- Z. Zhang, C. Zhang, H. Zheng and H. Xu, Plasmon-Driven Catalysis on Molecules and Nanomaterials, Acc. Chem. Res., 2019, 52, 2506–2515 CrossRef CAS PubMed.
- Y. Zhang, W. Guo, Y. Zhang and W. D. Wei, Plasmonic Photoelectrochemistry: In View of Hot Carriers, Adv. Mater., 2021, 33, 2006654–2006669 CrossRef CAS PubMed.
- Y. Zhang, S. He, W. Guo, Y. Hu, J. Huang, J. R. Mulcahy and W. D. Wei, Surface-Plasmon-Driven Hot Electron Photochemistry, Chem. Rev., 2018, 118, 2927–2954 CrossRef CAS PubMed.
- A. M. Brown, R. Sundararaman, P. Narang, W. A. Goddard and H. A. Atwater, Nonradiative Plasmon Decay and Hot Carrier Dynamics: Effects of Phonons, Surfaces, and Geometry, ACS Nano, 2015, 10, 957–966 CrossRef PubMed.
- Q. Hao, Z. Li, Y. Shi, R. Li, Y. Li, L. Wang, H. Yuan, S. Ouyang and T. Zhang, Plasmon-Induced Radical-Radical Heterocoupling Boosts Photodriven Oxidative Esterification of Benzyl Alcohol over Nitrogen-Doped Carbon-Encapsulated Cobalt Nanoparticles, Angew. Chem., Int. Ed., 2023, 62, e202312808 CrossRef CAS PubMed.
- A. J. Wilson, V. Mohan and P. K. Jain, Mechanistic Understanding of Plasmon-Enhanced Electrochemistry, J. Phys. Chem. C, 2019, 123, 29360–29369 CrossRef CAS.
- X. Zhang, A. Fu, X. Chen, L. Liu, L. Ren, L. Tong and J. Ye, Highly Efficient Cu Induced Photocatalysis for Visible-light Hydrogen Evolution, Catal. Today, 2019, 335, 166–172 CrossRef CAS.
- J. Xue, L. Wu, C. Deng, D. Tang, S. Wang, H. Ji, C. Chen, Y. Zhang and J. Zhao, Plasmon-Mediated Electrochemical Activation of Au/TiO2 Nanostructure-Based Photoanodes for Enhancing Water Oxidation and Antibiotic Degradation, ACS Appl. Nano Mater., 2022, 5, 11342–11351 CrossRef CAS.
- Y. Zhang, Y. Zhang, W. Guo, A. C. Johnston-Peck, Y. Hu, X. Song and W. D. Wei, Modulating Multi-hole Reaction Pathways for Photoelectrochemical Water Oxidation on Gold Nanocatalysts, Energy Environ. Sci., 2020, 13, 1501–1508 RSC.
- C. Zhan, Q.-X. Wang, J. Yi, L. Chen, D.-Y. Wu, Y. Wang, Z.-X. Xie, M. Moskovits and Z.-Q. Tian, Plasmonic Nanoreactors Regulating Selective Oxidation by Energetic Electrons and Nanoconfined Thermal Fields, Sci. Adv., 2021, 7, eabf0962 CrossRef CAS PubMed.
- A. Marimuthu, J. Zhang and S. Linic, Tuning Selectivity in Propylene Epoxidation by Plasmon Mediated Photo-Switching of Cu Oxidation State, Science, 2013, 339, 1590–1593 CrossRef CAS PubMed.
- L. Zhou, D. F. Swearer, C. Zhang, H. Robatjazi, H. Zhao, L. Henderson, L. Dong, P. Christopher, E. A. Carter, P. Nordlander and N. J. Halas, Quantifying Hot Carrier and Thermal Contributions in Plasmonic Photocatalysis, Science, 2018, 362, 69–72 CrossRef CAS PubMed.
- J. Wang, J. Heo, C. Chen, A. J. Wilson and P. K. Jain, Ammonia Oxidation Enhanced by Photopotential Generated by Plasmonic Excitation of a Bimetallic Electrocatalyst, Angew. Chem., Int. Ed., 2020, 59, 18430–18434 CrossRef CAS.
- F. Tong, X. Liang, Z. Wang, Y. Liu, P. Wang, H. Cheng, Y. Dai, Z. Zheng and B. Huang, Probing the Mechanism of Plasmon-Enhanced Ammonia Borane Methanolysis on a CuAg Alloy at a Single-Particle Level, ACS Catal., 2021, 11, 10814–10823 CrossRef CAS.
- F. Tong, X. Liang, M. Liu, Z. Wang, Y. Liu, P. Wang, H. Cheng, Y. Dai, Z. Zheng and B. Huang, Plasmon-Enhanced Water Activation for Hydrogen Evolution from Ammonia-Borane Studied at a Single-Particle Level, ACS Catal., 2022, 12, 3558–3565 CrossRef CAS.
- M. Lv, X. Zhang, B. Li, X. Gong, Y. Zhang, Z. Wang, Y. Liu, P. Wang, H. Cheng, Y. Dai, Y. Fan, B. Huang and Z. Zheng, Plasmonic Ag Interlayer Induced Direct Energy Transfer Studied at Single-Particle Level, ACS Energy Lett., 2023, 8, 4033–4042 CrossRef CAS.
- Y. Xin, K. Yu, L. Zhang, Y. Yang, H. Yuan, H. Li, L. Wang and J. Zeng, Copper-Based Plasmonic Catalysis: Recent Advances and Future Perspectives, Adv. Mater., 2021, 33, 2008145–2008170 CrossRef CAS PubMed.
- S. Jeong, Y. Liu, Y. Zhong, X. Zhan, Y. Li, Y. Wang, P. M. Cha, J. Chen and X. Ye, Heterometallic Seed-Mediated Growth of Monodisperse Colloidal Copper Nanorods with Widely Tunable Plasmonic Resonances, Nano Lett., 2020, 20, 7263–7271 CrossRef CAS.
- L. Li, Y. Zhang, T. Zhou, K. Wang, C. Wang, T. Wang, L. Yuan, K. An, C. Zhou and G. Lü, Mitigation of China's Carbon Neutrality to Global Warming, Nat. Commun., 2022, 13, 5315–5321 CrossRef CAS PubMed.
- Z. Liu, Z. Deng, G. He, H. Wang, X. Zhang, J. Lin, Y. Qi and X. Liang, Challenges and Opportunities for Carbon Neutrality in China, Nat. Rev. Earth Environ., 2021, 3, 141–155 CrossRef.
- T. Le, Y. Shao and B. Wang, Plasmon-Induced CO2 Conversion on Al@Cu2O: A DFT Study, J. Phys. Chem. C, 2021, 125, 6108–6115 CrossRef CAS.
- Z. Wang, H. Song, H. Pang, Y. Ning, T. D. Dao, Z. Wang, H. Chen, Y. Weng, Q. Fu, T. Nagao, Y. Fang and J. Ye, Photo-Assisted Methanol Synthesis via CO2 Reduction under Ambient Pressure over Plasmonic Cu/ZnO Catalysts, Appl. Catal., B, 2019, 250, 10–16 CrossRef CAS.
- M. P. S. Rodrigues, A. H. B. Dourado, A. G. Sampaio de Oliveira-Filho, A. P. de Lima Batista, M. Feil, K. Krischer and S. I. Córdoba de Torresi, Gold–Rhodium Nanoflowers for the Plasmon-Enhanced CO2 Electroreduction Reaction upon Visible Light, ACS Catal., 2022, 13, 267–279 CrossRef.
- Y. Kim, E. B. Creel, E. R. Corson, B. D. McCloskey, J. J. Urban and R. Kostecki, Surface-Plasmon-Assisted Photoelectrochemical Reduction of CO2 and NO3− on Nanostructured Silver Electrodes, Adv. Energy Mater., 2018, 8, 1800363 CrossRef.
- E. B. Creel, E. R. Corson, J. Eichhorn, R. Kostecki, J. J. Urban and B. D. McCloskey, Directing Selectivity of Electrochemical Carbon Dioxide Reduction Using Plasmonics, ACS Energy Lett., 2019, 4, 1098–1105 CrossRef CAS.
- W. Ou, B. Zhou, J. Shen, T. W. Lo, D. Lei, S. Li, J. Zhong, Y. Y. Li and J. Lu, Thermal and Nonthermal Effects in Plasmon-Mediated Electrochemistry at Nanostructured Ag Electrodes, Angew. Chem., Int. Ed., 2020, 59, 6790–6793 CrossRef CAS PubMed.
- X. Zhang, X. Li, M. E. Reish, D. Zhang, N. Q. Su, Y. Gutiérrez, F. Moreno, W. Yang, H. O. Everitt and J. Liu, Plasmon-Enhanced Catalysis: Distinguishing Thermal and Nonthermal Effects, Nano Lett., 2018, 18, 1714–1723 CrossRef CAS PubMed.
- W. Ou, Y. Fan, J. Shen, Y. Xu, D. Huang, B. Zhou, T. W. Lo, S. Li, Y. Y. Li, D. Lei and J. Lu, Plasmoelectric Potential in Plasmon-Mediated Electrochemistry, Nano Lett., 2022, 22, 8397–8405 CrossRef CAS PubMed.
- C. Zhan, B.-W. Liu, Y.-F. Huang, S. Hu, B. Ren, M. Moskovits and Z.-Q. Tian, Disentangling Charge Carrier from Photothermal Effects in Plasmonic Metal Nanostructures, Nat. Commun., 2019, 10, 2671–2678 CrossRef PubMed.
- E. Cortés, R. Grzeschik, S. A. Maier and S. Schlücker, Experimental Characterization Techniques for Plasmon-Assisted Chemistry, Nat. Rev. Chem., 2022, 6, 259–274 CrossRef PubMed.
- K. P. Kuhl, E. R. Cave, D. N. Abram and T. F. Jaramillo, New Insights into the Electrochemical Reduction of Carbon Dioxide on Metallic Copper Surfaces, Energy Environ. Sci., 2012, 5, 7050–7059 RSC.
- H. Xiong, Q. Sun, K. Chen, Y. Xu, X. Chang, Q. Lu and B. Xu, Correlating the Experimentally Determined CO Adsorption Enthalpy with the Electrochemical CO Reduction Performance on Cu Surfaces, Angew. Chem., Int. Ed., 2023, 62, e202218447 CrossRef CAS PubMed.
- Y. Yang, S. Louisia, S. Yu, J. Jin, I. Roh, C. Chen, M. V. Fonseca Guzman, J. Feijóo, P.-C. Chen, H. Wang, C. J. Pollock, X. Huang, Y.-T. Shao, C. Wang, D. A. Muller, H. D. Abruña and P. Yang, Operando Studies Reveal Active Cu Nanograins for CO2 Electroreduction, Nature, 2023, 614, 262–269 CrossRef CAS PubMed.
- J. L. Duan, T. W. Cornelius, J. Liu, S. Karim, H. J. Yao, O. Picht, M. Rauber, S. Müller and R. Neumann, Surface Plasmon Resonances of Cu Nanowire Arrays, J. Phys. Chem. C, 2009, 113, 13583–13587 CrossRef CAS.
- Y. Zhou, Y. Liang, J. Fu, K. Liu, Q. Chen, X. Wang, H. Li, L. Zhu, J. Hu, H. Pan, M. Miyauchi, L. Jiang, E. Cortés and M. Liu, Vertical Cu Nanoneedle Arrays Enhance the Local Electric Field Promoting C2 Hydrocarbons in the CO2 Electroreduction, Nano Lett., 2022, 22, 1963–1970 CrossRef CAS PubMed.
- C. Choi, S. Kwon, T. Cheng, M. Xu, P. Tieu, C. Lee, J. Cai, H. M. Lee, X. Pan, X. Duan, W. A. Goddard III and Y. Huang, Highly Active and Stable Stepped Cu Surface for Enhanced Electrochemical CO2 Reduction to C2H4, Nat. Catal., 2020, 3, 804–812 CrossRef CAS.
- J. Feijóo, Y. Yang, M. V. Fonseca Guzman, A. Vargas, C. Chen, C. J. Pollock and P. Yang, Operando High-Energy-Resolution X-ray Spectroscopy of Evolving Cu Nanoparticle Electrocatalysts for CO2 Reduction, J. Am. Chem. Soc., 2023, 145, 20208–20213 CrossRef PubMed.
- O. van der Heijden, S. Park, J. J. J. Eggebeen and M. T. M. Koper, Non-Kinetic Effects Convolute Activity and Tafel Analysis for the Alkaline Oxygen Evolution Reaction on NiFeOOH Electrocatalysts, Angew. Chem., Int. Ed., 2023, 62, e202216477 CrossRef CAS PubMed.
- S. Yu and P. K. Jain, The Chemical Potential of Plasmonic Excitations, Angew. Chem., Int. Ed., 2019, 59, 2085–2088 CrossRef PubMed.
- C. Xu, X. Zhang, M.-N. Zhu, L. Zhang, P.-F. Sui, R. Feng, Y. Zhang and J.-L. Luo, Accelerating Photoelectric CO2 Conversion with a Photothermal Wavelength-Dependent Plasmonic Local Field, Appl. Catal., B, 2021, 298, 120533 CrossRef CAS.
- Z. Lyu, S. Zhu, M. Xie, Y. Zhang, Z. Chen, R. Chen, M. Tian, M. Chi, M. Shao and Y. Xia, Controlling the Surface Oxidation of Cu Nanowires Improves Their Catalytic Selectivity and Stability toward C2+ Products in CO2 Reduction, Angew. Chem., Int. Ed., 2021, 60, 1909–1915 CrossRef CAS PubMed.
- Y. Zhao, X.-G. Zhang, N. Bodappa, W.-M. Yang, Q. Liang, P. M. Radjenovica, Y.-H. Wang, Y.-J. Zhang, J.-C. Dong, Z.-Q. Tian and J.-F. Li, Elucidating Electrochemical CO2 Reduction Reaction Processes on Cu(hkl) Single-Crystal Surfaces by in situ Raman Spectroscopy, Energy Environ. Sci., 2022, 15, 3968–3977 RSC.
- I. V. Chernyshova, P. Somasundaran and S. Ponnurangam, On the Origin of the Elusive First Intermediate of CO2 Electroreduction, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E9261–E9270 CrossRef CAS PubMed.
- Z. Lyu, S. Zhu, M. Xie, Y. Zhang, Z. Chen, R. Chen, M. Tian, M. Chi, M. Shao and Y. Xia, Controlling the Surface Oxidation of Cu Nanowires Improves Their Catalytic Selectivity and Stability toward C2+ Products in CO2 Reduction, Angew. Chem., Int. Ed., 2021, 60, 1909–1915 CrossRef CAS PubMed.
- B. Chang, Z. Min, N. Liu, N. Wang, M. Fan, J. Fan and J. Wang, Electrocatalytic CO2 Reduction to Syngas, Green Energy Environ., 2023 DOI:10.1016/j.gee.2023.05.005.
- Y. Xu, W. Liu, Z. Xu, Y. Zhou and X.-Y. Yu, In situ Reconstructed AgZn3 Nanoparticles Supported on Zinc Nanoplates for Efficient CO2 Electroreduction to Tunable Syngas, Chem. Commun., 2023, 59, 8596–8599 RSC.
- B. Zhao, X. Huang, Y. Ding and Y. Bi, Bias-Free Solar-Driven Syngas Production: A Fe2O3 Photoanode Featuring Single-Atom Cobalt Integrated with a Silver-Palladium Cathode, Angew. Chem., Int. Ed., 2022, 62, e202213067 CrossRef PubMed.
- Y. Kim, D. Dumett Torres and P. K. Jain, Activation Energies of Plasmonic Catalysts, Nano Lett., 2016, 16, 3399–3407 CrossRef CAS PubMed.
- Y. Jia, H.-S. Hsu, W.-C. Huang, D.-W. Lee, S.-W. Lee, T.-Y. Chen, L. Zhou, J.-H. Wang, K.-W. Wang and S. Dai, Probing the Roles of Indium Oxides on Copper Catalysts for Enhanced Selectivity during CO2-to-CO Electrochemical Reduction, Nano Lett., 2023, 23, 2262–2268 CrossRef CAS PubMed.
- S. Linic, P. Christopher and D. B. Ingram, Plasmonic-Metal Nanostructures for Efficient Conversion of Solar to Chemical Energy, Nat. Mater., 2011, 10, 911–921 CrossRef CAS PubMed.
- U. Aslam, V. G. Rao, S. Chavez and S. Linic, Catalytic Conversion of Solar to Chemical Energy on Plasmonic Metal Nanostructures, Nat. Catal., 2018, 1, 656–665 CrossRef.
- R. E. Vos, K. E. Kolmeijer, T. S. Jacobs, W. van der Stam, B. M. Weckhuysen and M. T. M. Koper, How Temperature Affects the Selectivity of the Electrochemical CO2 Reduction on Copper, ACS Catal., 2023, 13, 8080–8091 CrossRef CAS PubMed.
- S. T. Ahn, I. Abu-Baker and G. T. R. Palmore, Electroreduction of CO2 on Polycrystalline Copper: Effect of Temperature on Product Selectivity, Catal. Today, 2017, 288, 24–29 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental details, chemicals, formula derivation, PEC experiments of Cu NAs by CA and i–t measurements. See DOI: https://doi.org/10.1039/d3cp05450k |
| This journal is © the Owner Societies 2024 |