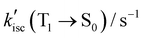Tailoring flavin-based photosensitizers for efficient photooxidative coupling of benzylic amines†
Huimin
Guo
 *,
Yang
Qiu
,
Siyu
Liu
,
Xiangyu
Zhang
and
Jianzhang
Zhao
*,
Yang
Qiu
,
Siyu
Liu
,
Xiangyu
Zhang
and
Jianzhang
Zhao

School of Chemistry, Dalian University of Technology, No. 2, Linggong Road, Dalian, 116024, P. R. China. E-mail: guohm@dlut.edu.cn
First published on 21st November 2023
Abstract
Photooxidative coupling of benzylic amines using naturally abundant O2 as an oxidant under visible light irradiation is an alternative green approach to synthesis imines and is of both fundamental and practical significance. We investigated the photophysical properties of flavin (FL) that is a naturally available sensitizer and its derivatives, i.e. 9-bromoflavin (MB-FL), 7,8-dibromoflavin (DB-FL) and 10-phenylflavin (Ph-FL), as well as the performance of these FL-based sensitizers (FLPSs) in the photooxidative coupling of benzylic amines to imines combining experimental and theoretical efforts. We showed that chemical functionalization with Br and phenyl effectively improves the photophysical properties of these FLPSs, in terms of absorption in the visible light range, singlet oxygen quantum yields, triplet lifetime, etc. Apart from nearly quantitative selectivity for the production of imines, the performance of DB-FL is superior to those of other FLPSs, and it is among the best photocatalysts for imine synthesis. Specifically, 0.5 mol% DB-FL is capable of converting 91% of 0.2 mmol benzylamine and more than 80% of 0.2 mmol fluorobenzylic amine derivatives into their corresponding imines in 5 h batch runs. Mechanistic investigation finely explained the observed photophysical properties of FLPSs and highlighted the dominant role of electron transfer in FLPS sensitized coupling of benzylic amines to imines. This work not only helps to understand the pathways for photocatalysis with FLPSs but also paves the way for the design of novel and efficient PSs to promote organic synthesis.
1. Introduction
Efficient tailoring of the structure and functionality of chemicals with inexpensive, abundant and renewable resources with the least energy expense and environmental impact is highly expected for the sustainable development of chemical synthesis.1 Imines are a class of important intermediates used for the synthesis of N-containing chemicals for pharmaceutical, biological and chemical applications.2,3 Though various synthesis procedures are being developed, it is always highly desirable to develop novel and efficient procedures and to improve the imine selectivity and yield of the current ones further.4–9 Photooxidative coupling of benzylic amines that utilizes naturally abundant O2 as an oxidant with visible light irradiation as an energy input is an alternative green approach to synthesise imines, and is of both fundamental and practical significance.4,10–12 In photocatalysis, the photosensitizer (PS) converts the absorbed light radiation into chemical energy through electron (ET) or/and energy transfer (EnT) with substrates, forming reactive intermediates (RIs) to keep the chemical transformations going.1,13–17 As the decay of PS from triplet excited states (Tn) to the ground state (S0) is a spin-forbidden process, triplet PSs are more efficient for EnT/ET with substrates.18–20 Tremendous efforts are devoted to develop novel triplet PSs for efficient chemical transformations.21Flavin is a naturally available triplet PS. It absorbs strongly in the visible light range and is also capable of initiating several redox processes in biological systems.22–27 Artificial monoamine oxidase based on FL has been developed and found effective for biomimetic amine oxidation even in the dark.28 Many flavin-based PSs (FLPSs) were also developed21,29–32 for photocatalytic conversion of chemicals, including photooxidation of aromatic compounds containing –OH, –NH2, alkyl and alkoxy groups, unsaturated fatty acids, esters, sulfides, hydrazine, triphenylphosphine, etc.,28,33–45 decarboxylative cyanation,46E/Z-isomerization,47,48 [2+2] cycloaddition,32,49 oxidative cycloelimination,50 chlorination/dechlorination,51 activation of N-bromosuccinimide,52 demethylation/debenzylation,53,54etc.21 In these processes, FLPSs were proposed to sensitize the formation of RIs through either EnT or ET or both.38–40,55,56 Feldmeier et al. also proposed that depending on the stability of the zwitterionic radical pair intermediates in different solvents, FLPSs may act as either a one- or a two-electron mediator during ET.45 For organic PSs like FL, spin–orbit coupling is weak and intrinsic intersystem crossing (ISC) rates from singlet excited states (Sn) to Tn are small, while the competing fluorescence emission (FE), nonradiative Sn → S0 internal conversion (IC) and ISC(Tn → S0) are too fast as compared to phosphorescence emission (PE), limiting the Tn quantum yield and lifetime of these FLPSs as well as the conversion efficiencies.43
Recently, many attempts have been made to improve the photophysical properties of FLPSs.21,31 By attaching FL with [Ru(bpy)3]2+, we enhanced ISC(Sn → Tn) rates and observed the room temperature phosphorescence of FL.57,58 With the heavy atom effect of Br, the ISC(Sn → Tn) rates, the 1O2 quantum yield and the performance in photooxidation of sulfides of flavin dibromide are enhanced by 2–5 times with respect to those of FL.42 We also showed that N-amidation and N-alkylation can effectively improve the performance of PSs of alloxazine and flavin frameworks.44,59 We recently fused flavin with naphthalimide as PS and achieved more than doubled visible light absorption, elongated T1 lifetime (up to 45.3 μs) and 1–5 times performance enhancement in the photooxidation of sulfide through ET.43 These results and reported photocatalysis with FLPSs suggest that molecular design would be feasible to improve the photophysical properties and the performance of related PSs in photocatalysis.27–57
As inspired by recent reports concerning the combination of photocatalysis with FLPSs,21,31 as well as our previous attempts,41–43,55–57 we investigated the photophysical properties of four FLPSs, namely FL, MB-FL, DB-FL and Ph-FL, as well as their performance in the photooxidative coupling of benzylic amines. We expect that enhanced ISC within these FLPSs would promote their population in Tn to benefit ET/EnT with O2 or organic substrates for the formation of RIs and boost the photooxidative coupling of benzylamine derivatives to imines. We also expect the findings would be helpful to rationalise the knowledge of photocatalysis with FLPSs.
2. Materials and methods
2.1. Materials and synthesis
The FLPSs, FL, MB-FL, DB-FL and Ph-FL were synthesized according to previous reports and fully characterized.40–43 More detailed information on the synthesis and characterization of FLPSs can be found in the ESI.†2.2. Theoretical methods
Density functional theory (DFT) and Time-dependent (TD)-DFT based calculations were performed to investigate the electronic structure of these FLPSs. The alkyl moieties in FLPSs were simplified as methyl moieties. FLPSs were fully relaxed at the B3LYP/6-311G(d) level of theory to obtain structures of the ground and excited states.60–64 Polarizable continuum model (PCM) developed and implemented by Scalmani and Frisch was used to study the impact of solvents.65–68 Frequency calculations were performed to verify the stability of ground and excited state structures of these FLPSs. The transition dipole moments of electronic transitions among Tn and Sn states and the spin–orbit coupling matrix elements (HSO) were calculated with Dalton based on the initial state structures of the electronic transitions.69–74 The photophysical properties of these FLPSs were investigated with the Thermal Vibration Correlation Function (TVCF) formalism proposed by Shuai, Peng and coworkers as implemented in MOMAP and integrated with Device Studio.75–803. Results and discussion
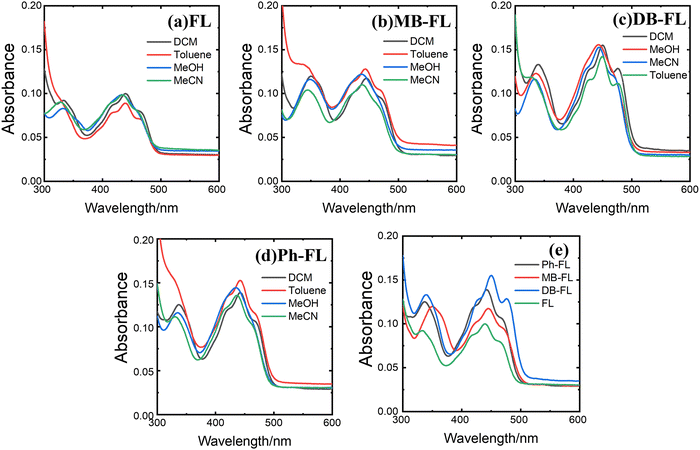
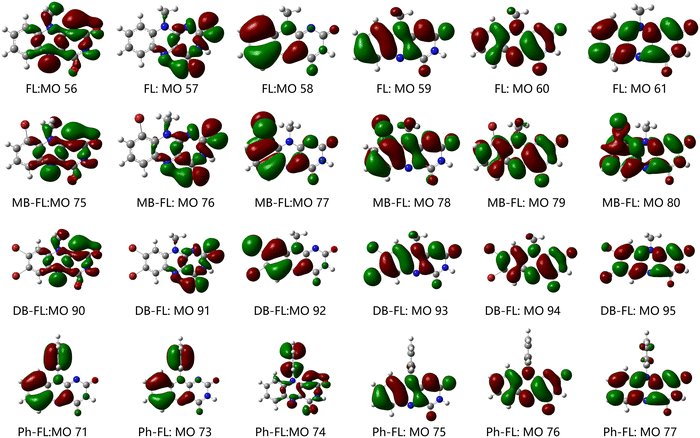
We synthesized and characterized FL, MB-FL, DB-FL and Ph-FL accordingly,40–43 and compared their photophysical properties as well as the performance in the photooxidative coupling of benzylic amines (Scheme S1, ESI†) combining experimental and theoretical efforts.The absorption spectra of these FLPSs are all characterized by two absorption bands, one centering at ∼330 nm and another one with shoulders centering at ∼440 nm (Fig. 1). The two absorption maxima (λabs) of FL are observed at 334 and 439 nm, respectively (Fig. 1a).42,57 Based on the TD-DFT calculated electronic transitions (Table 1 and Table S5–1, ESI†), the calculated λabs values of FL in DCM are 329 and 410 nm, respectively, finely reproducing the experimental results. Furthermore, FL absorption at 439 nm can be assigned to the S0 → S1 transition (involving MOs 59 and 60) while that at 334 nm can be assigned to the S0 → S4 transition (involving MOs 58, 59, 60 and 61) with both π → π* and n → π* characteristics (Fig. 2 and Table 1).42 As our experimental findings and theoretical predictions are consistent, and compare well with those reported previously, the current experimental and theoretical approaches are reliable and adequate to investigate the photophysical properties of FLPSs.
| Transitions | Energyb | f | Compositiond | CIe | Character |
|---|---|---|---|---|---|
| a The full lists of possible low-lying electronic transitions of FL, MB-FL, DB-FL and Ph-FL can be found in Tables S5-1–4 in the ESI. b Energy of electronic transitions. Only electronic transitions contributing mainly to the absorption spectra are presented. c Oscillator strength (>0.02). d Composition of transitions. e Absolute values of the CI coefficients corresponded to the orbital transitions. | |||||
| FL:S0 → S1 | 3.03 eV/410 nm | 0.2154 | 59 → 60 | 0.6960 | π → π*, n → π* |
| FL:S0 → S4 | 3.77 eV/329 nm | 0.1849 | 58 → 60 | 0.6811 | π → π*, n → π* |
| 59 → 61 | 0.1457 | π → π*, n → π* | |||
| MB-FL:S0 → S1 | 2.94 eV/422 nm | 0.1266 | 75 → 77 | 0.1311 | π → π*, n → π* |
| 76 → 77 | 0.6874 | π → π*, n → π* | |||
| MB-FL:S0 → S3 | 3.44 eV/360 nm | 0.0678 | 73 → 77 | 0.4037 | n → π* |
| 74 → 77 | 0.3876 | n → π* | |||
| 75 → 77 | 0.4009 | π → π*, n → π* | |||
| MB-FL:S0 → S4 | 3.47eV/357 nm | 0.1751 | 73 → 77 | 0.2878 | n → π* |
| 74 → 77 | 0.2847 | n → π* | |||
| 75 → 77 | 0.5471 | π → π*, n → π* | |||
| 76 → 77 | 0.1092 | π → π*, n → π* | |||
| 76 → 78 | 0.1156 | π → π*, n → π* | |||
| DB-FL:S0 → S1 | 2.91 eV/427 nm | 0.2562 | 93 → 94 | 0.6961 | π → π*, n → π* |
| DB-FL:S0 → S4 | 3.61 eV/344 nm | 0.2527 | 92 → 94 | 0.6844 | π → π*, n → π* |
| 93 → 95 | 0.1271 | π → π*, n → π* | |||
| Ph-FL:S0 → S1 | 3.02 eV/410 nm | 0.2141 | 75 → 76 | 0.6944 | π → π*, n → π* |
| Ph-FL:S0 → S4 | 3.63 eV/342 nm | 0.0818 | 73 → 76 | 0.6931 | π → π*, n → π* |
| Ph-FL:S0 → S6 | 3.80 eV/327 nm | 0.1195 | 71 → 76 | 0.6882 | π → π*, n → π* |
| 75 → 77 | 0.1135 | π → π*, n → π* | |||
Absorption bands of FL derivatives are all redshifted due to the attachment of Br and phenyl. In the absorption spectra, λabs of MB-FL appears at 350 and 450 nm, respectively, while those of DB-FL show up at 340 and 450 nm, respectively (Fig. 1b and c).41 The absorption bands of MB-FL, DB-FL and Ph-FL can all be assigned to the corresponding S0 → S4 and S0 → S1 transitions, respectively.40,42 A careful examination of the isosurface plots of MOs involved in the electronic transitions that contribute to UV-vis spectra shows that the characteristics of FL frontier MOs are well inherited in those of FLPSs. The orbitals of Br do contribute to the frontier MOs of MB-FL and DB-FL of π, n and π* symmetry (Fig. 2). This can be found in MOs 77, 78, 79 and 80 of MB-FL and in MOs 92, 93, 94 and 95 of DB-FL. Similar cases can also be found in MOs 71, 73, 74 and 77 of Ph-FL with a significant contribution of phenyl states (Fig. 2). The interaction of states of functional groups, such as –Br, –Ph, etc., with the MOs of the FL framework changes the spatial distribution of frontier MOs of these FLPSs as well as the energy required for the electronic transitions to take place, and may account for the observed redshifted λabs with respect to those of FL (Table 1). As no new MOs originated from the functional groups shift into the fundamental gap of FLPSs, such redshifts are limited. Benchmark calculations were also performed for DB-FL with long-range-corrected CAM-B3LYP81 and ωB97XD82 functionals (Tables S5-5–6 and Fig. S5-5, ESI†), yielding essentially the same findings. However, it should also be noted that the energies of electronic transitions and spectra calculated with the B3LYP function compare well with experimental findings, and the difference between results from CAM-B3LYP and ωB97XD and experiments are too large for predictive calculations.
Though the variations of λabs for these four FLPSs are limited, the corresponding molar absorption coefficients change significantly with the molecular structures. The measured molecular absorption coefficient of FL at 439 nm is 7.5 × 103 M−1 cm−1, and is nearly doubled to 1.17, 1.55 and 1.39 × 104 M−1 cm−1 for MB-FL, DB-FL and Ph-FL at the first λabs at a longer wavelength, respectively. Similar cases were previously reported for alkylated and amidated alloxazine derivatives, bromo FL and phenyl FL.40–42,44 We attributed this enhanced adsorption to variation of the electronic structure of these FLPSs with respect to FL which impacts the electronic transition dipole moments and the absorbance. Such changes may also have an effect on the FE and ISC properties of these FLPSs.
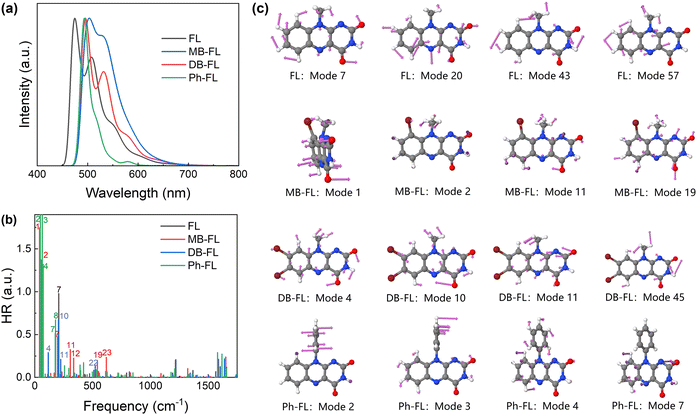
The emission properties of these FLPSs were also investigated (Fig. 3). The FE of FL becomes significant from ∼450 nm with reasonable intensity from 450 to 800 nm (Fig. 3a). The characteristics of vibrational fine structures of isoalloxazine can be recognized as 2 bands at 494 and 524 nm, respectively. These features are observable on the spectra of FL in non-polar solvents like toluene and DCM but disappear in MeOH and MeCN (Fig. 3a),42 and are well inherited by other FLPSs (Fig. 3b–d). The first emission maxima (λemi) of MB-FL, DB-FL and Ph-FL in 1.0 × 10−5 M DCM solution were found at 537, 537 and 532 nm, respectively. These compare well with the reported photophysical properties of these FLPSs.40–43
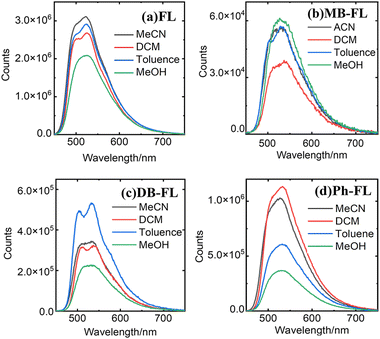 | ||
| Fig. 3 Fluorescence emission spectra of FL (a), MB-FL (b), DB-FL (c) and Ph-FL (d) in MeCN, DCM, toluene and MeOH, recorded in 1 × 10−5 M solution at 20 °C with λex at 435 nm. | ||
The absorption and FE of four FLPSs were found to be strongly solvent dependent (Fig. 1 and 3). The strongest emission of FL, MB-FL, DB-FL and Ph-FL was discovered in MeCN, MeOH, toluene and DCM, respectively (Fig. 3). This solvent dependent FE feature can be attributed to various PS-solvent interactions, such as π–π stacking, hydrogen bonds, etc., considering the functional groups on these FLPSs.25,42,58,83 A similar feature was previously reported for other FLPSs.40–43 It should also be noticed that the emission of FL is the strongest in all solvents investigated. In the same solvent, the strongest emission was always found for FL, while Ph-FL ranks the second, followed by DB-FL with MB-FL as the weakest. In principle, these features are also related to the electronic structure and can be correlated with the fluorescence quantum yields of these FLPSs (Fig. 4 and Table 2).
| k f(S1 → S0)/s−1 | k ic(S1 → S0)/s−1 | k isc(S1 → T1)/s−1 | k p(T1 → S0)/s−1 | ||
|---|---|---|---|---|---|
| FL | 5.50 × 107 | 9.61 × 108 | 1.60 × 102 | 1.82 × 102 | 1.42 × 10−1 |
| MB-FL | 3.44 × 107 | 4.91 × 108 | 4.17 × 107 | 4.92 × 107 | 3.42 |
| DB-FL | 7.52 × 107 | 1.35 × 108 | 2.24 × 105 | 7.67 × 10−2 | 1.72 × 10−2 |
| Ph-FL | 1.31 × 106 | 6.62 × 1011 | 3.95 × 108 | 1.24 × 103 | 3.57 × 10−2 |
The fluorescence emission properties of these FLPSs were investigated with TVCF formalism as proposed by Peng and Shuai et al.84,85 The calculated λemi values of FL are 474 and 508 nm at 298 K, respectively. As for the other FLPSs investigated, the λemi values were found to be 504 and 531 nm, respectively, for MB-FL, 497 and 531 nm, respectively, for DB-FL, and 495 and 520 nm, respectively, for Ph-FL (Fig. 3 and 4a).42,43,86 Huang–Rhys (HR) factors of FLPSs that characterize the vibronic coupling between S1 and S0 and can be directly correlated with the UV-absorbance, FE and IC behaviors were also calculated.76 The total HR factors of FL, MB-FL, DB-FL and Ph-FL are 2.41, 6.03, 2.43 and 31.45, respectively. HR factors were then projected back to S0 normal modes to correlate the molecular structures with the absorption and emission properties of these FLPSs (Fig. 4b and c). For FL, normal modes 7, 20, 43, 57, 58 and 59 contribute dominantly to HR factors.42 These modes are intrinsic vibration modes of the isoalloxazine framework and can be assigned as in-plane symmetrically and asymmetrically coupled stretching modes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (Fig. 4b and c). It should be noted that Mode 7, corresponding to the coupled mode of in-plane symmetric C
N (Fig. 4b and c). It should be noted that Mode 7, corresponding to the coupled mode of in-plane symmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations within benzene and pyrimidine moieties, contributes 40.6% to the total HR factors and 10.9% to the total Ereorg. The case of DB-FL is similar to that of FL. Normal modes corresponding to the coupling of Br–C stretching (Mode 4) and C–N stretching of the alkyl moiety at N10 (Modes 10 and 11) with the intrinsic vibration modes of isoalloxazine framework contribute 48.1% to total HR factors (Fig. 4c).
N stretching vibrations within benzene and pyrimidine moieties, contributes 40.6% to the total HR factors and 10.9% to the total Ereorg. The case of DB-FL is similar to that of FL. Normal modes corresponding to the coupling of Br–C stretching (Mode 4) and C–N stretching of the alkyl moiety at N10 (Modes 10 and 11) with the intrinsic vibration modes of isoalloxazine framework contribute 48.1% to total HR factors (Fig. 4c).
The case for MB-FL is slightly different. The alkyl moiety at N10 and Br at C9 are highly repulsive, and MB-FL is no longer planar (Fig. 4c). New out-of-plane normal modes appear at frequencies less than 300 cm−1, such as Modes 1 and 2, contributing 51.7% to the total HR factors. They also account for the nearly tripled HR factors with respect to FL and DBFL. In Ph-FL, the phenyl moiety is nearly vertical to isoalloxazine plane. The vibration modes of the phenyl moiety contribute to the drastic increase of the total HR factor from 2.41 for FL to 31.45. A close look at these normal modes shows that Mode 2 corresponds to out-of-plane vibration within the phenyl moiety at 47.8 cm−1 and the calculated HR factor is 22.65. Together with Mode 3 at 64.55 cm−1 with a HR factor of 3.73 resulting from the coupling of the bending mode of the phenyl moiety with the intrinsic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C breath mode of the isoalloxazine framework, and Mode 4 at 73.17 cm−1 that corresponds to the in-plane rotation of the phenyl moiety, these low frequency normal modes related to the phenyl moiety contribute to 88.1% total HR factor (Fig. 4c). We also analyzed the normal modes above 300 cm−1 of these FLPSs and found that they are mainly intrinsic normal modes of the isoalloxazine framework with limited contribution to HR factors.59 The aforementioned low frequency normal modes originate from functional groups contributing dominantly to HR factors and are the major pathways for the nonradiative decay of excited FLPSs.76 Apart from the normal modes introduced by -Br and -Phenyl moieties, the contributions of intrinsic framework normal modes to HR factors remain or even decrease.59 Considering the large HR factor, the photophysical properties of Ph-FL would be different from those of other FLPSs.
C breath mode of the isoalloxazine framework, and Mode 4 at 73.17 cm−1 that corresponds to the in-plane rotation of the phenyl moiety, these low frequency normal modes related to the phenyl moiety contribute to 88.1% total HR factor (Fig. 4c). We also analyzed the normal modes above 300 cm−1 of these FLPSs and found that they are mainly intrinsic normal modes of the isoalloxazine framework with limited contribution to HR factors.59 The aforementioned low frequency normal modes originate from functional groups contributing dominantly to HR factors and are the major pathways for the nonradiative decay of excited FLPSs.76 Apart from the normal modes introduced by -Br and -Phenyl moieties, the contributions of intrinsic framework normal modes to HR factors remain or even decrease.59 Considering the large HR factor, the photophysical properties of Ph-FL would be different from those of other FLPSs.
The kinetics for the evolution of excited FL, MB-FL, DB-FL and Ph-FL were investigated (Table 2).84,85 For FL, the calculated values are at the same level as the experimental results.42 The calculated FE (kf(S1 → S0)) and IC (kic(S1 → S0)) rate constants of FL are 5.50 × 107 and 9.61 × 108 s−1, respectively, and S1 → T1 ISC (kisc(S1 → T1)), T1 → S0 ISC (kisc(T1 → S0)) and PE (kp(T1 → S0)) rate constants are only 1.60 × 102, 1.82 × 102 and 1.42 × 10−1 s−1, respectively. Therefore, FE and IC of FL are the major decay processes of excited FL and this is in reasonable agreement with the experimentally measured fluorescence quantum yield (ΦF) of FL (Table 3).42,43 The other FLPSs were also investigated with the same approach. The calculated kic(S1 → S0) values of FL, MB-FL and DB-FL remain in the same order. The calculated kf(S1 → S0) values of FL, MB-FL, and DB-FL are similar, but kf(S1 → S0) of Ph-FL is one level of magnitude lower as compared to FL (Table 2). Apart from the significantly enlarged rate constant of S1 → S0 IC of Ph-FL, that of S1 → T1 ISC is also enlarged, making the photophysical properties of Ph-FL different from other FLPSs but more similar to that of FL considering the minor effect of the phenyl moiety nearly orthogonal to isoalloxazine (Table 3).
| λ abs /nm | ε (×105) | λ emi /nm | Φ F | τ F /ns | Φ Δ | τ T /μs | |
|---|---|---|---|---|---|---|---|
| a Absorption wavelengths, measured in 1.0 × 10−5 M DCM solution. b Molar absorption coefficient. c Emission wavelength, measured in DCM 1.0 × 10−5 M solution. d Fluorescence quantum yield of sensitizers. e Fluorescence lifetime at 293 K, in air and DCM. f Singlet oxygen quantum yield of sensitizers, measured in toluene with respect to anthracene (An, ΦΔ = 0.60 in ACN). g Triplet excited state lifetime, measured in 4.0 × 10−5 M toluene solution and determined with decay trace in transient absorption. | |||||||
| FL | 333;440 | 0.092;0.100 | 525 | 0.428 | 6.37 | 32.9 | 2.74 |
| MB-FL | 350;450 | 0.120;0.117 | 537 | 0.012 | 0.02 | 78.6 | 5.67 |
| DB-FL | 340;450 | 0.133;0.155 | 537 | 0.052 | 0.78 | 46.5 | 4.91 |
| Ph-FL | 337;442 | 0.125;0.139 | 532 | 0.218 | 3.28 | 2.4 | 2.76 |
For FL, kp(T1 → S0) is three orders of magnitude smaller than kisc(S1 → T1) and competing non-emissive decay rate constants 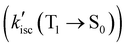 , showing that non-emissive decay is the dominant pathway for the decay of FL from T1. The calculated HSO values for S1 → T1 ISC are 1.27 × 10−3, 4.01, 0.26 and 4.84 cm−1, for FL, MB-FL, DB-FL and Ph-FL, respectively (Table S5-7, ESI†). The significantly larger HSOs of MB-FL, DB-FL and Ph-FL show that the introduction of both -Br and -phenyl significantly enhance the spin–orbit coupling within these FLPSs. This can be attributed to the heavy atom effect of Br, and the orthogonal states of the phenyl moiety to that of FL in Ph-FL that satisfy El-Sayed's rule (Table 1 and Tables S5-1–4, ESI†).40–43 Originating from this, the calculated kisc(S1 → T1) values increase by at least 3 orders of magnitude to 4.17 × 107, 2.24 × 105, 3.95 × 108 s−1, for MB-FL, DB-FL and Ph-FL, respectively. As the kisc(S1 → T1) value is comparable with kic(S1 → S0) and kf(S1 → S0), a reasonable amount of MB-FL and DB-FL at S1 would evolve into T1, leading to a decrease of ΦF (Table 3). At the same time, the
, showing that non-emissive decay is the dominant pathway for the decay of FL from T1. The calculated HSO values for S1 → T1 ISC are 1.27 × 10−3, 4.01, 0.26 and 4.84 cm−1, for FL, MB-FL, DB-FL and Ph-FL, respectively (Table S5-7, ESI†). The significantly larger HSOs of MB-FL, DB-FL and Ph-FL show that the introduction of both -Br and -phenyl significantly enhance the spin–orbit coupling within these FLPSs. This can be attributed to the heavy atom effect of Br, and the orthogonal states of the phenyl moiety to that of FL in Ph-FL that satisfy El-Sayed's rule (Table 1 and Tables S5-1–4, ESI†).40–43 Originating from this, the calculated kisc(S1 → T1) values increase by at least 3 orders of magnitude to 4.17 × 107, 2.24 × 105, 3.95 × 108 s−1, for MB-FL, DB-FL and Ph-FL, respectively. As the kisc(S1 → T1) value is comparable with kic(S1 → S0) and kf(S1 → S0), a reasonable amount of MB-FL and DB-FL at S1 would evolve into T1, leading to a decrease of ΦF (Table 3). At the same time, the 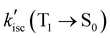 values of MB-FL and Ph-FL also increased. These can be attributed to the heavy atom effect of Br, and the orthogonal states of the phenyl moiety to that of FL in Ph-FL that satisfy El-Sayed's rule (Table 1 and Tables S5-1~4, ESI†) enhancing the spin–orbit coupling and promoting the non-radiative decay to S0.40–43,59 It should also be noticed that the
values of MB-FL and Ph-FL also increased. These can be attributed to the heavy atom effect of Br, and the orthogonal states of the phenyl moiety to that of FL in Ph-FL that satisfy El-Sayed's rule (Table 1 and Tables S5-1~4, ESI†) enhancing the spin–orbit coupling and promoting the non-radiative decay to S0.40–43,59 It should also be noticed that the 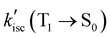 value of DB-FL changes differently from those of MB-FL and Ph-FL for its limited HSO, turning both the radiative and non-radiative T1 → S0 transition of DB-FL slower. Both the S1 →T1 and T1 → S0 are mainly of π → π* character with limited contribution of Br states, and this limits the heavy atom effect of Br to S1 →T1 and T1 → S0 transitions. This behavior can be attributed to the different bonding of functional groups with FL resulting in frontier Mos of different symmetry that determine HSO and kisc according to El-Sayed's rule (Fig. 2 and Fig. S5-14, ESI†).87,88 As kisc(S1 → T1) of MB-FL, DB-FL and Ph-FL all increase,
value of DB-FL changes differently from those of MB-FL and Ph-FL for its limited HSO, turning both the radiative and non-radiative T1 → S0 transition of DB-FL slower. Both the S1 →T1 and T1 → S0 are mainly of π → π* character with limited contribution of Br states, and this limits the heavy atom effect of Br to S1 →T1 and T1 → S0 transitions. This behavior can be attributed to the different bonding of functional groups with FL resulting in frontier Mos of different symmetry that determine HSO and kisc according to El-Sayed's rule (Fig. 2 and Fig. S5-14, ESI†).87,88 As kisc(S1 → T1) of MB-FL, DB-FL and Ph-FL all increase, 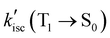 are at least at the same order of magnitude as kisc(S1 → T1) or even smaller and it is reasonable to consider that a reasonable amount of these FLPSs in T1 would be accumulated under continuous irradiation benefiting their interaction with the substrate for enhanced ET and EnT. This is also in reasonable agreement with the measured ΦΔ and τT values of these FLPSs (Table 3).
are at least at the same order of magnitude as kisc(S1 → T1) or even smaller and it is reasonable to consider that a reasonable amount of these FLPSs in T1 would be accumulated under continuous irradiation benefiting their interaction with the substrate for enhanced ET and EnT. This is also in reasonable agreement with the measured ΦΔ and τT values of these FLPSs (Table 3).
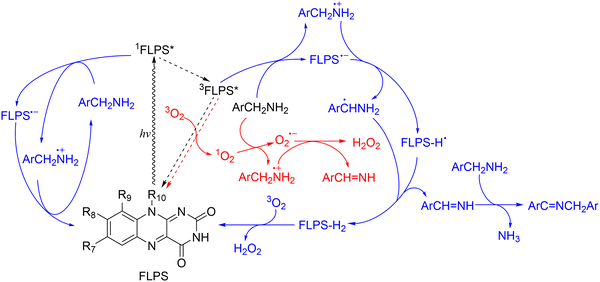
The performance of these FLPSs in the photooxidative coupling of benzylamine for the synthesis of imine was investigated. We started with FL catalyzed photooxidative coupling of benzylamine in MeCN and investigated the impact of factors, such as solvent, PS concentration, reaction duration, etc. on the yield of desired products (Table 4). The imine yield increases gradually with the FL concentration from 0.2 to 1.0 mol% in the reaction mixtures (Table 4, entries 1–3). Specifically, the imine yield reaches 67% in 9 h batch reactions when 0.5 mol% FL was used, but increases only slightly to 70% when 1.0 mol% FL was used as a PS. Therefore, an FL concentration of 0.5 mol% was considered optimal for subsequent investigation. In parallel runs, imine yields in solvents other than MeCN are all lower than that in MeCN (Table 4, entries 4–7), showing the superior role of MeCN as a solvent in the photooxidative coupling of benzylamine. This is in good agreement with the only reported example for imine synthesis with a FLPS.54 The solvent dependent behavior of imine yield can be attributed to the role of flavin as either a one- or a two-electron mediator when the stability of the zwitterionic radical pair intermediates is modulated by solvents as proposed by Feldmeier and coworkers.45 Based on the optimized PS concentration and solvent, the impact of the reaction duration was also investigated. The imine yields were 48, 55, 61, 67 and 70%, respectively, in parallel batch runs with durations of 3, 5, 7, 9 and 11 h (Table 4, entries 8–11). As the imine yield only increases by 3% from 67% to 70% when the reaction duration increases from 9 to 11 h, 9 h was considered optimal for subsequent investigation. The yields of reactions without FL, O2 or light radiation are negligible, showing that imine is only formed by FL sensitized photooxidative coupling of benzylamine, and FL, O2 or light radiation are all vital (Table 4, entries 12–14).
| Entry | Sensitizer | Sensitizer concentration (mol%) | Duration (h) | Solvent | Yield (%) |
|---|---|---|---|---|---|
a Benzylamine (0.2 mmol) as the substrate, the light wavelength was 451 nm, and the light intensity was 1400 W m−2. Further experimental details and 1H-NMR data of product mixtures are included in the ESI.
b The composition of mixed MeCN/MeOH, DCM/MeOH and MeCN/H2O solvents is v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9 v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, otherwise specified. 1, otherwise specified.
|
|||||
| 1 | FL | 0.2 | 9 | MeCN | 43 |
| 2 | FL | 0.5 | 9 | MeCN | 67 |
| 3 | FL | 1.0 | 9 | MeCN | 70 |
| 4 | FL | 0.5 | 9 | MeOH | 30 |
| 5 | FL | 0.5 | 9 | DCM | 24 |
| 6 | FL | 0.5 | 9 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH MeOH |
48 |
| 7 | FL | 0.5 | 9 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
63 |
| 8 | FL | 0.5 | 3 | MeCN | 48 |
| 9 | FL | 0.5 | 5 | MeCN | 55 |
| 10 | FL | 0.5 | 7 | MeCN | 61 |
| 11 | FL | 0.5 | 11 | MeCN | 70 |
| 12 | None | None | 9 | MeCN | 0 |
| 13 | FL | 0.5 | 9 | MeCN, No light | 0 |
| 14 | FL | 0.5 | 9 | MeCN, No O2 | 0 |
The reaction conditions when MB-FL was used as a sensitizer (Table S3-1 and Fig. S4-21–29, ESI†) were determined with the same approach. The optimal conditions for MB-FL sensitized photooxidative coupling of benzylamine were determined as using 0.5 mol% MB-FL with MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O(v
H2O(v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) = 9
v) = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the solvent and the reaction duration is 9 h. The reaction conditions were also optimized for DB-FL and Ph-FL (Table 5 and Table S3-2, and Fig. S4-30–47, ESI†). The optimal reaction conditions for DB-FL are using 0.5 mol% sensitizer, in MeCN
1 as the solvent and the reaction duration is 9 h. The reaction conditions were also optimized for DB-FL and Ph-FL (Table 5 and Table S3-2, and Fig. S4-30–47, ESI†). The optimal reaction conditions for DB-FL are using 0.5 mol% sensitizer, in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v
H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9
v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent and the reaction duration is 5 h (Table 5), while those for Ph-FL are using 0.5 mol% sensitizer, in the MeCN solvent and the reaction duration is 9 h (Table S3-2, ESI†). The imine yields under optimal conditions for each FLPS are 67, 86, 100 and 53% for FL, MB-FL, DB-FL and Ph-FL, respectively. Obviously, the performance of DB-FL is superior to other FLPSs in sensitizing the photooxidative coupling of benzylamine. Specifically, the product yield is already 91% for DB-FL in a 5 h batch run in MeCN
1) solvent and the reaction duration is 5 h (Table 5), while those for Ph-FL are using 0.5 mol% sensitizer, in the MeCN solvent and the reaction duration is 9 h (Table S3-2, ESI†). The imine yields under optimal conditions for each FLPS are 67, 86, 100 and 53% for FL, MB-FL, DB-FL and Ph-FL, respectively. Obviously, the performance of DB-FL is superior to other FLPSs in sensitizing the photooxidative coupling of benzylamine. Specifically, the product yield is already 91% for DB-FL in a 5 h batch run in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v
H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9
v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This can be attributed to the aforementioned superior photophysical properties of DB-FL and the solvent used.42,45 Furthermore, the synthesis of imine with DB-FL is different from the reported example that such reactions only take place in non-hydrous solvents.54 In this sense, the current work also highlights the possibility of photooxidative coupling of benzylic amines in protonic hydrous solvents.
1). This can be attributed to the aforementioned superior photophysical properties of DB-FL and the solvent used.42,45 Furthermore, the synthesis of imine with DB-FL is different from the reported example that such reactions only take place in non-hydrous solvents.54 In this sense, the current work also highlights the possibility of photooxidative coupling of benzylic amines in protonic hydrous solvents.
| Entry | Sensitizer | Sensitizer concentration (mol%) | Duration (h) | Solvent | Yield (%) |
|---|---|---|---|---|---|
a Benzylamine (0.2 mmol) as the substrate, the light wavelength was 451 nm, and the light intensity was 1400 W m−2. Further experimental details and 1H-NMR data of product mixtures are included in the ESI.
b The composition of mixed MeCN/MeOH, DCM/MeOH and MeCN/H2O solvents is v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9 v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, otherwise specified. 1, otherwise specified.
|
|||||
| 1 | DB-FL | 0.5 | 7 | MeOH | 84 |
| 2 | DB-FL | 0.5 | 7 | MeCN | 94 |
| 3 | DB-FL | 0.5 | 7 | DCM | 46 |
| 4 | DB-FL | 0.5 | 7 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH MeOH |
92 |
| 5 | DB-FL | 0.5 | 7 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
100 |
| 6 | DB-FL | 0.5 | 1 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
40 |
| 7 | DB-FL | 0.5 | 3 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
72 |
| 8 | DB-FL | 0.5 | 5 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
91 |
| 9 | DB-FL | 0.5 | 9 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
100 |
| 10 | None | None | 5 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
0 |
| 11 | DB-FL | 0.5 | 5 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, No light H2O, No light |
0 |
| 12 | DB-FL | 0.5 | 5 | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, No O2 H2O, No O2 |
0 |
Inspired by the performance of FLPSs in sensitizing the photooxidative coupling of benzylamines, they were also used as a catalyst to sensitize the coupling of various benzylic amine derivatives, including 2-fluorobenzylamine, 3-fluorobenzylamine, 4-fluorobenzylamine, 4-methylbenzylamine, 3,5-bis(trifluoromethyl)benzylamine, etc. (Table 6). We focused on benzylamine derivatives with strong electron withdrawing moieties such as –F, –CF3, etc., due to the fact that the electron-withdrawing functional group on the benzene rings of substrates would slow down the conversion.35,54,89,90 According to the product yields (Table 6), these FLPSs already outperform the reported PSs and photocatalysts for photooxidative coupling of fluorobenzylamines, such as NH2-MIL-125(Ti), Pd/NH2-MIL-125(Ti) nanosheets,91,92 TiO2 nanoparticles and Au/TiO2 nanocomposites,93 Ru(bpy)3@MIL-125,94 nanoparticles of Cu2O, carbon quantum dots and their nanocomposites,95 dye-sensitized TiO2,96 CdS@C3N4,97 acetylene-bridged donor-π-acceptor covalent triazine frameworks,98etc., as well as most of the recently developed homogeneous photocatalytic reaction systems (Table 7 and Fig. S4-48–67, ESI†).99
| Entry | Product | Sensitizer | Duration (h) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: benzylamine derivatives (0.2 mmol) as the substrate, a photosensitizer concentration of 0.5 mol%, a light wavelength of 451 nm, and a light intensity of 1400 W m−2, and 1H NMR data were used to quantify the reaction yield. The experimental details and 1H-NMR data are provided in the ESI. | ||||
| 1 |
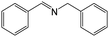
|
FL | 9 | 67 |
| MB-FL | 9 | 86 | ||
| DB-FL | 5 | 91 | ||
| Ph-FL | 9 | 53 | ||
| 2 |
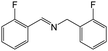
|
FL | 9 | 50 |
| MB-FL | 9 | 66 | ||
| DB-FL | 5 | 74 | ||
| Ph-FL | 9 | 43 | ||
| 3 |
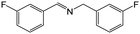
|
FL | 9 | 50 |
| MB-FL | 9 | 79 | ||
| DB-FL | 5 | 87 | ||
| Ph-FL | 9 | 41 | ||
| 4 |
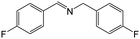
|
FL | 9 | 62 |
| MB-FL | 9 | 81 | ||
| DB-FL | 5 | 85 | ||
| Ph-FL | 9 | 48 | ||
| 5 |
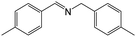
|
FL | 9 | 74 |
| MB-FL | 9 | 89 | ||
| DB-FL | 5 | 87 | ||
| Ph-FL | 9 | 58 | ||
| 6 |
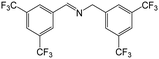
|
FL | 9 | 43 |
| MB-FL | 9 | 73 | ||
| DB-FL | 5 | 81 | ||
| Ph-FL | 9 | 51 | ||
| Entry | Sensitizer | Solvent | Scavengers | Duration (h) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: benzyl amine (0.2 mmol) as the substrate, a photosensitizer concentration of 0.5 mol%, a light wavelength of 451 nm, and a light intensity of 1400 W m−2, and 1H NMR spectroscopy was used to quantify the reaction yield. The experimental details and 1H-NMR data can be found in the ESI. | |||||
| 1 | FL | MeCN | None | 9 | 67 |
| 2 | DB-FL | CD3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O = 9 D2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
None | 3 | 94 |
| 3 | DB-FL | CD3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O = 9 D2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
None | 5 | 98 |
| 4 | FL | MeCN | 0.5 mol% DABCO | 9 | 48 |
| 5 | DB-FL | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 9 H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.5 mol% DABCO | 5 | 63 |
| 6 | FL | MeCN | 5 mol% BQ | 9 | 52 |
| 7 | DB-FL | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 9 H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 mol% BQ | 5 | 78 |
| 8 | FL | MeCN | 15 mol% TEMPO | 9 | 41 |
| 9 | FL | MeCN | 15 mol% BHT | 9 | 65 |
| 10 | FL | MeCN | 15 mol% DDUN | 9 | 66 |
| 11 | FL | MeCN | 0.5 mol% AgNO3 | 9 | 61 |
| 12 | FL | MeCN | 15 mol% AgNO3 | 9 | 30 |
| 13 | FL | MeCN | 25 mol% AgNO3 | 9 | 24 |
| 14 | Ph-FL | MeCN | 25 mol% AgNO3 | 9 | 10 |
| 15 | MB-FL | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 9 H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 mol% AgNO3 | 9 | 42 |
| 16 | DB-FL | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 9 H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 mol% AgNO3 | 5 | 32 |
These FLPSs are capable of sensitizing the photooxidative coupling of fluorobenzylamine derivatives and the product yields are more than 50% in most entries in batch runs. The product yields when DB-FL was used were always superior to those of the other FLPSs, independent of the substrate used (Table 7). Specifically, the product yields would be ∼80% in all entries with DB-FL used as a PS in 5 h batch runs. Considering the short duration for DB-FL (5 h), the above-mentioned performance results provide direct evidence for the superior performance of DB-FL as a PS for the photooxidative coupling of fluorobenzylamine derivatives (Table 3) and is in reasonable agreement with previously reported performance of DB-FL in sensitizing the photooxidation of sulfides.42,44 It is also worth noting that the contents of the reaction mixture were monitored with the 1H-NMR spectra where there are only NMR peaks of the benzylamine derivative substrate and product imines, and there is no observed variation of peaks of secondary products after the photooxidative coupling of benzylamines (ESI†), showing nearly quantitative selectivity for these FLPSs.
There is no direct correlation between the yields of imines with ΦΔ of these FLPSs (Tables 3 and 6), suggesting that the reaction network would be complex and 1O2 formation as ROSs via EnT may not be the only mechanism to keep the photocatalytic conversion efficient. It is generally accepted that photooxidation with 1O2 as the ROS may be accelerated in protonic solution, as a proton may stabilize the reaction intermediate and accelerate the formation of 1O2.39,100,101 However, the yield of the imine with FL as a PS in MeCN, the MeCN/MeOH (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9
v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture and MeOH are 67, 48 and 30%, respectively (Table 4, entries 2, 4 and 6). The case of DB-FL is similar, where the imine yields in MeCN, the MeCN/MeOH (v
1) mixture and MeOH are 67, 48 and 30%, respectively (Table 4, entries 2, 4 and 6). The case of DB-FL is similar, where the imine yields in MeCN, the MeCN/MeOH (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9
v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture and MeOH are 94, 92 and 84%, respectively (Table 5, entries 1,2 and 4). The increase of the product yield in MeCN with respect to that in MeOH and the mixture does not suggest 1O2 as a major ROS for the photooxidative coupling of benzylamines.56 This is also further evidenced by the decrease of imine yields in other solvents and solvent mixtures (Table 4, entries 2–7 and Table 5). To confirm the participation of 1O2 as the ROS, parallel reactions with DB-FL as a PS were also performed in a mixture of deuterated MeCN and H2O (v
1) mixture and MeOH are 94, 92 and 84%, respectively (Table 5, entries 1,2 and 4). The increase of the product yield in MeCN with respect to that in MeOH and the mixture does not suggest 1O2 as a major ROS for the photooxidative coupling of benzylamines.56 This is also further evidenced by the decrease of imine yields in other solvents and solvent mixtures (Table 4, entries 2–7 and Table 5). To confirm the participation of 1O2 as the ROS, parallel reactions with DB-FL as a PS were also performed in a mixture of deuterated MeCN and H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9
v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the imine yields increased from 72 and 91% (Table 5, entries 7 and 8) to 94 and 98% in 3 and 5 h batch runs (Table 7, entries 2 and 3). The increase of imine yield by ∼20% in a 3 h batch run in a deuterated solvent does suggest the participation of 1O2 as a ROS in the photooxidative coupling of benzylic amines.56 With the addition of 0.5 mol% 1,4-diazabicyclo[2.2.2]octane (DABCO) as a selective scavenger for 1O2, the decrease of imine yield from 67% to 48% when FL is used, and from 91 to 63% when DB-FL is used (Table 7, entries 4 and 5) also confirm the limited role of 1O2.102 In parallel experiments, the addition of 5 mol% benzoquinone (BQ) as a selective scavenger for O2−˙ radicals leads to imine yield decreasing from 67% to 52% when FL is used and from 91% to 78% when DB-DL is used, confirming the limited contribution of O2−˙ radicals in the photooxidative coupling (Table 7, entries 6 and 7).101,103,104 As the product yields are still significant with the addition of BQ, DABCO or without protonic solvents (Tables 4, 5 and 7, entries 1 and 7) when FL and DB-FL are used, there would be alternative pathways competing with EnT and dominating the photooxidative coupling of benzylamine.45
1) and the imine yields increased from 72 and 91% (Table 5, entries 7 and 8) to 94 and 98% in 3 and 5 h batch runs (Table 7, entries 2 and 3). The increase of imine yield by ∼20% in a 3 h batch run in a deuterated solvent does suggest the participation of 1O2 as a ROS in the photooxidative coupling of benzylic amines.56 With the addition of 0.5 mol% 1,4-diazabicyclo[2.2.2]octane (DABCO) as a selective scavenger for 1O2, the decrease of imine yield from 67% to 48% when FL is used, and from 91 to 63% when DB-FL is used (Table 7, entries 4 and 5) also confirm the limited role of 1O2.102 In parallel experiments, the addition of 5 mol% benzoquinone (BQ) as a selective scavenger for O2−˙ radicals leads to imine yield decreasing from 67% to 52% when FL is used and from 91% to 78% when DB-DL is used, confirming the limited contribution of O2−˙ radicals in the photooxidative coupling (Table 7, entries 6 and 7).101,103,104 As the product yields are still significant with the addition of BQ, DABCO or without protonic solvents (Tables 4, 5 and 7, entries 1 and 7) when FL and DB-FL are used, there would be alternative pathways competing with EnT and dominating the photooxidative coupling of benzylamine.45
FLPSs are also capable of sensitizing photooxidation through ET, leading to the formation of radicals as RIs, and photooxidation of benzylic alcohols was confirmed to proceed mainly through this pathway.38 This was also proposed as the major pathway for photooxidation of other organic substrates.31,36,56 Recently, we reported a flavin derivative that is capable of efficiently sensitising the photooxidation of sulfides by ET.43 Inspired by these, parallel reactions were then carried out to investigate the dominant pathways for the photooxidative coupling of benzylic amines (Table 7 and Fig. S4-68–78, ESI†). The oxidation initiated by ET between excited FLPSs and substrates would form substrate radicals as RIs to make the conversion efficient. The contribution of RIs was firstly investigated by adding 2,2,6,6-tetramethylpiperidinyl-1-oxy (TEMPO) as a selective scavenger of radicals105,106 (Table 7, entry 8). The product yield decreased from 67% to 41%. This evidences the contribution of radicals and triplet FL as RI for the conversion of benzyl amines. To determine the contribution of radical RIs, radical scavengers, such as butylated hydroxytoluene (BHT)107,108 and 1,1-diphenylethylene (DDUN),109–112 were also used in parallel reactions. However, the product yields are still 65 and 66%, respectively, when 15 mol% BHT or DDUN was added to the reaction mixtures (Table 7, entries 9 and 10). These suggest that radical RIs may be important, but the scavengers used may not be capable of inhibiting reactions due to the high reactivity of RIs.
Previous investigations on FL sensitized photooxidation of benzylic alcohols and sulfides concluded that these reactions can be initiated by ET from the organic substrate to excited FL sensitizers and ET would be vital for photooxidation of this kind.38,43 Parallel experiments were then carried out using AgNO3 as an electron acceptor.113,114 With an increase of the amount of AgNO3 introduced from 0 to 25 mol%, the imine yield decreases drastically from 67% to 24% in 9 h runs, showing the vital role of ET in the photooxidative coupling of benzylamine (Table 7, entries 11–13). Such a significant decrease (>50%) in imine yields was also observed in parallel reactions using MB-FL, DB-FL and Ph-FL as photosensitizers with the addition of 25 mol% AgNO3 (Table 7, entries 14–16), confirming the significant role of ET in the conversion of benzylamine.
To this end, ET from benzylic amine substrates is vital on the pathways for FLPS-sensitized photooxidative coupling of benzylic amines to imines, followed by reactions of charged substrate radicals as RIs generating imines with nearly quantitative selectivity (Scheme 1). The product imine yields were not totally quenched with the addition of each scavenger, suggesting that the photooxidative coupling of benzylic amines may proceed through a complex reaction network, where ET plays a dominant role (Scheme 1). This is different from the previous proposal that 1O2 sensitized by DB-FL works as the major ROS for sulfide photooxidation.42 The mechanism investigation proposes a more comprehensive view of the reaction network of FLPS-sensitized photooxidative coupling of benzylic amines, where ET for the formation of substrate RIs and EnT for generation of ROSs, such as 1O2, O2−˙, etc., are all working, but ET from the substrate plays a vital role in keeping the reaction efficient.
4. Conclusions
Inspired by the capability of FL based enzymes to initiate redox reactions in biological systems and to develop a green and efficient approach for the synthesis of imines, we investigated the photophysical properties of FL, MB-FL, DB-FL and Ph-FL, and their performance in the photooxidative coupling of benzylic amines to imines. We showed that the photophysical properties of these FLPSs can be effectively improved by chemical functionalization with Br, phenyl, etc., leading to enhanced absorption in the visible range, increased singlet oxygen quantum yields, elongated triplet lifetimes, etc. Resulting from these, the performances of FLPSs in the photooxidative coupling of benzylic amines to imines are also enhanced. Among these FLPSs, the performance of DB-FL is superior to those of other FLPSs. Specifically, imine yields of DB-FL in 5 h batch runs are even superior to those obtained with other FLPSs in 9 h batch runs, making DB-FL among the best PSs for imine synthesis. As the imine yields were not totally quenched with the addition of scavengers in mechanism investigations, the photooxidative coupling of benzylic amines may proceed through a complex reaction network, including EnT for the generation of ROSs, such as 1O2 and O2−˙, as well as ET for the formation of substrate RIs, etc., with ET from substrates to excited FLPSs playing a significant role. This work highlights the feasibility of improving the photophysical properties and catalytic performance of FLPSs by functionalization as well as the complex reaction network for photocatalytic conversion of benzylic amines. We expect the findings may help to understand photocatalysis with FLPSs and benefit the design of novel efficient PSs to promote organic synthesis reactions. Though we did not assess the toxicity of MB-FL and DB-FL, their enhanced performance in photooxidation does suggest that the attachment of Br to the FL framework may impact the biorelated properties of these FLPSs drastically. We are designing novel FLPSs with photophysical properties enhanced through mechanisms other than heavy atom effects.Author contributions
H. M. Guo designed this research after a discussion with J. Z. Zhao and drafted the manuscript. H. M. Guo and J. Z. Zhao acquired the funding and provided resources and research tools. Y. Qiu performed the experimental research with the guidance of H. M. Guo and J. Z. Zhao. S. Liu performed the theoretical calculations with the guidance of H. M. Guo. Y. Qiu and S. Liu are responsible for the results presented. H. M. Guo and J. Z. Zhao commented on the manuscript. H. M. Guo finalized the manuscript. All authors have given approval to the final version of the manuscript. The authors declare no competing financial interest.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2023YFE0197600) and the National Natural Science Foundation of China (NSFC, No: 21573034, 21771029, 11811530631, 21373036 and 21103015). We thank HZWTECH for providing the computation facilities.References
- L. Revathi, L. Ravindar, W. Y. Fang, K. P. Rakesh and H. L. Qin, Adv. Synth. Catal., 2018, 360, 4652–4698 CrossRef CAS.
- L. H. Choudhury and T. Parvin, Tetrahedron, 2011, 67, 8213–8228 CrossRef CAS PubMed.
- S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626–2704 CrossRef CAS PubMed.
- R. D. Patil and S. Adimurthy, Asian J. Org. Chem., 2013, 2, 726–744 CrossRef CAS.
- M. Largeron, Eur. J. Org. Chem., 2013, 5225–5235 CrossRef CAS.
- R. D. Patil and S. Adimurthy, Adv. Synth. Catal., 2011, 353, 1695–1700 CrossRef CAS.
- Z. Z. Hu and F. M. Kerton, Org. Biomol. Chem., 2012, 10, 1618–1624 RSC.
- S. Zhao, C. Liu, Y. Guo, J. C. Xiao and Q. Y. Chen, J. Org. Chem., 2014, 79, 8926–8931 CrossRef CAS PubMed.
- L. H. Liu, Z. K. Wang, X. F. Fu and C. H. Yan, Org. Lett., 2012, 14, 5692–5695 CrossRef CAS PubMed.
- B. L. Ryland and S. S. Stahl, Angew. Chem., Int. Ed., 2014, 53, 8824–8838 CrossRef CAS PubMed.
- F. Z. Su, S. C. Mathew, L. Mohlmann, M. Antonietti, X. C. Wang and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 657–660 CrossRef CAS PubMed.
- J. H. Park, K. C. Ko, E. Kim, N. Park, J. H. Ko, D. H. Ryu, T. K. Ahn, J. Y. Lee and S. U. Son, Org. Lett., 2012, 14, 5502–5505 CrossRef CAS PubMed.
- D. Ravelli, M. Fagnoni and A. Albini, Chem. Soc. Rev., 2013, 42, 97–113 RSC.
- D. Cambie and T. Noel, Top. Curr. Chem., 2018, 376, 27 CrossRef PubMed.
- M. Oelgemoller, Chem. Rev., 2016, 116, 9664–9682 CrossRef CAS PubMed.
- M. D. Karkas, J. A. Porco and C. R. J. Stephenson, Chem. Rev., 2016, 116, 9683–9747 CrossRef CAS PubMed.
- S. Schaefer-Ramadan, S. A. Gannon and C. Thorpe, Biochemistry, 2013, 52, 8323–8332 CrossRef CAS PubMed.
- J. Z. Zhao, W. H. Wu, J. F. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323–5351 RSC.
- S. E. Braslavsky, Pure Appl. Chem., 2007, 79, 293–465 CrossRef CAS.
- J. D. Bell and J. A. Murphy, Chem. Soc. Rev., 2021, 50, 9540–9685 RSC.
- A. Rehpenn, A. Walter and G. Storch, Synthesis, 2021, 2583–2593 CrossRef CAS.
- C. Walsh, Acc. Chem. Res., 1980, 13, 148–155 CrossRef CAS.
- C. Walsh, Acc. Chem. Res., 1986, 19, 216–221 CrossRef CAS.
- T. C. Bruice, Acc. Chem. Res., 1980, 13, 256–262 CrossRef CAS.
- P. F. Heelis, Chem. Soc. Rev., 1982, 11, 15–39 RSC.
- P. F. Heelis, R. F. Hartman and S. D. Rose, Chem. Soc. Rev., 1995, 24, 289–297 RSC.
- W. Kaim and B. Schwederski, Pure Appl. Chem., 2004, 76, 351–364 CrossRef CAS.
- A. T. Murray, M. J. H. Dowley, F. Pradaux-Caggiano, A. Baldansuren, A. J. Fielding, F. Tuna, C. H. Hendon, A. Walsh, G. C. Lloyd-Jones, M. P. John and D. R. Carbery, Angew. Chem., Int. Ed., 2015, 54, 8997–9000 CrossRef CAS PubMed.
- R. E. Frederick, J. A. Mayfield and J. L. DuBois, J. Am. Chem. Soc., 2011, 133, 12338–12341 CrossRef CAS PubMed.
- H. Iida, Y. Imada and S. I. Murahashi, Org. Biomol. Chem., 2015, 13, 7599–7613 RSC.
- B. Konig, S. Kummel, E. Svobodova and R. Cibulka, Phys. Sci. Rev., 2018, 3, 17 Search PubMed.
- J. Spackova, E. Svobodova, T. Hartman, I. Stibor, J. Kopecka, J. Cibulkova, J. Chudoba and R. Cibulka, ChemCatChem, 2017, 9, 1177–1181 CrossRef CAS.
- M. Marz, J. Chudoba, M. Kohout and R. Cibulka, Org. Biomol. Chem., 2017, 15, 1969–1974 RSC.
- M. Marz, M. Kohout, T. Nevesely, J. Chudoba, D. Prukata, S. Nizinski, M. Sikorski, G. Burdzinski and R. Cibulka, Org. Biomol. Chem., 2018, 16, 6809–6817 RSC.
- W. A. Massad, Y. Barbieri, M. Romero and N. A. Garcia, Photochem. Photobiol., 2008, 84, 1201–1208 CrossRef CAS PubMed.
- R. Lechner, S. Kuemmel and B. Koenig, Photochem. Photobiol. Sci., 2010, 9, 1367–1377 CrossRef CAS PubMed.
- B. Muhldorf and R. Wolf, Chem. Commun., 2015, 51, 8425–8428 RSC.
- U. Megerle, M. Wenninger, R. J. Kutta, R. Lechner, B. Konig, B. Dick and E. Riedle, Phys. Chem. Chem. Phys., 2011, 13, 8869–8880 RSC.
- T. Nevesely, E. Svobodova, J. Chudoba, M. Sikorski and R. Cibulka, Adv. Synth. Catal., 2016, 358, 1654–1663 CrossRef CAS.
- J. Dadova, S. Kummel, C. Feldmeier, J. Cibulkova, R. Pazout, J. Maixner, R. M. Gschwind, B. Konig and R. Cibulka, Chem. – Eur. J., 2013, 19, 1066–1075 CrossRef CAS PubMed.
- K. A. Korvinson, G. N. Hargenrader, J. Stevanovic, Y. Xie, J. Joseph, V. Maslak, C. M. Hadad and K. D. Glusac, J. Phys. Chem. A, 2016, 120, 7294–7300 CrossRef CAS PubMed.
- C. Dang, L. J. Zhu, H. M. Guo, H. Y. Xia, J. Z. Zhao and B. Dick, ACS Sustainable Chem. Eng., 2018, 6, 15254–15263 CrossRef CAS.
- H. M. Guo, Z. W. Lei, X. L. Ma, S. Y. Liu, Y. Qiu and J. Z. Zhao, Phys. Chem. Chem. Phys., 2022, 24, 15255–15264 RSC.
- H. M. Guo, H. Y. Xia, X. L. Ma, K. P. Chen, C. Dang, J. Z. Zhao and B. Dick, ACS Omega, 2020, 5, 10586–10595 CrossRef CAS PubMed.
- C. Feldmeier, H. Bartling, K. Magerl and R. M. Gschwind, Angew. Chem., Int. Ed., 2015, 54, 1347–1351 CrossRef CAS PubMed.
- N. P. Ramirez, B. Konig and J. C. Gonzalez-Gomez, Org. Lett., 2019, 21, 1368–1373 CrossRef CAS PubMed.
- T. Morack, J. B. Metternich and R. Gilmour, Org. Lett., 2018, 20, 1316–1319 CrossRef CAS PubMed.
- J. B. Metternich and R. Gilmour, J. Am. Chem. Soc., 2016, 138, 1040–1045 CrossRef CAS PubMed.
- V. Mojr, G. Pitrova, K. Strakova, D. Prukala, S. Brazevic, E. Svobodova, I. Hoskovcova, G. Burdzinski, T. Slanina, M. Sikorski and R. Cibulka, ChemCatChem, 2018, 10, 849–858 CrossRef CAS.
- T. Hartman and R. Cibulka, Org. Lett., 2016, 18, 3710–3713 CrossRef CAS PubMed.
- T. Hering, B. Muhldorf, R. Wolf and B. Konig, Angew. Chem., Int. Ed., 2016, 55, 5342–5345 CrossRef CAS PubMed.
- G. Tang, Z. Y. Gong, W. J. Han and X. L. Sun, Tetrahedron Lett., 2018, 59, 658–662 CrossRef CAS.
- L. J. Xie, R. L. Wang, D. Wang, L. Liu and L. Cheng, Chem. Commun., 2017, 53, 10734–10737 RSC.
- R. Lechner and B. Konig, Synthesis, 2010, 1712–1718, DOI:10.1055/s-0029-1218709.
- R. L. Jensen, J. Arnbjerg and P. R. Ogilby, J. Am. Chem. Soc., 2010, 132, 8098–8105 CrossRef CAS PubMed.
- J. Dad'ova, E. Svobodova, M. Sikorski, B. Konig and R. Cibulka, ChemCatChem, 2012, 4, 620–623 CrossRef.
- H. Guo, L. Zhu, C. Dang, J. Zhao and B. Dick, Phys. Chem. Chem. Phys., 2018, 20, 17504–17516 RSC.
- H. M. Guo, C. Dang, J. Z. Zhao and B. Dick, Inorg. Chem., 2019, 58, 8486–8493 CrossRef CAS PubMed.
- H. M. Guo, X. L. Ma, Z. W. Lei, Y. Qiu, J. Z. Zhao and B. Dick, Phys. Chem. Chem. Phys., 2021, 23, 13734–13744 RSC.
- R. C. Binning and L. A. Curtiss, J. Comput. Chem., 1990, 11, 1206–1216 CrossRef CAS.
- M. M. Francl, W. J. Pietro, W. J. Hehre, J. S. Binkley, M. S. Gordon, D. J. Defrees and J. A. Pople, J. Chem. Phys., 1982, 77, 3654–3665 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- S. Miertus, E. Scrocco and J. Tomasi, Chem. Phys., 1981, 55, 117–129 CrossRef CAS.
- R. Cammi and B. Mennucci, J. Chem. Phys., 1999, 110, 9877–9886 CrossRef CAS.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3093 CrossRef CAS PubMed.
- G. Scalmani and M. J. Frisch, J. Chem. Phys., 2010, 132, 15 CrossRef PubMed.
- O. Vahtras, H. Agren, P. Jorgensen, H. J. A. Jensen, T. Helgaker and J. Olsen, J. Chem. Phys., 1992, 97, 9178–9187 CrossRef CAS.
- H. Hettema, H. J. A. Jensen, P. Jorgensen and J. Olsen, J. Chem. Phys., 1992, 97, 1174–1190 CrossRef CAS.
- H. Agren, O. Vahtras, H. Koch, P. Jorgensen and T. Helgaker, J. Chem. Phys., 1993, 98, 6417–6423 CrossRef CAS.
- J. Olsen, D. L. Yeager and P. Jorgensen, J. Chem. Phys., 1989, 91, 381–388 CrossRef CAS.
- P. Jorgensen, H. J. A. Jensen and J. Olsen, J. Chem. Phys., 1988, 89, 3654–3661 CrossRef.
- K. Aidas, C. Angeli, K. L. Bak, V. Bakken, R. Bast, L. Boman, O. Christiansen, R. Cimiraglia, S. Coriani, P. Dahle, E. K. Dalskov, U. Ekstrom, T. Enevoldsen, J. J. Eriksen, P. Ettenhuber, B. Fernandez, L. Ferrighi, H. Fliegl, L. Frediani, K. Hald, A. Halkier, C. Hattig, H. Heiberg, T. Helgaker, A. C. Hennum, H. Hettema, E. Hjertenaes, S. Host, I. M. Hoyvik, M. F. Iozzi, B. Jansik, H. J. A. Jensen, D. Jonsson, P. Jorgensen, J. Kauczor, S. Kirpekar, T. Kjrgaard, W. Klopper, S. Knecht, R. Kobayashi, H. Koch, J. Kongsted, A. Krapp, K. Kristensen, A. Ligabue, O. B. Lutnaes, J. I. Melo, K. V. Mikkelsen, R. H. Myhre, C. Neiss, C. B. Nielsen, P. Norman, J. Olsen, J. M. H. Olsen, A. Osted, M. J. Packer, F. Pawlowski, T. B. Pedersen, P. F. Provasi, S. Reine, Z. Rinkevicius, T. A. Ruden, K. Ruud, V. V. Rybkin, P. Salek, C. C. M. Samson, A. S. de Meras, T. Saue, S. P. A. Sauer, B. Schimmelpfennig, K. Sneskov, A. H. Steindal, K. O. Sylvester-Hvid, P. R. Taylor, A. M. Teale, E. I. Tellgren, D. P. Tew, A. J. Thorvaldsen, L. Thogersen, O. Vahtras, M. A. Watson, D. J. D. Wilson, M. Ziolkowski and H. Agren, Wiley Interdiscip. Rev.-Comput. Mol. Sci., 2014, 4, 269–284 CrossRef CAS PubMed.
- Q. Peng, Y. P. Yi, Z. G. Shuai and J. S. Shao, J. Chem. Phys., 2007, 126, 114302 CrossRef PubMed.
- Q. Peng, Y. P. Yi, Z. G. Shuai and J. S. Shao, J. Am. Chem. Soc., 2007, 129, 9333–9339 CrossRef CAS PubMed.
- Y. L. Niu, Q. A. Peng, C. M. Deng, X. Gao and Z. G. Shuai, J. Phys. Chem. A, 2010, 114, 7817–7831 CrossRef CAS PubMed.
- Y. Niu, Q. Peng and Z. Shuai, Sci. China, Ser. B: Chem., 2008, 51, 1153–1158 CrossRef CAS.
- Q. Peng, Y. L. Niu, Q. H. Shi, X. Gao and Z. G. Shuai, J. Chem. Theory Comput., 2013, 9, 1132–1143 CrossRef CAS PubMed.
- Hongzhiwei Technology, Device Studio, Version 2021A, Available online: https://iresearch.net.cn/cloudSoftware (accessed on 2021-10-12).
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- J. D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- H. M. Guo, L. J. Zhu, C. Dang, J. Z. Zhao and B. Dick, Phys. Chem. Chem. Phys., 2018, 20, 17504–17516 RSC.
- Z. G. Shuai, Chin. J. Chem., 2020, 38, 1223–1232 CrossRef CAS.
- Y. L. Niu, W. Q. Li, Q. Peng, H. Geng, Y. P. Yi, L. J. Wang, G. J. Nan, D. Wang and Z. G. Shuai, Mol. Phys., 2018, 116, 1078–1090 CrossRef CAS.
- J. Yeow, A. Kaur, M. D. Anscomb and E. J. New, Chem. Commun., 2014, 50, 8181–8184 RSC.
- M. A. El-Sayed, Acc. Chem. Res., 1968, 1, 8–16 CrossRef CAS.
- C. M. Marian, Wiley Interdiscip. Rev.-Comput. Mol. Sci., 2012, 2, 187–203 CrossRef CAS.
- H. Schmaderer, P. Hilgers, R. Lechner and B. Koenig, Adv. Synth. Catal., 2009, 351, 163–174 CrossRef CAS.
- J. Svoboda, H. Schmaderer and B. Koenig, Chem. – Eur. J., 2008, 14, 1854–1865 CrossRef CAS PubMed.
- H. T. Wang, J. N. Yu, S. Wei, M. M. Lin, Y. J. Song and L. Wu, Chem. Eng. J., 2022, 441, 13 Search PubMed.
- J. G. Vitillo, D. Presti, I. Luz, F. Xamena and S. Bordiga, J. Phys. Chem. C, 2020, 124, 23707–23715 CrossRef CAS.
- K. Y. Zhang, G. L. Lu, F. Chu and X. B. Huang, Catal. Sci. Technol., 2021, 11, 7060–7071 RSC.
- X. Yang, T. Huang, S. Y. Gao and R. Cao, J. Catal., 2019, 378, 248–255 CrossRef CAS.
- A. Kumar, A. Hamdi, Y. Coffinier, A. Addad, P. Roussel, R. Boukherroub and S. L. Jain, J. Photochem. Photobiol., A, 2018, 356, 457–463 CrossRef CAS.
- Z. Wang and X. J. Lang, Appl. Catal., B, 2018, 224, 404–409 CrossRef CAS.
- Y. Xu, Y. Chen and W. F. Fu, Appl. Catal., B, 2018, 236, 176–183 CrossRef CAS.
- X. W. Lan, X. P. Liu, Y. Z. Zhang, Q. Li, J. Wang, Q. F. Zhang and G. Y. Bai, ACS Catal., 2021, 11, 7429–7441 CrossRef CAS.
- Y. Yamamoto, S. Kodama, A. Nomoto and A. Ogawa, Org. Biomol. Chem., 2022, 20, 9503–9521 RSC.
- E. Baciocchi, T. Del Giacco, F. Elisei, M. F. Gerini, M. Guerra, A. Lapi and P. Liberali, J. Am. Chem. Soc., 2003, 125, 16444–16454 CrossRef CAS PubMed.
- S. M. Bonesi, I. Manet, M. Freccero, M. Fagnoni and A. Albini, Chem. – Eur. J., 2006, 12, 4844–4857 CrossRef CAS PubMed.
- A. Casado-Sanchez, R. Gomez-Ballesteros, F. Tato, F. J. Soriano, G. Pascual-Coca, S. Cabrera and J. Aleman, Chem. Commun., 2016, 52, 9137–9140 RSC.
- S. M. Bonesi, M. Fagnoni and A. Albini, J. Sulfur Chem., 2008, 29, 367–376 CrossRef CAS.
- S. M. Bonesi, S. Protti and A. Albini, J. Org. Chem., 2016, 81, 11678–11685 CrossRef CAS PubMed.
- P. J. Wright and A. M. English, J. Am. Chem. Soc., 2003, 125, 8655–8665 CrossRef CAS PubMed.
- G. G. Borisenko, I. Martin, Q. Zhao, A. Amoscato and V. E. Kagan, J. Am. Chem. Soc., 2004, 126, 9221–9232 CrossRef CAS PubMed.
- W. A. Yehye, N. A. Rahman, A. Ariffin, S. B. A. Hamid, A. A. Alhadi, F. A. Kadir and M. Yaeghoobi, Eur. J. Med. Chem., 2015, 101, 295–312 CrossRef CAS PubMed.
- S. Fujisawa, Y. Kadoma and I. Yokoe, Chem. Phys. Lipids, 2004, 130, 189–195 CrossRef CAS PubMed.
- Z. Q. Sun, W. J. Ma, Y. H. Cao, T. Y. Wei, X. Y. Mo, H. Y. Chow, Y. Tan, C. H. P. Cheung, J. M. Liu, H. K. Lee, E. C. M. Tse, H. Liu and X. C. Li, Chem, 2022, 8, 2542–2557 CAS.
- S. Dutta, B. Prabagar, R. Vanjari, V. Gandon and A. K. Sahoo, Green Chem., 2020, 22, 1113–1118 RSC.
- M. A. Carroll, J. Nairne, G. Smith and D. A. Widdowson, J. Fluorine Chem., 2007, 128, 127–132 CrossRef CAS.
- D. J. McPhee, M. Campredon, M. Lesage and D. Griller, J. Am. Chem. Soc., 1989, 111, 7563–7567 CrossRef CAS.
- P. Zheng, Z. Pan, H. Y. Li, B. Bai and W. S. Guan, J. Mater. Sci.: Mater. Electron., 2015, 26, 6399–6410 CrossRef CAS.
- J. Schneider and D. W. Bahnemann, J. Phys. Chem. Lett., 2013, 4, 3479–3483 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed information on synthesis and characterization of FLPSs and the photocatalytic reactions, structure characterization, the NMR and HRMS spectra, the DFT/TD-DFT results and references (PDF). See DOI: https://doi.org/10.1039/d3cp04579j |
| This journal is © the Owner Societies 2024 |