Enhancing the upconversion of Er3+ incorporated BaTiO3 by introducing oxygen vacancies†
Young Gwon
Jung‡
 ,
Hyeongyu
Bae‡
,
Hyeongyu
Bae‡
 and
Kang Taek
Lee
and
Kang Taek
Lee
 *
*
Department of Chemistry, Gwangju Institute of Science and Technology (GIST), Gwangju 61005, Republic of Korea. E-mail: ktlee@gist.ac.kr
First published on 28th November 2023
Abstract
Lanthanide-incorporated crystals display the phenomenon of upconversion (UC), wherein near-infrared (NIR) light is converted into ultraviolet-visible (UV-Vis) emission with a narrow bandwidth. This unique photophysical property renders lanthanide UC materials highly promising for diverse applications. However, the limited quantum efficiency (∼3%) hinders the broader utilization of UC materials. Consequently, numerous studies have focused on overcoming this low efficiency. Notably, it has been observed that manipulation of the site symmetry in UC materials significantly enhances their UC efficiency. In this study, we investigate the UC enhancement of Er3+ incorporated BaTiO3 (E-BT) crystals through the introduction of oxygen vacancies (OV). The OV were created using a post-heat treatment method, and the annealing time was varied to control the quantity of OV. An optimal annealing time of 6 hours was determined for efficient OV generation, beyond which the OV content decreased. Remarkably, E-BT crystals with OV exhibited up to three-fold greater UC compared to those without OV. This outcome suggests that OV induce symmetry changes in the E-BT crystal structure. Furthermore, the degree of UC enhancement in E-BT was found to be proportional to the amount of OV present.
Introduction
Upconversion (UC) is a non-linear phenomenon that generates higher energy photons by sequentially absorbing two or more photons with lower energy. This unique phenomenon can be observed in lanthanide (Ln3+)-incorporated crystal systems. In particular, Ln3+ show sharp ultraviolet-visible (UV-Vis) UC emission bands under near-infrared (NIR) irradiation. The UC materials have great potential for applications in anti-counterfeiting,1 light-emitting materials,2 photovoltaics,3–6 and bio-imaging.7–10 Unfortunately, the UC materials possess low quantum efficiency (∼3%) due to the Laporte forbidden 4f–4f transition.10–12 Recently, efforts to increase the UC efficiency by adopting additional photonic nanostructures and site symmetry manipulation of host materials have been reported.7,8,11,13–15 The photonic nanostructures are widely used for UC enhancement. In particular, the enhanced local electromagnetic field could increase the rate of energy transfer in the Ln3+-incorporated system.14 However, the photonic nanostructures are highly dependent on environmental conditions. Additionally, the size of the enhanced site (hot spot) is too small to be used in large-scale applications. On the other hand, manipulating the site symmetry of the host has no limit on atmospheric conditions. Indeed, the UC materials with low symmetry sites show enhanced UC due to the increased intra-4f transitions.10 In a previous study, UC efficiency changing with the phase transition of the host was highlighted.15 Moreover, co-doping Gd3+ and Lu3+ into NaYF4:Er3+,Yb3+ showed greatly enhanced UC, that is, an indication of symmetry-breaking for the generation of UC up to 1.6 fold.11 The Judd–Ofelt theory suggests that the non-centrosymmetric interaction of host materials is highly related to the 4f–4f transitions of Ln3+.10,16–18 Given the profound interest, the research on non-centrosymmetric host materials should become important for both UC efficiency enhancement and advanced UC studies.Oxygen vacancies (OV) are inevitable structural defects in nature. It is known that the OV significantly influence the chemical and physical properties of materials. Numerous studies have reported that introducing OV in photonic materials influences their optical properties. Wang et al. reported enhanced UC by introducing OV into Er3+ incorporated BiOCl nanosheets.19 However, OV not only cause structural distortions but also alter the electrical properties. OV in the host material create the localized state in the host material, facilitating energy transfer between OV and Ln3+. In other words, it is still hard to fundamentally determine the relationship between UC enhancement and OV in terms of symmetry dependence.
Barium titanate (BaTiO3) is an oxide perovskite that is highly stable in the atmosphere, optically transparent and has low phonon energy (0.087 eV).15,20–23 Upon incorporating Ln3+, distortion on the doped site is induced and the UC emission is observed under NIR irradiation. Notably, the introduction of the OV into BaTiO3 does not create the localized state in the band gap.24 Taking these advantages into account, BaTiO3 would be an ideal material to investigate the correlation between UC enhancement and the OV solely in terms of the symmetric interactions. Our previous study reported that Er3+ in a non-centrosymmetric environment shows a more intense UC emission than that in a centrosymmetric environment.15 In this work, we investigated the effect of the amount of OV on the UC of Ba2+-site Er3+ incorporated BaTiO3 (E-BT). The post-annealing process was conducted to create OV in E-BT. Based on our hypothesis, the OV in the UC material would break the symmetric structure and induce the UC enhancement. The annealing time was controlled to investigate the relationship between the reaction time and the OV generation.21
Methods
E-BT was synthesized via solid-state reactions. Since the solid-state reaction is an ionic size-dependent method, it is a preferred method to synthesize E-BT. Precisely calculated amounts of chemicals, barium carbonate (BaCO3, ≥99%), titanium(IV) oxide (TiO2, 99.8%), and erbium(III) oxide (Er2O3, 99.9%) = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mol%, were mixed with 100 mol% sodium chloride (NaCl, 99.9%). The role of sodium chloride is to increase the surface area for the reaction. The mixture was ground for 1 h in an agate mortar and pestle. The mixed reagents were loaded in a muffle furnace with an alumina crucible and sintered for 5 h at 1000 °C. The product E-BT was pinkish-white. After cooling to room temperature, E-BT was treated by post-annealing at 600 °C for OV effect investigation.
2 mol%, were mixed with 100 mol% sodium chloride (NaCl, 99.9%). The role of sodium chloride is to increase the surface area for the reaction. The mixture was ground for 1 h in an agate mortar and pestle. The mixed reagents were loaded in a muffle furnace with an alumina crucible and sintered for 5 h at 1000 °C. The product E-BT was pinkish-white. After cooling to room temperature, E-BT was treated by post-annealing at 600 °C for OV effect investigation.
Results and discussion
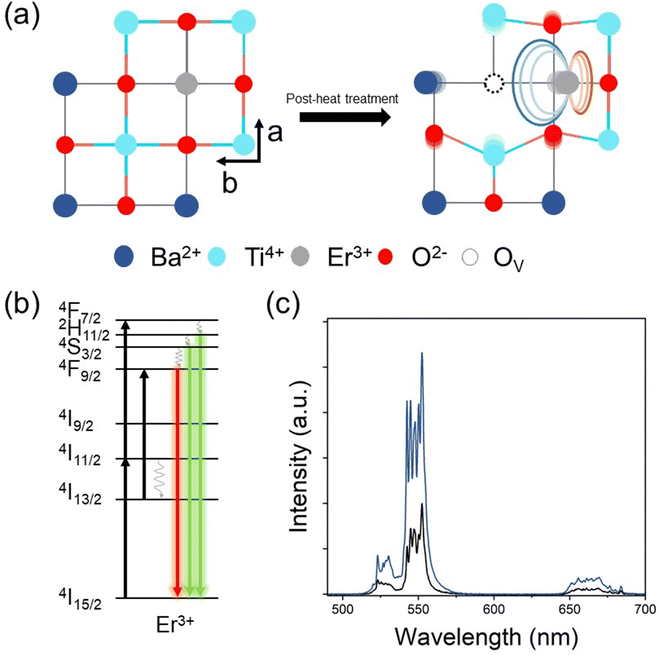
The illustrative figures for comparison between pure (pristine) E-BT and oxygen vacancy (OV) introduced E-BT are shown in Fig. 1(a). On the left-hand side, Er3+ in the pristine E-BT interacts with the surrounding ions equally (centrosymmetric), whereas Er3+ in the OV introduced E-BT, on the right-hand side, does not interact with the surrounding ions equally (non-centrosymmetric). The Coulomb interaction between the OV and other ions leads to the symmetry breaking of the E-BT crystal. As mentioned, the non-centrosymmetric interaction would induce the enhanced UC of lanthanides. Therefore, a centrosymmetric environment around Er3+ is less desirable for UC generation compared with a non-centrosymmetric environment. In Fig. 1(b) we illustrated a scheme for photophysical pathways of Er3+ UC, under 980 nm irradiation. The UC of Er3+ is mainly contributed by the sequential absorption of photons. In particular, Er3+ would emit bright green UC luminescence derived from the 2H11/2, 4S3/2 → 4I15/2 energy level pathways. To begin with, we investigate the UC properties of pristine E-BT (0 h) and post-thermally treated (6 h, 600 °C) E-BT. As shown in Fig. 1(c), post-treated E-BT shows up to 3 times stronger UC signals compared with the one untreated.To verify the presence of OV in E-BT, X-ray photoelectron spectroscopy (XPS) was conducted. The XPS spectrum contains the elemental information of various ions so that we can verify the presence of OV. The O 1s XPS spectra of post-annealed E-BT for 0, 2, 4, 6, 8, and 12 h at 600 °C are shown in Fig. 2. The Gaussian fitting of O 1s XPS was performed to characterize the OV in a series of E-BT samples. In XPS spectra, the presence of OV induces two different spectral changes: shifting of the peak position and generation of an additional peak of ions.25 Interestingly, 0 h (non-treated) E-BT showed only two oxygen peaks of O (I) and O (II) (Fig. 2(a)). Ideally speaking, defects like vacancies usually exist in crystals. However, only two fitted peaks are observed in the XPS spectrum of 0 h E-BT and they do not correspond to the OV. After annealing, O 1s spectra show three peaks centered at about 529 eV for O (I), 530 eV for O (II), and 532 eV for O (III) in Fig. 2(b)–(f). The peak positions of annealed E-BT are shifted to a higher binding energy compared to those of 0 h E-BT. In addition, it is known that the OV peak of TiO6 octahedra in BaTiO3 is at ∼532 eV.19–21,25–30 Therefore, the O (III) peak corresponds to the OV in E-BT. The integrated area values (Ax–y, where x and y represent the element and the peak number, respectively) of XPS spectra are summarized in Table S1 (ESI†). The AO-III values are related to the amount of OV. It is to be noted that (1) post-annealing generates the OV and (2) the OV increase as time increases and decrease sharply after 8 hours of post-treatment (AO-III: 3365 → 11980 → 13707 → 4904 → 2997, 2 to 12 h in order). These values imply that post-treatment generates OV in complex ways, and the optimum time for the generation of OV exists. In addition, the XPS spectra of Ti 2p are shown in Fig. S1 (ESI†). The XPS spectra of Ti 2p show four peaks at ∼457 eV (I), ∼458 eV (II), ∼462.5 eV (III), and ∼464 eV (IV). The presence of Ti3+ peaks indicates that the OV would be generated by charge compensation in the whole crystal.26,31 Each of the Ti3+ peaks (Ti3+ (I) and Ti3+ (III)) implies the amount of OV (Fig. S1(a), ESI†). It was expected that there would be no Ti3+ peaks in the Ti 2p XPS spectrum of 0 h E-BT. However, two Ti3+ peaks of Ti3+ (I) and Ti3+ (II) are shown in Fig. S1(a) (ESI†). Perhaps, Ti3+ is generated to maintain the electrostatic balance because of positive charge excess created by doping Er3+ in the Ba2+-site. After heat-treatment, Ti3+ peaks are enhanced (Table S1, ESI†) and show the same tendencies as O 1s XPS. These results demonstrate that the post-heating treatment method effectively worked to create the OV in E-BT. It is noteworthy that the amount of OV is decreased after 6 h of annealing.
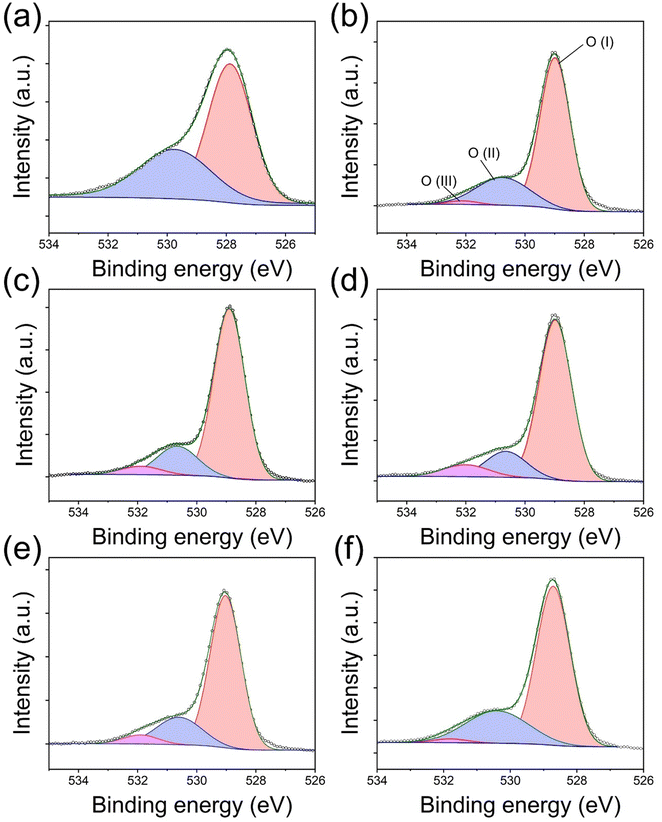 | ||
| Fig. 2 O 1s XPS spectra of E-BT annealed at 600 °C. (a)–(f): annealing time: 0, 2, 4, 6, 8, and 12 h, respectively (O (I): ∼529 eV, O (II): ∼530 eV, and O (III): ∼532 eV). | ||
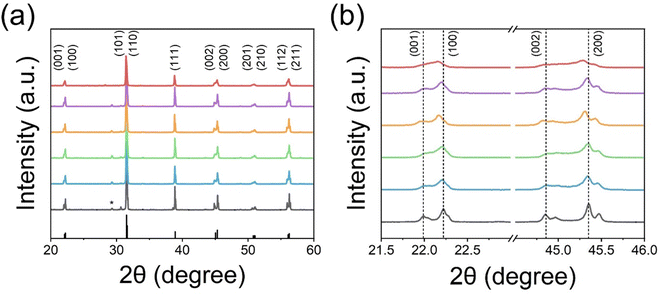
The X-ray diffraction (XRD) patterns of post-annealed E-BT are shown in Fig. 3. In diffraction patterns, all E-BT (0–12 h annealed) show the tetragonal phase (p4mm). The main diffraction peaks were at about 21.99°, 22.23°, 30.65°, 31.47°, 38.87°, 44.85°, 45.35°, 50.96°, 51.07°, 55.93°, and 57.92° corresponding to the (001), (100), (101), (110), (111), (002). (200), (201), (210), (112), and (211) diffraction planes, respectively (ISCD: 01-076-0744, Fig. 3(a)). This result indicates that the tetragonality of E-BT is sustained with the heat-treatment. A minor, anonymous phase peak at ∼30° was generated with dominant phase patterns of E-BT. The relatively small amounts of impurities could be considered negligible. In addition, the direct evidence of the tetragonal phase corresponding to the diffraction patterns of the (001), (100), (002), and (200) planes is shown in Fig. 3(b). Notably, the pattern's intensities degraded with increasing post-annealing time. The peak positions of the (100) and (200) planes shifted to a smaller angle with the increment of OV, indicating the expansion of the crystal lattice. This shift is attributed to the outward distortion of Ba2+ and Ti4+ ions from the OV site due to Coulomb interactions.24,32,33 These results suggest that crystallinity decreased with annealing and the creation of the OV. Therefore, we consider that the presence of the OV would give rise to an expansion of the E-BT unit cells. To verify the unit cell expansion, the lattice constants of each E-BT were calculated. Since E-BT has a tetragonal structure, eqn (1) is used to calculate lattice constants. As shown in Fig. 3 and Table S1 (ESI†), the amount of OV started to decrease after 8 h of annealing. Thus, a comparison of 6 h and 8 h E-BT with untreated E-BT would prove the effect of the amount of OV on the unit cells. Here, the 6 h annealed E-BT contains the highest amount of OV compared to the 8 h annealed E-BT. The calculated values of lattice constants (a and c) and volume of unit cells (vol) for 0, 6, and 8 h annealed E-BT are listed in Table 1.
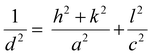 | (1) |
| Annealing time (h) | Lattice constants and volume | ||
|---|---|---|---|
| a (Å) | c (Å) | Vol (Å3) | |
| 0 | 4.00833 | 4.02576 | 64.68072 |
| 6 | 4.01328 | 4.03077 | 64.92126 |
| 8 | 4.00956 | 4.02702 | 64.74067 |
Overall, the lattice constants of 6 and 8 h E-BT were larger than those of 0 h E-BT. In particular, the 6 h annealed E-BT showed the most expanded unit cell volume. The dislocation of ions from their original location results in the expansion of the E-BT unit cell and the crystallinity is decreased with the OV increment. In the case of the annealing time being longer than 8 h, it is considered that the excess OV induce the degradation of the E-BT crystal. The excess number of OV makes the E-BT lattice unstable and induces the collapse of the E-BT crystal. Due to the collapse of the crystal, the OV in the E-BT lattice also disappeared. Therefore, the O 1s XPS spectra of 8 and 12 h annealed E-BT show a smaller amount of OV than those of 6 h annealed E-BT. This also explains the broad peaks of (001), (100), (002), and (200) planes of 12 h annealed E-BT.
We conducted additional heat-treatment for E-BT for 6 and 12 h at 600, 800, and 1000 °C. Fig. S2 and S3 (ESI†) show the O 1s and Ti 2p XPS spectra of additional heat-treatment, respectively. As mentioned, OV was generated efficiently at 6 h treatment than at 12 h treatment (Table S2, ESI†). In addition, it turns out that the annealing temperature also affects the creation of OV. The amount of OV decreased when the temperature increased to 800 °C. In general, 800 °C heat is sufficient for enhancing the crystallinity of bulk phase polycrystalline BaTiO3 (coarsening). Therefore, 800 °C heat-treatment could enhance the crystallinity of E-BT, which is directly shown by the OV decrement. In the same context, a higher contribution of crystallinity enhancement than the OV generation at 1000 °C annealing could lead to a significant decrease in OV. However, the experimental result of 1000 °C treatment differed from the expected result, as a significant amount of OV was observed (Fig. S2(e), ESI†). The cause of OV enhancement is not clear; however, we assume that the high temperature (1000 °C) induced the migration of the oxygen ion on the surface of the largely coarsened grain.
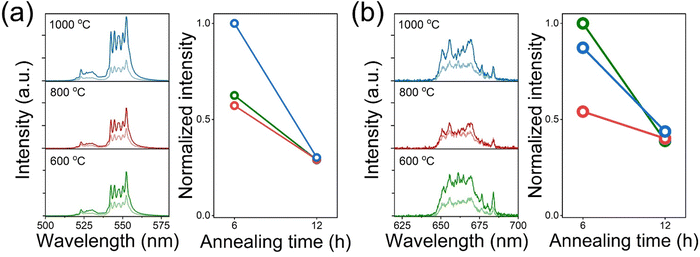
The OV in the E-BT is deeply related to the crystal structure of the E-BT. Therefore, OV could affect UC through a crystal field change. Fig. 4 shows the UC emission spectra of heat-treated E-BT at 600, 800, and 1000 °C. These results show that bright green UC is attributed to 2H11/2, 4S3/2 → 4I15/2. In addition, the intensity of green UC is proportional to the amount of OV (Fig. 4(a)). However, the 4F9/2 → 4I15/2 (red UC) (Fig. 4(b)) showed a different tendency from the green UC. Although the amount of OV is the largest at 1000 °C annealing for 6 h, the 600 °C annealed E-BT for 6 h shows the highest red UC intensity. Moreover, the specific UC peaks of the 655–665 nm region show strong emission for 600 °C annealing, but it decreases with the annealing temperature increment (Fig. 4(b)). Perhaps, these results imply that the crystal structures of 800 and 1000 °C annealed E-BT are slightly different from that of 600 °C annealed E-BT owing to the coarsening. Therefore, conducting the post-treatment at 600 °C would provide clear evidence of the relationship between the amount of OV and UC. Fig. S4 and S5 (ESI†) show the UC spectra of annealed E-BT.
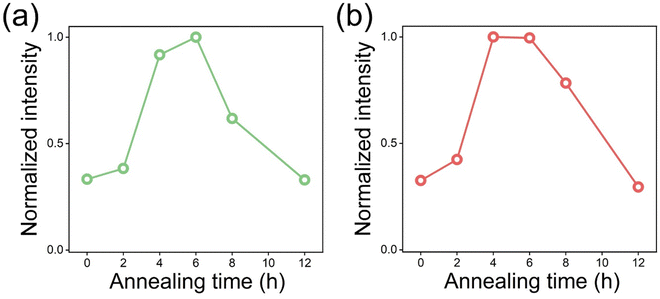
The UC intensity sequentially increases until 6 h of annealing, and after that, it decreases (Fig. S4–S6, ESI†). The UC spectra indicate that the OV do not affect the spectral changes but the UC enhancement. In addition, the sequential increment of UC intensity is responsive to the amount of OV. The intensity of green UC is the highest for 6 h annealed E-BT followed by 4, 8, and 12 h annealed E-BT (Fig. 5(a)). It is assumed that the reason for the slightly lower UC of 12 h annealed E-BT than 0 h annealed E-BT is due to the low crystallinity (Fig. 3). The order of the green UC intensity from the highest to the lowest shows a similar tendency to the amount of OV in E-BT. However, the red UC intensity is the highest for 4 h annealed E-BT and followed by 6, 8, and 12 h annealed E-BT (Fig. 5(b)). The UC of 4 h annealed E-BT is slightly higher than that of the 6 h annealed one. The red UC shows a different tendency compared to the green UC. It has been argued that the red UC has different photophysical pathways from the green UC, such as cross-relaxation among the neighboring Er3+ ions.34,35 Therefore, the cross-relaxation of 4F7/2, 4I11/2 → 2 × 4F9/2 between neighboring Er3+ ions might affect the red UC and then a tendency different than green UC appeared. To investigate the effect of OV on the photophysical pathways of UC, the pump–power dependence of the green and red UC was measured under 980 nm excitation. The pump–power dependence data are plotted in Fig. S7 (ESI†) with a relationship of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I ∝ n
I ∝ n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, where I is the UC intensity, P is the pump–power and n is the slope of the plot. The n values in the plots indicate the number of photons involved in the UC.36 The UC in Er3+ occurs with the sequential absorption of 980 nm photons. In both green and red UC, the n values are close to 2, which demonstrates that the sequential absorption of two photons mainly contributed to the UC. Therefore, both green and red UC are generated through the photophysical pathway described in Fig. 1(b). Although in red UC, the slope values of annealed E-BT are slightly smaller than those of the un-annealed E-BT (Fig. S7(g)–(l), ESI†), the influence of possible cross-relaxation is negligible. In addition, the intensity difference of red UC for the 4 and 6 h E-BT is negligibly small. Considering these results, the OV in E-BT rarely influence the photophysical pathways of UC, which means that the OV only affect the symmetry of E-BT.
P, where I is the UC intensity, P is the pump–power and n is the slope of the plot. The n values in the plots indicate the number of photons involved in the UC.36 The UC in Er3+ occurs with the sequential absorption of 980 nm photons. In both green and red UC, the n values are close to 2, which demonstrates that the sequential absorption of two photons mainly contributed to the UC. Therefore, both green and red UC are generated through the photophysical pathway described in Fig. 1(b). Although in red UC, the slope values of annealed E-BT are slightly smaller than those of the un-annealed E-BT (Fig. S7(g)–(l), ESI†), the influence of possible cross-relaxation is negligible. In addition, the intensity difference of red UC for the 4 and 6 h E-BT is negligibly small. Considering these results, the OV in E-BT rarely influence the photophysical pathways of UC, which means that the OV only affect the symmetry of E-BT.
Comparing the integrated UC intensity, the UC of 6 h annealed E-BT is 3 times greater than the UC of un-annealed E-BT (Fig. S4, ESI†). It is noteworthy that the amount of Er3+ in E-BT is 2 mol%, and the only difference is the amount of OV. From Fig. 3 and Table 1, it is found that the OV expand the unit cell of E-BT. Among the E-BT samples, the 6 h annealed E-BT, in which the amount of OV is the largest, shows the most expanded unit cell. The dislocation of Ti4+ and Ba2+ from their original position induces symmetry breaking. The symmetry distorted E-BT would have a different Coulomb interaction than the un-annealed E-BT. The Coulomb and the crystal fields are known to be important factors in determining the energy levels of the free ion Er3+ in the 4f orbital.13 In addition, the UC of both un-/annealed E-BT mainly occurred by the sequential photon absorption (Fig. S5, ESI†). Therefore, the enhancement of UC efficiency is the result of the more allowed 4f–4f transitions of Er3+ due to the symmetry breaking of E-BT with OV introduction.
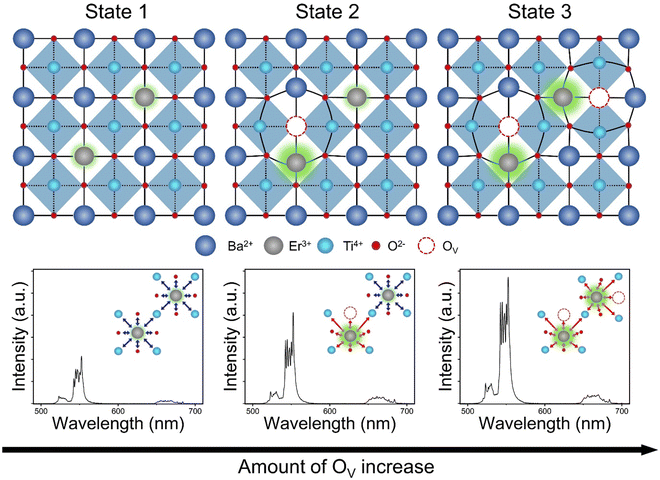
Fig. 6 illustrates the cross-sectional views of the E-BT crystal structures and their corresponding UC spectra: state 1 is pure E-BT (without OV) and states 2 and 3 are E-BT with different amounts of OV. The illustration contains the implications from various experimental results of the amount of OV, site symmetry changes, and the UC. In state 1, relatively low UC was observed due to the centrosymmetric field (blue arrows) surrounding the Er3+. In state 2, the OV started to be introduced into E-BT. Because of the Coulomb interaction among the OV site and surrounding ions, state 2 has a distorted crystal structure. The incorporated Er3+ would be surrounded by a non-centrosymmetric field (red arrows). Meanwhile, Er3+ without the neighboring OV would not be influenced by the non-centrosymmetric field. The non-centrosymmetric field induces more 4f–4f transitions in Er3+, and state 2 emits more intense UC than state 1. Finally, state 3 has more OV than state 2. From Fig. 3 and Table 1, it is found that the expansion of the E-BT unit cell is related to the amount of OV. Therefore, state 3 has a much more distorted crystal structure than state 2. In other words, more Er3+ would experience a non-centrosymmetric field in state 3. The UC spectra corresponding to state 3 show a more enhanced UC intensity than those corresponding to states 1 and 2. These results demonstrated that the OV are deeply related to the UC enhancement by manipulating the symmetry of E-BT. Furthermore, from the pump–power dependence measurement, it is found that there is no change in the photon number that is involved in the UC. This result implies that there is no significant photophysical change in OV introduced E-BT. Therefore, UC enhancement is only influenced by symmetry changes. That is, the OV purely influence the site symmetry of E-BT and do not change the photophysical pathway of UC. Therefore, we can conclude that the amount of OV influences the local symmetry of E-BT and its UC efficiency.
Conclusion
In this work, we experimentally verified the effect of oxygen vacancies (OV) on the upconversion (UC) of 2 mol% Er3+ doped BaTiO3 (E-BT). The OV were introduced by the post-heating treatment method at 600 °C with time variation. We confirmed that the annealing time variation could control the amount of OV from the XPS analysis. The XRD analysis indicates that the amount of OV corresponds to the degree of distortion in E-BT. Overall, E-BT with the highest amount of OV showed the highest UC enhancement. The OV in E-BT induced the non-centrosymmetric environment around Er3+ so that the enhanced UC was observed. Furthermore, from the pump–power dependence measurement, we have confirmed that the UC enhancement originated from the induced non-centrosymmetric environment. Consequently, we observed three times enhanced UC emission from the OV introduced E-BT. Therefore, the non-centrosymmetric manipulation would be the way to enhance the UC efficiency. We hope that this work inspires the development of further UC research.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
K. T. L. was supported by the grant awarded by the National Research Foundation (NRF) of South Korea (2021R1A2C2010557) and by the GIST in 2022 through the GIST Research Institute (GRI) program.References
- H. Xiao, B. Liu, L. Qiu, G. Li, G. Zhang, D. Huang, Y. Zhao, C. Yang, F. Jiang, P. Dang, H. Lian, Z. Cheng and J. Lin, Core-Shell Structured Upconversion/Lead-Free Perovskite Nanoparticles for Anticounterfeiting Applications, Angew. Chem., Int. Ed., 2022, 61(8), e202115136, DOI:10.1002/anie.202115136.
- Z. Xia and A. Meijerink, Ce3+-Doped garnet phosphors: composition modification, luminescence properties and applications, Chem. Soc. Rev., 2017, 46(1), 275–299, 10.1039/c6cs00551a.
- X. Zhang, Y. Zhang, X. Zhang, W. Yin, Y. Wang, H. Wang, M. Lu, Z. Li, Z. Gu and W. W. Yu, Yb3+ and Yb3+/Er3+ Doping for Near-Infrared Emission and Improved Stability of CsPbCl3 Nanocrystals, J. Mater. Chem. C, 2018, 6(37), 10101–10105, 10.1039/c8tc03957g.
- J. Shi, F. Li, J. Yuan, X. Ling, S. Zhou, Y. Qian and W. Ma, Efficient and stable CsPbI3 perovskite quantum dots enabled by in situ ytterbium doping for photovoltaic applications, J. Mater. Chem. A, 2019, 7(36), 20936–20944, 10.1039/c9ta07143a.
- L. Wang, H. Zhou, J. Hu, B. Huang, M. Sun, B. Dong, G. Zheng, Y. Huang, Y. Chen, L. Li, Z. Xu, N. Li, Z. Liu, Q. Chen, L.-D. Sun and C.-H. Yan, A Eu3+–Eu2+ ion redox shuttle imparts operational durability to Pb–I perovskite solar cells, Science, 2019, 363(6424), 265–270, DOI:10.1126/science.aau5701.
- C. Peng, G. Reid, H. Wang and P. Hu, Perspective: photocatalytic reduction of CO2 to solar fuels over semiconductors, J. Chem. Phys., 2017, 147(3), 030901, DOI:10.1063/1.4985624.
- W. You, D. Tu, W. Zheng, P. Huang and X. Chen, Lanthanide-doped disordered crystals: site symmetry and optical properties, J. Lumin., 2018, 201, 255–264, DOI:10.1016/j.jlumin.2018.04.057.
- Q. Sun, X. Chen, Z. Liu, F. Wang, Z. Jiang and C. Wang, Enhancement of the upconversion luminescence intensity in Er3+ doped BaTiO3 nanocrystals by codoping with Li+ ions, J. Alloys Compd., 2011, 509(17), 5336–5340, DOI:10.1016/j.jallcom.2010.12.212.
- H. Dong, S. R. Du, X. Y. Zheng, G. M. Lyu, L. D. Sun, L. D. Li, P. Z. Zhang, C. Zhang and C. H. Yan, Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy, Chem. Rev., 2015, 115(19), 10725–10815, DOI:10.1021/acs.chemrev.5b00091.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Upconversion nanoparticles: design, nanochemistry, and applications in theranostics, Chem. Rev., 2014, 114(10), 5161–5214, DOI:10.1021/cr400425h.
- M. D. Wisser, S. Fischer, P. C. Maurer, N. D. Bronstein, S. Chu, A. P. Alivisatos, A. Salleo and J. A. Dionne, Enhancing Quantum Yield via Local Symmetry Distortion in Lanthanide-Based Upconverting Nanoparticles, ACS Photonics, 2016, 3(8), 1523–1530, DOI:10.1021/acsphotonics.6b00166.
- J.-C. Boyer and F. C. J. M. van Veggel, Absolute quantum yield measurements of colloidal NaYF4:Er3+,Yb3+ upconverting nanoparticles, Nanoscale, 2010, 2(8), 1417–1419, 10.1039/C0NR00253D.
- S. Han, R. Deng, X. Xie and X. Liu, Enhancing luminescence in lanthanide-doped upconversion nanoparticles, Angew. Chem., Int. Ed., 2014, 53(44), 11702–11715, DOI:10.1002/anie.201403408.
- Q. C. Sun, H. Mundoor, J. C. Ribot, V. Singh, I. I. Smalyukh and P. Nagpal, Plasmon-enhanced energy transfer for improved upconversion of infrared radiation in doped-lanthanide nanocrystals, Nano Lett., 2014, 14(1), 101–106, DOI:10.1021/nl403383w.
- H. Bae and K. T. Lee, Effect of tetragonal to cubic phase transition on the upconversion luminescence properties of A/B site erbium-doped perovskite BaTiO3, RSC Adv., 2019, 9(5), 2451–2457, 10.1039/c8ra09783f.
- B. R. Judd, Optical Absorption Intensities of Rare-Earth Ions, Phys. Rev., 1962, 127(3), 750–761, DOI:10.1103/PhysRev.127.750.
- G. S. Ofelt, Intensities of Crystal Spectra of Rare-Earth Ions, J. Chem. Phys., 2004, 37(3), 511–520, DOI:10.1063/1.1701366 , (acccessed 9/1/2023).
- J.-C. G. Bünzli and S. V. Eliseeva, Basics of Lanthanide Photophysics, in Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects, ed. P. Hänninen and H. Härmä, Springer Berlin, Heidelberg, 2011, pp. 1–45 Search PubMed.
- T. Wang, L. Xu, Z. Wu, Y. Li, Z. Yin, J. Han, Z. Yang, J. Qiu and Z. Song, Self-doping induced oxygen vacancies and lattice strains for synergetic enhanced upconversion luminescence of Er3+ ions in 2D BiOCl nanosheets, Nanoscale, 2022, 14(35), 12909–12917, 10.1039/d2nr02624d.
- H. J. Clabel, I. T. Awan, G. Lozano, M. A. Pereira-da-Silva, R. A. Romano, V. A. G. Rivera, S. O. Ferreira and E. Marega, Jr., Understanding the electronic properties of BaTiO3 and Er3+ doped BaTiO3 films through confocal scanning microscopy and XPS: the role of oxygen vacancies, Phys Chem Chem Phys, 2020, 22(26), 15022–15034, 10.1039/d0cp01010c.
- J. Gu, W. Tian, Z. Wang, N. Ma and P. Du, Control of Oxygen Vacancies in TiO6 Octahedra of Amorphous BaTiO3 Thin Films with Tunable Built-In Electric Field in a BaTiO3/p-Si Heterojunction for Metal–Oxide-Semiconductor Applications, Phys. Status Solidi A, 2020, 217, 1900941, DOI:10.1002/pssa.201900941.
- A. Sagdeo, A. Nagwanshi, P. Pokhriyal, A. K. Sinha, P. Rajput, V. Mishra and P. R. Sagdeo, Disappearance of dielectric anomaly in spite of presence of structural phase transition in reduced BaTiO3: effect of defect states within the bandgap, J. Appl. Phys., 2018, 123, 161424, DOI:10.1063/1.5010870.
- M. Acosta, N. Novak, V. Rojas, S. Patel, R. Vaish, J. Koruza, G. A. Rossetti and J. Rödel, BaTiO3-based piezoelectrics: fundamentals, current status, and perspectives, Appl. Phys. Rev., 2017, 4, 041305, DOI:10.1063/1.4990046.
- D. D. Cuong and J. Lee, Electronic Structure of Oxygen Deficient BaTiO3, Integr. Ferroelectr., 2011, 84(1), 23–30, DOI:10.1080/10584580601077849.
- Q. Ji, L. Bi, J. Zhang, H. Cao and X. S. Zhao, The role of oxygen vacancies of ABO3 perovskite oxides in the oxygen reduction reaction, Energy Environ. Sci., 2020, 13(5), 1408–1428, 10.1039/d0ee00092b.
- L. Yang, Y. Peng, Y. Yang, J. Liu, Z. Li, Y. Ma, Z. Zhang, Y. Wei, S. Li, Z. Huang and N. V. Long, Green and Sensitive Flexible Semiconductor SERS Substrates: Hydrogenated Black TiO2 Nanowires, ACS Appl. Nano Mater., 2018, 1(9), 4516–4527, DOI:10.1021/acsanm.8b00796.
- Z. Wang and L. Wang, Role of oxygen vacancy in metal oxide based photoelectrochemical water splitting, EcoMat, 2021, 3, e12075, DOI:10.1002/eom2.12075.
- Z. Wang, X. Mao, P. Chen, M. Xiao, S. A. Monny, S. Wang, M. Konarova, A. Du and L. Wang, Understanding the Roles of Oxygen Vacancies in Hematite-Based Photoelectrochemical Processes, Angew. Chem., Int. Ed., 2019, 58(4), 1030–1034, DOI:10.1002/anie.201810583.
- X. Zhang, J. Qin, Y. Xue, P. Yu, B. Zhang, L. Wang and R. Liu, Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods, Sci. Rep., 2014, 4(1), 4596, DOI:10.1038/srep04596.
- M. K. Yang, G. H. Kim, H. Ju, J.-K. Lee and H.-C. Ryu, An analysis of “non-lattice” oxygen concentration effect on electrical endurance characteristic in resistive switching MnOx thin film, Appl. Phys. Lett., 2015, 106, 053504, DOI:10.1063/1.4907704.
- X. Jiang, Y. Zhang, J. Jiang, Y. Rong, Y. Wang, Y. Wu and C. Pan, Characterization of Oxygen Vacancy Associates within Hydrogenated TiO2: A Positron Annihilation Study, J. Phys. Chem. C, 2012, 116(42), 22619–22624, DOI:10.1021/jp307573c.
- M. Choi, F. Oba and I. Tanaka, Electronic and structural properties of the oxygen vacancy in BaTiO3, Appl. Phys. Lett., 2011, 98, 172901, DOI:10.1063/1.3583460.
- R. Scharfschwerdt, A. Mazur, O. F. Schirmer, H. Hesse and S. Mendricks, Oxygen vacancies in BaTiO3, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54(21), 15284–15290, DOI:10.1103/PhysRevB.54.15284.
- J. P. Wittke, I. Ladany and P. N. Yocom, Y2O3:Yb:Er-New Red-Emitting Infrared-Excited Phosphor, J. Appl. Phys., 1972, 43(2), 595–600, DOI:10.1063/1.1661163.
- F. Vetrone, J.-C. Boyer, J. A. Capobianco, A. Speghini and M. Bettinelli, Significance of Yb3+ concentration on the upconversion mechanisms in codoped Y2O3:Er3+,Yb3+ nanocrystals, J. Appl. Phys., 2004, 96(1), 661–667, DOI:10.1063/1.1739523.
- M. Pollnau, D. R. Gamelin, S. R. Lüthi, H. U. Güdel and M. P. Hehlen, Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61(5), 3337–3346, DOI:10.1103/PhysRevB.61.3337.
Footnotes |
| † Electronic supplementary information (ESI) available: Instruments and additional experimental data including XPS, UC spectra, and pump–power dependent measurement of E-BT. See DOI: https://doi.org/10.1039/d3cp02133e |
| ‡ These authors contributed equally. |
| This journal is © the Owner Societies 2024 |