Open Access Article
Open Access ArticleA serendipitous one-pot synthesis of the octahydro-2H-pyrazino[1,2-a]pyrazine core†
Claudio
Maestri
 ab,
Paolo
Minazzi
c,
Toni
Grell
ab,
Paolo
Minazzi
c,
Toni
Grell
 d,
Valentina
Colombo
d,
Valentina
Colombo
 d,
Luciano
Lattuada
d,
Luciano
Lattuada
 e,
Giovanni B.
Giovenzana
e,
Giovanni B.
Giovenzana
 ac and
Fabio
Travagin
ac and
Fabio
Travagin
 *a
*a
aDipartimento di Scienze del Farmaco (DSF), Università del Piemonte Orientale, Largo Donegani 2, I-28100 Novara, Italy. E-mail: fabio.travagin@uniupo.it; claudio.maestri@uniupo.it
bPRC Ticinum Lab srl, Via Bovio 6, 28100 Novara, NO, Italy
cCAGE Chemicals srl, Via Bovio 6, 28100 Novara, NO, Italy
dDipartimento di Chimica, Università degli Studi di Milano, Via Golgi 19, 20133 Milano, Italy
eBracco Imaging SpA, Via Egidio Folli 50, 20134 Milano, Italy
First published on 6th August 2024
Abstract
An unexpected nitro group displacement during a nitro-Mannich reaction led to the one-pot formation of the octahydro-2H-pyrazino[1,2-a]pyrazine core, representing the shortest access to date to this pharmacologically relevant heterobicyclic system. A mechanistic hypothesis is suggested and supported by specific experiments and HRMS analysis of reaction mixtures.
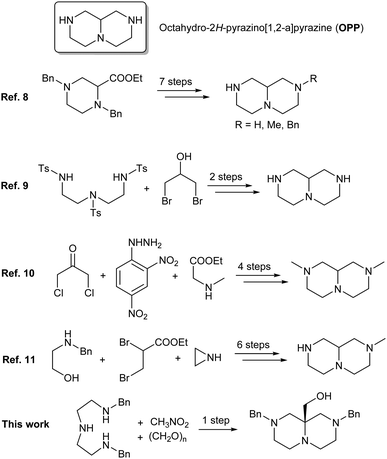
The heterobicyclic core of octahydro-2H-pyrazino[1,2-a]pyrazines (OPPs) (Scheme 1) is the subject of an increasing number of medicinal chemistry studies, focused on its pharmacophoric properties. OPPs have been extensively investigated as putative β-turn mimetics1,2 and as 5-HT2C receptor agonists.3 OPP derivatives were patented as IgE inhibitors,4 while inhibitor activity was ascribed to OPPs against renal outer medullary potassium channel (ROMK),5 ubiquitin specific peptidase 30 (USP30)6 and the important lung adenocarcinoma-related mutant oncogene KRASG12C.7
The synthesis of the OPP framework is not straightforward, and the few procedures reported in the literature usually require several steps. Gubert et al.8 described a 7-step synthesis of the unsubstituted OPP, starting from ethyl 1,4-dibenzylpiperazine-2-carboxylate, while Wu et al. reported a transannular reaction leading to the unsubstituted OPP during the HBr-mediated detosylation of 1,4,7-tritosyl-1,4,7-triazadecan-9-ol for which fractional crystallization is required to separate the native OPP from the 10-membered monocyclic isomeric byproduct.9
Different approaches have been developed for the preparation of octahydro-2H-pyrazino[1,2-a]-pyrazinones (including -diones and -triones), with the eventual need for an additional reduction step to access the fully reduced OPP. An early approach involves 4 steps, starting from 1,3-dichloroacetone, 2,4-dinitrophenylhydrazine and sarcosine ethyl ester and leading to N-methyloctahydro-2H-pyrazino[1,2-a]pyrazine.10 A 6-step preparation of the same derivative starting from N-benzylethanolamine was reported in 1977 by Beim and Day.11 Peptide synthesis approaches were employed for the preparation of the OPP core, sequentially forming the piperazine rings by ex novo lactamization of α-amino acid precursors, either by solution-phase12 or by solid-phase2 protocols. The first piperazine ring of OPPs was alternatively accessed through the Castagnoli–Cushman reaction of cyclic anhydrides with imines.1
The nitro-Mannich reaction is a well-established C–C bond forming transformation leading to useful synthons bearing vicinal nitrogen-based functional groups.13 Our research group has routinely employed the nitro-Mannich reaction for the synthesis of precursors of mesocyclic chelating agents, by reacting a vicinal secondary diamine (e.g.: N,N′-dibenzyl-1,2-ethylenediamine, 1) with formaldehyde and a suitable nitroalkane (Scheme 2).14
In continuation of our studies on functionalized analogues of mesocyclic chelating agents, we explored the reactivity of a secondary triamine, observing an unexpected behaviour, dependent on the reaction conditions. When N,N′′-dibenzyldiethylenetriamine 315 is refluxed in ethanol with nitromethane and para-formaldehyde, a vigorous evolution of a red-brown gas (identified as NO2 by absorption in aqueous NaOH solution, the latter tested positive to nitrite by semiquantitative nitrite-test strips) occurred and the formation of a main product was observed by TLC analysis. This product was isolated in 75% yield as a white solid and to our surprise its NMR and MS spectra are not compatible to the expected nitro-Mannich product 4 (Scheme 2). The NMR spectra suggest a symmetric structure, lacking the nitro group as confirmed by IR and HRMS analysis, the latter providing the molecular formula C22H29N3O (Fig. S7–S12, S27 and S29, ESI†).
Crystallization from diethyl ether provided single crystals suitable for X-ray diffractometric analysis (Fig. 1 and Table S3, ESI†), which identified the new compound as (2,8-dibenzylocta-hydro-9aH-pyrazino[1,2-a]pyrazin-9a-yl)-methanol 5a (Scheme 2 and Fig. 1).
The unexpected elimination of the nitro group and the concomitant formation of compound 5a prompted us to investigate the mechanism of this unprecedented reaction.
The nitro group is known to act as a leaving group,16,17 for example in β-eliminations,18 generally triggered by the presence of an acidic hydrogen in the β-position, and in SRN1 substitutions,19 requiring a radical-stabilising substitution pattern.
Compound 5a may be originated by the loss of the nitro group from the expected nitro-Mannich product 4 preformed under the reaction conditions or directly by an alternative pathway. The expected nitro-Mannich product 4 is in fact detected in the reaction mixture and can be isolated in 51% yield by running the reaction under milder conditions (methanol, 50 °C), along with minor amounts of 5a (Scheme 2).
The comparison between the molecular formula of compounds 4 and 5a points to the loss of the nitro group and the following introduction of a hydroxyl group. The clear change in connectivity may be traced back to a rearrangement process, likely concomitant with the loss of the nitro group. The complete loss of the nitro group strongly suggested by the copious evolution of gaseous NO2 points to an intermolecular origin of the incoming OH group, presumably ascribed to H2O, either adventitious or produced by the initial nitro-Mannich steps (3 eq. of H2O are generated in the condensation 3 + 3CH2O + CH3NO2 → 4 + 3H2O).
A possible reaction mechanism for this unprecedented transformation is proposed in Scheme 3.
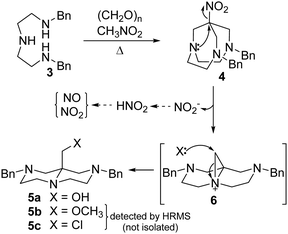 | ||
| Scheme 3 Proposed mechanism for the formation of compound 5a and two side products 5b and 5c, detected by HRMS. | ||
The nitrotriamine 4 is initially formed by a classic nitro-Mannich condensation. On heating, compound 4 undergoes an intramolecular nucleophilic displacement of the nitro group by the central nitrogen atom, leading to the intermediate tricyclic aziridinium ion 6. Nucleophilic ring opening of the strained three-membered ring takes place at its unhindered methylene group, leading to compound 5a, with the role of the nucleophile played by water formed during the condensation step. The nitro group acts as the leaving group and is lost as NO2−, the latter in equilibrium with the highly unstable nitrous acid, finally undergoing decomposition to nitrogen oxides (Scheme 3).
To assess the role of water, triamine 3, para-formaldehyde and nitromethane were heated in an aprotic solvent (THF), kept dry by an excess of preactivated 3 Å molecular sieves. TLC analysis of the reaction mixture clearly showed the formation of 4, the total absence of 5a and the formation of minor amounts of highly polar compounds. The reaction mixture was then cooled to room temperature and filtered under vacuum to remove the molecular sieves. Water was added, and the solution refluxed for additional 4 hours. TLC analysis showed the progressive formation of 5a, confirming the direct role of water in this reaction.
Careful analysis by HPLC-HRMS of the reaction mixture run in methanol (Scheme 2) and leading to 4 allowed to detect two additional compounds (5b and 5c), identified on the base of their molecular weight and fragmentation patterns (Fig. S16, ESI†). The methoxy derivative 5b likely arises from solvolytic ring opening of aziridinium 6. The formation of compound 5c could be ascribed to the presence of residual chloride ions in the starting triamine 3, as the latter is prepared and isolated as the trihydrochloride following a literature procedure.15
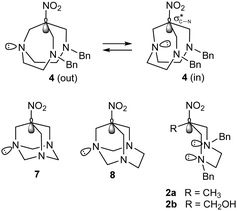
A rational underlying the unusual nucleophilic displacement may be proposed on the base of stereoelectronic effects. The instability of nitrotriamine 4 on heating could arise from the possibility for this flexible bicyclic structure to undergo a pyramidal inversion of the (central) nitrogen atom, leading its lone pair free to flip outward/inward (Scheme 4). The lone pair, once directed inward, can approach the carbon atom bearing the nitro group to start the nucleophilic displacement en route to the intermediate aziridinium 6.
Obviously, pyramidal inversion of the nitrogen atom is precluded in the nitrotriamines 720 and 821 (Scheme 4), previously reported in the literature, as they are stable at temperatures higher than those involved in the formation of 5 (compound 7 decomposes only at T > 260 °C while compound 8 withstands recrystallization from boiling 1-butanol (bp 117.7 °C) and sublimes unchanged at 191–192 °C).
A similar nitro group displacement could be expected in monocyclic nitrodiamines 2a–b22,23 (Scheme 4), as they are free to undergo pyramidal inversion of the nitrogen atoms. However, compounds 2a–b are stable under the reaction conditions (i.e. refluxing ethanol), actually employed for their synthesis in almost quantitative yield. Nevertheless, HRMS of 2a–b clearly shows fragmentation peaks corresponding to the formation of the bicyclic aziridinium ion with loss of the nitro group in the mass spectrometer ion source (Fig. S17 and S18, ESI†), supporting the possibility of this reaction pathway. The lower propensity of the monocyclic nitrodiamines 2a–b to the loss of the nitro group could be explained by the need for assuming high energy conformations to allow the nitrogen lone pair to approach the ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) –NO2 carbon atom, while the bicyclic structure of 4, when in the “in” conformation, forces the lone pair correctly pointed towards the
–NO2 carbon atom, while the bicyclic structure of 4, when in the “in” conformation, forces the lone pair correctly pointed towards the  orbital.
orbital.
The easy access to the OPP core represented by this unexpected transformation paves the way for the preparation of a wide range of OPP derivatives, taking advantage of the functionalization pattern of compound 5a.
We demonstrated the versatility of the latter in accessing the OPP chemical space with a series of simple reactions, summarized in Scheme 5.
The OH-group of compound 5a shows the expected reactivity of a primary alcohol. Quantitative acetylation to 9 is obtained by treatment with acetic anhydride in DCM at r.t., while reaction in the same conditions with 2-phenethyl isocyanate provides the carbamate 10. The reaction of 5a with IBX in DMSO leads to the oxidation to the interesting triaminoaldehyde 11 in 45% yield.
Synthetic work on the distal nitrogen atoms of 5a requires their preliminary debenzylation, cleanly obtained by catalytic hydrogenolysis in methanol at r.t., to give compound 12. Selective acylation of the resulting secondary amines is achieved by treatment with stoichiometric acetic anhydride in DCM at r.t., quantitatively yielding diacetamide 13. An additional equivalent of acetic anhydride in the same experimental conditions allows exhaustive acylation of the secondary amines and of the primary alcoholic group to give the diamidoester 14 in 78% yield.
Treatment of compound 12 with an alkylating agent (t-butyl bromoacetate) in acetonitrile in the presence of DIPEA at r.t. leads to the alkylation of the secondary amines, yielding the highly functionalized triaminohydroxydiester 15 in 52% yield.
Finally, the reaction of 1,4,7-trimethyldiethylenetriamine with nitromethane and para-formaldehyde in refluxing ethanol for 5 h, the same conditions leading to the formation of compound 5a, formed a complex mixture, possibly due to an extensive polymerization.
The reaction described in this manuscript represents an unprecedented, simple and efficient approach to pharmacologically relevant octahydro-2H-pyrazino[1,2-a]pyrazines (OPPs).
A possible mechanism for this transformation involving a combination of nitro-group displacement-rearrangement is proposed and supported by preliminary experimental data.
The functionalized OPP obtained by this unexpected transformation has been converted to a series of OPP derivatives, demonstrating its versatility.
The nitro-Mannich-rearrangement sequence described in this manuscript represents a short and efficient synthesis to OPPs core and a useful entry into the chemical space of this pharmacologically relevant heterobicyclic compounds.
Data availability
The data supporting this article have been included as a part of ESI.† CCDC 2327990 (5a) contain the supplementary crystallographic data for this paper.Conflicts of interest
There are no conflicts to declare.Notes and references
- L. Usmanova, D. Dar’in, M. S. Novikov, M. Gureev and M. Krasavin, J. Org. Chem., 2018, 83, 5859–5868 CrossRef CAS PubMed.
- A. Golebiowski, J. Jozwik, S. R. Klopfenstein, A.-O. Colson, A. L. Grieb, A. F. Russell, V. L. Rastogi, C. F. Diven, D. E. Portlock and J. J. Chen, J. Comb. Chem., 2002, 4, 584–590 CrossRef CAS PubMed.
- G. S. Welmaker, J. A. Nelson, J. E. Sabalski, A. L. Sabb, J. R. Potoski, D. Graziano, M. Kagan, J. Coupet, J. Dunlop, H. Mazandarani, S. Rosenzweig-Lipson, S. Sukoff and Y. Zhang, Bioorg. Med. Chem. Lett., 2000, 10, 1991–1994 CrossRef CAS PubMed.
- Kowa Co. Ltd. (JP), US Pat., US6340682B1, 2002 Search PubMed.
- Y. Zhu, R. K. de Jesus, H. Tang, S. P. Walsh, J. Jiang, X. Gu, N. Teumelsan, A. Shahripour, B. Pio, F.-X. Ding, S. Ha, B. T. Priest, A. M. Swensen, M. Alonso-Galicia, J. P. Felix, R. M. Brochu, T. Bailey, B. Thomas-Fowlkes, X. Zhou, L.-Y. Pai, C. Hampton, M. Hernandez, K. Owens, J. Ehrhart, S. Roy, G. J. Kaczorowski, L. Yang, E. R. Parmee, K. Sullivan, M. L. Garcia and A. Pasternak, Bioorg. Med. Chem. Lett., 2016, 26, 5695–5702 CrossRef CAS PubMed.
- Forma Therapeutics Inc. (US), WO Pat., WO2019222468A1, 2019 Search PubMed.
- J. G. Kettle, S. K. Bagal, S. Bickerton, M. S. Bodnarchuk, J. Breed, R. J. Carbajo, D. J. Cassar, A. Chakraborty, S. Cosulich, I. Cumming, M. Davies, A. Eatherton, L. Evans, L. Feron, S. Fillery, E. S. Gleave, F. W. Goldberg, S. Harlfinger, L. Hanson, M. Howard, R. Howells, A. Jackson, P. Kemmitt, J. K. Kingston, S. Lamont, H. J. Lewis, S. Li, L. Liu, D. Ogg, C. Phillips, R. Polanski, G. Robb, D. Robinson, S. Ross, J. M. Smith, M. Tonge, R. Whiteley, J. Yang, L. Zhang and X. Zhao, J. Med. Chem., 2020, 63, 4468–4483 CrossRef CAS PubMed.
- S. Gubert, C. Braojos, A. Sacristán and J. A. Ortiz, Synthesis, 1991, 318–320 CrossRef CAS.
- F. Liang, X. Wu, S. Zhang and C. Wu, Synth. Commun., 2004, 34, 845–851 CrossRef CAS.
- M. Rink and K. Feiden, Arch. Pharm., 1962, 295, 121–126 CrossRef CAS PubMed.
- H. J. Beim and A. R. Day, J. Heterocycl. Chem., 1977, 14, 307–308 CrossRef CAS.
- V. N. Belov, C. Funke, T. Labahn, M. Es-Sayed and A. de Meijere, Eur. J. Org. Chem., 1999, 1345–1356 CrossRef CAS.
- A. Noble and J. C. Anderson, Chem. Rev., 2013, 113, 2887–2939 CrossRef CAS PubMed.
- F. Travagin, L. Lattuada and G. B. Giovenzana, Coord. Chem. Rev., 2021, 438, 213908–213931 CrossRef CAS.
- J. A. Sclafani, M. T. Maranto, T. M. Sisk and S. A. Van Arman, J. Org. Chem., 1996, 61, 3221–3222 CrossRef CAS PubMed.
- N. Ono, The Nitro Group in Organic Synthesis, Wiley-VCH, New York, 2001, pp. 182–230 Search PubMed.
- R. Tamura, A. Kamimura and N. Ono, Synthesis, 1991, 423–434 CrossRef CAS.
- R. Ballini and A. Palmieri, Adv. Synth. Catal., 2019, 361, 5070–5097 CrossRef CAS.
- N. Kornblum, S. D. Boyd and F. W. Stuchal, J. Am. Chem. Soc., 1970, 92, 5783–5784 CrossRef CAS.
- E. B. Hodge, J. Org. Chem., 1972, 37, 320–321 CrossRef CAS.
- A. I. Kuznetsov, V. A. Kosmakov and A. S. Moskovkii, Chem. Heterocycl. Compd., 1990, 26, 578–581 CrossRef.
- S. Aime, L. Calabi, C. Cavallotti, E. Gianolio, G. B. Giovenzana, P. Losi, A. Maiocchi, G. Palmisano and M. Sisti, Inorg. Chem., 2004, 43, 7588–7590 CrossRef CAS PubMed.
- G. Gugliotta, M. Botta, G. B. Giovenzana and L. Tei, Bioorg. Med. Chem. Lett., 2009, 19, 3442–3444 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2327990 (5a). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc03596h |
| This journal is © The Royal Society of Chemistry 2024 |