Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cubane-1,3-dicarboxamides as structural isosteres for isophthalamides in hydrogen bond templated interlocked molecules†
Sean R.
Barlow
 a,
Nathan R.
Halcovitch
a,
Nathan R.
Halcovitch
 a,
Geoffrey R.
Akien
a,
Geoffrey R.
Akien
 a,
Susannah C.
Coote
a,
Susannah C.
Coote
 *b and
Nicholas H.
Evans
*b and
Nicholas H.
Evans
 *a
*a
aDepartment of Chemistry, Lancaster University, Lancaster, LA1 4YB, UK. E-mail: n.h.evans@lancaster.ac.uk
bDepartment of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK
First published on 13th September 2024
Abstract
The synthesis and characterization of the first examples of cubane containing interlocked molecules are reported. Catenanes and rotaxanes have been prepared by hydrogen bond templation with cubane-1,3-dicarboxamides replacing isophthalamide motifs.
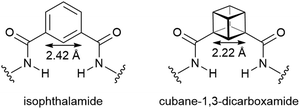
Due to the geometric correlation between a cubane and a benzene ring, there has been considerable interest amongst organic and medicinal chemists into whether cubanes may act as bioisosteres for benzene rings in biologically active compounds.1 With the notable exception of a cubane analogue of a 1,4-benzene-dicarboxylic acid containing metal organic framework,2 cubanes have yet to be incorporated into self-assembled supramolecular structures—despite the potential to exploit the three-dimensional vectors of the cubic structure. Very recently, MacMillan3 and Coote4 have disclosed syntheses of cubane-1,3-dicarboxylic acid derivatives, which may be considered structural isosteres of isophthalic acid derivatives (Fig. 1). In the field of mechanically interlocked molecules (MIMs), following upon the work of Hunter,5 Vögtle,6 Leigh7 and Philp,8 Evans and co-workers have prepared hydrogen bond templated catenanes and rotaxanes that include isophthalamide macrocycles.9,10 Here we report that it is possible to synthesize and spectrally characterize hydrogen bond templated MIMs where 1,3-substituted cubanes are replacing isophthalic groups. These are the first reported examples of cubane containing catenanes and rotaxanes.
We chose to investigate a range of hydrogen bond templated catenane and rotaxane syntheses as reported by Leigh7a,c and Evans9c,g (Scheme 1). Our initial target was to prepare a cubane analogue of Leigh's serendipitous [2]catenane (Scheme 1a).7a To allow for confident yield comparisons, Leigh's original synthesis was repeated; in our hands we obtained the previously reported all-isophthalamide [2]catenane in 17% yield (20% lit. yield, see ESI† pp. S6 and S36). To prepare the cubane analogue, solutions of cubane-1,3-diacyl chloride 111 and p-xylylenediamine in dry CHCl3 were simultaneously added dropwise to a solution of Et3N in CHCl3 over three hours and then the reaction was stirred for a further 16 hours. After work-up, rather than being able to isolate a pure sample of the target [2]catenane 2, a crude mixture was obtained, which by analytical silica TLC and 1H NMR analysis indicated the presence of at least two cubane containing species. Silica gel column chromatography and preparative TLC allowed for separation of an analytical sample (isolated 4% yield) of [2]catenane 2 for spectral characterization. In addition to detection of the molecular ion peak by electrospray mass spectrometry (see ESI† p. S21), isolation of [2]catenane 2 (rather than non-interlocked macrocycle) is evidenced by the 1H NMR spectrum (Fig. 2). In particular, the splitting of aromatic protons 8 (as well as protons 1 and 6) indicates the presence of one macrocyclic ring passing through the cavity of its interlocked partner.
 | ||
| Scheme 1 Synthesis of cubane containing interlocked molecules. Inset: X-ray structure of macrocycle 5. | ||
The second target was to prepare a cubane analogue of Leigh's furamide [2]rotaxane (Scheme 1b).7c As for the catenane, Leigh's original synthesis was repeated; in our hands we synthesized the all-isophthalamide [2]rotaxane in 75% yield (calculated from the 1H NMR spectrum of the purified rotaxane contaminated with free axle, c.f. lit. yield: 97%). To prepare the cubane analogue 4, solutions of cubane-1,3-diacyl chloride 1 and p-xylylenediamine in dry CHCl3 were simultaneously added dropwise to a solution of axle 3 and Et3N in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of dry CHCl3/CH3CN and then the reaction was stirred for a further 16 hours. After aqueous work-up, the crude material was then subjected to careful silica gel column chromatography, which allowed for the isolation of the target [2]rotaxane 4 in 35% yield.
1 mixture of dry CHCl3/CH3CN and then the reaction was stirred for a further 16 hours. After aqueous work-up, the crude material was then subjected to careful silica gel column chromatography, which allowed for the isolation of the target [2]rotaxane 4 in 35% yield.
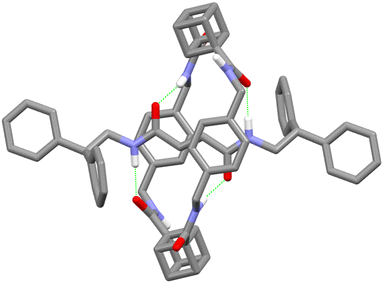
Evidence for rotaxane formation is provided by comparison of the 1H NMR spectra of axle 3 and [2]rotaxane 4 (see ESI† p. S37): for example, there is a dramatic upfield shift in the sp2 C–H furamide axle protons consistent with shielding due to the presence of the macrocyclic ring.12 Irrefutable demonstration of interlocked structure formation was secured by the growth of single crystals of sufficient quality for X-ray diffraction from chloroform/methanol solution. The solved crystal structure (Fig. 3) reveals that the cubane-1,3-dicarboxamides are syn–anti with hydrogen bonding of the axle amide oxygen atoms to a cubane carboxamide N–H, while the oxygen atom of the other cubane carboxamides are hydrogen bonding to the axle amide N–Hs. This contrasts with Leigh's report of the all-isophthalamide analogue, where all four N–Hs of the syn–syn isophthalamides hydrogen bond to axle amide oxygen atoms.7c This variation in conformation is attributed to the differences in strength of the possible inter-component hydrogen bonds in the [2]rotaxanes.
Our attention then turned to studying the use of cubane-1,3-dicarboxamides in examples of Evans style hydrogen bond templated synthesis. We first sought to trial novel macrocycle 513 in the synthesis of a [2]catenane using our recently published methodology, specifically seeking to generate the cubane containing analogue of the highest yielding reported example (afforded in a yield of 70%).9g Following the published methodology, cubane macrocycle 5 and alkyne–azide 69g were dissolved in dry CH2Cl2, and stirred for 15 minutes to allow for the formation of the pseudorotaxane complex. Then, Cu(CH3CN)4BF4, TBTA and DIPEA were added, and the reaction stirred for 16 hours under an inert atmosphere. After aqueous work-up, the crude material was purified by silica gel column chromatography and preparative TLC to afford the cubane containing [2]catenane 7 in 23% yield.
The formation of cubane containing [2]catenane 7 is evident by comparison of the 1H NMR spectra of alkyne–azide 6, [2]catenane 7 and macrocycle 5 (Fig. 4). For example, the upfield shifts of both sets of aromatic protons 8/9 and j/k are consistent with intercalation of aromatic rings within the interlocked molecule. In addition, cubane proton environments 1 and 3 split as the two faces of cubane macrocycle 5 become inequivalent upon formation of the mechanical bond, due to the second macrocycle being rotationally asymmetric.12
 | ||
| Fig. 4 1H NMR spectra of (a) alkyne–azide 6, (b) [2]catenane 7 and (c) macrocycle 5 (CDCl3, 400 MHz, 298 K). | ||
Finally we targeted the synthesis of an Evans style [2]rotaxane, specifically a cubane analogue of a single amide containing axle [2]rotaxane (reported 47% yield).9c Once again following the published methodology, a pseudorotaxane was formed by stirring a solution of cubane macrocycle 5 and azide 89c in dry CH2Cl2 for 15 minutes. Then, alkyne 9,9c Cu(CH3CN)4BF4, TBTA and DIPEA were added, and the reaction stirred for 16 hours under an inert atmosphere. The reaction was then submitted to aqueous work-up and the crude material purified by column chromatography and preparative TLC to afford the cubane containing [2]rotaxane 10 in 10% yield.12
In all cases, the yields were lower for the cubane-containing MIMs in comparison with their all-isophthalamide analogues.14,15 This is tentatively attributed to a combination of (i) the amides being less optimally arranged spatially to support the hydrogen bonding interactions required to create the necessary self-assembled complexes prior to covalent capture of the interlocked structure and (ii) the cubane-1,3-dicarboxamide N–Hs being less acidic than the isophthalamide N–Hs.16
Evidence for weaker intercomponent hydrogen bonds in at least one of the cubane-containing MIMs is provided by NMR spectroscopy. At room temperature, the all-isophthalamide analogue of the Evans style cubane [2]catenane 7 has a broad 1H NMR spectrum in CDCl3 indicating ring rotation occurs at a rate between that of the fast and slow exchange regimes on the NMR timescale. In comparison, the cubane-containing [2]catenane 7 has a sharp 1H NMR spectrum in CDCl3 at room temperature, consistent with ring rotation being in the fast exchange regime and weaker inter-ring hydrogen bonding (see ESI† pp. S40 and S41).
While generated in modest yields, the successful preparation of cubane containing MIMs is nevertheless exciting. It has been demonstrated that Coote's synthesis4 does allow for production of sufficient quantities of 1,3-disubstituted cubanes for useful synthetic studies. Aided by further work optimizing the synthesis of cubane-containing MIMs, we envisage incorporating cubanes into more sophisticated MIMs for host–guest17 or catalytic18 applications that exploit the three-dimensional vector potential of the cubane structure to augment the inherent three-dimensional architectures of MIMs. In addition, an ability to alter the hydrogen bonding interactions between interlocked components by substituting isophthalamide(s) for cubane dicarboxamide(s), provides supramolecular chemists with another strategy to adjust the dynamic co-conformational behaviour of future MIM-based molecular machines.19
NHE and SCC proposed the study. SRB and SCC conducted the synthesis and characterization of materials (with contributions from NRH, GRA and NHE). NHE supervised SRB. SRB, SCC and NHE wrote the manuscript. All authors discussed and commented on the manuscript.
S. R. B. acknowledges EPSRC DTP PhD funding from the Engineering and Physical Sciences Research Council [EP/T518037/1] and a Sydney Andrew Scholarship from the Society of Chemical Industry. VT NMR spectra were recorded on a spectrometer supported by an Unmet Demand Funding allocation associated with an EPSRC Core Equipment Award [EP/X034844/1]. We thank Dr Rebecca Spicer (Lancaster University) for assistance in the preparation of chemical structure diagrams containing cubanes.
Data availability
Underlying data for this paper are provided in the ESI.† Electronic copies of NMR spectra (including fid files) will be available upon publication from: DOI: https://doi.org/10.17635/lancaster/researchdata/662 and DOI: https://doi.org/10.17635/lancaster/researchdata/682. Crystallographic data for 4 and 5 have been deposited at CCDC (https://ccdc.cam.ac.uk) under structure numbers 2335982 and 2335981.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) J. Wlochal, R. D. M. Davies and J. Burton, Org. Lett., 2014, 16, 4094–4097 CrossRef CAS PubMed; (b) B. A. Chalmers, H. Xing, S. Houstan, C. Clark, S. Ghassabian, A. Kuo, B. Cao, A. Reitsma, C.-E. P. Murray, J. E. Stok, G. M. Boyle, C. J. Pierce, S. W. Littler, D. A. Winkler, P. V. Bernhardt, C. Pasay, J. J. De Voss, J. McCarthy, P. G. Parsons, G. H. Walter, M. T. Smith, H. M. Cooper, S. K. Nilsson, J. Tsanaktsidis, G. P. Savage and C. M. Williams, Angew. Chem., Int. Ed., 2016, 55, 3580–3585 CrossRef CAS PubMed; (c) T. A. Reekie, C. M. Williams, L. M. Redina and M. Kassiou, J. Med. Chem., 2019, 62, 1078–1095 CrossRef CAS PubMed.
- L. K. Macreadie, E. J. Mensforth, R. Babarao, K. Konstas, S. G. Telfer, C. M. Doherty, J. Tsanaktsidis, S. R. Batten and M. R. Hill, J. Am. Chem. Soc., 2019, 141, 3828–3832 CrossRef CAS PubMed.
- M. P. Wiesenfeldt, J. A. Rossi-Ashton, I. B. Perry, J. Diesel, O. L. Garry, F. Bertels, S. C. Coote, X. Ma, C. S. Yeung, D. J. Bennett and D. W. C. MacMillan, Nature, 2023, 618, 513–518 CrossRef CAS.
- N. Kazi, M. C. Aublette, S. L. Allinson and S. C. Coote, Chem. Commun., 2023, 59, 7971–7973 RSC.
- C. A. Hunter, J. Am. Chem. Soc., 1992, 114, 5303–5311 CrossRef CAS.
- (a) F. Vögtle, S. Meier and R. Hoss, Angew. Chem., Int. Ed. Engl., 1992, 31, 1619–1622 CrossRef; (b) F. Vögtle, M. Händel, S. Meier, S. Ottens-Hildebrandt, F. Ott and T. Schmidt, Liebigs Ann., 1995, 739–743 CrossRef.
- (a) A. G. Johnston, D. A. Leigh, R. J. Pritchard and M. D. Deegan, Angew. Chem., Int. Ed. Engl., 1995, 34, 1209–1212 CrossRef CAS; (b) D. A. Leigh, A. Murphy, J. P. Smart and A. M. Z. Slawin, Angew. Chem., Int. Ed. Engl., 1997, 36, 728–732 CrossRef CAS; (c) F. G. Gatti, D. A. Leigh, S. A. Nepogodiev, A. M. Z. Slawin, S. J. Teat and J. K. Y. Wong, J. Am. Chem. Soc., 2001, 123, 5983–5989 CrossRef CAS PubMed; (d) A. Altieri, G. Bottari, F. Dehez, D. A. Leigh, J. K. Y. Wong and F. Zerbetto, Angew. Chem., Int. Ed., 2003, 42, 2296–2300 CrossRef CAS.
- (a) A. Vidonne and D. Philp, Tetrahedron, 2008, 64, 8464–8475 CrossRef CAS; (b) N. I. Hassan, V. del Amo, E. Calder and D. Philp, Org. Lett., 2011, 13, 458–461 CrossRef CAS; (c) T. Kosikova, N. I. Hassan, D. B. Cordes, A. M. Z. Slawin and D. Philp, J. Am. Chem. Soc., 2015, 137, 16074–16083 CrossRef CAS PubMed; (d) A. Vidonne, T. Kosikova and D. Philp, Chem. Sci., 2016, 7, 2592–2603 RSC.
- (a) C. N. Marrs and N. H. Evans, Org. Biomol. Chem., 2015, 13, 11021–11025 RSC; (b) N. H. Evans, C. E. Gell and M. J. G. Peach, Org. Biomol. Chem., 2016, 14, 7972–7981 RSC; (c) B. E. Fletcher, M. J. G. Peach and N. H. Evans, Org. Biomol. Chem., 2017, 15, 2797–2803 RSC; (d) N. H. Evans and G. R. Akien, Supramol. Chem., 2018, 30, 758–764 CrossRef; (e) C. E. Gell, T. A. Mcardle-Ismaguilov and N. H. Evans, Chem. Commun., 2019, 55, 1576–1579 RSC; (f) M. J. Young, G. R. Akien and N. H. Evans, Org. Biomol. Chem., 2020, 18, 5203–5209 RSC; (g) S. R. Barlow, G. R. Akien and N. H. Evans, Org. Biomol. Chem., 2023, 21, 402–414 RSC; (h) R. L. Spicer, C. C. Shearman and N. H. Evans, Chem. – Eur. J., 2023, 29, e2022030502 CrossRef; (i) S. R. Barlow, N. R. Halcovitch and N. H. Evans, Org. Biomol. Chem., 2024, 22, 3001–3008 RSC.
- Lewis has also recently reported upon similar MIMs: (a) J. E. M. Lewis, Org. Biomol. Chem., 2019, 17, 2442–2447 RSC; (b) N. Hoyas Pérez, P. S. Sherin, V. Posligua, J. L. Greenfield, M. J. Fuchter, K. E. Jelfs, M. K. Kuimova and J. E. M. Lewis, Chem. Sci., 2022, 13, 11368–11375 RSC.
- Successful activation of cubane-1,3-dicarboxylic acid by use of oxalyl chloride was confirmed by preparation of a simple model system ESI-1 see ESI† pp. S5, S16 and S17.
- In addition to comparison of 1H NMR spectra, spectral evidence of interlocked structure formation is provided by multiple intercomponent through-space correlations in the 1H–1H ROESY NMR spectrum and detection of the molecular ion peak by electrospray mass spectroscopy (see ESI†).
- The synthesis and characterization of macrocycle 5 is included in ESI,† pp. S10, S26, S27, S42 & S43. The crystal structure of 5 (omitting C–H bonds for clarity) is depicted in Scheme 1.
- Similar trends in yield have been observed by Martinez-Cuezva and Berna in preparation of adamantane-containing analogues of furamide and succinimide rotaxanes: (a) A. Martinez-Cuezva, F. Morales, G. R. Marley, A. Lopez-Lopez, J. C. Martinez-Costa, D. Bautista, M. Alajarin and J. Berna, Eur. J. Org. Chem., 2019, 3480–3488 CrossRef CAS; (b) A. Martinez-Cuezva, A. Pastor, M. Marin-Luna, C. Diaz-Marin, D. Bautista, M. Alajarin and J. Berna, Chem. Sci., 2021, 12, 747–756 RSC; (c) J. de Maria Perez, M. Alajarin, A. Martinez-Cuezva and J. Berna, Org. Chem. Front., 2022, 9, 2690–2696 RSC.
- Highlighted by one of the reviewers, a recent report of modifying previously reported MIM synthesis by motif substitution: A. Sarwa, A. Białońska, M. Sobieraj, J. P. Martínez, B. Trzaskowski and B. Szyszko, Angew. Chem., Int. Ed., 2024, 62, e202316489 Search PubMed.
- We believe the lower yields for the cubane-containing MIMs cannot be attributed solely to losses in purification, even though–most notably for the Leigh style [2]catenane 2–challenging chromatographic separation may play a role.
- J. T. Wilmore and P. D. Beer, Adv. Mater., 2024, 2309098 CrossRef CAS.
- R. W. Heard, J. M. Suárez and S. M. Goldup, Nat. Rev. Chem., 2022, 6, 182–196 CrossRef PubMed.
- (a) J.-P. Sauvage, Angew. Chem., Int. Ed., 2017, 56, 11080–11093 CrossRef CAS; (b) J. F. Stoddart, Angew. Chem., Int. Ed., 2017, 56, 11094–11125 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data. CCDC2335981 and 2335982. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc01859a |
| This journal is © The Royal Society of Chemistry 2024 |