 Open Access Article
Open Access ArticleRh(III)-catalyzed atroposelective C–H alkynylation of 1-aryl isoquinolines with hypervalent iodine–alkyne reagents†
Dong-Song
Zheng
ab,
Fangnuo
Zhao
b,
Qing
Gu
*b and
Shu-Li
You
 *ab
*ab
aChang-Kung Chuang Institute, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062, China
bNew Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China. E-mail: qinggu@sioc.ac.cn; slyou@sioc.ac.cn
First published on 5th June 2024
Abstract
An efficient Rh(III)-catalyzed enantioselective C–H alkynylation of isoquinolines is disclosed. The C–H alkynylation of 1-aryl isoquinolines with hypervalent iodine–alkyne reagents proceeded in DMA at room temperature in the presence of 2.5 mol% chiral SCpRh(III) complex along with 20 mol% AgSbF6, providing axially chiral alkynylated 1-aryl isoquinolines in excellent yields (up to 93%) and enantioselectivity (up to 95% ee). The diverse transformations of the product further enhance the potential utility of this reaction.
Hypervalent iodine reagents (HIRs) have been established as versatile and useful reagents for synthetic organic transformations due to their exceptional reactivity.1 Among them, ethynylbenziodoxolone (EBX) reagent is one of the most widely used hypervalent iodine reagents for the direct alkynylation of heteroatom or carbon nucleophiles.2 In particular, great achievements have been made in transition-metal-catalyzed C–H alkynylation reactions by taking advantage of EBX reagents as the coupling partners (Scheme 1a).3 In 2009, pioneering works on gold or palladium-catalyzed C–H alkynylation of electron-rich aromatic substrates including indoles, pyrroles and anilines with hypervalent alkynyl iodine reagents were developed by Waser and co-workers.3d Later on, Loh and coworkers reported an elegant example of rhodium-catalyzed electronically reversed Sonogashira coupling of electron-poor arenes, affording the alkynyl benzamide products in up to 99% yield.3e Shortly afterwards, Li and coworkers developed efficient Rh(III)- and Ir(III)-catalyzed ortho C–H alkynylation reactions of diversified arenes using hypervalent iodine–alkyne reagents.3j Both electron-poor and electron-rich arenes bearing different directing groups are viable substrates in this transformation. Although much progress has been accomplished, it remains challenging to develop Rh(III)-catalyzed enantioselective C–H alkynylation by employing EBX reagents.
The synthesis of axially chiral biaryls has been extensively investigated since they broadly exist in natural products, pharmaceuticals, biologically active molecules, chiral ligands, and organocatalysts.4 Among these methods, the atroposelective C–H functionalization of achiral biaryls represents one of the most efficient and straightforward approaches to access axially chiral biaryls.5 In this regard, the synthesis of axially chiral alkyne-functionalized biaryls has been widely explored via transition-metal-catalyzed atroposelective C–H alkynylation.6 In 2018, Shi and coworkers demonstrated a highly practical and efficient strategy for the construction of a broad range of enantiomerically enriched axially chiral biaryls in excellent yields (up to 99%) and enantioselectivity (up to >99% ee) through Pd-catalyzed atroposelective C–H alkynylation.7 Later on, they found that the atropisomers containing one or even two five-membered rings connected through C–C or C–N bonds were compatible with the same strategy.8 While these elegant achievements exhibited the feasibility of catalytic asymmetric synthesis of alkynyl-containing atropisomers, the application of EBX reagents in transition-metal-catalyzed atroposelective C–H alkynylation remains undeveloped. With our continuous interest in Rh-catalyzed asymmetric C–H functionalization and synthesis of biaryl atropisomers,9 we envisioned that Rh-enabled enantioselective C–H alkynylation of 1-aryl isoquinoline with EBX might efficiently furnish enantio-enriched alkynyl biaryls. Herein, we report the highly atroposelective synthesis of 1-aryl isoquinolines via CpRh-catalyzed C–H alkynylation reaction. This strategy provides efficient access to a wide range of axially chiral alkynylated isoquinolines.
We commenced our studies with the reaction of 1-(naphthalen-1-yl)benzo[h]isoquinoline 1a with 1-[(triisopropylsilyl)ethynyl]-1,2-benziodoxol-3(1H)-one (TIPS-EBX) 2a at 80 °C in the presence of 5 mol% of (S)-Rh1,9d 20 mol% of AgSbF6, and 2 equivalents of Cu(OAc)2 in tAmylOH. Gratifyingly, the desired axially chiral alkynylated isoquinoline 3aa was obtained in 97% yield and 59% ee (Table 1, entry 1). 3aa was proved to be conformationally stable, and no erosion of its ee value was observed after heating at 170 °C for 10 h. Encouraged by these preliminary results, various chiral CpRh catalysts were screened, and (S)-Rh1 was confirmed to be the optimal one (entries 2–3, for complete catalyst screening, see Table S1, ESI†). The solvent effect revealed that DMA was the optimal choice, giving rise to 3aa in 86% yield and 72% ee (see Table S2 for details, ESI†). The utilization of other silver salts including AgNTf2, AgOTf and AgPF6etc. has no obvious influence on the enantioselectivity (see Table S3 for details, ESI†). In addition, other reaction parameters such as copper salts, concentration, and additives were investigated, and the results in terms of yield and enantioselectivity were not further improved (see Tables S4–S6 for details, ESI†). Notably, when the reaction was performed at room temperature, the enantioselectivity was increased to 78% ee. Further lowering the reaction temperature to 0 °C resulted in a dramatically decreased yield (51%, 88% ee) (see Table S7 for details, ESI†). Subsequently, the TIPS-protected alkynylation reagents 2b–2d were screened (entries 5–7, see Table S8 for other alkynylation reagents, ESI†). The use of EBX reagent 2c10 bearing gem-ditrifluoromethyl groups gave the best results in terms of yield and enantioselectivity (entry 6, 88% yield, 84% ee). It is noteworthy that TIPS-protected alkynyl bromide (2d) was also compatible in this reaction with slightly decreased yield and enantioselectivity (entry 7, 63% yield, 80% ee). The ee value of 3aa could be further improved when this C–H alkynylation reaction with 2c was carried out in the absence of Cu(OAc)2. (Entry 8, 82% yield, 87% ee. See Table S10 for details, ESI†) Prolonging the reaction time to 48 h gave the optimal reaction conditions, affording 3aa in 88% yield and 87% ee (entry 9). It is noteworthy that this reaction could be accomplished under an air atmosphere, leading to the alkynylated product in 52% yield and 90% ee (entry 10). Overall, the optimal reaction conditions were identified as the following: 1a (1 equiv.), 2c (2 equiv.), (S)-Rh1 (2.5 mol%), AgSbF6 (20 mol%) under argon in DMA at room temperature for 48 h.
| Entry | [Rh] | 2 | Solvent | T (°C) | t (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.05 mmol), 2 (0.1 mmol), [Rh] (5 mol%), AgSbF6 (20 mol%), Cu(OAc)2 (0.1 mmol) in solvent (0.25 mL). b Isolated yield. c Determined by HPLC analysis with a chiral stationary phase. d No Cu(OAc)2. e Under air. | |||||||
| 1 | (S)-Rh1 | 2a | t AmylOH | 80 | 12 | 97 | 59 |
| 2 | (S)-Rh2 | 2a | t AmylOH | 80 | 12 | 91 | 33 |
| 3 | (S)-Rh3 | 2a | t AmylOH | 80 | 12 | 40 | −13 |
| 4 | (S)-Rh1 | 2a | DMA | 80 | 12 | 86 | 72 |
| 5 | (S)-Rh1 | 2b | DMA | 80 | 24 | 30 | 12 |
| 6 | (S)-Rh1 | 2c | DMA | rt | 24 | 88 | 84 |
| 7 | (S)-Rh1 | 2d | DMA | rt | 24 | 63 | 80 |
| 8d | (S)-Rh1 | 2c | DMA | rt | 24 | 82 | 87 |
| 9d | (S)-Rh1 | 2c | DMA | rt | 48 | 88 | 87 |
| 10de | (S)-Rh1 | 2c | DMA | rt | 48 | 52 | 90 |
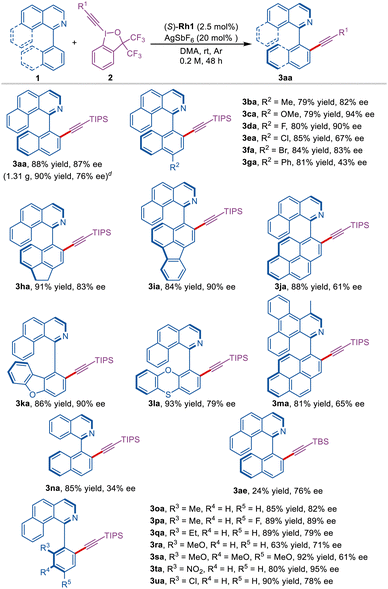
With the optimal reaction conditions in hand, the atroposelective C–H alkynylation of substituted 1-aryl isoquinolines 1 with 2c was first investigated (Scheme 2). The isoquinolines bearing either a 4-methyl or 4-methoxy group on the naphthalene ring gave the desired products 3ba–3ca in 79% yield and good enantioselective control (82–94% ee). The substrates bearing F, Cl, Br and a phenyl group on the 4-position of the naphthalene ring were also well compatible, affording 3da–3ga with good yields and moderate to excellent enantioselectivity (80–85% yields, 43–90% ee). It is noteworthy that the polycyclic naphthalene substrates were also tolerated, furnishing products in good to excellent yields and moderate to excellent enantioselectivity (3ha–3ja, 84–91% yields, 61–90% ee). The benzoisoquinoline substrates containing heteroaromatic rings were compatible with this reaction, giving 3ka in 86% yield with 90% ee and 3la in 93% yield with 79% ee, respectively. Employing the π-extended benzoisoquinoline 1m as a substrate led to the alkynylated atropisomer 3ma in 81% yield with 65% ee. In addition, the isoquinoline substrate 1n furnished 3na with poor enantioselectivity, likely due to the less steric effect (3n, 85% yield, 34% ee). Notably, a gram-scale reaction of 1a with 2c was conducted under the standard conditions, providing 3aa in 90% yield (1.31 g) with 76% ee.
Next, the benzoisoquinolines bearing ortho-substituted phenyl groups were explored (Scheme 2). When substrates bearing 2-methyl or 2-ethyl groups on the benzene ring were applied in this reaction, 3oa–3qa were obtained smoothly with moderate to excellent enantioselectivity (85–89% yields, 79–89% ee). The ortho-methoxy-substituted substrates 1r–1s gave the desired products 3ra–3sa in 63–92% yields and 61–71% ee. Interestingly, the ortho NO2-substituted substrate gave the best ee value, and this is probably due to the nitro group being coordinated with rhodium (3ta, 80% yield, 95% ee). It should be noted that the ortho Cl-substituted substrate was also compatible with this reaction (3ua, 90% yield, 78% ee). In addition, the protecting group TIPS of 2c replaced by TBS (t-butyldimethylsilyl) resulted in a dramatically decreased yield (3ae, 24% yield, 76% ee).
To demonstrate the potential utility of 3aa, the synthetic applications of the alkynylation product were investigated. By treatment of 3aa with TBAF in THF at room temperature, the TIPS group could be readily removed to afford the terminal alkyne 4 in 97% yield with 87% ee (Scheme 3a). Then, 4 was utilized as a key intermediate for the synthesis of various axially chiral 1-aryl isoquinolines. The Sonogashira coupling reaction of 4 was carried out to afford the phenylacetylene substituted product 5 in 96% yield. The absolute configuration of 5 was assigned as Ra according to the reported literature (Scheme 3b).9i The configurations of other products were assigned by analogy. Highly selective semi-hydrogenation of alkyne was achieved in 74% yield (Scheme 3c).11 Moreover, the Cu(I)-catalyzed click reaction of 4 with benzyl azide was performed under mild conditions to give triazole product 7 in 86% yield and 86% ee (Scheme 3d).12 Finally, the N-oxidation reaction of 4 treated with m-CPBA furnished N-oxide 8 in 93% yield without loss of enantioselectivity (Scheme 3e).
To shed light on the mechanism of this C–H alkynylation reaction, H/D exchange of 1a was performed in the presence of D2O under standard conditions. No deuterium incorporation indicated that the C–H cleavage is irreversible (Scheme 4a). The kinetic isotope effect was then measured from the alkynylation reaction of 1a-D1 and 1a, and a large KIE value of 4.05 suggests that the C–H bond activation might be involved in the turnover-limiting step (Scheme 4b).13
Based on the above mechanistic studies and previous report,14 a plausible mechanism for atroposelective C–H alkynylation is proposed (Fig. S3, ESI†). Following path a, the chiral CpRh complex firstly undergoes oxidative addition with 2c to form the CpRh(V) complex I. Subsequently, the cyclometalation of 1a with the active CpRh(V) complex I affords an intermediate III, along with the generation of alcohol 9. Finally, the alkanylated product 3aa is released after the reductive elimination of rhodacycle III and regenerates the Rh(III) catalyst. Another pathway involving the C–H activation of 1-aryl benzoisoquinoline first, followed by oxidative addition with 2c cannot be completely excluded at present.
In summary, we have developed highly atroposelective synthesis of alkynylated 1-aryl isoquinolines based on Rh(III)-catalyzed C–H alkynylation of 1-aryl isoquinolines with hypervalent iodine–alkyne reagents. A broad scope of substrates has been defined, and the C–H Sonogashira coupling reaction proceeded in excellent yields and enantioselectivity under mild reaction conditions. It is noteworthy that diverse transformations were realized for the synthesis of structurally diverse 1-aryl isoquinolines with potential applications. Further development of transition-metal-catalyzed enantioselective C–H functionalization reactions for the construction of axially chiral biaryls is underway in our laboratory.
This work is supported by the National Key R&D Program of China (2021YFA1500100) and the National Natural Science Foundation of China (21821002, 92256302, 22071260). S.-L. Y. acknowledges the support from the New Cornerstone Science Foundation.
Conflicts of interest
The authors declare no competing financial interest.Notes and references
- For reviews on hypervalent iodine reagents, see: (a) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2002, 102, 2523–2584 CrossRef CAS PubMed; (b) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299–5358 CrossRef CAS PubMed; (c) J. Charpentier, N. Fruh and A. Togni, Chem. Rev., 2015, 115, 650–682 CrossRef CAS PubMed; (d) A. Yoshimura and V. V. Zhdankin, Chem. Rev., 2016, 116, 3328–3435 CrossRef CAS PubMed; (e) D. P. Hari, P. Caramenti and J. Waser, Acc. Chem. Res., 2018, 51, 3212–3225 CrossRef CAS PubMed; (f) A. Parra, Chem. Rev., 2019, 119, 12033–12088 CrossRef CAS PubMed; (g) X. Zhang, K. Zhao and Z. Gu, Acc. Chem. Res., 2022, 55, 1620–1633 CrossRef CAS PubMed; (h) X. Peng, A. Rahim, W. Peng, F. Jiang, Z. Gu and S. Wen, Chem. Rev., 2023, 123, 1364–1416 CrossRef CAS PubMed.
- For reviews on ethynylbenziodoxolone (EBX) reagents, see: (a) S. Banerjee, V. W. Bhoyare and N. T. Patil, Chem. Commun., 2020, 56, 2677–2690 RSC; (b) E. Le Du, T. Duhail, M. D. Wodrich, R. Scopelliti, F. Fadaei-Tirani, E. Anselmi, E. Magnier and J. Waser, Chem. – Eur. J., 2021, 27, 10979–10986 CrossRef CAS PubMed; (c) I. A. Mironova, D. M. Noskov, A. Yoshimura, M. S. Yusubov and V. V. Zhdankin, Molecules, 2023, 28, 2136 CrossRef CAS PubMed; (d) E. Le Du and J. Waser, Chem. Commun., 2023, 59, 1589–1604 RSC . For selected examples on ethynylbenziodoxolone (EBX) reagents, see: ; (e) J. P. Brand, C. Chevalley, R. Scopelliti and J. Waser, Chem. – Eur. J., 2012, 18, 5655–5666 CrossRef CAS PubMed; (f) C. C. Chen and J. Waser, Chem. Commun., 2014, 50, 12923–12926 RSC; (g) F. Le Vaillant, T. Courant and J. Waser, Angew. Chem., Int. Ed., 2015, 54, 11200–11204 CrossRef CAS PubMed; (h) D. P. Hari and J. Waser, J. Am. Chem. Soc., 2016, 138, 2190–2193 CrossRef CAS PubMed; (i) S. Banerjee and N. T. Patil, Chem. Commun., 2017, 53, 7937–7940 RSC; (j) D. P. Hari and J. Waser, J. Am. Chem. Soc., 2017, 139, 8420–8423 CrossRef CAS PubMed; (k) E. Voutyritsa, M. Garreau, M. G. Kokotou, I. Triandafillidi, J. Waser and C. G. Kokotos, Chem. – Eur. J., 2020, 26, 14453–14460 CrossRef CAS PubMed.
- For reviews on transition-metal-catalyzed C-H alkynylation reactions by employing EBX reagents, see: (a) X. Li, W. Ouyang, J. Nie, S. Ji, Q. Chen and Y. Huo, ChemCatChem, 2020, 12, 2358–2384 CrossRef CAS; (b) A. Suseelan Sarala, S. Bhowmick, R. L. de Carvalho, S. A. Al-Thabaiti, M. Mokhtar, E. N. da Silva Junior and D. Maiti, Adv. Synth. Catal., 2021, 363, 4994–5027 CrossRef CAS; (c) D. Chandra, A. K. Dhiman, D. Parmar and U. Sharma, Cat. Rev.-Sci. Eng., 2022, 64, 716–788 CrossRef CAS . For selected examples on transition-metal-catalyzed C–H alkynylation reactions by employing EBX reagents, see: ; (d) J. P. Brand, J. Charpentier and J. Waser, Angew. Chem., Int. Ed., 2009, 48, 9346–9349 CrossRef CAS PubMed; (e) C. Feng and T.-P. Loh, Angew. Chem., Int. Ed., 2014, 53, 2722–2726 CrossRef CAS PubMed; (f) K. D. Collins, F. Lied and F. Glorius, Chem. Commun., 2014, 50, 4459–4461 RSC; (g) C. Feng, D. Feng and T.-P. Loh, Chem. Commun., 2014, 50, 9865–9868 RSC; (h) J. Jeong, P. Patel, H. Hwang and S. Chang, Org. Lett., 2014, 16, 4598–4601 CrossRef CAS PubMed; (i) C. Feng, D. Feng, Y. Luo and T.-P. Loh, Org. Lett., 2014, 16, 5956–5959 CrossRef CAS PubMed; (j) F. Xie, Z. Qi, S. Yu and X. Li, J. Am. Chem. Soc., 2014, 136, 4780–4787 CrossRef CAS PubMed; (k) H. Wang, F. Xie, Z. S. Qi and X. W. Li, Org. Lett., 2015, 17, 920–923 CrossRef CAS PubMed; (l) P. Finkbeiner, U. Kloeckner and B. J. Nachtsheim, Angew. Chem., Int. Ed., 2015, 54, 4949–4952 CrossRef CAS PubMed; (m) Y. Li and J. Waser, Angew. Chem., Int. Ed., 2015, 54, 5438–5442 CrossRef CAS PubMed; (n) A. C. Shaikh, D. R. Shinde and N. T. Patil, Org. Lett., 2016, 18, 1056–1059 CrossRef CAS PubMed; (o) G. N. Hermann, M. T. Unruh, S.-H. Jung, M. Krings and C. Bolm, Angew. Chem., Int. Ed., 2018, 57, 10723–10727 CrossRef CAS PubMed; (p) J. Zhang, M. Wang, H. Wang, H. Xu, J. Chen, Z. Guo, B. Ma, S.-R. Ban and H.-X. Dai, Chem. Commun., 2021, 57, 8656–8659 RSC.
- For reviews, see: (a) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029–3070 CrossRef CAS PubMed; (b) J. Chang, J. Reiner and J. Xie, Chem. Rev., 2005, 105, 4581–4609 CrossRef CAS PubMed; (c) M. C. Kozlowski, B. J. Morgan and E. C. Linton, Chem. Soc. Rev., 2009, 38, 3193–3207 RSC; (d) G. Bringmann, T. Gulder, T. A. M. Gulder and M. Breuning, Chem. Rev., 2011, 111, 563–639 CrossRef CAS PubMed; (e) L. Pu, Acc. Chem. Res., 2012, 45, 150–163 CrossRef CAS PubMed; (f) M. P. Carroll and P. J. Guiry, Chem. Soc. Rev., 2014, 43, 819–833 RSC; (g) E. Kumarasamy, R. Raghunathan, M. P. Sibi and J. Sivaguru, Chem. Rev., 2015, 115, 11239–11300 CrossRef CAS PubMed.
- For reviews on the synthesis of axially chiral biaryls by asymmetric C–H functionalizations, see: (a) J. Wencel-Delord, A. Panossian, F. R. Leroux and F. Colobert, Chem. Soc. Rev., 2015, 44, 3418–3430 RSC; (b) G. Liao, T. Zhou, Q. J. Yao and B.-F. Shi, Chem. Commun., 2019, 55, 8514–8523 RSC; (c) Q. Wang, Q. Gu and S.-L. You, Acta Chim. Sin., 2019, 77, 690–704 CrossRef CAS.
- For reviews, see: (a) S. Toyota, Chem. Rev., 2010, 110, 5398–5424 CrossRef CAS PubMed; (b) Z.-F. Zhou, M. Menna, Y. S. Cai and Y. W. Guo, Chem. Rev., 2015, 115, 1543–1596 CrossRef CAS PubMed . For selected examples, see: ; (c) C. J. Smedley, G. Li, A. S. Barrow, T. L. Gialelis, M.-C. Giel, A. Ottonello, Y. Cheng, S. Kitamura, D. W. Wolan, K. B. Sharpless and J. E. Moses, Angew. Chem., Int. Ed., 2020, 59, 12460–12469 CrossRef CAS PubMed; (d) B. Song, R. Zhang, R. Hu, X. Chen, D. Liu, J. Guo, X. Xu, A. Qin and B. Z. Tang, Adv. Sci., 2020, 7, 2000465 CrossRef CAS PubMed; (e) A. S.-Y. Law, L. C.-C. Lee, K. K.-W. Lo and V. W.-W. Yam, J. Am. Chem. Soc., 2021, 143, 5396–5405 CrossRef CAS PubMed.
- G. Liao, Q.-J. Yao, Z.-Z. Zhang, Y.-J. Wu, D.-Y. Huang and B.-F. Shi, Angew. Chem., Int. Ed., 2018, 57, 3661–3665 CrossRef CAS PubMed.
- S. Zhang, Q.-J. Yao, G. Liao, X. Li, H. Li, H.-M. Chen, X. Hong and B.-F. Shi, ACS Catal., 2019, 9, 1956–1961 CrossRef CAS.
- For reviews, see: (a) C.-X. Liu, W.-W. Zhang, S.-Y. Yin, Q. Gu and S.-L. You, J. Am. Chem. Soc., 2021, 143, 14025–14040 CrossRef CAS PubMed; (b) C.-X. Liu, S.-Y. Yin, F. Zhao, H. Yang, Z. Feng, Q. Gu and S.-L. You, Chem. Rev., 2023, 123, 10079–10134 CrossRef CAS PubMed; (c) Y.-M. He, Y.-Z. Cheng, Y. Duan, Y.-D. Zhang, Q.-H. Fan, S.-L. You, S. Luo, S.-F. Zhu, X.-F. Fu and Q.-L. Zhou, CCS Chem., 2023, 5, 2685–2716 CrossRef CAS . For selected examples, see: ; (d) J. Zheng, W.-J. Cui, C. Zheng and S.-L. You, J. Am. Chem. Soc., 2016, 138, 5242–5245 CrossRef CAS PubMed; (e) Q. Wang, Z.-J. Cai, C.-X. Liu, Q. Gu and S.-L. You, J. Am. Chem. Soc., 2019, 141, 9504–9510 CrossRef CAS PubMed; (f) Q. Wang, W.-W. Zhang, H. Song, J. Wang, C. Zheng, Q. Gu and S.-L. You, J. Am. Chem. Soc., 2020, 142, 15678–15685 CrossRef CAS PubMed; (g) W.-W. Zhang, C.-X. Liu, P. Yang, S.-Z. Zhang, Q. Gu and S.-L. You, Org. Lett., 2022, 24, 564–569 CrossRef CAS PubMed; (h) W.-W. Zhang, Q. Wang, S.-Z. Zhang, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2023, 62, e202214460 CrossRef CAS PubMed; (i) D.-S. Zheng, W.-W. Zhang, Q. Gu and S.-L. You, ACS Catal., 2023, 13, 5127–5134 CrossRef CAS; (j) D.-S. Zheng, J. Zheng, Q. Wang, Q. Gu and S.-L. You, Chin. J. Chem., 2023, 41, 2684–2690 CrossRef CAS; (k) D.-S. Zheng, P.-P. Xie, F. Zhao, C. Zheng, Q. Gu and S.-L. You, ACS Catal., 2024, 14, 6009–6015 CrossRef CAS.
- V. V. Zhdankin, C. J. Kuehl, A. P. Krasutsky, J. T. Bolz and A. J. Simonsen, J. Org. Chem., 1996, 61, 6547–6551 CrossRef CAS PubMed.
- (a) A. M. Whittaker and G. Lalic, Org. Lett., 2013, 15, 1112–1115 CrossRef CAS PubMed; (b) K. Semba, T. Fujihara, T. Xu, J. Terao and Y. Tsuji, Adv. Synth. Catal., 2012, 354, 1542–1550 CrossRef CAS.
- V. Hornillos, A. Ros, P. Ramírez-López, J. Iglesias-Sigüenza, R. Fernández and J. M. Lassaletta, Chem. Commun., 2016, 52, 14121–14124 RSC.
- E. M. Simmons and J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 3066–3072 CrossRef CAS PubMed.
- (a) S. Liu, X. Qi, L.-B. Qu, R. Bai and Y. Lan, Catal. Sci. Technol., 2018, 8, 1645–1651 RSC; (b) S. Vasquez-Cespedes, X. Wang and F. Glorius, ACS Catal., 2018, 8, 242–257 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data of all compounds. See DOI: https://doi.org/10.1039/d4cc01785d |
| This journal is © The Royal Society of Chemistry 2024 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions: 1 (0.2 mmol), 2 (0.4 mmol), (
Reaction conditions: 1 (0.2 mmol), 2 (0.4 mmol), (
