Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
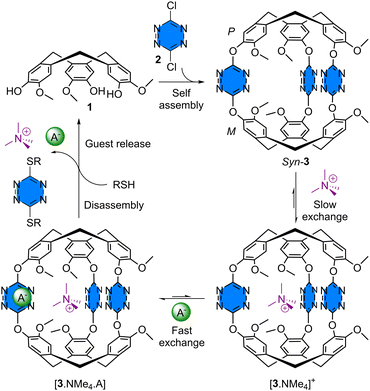
Self-assembled tetrazine cryptophane for ion pair recognition and guest release by cage disassembly†
Louise
Miton
a,
Elise
Antonetti
a,
Marie
Poujade
a,
Jean-Pierre
Dutasta
b,
Paola
Nava
 a,
Alexandre
Martinez
a,
Alexandre
Martinez
 *a and
Yoann
Cotelle
*a and
Yoann
Cotelle
 *a
*a
aAix Marseille Université, CNRS, Centrale Marseille, iSm2, UMR 7313, 13397 Marseille, France. E-mail: alexandre.martinez@centrale-marseille.fr; yoann.cotelle@univ-amu.fr
bENS Lyon, CNRS, Laboratoire de Chimie, UMR 5182, 46 Allée d’Italie, 69364 Lyon, France
First published on 24th April 2024
Abstract
Hereby, we describe the synthesis of a self-assembled syn-cryptophane using dynamic nucleophilic aromatic substitution of tetrazines. 1H NMR cage titrations reveal that the tetramethylammonium cation binds under slow exchange conditions while counter-anions show a fast exchange regime. Finally, the cryptophane can be disassembled by the addition of thiols allowing guest release.
Cryptophanes are a class of cage molecules that are built from two cyclotriveratrylene (CTV) moieties usually linked by alkylene-dioxy arms,1 although other linkers such as disulfide bridges,2p-phenylene groups3 or triazole rings4 have been reported. These molecular hosts attract great interest as they display remarkable recognition properties toward small molecules like epoxides,5 methane and halogenomethane,2a,6 anions,7 cations,8 or even atoms like radon or xenon.7b,9 However, cryptophanes have not yet been used for ion-pair recognition due to their homotopicity. Promising related applications are currently being investigated; for instance, the development of hyperpolarized 129Xe biosensors for MRI,10 the design of devices for methane sensing,11 and the trapping of radioactive Cs+ found in nuclear waste.12 Both covalent and self-assembled cryptophane cages13 have been reported and the photoswitching of metallo cryptophanes has also been described; however, without the release of a guest.14 Nevertheless, controlled guest release triggered by the disassembly of a cryptophane host remains unexplored. To avoid the possible dissociation of cryptophane-like coordination cages at low concentrations, which could limit their applications, we turned our attention to the construction of covalent cryptophane cages by subcomponent self-assembly using reversible covalent bonds. We hypothesized that a cryptophane bearing tetrazine units as linkers should solve both issues, i.e. heterotopicity and controlled guest release, simultaneously. Indeed, 1,2,4,5-tetrazines are known to bind cations15 by nitrogen lone pairs and anions16 through anion–π interactions. Thus, their embedment in a receptor should raise ion pair recognition. Moreover, the dynamic nucleophilic aromatic substitution of tetrazines (SNTz), recently reported by the group of Carrillo, facilitates their assembly into a functional receptor and the subsequent addition of thiols was shown to promote their disassembly.17 Intrigued by the versatility of such a simple building block, we therefore set out to investigate its incorporation into a heteroditopic self-assembled cryptophane for ion pair recognition, capable of guest release using a simple chemical stimulus. The dynamic nature of dative bonds has already been employed for guest release in metallacages;18 however, this is the first example of a covalent cage using SNTz. In this communication, we describe the synthesis of a tris-tetrazine syn-cryptophane 3, the binding of the tetramethylammonium cation in slow exchange, together with the recognition of a counter anion in fast exchange harnessed by anion–π and coulombic interactions. Finally, on-demand guest release was achieved by the disassembly of the receptor using alkanethiols (Scheme 1).
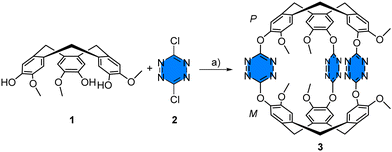
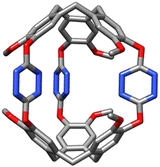
The synthesis of cryptophane 3 is straightforward and involves the reaction of two equivalents of rac-CTV-OH 1 and three equivalents of dichloro-s-tetrazine 2 in acetonitrile in the presence of triethylamine at room temperature for 3.5 h (15% yield) (Scheme 2). Cryptophane 3 was fully characterized by NMR and HRMS techniques (see ESI†). The 1H NMR spectrum of 3 in acetonitrile-d3 displays OMe protons (c) as a singlet at 3.58 ppm, two AB doublets for the He and Ha protons at 3.73 and 4.87 ppm, respectively, and finally aromatic protons (b) at 7.08 ppm and (a) at 7.42 ppm (Fig. 2(b), 0.0 equiv.). It is important to note that cryptophane 3 was obtained exclusively as the achiral syn-diastereomer after silica gel chromatography. Experiments starting from enantiopure CTV 1 did not gave any trace of anti-cryptophane, but led to the formation of oligomers. Single crystals of 3 were grown from slow evaporation of an acetonitrile solution at 4 °C. The resolution is only partial, as the crystals degrade due to fast desolvatation and the cif file could not pass the checkcif process. However, the resolution was sufficient enough to assess the stereochemistry of cryptophane 3 (Fig. 1). Optimization of the syn-cryptophane 3 was also performed by DFT calculations in the gas phase (Fig. S2, ESI†) and is in accordance with the partial structure determined by X-ray analysis.
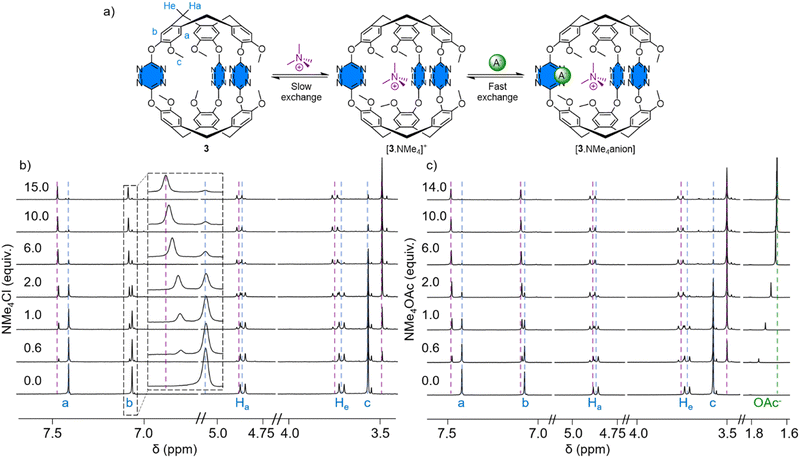
Next, we evaluated the affinity of cryptophane 3 for the tetramethylammonium chloride (NMe4Cl) ion pair. A solution of 3 (0.79 mM) was titrated by aliquots of an NMe4Cl solution (19 mM) in acetonitrile-d3 followed by 1H NMR (Fig. 2(b)). From this titration, we first observed the appearance of a new set of signals corresponding to the formation of a complex in slow exchange compared to the NMR time scale. Such behavior is expected for the recognition of tetramethylammonium by cryptophanes.1c Cryptophane 3 can interact with cations, not only through the usual cation–π interactions with the CTV unit, but also engage in additional interactions with the lone pair of the tetrazine's nitrogens.15 Protons corresponding to [3·NMe4]+ can be observed at 3.50 ppm for protons (c), at 3.76 and 4.89 ppm for protons He and Ha respectively and at 7.09 and 7.48 ppm for aromatic protons (b) and (a), after the addition of one equivalent of NMe4Cl. A binding constant of Ka = 460 was calculated for the recognition of the tetramethylammonium cation considering a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. Following the addition of more aliquots (Fig. 2(b)), the signals (a) and (b) corresponding to the complex [3·NMe4]+ are shifted towards lower fields, corresponding to the formation of a second species, a ternary complex with chloride [3·NMe4Cl]. These shifts are modest; however, they support the recognition of the anion. By plotting the variation of the [3·NMe4Cl] chemical shifts against the host guest ratio, we obtained a binding constant of Ka = 124 considering a 1
1 stoichiometry. Following the addition of more aliquots (Fig. 2(b)), the signals (a) and (b) corresponding to the complex [3·NMe4]+ are shifted towards lower fields, corresponding to the formation of a second species, a ternary complex with chloride [3·NMe4Cl]. These shifts are modest; however, they support the recognition of the anion. By plotting the variation of the [3·NMe4Cl] chemical shifts against the host guest ratio, we obtained a binding constant of Ka = 124 considering a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
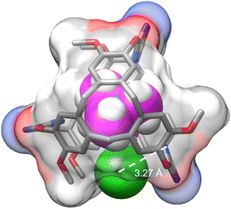
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Fig. S9, ESI†). This behavior is remarkable as it accounts for following the recognition of ion pairs using two different 1H NMR probes and thus allows the determination of the two binding constants separately without additional experiments. In addition, the DFT optimized structure of [3·NMe4Cl] gives valuable information on the interactions stabilizing the complex. Multiple short contacts are observed between the tetramethylammonium guest and the aromatic rings of the CTVs, which can be described as cation π-interactions (dH–C = 2.74 and 2.78 Å), as well as with the nitrogen of the tetrazines (dH–N = 2.14, 2.44 and 2.38 Å) (Fig. S3, ESI†). An additional interaction between the chloride and the tetrazine (dC–N = 3.27 Å) can be observed characteristic of an anion–π interaction (Fig. 3).
1 stoichiometry (Fig. S9, ESI†). This behavior is remarkable as it accounts for following the recognition of ion pairs using two different 1H NMR probes and thus allows the determination of the two binding constants separately without additional experiments. In addition, the DFT optimized structure of [3·NMe4Cl] gives valuable information on the interactions stabilizing the complex. Multiple short contacts are observed between the tetramethylammonium guest and the aromatic rings of the CTVs, which can be described as cation π-interactions (dH–C = 2.74 and 2.78 Å), as well as with the nitrogen of the tetrazines (dH–N = 2.14, 2.44 and 2.38 Å) (Fig. S3, ESI†). An additional interaction between the chloride and the tetrazine (dC–N = 3.27 Å) can be observed characteristic of an anion–π interaction (Fig. 3).
In order to get more insights into the two equilibria, we decided to titrate cryptophane 3 with tetramethylammonium acetate (NMe4OAc), providing a counter anion with a trigonal geometry. Satisfyingly, the 1H NMR titration also shows a slow exchange compared to the NMR time scale characterized by the appearance of a new set of signals upon the addition of the titrating solution (Fig. 2(c)). Integration of the chemical shifts corresponding to the complex and the free cryptophane 3 gives a binding constant of Ka = 2000 for NMe4+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry). The difference with the binding constant obtained with NMe4Cl probably arises from a less tight ion pair in NMe4OAc. After the addition of more aliquots, the signals of protons (a) and (b) shift downfield, corresponding to an ion pair ternary complex [3·NMe4OAc]. At the early stage of the titration, the chemical shift of the methyl of the acetate moiety is deshielded (1.76 ppm) due to the formation of the ternary complex, but will eventually match the chemical shift of acetate in solution (1.65 ppm) due to the large number of equivalents added (14 equiv.). The binding constant Ka = 67 of [3·NMe4]+ for acetate was determined from the variation of the chemical shift of proton (a) assuming a 1
1 stoichiometry). The difference with the binding constant obtained with NMe4Cl probably arises from a less tight ion pair in NMe4OAc. After the addition of more aliquots, the signals of protons (a) and (b) shift downfield, corresponding to an ion pair ternary complex [3·NMe4OAc]. At the early stage of the titration, the chemical shift of the methyl of the acetate moiety is deshielded (1.76 ppm) due to the formation of the ternary complex, but will eventually match the chemical shift of acetate in solution (1.65 ppm) due to the large number of equivalents added (14 equiv.). The binding constant Ka = 67 of [3·NMe4]+ for acetate was determined from the variation of the chemical shift of proton (a) assuming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Fig. S8, ESI†). Hence, tetramethylammonium ion pairs are recognized by cryptophane 3, which exhibit different exchange rates depending on the nature of the ion.
1 stoichiometry (Fig. S8, ESI†). Hence, tetramethylammonium ion pairs are recognized by cryptophane 3, which exhibit different exchange rates depending on the nature of the ion.
Tetrazines are good acceptors for anion–π interactions, we thus decided to evaluate if the concomitant complexation of the tetramethylammonium cation was necessary for the complexation of the anion by the cryptophane cage or if the affinity of cryptophane 3 for anions remains without tetramethylammonium trapped inside. Titrations of 3 with tetrabutylammonium chloride or acetate did not show any binding event in acetonitrile-d3 (Fig. S6 and S7, ESI†). Since anions are recognized by the cationic complex [3·NMe4]+ but not by 3 alone, we have clear evidence for a positive allosteric effect which could be attributed to synergistic coulombic and anion–π interactions.
Lastly, we investigated the disassembly of the cryptophane 3 taking advantage of the dynamic nucleophilic aromatic substitution of tetrazines. In the presence of alkanethiols and a base, the group of Carrillo demonstrated the disassembly of tetrazine cages.17 However, this has never been achieved on an inclusion complex. To this end, 1-hexanethiol (10 equiv.) and triethylamine (10 equiv.) were added to an equimolar mixture of 3 and NMe4OAC (Fig. 4). After stirring for one hour at room temperature, we observed the 1H NMR signals of the CTV 1 confirming the disassembly of cryptophane 3. In addition, the chemical shift of NMe4+ is at 3.00 ppm, close to the 3.15 ppm observed for NMe4+ in acetonitrile-d3. This experiment demonstrates the possibility of disassembling cryptophane 3 in the presence of alkanethiol and a base leading to the release of the tetramethylammonium cation.
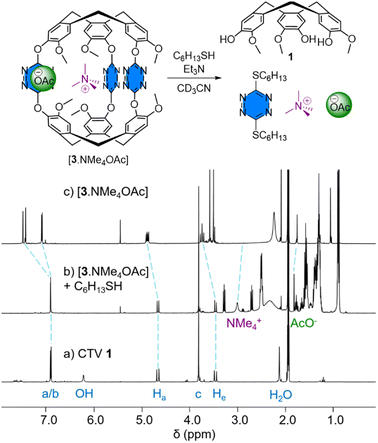 | ||
| Fig. 4 Superposition of the 1H NMR spectra (400 MHz, acetonitrile-d3, 298 K) of (a) CTV 1 (see atom labelling on Fig. 1), (b) the mixture obtained after one hour of stirring of an equimolar solution of 3 (1.6 mM, acetonitrile-d3) and NMe4OAc (1.6 mM, acetonitrile-d3) in the presence of 1-hexanethiol (10 equivalents) and triethylamine (10 equivalents) and (c) the [3·NMe4OAc] complex. | ||
In conclusion, a self-assembled tetrazine cryptophane receptor 3 was obtained in one step. This achiral heteroditopic molecular cage forms an inclusion complex with the tetramethylammonium cation, which can simultaneously bind anionic species to provide [3·NMe4Anion] complexes. No affinity for anionic guests without a cation inside the cavity was measured, suggesting a positive allosteric effect. This result can be explained by a synergy between anion–π, cation–π and coulombic interactions. Moreover, the slow and fast exchange kinetics observed, respectively, with cationic and anionic guests, allowed the determination of both binding constants in a single titration. Cryptophane disassembly was achieved by exchange reaction in the presence of thiols and a base, triggering the release of the encapsulated guest molecule. Finally, post-functionalization of the tetrazine units via inverse electron demand Diels–Alder [4+2] cycloadditions would be of interest to modify the cryptophane structure. Studies are currently in progress in our group.
This work was supported by the ANR, grant ANR-19-CE07-0024 and ANR-21-CE07-0011.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) J. Gabard and A. Collet, J. Chem. Soc., Chem. Commun., 1981, 1137 RSC; (b) A. Collet, Tetrahedron, 1987, 43, 5725 CrossRef CAS; (c) T. Brotin and J.-P. Dutasta, Chem. Rev., 2009, 109, 88 CrossRef CAS PubMed; (d) S. Kancherla and J. H. Hansen, Eur. J. Org. Chem., 2024, e202301050 CrossRef CAS.
- (a) M. A. Little, J. Donkin, J. Fisher, M. A. Halcrow, J. Loder and M. J. Hardie, Angew. Chem., Int. Ed., 2012, 51, 764 CrossRef CAS PubMed; (b) F. Brégier, O. Hudeček, F. Chaux, M.-J. Penouilh, J.-C. Chambron, P. Lhoták, E. Aubert and E. Espinosa, Eur. J. Org. Chem., 2017, 3795 CrossRef.
- F. L. Thorp-Greenwood, M. J. Howard, L. T. Kuhn and M. J. Hardie, Chem. – Eur. J., 2019, 25, 3536 CrossRef CAS PubMed.
- P. Satha, G. T. Illa, S. Hazra and C. S. Purohit, ChemistrySelect, 2017, 2, 10699 CrossRef CAS.
- (a) A. Bouchet, T. Brotin, M. Linares, H. Ågren, D. Cavagnat and T. Buffeteau, J. Org. Chem., 2011, 76, 4178 CrossRef CAS PubMed; (b) N. De Rycke, M. Jean, N. Vanthuyne, T. Buffeteau and T. Brotin, Eur. J. Org. Chem., 2018, 1601 CrossRef CAS.
- (a) L. Garel, J. P. Dutasta and A. Collet, Angew. Chem., Int. Ed. Engl., 1993, 32, 1169 CrossRef; (b) J. Crassous, F. Monier, J.-P. Dutasta, M. Ziskind, C. Daussy, C. Grain and C. Chardonnet, ChemPhysChem, 2003, 4, 541 CrossRef CAS PubMed; (c) E. Chaffee, H. Fogarty, T. Brotin, B. M. Goodson and J.-P. Dutasta, J. Phys. Chem. A, 2009, 113, 1367 CrossRef PubMed.
- (a) R. M. Fairchild and K. T. Holman, J. Am. Chem. Soc., 2005, 127, 16364 CrossRef CAS PubMed; (b) R. M. Fairchild, A. I. Joseph, K. T. Holman, H. A. Fogarty, T. Brotin, J.-P. Dutasta, C. Boutin, G. Huber and P. Berthault, J. Am. Chem. Soc., 2010, 132, 15505 CrossRef CAS PubMed.
- (a) T. Brotin, R. Montserret, A. Bouchet, D. Cavagnat, M. Linares and T. Buffeteau, J. Org. Chem., 2012, 77, 1198 CrossRef CAS PubMed; (b) D. Zhang, T. K. Ronson, J. L. Greenfield, T. Brotin, P. Berthault, E. Léonce, J.-L. Zhu, L. Xu and J. R. Nitschke, J. Am. Chem. Soc., 2019, 141, 8339 CrossRef CAS PubMed.
- (a) D. R. Jacobson, N. S. Khan, R. Colle, R. Fitzgerald, L. Laureano-Perez, Y. Bai and I. J. Dmochowski, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 10969 CrossRef CAS PubMed; (b) K. Bartik, M. Luhmer, J.-P. Dutasta, A. Collet and J. Reisse, J. Am. Chem. Soc., 1998, 120, 784 CrossRef CAS; (c) R. M. Fairchild, A. I. Joseph, K. T. Holman, H. A. Fogarty, T. Brotin, J.-P. Dutasta, C. Boutin, G. Huber and P. Berthault, J. Am. Chem. Soc., 2010, 132, 15505 CrossRef CAS PubMed; (d) A. I. Joseph, G. El-Ayle, C. Boutin, E. Léonce, P. Berthault and K. T. Holman, Chem. Commun., 2014, 50, 15905 RSC; (e) A. I. Joseph, S. H. Lapidus, C. M. Kane and K. T. Holman, Angew. Chem., Int. Ed., 2015, 54, 1471 CrossRef CAS PubMed; (f) C. Vigier, D. Fayolle, H. El Siblani, J. Sopkova de Oliveira Santos, F. Fabis, T. Cailly and E. Dubost, Angew. Chem., Int. Ed., 2022, 61, e202208580 CrossRef CAS PubMed.
- (a) S. D. Zemerov, Y. Lin and I. J. Dmochowski, Anal. Chem., 2021, 93, 1507 CrossRef CAS PubMed; (b) H. Zhang, Q. Yu, Y. Li, Z. Yang, X. Zhou, S. Chen and Z.-X. Jiang, Chem. Commun., 2020, 56, 3617 RSC; (c) E. Mari, Y. Bousmah, C. Boutin, E. Léonce, G. Milanole, T. Brotin, P. Berthault and M. Erard, ChemBioChem, 2019, 20, 1450 CrossRef CAS PubMed; (d) H. Zhang, S. Chen, Y. Yuan, Y. Li, Z. Jiang and X. Zhou, ACS Appl. Bio Mater., 2019, 2, 27 CrossRef CAS PubMed; (e) E. Mari and P. Berthault, Analyst, 2017, 142, 3298 RSC; (f) Y. Wang and I. J. Dmochowski, Acc. Chem. Res., 2016, 49, 2179 CrossRef CAS PubMed; (g) G. K. Seward, Y. Bai, N. S. Khan and I. J. Dmochowski, Chem. Sci., 2011, 2, 1103 RSC; (h) G. K. Seward, Q. Wei and I. J. Dmochowski, Bioconjugate Chem., 2008, 19, 2129 CrossRef CAS PubMed.
- (a) F. T. Dullo, S. Lindecrantz, J. Jágerská, J. H. Hansen, M. Engqvist, S. A. Solbø and O. G. Hellesø, Opt. Express, 2015, 23, 31564 CrossRef CAS PubMed; (b) A. Estelmann and R. Prien, IEEE Sens. J., 2023, 23, 4785 CAS; (c) J. Yang, L. Zhou, X. Che, J. Huang, X. Li and W. Chen, Sens. Actuators, B, 2016, 235, 717 CrossRef CAS; (d) C. Boulart, M. C. Mowlem, D. P. Connelly, J.-P. Dutasta and Ch. R. German, Opt. Express, 2008, 16, 12607 CrossRef CAS PubMed; (e) M. Benounis, N. Jaffrezic-Renault, J.-P. Dutasta, K. Cherif and A. Abdelghani, Sens. Actuators, B, 2005, 107, 32 CrossRef CAS; (f) T. Hong, J. T. Culp, K.-J. Kim, J. Devkota, C. Sun and P. R. Ohodnicki, TrAC, Trends Anal. Chem., 2020, 125, 115820 CrossRef CAS.
- (a) T. Brotin, P. Berthault, D. Pitrat and J.-C. Mulatier, J. Org. Chem., 2020, 85, 9622 CrossRef CAS PubMed; (b) L.-L. Chapellet, J.-P. Dognon, M. Jean, N. Vanthuyne, P. Berthault, T. Buffeteau and T. Brotin, ChemistrySelect, 2017, 2, 5292 CrossRef CAS.
- (a) M. J. Hardie, Chem. Lett., 2016, 45, 1336 CrossRef CAS; (b) J. J. Henkelis and M. J. Hardie, Chem. Commun., 2015, 51, 11929 RSC; (c) S. Kai, T. Kojima, F. L. Thorp-Greenwood, M. J. Hardie and S. Hiraoka, Chem. Sci., 2018, 9, 4104 RSC; (d) V. E. Pritchard, D. Rota Martir, S. Oldknow, S. Kai, S. Hiraoka, N. J. Cookson, E. Zysman-Colman and M. J. Hardie, Chem. – Eur. J., 2017, 23, 6290 CrossRef CAS PubMed; (e) J. J. Henkelis, C. J. Carruthers, S. E. Chambers, R. Clowes, A. I. Cooper, J. Fisher and M. J. Hardie, J. Am. Chem. Soc., 2014, 136, 14393 CrossRef CAS PubMed; (f) A. Schaly, Y. Rousselin, J.-C. Chambron, E. Aubert and E. Espinosa, Eur. J. Inorg. Chem., 2016, 832 CrossRef CAS; (g) A. Schaly, M. Meyer, J.-C. Chambron, M. Jean, N. Vanthuyne, E. Aubert, E. Espinosa, N. Zorn and E. Leize-Wagner, Eur. J. Inorg. Chem., 2019, 2691 CrossRef CAS.
- (a) E. Britton, R. J. Ansell, M. J. Howard and M. J. Hardie, Inorg. Chem., 2021, 60, 12912 CrossRef CAS PubMed; (b) S. Oldknow, D. R. Martir, V. E. Pritchard, M. A. Blitz, C. W. G. Fishwick, E. Zysman-Colman and M. J. Hardie, Chem. Sci., 2018, 9, 8150 RSC.
- O. Stetsiuk, A. Abhervé and N. Avarvari, Dalton Trans., 2020, 49, 5759 RSC.
- M. Savastano, C. Bazzicalupi, C. Giorgi, C. García-Gallarín, M. D. López de la Torre, F. Pichierri, A. Bianchi and M. Melguizo, Inorg. Chem., 2016, 55, 8013 CrossRef CAS PubMed.
- T. Santos, D. S. Rivero, Y. Pérez-Pérez, E. Martín-Encinas, J. Pasán, A. H. Daranas and R. Carrillo, Angew. Chem., Int. Ed., 2021, 60, 18783 CrossRef CAS PubMed.
- (a) T. Y. Kim, R. A. S. Vasdev, D. Preston and J. D. Crowley, Chem. – Eur. J., 2018, 24, 14878 CrossRef CAS PubMed; (b) A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc01421a |
| This journal is © The Royal Society of Chemistry 2024 |