Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unlocking catalytic potential: a rhodium(II)-based coordination polymer for efficient carbene transfer reactions with donor/acceptor diazoalkanes†
Claire
Empel‡
 a,
Marcus N. A.
Fetzer‡
b,
Suman
Sasmal
a,
Till
Strothmann
a,
Marcus N. A.
Fetzer‡
b,
Suman
Sasmal
a,
Till
Strothmann
 b,
Christoph
Janiak
b,
Christoph
Janiak
 b and
Rene M.
Koenigs
b and
Rene M.
Koenigs
 *a
*a
aInstitute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany. E-mail: rene.koenigs@rwth-aachen.de
bInstitut für Anorganische Chemie und Strukturchemie, Heinrich-Heine-Universität Düsseldorf, Universitätsstraße 1, 40225 Düsseldorf, Germany
First published on 17th June 2024
Abstract
Herein, we report the use of a molecular-defined rhodium(II) coordination polymer (Rh-CP) as a heterogeneous, recyclable catalyst in carbene transfer reactions. We showcase the application of this heterogeneous catalyst in a range of carbene transfer reactions and conclude with the functionalization of natural products and drug molecules.
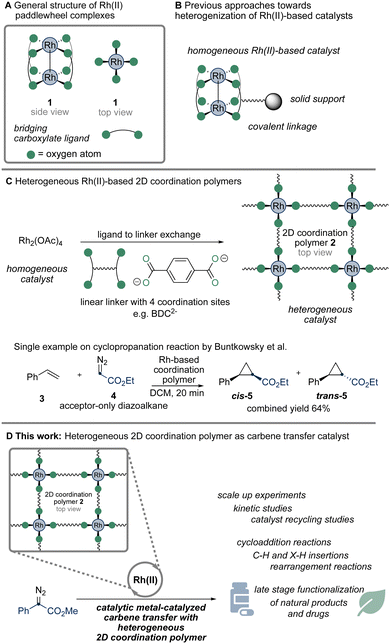
Rhodium(II) carboxylate paddlewheel complexes feature two rhodium centers, a rhodium–rhodium single bond and four bridging carboxylate ligands. They represent a privileged class of catalysts, primarily due to each rhodium center having a free coordination site, central for facilitating for example carbene or nitrene transfer reactions (Scheme 1A).1–4 While the development of homogeneous rhodium paddlewheel catalysts has received significant attention,3,4 the use of their heterogeneous counterparts has been much less explored.5–8 Previous efforts primarily focused on the immobilization of homogeneous catalysts on a surface or polymer.5 The catalytic activity of this material, however, is achieved through a single-molecule catalyst grafted onto the heterogeneous support (Scheme 1B). Studies on the application of heterogeneous rhodium-based carbene transfer catalysts, where the catalytically active site is an integral part of the heterogeneous material, however, received only little attention (Scheme 1C).6–8 Studies on such heterogeneous catalysts are in high demand as these would allow facile catalyst recovery from the reaction mixture and may result in enhanced catalyst lifetime, which—given the low abundancy and high price of rhodium—would have a significant impact on the environmental footprint of rhodium-catalyzed reactions.4
Building on our interest in metal-catalyzed carbene transfer reactions using,9 we considered that a Rh(II)-based coordination polymer (Rh-CP) could serve as a viable starting point to access a heterogeneous, molecular-defined rhodium catalyst with a repetitive paddlewheel structure (Scheme 1D). Such Rh-CP would differentiate from other approaches, where molecular-defined rhodium catalysts are heterogenized by immobilization to surfaces.5 We got intrigued by a previous report by Buntkowsky and co-workers, who described a straightforward synthesis of such a Rh(II)-based coordination polymer by a ligand exchange reaction of Rh2(OAc)4 (Scheme 1C).6 A related report by Furukawa et al. reports on the formation of octahedral clusters using benzene-1,3-dicarboxylic acid.7 Very much to our surprise, only limited applications in catalysis were described, which focus on the reaction of ethyl diazoacetate.6
We commenced our studies by performing a ligand exchange reaction between Rh2(OAc)4 and terephthalic acid (H2BDC) to obtain the Rh-CP as a green powder in excellent yield (Fig. 1A, 97%). The Rh-CP was authenticated to the literature6 by PXRD analysis (see Fig. S1, ESI†), TGA analysis (Fig. S2 and S3, ESI†) ATR-IR (Fig. S4, ESI†), and diffuse reflectance spectra (Fig. 1B). Importantly, diffuse reflectance spectra in an Ulbricht sphere (Fig. 1B) indicate the persistence of the paddlewheel structure within the Rh-CP. This structural motif is crucial for the catalytic properties.10
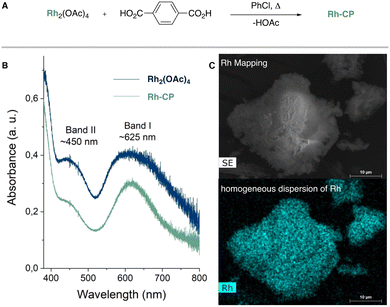 | ||
| Fig. 1 (A) Synthesis of Rh-CP. (B) Optical Kubelka–Munk spectra of Rh2(OAc)4 and Rh-CP obtained from diffuse reflectance spectra. (C) EDX Rh mapping of Rh-CP. | ||
1H NMR measurements were carried out to determine the degree of ligand exchange. For this purpose, Rh-CP was digested in a mixture of DMSO-d6 and D2SO4, whereby the integrals of the BDC linker and acetate gave a ratio of about 82 to 3 corresponding to a molar BDC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OAc ratio of 20
OAc ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S7 and S8, ESI†). Scanning electron microscopy (SEM) shows our Rh-CP as comparatively small agglomerates, which are characterized by a lamellar morphology at higher magnification (Fig. S5 and S6, ESI†). EDX elemental mapping indicates a homogeneous dispersion of rhodium throughout the synthesized Rh-CP, confirming a consistent elemental distribution within the compound (Fig. 1C).
1 (Fig. S7 and S8, ESI†). Scanning electron microscopy (SEM) shows our Rh-CP as comparatively small agglomerates, which are characterized by a lamellar morphology at higher magnification (Fig. S5 and S6, ESI†). EDX elemental mapping indicates a homogeneous dispersion of rhodium throughout the synthesized Rh-CP, confirming a consistent elemental distribution within the compound (Fig. 1C).
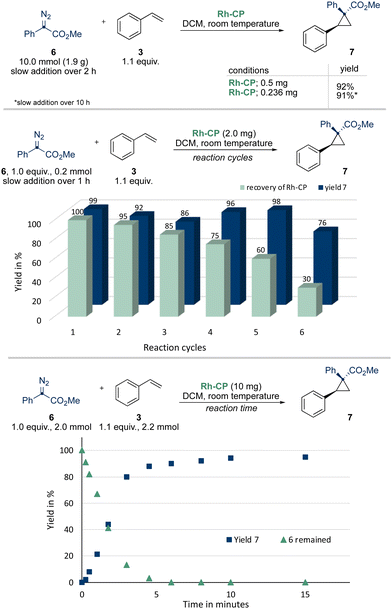
To evaluate the catalytic efficiency and stability in catalysis of the Rh-CP and to probe differences to homogeneous, achiral rhodium(II) catalysts, we investigated the model reaction of donor/acceptor diazoalkane 6 with styrene 3 to give cyclopropane 7 (Scheme 3). In a first step, we performed the reaction on 10 mmol scale of diazoalkane 6 (1.9 g) employing 0.5 mg Rh-CP (23.1% Rh-content) to evaluate stability and activity of Rh-CP. Complete consumption of the diazoalkane was observed and the desired cyclopropane was isolated in 92% yield (2.31 g) in high diastereoselectivity. The diastereoselectivity was comparable to achiral, homogeneous Rh(II) catalysts, which is suggestive that the coordination polymer backbone has little influence on the geometry of transition state in this cyclopropanation reaction. A further reduction of the Rh-CP catalyst loading to 0.236 mg gave the desired cyclopropane 7 in slightly reduced yield of 91% (2.29 g).
Recycling studies further showed that the first five cycles gave a relatively constant yield of >86% of cyclopropane 7, while the amount of recovered Rh-CP constantly decreased to 60%. This reduction might be attributed to mechanical forces on the Rh-CP catalyst during stirring the reaction mixture that results reduction of particle sizes and reduced catalyst recovery. The increase of product yield in the fourth and fifth catalytic cycle might result from this particle size reduction that would also result in an increased catalytically active surface of the heterogeneous catalyst. Notably, the amount of recovered Rh-CP significantly decreased after the fifth cycle (30%) resulting in a reduced yield of 76% of the desired cyclopropane. After the sixth cycle only traces of Rh-CP were recovered, and no further reactions were performed.11 Kinetic studies of the Rh-CP were investigated next. Using 10 mg of Rh-CP a rapid and linear consumption of diazoalkane 6 was observed. The reaction rate only slowed down after a consumption of >90% of diazoalkane 6 (Scheme 2 and Table S1, ESI†).
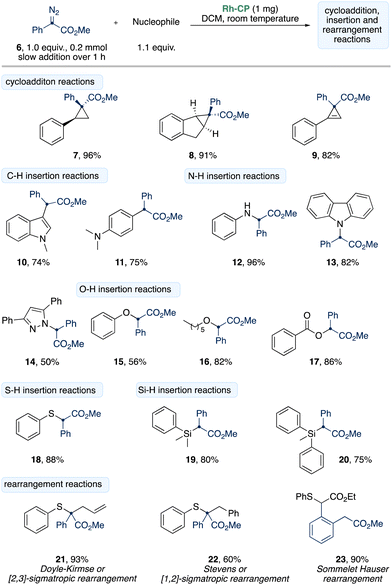
In a next step, we investigated the applicability of the Rh-CP in the reaction of donor/acceptor diazoalkane 6 in carbene transfer reactions (Scheme 3). Only a minor excess of the corresponding nucleophile was used to emphasize the high efficiency of the Rh-CP. To our delight, the Rh-CP is compatible with a broad range of different substrates and performs excellent in cyclopropa(e)nation reactions (7–9). N-Methyl indole gave the product of C3-functionalization 10 in 74% yield and N,N-dimethyl aniline reacted smoothly in para-C–H functionalization to give 11 75% yield.
X–H reactions (X = N, O, S, Si) gave the desired reaction products (12–20) in up to 96%. Yet, a longer reaction time of up to 24 h was required to achieve the full conversion of diazoalkane 6. This observation could be explained by a potential catalyst poisoning as the desorption of products or reagents might be energetically disfavored. We further examined rearrangement reactions of sulfur ylides and were able to isolate the products of Doyle–Kirmse (21), Stevens rearrangement (22) and Sommelet–Hauser (23) rearrangement reactions in up to 93% yield (Scheme 3).
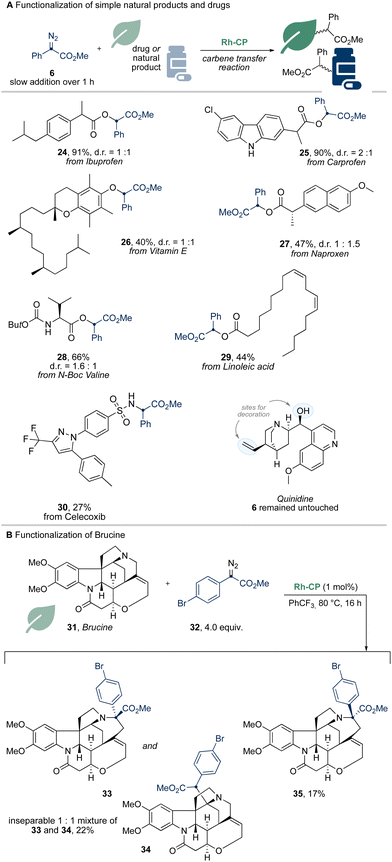
To further underline the applicability of Rh-CP, we studied more complex natural products and drugs in the reaction with donor/acceptor diazoalkanes (Scheme 4). Simple drug molecules such as Ibuprofen, Naproxen or Carprofen gave the desired O–H functionalization products (24, 25, 27) in high yield. Notably, only the O–H functionalization product of Carprofen was observed, while the free N–H function remained untouched.
We further explored natural products; vitamin E reacted in an O–H functionalization reaction to give the desired O–H functionalization product 26 in moderate yield. Derivatives of amino acids (28) and celecoxib (30) underwent X–H functionalization with Rh-CP catalyst. Quinidine, however, remained unreactive and no reaction was observed with the diazoalkane 6 staying untouched, which is indicative of catalyst poisoning (Scheme 4A).
In a last step, we studied the functionalization of brucine 31 in Rh-CP catalyzed reaction with diazoalkane 32. Under similar reaction conditions as reported by Beckwith and co-workers,12 we were able to obtain three different reaction products (33–35) in a total yield of 39% using 4 equivalents of diazoalkane 32 (Scheme 4B).13
In summary, we described here the application of a Rh(II)-based coordination polymer as a heterogeneous catalyst in carbene transfer reactions. Based on the IR and UV-Vis data, we were able to prove that the paddlewheel structure of the Rh2(OAc)4 precursor is also present in the synthesized Rh-based coordination polymer. Moreover, detailed studies on catalyst recycling and catalyst efficiency were performed. We were able to demonstrate the broad applicability ranging from X–H and C–H functionalization reactions towards rearrangement reactions and the functionalization of natural products and drugs.
R. M. K. thanks the Deutsche Forschungsgemeinschaft (DFG, for grant KO5659/2-1), C. J. for grant Ja466/43-1 within SPP 1928/2 COORNETs). C. E. thanks RWTH Aachen University for a Doctoral Scholarship. We thank Jun.-Prof. Dr Markus Suta for carrying out optical measurements.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) R. Hrdina, Eur. J. Inorg. Chem., 2021, 501 CrossRef CAS; (b) X. Tang, H. Noda and M. Shibasaki, Angew. Chem., Int. Ed., 2023, 62, e202311027 CrossRef CAS PubMed; (c) A. Mang, M. Linseis and R. F. Winter, Z. Anorg. Allg. Chem., 2022, 648, e20220019 CrossRef.
- Selected review articles: (a) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981 CrossRef CAS PubMed; (b) H. U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151 CrossRef CAS PubMed; (c) H. M. L. Davies and J. R. Manning, Nature, 2018, 451, 417 CrossRef PubMed; (d) M. P. Doyle, R. Duffy, M. Ratnikov and Z. Zhou, Chem. Rev., 2010, 110, 704 CrossRef CAS PubMed; (e) Y. Xia, D. Qiu and J. Wang, J. Chem. Rev., 2017, 117, 13810 CrossRef CAS PubMed; (f) H. M. L. Davies and J. R. Denton, Chem. Soc. Rev., 2009, 38, 3061 RSC; (g) J. Hansen and H. M. L. Davies, Coord. Chem. Rev., 2008, 252, 545 CrossRef CAS PubMed; (h) R. Wu, D. Zhu and S. Zhu, Org. Chem. Front., 2023, 10, 2849 RSC; (i) Y. Luo, X. Zhang and Y. Xia, Chin. Chem. Lett., 2024, 35, 108778 CrossRef CAS; (j) L. Mertens and R. M. Koenigs, Org. Biomol. Chem., 2016, 14, 10547 RSC; (k) Y. Xia, D. Qiu and J. Wang, Chem. Rev., 2017, 117, 13810 CrossRef CAS PubMed; (l) M. Akter, K. Rupa and P. Anbarasan, Chem. Rev., 2020, 122, 13108 CrossRef PubMed.
- Selected articles: (a) H. M. L. Davies and T. Hansen, J. Am. Chem. Soc., 1997, 119, 9075 CrossRef CAS; (b) R. P. Reddy and H. M. L. Davies, Org. Lett., 2006, 8, 5013 CrossRef CAS PubMed; (c) F. Xiao and J. Wang, J. Org. Chem., 2006, 71, 5789 CrossRef CAS PubMed; (d) H. Wang, D. Guptill, A. Varela-Alveraz, D. G. Musaev and H. M. L. Davies, Chem. Sci., 2013, 4, 2844 RSC; (e) W. Liu, Z. Ren, A. T. Bosse, K. Liao, E. L. Goldstein, J. Basca, D. H. Musaev, B. M. Stoltz and H. M. L. Davies, J. Am. Chem. Soc., 2018, 140, 12247 CrossRef CAS PubMed.
- Selected examples: (a) F. P. Caló, A. Zimmer, G. Bistoni and A. Fürstner, J. Am. Chem. Soc., 2022, 144, 7465 CrossRef PubMed; (b) T. A. C. A. Bayrakda and C. Lescot, ChemSusChem, 2023, 16, e202300596 CrossRef PubMed; (c) K. Makino, Y. Kumagai, T. Yoshino, M. Kojima and S. Masunaga, Org. Lett., 2023, 25, 3234 CrossRef CAS PubMed; (d) J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6802 CrossRef CAS PubMed; (e) M. Lee, G. D. Musaev and H. M. L. Davies, ACS Catal., 2020, 10, 6240 CrossRef CAS PubMed; (f) Z. Zhang, Z. Sheng, W. Yu, G. Wu, R. Zhang, W.-D. Chu, Y. Zhang and J. Wang, Nat. Chem., 2017, 9, 970 CrossRef CAS PubMed; (g) Z. Gao, D. Jiang, B. Li and B. Wang, Chem. Commun., 2022, 58, 1017 RSC.
- Selected examples: (a) H. M. L. Davies and A. M. Walji, Org. Lett., 2003, 5, 479 CrossRef CAS PubMed; (b) H. M. L. Davies and A. M. Walji, Org. Lett., 2003, 7, 2941 CrossRef PubMed; (c) C.-J. Yoo, D. Rackl, W. Liu, C. B. Hoyt, B. Pimentel, R. P. Lively, H. M. L. Davies and C. Jones, Angew. Chem., Int. Ed., 2018, 57, 10923 CrossRef CAS PubMed; (d) T. A. Hatridge, W. Liu, C.-J. Yoo, H. M. L. Davies and C. Jones, Angew. Chem., Int. Ed., 2020, 59, 19525 CrossRef CAS PubMed; (e) K. T. T. Oohar, M. Anada, H. Nambu and S. A. Hashimoto, Angew. Chem., Int. Ed., 2010, 49, 6979 CrossRef PubMed; (f) Z. Li, H. Jiang, M. Zhu and F. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 19003 CrossRef CAS PubMed; (g) Z. Li, L. Rösler, H. Breitzke, T. Gutmann and G. Buntkowsky, J. CO2 Util., 2021, 52, 101682 CrossRef CAS.
- L. Liu, C. Fasel, P. Braga-Groszewicz, N. Rothermel, A. S. L. Thankamony, G. Sauer, Y. Xu, T. Gutmann and G. Buntkowsky, Catal. Sci. Technol., 2016, 6, 7830 RSC.
- S. Furukawa, N. Horike, M. Kondo, Y. Hijikata, A. Carné-Sánchez, P. Larpent, N. Louvain, S. Diring, H. Sato, R. Matsuda, R. Kawano and S. Kitagawa, Inorg. Chem., 2016, 55, 10843 CrossRef CAS PubMed.
- G. Nickerl, U. Stoeck, U. Burkhardt, I. Senkovska and S. A. Kaskel, J. Mater. Chem. A, 2014, 2, 144 RSC.
- Selected examples: (a) F. Li, C. Pei and R. M. Koenigs, Chem. Sci., 2021, 12, 6362 RSC; (b) S. Jana, C. Empel, C. Pei, P. Aseeva, T. V. Nguyen and R. M. Koenigs, ACS Catal., 2020, 362, 5721 CAS; (c) K. J. Hock, A. Knorrscheidt, R. Hommelsheim, J. Ho, M. J. Weissenborn and R. M. Koenigs, Angew. Chem., Int. Ed., 2019, 58, 3630 CrossRef CAS PubMed; (d) U. P. N. Tran, R. Hommelsheim, Z. Yang, C. Empel, K. J. Hock, T. V. Nguyen and R. M. Koenigs, Chem. – Eur. J., 2020, 26, 1254 CrossRef CAS PubMed; (e) Z. Yang, M. Möller and R. M. Koenigs, Angew. Chem., Int. Ed., 2020, 59, 5572 CrossRef CAS PubMed; (f) C. Empel, S. Jana, T. Langletz and R. M. Koenigs, Chem. – Eur. J., 2022, 28, e202104321 CrossRef CAS PubMed; (g) C. Empel, S. Jana and R. M. Koenigs, Molecules, 2020, 25, 880 CrossRef CAS PubMed; (h) C. Empel, K. J. Hock and R. M. Koenigs, Org. Biomol. Chem., 2018, 16, 7129–7133 RSC.
- (a) R. L. Firor and K. Seff, J. Am. Chem. Soc., 1978, 100, 978 CrossRef CAS; (b) D. S. Martin, T. R. Webb, G. A. Robbins and P. E. Fanwick, Inorg. Chem., 1979, 18, 475 CrossRef CAS; (c) J. Kitchens and L. J. Bear, J. Inorg. Nucl. Chem., 1969, 31, 2415 CrossRef CAS.
- The recycling may proof difficult due to the small reaction scale and an improved recovery of Rh-CP could be possible on a larger reaction scale.
- J. He, L. G. Hamann, H. M. L. Davies and E. E. Beckwith, Nat. Commun., 2015, 6, 5943 CrossRef PubMed.
- Structural assignment of 33–35 according to ref. 11.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc01386g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |