Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorogenic cell surface glycan labelling with fluorescence molecular rotor dyes and nucleic acid stains†
Alen
Koçak
 a,
Amal K.
Homer
a,
Antonia
Feida
a,
Florian
Telschow
b,
Jacob L.
Gorenflos López
c,
Cihan
Baydaroğlu
d,
Michael
Gradzielski
a,
Amal K.
Homer
a,
Antonia
Feida
a,
Florian
Telschow
b,
Jacob L.
Gorenflos López
c,
Cihan
Baydaroğlu
d,
Michael
Gradzielski
 d,
Christian P. R.
Hackenberger
d,
Christian P. R.
Hackenberger
 ac,
Ulrike
Alexiev‡
ac,
Ulrike
Alexiev‡
 b and
Oliver
Seitz
b and
Oliver
Seitz
 *a
*a
aDepartment of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Straße 2, 12489 Berlin, Germany. E-mail: oliver.seitz@chemie.hu-berlin.de
bInstitut für Experimentalphysik, Freie Universität Berlin, Arnimallee 14, 14195 Berlin, Germany
cLeibniz-Forschungsinstitut für Molekulare Pharmakologie, Robert-Rössle-Straße 10, 13125 Berlin, Germany
dInstitute of Chemistry, Technische Universität Berlin, Straße des 17. Juni 124, 10623 Berlin, Germany
First published on 4th April 2024
Abstract
We show that covalent labelling of sialic acids on live cell surfaces or mucin increases the fluorescence of the fluorescence molecular rotors (FMRs) CCVJ, Cy3 and thioazole orange, enabling wash-free imaging of cell surfaces. Dual labelling with an FMR and an environmentally insensitive dye allows detection of changes that occur, for example, when cross-linking is altered.
Fluorescence molecular rotor (FMR) dyes are a class of dyes which are exceptionally responsive to the environment.1 Their responsiveness is based on twisting motions which non-radiatively deplete the excited state. Environments that hinder twisting can induce strong enhancements of fluorescence. This property has been used to measure nanoviscosity2–4 and interactions of FMR-labelled ligands with proteins5 and nucleic acids.6,7 We envisioned that FMR dyes should also be able to show enhanced fluorescence upon labelling of cell surface glycans. The glycocalyx and mucus layers on the cell surface are dense materials, which should provide crowded environments imposing friction on FMR dyes. The resulting fluorogenic labelling is advantageous, because wash-steps typically used for the removal on non-bound probes are no longer required, facilitating the analysis of fast processes. We furthermore envisaged that FMRs, when attached to cell surface glycans, could respond to changes occurring in disease and therapeutic intervention.
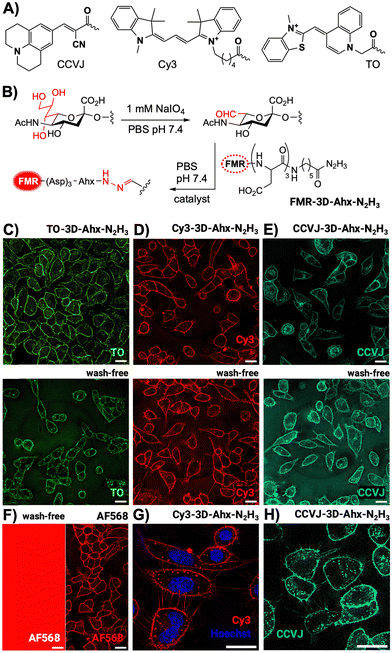
For covalent labelling of cell surface glycans, we selected the FMRs CCVJ and Cy3 (Fig. 1A), which show increased fluorescence when high viscosity or the crowded environment of a binding partner hinders twisting motions.3,8 So far, binding induced enhancements of CCVJ or Cy3 emission have been demonstrated for interactions with proteins9–11 and nucleic acids12 but to the best of our knowledge increased fluorescence upon attachment to glycans has not been reported. In addition, we explored the use of thiazole orange (TO) previously not considered as a cell surface stain. TO is virtually non-fluorescent in free form but experiences dramatic fluorescence enhancements upon DNA intercalation because rotations around a central methine bridge are hindered.13 TO dyes are therefore frequently used as nucleic stains.14,15 In addition, TO has been used to sense the vicinity of proteins that hinder twisting.16,17 Assuming that glycan labelling and cell surface viscosity would induce a similar effect, we repurposed TO to enable staining of the cell surface. For this purpose, TO as well as CCVJ and Cy3 were attached to a triaspartate peptide hydrazide (FMR-3D-Ahx-N2H3, Fig. 1B). The negative charges hinder cell membrane permeation of the hydrophobic dyes (Fig. S8, ESI†). The hydrazide group allowed conjugation with aldehyde functions installed at cell surface sialic acid residues upon mild periodate treatment (Fig. 1B and Fig. S8, ESI†).18,19 This method has frequently been used to stain cell surfaces. However, fluorogenic labelling with FMR dyes has not been reported. Published labelling protocols involve a total of 0.5–2 h incubation of cells at 0–4 °C, and wash steps to remove excess labelling reagents prior to fluorescence microscopic imaging. This can induce cold stress potentially altering cellular surfaces (Fig. S6, ESI†). We added 5-methoxyanthranilic acid (5MA) as an organocatalyst20 and performed both the periodate treatment and the hydrazone ligation with the acyl hydrazides at 25 °C for 5 minutes only (Fig. S7 and S8, ESI†). This way, delicate cells such as A549 cells were labelled with little, if any, blebbing.
Labelling with TO-3D-Ahx-N2H3 allowed clear imaging of cell surfaces (Fig. 1C). Control experiments showed that periodate treatment and the presence of 5MA was required for cell surface staining (Fig. S9, ESI†). Considering that TO in free form is essentially non-florescent in aqueous solution,21 we attribute the signals provided by the glycan-bound TO probe to properties of the cell surface matrix (vide infra). As anticipated, washing is not required after treatment with the TO probe (Fig. 1C, low). Similarly, A549 cells were treated, after rapid periodate treatment, with peptide hydrazides containing Cy3 and CCVJ. Again, a short (5 min) 5MA-catalyzed labelling with Cy3-3D-Ahx-N2H3 and CCVJ-3D-Ahx-N2H3 was sufficient to stain the surface of living A549 cells (Fig. 1D and E). Even filopodial protrusions were clearly visible (Fig. 1G and H). Again, no washing was required indicating that the fluorescence of non-reacted Cy3 and CCVJ probes is lower than the fluorescence of probes on the cell surface. In a control experiment, we labelled cells with AF568 hydrazide (Fig. 1F). While clear cell surface staining was achieved after removal of non-reacted probe by washing, the no-wash protocol failed to provide contrast. Regardless of the imaging probe used, staining by direct labelling of the surface glycans was never exclusive for the surface of A549 cells. The punctuate signals inside cells are likely due to rapid internalization as a result of constitutively active endocytotic processes. Of note, rapid room temperature labelling with the FMR-3D-Ahx-N2H3 probes (FMR = Cy3, CCVJ and TO) also succeeded with CEM cells as an example of a suspension cell line (Fig. S12, ESI†).
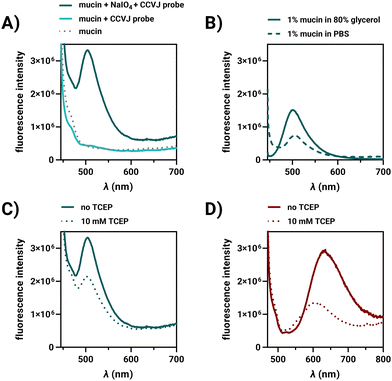
In studies of porcine mucin, we investigated the factors responsible for enhancing the fluorescence of covalently bound FMR on the cell surface. Porcine mucin in water is viscous and can be labelled with FMR hydrazides due to its approx. 1.2% sialic acid content. The fluorescence of the CCVJ-3D-Ahx-N2H3 probe increased substantially when allowed to react with periodate-treated mucin (Fig. 2A). For comparison, the reactive CCVJ-3D-Ahx-N2H3 probe was added to mucin without prior periodate activation. As a further control, fluorescence of an unreactive CCVJ–OH was measured in PBS and after addition of the periodate-activated mucin (Fig. S16, ESI†). Fluorescence remained low in both cases. This suggests that the close proximity to glycans induced upon formation of covalent bonds is very efficient in hindering CCVJ torsional motions, demonstrating, for the first time, that CCVJ can respond to interactions with glycans. This raises the question of whether mucin-conjugated CCVJ would still be able to sense changes in viscosity. Glycerol (80% v/v) was included in the buffer, and an increase in CCVJ emission was observed (Fig. 2B). In a second experiment, TCEP was added, which was reported to decrease viscosity of mucin by cleavage of disulfide crosslinks.22 Intensity of CCVJ emission decreased upon addition of TCEP (Fig. 2C). Rheological measurements showed that TCEP treatment affected both the storage modulus G′ and the loss modulus G′′ (Fig. S19, ESI†). The decrease in fluorescence might therefore be caused by both decreases of molecular crowding around CCVJ and reduced viscosity. Control measurements involving addition of TCEP to CCVJ in glycerol (a matrix which lacks cleavable disulfides), showed that chemical quenching contributed only little to the decrease of dye emission (Fig. S18A, ESI†). We concluded that CCVJ on the glycans of porcine mucin remains responsive to changes of the environment that alter friction.
Labelling of porcine mucin was also performed with TO-3D-Ahx-N2H3. The emission from TO on mucin was red-shifted with a maximum at 635 nm (Fig. 2D), indicative of TO aggregation.16 As observed for CCVJ, treatment of the TO-labelled mucin with TCEP led to a decrease of fluorescence, which did not occur when TCEP was added to TO in glycerol (Fig. S18B, ESI†). For Cy3-3D-Ahx-N2H3 we also observed a fluorescence increase upon covalent labelling and a viscosity responsiveness (Fig. S17, ESI†), both of which may contribute to the fluorogenicity of cell surface glycan labelling.
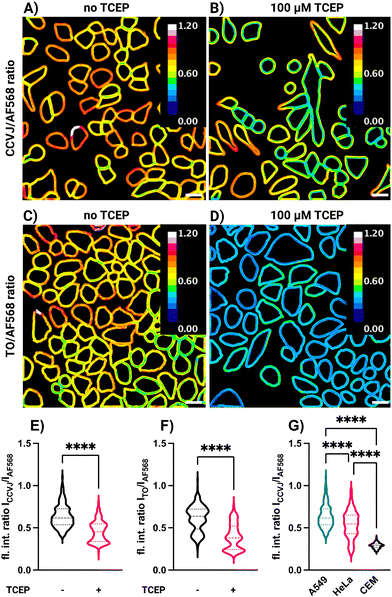
Based on the results of the mucin labelling experiments, we hypothesized that conjugation of FMR dyes with cell surface glycans might allow detection of changes in the glycocalyx environment, such as might be caused by modifications/remodelling of cell surface components. However, in cell measurements, low fluorescence intensity does not necessarily have to be due to such changes, but can also be caused by low occupancy, i.e., a lower amount of dye. We, therefore, investigated simultaneous labelling of A549, HeLa and CEM cells with two dyes (Fig. 3). The FMR dye would serve as friction sensor, while Alexa Fluor 568 hydrazide, would function as an environmentally insensitive concentration reporter. A mixture of both dyes was used for labelling and the ratio of staining intensities in the FMR and the AF568 channels was determined for each cell surface. In control experiments, we verified that AF568 fluorescence is not affected by viscosity (Fig. S15, ESI†). The analysis was focused on cell surface regions (Fig. 3A–D and Fig. S14, ESI†). In one set of experiments, A549 cells were treated with TCEP and washed prior to labelling. As shown in Fig. 2C and D, TCEP treatment reduced the degree of molecular crowding/viscosity of mucus, and we reasoned that it might also act on the glycocalyx. Remarkably, cells which were incubated for 60 min with 100 μM TCEP before labelling showed significantly reduced CCVJ/AF568 (compare Fig. 3A, B and E) and TO/AF568 (compare Fig. 3C, D and F) fluorescence ratio values compared to control cells. Quantitative analysis of a total of 400–600 cells (Fig. 3E and F) showed that labelling of TCEP-treated cells resulted in mean FMR/AF568 ratios that were 29% (FMR = CCVJ) or 35% (FMR = TO) lower than the ratio determined for untreated cells. TCEP is a reducing agent, and cleavage of accessible disulfide bridges could cause partial shedding of cell surface glycans. This could reduce the amount of sialic acid residues available for reactions with the rotor dyes. Nonetheless, this should affect both, the FMR and AF568 dyes. It is therefore plausible to assume that the decrease in FMR/AF568 intensity ratio is due to a decrease in steric hindrance experienced by the FMR. This decrease could be due to a reduced viscosity or an increase of degrees of freedom when reductive cleavage of the disulfide bridges reduces crowding in the FMR's environment.
Interestingly, simultaneous labelling with AF568-N2H3 and CCVJ-3D-Ahx-N2H3 showed cell type-specific emission ratios. Compared to A549 and HeLa cells, CEM cells were characterized by a significantly lower ratio value (Fig. 3G). We speculate that this difference can be caused by different compositions of the cell surfaces. A549 cells originate from the lung epithelium and HeLa from the cervix. These epithelial cell types might form a thicker glycocalyx than CEM cells, which originate from circulating lymphocytes.
The data shown in Fig. 2 and 3 suggest that direct labelling of neuraminic acid with an FMR dye such as CCVJ can detect changes of the cell surface composition. For further validation we assessed the CCVJ-labelled cells by fluorescence lifetime imaging microscopy (FLIM).23 The mean fluorescence lifetime of 0.37 ns determined for CCVJ-3D-Ahx-N2H3 in PBS at pH 7.4 increased after labelling of the cell surface to 1.9 ± 0.2 ns (Table S1 and Fig. S20, ESI†). This confirms that the signals measured by fluorescence intensity microscopy (Fig. 2) are not due to dye enrichment but to interactions of the dye with the cell surface layer. Of note, the lifetime of CCVJ emission on the cell is much longer than the lifetime measured in highly viscous glycerol, which is in the subnanosecond range.24 This might be attributed to the additional contribution of interactions with glycans induced upon covalent attachment. Cells that were treated with TCEP prior to labelling with CCVJ-3D-Ahx-N2H3 showed a decrease in the mean fluorescence lifetime to 1.3 ns (Table S1 and Fig. S20B, ESI†). Thus, we conclude that conjugation of CCVJ with cell surface sialic acids allows detection of changes in the glycocalyx layers that occur, for example, when disulfide bridges are cleaved.
In summary, we explored covalent labelling of cell surface sialic acids with the FMRs CCVJ, Cy3 and thiazole orange (TO). TO is a nucleic acid stain, which, to our knowledge, has not been used for staining cell surfaces. Our data shows that sialic acid labelling of cell surfaces or isolated mucin via the periodate oxidation/peptidyl hydrazone ligation sequence guides the FMRs into an environment where the free volume available for twisting motions is effectively restricted. This can be caused by viscosity describing the resistance of solvent molecules to displacements and/or molecular crowding/confinement referring to the steric effect of biomolecules. To the best of our knowledge, this is the first example showing that FMR dyes can be used to detect interactions with glycans. Both CCVJ and TO are essentially dark when free in aqueous solution, and their fluorogenic response allows omission of washing after labelling. Co-labelling of cell surfaces with an environmentally insensitive dye and intensity ratio imaging proved that CCVJ and TO fluorescence is affected by alterations induced upon the treatment of cells with the reducing agent TCEP. This suggests that treatment of cells, and also mucin, with TCEP reduced frictional forces acting on the glycan-bound dyes, most likely by decreasing crowding in their environment through disulfide cleavage. In diseases such as mucoviscidosis or chronic obstructive pulmonary disease (COPD), the surface of airway epithelial cells is covered by abnormally thick mucus. Our feasibility study suggests that it should be possible to monitor changes of cell surface properties using equipment commonly available to cell biology labs. We envision applications, such as in screening campaigns searching for mucolytic agents or to decipher the effects that occur upon bacterial/viral challenge. In this context, it is beneficial that direct labelling of glycans allows studies of wildtype cells.
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project ID 431232613 – SFB 1449.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Paez-Perez and M. K. Kuimova, Angew. Chem., Int. Ed., 2024, 63, e202311233 CrossRef CAS PubMed.
- R. O. Loutfy and B. A. Arnold, J. Phys. Chem., 1982, 86, 4205–4211 CrossRef CAS.
- S.-C. Lee, J. Heo, H. C. Woo, J.-A. Lee, Y. H. Seo, C.-L. Lee, S. Kim and O.-P. Kwon, Chem. – Eur. J., 2018, 24, 13706–13718 CrossRef CAS PubMed.
- M. K. Kuimova, G. Yahioglu, J. A. Levitt and K. Suhling, J. Am. Chem. Soc., 2008, 130, 6672–6673 CrossRef CAS PubMed.
- U. Alexiev, P. Volz, A. Boreham and R. Brodwolf, Eur. J. Pharm. Biopharm., 2017, 116, 111–124 CrossRef PubMed.
- O. Köhler, D. V. Jarikote and O. Seitz, ChemBioChem, 2005, 6, 69–77 CrossRef PubMed.
- R. W. Sinkeldam, A. J. Wheat, H. Boyaci and Y. Tor, ChemPhysChem, 2011, 12, 567–570 CrossRef CAS PubMed.
- M. A. Haidekker and E. A. Theodorakis, Org. Biomol. Chem., 2007, 5, 1669–1678 RSC.
- W. L. Goh, M. Y. Lee, T. L. Joseph, S. T. Quah, C. J. Brown, C. Verma, S. Brenner, F. J. Ghadessy and Y. N. Teo, J. Am. Chem. Soc., 2014, 136, 6159–6162 CrossRef CAS PubMed.
- W.-T. Yu, T.-W. Wu, C.-L. Huang, I. C. Chen and K.-T. Tan, Chem. Sci., 2016, 7, 301–307 RSC.
- E. M. S. Stennett, M. A. Ciuba, S. Lin and M. Levitus, J. Phys. Chem. Lett., 2015, 6, 1819–1823 CrossRef CAS PubMed.
- B. J. Harvey and M. Levitus, J. Fluoresc., 2009, 19, 443–448 CrossRef CAS PubMed.
- V. Karunakaran, J. L. Pérez Lustres, L. Zhao, N. P. Ernsting and O. Seitz, J. Am. Chem. Soc., 2006, 128, 2954–2962 CrossRef CAS PubMed.
- F. Hövelmann and O. Seitz, Acc. Chem. Res., 2016, 49, 714–723 CrossRef PubMed.
- L. G. Lee, C. H. Chen and L. A. Chiu, Cytometry, 1986, 7, 508–517 CrossRef CAS PubMed.
- O. Suss, L. Motiei and D. Margulies, Molecules, 2021, 26, 2828 CrossRef CAS PubMed.
- L. Unger-Angel, B. Rout, T. Ilani, M. Eisenstein, L. Motiei and D. Margulies, Chem. Sci., 2015, 6, 5419–5425 RSC.
- P. A. De Bank, B. Kellam, D. A. Kendall and K. M. Shakesheff, Biotechnol. Bioeng., 2003, 81, 800–808 CrossRef CAS PubMed.
- Y. Zeng, T. N. C. Ramya, A. Dirksen, P. E. Dawson and J. C. Paulson, Nat. Methods, 2009, 6, 207–209 CrossRef CAS PubMed.
- P. Crisalli and E. T. Kool, J. Org. Chem., 2013, 78, 1184–1189 CrossRef CAS PubMed.
- T. L. Netzel, K. Nafisi, M. Zhao, J. R. Lenhard and I. Johnson, J. Phys. Chem., 1995, 99, 17936–17947 CrossRef CAS.
- L. E. Morgan, A. M. Jaramillo, S. K. Shenoy, D. Raclawska, N. A. Emezienna, V. L. Richardson, N. Hara, A. Q. Harder, J. C. NeeDell, C. E. Hennessy, H. M. El-Batal, C. M. Magin, D. E. Grove Villalon, G. Duncan, J. S. Hanes, J. S. Suk, D. J. Thornton, F. Holguin, W. J. Janssen, W. R. Thelin and C. M. Evans, Nat. Commun., 2021, 12, 249 CrossRef CAS PubMed.
- P. Volz, R. Brodwolf, C. Zoschke, R. Haag, M. Schäfer-Korting and U. Alexiev, Z. Phys. Chem., 2018, 232, 671–688 CrossRef CAS.
- C. Rumble, K. Rich, G. He and M. Maroncelli, J. Phys. Chem. A, 2012, 116, 10786–10792 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic protocols, UPLC-ESI-MS, cell biological experiments, fluorescence microscopy and image analysis. See DOI: https://doi.org/10.1039/d4cc00884g |
| ‡ Deceased |
| This journal is © The Royal Society of Chemistry 2024 |