Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Passerini polymerization of α-lipoic acid for dynamically crosslinking 1,2-dithiolane-functionalized polymers†‡
Yasuyuki
Nakamura§
 *a,
Yi-Shen
Huang§
b,
Chih-Feng
Huang
*a,
Yi-Shen
Huang§
b,
Chih-Feng
Huang
 *b and
Sadaki
Samitsu
*b and
Sadaki
Samitsu
 a
a
aData-driven Polymer Design Group, Research Center for Macromolecules and Biomaterials, National Institute for Materials Science, 1-2-1 Sengen, Tsukuba, Ibaraki 305-0047, Japan. E-mail: NAKAMURA.Yasuyuki@nims.go.jp
bDepartment of Chemical Engineering, i-Center for Advanced Science and Technology (iCAST), National Chung Hsing University, 145 Xingda Road, South District, Taichung 40227, Taiwan. E-mail: HuangCF@dragon.nchu.edu.tw
First published on 2nd April 2024
Abstract
Passerini polymerization using naturally occurring α-lipoic acid as a raw material yields polyamides with 1,2-dithiolane functional groups in a one-step reaction. The polyamide exhibits characteristics of an adaptable dynamically crosslinked network through reversible ring-opening reaction of 1,2-dithiolane, enabling self-healing, reusable strong adhesion, and regeneration through decrosslinking and re-crosslinking.
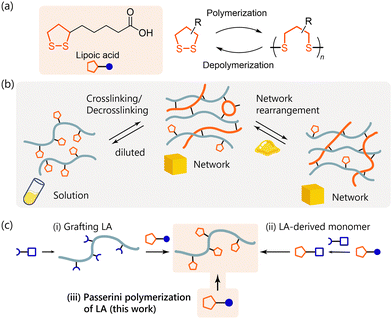
1,2-Dithiolane is a five-membered ring structure with adjacent sulfur atoms. This chemical motif is inherent in naturally occurring α-lipoic acid (LA), also known as thioctic acid, which exhibits biological activities such as robust antioxidant properties (Fig. 1a).1,2 In addition, LA derivatives are recognized for various applications including the stabilization of quantum dots and metal nanoparticles.3–5 In recent years, considerable attention has been directed towards the utilization of 1,2-dithiolanes in polymer science as a pivotal motif for creating “circular” plastics with recyclability,6,7 and adaptable networks with functionalities like self-healing.8–11 These attributes are based on the reversible ring-opening reaction of 1,2-dithiolane and the resulting disulfide-containing chain which has the dynamic covalent bonding character (Fig. 1a). The development of methods for introducing the 1,2-dithiolane moiety into a polymer structure enables the creation of polymer materials with controllable crosslinking and the resulting material properties (Fig. 1b).
Due to its natural occurrence and abundance, LA is highly attractive as a source of the 1,2-dithiolane moiety, making it a sustainable raw material for functional polymers. Recently, methods employing LA for polymer synthesis have been extensively studied, with most of them utilizing the ring-opening reaction of 1,2-dithiolane to form the S–S backbone of poly(disulfide)s.12–15 On the other hand, the 1,2-dithiolane-functionalized (pendant) polymers have high material potential due to the broad range of polymer chain structure. The syntheses of such polymers were achieved through post-modification of polymers using LA, such as amide condensation between the CO2H group in LA and NH2 group in the polymer (Fig. 1c).3,10,16,17 LA-derived monomers with the polymerizable functional groups have also been developed.9,11,18–20 However, there has been a lack of simple methods for directly utilizing LA as a raw material for polymers possessing the 1,2-dithiolane moiety as a functional group.
The Passerini reaction is a multicomponent reaction that affords a three-component adduct from a carboxylic acid, an aldehyde, and an isocyanide.21,22 Passerini polymerization, which extends the Passerini reaction to polymerization by using di-functional components, enables polymer synthesis with a versatile carboxylic acid when dialdehyde and diisocynanide are employed.23–26 1,2-Dithiolane has rich reactivity, undergoing ring-opening reactions induced by temperature, light, and external radicals, nucleophiles, and electrophiles. These features are the origin of 1,2-dithiolane's chemistry and functions; however, they pose a challenge to polymerizing 1,2-dithiolane-containing monomers while retaining the 1,2-dithiolane moiety.27,28 To address this challenge, Passerini polymerization provides an opportunity for the direct polymerization of LA, owing to the mild reaction conditions and the reaction mechanism without radicals, strong nucleophilic or electrophilic species (Fig. 1c).
In this study, we demonstrated the synthesis of 1,2-dithiolane-functionalized polymers directly using LA as a raw material through Passerini polymerization, and the resulting polymer exhibited characteristics of a dynamically crosslinked polymer network.
First, we conducted the Passerini reaction using LA, butyraldehyde, and 1,6-diisocyanohexane to test the compatibility of the reaction condition for the 1,2-dithiolane moiety. The mixture of the three compounds afforded the adduct within 1 h at 40 °C, and the 1H NMR analysis of the product confirmed the retention of 1,2-dithiolane moiety (Fig. S1 in ESI‡). Next, we conducted Passerini polymerization using LA, glutaraldehyde, and 1,6-diisocyanohexane (Fig. 2). The reaction in THF solvent afforded the polyamide PA-LA with a 1,2-dithiolane pendant group derived from LA, whose structure was characterized by IR and NMR spectroscopies (Table 1 and ESI‡). ATR-IR measurement of the polyamide exhibited distinctive vibration signals of νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O,amide of the main chain amide moiety at 1658 cm−1. In addition, νC
O,amide of the main chain amide moiety at 1658 cm−1. In addition, νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O,ester at 1738 cm−1 indicated the presence of an ester group, which corresponds to the α-acyloxy polyamide structure (Fig. S2, ESI‡). These observations confirmed the incorporation of LA into the polymer main chain through the conversion of the CO2H group. The 1H NMR spectra of PA-LA confirmed proton signals of CH of α-carbonyloxy amide (5.05 ppm), amide NH (6.45 ppm), and those in LA-derived 1,2-dithiolane moiety, indicating the successful formation of polyamide with 1,2-dithiolane pendant group (Fig. 2). The signal observed at 9.76 ppm was assigned to the remaining CHO at the polymer chain end, while no peaks assigned to the remaining isocyanide group in the polymer were observed. This indicated that the chain end structure of the polymer would be CHO group derived from glutaraldehyde. GPC analysis of the PA-LA taken from the reaction mixture resulted in Mn = 6590 and Đ = 1.95 (Fig. 3a). From this result, the number of 1,2-dithiolane groups was roughly determined to be 20 in one PA-LA chain on average according to calculations based on the molecular weight of the repeating unit.
O,ester at 1738 cm−1 indicated the presence of an ester group, which corresponds to the α-acyloxy polyamide structure (Fig. S2, ESI‡). These observations confirmed the incorporation of LA into the polymer main chain through the conversion of the CO2H group. The 1H NMR spectra of PA-LA confirmed proton signals of CH of α-carbonyloxy amide (5.05 ppm), amide NH (6.45 ppm), and those in LA-derived 1,2-dithiolane moiety, indicating the successful formation of polyamide with 1,2-dithiolane pendant group (Fig. 2). The signal observed at 9.76 ppm was assigned to the remaining CHO at the polymer chain end, while no peaks assigned to the remaining isocyanide group in the polymer were observed. This indicated that the chain end structure of the polymer would be CHO group derived from glutaraldehyde. GPC analysis of the PA-LA taken from the reaction mixture resulted in Mn = 6590 and Đ = 1.95 (Fig. 3a). From this result, the number of 1,2-dithiolane groups was roughly determined to be 20 in one PA-LA chain on average according to calculations based on the molecular weight of the repeating unit.
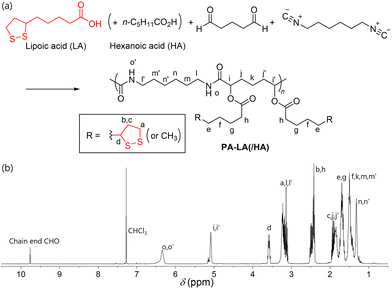 | ||
| Fig. 2 Reaction scheme and 1H NMR spectrum of PA-LA (sample of Table 1, run 3). | ||
| Run | Carboxylic acidb | Solvent | Conversion: CHO, NCc (%) | M n | Đ |
|---|---|---|---|---|---|
a [Carboxylic acid]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [glutaraldehyde] [glutaraldehyde]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [1,6-diisocyanohexane] = 2.2 [1,6-diisocyanohexane] = 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. [Carboxylic acid] = 2.0 M. For the detailed conditions, see ESI.
b Molar ratio is given for lipoic acid (LA) and hexanoic acid (HA) mixture.
c Conversions of aldehyde and isocyanide groups calculated by the 1H NMR signal intensity of CHO in the starting dialdehyde.
d Calculated against PMMA standards.
e The reaction mixture became a gel during the reaction.
f [Carboxylic acid] = 1.0 M.
g [Carboxylic acid] = 2.5 M. 1. [Carboxylic acid] = 2.0 M. For the detailed conditions, see ESI.
b Molar ratio is given for lipoic acid (LA) and hexanoic acid (HA) mixture.
c Conversions of aldehyde and isocyanide groups calculated by the 1H NMR signal intensity of CHO in the starting dialdehyde.
d Calculated against PMMA standards.
e The reaction mixture became a gel during the reaction.
f [Carboxylic acid] = 1.0 M.
g [Carboxylic acid] = 2.5 M.
|
|||||
| 1 | LA | THF | 97, 100 | 6590 | 1.95 |
| 2 | LA | THFf | 88, 100 | 2980 | 2.29 |
| 3 | LA | THFg | 98, 100 | 8740 | 2.01 |
| 4 | LA | CHCl3 | —e | — | — |
| 5 | LA/HA (1/1) | THF | 99, 100 | 8860 | 1.98 |
| 6 | LA/HA (1/2) | THF | 98, 100 | 8750 | 2.02 |
| 7 | LA/HA (1/4) | THF | 98, 100 | 8920 | 2.00 |
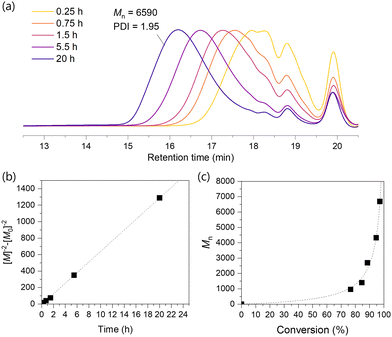 | ||
| Fig. 3 (a) GPC profiles in the synthesis of PA-LA, (b) the kinetic curve, and (c) Mn evolution against the conversion of CHO group (Table 1, run 1). | ||
The study of the polymerization progress found that both CHO and isocyanide groups were rapidly consumed in the early stage of polymerization, following third-order reaction kinetics (Fig. 3b). The molecular weight increased with the conversion of functional groups, and nearly reached a plateau after around 20–40 hours. The concentration of starting materials correlated to the molecular weight of polymers, with higher concentrations yielded longer polymers (run 2–3). However, excessively high concentrations led to gelation during the reaction, making the analysis difficult. Such gelation was also observed when CHCl3 was used as a solvent (run 4).
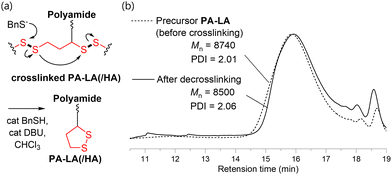
After precipitating from a selective solvent (Et2O), the isolated PA-LA was crosslinked and insoluble in the solvent used in the polymerization. Slow evaporation of sodium lipoate aqueous solution is reported to result in the concentration-induced formation of poly(disulfide) via ring-opening reaction of 1,2-dithiolane, which indicates that the reaction occurs even at room temperature.13 Thus, the isolation of PA-LA caused such concentration-induced crosslinking. However, the crosslinked PA-LA could be applied for hot-press molding at 150 °C (Fig. 4a). This experiment indicated that the dynamic covalent bond nature of 1,2-dithiolane-derived disulfide bonds in crosslinked PA-LA (Fig. 4b).
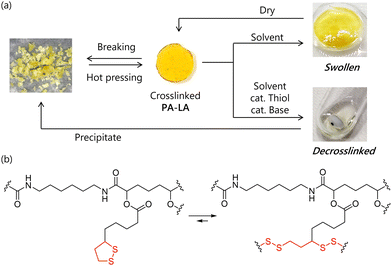 | ||
| Fig. 4 (a) Cycle of crosslinked PA-LA: molding, swelling, decrosslinking, and recovery. (b) A plausible mechanism of bond exchanging. | ||
To study the effect of the number of crosslinking 1,2-dithiolane groups in the polymer chain, copolymerization using another carboxylic acid was examined. We conducted Passerini polymerization using LA and hexanoic acid (HA) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, and glutaraldehyde and 1,6-diisocyanohexane (Table 1, run 5). The polymerization successfully afforded PA-LA/HA with high monomer conversion. Polymerization monitoring by 1H NMR measurements indicated a random incorporation of LA and HA units into the polymer chain. The structure of PA-LA/HA was further identified, as well as PA-LA (Fig. S3, ESI‡). The Mn = 8860 of PA-LA/HA corresponded to twelve 1,2-dithiolane groups, which is half of the dithiolane density compared to PA-LA. Similarly, PA-LA/HA from different feeding ratios of LA and HA were synthesized in the same manner (Table 1, runs 6 and 7).
1 ratio, and glutaraldehyde and 1,6-diisocyanohexane (Table 1, run 5). The polymerization successfully afforded PA-LA/HA with high monomer conversion. Polymerization monitoring by 1H NMR measurements indicated a random incorporation of LA and HA units into the polymer chain. The structure of PA-LA/HA was further identified, as well as PA-LA (Fig. S3, ESI‡). The Mn = 8860 of PA-LA/HA corresponded to twelve 1,2-dithiolane groups, which is half of the dithiolane density compared to PA-LA. Similarly, PA-LA/HA from different feeding ratios of LA and HA were synthesized in the same manner (Table 1, runs 6 and 7).
PA-LA and PA-LA/HA (LA/HA = 1/1) exhibited a swelling property with a swelling ratio of 120% for PA-LA and 308% for PA-LA/HA using CHCl3 (Fig. 4a). The higher swelling ratio for PA-LA/HA is attributed to the lower crosslink density in the network. These swelling behaviours without dissolution indicates the presence of covalent crosslinks in the material. Thermal gravimetry analysis (TGA) of these polymers exhibited the 5% thermal decomposition temperatures (Tdeg,5%) above 278 °C, showing their thermal robustness (Fig. S4, ESI‡). Differential scanning calorimetry (DSC) measurements revealed the glass transition temperature (Tg) at 29 °C and 24 °C for crosslinked PA-LA and PA-LA/HA, respectively (Fig. S5, ESI‡). The lower Tg of PA-LA/HA is likely due to the incorporation of a less sterically bulky C5-alkyl chain instead of a tetramethylene 1,2-dithiolane chain.
The viscoelastic properties of the crosslinked PA-LA and PA-LA/HA (LA/HA = 1/1) were characterized by rheology measurements. These materials exhibited typical curves of crosslinked polymers in temperature ramp measurements, with a notable modulus at low temperature and a rubbery plateau at high temperature (Fig. S6 and S7, ESI‡). While both materials showed almost identical moduli (approximately 100 MPa) at the temperature below their respective Tg, PA-LA/HA exhibited an order of magnitude lower modulus (0.3 MPa) compared to that of PA-LA (2.3 MPa) at the temperature above their respective Tg. These experiments suggest the potential for tuning viscoelastic properties of these materials using additional carboxylic acid. The mechanical property of the material was further examined by a uniaxial tensile test, and PA-LA/HA (1/1) exhibited a stretch ratio of over 500% (Fig. S10, ESI‡).
The dynamic covalent bonding nature of S–S bonds in PA-LA(/HA) imparted the characteristics of healable elastomers (Fig. S11, ESI‡) and recyclable adhesives. These polymers had almost no tackiness and lacked adhesive strength when they were simply sandwiched between stainless steel plates under pressure. On the other hand, when these specimens were heated at 180 °C under pressure for 10–20 min, they displayed significant adhesive properties with a shear strength of 10.8 MPa for PA-LA/HA (LA/HA = 1/1, Fig. 5). After the break of the specimen, the polymer was collected and used for a new specimen under the same preparation procedure. The specimen exhibited adhesion strength again, and this recycling process could be repeated (Fig. 5). This strong adhesion can be attributed to the polar and flexible main chain of polyamide that enhances the interaction at the interface, the flexible alkyl side chain derived from LA and HA, and dynamic and robust disulfide crosslinks that reconstruct the network structure enabling a large and optimal contact area.
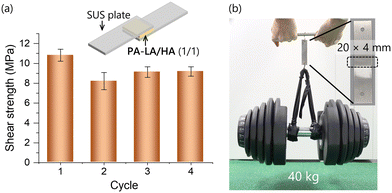 | ||
| Fig. 5 Adhesion cyclic test of PA-LA/HA (1/1) (a) and a demonstrating loading test (b). The standard errors are shown on the bars. | ||
Finally, we studied the solubilization and recovery of these crosslinked polymers via catalytic decrosslinking of disulfide bonding. The crosslinked PA-LA was immersed in CHCl3 and added a catalytic amount of benzyl sulfide (BnSH) and 1,8-diazabicyclo[5.4.0]-7-undecene (DBU).17 The crosslinked polymer underwent dissolution in CHCl3, yielding a homogeneous solution. GPC analysis of the resulting polymer solution revealed a profile consistent with that of the precursor PA-LA before crosslinking, showing successful decrosslinking (Fig. 6). The recovered PA-LA were crosslinked again by concentrating the solution or precipitating the polymer using Et2O. Similarly, the decrosslinking of crosslinked PA-LA/HA was achieved in the same manner (Fig. S12, ESI‡). These experiments suggest that the 1,2-dithiolane pendant group allowed facile manipulation of the crosslinked and non-crosslinked structure of the polymers, highlighting the recyclability of these polymers.
In conclusion, we have developed a synthesis of 1,2-dithiolane-functionalized polymer directly using naturally abundant LA as a raw material by Passerini polymerization. The resulting dynamically crosslinked polymer exhibited characteristics of adaptable elastomers, such as re-processability and recyclable adhesion. This work provides easy access to a variety of polymers with 1,2-dithiolane functionalities, which encompass not only dynamic crosslinking presented in this study but also inherent features of 1,2-dithiolane, such as antioxidant activity and applications in nanomaterials.
Y. N., Y.-S. H. and C.-F. H. designed and conceived the experiments; Y. N., Y.-S. H., and S. S. conducted the experiments.
This work was partially supported by the Japan Society for the Promotion of Science KAKENHI Grand No. 23K04845 (Y. N.). Y.-S. H. acknowledges the NIMS Internship Program for supporting his research at NIMS. The authors thank N. Yamamoto for the experimental support.
Conflicts of interest
There are no conflicts to declare.References
- G. P. Biewenga, G. R. Haenen and A. Bast, Gen. Pharmacol., 1997, 29, 315–331 CrossRef CAS PubMed.
- L. Packer, E. H. Witt and H. J. Tritschler, Free Radicals Biol. Med., 1995, 19, 227–250 CrossRef CAS PubMed.
- W. Wang, A. Kapur, X. Ji, M. Safi, G. Palui, V. Palomo, P. E. Dawson and H. Mattoussi, J. Am. Chem. Soc., 2015, 137, 5438–5451 CrossRef CAS PubMed.
- B. C. Mei, K. Susumu, I. L. Medintz, J. B. Delehanty, T. J. Mountziaris and H. Mattoussi, J. Mater. Chem., 2008, 18, 4949–4958 RSC.
- H. T. Uyeda, I. L. Medintz, J. K. Jaiswal, S. M. Simon and H. Mattoussi, J. Am. Chem. Soc., 2005, 127, 3870–3878 CrossRef CAS PubMed.
- Q. Zhang, Y. Deng, C.-Y. Shi, B. L. Feringa, H. Tian and D.-H. Qu, Matter, 2021, 4, 1352–1364 Search PubMed.
- C.-Y. Shi, Q. Zhang, B.-S. Wang, M. Chen and D.-H. Qu, ACS Appl. Mater. Interfaces, 2021, 13, 44860–44867 CrossRef CAS PubMed.
- S. Huang, Y. Shen, H. K. Bisoyi, Y. Tao, Z. Liu, M. Wang, H. Yang and Q. Li, J. Am. Chem. Soc., 2021, 143, 12543–12551 Search PubMed.
- X. Zhang and R. M. Waymouth, J. Am. Chem. Soc., 2017, 139, 3822–3833 CrossRef CAS PubMed.
- W. Yang, Y. Zou, F. Meng, J. Zhang, R. Cheng, C. Deng and Z. Zhong, Adv. Mater., 2016, 28, 8234–8239 CrossRef CAS PubMed.
- G. A. Barcan, X. Zhang and R. M. Waymouth, J. Am. Chem. Soc., 2015, 137, 5650–5653 Search PubMed.
- Q. Zhang, C.-Y. Shi, D.-H. Qu, Y.-T. Long, B. L. Feringa and H. Tian, Sci. Adv., 2018, 4, eaat8192 CrossRef CAS PubMed.
- Q. Zhang, Y.-X. Deng, H.-X. Luo, C.-Y. Shi, G. M. Geise, B. L. Feringa, H. Tian and D.-H. Qu, J. Am. Chem. Soc., 2019, 141, 12804–12814 Search PubMed.
- Y. Deng, Q. Zhang, B. L. Feringa, H. Tian and D.-H. Qu, Angew. Chem., Int. Ed., 2020, 59, 5278–5283 CrossRef CAS PubMed.
- C. Cai, S. Wu, Y. Zhang, F. Li, Z. Tan and S. Dong, Adv. Sci., 2022, 9, 2203630 CrossRef CAS PubMed.
- Y. Xu, B.-W. Zhu, R. Sun, X. Li, D. Wu and J.-N. Hu, ACS Appl. Mater. Interfaces, 2023, 15, 26298–26315 CrossRef CAS PubMed.
- B. Sieredzinska, Q. Zhang, K. J. van den Berg, J. Flapper and B. L. Feringa, Chem. Commun., 2021, 57, 9838–9841 Search PubMed.
- S. Maes, V. Scholiers and F. E. Du Prez, Macromol. Chem. Phys., 2022, 2100445 Search PubMed.
- K. Margulis, X. Zhang, L.-M. Joubert, K. Bruening, C. J. Tassone, R. N. Zare and R. M. Waymouth, Angew. Chem., Int. Ed., 2017, 56, 16357–16362 CrossRef CAS PubMed.
- S. M. Page, S. Parelkar, A. Gerasimenko, D. Y. Shin, S. R. Peyton and T. Emrick, J. Mater. Chem. B, 2014, 2, 620–624 RSC.
- M. Passerini and L. Simone, Gazz. Chim. Ital., 1921, 51, 126–129 Search PubMed.
- R. Ramozzi and K. Morokuma, J. Org. Chem., 2015, 80, 5652–5657 CrossRef CAS PubMed.
- O. Kreye, T. Toth and M. A. R. Meier, J. Am. Chem. Soc., 2011, 133, 1790–1792 CrossRef CAS PubMed.
- Y.-Z. Wang, X.-X. Deng, L. Li, Z.-L. Li, F.-S. Du and Z.-C. Li, Polym. Chem., 2013, 4, 444–448 RSC.
- L.-C. Chou, M. G. Mohamed, S.-W. Kuo, Y. Nakamura and C.-F. Huang, Chem. Commun., 2022, 58, 12317–12320 RSC.
- M. Li, X. Fu, J. Wang, A. Qin and B. Z. Tang, Macromol. Chem. Phys., 2023, 224, 2200352 Search PubMed.
- T. Suzuki, Y. Nambu and T. Endo, Macromolecules, 1990, 23, 1579–1582 Search PubMed.
- H. Tang and N. V. Tsarevsky, Polym. Chem., 2015, 6, 6936–6945 RSC.
Footnotes |
| † This paper is dedicated to Professor Atsuhiro Osuka on the occasion of his 70th birthday |
| ‡ Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc00751d |
| § These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |