Open Access Article
Open Access ArticleEnhanced photocatalytic hydrogen evolution through MoS2 quantum dots modification of bismuth-based perovskites†
Yunjian
Fan
a,
Jingmiao
Hu
bc,
Tianyang
Li
d,
Shuang
Xu
d,
Shan
Chen
 *a and
Huajie
Yin
*a and
Huajie
Yin
 *b
*b
aInstitutes of Physical Science and Information Technology, Anhui University, Hefei, 230039, China. E-mail: chenshan@ahu.edu.cn
bKey Laboratory of Materials Physics, Centre for Environmental and Energy nanomaterials, Anhui Key Laboratory of Nanomaterials and Nanotechnology, CAS Center for Excellence in Nanoscience, Institute of Solid State Physics, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei 230031, China, Hefei, 230031, China. E-mail: yinhj@issp.ac.cn
cUniversity of Science and Technology of China, Hefei, Anhui 230026, People's Republic of China
dSchool of Materials Science and Engineering, Anhui University, Hefei, 230039, China
First published on 22nd December 2023
Abstract
Efficient and cost-effective photocatalysts are pivotal for advancing large-scale solar hydrogen generation. Herein, we report a composite photocatalyst by incorporating MoS2 quantum dots (MoS2 QDs) as a cocatalyst into Cs3Bi2I9, resulting in a high enhancement in photocatalytic performance. Remarkably, the optimum MoS2 QDs/Cs3Bi2I9 composite achieves an impressive hydrogen evolution rate (6.09 mmol h−1 g−1) in an ethanol and HI/H3PO2 mixed solution. This rate is 8.8 times higher than pristine Cs3Bi2I9 (0.69 mmol h−1 g−1) and notably surpasses Pt/Cs3Bi2I9 (2.47 mmol h−1 g−1). Moreover, the composite displays exceptional stability during an 18-hour reaction, showcasing its potential for sustainable photocatalytic hydrogen evolution.
The urgent need for sustainable energy due to extensive fossil fuel use has sparked interest in harnessing solar energy as a renewable solution.1 Photocatalytic hydrogen evolution, a promising pathway for generating clean hydrogen from sunlight, faces challenges like efficiency, stability, and cost, underscoring the crucial importance of developing high-performance and cost-effective photocatalysts to drive innovation in this technology.2–5 Lead halide perovskites (LHPs) have gained significant attention as efficient photocatalysts in the past five years, exhibiting favorable visible light absorption, exceptionally-long charge-carrier diffusion length when employed for photocatalytic fuel production and chemical synthesis.6–11 However, the presence of toxic lead (Pb) in LHPs severely limits their potential for practical application. Therefore, researchers are exploring alternatives like Sn and Bi metals to replace Pb within halide perovskite structures. Despite this, the introduction of Sn2+ often leads to instability, while Bi3+ shows promise due to its similar isoelectric state to Pb2+ and success in stabilizing bismuth-based halide perovskites.12–16 However, the severe charge recombination due to the high exciton binding energy of Bi-based perovskite photocatalysts, results in their poor photocatalytic activity. For example, Zhao et al. reported the MA3Bi2I9 photocatalyst for photocatalytic hydrogen evolution reaction (HER) in the saturated HI solution, which initially exhibited low hydrogen generation activity (12.19 μmol h−1 g−1) and was subsequently improved (169.21 μmol h−1 g−1) with Pt as a cocatalyst.17 Nonetheless, its activity remains notably lower than that reported for LHP photocatalysts (1–20 mmol h−1 g−1).
Recently, several non-noble metal co-catalysts, including rGO,12 CoP,18 BP,9 Ni3C,10 and MoS2 nanosheet,19 have been introduced to the LHP photocatalysts and the resulting composite demonstrated an impressive photocatalytic performance improvement. It is widely acknowledged that their beneficial impact stems from the creation of various heterojunctions between lead perovskites and the co-catalyst, which effectively enhances the electron–hole separation efficiency and augments photocatalyst carrier transport rates.20–22 Notably, although MoS2 quantum dots (QDs) have proven to be a high-performance, sustainable, and cost-effective alternative to Pt for electrocatalytic and photocatalytic HER applications,23–25 their potential as a cocatalyst to enhance the photocatalytic HER activity of Bi-based perovskite photocatalysts remains unexplored.
Herein, we present a composite of Cs3Bi2I9 perovskite microcrystals modified with MoS2 QDs for efficient photocatalytic hydrogen generation via HI splitting in an HI/ethanol solution. The photocatalytic performance of the MoS2 QDs/Cs3Bi2I9 composite demonstrated an impressive rate of 6.09 mmol h−1 g−1 for hydrogen evolution, surpassing pristine Cs3Bi2I9 (0.69 mmol h−1 g−1) by 8.8 times and Pt/Cs3Bi2I9 (2.47 mmol h−1 g−1) by 2.5 times. Notably, this activity level represents a new record among Bi-based perovskite photocatalysts under visible-light conditions. This heightened activity is attributed to the superior HER active sites provided by MoS2 QDs as co- catalysts, combined with the unique crystal structure of Cs3Bi2I9, ensuring the creation of MoS2 QDs/Cs3Bi2I9 heterojunctions of a type II nature.
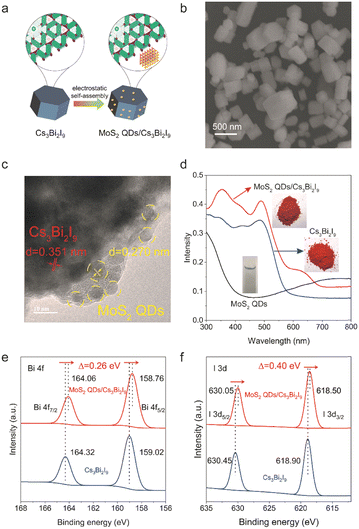
We employed a modified solvothermal approach to synthesize high-quality Cs3Bi2I9 microcrystals (method details in the supporting information).26 Additionally, MoS2 QDs were synthesized via a solvothermal treatment of bulk MoS2 in isopropanol, followed by a sequence of centrifugal separation.27 The MoS2 QDs/Cs3Bi2I9 heterostructure composites were obtained by ultrasonic mixing Cs3Bi2I9 microcrystals into the isopropanol dispersion of MoS2 QDs followed by solvent evaporation. The zeta potential of Cs3Bi2I9 microcrystals and MoS2 QDs is 10.57 and −9.39 mV, respectively (Fig. S1, ESI†). Thus, the negatively charged MoS2 QDs were electrostatic self-assembly onto the surface of the positively charged Cs3Bi2I9 after ultrasonication and solvent evaporation, as illustrated in Fig. 1a. The resulting composite contained MoS2 QDs with a mass percentage of 5.4 wt%. Pristine Cs3Bi2I9, as illustrated in Fig. S2 (ESI†), exhibited a regular hexagonal prism shape with sizes ranging from 500 nm to 1 μm. The XRD pattern (Fig. S3, ESI†) confirms its excellent crystallinity, with peak positions matching the standard perovskite structure of Cs3Bi2I9 (JCPDS#23-0847). TEM images of MoS2 QDs revealed their size to be between 2 nm and 5 nm (Fig. S4, ESI†), and XPS spectra illustrated the presence of both the 1T phase and 2H phase (Fig. S5, ESI†).28 After incorporating MoS2 QDs, the resulting MoS2 QDs/Cs3Bi2I9 composites maintained their hexagonal prism shape (Fig. 1b) and crystallinity. TEM and HRTEM images revealed tight attachment of MoS2 QDs (yellow circles) to the surface of Cs3Bi2I9 crystals (Fig. 1c and Fig. S6, ESI†).
In the inset image of Fig. 1d, Cs3Bi2I9 exhibited a vibrant red color, while the color of MoS2 QDs/Cs3Bi2I9 was slightly deepened. Their UV-vis diffuse reflectance spectra (UV-vis-DRS) further indicated extended light absorption in the range of 600–800 nm due to the presence of MoS2 QDs. In comparison to pristine Cs3Bi2I9, the measured band gap of MoS2 QDs/Cs3Bi2I9 was reduced to 1.81 eV from the Kubelka–Munk plot (Fig. S7, ESI†). The presence of MoS2 QDs in the Cs3Bi2I9/MoS2 QDs composite was further confirmed by XPS survey spectra, including the Bi 4f and I 3d regions (Fig. 1e, f and Fig. S8, ESI†). The high-resolution Bi 4f and I 3d peaks of MoS2 QDs/Cs3Bi2I9 shifted towards lower binding energies (0.26 eV for Bi 4f and 0.40 eV for I 3d), respectively, compared to pristine Cs3Bi2I9. These shifts could be attributed to the strong interfacial interaction between MoS2 QDs and Cs3Bi2I9, as reported in previous studies.20–22,29
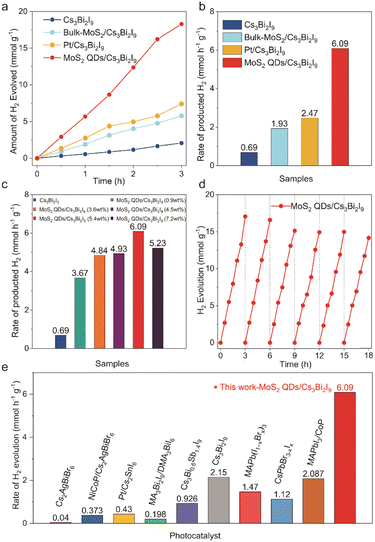
The photocatalytic hydrogen evolution performance of MoS2 QDs/Cs3Bi2I9 (5 mg) and the comparison samples was measured in a 20 mL ethanol solution containing 0.2 mL of HI and 0.2 mL of H3PO2 under visible light irradiation (λ > 420 nm, 300-W Xe lamp). H3PO2 is used as a sacrificial reagent in the reaction system to reduce I3− ions to form I-. The generated H2 increased linearly with irradiation time, reaching a peak hydrogen evolution rate of 6.09 mmol h−1 g−1 over a 3-hour reaction period. The apparent quantum efficiency (AQE) of MoS2 QDs/Cs3Bi2I9 for H2 evolution at 500 nm was determined to be 4.71%.30 When the MoS2 QDs/Cs3Bi2I9 composite was replaced with an equal amount of pristine Cs3Bi2I9 while maintaining other experimental conditions, the average achieved H2 evolution rate dramatically dropped to 0.69 mmol h−1 g−1. This observation indicates that the enhanced photocatalytic activity of MoS2 QDs/Cs3Bi2I9 originates from the incorporation of MoS2 QDs. Furthermore, Pt-loaded Cs3Bi2I9 (Pt/Cs3Bi2I9) and bulk MoS2-modified Cs3Bi2I9 exhibited H2 evolution rates of 2.47 (Fig. S9, ESI†) and 1.93 mmol h−1 g−1, respectively, which were notably lower than that of MoS2 QDs/Cs3Bi2I9, highlighting the superior cocatalytic performance of MoS2 QDs over Pt and bulk MoS2. Consequently, the photocatalytic HER activity of MoS2 QDs/Cs3Bi2I9 is over 8.8 and 2.4 times higher than that of Cs3Bi2I9 and Pt/Cs3Bi2I9, respectively. As illustrated in Fig. 2c, the effect of different mass percentages of MoS2 QDs in the MoS2 QDs/Cs3Bi2I9 composite was investigated. Notably, the incorporation of just 0.9 wt% MoS2 QDs into Cs3Bi2I9 led to a significant increase in the H2 evolution rate to 3.67 mmol h−1 g−1. With higher MoS2 QDs loading, the H2 evolution rate progressively increased, peaking at 6.09 mmol h−1 g−1 for the MoS2 QDs/Cs3Bi2I9 composite with 5.4 wt% MoS2 QDs. However, at 7.2 wt% MoS2 QDs, the photocatalytic HER activity showed a decline.
In addition to its impressive photocatalytic hydrogen evolution activity, the MoS2 QDs/Cs3Bi2I9 composite also exhibited remarkable stability (Fig. 2d). Throughout six cycles of 3-hour experiments, the rate of photocatalytic hydrogen evolution remained nearly constant. XRD patterns displayed no alterations in the crystalline phase of the MoS2 QDs/Cs3Bi2I9 composite after an 18-hour reaction, and SEM analysis confirmed the preservation of its morphology (Fig. S10, ESI†). Furthermore, the MoS2 QDs/Cs3Bi2I9 composite outperformed recently reported Bi-based perovskite photocatalysts and some of Pb-based perovskite photocatalysts in terms of photocatalytic hydrogen evolution (Fig. 2e and Table. S1, ESI†).7,13,23–25,29,31,32
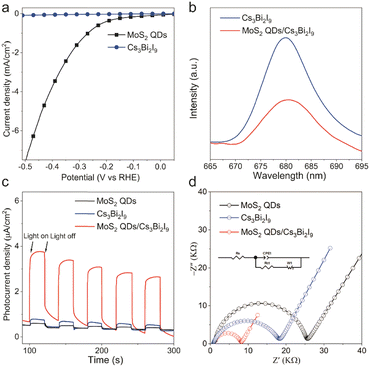
Both electrochemical and photoelectrochemical experiments were employed to elucidate the significant effect of MoS2 QDs in enhancing the photocatalytic hydrogen evolution activity of Cs3Bi2I9. From Fig. 3a, it can be found that the electrocatalytic HER onset potential of MoS2 QDs could reach ∼−0.2 V vs. RHE indicating a superior HER activity of MoS2 QDs compared with pristine Cs3Bi2I9. Moreover, the photoluminescence (PL) emission intensity of MoS2 QDs/Cs3Bi2I9 exhibited a notable reduction compared to that of pristine Cs3Bi2I9 (Fig. 3b). Time-resolved photoluminescence (TRPL) results show that the average lifetime for MoS2 QDs/Cs3Bi2I9 is greater than Cs3Bi2I9 (Fig. S11, ESI†). This suggests that the association with MoS2 QDs significantly enhances the separation efficiency of electrons and holes within Cs3Bi2I9, thereby substantially boosting the photocatalytic HER efficacy of MoS2 QDs/Cs3Bi2I9. Notably, the MoS2 QDs/Cs3Bi2I9 composite demonstrated the highest photocurrent, directly correlating with its most favorable catalytic H2 evolution activity (Fig. 3c). Furthermore, for a deeper understanding of the improved HER activity of the photocatalyst, we employed electrochemical impedance spectroscopy (EIS) to characterize and analyze the opto-electronic performance of the photocatalysts in terms of the separation and transfer of photogenerated charges. EIS analysis revealed that the MoS2 QDs/Cs3Bi2I9 composite displayed significantly lower charge transfer resistance compared to pristine Cs3Bi2I9 (Fig. 3d).
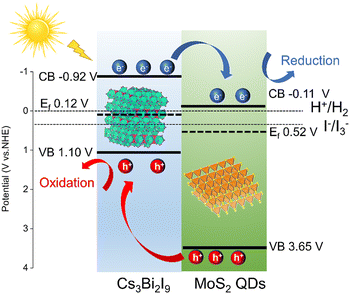
Based on the ultraviolet photoelectron spectroscopy (UPS), we obtained EVB and ECB values of 1.1 V (NHE), -0.92 V (NHE) and 3.54 V (NHE), −0.11 V (NHE) for Cs3Bi2I9 and MoS2 QDs, respectively (Fig. S12 and S13, ESI†). The energy diagram of the MoS2 QDs/Cs3Bi2I9-based hydrogen generation system through HI splitting is illustrated in Fig. 4.10,18,19,33 According to the UPS calculation method, we obtained the Fermi levels (Ef) values of 0.12 V for Cs3Bi2I9 and 0.52 V for MoS2 QDs. A type II heterojunction forms at the interface of MoS2 QDs and Cs3Bi2I9, leading to efficient migration of photogenerated electrons to MoS2 QDs, thus facilitating the effective separation of photogenerated carriers. Simultaneously, these photogenerated electrons on the surface of MoS2 QDs diffuse toward active sites where protons undergo efficient reduction to generate H2. These heterojunctions play a pivotal role in promoting the separation of charge carriers, thereby contributing significantly to the remarkable enhancement in activity.
In summary, the incorporation of MoS2 QDs as cocatalysts has been demonstrated to significantly enhance the photocatalytic performance of Cs3Bi2I9 for hydrogen evolution. The optimized MoS2 QDs/Cs3Bi2I9 composite showcases an impressive 8.8-fold increase in the rate of H2 evolution compared to the unmodified Cs3Bi2I9. This study underscores the potential of harnessing zero-dimensional MoS2 QDs as remarkably efficient cocatalysts, to enhance the efficacy of the photocatalytic HER in Bi-based perovskite photocatalysts. Furthermore, the insights gleaned from this research pave the way for the development of exceptionally active photocatalysts based on lead-free perovskites.
This work was financially supported by the National Natural Science Foundation of China (52102197, 52102325), Excellent Research and Innovation Team Project of Anhui Province (2023AH010001) and Collaborative Innovation Program of Hefei Science Center, CAS (2022HSC-CIP016).
Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Sivula and R. Krol, Nat. Rev. Mater., 2016, 1, 15010 CrossRef CAS.
- Q. Wang and K. Domen, Chem. Rev., 2020, 120, 919–985 CrossRef CAS.
- M.-Y. Qi, M. Conte, M. Anpo, Z.-R. Tang and Y.-J. Xu, Chem. Rev., 2021, 121, 13051–13085 CrossRef CAS PubMed.
- S. Chen, H. Yin, P. Liu, Y. Wang and H. Zhao, Adv. Mater., 2023, 35, 2203836 CrossRef CAS.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed.
- S. Park, W. J. Chang, C. W. Lee, S. Park, H.-Y. Ahn and K. T. Nam, Nat. Energy, 2016, 2, 16185 CrossRef.
- Z. Guan, Y. Wu, P. Wang, Q. Zhang, Z. Wang, Z. Zheng, Y. Liu, Y. Dai, M.-H. Whangbo and B. Huang, Appl. Catal., B, 2019, 245, 522–527 CrossRef CAS.
- S. Bera, S. Ghosh, T. Maiyalagan and R. N. Basu, ACS Appl. Energy Mater., 2022, 5, 3821–3833 CrossRef CAS.
- X. Deng, X. Kuang, J. Zeng, B. Zi, Y. Ma, R. Yan, J. Zhang, B. Xiao and Q. Liu, Nanotechnology, 2022, 33, 175401 CrossRef.
- Z. Zhao, J. Wu, Y.-Z. Zheng, N. Li, X. Li and X. Tao, ACS Catal., 2019, 9, 8144–8152 CrossRef CAS.
- Y. Wu, P. Wang, X. Zhu, Q. Zhang, Z. Wang, Y. Liu, G. Zou, Y. Dai, M.-H. Whangbo and B. Huang, Adv. Mater., 2018, 30, 1704342 CrossRef PubMed.
- T. Wang, D. Yue, X. Li and Y. Zhao, Appl. Catal., B, 2020, 268, 118399 CrossRef CAS.
- P. Zhou, H. Chen, Y. Chao, Q. Zhang, W. Zhang, F. Lv, L. Gu, Q. Zhao, N. Wang, J. Wang and S. Guo, Nat. Commun., 2021, 12, 4412 CrossRef CAS PubMed.
- D. Wu, B. Huo, Y. Huang, X. Zhao, J. Yang, K. Hu, X. Mao, P. He, Q. Huang and X. Tang, Small, 2022, 18, e2106001 CrossRef PubMed.
- B.-M. Bresolin, P. Sgarbossa, D. W. Bahnemann and M. Sillanpää, Sep. Purif. Technol., 2020, 251, 117320 CrossRef CAS.
- Z. Zhang, Y. Yang, Y. Wang, L. Yang, Q. Li, L. Chen and D. Xu, Angew. Chem., Int. Ed., 2020, 132, 18293–18296 CrossRef.
- Y. Guo, G. Liu, Z. Li, Y. Lou, J. Chen and Y. Zhao, ACS Sustainable Chem. Eng., 2019, 7, 15080–15085 CrossRef CAS.
- C. Cai, Y. Teng, J.-H. Wu, J.-Y. Li, H.-Y. Chen, J.-H. Chen and D.-B. Kuang, Adv. Funct. Mater., 2020, 30, 2001478 CrossRef CAS.
- X. Zhao, S. Chen, H. Yin, S. Jiang, K. Zhao, J. Kang, P. F. Liu, L. Jiang, Z. Zhu, D. Cui, P. Liu, X. Han, H. G. Yang and H. Zhao, Matter, 2020, 3, 935–949 CrossRef.
- Y. Jiang, J.-F. Liao, H.-Y. Chen, H.-H. Zhang, J.-Y. Li, X.-D. Wang and D.-B. Kuang, Chemistry, 2020, 6, 766–780 CrossRef CAS.
- J. Ran, H. Zhang, S. Fu, M. Jaroniec, J. Shan, B. Xia, Y. Qu, J. Qu, S. Chen, L. Song, J. M. Cairney, L. Jing and S. Z. Qiao, Nat. Commun., 2022, 13, 4600 CrossRef CAS PubMed.
- B. Xia, B. He, J. Zhang, L. Li, Y. Zhang, J. Yu, J. Ran and S. Z. Qiao, Adv. Energy Mater., 2022, 12, 2201449 CrossRef CAS.
- Y. Ji, M. She, X. Bai, E. Liu, W. Xue, Z. Zhang, K. Wan, P. Liu, S. Zhang and J. Li, Adv. Funct. Mater., 2022, 32, 2201721 CrossRef CAS.
- Y. Tang, C. H. Mak, R. Liu, Z. Wang, L. Ji, H. Song, C. Tan, F. Barrière and H.-Y. Hsu, Adv. Funct. Mater., 2020, 30, 2006919 CrossRef CAS.
- Z. Zhao, J. Wu, Y.-Z. Zheng, N. Li, X. Li, Z. Ye, S. Lu, X. Tao and C. Chen, Appl. Catal., B, 2019, 253, 41–48 CrossRef CAS.
- M. Li, S. Xu, L. Wu, H. Tang, B. Zhou, J. Xu, Q. Yang, T. Zhou, Y. Qiu, G. Chen, G. I. N. Waterhouse and K. Yan, ACS Energy Lett., 2022, 7, 3370–3377 CrossRef CAS.
- L. Najafi, B. Taheri, B. Martín-García, S. Bellani, D. Di Girolamo, A. Agresti, R. Oropesa-Nuñez, S. Pescetelli, L. Vesce, E. Calabrò, M. Prato, A. E. Del Rio Castillo, A. Di Carlo and F. Bonaccorso, ACS Nano, 2018, 12, 10736–10754 CrossRef CAS PubMed.
- Z. Zhao, J. Wu, Y.-Z. Zheng, N. Li, X. Li, Z. Ye, S. Lu, X. Tao and C. Chen, Appl. Catal., B, 2019, 253, 41–48 CrossRef CAS.
- G. Chen, P. Wang, Y. Wu, Q. Zhang, Q. Wu, Z. Wang, Z. Zheng, Y. Liu, Y. Dai and B. Huang, Adv. Mater., 2020, 32, e2001344 CrossRef PubMed.
- J. Yang, A. Acharjya, M.-Y. Ye, J. Rabeah, S. Li, Z. Kochovski, S. Youk, J. Roeser, J. Grüneberg, C. Penschke, M. Schwarze, T. Wang, Y. Lu, R. van de Krol, M. Oschatz, R. Schomäcker, P. Saalfrank and A. Thomas, Angew. Chem., Int. Ed., 2021, 60, 19797–19803 CrossRef CAS PubMed.
- Q. Huang, Y. M. Guo, J. X. Chen, Y. B. Lou and Y. X. Zhao, New J. Chem., 2022, 46, 7395–7402 RSC.
- Y. Zhang, Z. Sun, Z. Wang, Y. Zang and X. Tao, Int. J. Hydrogen Energy, 2022, 47, 8829–8840 CrossRef CAS.
- S. A. Shah, I. Khan and A. Yuan, Molecules, 2022, 27, 3289 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc05781j |
| This journal is © The Royal Society of Chemistry 2024 |