Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermodynamic analysis of gate-opening carbon dioxide adsorption behavior of metal–organic frameworks†
Shino
Kannaka
,
Ayumi
Ohmiya
,
Chiho
Ozaki
and
Masataka
Ohtani
 *
*
School of Engineering Science, Kochi University of Technology, 185 Miyanokuchi, Tosayamada, Kami, Kochi 782-8502, Japan. E-mail: ohtani.masataka@kochi-tech.ac.jp
First published on 18th March 2024
Abstract
Thermodynamic analysis of gate-opening carbon dioxide (CO2) adsorption behavior of metal–organic frameworks (MOFs) was investigated using differential scanning calorimetry (DSC). Unlike measurements under nitrogen atmosphere, obvious exothermic and endothermic peaks were observed in DSC curves under CO2 flow. In situ heating X-ray diffraction and thermogravimetric analyses under CO2 revealed that reversible crystal structure and weight changes occurred upon CO2 adsorption/desorption. The thermodynamic parameters of the CO2 adsorption process by MOFs were determined by DSC analysis at various CO2 partial pressures.
Metal–organic frameworks (MOFs), which consist of alternating coordination bonds of metal ions and organic ligands, can be designed with pore structures. MOFs with pore sizes and shapes that are appropriate for different applications can be produced by changing the combination of metal ions and organic ligands.1,2 However, the robust structure of most MOFs makes it difficult to control the movement of gas molecules in the pores during gas adsorption and separate mixed gases efficiently. Fortunately, some MOFs are known to change their structure in response to certain stimuli. For example, flexible MOFs with crystalline structures that open up at a certain atmospheric pressure and abruptly adsorb gas molecules have been reported.3–6 As the pressure decreases, the MOF structures revert to their closed forms, releasing the gas molecules. This gas adsorption behavior, driven by crystal structure changes, is called gate-opening adsorption. The gas adsorption/desorption isotherms of gate-opening MOFs exhibit stepwise adsorption/desorption curves, which cannot be classified by the IUPAC classification for common porous materials.7 Such behavior can be used for the selective separation of mixed gases because different gas molecules have different threshold pressures for the gate opening of the MOF structure.
Zeolitic imidazole framework-7 (ZIF-7) is a well-known MOF that shows structural changes upon adsorption and desorption of carbon dioxide (CO2).8–10 ZIF-7 is composed of zinc(II) ions (Zn2+) and benzimidazole (BIm) and has three known crystal polymorphs (phases I, II, and III). These polymorphs have different coordination network structures because of their different bond angles of BIm with Zn2+. Phases I, II, and III of ZIF-7 have rhombohedral, triclinic, and monoclinic crystal systems, respectively. The structure of ZIF-7 changes to phase II upon desorption of N,N-dimethylformamide (DMF) from the pores of the phase-I polymorph. Phase I is recovered from phase II by the adsorption of DMF in the closed pores of phase II. These reversible conformational changes of ZIF-7 between phases I and II can also be induced by CO2 adsorption/desorption.11,12
In CO2 adsorption measurements, ZIF-7 shows a staircase adsorption isotherm as the CO2 gas pressure changes, which is characteristic of gate-opening MOFs.13–16 The structural change of ZIF-7 induced by CO2 adsorption has been examined by in situ heating powder X-ray diffraction (XRD).16,17 The CO2 adsorption process of ZIF-7 has also been measured under various pressures using synchrotron powder XRD.18 Kamili et al.19 traced structural changes of ZIF-7 with temperature and pressure using Raman spectroscopy. Molecular dynamics simulations have predicted how CO2 moves through adsorption sites of ZIF-7.17,18 In addition, neutron powder diffraction has been used to identify preferential sites of ZIF-7 for CO2 adsorption.18,20 Density functional theory (DFT) was used to estimate the thermodynamic energy change required for CO2 adsorption at each location.18
Although the above studies have investigated the relationship between CO2 adsorption and changes in ZIF-7 crystal structure, this relationship still has many unknown parameters. In particular, the interplay between the CO2 adsorption process, changes in ZIF-7 structure, and adsorption energy remains unclear. Further detailed experimental investigation of the CO2 adsorption process by ZIF-7 under atmospheric pressure should be conducted. In this study, differential scanning calorimetry (DSC) is performed under CO2 atmosphere to evaluate the calorimetric changes accompanying the gate-opening structural changes of ZIF-7 during CO2 adsorption/desorption. The behavior of ZIF-7 during CO2 adsorption/desorption under various conditions is analyzed by DSC to thermodynamically evaluate the adsorption mechanism. Changes in adsorption/desorption behavior associated with structural changes are also followed by in situ heating powder XRD and thermogravimetric analysis (TGA) measurements. Furthermore, DSC analysis under various CO2 partial pressures (p(CO2)) is used to evaluate the thermodynamic parameters of CO2 adsorption for ZIF-7.
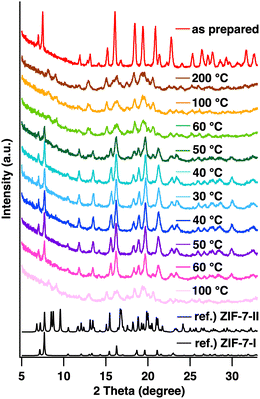
First, the synthetic conditions for ZIF-7 were thoroughly investigated by modifying the literature methods (see the ESI† for details).12Fig. 1a shows powder XRD patterns of the ZIF-7 product (denoted hereafter as ZIF-7-I, where I indicates phase I). The observed diffraction peaks were matched to reference peaks, confirming that highly crystalline ZIF-7-I was obtained. Next, CO2 adsorption measurements were performed to determine the CO2 adsorption capacity of ZIF-7-I. Prior to the adsorption measurements, DMF in the pores of ZIF-7-I was removed by heating at 200 °C for 20 h. TGA results confirmed the removal of DMF from ZIF-7-I (Fig. S1, ESI†). CO2 adsorption/desorption measurements were performed at 27 °C. The obtained ZIF-7-I showed CO2 adsorption/desorption isotherms consistent with gate-opening behavior (Fig. 1b), which rose sharply at a certain pressure. The maximum CO2 uptake of ZIF-7-I was 2.5 mmol g−1, which was almost identical to that reported in the literature.14
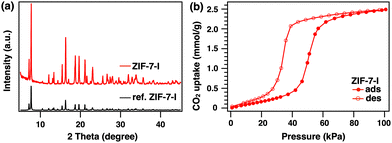 | ||
| Fig. 1 (a) XRD pattern of as-prepared ZIF-7-I (red) and simulated reference pattern of ZIF-7-I (black, CCDC 602541). (b) CO2 adsorption/desorption isotherms of ZIF-7-I at 27 °C. | ||
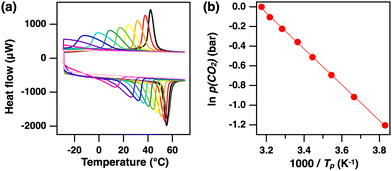
To observe the calorimetric change in ZIF-7 caused by CO2 adsorption, DSC measurements were performed under CO2 flow (Fig. 2a). Before the measurement, the temperature was increased from 25 to 200 °C at 20 K min−1 under a nitrogen (N2) flow rate of 50 mL min−1 to desorb DMF remaining in the pores. The temperature was maintained at 200 °C for 10 min. The temperature was then cooled from 200 to −30 °C at 2 K min−1 and subsequently heated from −30 to 200 °C under N2 flow. No characteristic endothermic or exothermic peaks were observed under N2 atmosphere (Fig. 2a, blue). After the same pretreatment as for the N2 measurements, the gas was switched to CO2 and the same DSC cooling/heating cycle was conducted. Under CO2 atmosphere, an exothermic peak appeared around 45 °C during the cooling cycle (Fig. 2a, red). In the heating cycle, an endothermic peak was observed around 60 °C. These peaks were ascribed to calorimetric changes originating from the structural changes of ZIF-7-I associated with CO2 adsorption and desorption. Next, TGA measurements were performed under similar conditions (Fig. 2b). No weight change was observed under N2 atmosphere (Fig. 2b, blue). Under CO2 atmosphere, weight changes attributed to CO2 adsorption and desorption were observed during the cooling and heating processes, respectively. These weight changes occurred at temperatures similar to those of the exo- and endothermic peaks in DSC measurements (Fig. 2b, red). These results indicate that the observed calorimetric changes in DSC measurements were caused by the adsorption/desorption of CO2 on ZIF-7-I.
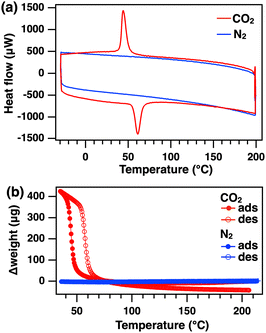 | ||
| Fig. 2 (a) DSC curves of ZIF-7-I heated at 2 K min−1 under CO2 (red) and N2 (blue). (b) Thermogravimetric analysis of ZIF-7-I under CO2 (red) and N2 (blue). | ||
The calorimetric changes of ZIF-7-I in CO2 atmosphere were observed repeatedly in DSC measurements (five cycles, Fig. S2, ESI†). The peaks shifted to lower temperature and peak area increased with rising cycle number. Similar behavior was also observed in the TGA of ZIF-7-I in CO2 (Fig. S3, ESI†). The first cycle showed a large difference between the weight before CO2 adsorption and that after CO2 desorption. As the number of cycles increased, this weight difference became smaller. In the first cycle, DMF and other gaseous substances remained in the pores of ZIF-7-I. The slight peak shifts observed in DSC curve and the increasing weight change in TGA curve during cycling are attributed to the gradual desorption of residual solvent and gas molecules. Judging from the negligible weight change upon completion of the fourth and fifth desorption cycles, the repeated weight change, which was accompanied by CO2 adsorption, was almost complete by the fifth cycle.
The exothermic peak area of the adsorption process was calculated from DSC analysis to be −1.78 × 10−4 kJ. Based on the maximum adsorption amount in CO2 adsorption/desorption measurements (Fig. 1b) and the isosteric heat of CO2 adsorption of a typical MOF (25 kJ mol−1), the theoretical adsorption heat was estimated to be −2.69 × 10−4 kJ. Judging from the difference between the observed exothermic heat in the DSC peak area and the predicted exothermic heat, the reaction heat was estimated to be +0.91 × 10−4 kJ. Since ZIF-7 undergoes a significant structural change from close form to open form upon CO2 adsorption, the origin of the estimated endothermic heat can be attributed to the reaction heat of the structural change. This indicates that the calorimetric change observed in DSC curve includes both the isosteric heat of CO2 adsorption and the reaction heat due to the structural change of ZIF-7.
In situ heating powder XRD measurements were performed to confirm the structural changes observed in DSC measurements (Fig. 3 and Fig. S4, ESI†). When heated to 200 °C under N2 flow, DMF in the pores of ZIF-7-I desorbed and its structure changed to ZIF-7-II. Then, the flow gas was switched to CO2 and the sample was cooled to 30 °C. The diffraction pattern of ZIF-7-II changed to that of ZIF-7-I at 50 °C, which is consistent with the temperature of the exothermic peak observed in DSC measurements under CO2. When the sample was heated to 100 °C in the second heating cycle, CO2 was completely desorbed, and the diffraction pattern indicated that ZIF-7-I converted to ZIF-7-II again. The same measurements were performed under N2 atmosphere (Fig. S4, ESI†). After the same pretreatment and cooling/heating cycle, no obvious change in the diffraction pattern of ZIF-7-II was observed, indicating that no structural changes occur under N2 atmosphere. Therefore, the in situ heating powder XRD patterns revealed that the calorimetric and weight changes observed in DSC and TGA curves were caused by a gate-opening CO2 adsorption/desorption process occurring in the crystalline ZIF-7 structure.
Because CO2 adsorption on ZIF-7 is an equilibrium reaction, we attempted to determine the thermodynamic parameters of this process by changing the CO2 concentration. For this purpose, DSC measurements were performed with different CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 ratios (Fig. 4a). As a pretreatment, a series of five cooling/heating cycles was performed under CO2. From the sixth cycle, the N2
N2 ratios (Fig. 4a). As a pretreatment, a series of five cooling/heating cycles was performed under CO2. From the sixth cycle, the N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 ratio was changed for each cycle to decrease p(CO2). The exothermic peak caused by CO2 adsorption shifted to the lower temperature side as p(CO2) decreased. Similarly, the endothermic peak caused by CO2 desorption also shifted to a lower temperature as p(CO2) decreased. Since CO2 adsorption on ZIF-7 is less likely to occur at low CO2 partial pressures, the adsorption equilibrium temperature is expected to change to a lower temperature. This suggests that changes in p(CO2) directly influence the chemical equilibrium process of gate-opening CO2 adsorption by ZIF-7.
CO2 ratio was changed for each cycle to decrease p(CO2). The exothermic peak caused by CO2 adsorption shifted to the lower temperature side as p(CO2) decreased. Similarly, the endothermic peak caused by CO2 desorption also shifted to a lower temperature as p(CO2) decreased. Since CO2 adsorption on ZIF-7 is less likely to occur at low CO2 partial pressures, the adsorption equilibrium temperature is expected to change to a lower temperature. This suggests that changes in p(CO2) directly influence the chemical equilibrium process of gate-opening CO2 adsorption by ZIF-7.
Recently, Takiishi and Fujishiro21 reported the thermodynamic analysis of CO2 adsorption by cubic perovskite-type BaFe1−xInxO3−δ. They determined the standard reaction enthalpy and standard reaction entropy from TGA measurements at various p(CO2), and estimated the standard reaction Gibbs energy change (ΔrG°) using the obtained parameters. Takamizawa et al.22,23 investigated the DSC behavior of one-dimensional coordination polymer crystals under CO2 at low temperatures (0 to −35 °C). According to these reports, ΔrG° of CO2 adsorption by ZIF-7-I was estimated from the exothermic peak temperature (Tp) in a series of DSC cooling curves at various p(CO2). Fig. 4b presents a plot of ln p(CO2) against 1000/Tp, which has a good linear correlation. The slope of the regression line of this plot gave ΔrG° = −15.1 kJ mol−1. This experimentally determined value was consistent with the theoretical value obtained from DFT calculations in a previous study.17 Such thermodynamic parameters are clearly associated with the structural changes of ZIF-7 upon CO2 adsorption/desorption.
The calorimetric changes in DSC at various CO2 partial pressures (1.0–0.5 bar) are summarized in Table S1 (ESI†). In the case of lower CO2 partial pressure, the observed calorimetric changes were slightly greater than that observed at 1.0 bar. As discussed above, since the calorimetric changes in the DSC curve are considered to include the exothermic heat of CO2 adsorption and the endothermic heat of structural change, it is expected that there is a slight difference in the degree of structural change associated with partial CO2 adsorption on the crystal surface or in the pore at lower CO2 partial pressures.
We then measured the DSC behavior of another MOF to examine its ability to adsorb CO2. Here, we focused on ZIF-9, which is a ZIF-7 analog with a similar pore structure (pore diameter (phase II, closed state), ZIF-7: 0.29 nm, ZIF-9: 0.32 nm; pore diameter (phase I, open state), ZIF-7 and ZIF-9: 0.37–0.42 nm)13–17 that contains cobalt(II) ions rather than Zn2+ and also exhibits gate-opening CO2 adsorption. ZIF-9 was synthesized in a similar manner to ZIF-7 (see the ESI† for details). Powder XRD measurements were performed, and the results were consistent with the formation of ZIF-9 (Fig. S5, ESI†). Fig. 5a shows a CO2 adsorption/desorption isotherm of ZIF-9 along with one of ZIF-7 for comparison. The total CO2 uptake for ZIF-9 was slightly lower than that for ZIF-7. The gate-opening CO2 adsorption process of ZIF-9 was observed at a higher pressure than that of ZIF-7, suggesting that ZIF-9 has a lower CO2 adsorption ability than that of ZIF-7. DSC measurements were performed for ZIF-9 under the same conditions as for ZIF-7 (Fig. 5b). Exothermic/endothermic peaks ascribed to CO2 adsorption/desorption were observed for ZIF-9. However, the exothermic peak appeared at a lower temperature than that of ZIF-7, indicating that the CO2 adsorption ability was lower for ZIF-9 than ZIF-7. Additionally, the DSC peak area for ZIF-9 (−0.71 × 10−4 kJ) was smaller than that for ZIF-7 (−1.78 × 10−4 kJ). This is consistent with the different CO2 adsorption capacities of the two MOFs identified in their adsorption isotherms. Furthermore, since the estimated exothermic heat of CO2 adsorption for ZIF-9 based on the adsorption isotherm is −2.00 × 10−4 kJ, the endothermic reaction heat due to the structural change is calculated to be +1.29 × 10−4 kJ, which value is higher than that of ZIF-7. This indicates the intrinsic energy change in the CO2-induced structural transition of ZIF-9 is greater than that of ZIF-7. These results reveal that DSC under CO2 is a useful thermodynamic analysis method for exploring the CO2 adsorption process of MOFs and can be used to quantitatively evaluate various CO2 adsorption materials.
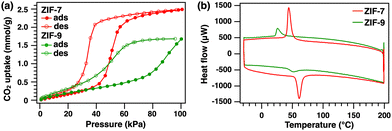 | ||
| Fig. 5 (a) CO2 adsorption isotherms of ZIF-7 (red) and ZIF-9 (green). (b) DSC curves of ZIF-7 (red) and ZIF-9 (green) heated at 2 K min−1 under CO2. | ||
In conclusion, DSC analysis of ZIF-7 under CO2 atmosphere was conducted to evaluate its gate-opening CO2 adsorption behavior. Calorimetric changes associated with the gate-opening structural changes of ZIF-7 occurred only under CO2. In situ heating powder XRD and TGA of ZIF-7 under CO2 atmosphere revealed that reversible weight and structural changes occurred during CO2 adsorption/desorption. The thermodynamic parameters of the structural changes of ZIF-7 associated with CO2 adsorption and desorption were calculated by analyzing the exothermic peak in DSC curves. The evaluation of gas adsorption capacity by DSC presented in this study for ZIF-7 and ZIF-9 is also applicable to other MOFs. The adsorption capacities of different types of MOFs can be compared based on peak areas and temperatures in DSC curves under CO2 atmosphere. This means DSC is a useful thermodynamic analysis method for evaluating the gas adsorption/desorption mechanisms of porous materials that undergo related structural changes. We are currently exploring the scope of DSC analysis with other gate-opening MOFs to better understand the thermodynamic parameters of the structural changes in MOFs that exhibit gate-opening gas adsorption behavior.
We acknowledge the financial support from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Numbers 19K05186 and 22K04857). We thank Natasha Lundin, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- S. Kitagawa and R. Matsuda, Coord. Chem. Rev., 2007, 251, 2490–2509 CrossRef CAS.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- L. Chen, J. P. S. Mowat, D. Fairen-Jimenez, C. A. Morrison, S. P. Thompson, P. A. Wright and T. Duren, J. Am. Chem. Soc., 2013, 135, 15763–15773 CrossRef CAS PubMed.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- N. Yanai, K. Kitayama, Y. Hijikata, H. Sato, R. Matsuda, Y. Kubota, M. Takata, M. Mizuno, T. Uemura and S. Kitagawa, Nat. Mater., 2011, 10, 787–793 CrossRef CAS PubMed.
- S. Hiraide, Y. Sakanaka, Y. lida, H. Arima, M. T. Miyahara and S. Watanabe, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, 31 CrossRef PubMed.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe–Romo, H. K. Chae, M. O’Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O’Keeffe and O. M. Yaghi, Science, 2008, 319, 939–943 CrossRef CAS PubMed.
- M. R. Ryder, B. Civalleri, T. D. Bennett, S. Henke, S. Rudic, G. Cinque, F. Fernandez–Alonso and J.-C. Tan, Phys. Rev. Lett., 2014, 113, 215502 CrossRef PubMed.
- C. Gucuyener, J. V. D. Bergh, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2010, 132, 17704–17706 CrossRef PubMed.
- P. Zhao, G. I. Lampronti, G. O. Lloyd, M. T. Wharmby, S. Facq, A. K. Cheetham and S. A. T. Redfern, Chem. Mater., 2014, 26, 1767–1769 CrossRef CAS PubMed.
- X. Wu, M. N. Shahrak, B. Yuan and S. Deng, Microporous Mesoporous Mater., 2014, 190, 189–196 CrossRef CAS.
- S. Aguado, G. Bergeret, M. P. Titus, V. Moizan, C. Nieto-Draghi, N. Bats and D. Farrusseng, New J. Chem., 2011, 35, 546–550 RSC.
- C. M. McGuirk, T. Runcevski, J. Oktawiec, A. Turkiewicz, M. K. Taylor and J. R. Long, J. Am. Chem. Soc., 2018, 140, 15924–15933 CrossRef CAS PubMed.
- W. Cai, T. Lee, M. Lee, W. Cho, D.-Y. Han, N. Choi, A. C. K. Yip and J. Choi, J. Am. Chem. Soc., 2014, 136, 7961–7971 CrossRef CAS PubMed.
- Y. Du, B. Wooler, M. Nines, P. Kortunov, C. S. Paur, J. Zengel, S. C. Weston and P. I. Ravikovitch, J. Am. Chem. Soc., 2015, 137, 13603–13611 CrossRef CAS PubMed.
- P. Zhao, H. Fang, S. Mukhopadhyay, A. Li, S. Rudic, I. J. McPherson, C. C. Tang, D. Fairen-Jimenez, S. C. E. Tsang and S. A. T. Redfern, Nat. Commun., 2019, 10, 999 CrossRef CAS PubMed.
- K. Kamali, S. Prasad, M. K. Sahoo, J. N. Behera, U. V. Waghmare and C. Narayama, Inorg. Chem., 2022, 61, 11571–11580 CrossRef CAS PubMed.
- P. Zhao, G. I. Lampronti, G. O. Lloyd, E. Suard and S. A. T. Redfern, J. Mater. Chem. A, 2014, 2, 620–623 RSC.
- K. Takiishi and F. Fujishiro, J. Phys. Chem. C, 2022, 126, 20395–20400 CrossRef CAS.
- S. Takamizawa, E. Nakata, H. Yokoyama, K. Mochizuki and W. Mori, Angew. Chem., Int. Ed., 2003, 42, 4331–4334 CrossRef CAS PubMed.
- S. Takamizawa, E. Nakata, T. Akatsuka, R. Miyaki, Y. Kakizaki, H. Takeuchi, G. Maruta and S. Takeda, J. Am. Chem. Soc., 2010, 132, 3783–3792 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc05700c |
| This journal is © The Royal Society of Chemistry 2024 |