Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Iridium-catalysed hydroamination of internal homoallylic amines†
An T.
Ho
a,
Evan P.
Vanable
b,
Chelsea San
Miguel
a and
Kami L.
Hull
 *ab
*ab
aDepartment of Chemistry, University of Texas at Austin, 100 East 24th Street, Austin, TX 78741, USA. E-mail: kamihull@utexas.edu
bDepartment of Chemistry, University of Illinois at Urbana-Champaign, 600 South Mathews Avenue, Urbana, Illinois 61801, USA
First published on 4th January 2024
Abstract
An Ir-catalysed regioselective hydroamination of internal homoallylic amines is reported. Both cyclic and acyclic internal olefins undergo directed hydroamination reactions with both aromatic and cyclic aliphatic amines to afford a variety of 1,4-diamines in fair to excellent yields. Diastereoselectivity and mechanistic investigations support that for cyclic substrates the reactions are proceeding via trans-aminoiridation to form a 5-membered metalacyclic intermediate.
Putrescine derivatives are an important class of polyamines due to their bioactivities as well as their utility as synthons for other nitrogenous moieties (Scheme 1a).1 Hydroamination, the addition of a N–H bond across an unsaturated C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or C
C or C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond, is an attractive approach to form these valuable products as it couples two readily accessible starting materials in an atom-economical fashion.2 Hydroamination methods commonly suffer from challenges with respect to reactivity, low binding affinity of the alkene to the transition metal catalyst relative to the amine, chemoselectivity as both hydro- and oxidative amination can occur, and regioselectivity as it is generally possible to form two regioisomers. These challenges are exacerbated in the hydroamination of internal olefins due to increased steric hindrance, additional β-hydrides to undergo elimination, and the lack of significant electronic and steric bias to promote regioselectivity.
C bond, is an attractive approach to form these valuable products as it couples two readily accessible starting materials in an atom-economical fashion.2 Hydroamination methods commonly suffer from challenges with respect to reactivity, low binding affinity of the alkene to the transition metal catalyst relative to the amine, chemoselectivity as both hydro- and oxidative amination can occur, and regioselectivity as it is generally possible to form two regioisomers. These challenges are exacerbated in the hydroamination of internal olefins due to increased steric hindrance, additional β-hydrides to undergo elimination, and the lack of significant electronic and steric bias to promote regioselectivity.
Several approaches are known for the hydroamination of strained internal olefins. Milstein reported the Ir-catalysed hydroamination of norbornene with aniline.3,4 In this reaction, cis-aminometalation occurs selectively across the norbornene and the lack of accessible cis β-hydrogens result in no oxidative amination. Reports of intermolecular hydroaminations of unactivated internal alkenes are limited and generally afford mixtures of regioisomers.5 Hydroamination of unactivated internal olefins is known to be promoted by a Cu catalyst. These reactions couple a nucleophilic hydride, generated by transmetalation with a silane, the olefin, and an electrophilic amine.5a–d Ir has also been shown to catalyse the hydroamination of unactivated internal alkenes with 6-amino-2-picoline.5e
The Hull group pioneered the utilization of directing groups to promote regioselective hydroamination of terminal alkenes.6 With homoallylic amines, primary amine directing groups prove excellent at promoting selective alkene hydroamination. This directing group promotes the aminometalation of the proximal alkene by increasing the affinity of the substrate to the catalyst. Further, these reactions have excellent chemoselectivity: upon aminometalation, a metallacycle is generated, which slows β-hydride elimination and promotes selective hydroamination over oxidative amination of terminal alkenes. We have showed that with Ir and cationic Rh, 5-membered metalacyclic intermediates are typically favoured: the Markovnikov product forms with allylic amines, while homoallylic amines afford anti-Markovnikov regioisomer.6e In a similar approach, 8-aminoquinoline (AQ) is used to control the regio- and chemo-selectivity in the Pd-catalysed hydroamination of terminal and internal alkenes with acidic amine nucleophiles7 and acetoxy groups promote the Ir-catalyzed regioselective hydroamination with primary amines (Scheme 1b and c).8 Given the complementarity of the nucleophiles which participate in our Rh/Ir-catalysed hydroamination, both aryl and aliphatic amines,2a we sought to develop the hydroamination of internal homoallylic amines (Scheme 1d).
Our initial efforts towards the development of this hydroamination focused on homoallylic amine 1a and aniline 2a (Table 1). Promisingly, employing an iridium catalyst under previously optimized conditions results in 34% yield of 3 with excellent regioselectivity for the 1,4-diamine (Table 1, entry 1). Changing from LiI to Mg(NO3)2 significantly improves in the yields of 3. A variety of phosphine ligands were screened (Table 1, entries 2–4 and Table S1, ESI†) and all showed similar reactivity with 1a, however tol-BINAP was moderately superior as it affords 3 in 80% yield (Table 1, entry 4) in the presence of Mg(NO3)2. Other Lewis acidic additives were explored and proved inferior (Table 1, entry 4–8, Table S2 and S3, ESI†). The additive is not essential, as in its absence we observe a 34% yield of 3. Notably, the addition of MgCl2 inhibits the reaction as only 9% yield of 3 is observed. The optimized condition are as follows: 1.5 mol% [Ir(cod)Cl]2, 3.3 mol% (±)-tol-BINAP, 25 mol% Mg(NO3)2, in neat aniline (2a, 7.5 equiv.), at 100 °C for 16 hours. Under these conditions, 1a undergoes hydroamination to afford 3 in 60% isolated yield (2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 d.r.) as a single regioisomer. When the trans-isomer (E-1a) (6.9
1.0 d.r.) as a single regioisomer. When the trans-isomer (E-1a) (6.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E:Z) is subjected to the hydroamination conditions 3 is observed in decreased yield (35%, 2
1 E:Z) is subjected to the hydroamination conditions 3 is observed in decreased yield (35%, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.), relative to the Z-1a, along with the remaining 1a (3.3
1 d.r.), relative to the Z-1a, along with the remaining 1a (3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.). We hypothesize that the reduced yield with (E)-1a is due to it being an inferior ligand for the catalyst compared to (Z)-1a. The epimerization of (E)-1a is consistent with olefin epimerization under the reaction conditions.
1 d.r.). We hypothesize that the reduced yield with (E)-1a is due to it being an inferior ligand for the catalyst compared to (Z)-1a. The epimerization of (E)-1a is consistent with olefin epimerization under the reaction conditions.
| Catalyst | Ligandb | Additive | Yieldc | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3′d 3′d |
|
|---|---|---|---|---|---|
| a General conditions: 0.20 mmol of 1a, 1.5 mol% catalyst, 3.3 mol% ligand, and 25 mol% additive, neat in aniline at 100 °C for 16 hours. b Structures are shown in SI. c Calibrated in situ yields as determined by GC using 1-methylnaphthalene as the internal standard. d As determined by 1H NMR and/or GC chromatography. | |||||
| 1 | [Ir(cod)Cl]2 | ± BINAP | LiI | 34% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | [Ir(cod)Cl]2 | ± BINAP | Mg(NO3)2 | 78% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | [Ir(cod)Cl]2 | SEGPHOS | Mg(NO3)2 | 77% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | [Ir(cod)Cl]2 | ± tol-BINAP | Mg(NO3)2 | 80% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | [Ir(cod)Cl]2 | ± tol-BINAP | LiNO3 | 74% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | [Ir(cod)Cl]2 | ± tol-BINAP | MgCl2 | 9% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | [Ir(cod)Cl]2 | ± tol-BINAP | AgNO3 | 47% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | [Ir(cod)Cl]2 | ± tol-BINAP | — | 34% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
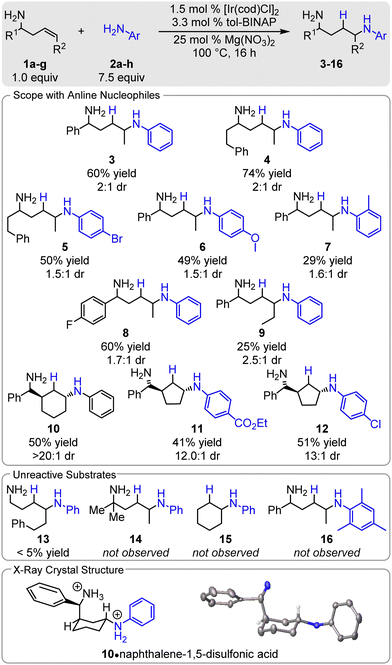
Next, we examined the scope of the reaction. Notably, all homoallylic amines which participate in the hydroamination reaction afford the 1,4-diamine as a single regioisomer (> 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The conditions are compatible with both electron-rich (6) and electron-deficient aryl amines (5, 11, 12), forming products in 41–51% yields. While the reactions are typically run neat when the nucleophile is a liquid, hexanes is an effective cosolvent. A variety of functional groups are tolerated in the reaction, including halogens (5, 12), esters (11), and ethers (6). Steric hindrance of the nucleophile has an adverse effect as o-toluidine affords 7 in a modest 29% yield and mesityl amine does not participate (16). Steric hindrance proximal to the directing group has a significant impact on the reaction: phenyl (3) to phenethyl (4), results in an increase in yield,60% and 74% respectively. More hindered directing groups, i.e. α,α-dimethyl, do not react (14). Likewise, increasing the size of R2 to a decrease in yield; when R2 is ethyl 9 is isolated in 25% yield and only trace 13 (R2 = phenethyl) is observed. On the other hand, cyclic olefins show good reactivity: α-(2-cycloalkyl)-amines readily undergo hydroamination as 10–12 are formed in 41–51% yield with excellent regio (> 20
1). The conditions are compatible with both electron-rich (6) and electron-deficient aryl amines (5, 11, 12), forming products in 41–51% yields. While the reactions are typically run neat when the nucleophile is a liquid, hexanes is an effective cosolvent. A variety of functional groups are tolerated in the reaction, including halogens (5, 12), esters (11), and ethers (6). Steric hindrance of the nucleophile has an adverse effect as o-toluidine affords 7 in a modest 29% yield and mesityl amine does not participate (16). Steric hindrance proximal to the directing group has a significant impact on the reaction: phenyl (3) to phenethyl (4), results in an increase in yield,60% and 74% respectively. More hindered directing groups, i.e. α,α-dimethyl, do not react (14). Likewise, increasing the size of R2 to a decrease in yield; when R2 is ethyl 9 is isolated in 25% yield and only trace 13 (R2 = phenethyl) is observed. On the other hand, cyclic olefins show good reactivity: α-(2-cycloalkyl)-amines readily undergo hydroamination as 10–12 are formed in 41–51% yield with excellent regio (> 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and diastereoselectivities (12
1) and diastereoselectivities (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 20
1 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
The cyclic alkene substrates offer mechanistic insight into the C–N bond formation. The crystal structure of Armstrong's acid salt of 10 shows that the aniline group is trans to the directing group (Scheme 2). This suggests either that trans-aminometalation occurs or that the catalyst selectively binds opposite of the directing group and that the C–N bond forms via migratory insertion into the [Ir]–NHAr bond. Both cyclohexene and sterically hindered homoallylic amines do not undergo the Ir-catalysed hydroamination (neither 14 nor 15 are observed), indicating that the directing group is required to promote the reaction and that trans-aminoiridation is occurring. Notably, other iridium-catalysed hydroaminations with anilines all proceed via cis-aminometalation – oxidative addition into the ArHN–H bond then migratory insertion. Trans-aminoiridation has been previously proposed with nucleophilic benzyl amines in the Ir-catalysed hydroamination of allyl acetates.8
The observation of the trans-relationship between the directing group and anilines prompted us to test the reaction conditions with aliphatic amines (Scheme 3). When morpholine is used, both cyclic and acyclic alkenes result in hydroamination products (17, 23). The acyclic alkene affords the hydroamination 17 in a 65% yield (1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.). The low d.r. may be attributed to olefin isomerization and/or poor facial control in the aminometalation step. Alternatively, cyclohexene-derived substrate affords 23 with higher diastereoselectivity (29% yield, >20
1 d.r.). The low d.r. may be attributed to olefin isomerization and/or poor facial control in the aminometalation step. Alternatively, cyclohexene-derived substrate affords 23 with higher diastereoselectivity (29% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.). The low yield is likely due to the sterically hindered homoallylic amine, while excellent d.r. can be attributed to the inability of the alkene to undergo cis/trans isomerization and highly organized aminometalation step. Again, a trans relationship between the directing group and the nucleophile in 23•2TsOH is observed. The reaction occurs with a variety of cyclic amines, including piperidine derivatives (18–21) and N-substituted piperazines (22). The conditions tolerate ethers (19), tertiary amines (22), and acetals (21). Unfortunately, acyclic primary and secondary amines do not participate (24, 25). Overall, this hydroamination reaction has promising synthetic utility due to its modularity; both coupling partners are easily modified, useful synthetic handles are tolerated, allowing for further functionalization at a specific position. This also allows rapid access to N,N′-differentially substituted diamines, which are challenging to synthesize using conventional methods. Moreover, diamine products can be transformed into other moieties of equal importance such as amide, cyclic urea, cyclic amide, and diazepane.9
1 d.r.). The low yield is likely due to the sterically hindered homoallylic amine, while excellent d.r. can be attributed to the inability of the alkene to undergo cis/trans isomerization and highly organized aminometalation step. Again, a trans relationship between the directing group and the nucleophile in 23•2TsOH is observed. The reaction occurs with a variety of cyclic amines, including piperidine derivatives (18–21) and N-substituted piperazines (22). The conditions tolerate ethers (19), tertiary amines (22), and acetals (21). Unfortunately, acyclic primary and secondary amines do not participate (24, 25). Overall, this hydroamination reaction has promising synthetic utility due to its modularity; both coupling partners are easily modified, useful synthetic handles are tolerated, allowing for further functionalization at a specific position. This also allows rapid access to N,N′-differentially substituted diamines, which are challenging to synthesize using conventional methods. Moreover, diamine products can be transformed into other moieties of equal importance such as amide, cyclic urea, cyclic amide, and diazepane.9
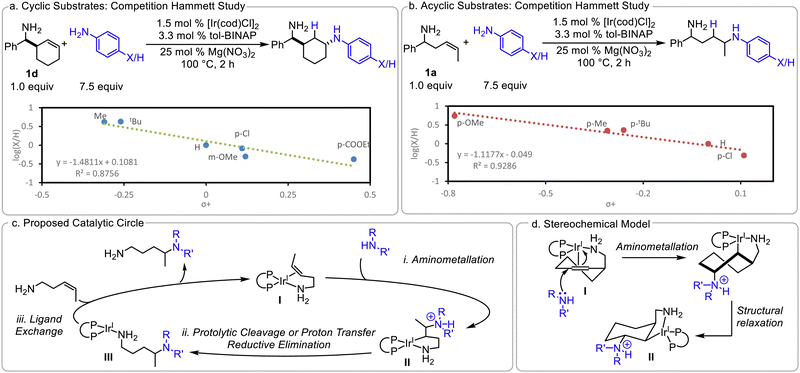
The trans-diastereoisomers formed with cyclic substrates (10–12 and 23) suggest that the C–N bond is formed via trans-aminometalation. This is further supported by a competition Hammett investigation10 between aniline and para-substituted anilines (Scheme 4a): a linear relationship is observed with an ρ of −1.5 with cyclic olefin 1d. This moderately negative ρ is consistent with the more nucleophilic amine reacting selectively in the product-determining step and trans-aminometalation of the olefin. Due to the observed olefin isomerization with acyclic alkenes, we are unable to determine definitively if a cis- or trans-aminoiridation is occurring under these conditions. An analogous competition Hammett investigation was conducted with 1a; similar ρ values with the cyclic and acyclic substrates would suggest that they may be going through analogous mechanisms. Indeed, a negative ρ of −1.1 is observed (Scheme 4b). This is consistent with a product-determining trans-aminoiridation, however we are unable to exclude cis-functionalization, which may have a similar ρ value.
Finally, we sought to determine the role of the Mg(NO3)2. While the hydroamination of 1a does occur without any additive to afford 3 in low yields, added Mg salts significantly increase the reaction efficiency. We hypothesized that these additives may abstract the chloride from the catalyst. To investigate this possibility, 10 mol% Bu4NCl was added to a hydroamination reaction to inhibit the proposed abstraction. Indeed, the addition of the Cl− impedes the reaction; only 10% in situ yield of 3 is observed after 16 hours (vs. 80% yield in the absence of added chloride, see ESI†). AgNO3, commonly used for halide abstraction, promotes hydroamination, albeit in a lower 47% yield. This suggests that Mg also coordinates to the diamine product to prevent product inhibition. Taking our observations into account, a catalytic cycle is proposed (Scheme 4c). The Mg(NO3)2 abstracts the Cl− from the catalyst and 1a binds to afford κ-2 amino olefin complex I. (i) aminometalation occurs to generate the 5-membered metalacyclic intermediate II, (ii) proteolytic cleavage of Ir–C bond affords the product bound complex III and, (iii) ligand exchange regenerates I. The trans relationship between the nucleophile and directing group is the result of the nucleophilic attack of κ-2 complex (Scheme 4d). While the proposed catalytic cycle, shown in Scheme 4c, invokes a Ir(I) catalyst and is overall redox neutral, an alternative mechanism would involve oxidative addition into an ArNH–H bond, to afford Ir(III), then proceeding through an analogous catalytic cycle.
In summary, we herein report an intermolecular iridium-catalysed hydroamination of internal homoallylic amines. The reaction is compatible with both aryl and aliphatic amines, affording 1,4-diamine products in fair to very good yields. Mechanistic evidence supports that an unusual trans-aminometalation with an Ir-catalyst and aniline is occurring.
The authors thank the NIH (R35 GM125029), the Welch Foundation (F-1994-20220331), Eli Lilly, and Novartis for their generous support. C. S. M. thanks the NSF (CHE-1945401 and CHE-2003735) for funding of the CREATE program.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) S. Sarhan, F. Dezeure and N. Seiler, Int. J. Biochem., 1987, 19, 1037 CrossRef CAS PubMed; (b) Eli Lilly and company, WO 01/46199 A1, 2001; (c) G. R. Labadie, S. R. Choi and M. A. Avery, Bioorg. Med. Chem. Lett., 2004, 14, 615–619 CrossRef CAS PubMed; (d) A. P. Caminos, E. A. Panozzo-Zenere, S. R. Wilkinson, B. L. Tekwani and G. R. Labadie, Bioorg. Med. Chem. Lett., 2012, 22, 1712–1715 CrossRef CAS PubMed.
- (a) T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795–3892 CrossRef PubMed; (b) L. Huang, M. Arndt, K. Gooßen, H. Heydt and L. J. Gooßen, Chem. Rev., 2015, 115, 2596–2697 CrossRef CAS PubMed.
- A. L. Casalnuovo, J. C. Calabrese and D. Milstein, J. Am. Chem. Soc., 1988, 110, 6738–6744 CrossRef CAS.
- (a) R. Dorta, P. Egli, F. Zürcher and A. Togni, J. Am. Chem. Soc., 1997, 119, 10857–10858 CrossRef CAS; (b) J. Zhou and J. F. Hartwig, J. Am. Chem. Soc., 2008, 130, 12220–12221 CrossRef CAS PubMed; (c) S. Pan, K. Endo and T. Shibata, Org. Lett., 2012, 14, 780–783 CrossRef CAS PubMed; (d) C. S. Sevov, J. Zhou and J. F. Hartwig, J. Am. Chem. Soc., 2012, 134, 11960–11963 CrossRef CAS PubMed.
- (a) S. Zhu, N. Niljianskul and S. L. Buchwald, J. Am. Chem. Soc., 2013, 135, 15746–15749 CrossRef CAS PubMed; (b) Y. Yang, S. L. Shi, D. Niu, P. Liu and S. L. Buchwald, Science, 2015, 62–66 CrossRef PubMed; (c) Y. Xi, T. W. Butcher, J. Zhang and J. F. Hartwig, Angew. Chem., Int. Ed., 2016, 55, 776–780 CrossRef CAS PubMed; (d) S. Ichikawa, X. J. Dai and S. L. Buchwald, Org. Lett., 2019, 21, 4370–4373 CrossRef CAS PubMed; (e) Y. Xi, S. Ma and J. F. Hartwig, Nature, 2020, 588, 254–260 CrossRef CAS PubMed.
- (a) A. R. Ickes, S. C. Ensign, A. K. Gupta and K. L. Hull, J. Am. Chem. Soc., 2014, 136, 11256–11259 CrossRef CAS PubMed; (b) S. C. Ensign, E. P. Vanable, G. D. Kortman, L. J. Weir and K. L. Hull, J. Am. Chem. Soc., 2015, 137, 13748–13751 CrossRef CAS PubMed; (c) E. P. Vanable, J. L. Kennemur, L. A. Joyce, R. T. Ruck, D. M. Schultz and K. L. Hull, J. Am. Chem. Soc., 2019, 141, 739–742 CrossRef CAS PubMed; (d) A. T. Ho, S. C. Ensign, E. P. Vanable, D. Portillo, J. N. Humke, G. D. Kortman and K. L. Hull, ACS Catal., 2022, 12, 8331–8340 CrossRef CAS PubMed; (e) B. J. Lee, A. R. Ickes, A. K. Gupta, S. C. Ensign, T. D. Ho, A. Tarasewicz, E. P. Vanable, G. D. Kortman and K. L. Hull, Org. Lett., 2022, 24, 5513–5518 CrossRef CAS PubMed.
- (a) J. A. Gurak, K. S. Yang, K. Liu and K. M. Engle, J. Am. Chem. Soc., 2016, 138, 5805–5808 CrossRef CAS PubMed; (b) J. A. Gurak, V. T. Tran, M. M. Sroda and K. M. Engle, Tetrahedron, 2017, 73, 3636–3642 CrossRef CAS.
- S. W. Kim, T. Wurm, G. A. Brito, W. O. Jung, J. R. Zbieg, C. E. Stivala and M. J. Krische, J. Am. Chem. Soc., 2018, 140, 9087–9090 CrossRef CAS PubMed.
- (a) J. E. Díaz, J. Á. Bisceglia, M. C. Mollo and L. R. Orelli, Tetrahedron Lett., 2011, 52, 1895–1897 CrossRef; (b) M. Šimunovic, I. Perkovic, B. Zorc, K. Ester, M. Kralj, D. Hadjipavlou-Litina and E. Pontiki, Bioorg. Med. Chem., 2009, 17, 5605–5613 CrossRef PubMed; (c) J. Á. Bisceglia, J. E. Díaz, R. A. Torres and L. R. Orelli, Tetrahedron Lett., 2011, 52, 5238–5240 CrossRef CAS.
- (a) L. P. Hammett, J. Am. Chem. Soc., 1937, 59, 96–103 CrossRef CAS; (b) C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1919599 and 2279637. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc05594a |
| This journal is © The Royal Society of Chemistry 2024 |