Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Layer-by-layer designer nanoarchitectonics for physical and chemical communications in functional materials
Katsuhiko
Ariga
 *ab,
Jingwen
Song
c and
Kohsaku
Kawakami
*ab,
Jingwen
Song
c and
Kohsaku
Kawakami
 cd
cd
aResearch Center for Materials Nanoarchitectonics, National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba 305-0044, Japan. E-mail: ARIGA.Katsuhiko@nims.go.jp
bGraduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwa-no-ha, Kashiwa 277-8561, Japan
cResearch Center for Functional Materials, National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba 305-0044, Ibaraki, Japan
dGraduate School of Pure and Applied Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba 305-8577, Ibaraki, Japan
First published on 18th January 2024
Abstract
Nanoarchitectonics, as a post-nanotechnology concept, constructs functional materials and structures using nanounits of atoms, molecules, and nanomaterials as materials. With the concept of nanoarchitectonics, asymmetric structures, and hierarchical organization, rather than mere assembly and organization of structures, can be produced, where rational physical and chemical communications will lead to the development of more advanced functional materials. Layer-by-layer assembly can be a powerful tool for this purpose, as exemplified in this feature paper. This feature article explores the possibility of constructing advanced functional systems based on recent examples of layer-by-layer assembly. We will illustrate both the development of more basic methods and more advanced nanoarchitectonics systems aiming towards practical applications. Specifically, the following sections will provide examples of (i) advancement in basics and methods, (ii) physico-chemical aspects and applications, (iii) bio-chemical aspects and applications, and (iv) bio-medical applications. It can be concluded that materials nanoarchitectonics based on layer-by-layer assembly is a useful method for assembling asymmetric structures and hierarchical organization, and is a powerful technique for developing functions through physical and chemical communication.
1. Introduction
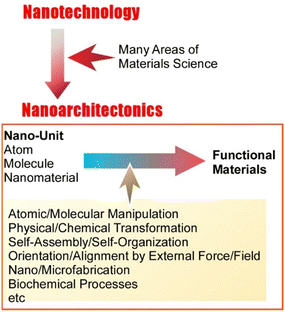
The development of humankind has been accompanied by the progress of materials science. The development of new functional materials has enriched human life. In particular, the chemistry has made a significant contribution in the past few decades. For example, organic chemistry,1 inorganic chemistry,2 polymer chemistry,3 supramolecular chemistry,4 coordination chemistry,5 biochemistry,6 and other materials chemistry7 have continuously developed new functional materials. Physics has also made a significant contribution. Physics-based analysis has revealed that the quality of functionality depends not on the material itself but on its structure (especially its internal structure).8 The biggest turning point was the rise of nanotechnology. Nanotechnology, proposed by Richard Feynman,9 has made the direct observation, manipulation, and characterization of structures down to the atomic and molecular level possible.10 As a post-nanotechnology,11 nanoarchitectonics was founded by Masakazu Aono.12 Based on the knowledge and techniques obtained from nanotechnology, nanoarchitectonics architects functional materials and structures using nano-units of atoms, molecules, and nanomaterials (Fig. 1).13Rather than creating a new and novel field, nanoarchitectonics can be thought as the integration of many areas of materials science with a focus on nanotechnology.14 In nanoarchitectonics approaches, the architecture of functional materials from nano-units is achieved through selection and combination of operations, such as atomic and molecular manipulation and their physical and chemical transformations, organizational processes such as self-assembly/self-organization and orientation and alignment by external forces and fields, and more advanced nano/microfabrication and biochemical processes.15 This methodology is not bound by material or application objectives and can be applied to a wide variety of objects. In fact, recent papers advocating nanoarchitectonics include multiple topics ranging from material synthesis,16 structure control,17 fundamental physical phenomena,18 and biochemistry-related research19 to applications such as catalysts,20 sensors,21 devices,22 energy,23 environment,24 and biomedical research.25 Since materials are originally formed from atoms and molecules, nanoarchitectonics, which architects functional materials from atoms and molecules, can be applied to all materials. It may correspond to the theory of everything,26 which could be the super unification theory (SUT) in the world of physics. Nanoarchitectonics can be a method for everything in materials science.27 In order to construct materials from atoms and molecules, it is necessary to deal with phenomena specifically. In particular, it is necessary to bridge the science specific to the nanoscale with the properties of the macroscale. These processes require the integration of many methodologies that have been developed to date. Nanoarchitectonics aims to establish such an integrated discipline.
In nanoarchitectonics, several processes are combined to form functional materials and structures, which are more suitable for forming asymmetric and hierarchical structures.28 Compared to self-assembly based on simple equilibrium, it is more suitable for forming hierarchical structures by multiple processes, including nonequilibrium processes. In addition, the nanoscale phenomena and interactions underlying nanoarchitectonics involve uncertainty and complexity. Compared to macroscopic phenomena, uncertainties such as thermal fluctuations, stochastic distributions, and quantum effects are difficult to ignore in the nanoscopic regime. Therefore, rather than simply integrating various interactions and responses, the overall response of the material or functional system is demonstrated in a harmonized way.29 This mode of functional expression in which various effects are harmonized under a hierarchical structure is common in biological systems.30 In other words, the creation of functional materials by nanoarchitectonics has the potential to realize advanced functional systems such as those found in living organisms.31
In biological systems, a number of functional units are hierarchically organized in a field such as a biological membrane, widely employed in photosynthesis and signal transduction systems.32 The coordination of these interactions results in sophisticated functions. The essence of these functions is seen in the rational physical and chemical communications of signals, energy, and molecules. In living systems, membrane tissues such as biomembranes are successfully used as a medium for these functions. In order to artificially mimic this, it is beneficial to apply methods to fabricate thin films with ideal controllable structures. The Langmuir–Blodgett method33 and layer-by-layer assembly34 are useful as such techniques. These two methods are also powerful tools for the fabrication of functional structures that can be taken to good use in nanoarchitectonics.35
The Langmuir–Blodgett method is a technique for creating highly oriented monolayers at the liquid interface and building them up in layers on a solid substrate.36 Generally speaking, this method is easy to obtain denser and more controlled thin-film structures, but it is not necessarily applicable to many types of materials. The layer-by-layer assembly method is a method to obtain multilayer thin films by sequentially adsorbing components into a film based on interactions between the materials.37 A typical method is as follows. A thin film is made by adsorbing a substance with opposite charge on a solid substrate. In this process, the surface charge is inverted so that another substance with an opposite charge can be adsorbed as a thin film. Using the principle of charge inversion, multilayers of various substances can be produced in any number of layers and any desired sequence. Since there are an extremely large number of substances with charge, many substances can be applied to this technique. The interactions are not limited to electrostatic interactions, but can be hydrogen bonds,38 coordination,39 charge transfer interactions,40 stereocomplexation,41 supramolecular inclusion,42 biospecific recognition,43 and many other interactions.44 Thereby, many materials ranging from various polymers45 to quantum materials,46 colloidal particles,47 two-dimensional materials,48 biomolecules,49 and viral particles50 can be applied in layer-by-layer assembly. In addition to the usual dipping method,51 engineering techniques such as spin-coating52 and spraying53 are also applied. In addition, layer-by-layer assembly on microscopic particles as well as layer-by-layer assembly fabrication of hollow capsules are widely used.54
Thus, layer-by-layer assembly is a promising method for organizing multi-components and constructing systems that control the physical and chemical communications between them. Layer-by-layer assembly is also an easy method to combine with other processes to create hierarchical structures. In the fabrication shown in Fig. 2, mesoporous silica capsules created by molecular assembly and template synthesis can be stacked together with other nanoparticles in a layer-by-layer organization.55 In this case, a hierarchical structure is built up, with nanometer-level mesopore structures and microcapsule spaces, which are built up macroscopically in layers. The layer-by-layer structure of the associated mesoporous materials can perform functions specific to the hierarchical structure, such as sensor functions that can be adjusted in various ways56 and automatic on/off material release functions.57
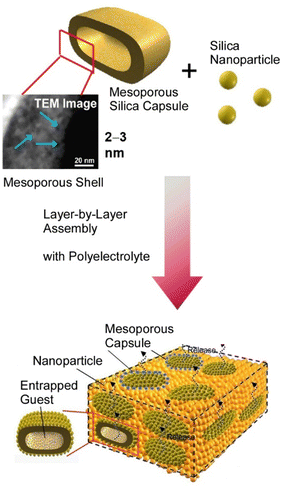 | ||
| Fig. 2 Layer-by-layer organization of mesoporous silica capsules together with other nanoparticles as a hierarchical structure with nanometer-level mesopore structures and microcapsule spaces. Reproduced with permission from ref. 55. Copyright 2008 American Chemical Society. | ||
Nanoarchitectonics has the potential to architect highly functional systems such as living organisms. A key to this is the realization of physical and chemical communications of signals, energy, and matter in structures with properties such as asymmetry, hierarchy, and so on. Layer-by-layer assembly is a promising structure fabrication method for this purpose. Based on this background, this feature article explores the possibility of constructing advanced functional systems based on recent examples of layer-by-layer assembly. In the following sections, we will illustrate the development of more basic methods and the development of nanoarchitectonics systems that bring about functional expression from structural understanding. Specifically, the following sections will provide examples of (i) advancement in basics and methods, (ii) physico-chemical aspects and applications, (iii) bio-chemical aspects and applications, and (iv) bio-medical applications.
2. Advancement in basics and method
In order to reasonably communicate physically and chemically in functional structures, precise structures must be controlled and evaluated. As mentioned above, a promising method for nanoarchitectonics of materials with such functional properties is the layer-by-layer method described above. Composite nanoarchitectures can be fabricated from a wide variety of materials, ranging from quantum inorganic objects to organic polymers and biomaterials. It is also an environmentally friendly, simple, and economical method of fabrication. In contrast to the high potential of the layer-by-layer method, there is still much room for further study on how to analyze the nanoscopic structure. In the development of devices involving physical and chemical communications, prediction and understanding of their performance are important keys.Gutfreund et al. used neutron scattering to study the average conformation of polymer chains in layer-by-layer film structures consisting of deuterated polyelectrolyte chains.58 Layer-by-layer films of poly(sodium 4-styrenesulfonate) and poly(allylamine hydrochloride) were prepared by dipping, spray, and spin-assisted methods and compared. The three-dimensional nanostructure of the layer-by-layer assembled polyelectrolyte multilayers was determined under various conditions. The out-of-plane radius of rotation of layer-by-layer films prepared at different salt concentrations was compared, and the lower the salt concentration, the less equilibrated the structure. The structure of the resulting solid-state polyelectrolyte multilayer strongly depends on the deposition process, in the following order: spin-assisted method > spray-assisted method > dipping method, which affected the asymmetry. Off-specular neutron reflectometry revealed that complexation at the polyanion/polycation interface occurs at the molecular level, not at the monomer level. The poly(sodium 4-styrenesulfonate) chain exhibits a flattened coil structure with an asymmetry coefficient of about 7. Despite being a non-equilibrium polymer chain, its density profile follows a Gaussian distribution occupying approximately the same volume as the bulk complex. The deformation of the chain is equal on all length scales, preserving the Gaussian nature of the segmental density. Such an analytical approach provides a better understanding of the formation of layer-by-layer multilayers composed of polyelectrolytes, their internal structures, and the properties they confer. Tuning of these structural details affects the efficiency and anisotropy of physical and scientific communications. Varying layer-by-layer assembly methods can be viewed as a well-stocked toolbox for fine-tuning to meet the needs of specific applications.
Decher and co-workers reported a very simple method to create anisotropic layer-by-layer films (Fig. 3).59 This is a spray layer-by-layer method by controlling the angle between the spray jet and the surface in a grazing incidence spray. This is a simple and efficient method for spray-assisted orientation of nanofibrils in layer-by-layer assembly of cellulose nanofibrils. Spraying at an angle of 90° to the deposition surface produces films with uniform in-plane orientation. On the other hand, spraying at a smaller angle produces a macroscopic directional surface flow of liquid on the receiving surface of the spray. As a result, films with in-plane anisotropy are obtained. Cellulose nanofibrils are oriented parallel to the spray direction. Furthermore, the orientation order depends on the distance of the deposition surface from the spray nozzle. In a layer-by-layer multilayer film nanoarchitected in this way, the apparent refractive index of the film has a minimum in the orientation direction and a maximum in the direction perpendicular to the orientation direction. This technique provides a simple method for creating optically birefringent films over a large surface area. This method utilizes shear alignment when depositing layer-by-layer collective films. This method of forming highly anisotropic structures by spray alignment, combined with the ability to form further layered structures by further layer-by-layer methods, may yield materials with a higher degree of anisotropy and hierarchical structure. With grazing incidence spraying, the orientation direction in individual layers can be easily selected. Different chemical compounds can be used for different layers. As a result, it is possible to prepare materials with a hierarchical structure with more complex anisotropy than one direction of orientation.
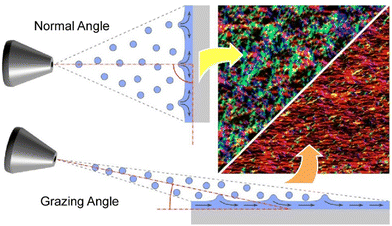 | ||
| Fig. 3 Simple and efficient method for spray-assisted orientation of nanofibrils in layer-by-layer assembly of cellulose nanofibrils: less oriented film (top); anisotropic films fabricated with a grazing incidence spray (bottom). Reproduced from ref. 59, under the terms of the CC BY. | ||
The nanoarchitectonics of artificial layer-by-layer structures will also lead to the development of materials that mimic natural layered structures and exhibit higher functionality. For example, certain biomaterials are known to exhibit superior strength, modulus, and toughness due to mechanical communication in their layered structures.60 Mechanical communication of inorganic reinforced nanofillers within a ductile organic matrix leads to such properties. In particular, the two-dimensional nanosheets and nanoplatelets are attributed to the elaborate formation of layered microstructures. Liu and co-workers reported a method for nanoarchitectonics of nanocomposite materials with highly ordered layered structures.61 This strategy exploits the shear flow-induced orientation of two-dimensional nanosheets at the interface between the immiscible hydrogel and oil. This can be thought of as the fabrication of bulk artificial pearl layers using graphene oxide, clay, and carbon nanotubes. Layered nanocomposites based on graphene oxide and clay nanosheets yielded high strength, such as 9.0 times the tensile strength and 2.8 times the Young's modulus equivalent of natural mother-of-pearl. When clay nanosheets were used, nanocomposites exhibiting 20.4 times the toughness of natural mother-of-pearl were obtained. Such critical interfaces have a very high regime for crack formation and propagation under high tensile stress. In particular, the small aspect ratio of the clay nanosheets allows the transition of the deformation mode from nanosheet fracture to nanosheet pullout. The method is a layered nanoarchitectonic based on super-diffusive shear flow-induced orientation of nanosheets at the immiscible hydrogel/oil interface. It is a scalable layering method that can be universally applied to many materials. It will be a simple methodology to fabricate layered nanocomposite films from a wide range of polymers and two-dimensional inorganic materials that can encapsulate mechanical communication. It is a promising nanoarchitectonics method for the development of advanced layered nanocomposites for practical applications.
The nanoarchitectonics of laminated membranes often takes place at stable interfaces between immiscible solvents. Using interfaces between immiscible solvents, Zhao and co-workers reported spontaneous water-on-water diffusion and self-assembly of polyelectrolyte membranes (Fig. 4).62 This is an example of water-on-water diffusion applied to materials engineering and other fields. In this example, the process of spreading an aqueous solution mixture containing poly(ethyleneimine) and poly(sodium 4-styrenesulfonate) over acidic water is used to create a hierarchical porous membrane. The decrease in surface tension of the polyelectrolyte mixture solution promotes surface diffusion. Complexation of the polyelectrolytes at the interface, caused by the low pH of the water, moderates water–water mixing. In this design, poly(sodium 4-styrenesulfonate) gives the mixed solution a lower surface tension than water. This is the driving force behind surface diffusion. Poly(ethyleneimine) is a weak polyelectrolyte that takes on a positive charge under acidic conditions, leading to complexation. This synergistic approach of surface tension and pH-dependent complex formation does not require surfactants or sophisticated equipment. It does not require small droplets or complex techniques, and can be easily manipulated using a pipette at room temperature. It is also applicable to various polyelectrolytes and nanomaterials, and can be a general methodology for functional materials engineering.
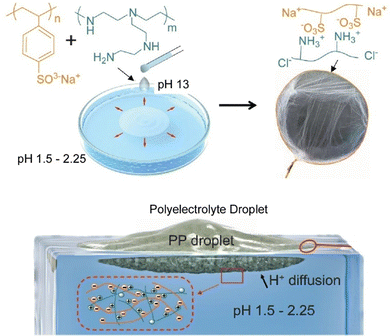 | ||
| Fig. 4 Spontaneous water-on-water diffusion and self-assembly of polyelectrolyte membranes: the process of spreading of an aqueous solution mixture containing poly(ethyleneimine) and poly(sodium 4-styrenesulfonate) over acidic water to create a hierarchical porous membrane. Reproduced from ref. 62, under the terms of the CC BY. | ||
Efforts are also being devoted to the nanoarchitectonics of two-dimensional structures that form the cornerstone of layered structures. Beyond Staudinger's concept based on polymers on linear chains, sheet polymers with long-range ordering along two orthogonal directions are attracting attention as two-dimensional polymers.63 Methodologies for nanoarchitectonics of reliable structures of new classes of organic two-dimensional materials, rather than inorganic two-dimensional materials such as graphene, are needed. Kaiser, Zheng, Feng, and co-workers reported surfactant-monolayer-assisted interface synthesis.64 It is a controlled synthesis of few-layer two-dimensional polymer crystals with high crystallinity and domain sizes of a few micrometers. In this method, rigid and symmetric monomer arrangements and two-dimensional polymerization were achieved using surfactant monolayers on the water surface. The thickness of the nanoarchitectonized two-dimensional polyimide nanosheets is about 2 nm, which corresponds to about 5 layers. The average crystalline domain size is about 3.5 μm2. This surfactant monolayer-assisted interfacial synthesis strategy has also been extended to polycondensation reactions to develop crystalline few-layer two-dimensional polyamides with a double-pore lattice structure. These methods are strategies for the synthesis of two-dimensional polymer crystals, two-dimensional polyimides and two-dimensional polyamides, under ambient temperature conditions. Extensions to other two-dimensional polymerizations involving reversible or irreversible covalent bonds are expected.
Similarly, nanoarchitectonics has also been investigated through the self-assembly of peptides and other molecules on surfaces. Understanding the mechanisms by which short peptides self-assemble into two- and three-dimensional structures is of great interest in the formation of crystalline biomolecular systems and their practical applications. Yurtsever, Sun, Sarikaya, and co-workers used graphite-bound dodecapeptides for a highly oriented self-assembly process in aqueous solution observed in situ using frequency-modulated atomic force microscopy.65 The main observations suggest that the first layer forms homogeneously and produces self-assembled crystals with a lattice structure in contact with the underlying graphite. In detail, peptide crystals formed on the surface have a chiral crystallographic orientation relationship with the underlying graphite lattice, forming a six-fold symmetric domain. Thus, molecular surface self-assembly nanoarchitectonics depends on a series of molecular interactions, including conformational states, molecule–substrate and intermolecular interactions. These studies are helpful in the scientific understanding and construction of coherent bio/nano hybrid interfaces through the surface behavior of short peptides on solids. Detailed computational modelling studies, such as controlled molecular dynamics, should be performed in hierarchical dimensions, through which peptides are likely to take on various conformational structures while behaving at the surface and folding as they form various two-dimensional molecular crystals at the surface. This provides quantitative guidelines for the elucidation of molecular mechanisms of self-assembly. It is also a fundamental guideline for designing nanostructures for hybrid surface technologies such as biomolecular logic devices.
Materials nanoarchitectonics based on the layer-by-layer method has been widely studied because of its simplicity and wide range of applications. It is an easy method to assemble asymmetric structures and hierarchical organization, and is a powerful technique for developing functions through physical and chemical communications. However, not everything has been clarified by such a popular method, and research continues to analyze basic issues such as the average conformation of polymer chains in layer-by-layer membrane structures. On the other hand, the fabrication of highly oriented layer-by-layer film structures by spraying from low angles and the creation of spontaneous multilayer films using miscible and immiscible interfaces are also being investigated. Two-dimensional materials and aggregate structures, which are also the basic structures of layered structures, are also being investigated. The analysis and exploration of nanoscopic structures are considered to be key to predicting and understanding their performance in device development involving physical and chemical communications.
3. Physico-chemical aspects and applications
The cleverly designed layer-by-layer structure creates physical and chemical communications of signals and properties. This leads to more advanced functionality. Various studies are being promoted from this perspective. These functional structures are often done with various application developments in mind.Wang and co-workers developed a material in which highly effective heat transmission can be controlled by a layer-by-layer structure.66 They nanoarchitectonized sodium silicate-derived silica aerogels by a one-pot, continuous and natural solvent evaporation method of hydrophobization, solvent exchange, sodium purification, and atmospheric pressure drying (Fig. 5). Using inexpensive sodium silicate as the silica precursor, a continuous and spontaneous progressive hierarchical sub-sensorial alignment was obtained. Silica aerogels with high contact angle, high specific surface area, low density, and low thermal conductivity were synthesized by this one-pot nanoarchitectonics approach. A layer-by-layer structure containing this silica aerogel layer and an additional phase change material layer was designed. Experimental and simulation results show that this layer-by-layer structure has dual-function thermoregulatory performance to control thermal communication and postpone the time to reach equilibrium in both severe cold and hot environments. It has been shown that when the outside temperature is −30 °C, the internal temperature can be maintained above 20 °C for extended periods, and when the outside temperature is 70 °C, the internal temperature can be maintained at 30 °C. A proof-of-concept experimental setup simulating sunlight exposure was performed. It was proven that a model car protected by a layer-by-layer structure can maintain an interior temperature of 28 °C or lower, even when the outside temperature is 70 °C. Through layer-by-layer nanoarchitectonics, thermal communication in both harsh hot and cold environments can be controlled, enabling the development of robust dual-function personal thermal management systems for thermoregulation.
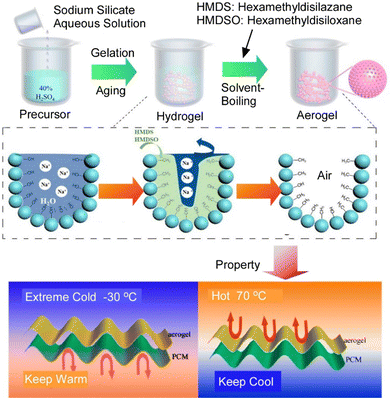 | ||
| Fig. 5 A layer-by-layer structure containing this silica aerogel layer and an additional phase change material layer with dual-function thermoregulatory performance to control thermal communication and postpone the time to reach equilibrium in both severe cold and hot environments. Reproduced with permission from ref. 66. Copyright 2021 American Chemical Society. | ||
Technologies such as 5G communication, electric vehicles, and wearable electronics are developing. Accordingly, communication of electromagnetic radiation has become more and more important. For example, ultra-high performance and cost-effective shielding materials are required to avoid potentially harmful effects of electromagnetic interference on the human body. Kim and co-workers have shown that layer-by-layer assembly using copper nanosheets can be a practical and promising electromagnetic interference shielding technology (Fig. 6).67 In this study, hierarchical porous copper foils with layer-by-layer assembly of single-crystal, nanometer-thick, and micrometer-long copper nanosheets were nanoarchitectonically investigated for their use in electromagnetic interference shielding. Layer-by-layer assembly of Cu nanosheets can be used for multilayer stacking, two-dimensional networking, and layer-by-layer sheet void structures, and hierarchically structured porous Cu films can be formed. Large metal sheets can be vertically stacked to form multilayer two-dimensional structures and sheet-like voids simultaneously. The layer-by-layer structure of porous Cu foil fabricated in this way exhibited superior electromagnetic interference shielding performance compared to dense copper and other materials of the same thickness. The layer-by-layer layering inside the Cu nanosheet and its interaction with the incoming electromagnetic radiation is responsible for this strong absorption of radiation. Controlled electromagnetic communication through hierarchical layer-by-layer structures has numerous advantages, including light weight, thinness, mechanical softness, low cost, ease of synthesis, and ease of fabrication. It would be useful for performance in a wide range of electronic applications.
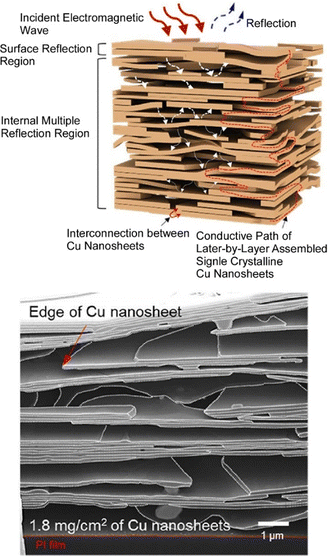 | ||
| Fig. 6 A layer-by-layer assembly of single-crystal, nanometer-thick, and micrometer-long copper nanosheets for promising electromagnetic interference shielding technology: design (top) and image (bottom). Reproduced with permission from ref. 67. Copyright 2021 American Chemical Society. | ||
It can also control the physicochemical properties of substances that have been widely distributed since ancient times. Cotton and its blends are among the most commonly used fabrics. However, cotton is known to be highly flammable. Lvov and co-workers have dramatically restricted the flammability of those materials by coating them with a flame retardant layer-by-layer process (Fig. 7).68 Flame retardancy was imparted by layer-by-layer assembly of cationic polyethyleneimine and anionic halloysite clay nanotubes on the raw cotton as a nanoarchitectural structure of organic/inorganic coating. From the overall structure, only two layers of polyethyleneimine/halloysite clay nanotube layer-by-layer coating with a weight ratio of approximately 7% were needed to give the processed cotton an optimal level of flame retardancy. The flame retardant properties are such that the flame would self-extinguish when burned. The halloysite clay nanotubes used in the coating have a hollow structure with a diameter of 50 nm, composed of biocompatible SiO2/Al2O3. Chemical substances can be held in this nanospace, which guarantees the addition of various functionalities and safe interaction with living organisms. For instance, color-enhancing dyes, antimicrobial chloramphenicol or silver can be loaded in the halloysite clay nanotubes. They can also be architectural coatings with complex functionality. Halloysite clay nanotube/polyethyleneimine layer-by-layer multilayers have been shown to withstand multiple washings. The nanostructural design of integrated nano-, micro-, and macrostructural composites is a promising nano-architectonics method for practical fabric material development.
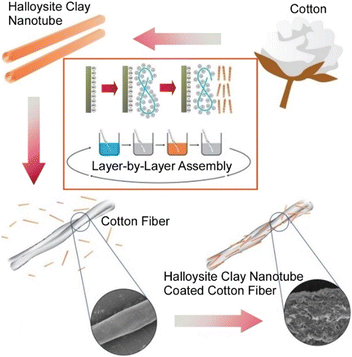 | ||
| Fig. 7 Flame retardancy imparted by layer-by-layer assembly of cationic polyethyleneimine and anionic halloysite clay nanotubes on the raw cotton as a nanoarchitectural structure of organic/inorganic coating. Reproduced with permission from ref. 68. Copyright 2023 Wiley-VCH. | ||
Communication of physical information by reflection of light rays depends on the design of the mirror. Conventional mirrors invert the circular polarization handedness during reflection. Pauly and co-workers fabricated chiral mirrors by layer-by-layer assembly of oriented silver nanowire layers fabricated using the aforementioned grazing incidence method on a semi-reflective silver layer (Fig. 8).69 The chiral mirror function is achieved by the formation of twisted Ag nanowire superstructures on a reflective metal surface. By tuning the number of oriented Ag nanowire layers and the spacing between the layers, optical properties such as total reflectance, differential reflectance, and spectral shape can be tuned. The nanoarchitected chiral metasurfaces exhibit structure-dependent differential reflectance for circularly polarized light over a wide wavelength range in the UV, visible, and near-infrared regions. They also have extremely high merit indices. Most chiral mirrors created by conventional techniques are fabricated by top-down techniques such as electron beam lithography. This conventional technique is very costly and difficult to scale up to macroscopic devices. In contrast, the method presented here only uses aqueous suspensions/solutions of commercially available nanowires and polymers and is based on wet chemistry lab techniques, which are very inexpensive and easy compared to vacuum-based lithography processes. Layer-by-layer nanoarchitectonically oriented nanowire layers not only have sufficient optical quality but also benefit from low cost and simplicity. They can also be created on the surface of various optical elements. Such large-area chiral mirrors have many potential applications, including optics, sensing, and chiral light-matter interactions. Adding another chiral mirror on top of the chiral superstructure could result in a closed chiral resonator, generating tunable chiral resonator modes. Alternatively, it would contribute to chiral catalytic reactions and enantioselective synthesis.
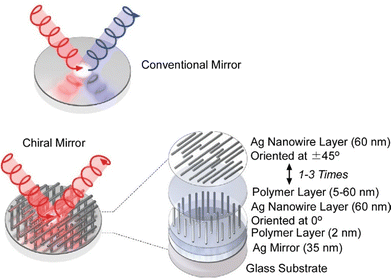 | ||
| Fig. 8 Fabrication of chiral mirrors by layer-by-layer assembly of oriented silver nanowire layers fabricated using the grazing incidence method on a semi-reflective silver layer. Reproduced from ref. 69, under the terms of the CC BY. | ||
Important examples of physical property conversion and functional communication can be found in the design of various energy-related devices. For example, solution-processed organic solar cells have become an extremely promising methodology for inexpensive and sustainable light-energy conversion with increasing photovoltaic efficiency.70 However, in many cases, the efficiency of large-scale organic solar cell modules fabricated by the donor–acceptor bulk heterojunction strategy is not always satisfactory. Focusing on the excellent physical dynamics and good surface uniformity of layer-by-layer blends, Min and co-workers applied layer-by-layer nanoarchitectonics to the fabrication of organic photovoltaic modules with larger active areas (Fig. 9).71 A layer-by-layer approach was introduced to the fabrication of organic solar cells by blade-coating and demonstrated to obtain higher photovoltaic performance compared to bulk heterojunctions. The layer-by-layer fabrication strategy can significantly reduce the scaling gap. Printing techniques that process photoactive layers by a layer-by-layer strategy have proven to be useful for large-scale production and industrial application of high-performance organic solar cells.
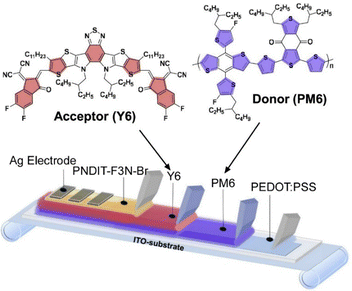 | ||
| Fig. 9 Layer-by-layer nanoarchitectonics to the fabrication of organic photovoltaic modules with larger active areas by blade-coating to obtain higher photovoltaic performance compared to bulk heterojunctions. Reproduced with permission from ref. 71. Copyright 2020 Elsevier. | ||
Layer-by-layer methods are used for coating micro- and nanoparticles and fabricating hollow capsules,72 as well as for coating surfaces with flat multilayers. Cui, Caruso, and co-workers proposed the coordination of metal ions to quinones via metal-acetylacetone coordination bonds to design structurally tunable, universally adhesive, hydrophilic, and pH-degradable capsules and other materials (Fig. 10).73 A library of metal-quinone networks is formed from nine metal ions and five paired model quinone ligands. The structures to be nanoarchitectonized are diverse, including particles, tubes, capsules, and films. The networks can also serve as reactive platforms for functionalization with molecules that can improve fluorescence, enzyme catalysis, and colloidal stability. The result is a network in which physical, chemical, and biological functions can communicate. A notable property of this network is that it exhibits bidirectional pH-responsive degradation in acidic and alkaline solutions. Quinone ligands mediate the degradation kinetics, allowing temporal and spatial control of the release of multiple components through the multilayer metal-quinone network. Since quinones are themselves functional molecules, they can be used as prodrugs for cancer therapy. High drug-loading metal-quinone network prodrugs have been designed using doxorubicin for anticancer therapy and shikonin for inhibition of the major protease of the SARS-CoV-2 virus. Metal-quinone networks, as hydrophilic coatings on a wide range of substrates, are beneficial structures for the creation of complex and tunable stimuli-responsive organometallic films and particles for a variety of applications.
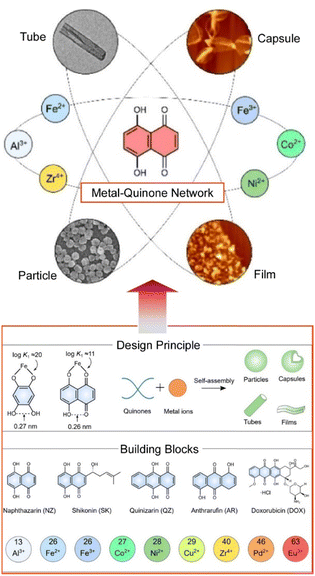 | ||
| Fig. 10 Fabrication of a metal-quinone network upon coordination of metal ions to quinones via metal-acetylacetone coordination bonds to design structurally tunable materials including particles, tubes, capsules, and films from a library of nine metal ions and five paired model quinone ligands. Reproduced from ref. 73, under the terms of the CC BY. | ||
As shown in some of the examples above, cleverly designed layer-by-layer structures create various types of physical and chemical communication. Controlling thermal exchange allows materials to block external temperature changes and to be highly flame retardant. Similarly, materials that block electromagnetic interference have been developed. Layer-by-layer structures aimed at controlling optical management will give rise to new materials called chiral mirrors and highly efficient light-energy conversion devices. Thus, nanoarchitectonics with layer-by-layer structures are useful for controlling the communication of physical and chemical signals. New strategies such as multilayer metal-quinone networks have been proposed to complement layer-by-layer structures, and the exploration of physical functions through hierarchical and oriented layered structures will continue to be active.
4. Bio-chemical aspects and applications
The advantages of forming orientational, anisotropic, and hierarchical nanostructures of layer-by-layer assembly go beyond bringing about modulation of physical properties through physical and chemical interactions. It is also possible to control biological events through chemical stimulus of signals and molecules. Originally, sophisticated functions in living organisms are due to vectorial and oriented transportation of signals and substances. This is brought about by the hierarchical organization of functional components. The hierarchical and anisotropic arrangement of functional units allows for rational and efficient communication of signals, energy, and chemicals. In layer-by-layer nanoarchitectonics, the formation of anisotropic and hierarchical structures also leads to the regulation of biochemical phenomena through efficient chemical communication.Artificial nanoarchitectonics of highly organized skeletal muscle tissue is promising for a variety of bioapplications, e.g., treatment of muscle damage and muscle diseases, alternative medicine, or pharmacological research. To this end, myoblasts must differentiate into parallel-oriented myotubes and form aligned myofibers. Boulmedais and co-workers developed hydrogen-bonded tannic acid/collagen layer-by-layer nanofilms by the brushing method as a substrate to induce such a tissue structure.74 The brushed tannic acid/collagen films were highly oriented compared to films obtained by conventional dipping. An orientation of collagen fibers with a diameter of 60 nm was observed along the brushing direction. Human myoblasts were cultured on oriented tannic acid/collagen films terminated with collagen. This aligned tannic acid/collagen nanofilm improves adhesion to the substrate. Cell behavior is regulated by its effects on cell morphology, alignment, and differentiation. When cultured in a differentiation medium without other supplements, human myoblasts aligned on brushed tannic acid/collagen films. Differentiation into long-aligned myotubes was induced by two properties: collagen fiber orientation, which induces myoblast alignment, and tannic acid release, which promotes differentiation. The oriented structures generated by layer-by-layer nanoarchitectonics with the brushing method can mimic complex in vivo states by taking advantage of topographic cues and strong cell/collagen interconnections. This methodology holds promise for designing model tissues for anisotropic tissue regeneration, injury and disease treatment, and pharmacological research.
When nanoparticles are exposed to serum or plasma, proteins from the blood adsorb onto the nanoparticle surface to form a protein corona. Chan and co-workers have investigated the basic organization and binding function of these adsorbed proteins based on a layer-by-layer structure.75 This study provides detailed functional and structural insights into the protein corona on nanomaterials and clues to strategies for corona to control interactions with biological systems. Fig. 11 proposes a mechanism for the structure and function of the protein corona. This is a layer-by-layer structure based on protein–protein–protein interactions in which this is a three-layer structure. When nanoparticles are exposed to serum or plasma, the interaction between the protein and the nanoparticle causes the protein to adsorb to the surface. This forms the underlying layer. The chemical composition and surface chemistry of the nanoparticles may determine the specific serum protein that is adsorbed. Based on further protein–protein interactions, an aggregation layer is formed that is bound to the protein base layer. The functional proteins of the final bound layer will determine the interaction function of the nanoparticle protein corona with the cellular receptor. Successful control of this structure makes it possible to tailor the protein corona. It can be used to design precoated coronas with various biological identities in nanomaterials.
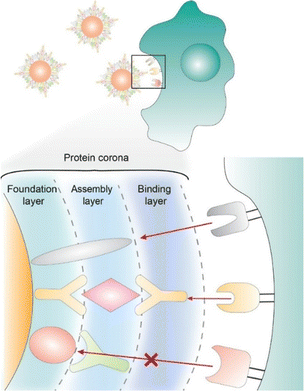 | ||
| Fig. 11 An organization model of the adsorbed proteins based on a layer-by-layer structure to provide detailed functional and structural insights into the protein corona on nanomaterials and clues to strategies for corona to control interactions with biological systems. Reproduced with permission from ref. 75. Copyright 2020 American Chemical Society. | ||
The nanoarchitectonics of nanoparticles with proper interaction with biofilms is also important in terms of antimicrobial drug development.76 Microorganisms that are established in biofilms tolerate high concentrations of antibiotics because of the viscous extracellular matrix that encapsulates their antimicrobial activity. Compared to such free drugs alone, nanoparticle-based therapeutics achieve higher local concentrations in the biofilm, thereby increasing their efficacy. In particular, positively charged nanoparticles bind multivalently to anionic biofilm components, enhancing their penetration into the biofilm. Unfortunately, cationic particles are toxic and are rapidly expelled from the circulation in vivo, limiting their use.
Hammond and co-workers have developed pH-responsive nanoparticles that change their surface charge from negative to positive in response to a decrease in the pH microenvironment of the biofilm nanoarchitectonics (Fig. 12).77 Four pH-dependent hydrolysable polymers were synthesized and biocompatible nanoparticles were prepared using a layer-by-layer electrostatic assembly method. These polymers differ in the hydrophilicity of their backbones and the flexibility of their side chains. Accordingly, each resulted in a different and predictable charge reversal rate. This reversal rate determines penetration and permeation into the biofilm. The rates ranged from several hours to undetectable within the experimental time frame. Tobramycin, an antibiotic known to be trapped by anionic biofilm components, was loaded into the final layer of layer-by-layer nanoparticles. By loading tobramycin, a clinically relevant antibiotic that is attenuated in the biofilm matrix, onto the nanoparticle surface, the increased accumulation of nanoparticles due to the surface charge reversal rate leads to more effective drug delivery. The fastest charge-converting nanoparticles resulted in a 3.2-fold reduction in bacterial colony-forming units compared to free tobramycin. State communication, i.e., the layer-by-layer structure change over time and return of charge state, led to the elimination of biofilm-based infections. This will be useful in providing structure-function guidance for future design of related pH-responsive nanocarriers.
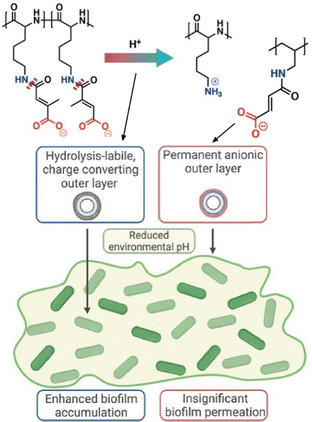 | ||
| Fig. 12 pH-Responsive nanoparticles that change their surface charge from negative to positive in response to a decrease in the pH microenvironment of the biofilms. Reproduced with permission from ref. 77. Copyright 2023 American Chemical Society. | ||
In a biofunctional system, various functional units are organized to perform sophisticated functions through directed chemical communication. As an artificial construct of such a system, Dong, Li, and co-workers developed a supramolecular assembly of bacteriorhodopsin and ATP synthase co-assembled in an artificial biomimetic system (Fig. 13).78 This system allows cascade reactions and ATP synthesis under illumination. The directed transport of protons is used to generate a high proton gradient, which facilitates energy conversion under illumination. First, oriented bacteriorhodopsin is constructed by layer-by-layer assembly in a polyelectrolyte-based microcapsule. Bacteriorhodopsin maintains a highly uniform orientation due to its high loading capacity and accelerated phosphorylation. Proteoliposomes then introduce FoF1-ATPase onto the microcapsules by fusion. Bacteriorhodopsin in the microcapsules pumps protons from the inside to the outside only under light irradiation. A proton gradient is formed in the proteoliposome and FoF1-ATPase is activated sequentially to synthesize ATP. To further promote photosynthetic activity, CdSe/ZnS quantum dots were introduced. The introduction of optically matched quantum dots significantly enhanced the light-induced phosphorylation and further improved the ATP production. Rational nanoarchitectonics of tissue bodies using layer-by-layer assembly enabled multiple functional communications in favor of light to chemical energy.
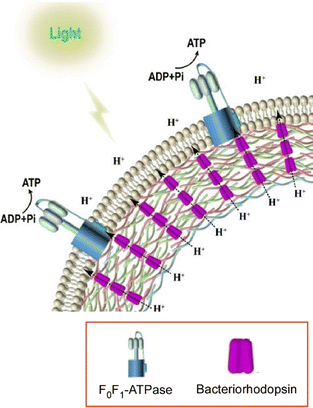 | ||
| Fig. 13 Supramolecular assembly of bacteriorhodopsin and ATP synthase in an artificial biomimetic system to allow cascade reactions and ATP synthesis under illumination. Reproduced with permission from ref. 78. Copyright 2022 Wiley-VCH. | ||
Advanced functions in living organisms depend on vectorial and oriented communication of signals and substances. In layer-by-layer nanoarchitectonics, anisotropic and hierarchical structures are formed to regulate biochemical phenomena through efficient chemical communication. For example, the oriented structure of hydrogen-bonded tannic acid/collagen layer-by-layer nanofilms by the brushing method can mimic complex states in vivo by using topographic cues and strong cell/collagen interactions. The structure of the biofilms can be analyzed based on a layer-by-layer structure to devise strategies for corona control to control interactions with biological systems, and nanoarchitectonics of pH-responsive nanoparticles that change their surface charge from negative to positive in response to a decrease in the pH microenvironment of the biofilm, and enhancing antimicrobial activity. In this nanoarchitectonics of supramolecular assembly, bacteriorhodopsin and ATP synthase are co-assembled in an artificial biomimetic system, enabling cascade reactions and ATP synthesis under illumination. In the latter system, multiple species of functional units are organized in layer-by-layer nanoarchitectonics, and the conversion of light energy into chemical energy is achieved by facilitating functional communication among them. The above example shows that the development of chemical communication by layer-by-layer nanoarchitectonics is also useful for mimicking biological functions.
5. Biomedical applications
As emphasized in the previous section, asymmetric or hierarchical organization through layer-by-layer nanoarchitectonics allows for the expression of functions through controlled chemical communication. The effect is more suitable for mimicry and application of various biological functions. Such an approach is useful not only in basic biochemistry, but also in applicable biomedical fields such as drug delivery.For example, layer-by-layer assemblies based on nanoparticles can be important tools in the field of drug delivery.79 Applications include the delivery of chemotherapeutic agents, small molecule inhibitors, and nucleic acids. Potentially useful is the ability to encapsulate multiple functional units. Nanoarchitectonics of nanocarriers, such as the stepwise achievement of complex combination therapies, will be possible. Compared to the already well-established planar film-type layer-by-layer assemblies, the multi-step process of organizing a series of functional units into a nanoscopic template is inherently complex. The nanoarchitectonics of these materials requires appropriate conditions for each synthesis step and adsorbed material. Hammond and co-workers examined the requirements for a reliable method for the synthesis of layer-by-layer liposomes containing nucleic acids: the influence of solution conditions such as pH, ionic strength, salt composition, and valence on the preparation of layer-by-layer nanoparticles.80 The results revealed the importance of optimizing the parameters for the selection of solution conditions to control the degree of ionization and electrostatic screening length suitable for the adsorption of nucleic acids and synthetic polypeptides. Moreover, the amount of nucleic acids in layer-by-layer liposomes was improved by about 8-fold. It was shown that the optimization of the solution conditions for the preparation of layer-by-layer nanoparticles for therapeutic use is actually a fundamental part of the preparation. Despite the above example being a fundamental study, it is obvious that the functionalization of liposomes is a practical and important matter, with layer-by-layer membranes to extend their biomedical applications, because liposomes are important drug delivery entities. Layer-by-layer nanoarchitectonics also suggests that optimization of practicality must go back to basics.
Nanoarchitectonics of biosynthetic bioparts in artificial cell-like hollow structures is very useful to advance research in biomolecular synthesis, biosensing, and biomedical applications.81 As a provider of such a venue, microcapsules nanoarchitectonized by templated layer-by-layer assembly using natural polymers are a powerful tool. In one such attempt, Drachuk et al. immobilized DNA templates encoding translationally activated riboswitches and RNA aptamers in layer-by-layer microcapsules prepared from regenerated silk fibroin protein (Fig. 14).82 To successfully meet the appropriate biocompatibility and semipermeability, several important parameters were controlled, including the presence of polymer primers, the concentration of silk protein, and the amount of DNA in each layer during assembly. The properties of the material, regenerated silk fibroin protein, were also successfully used. Acute treatment with methanol induced a transition in the secondary structure of silk. The use of organic solvents facilitated the formation of β-sheets in the shell structure of the protein, allowing stacking through multiple hydrogen bonds and hydrophobic interactions. The resulting microcapsules were DNA immobilized, uniformly sized, and robust. The capsule shell was further functionalized by modifying it with gold nanoparticles and IgG antibodies. This organizes a micro-compartmentalized nanoarchitecture with sensing elements for remote sensing and targeted delivery. Such a design could potentially be used as a target cell-specific biosensor. Furthermore, the incorporation of various RNA sensors could lead to the design of multiplexed biosensors that track multiple biomarkers in complex media.
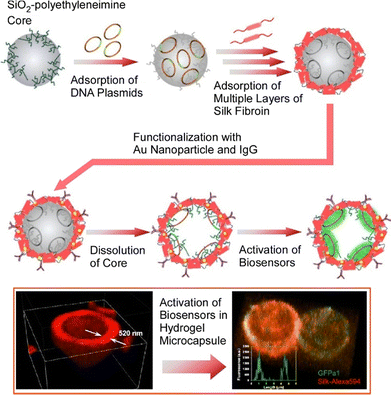 | ||
| Fig. 14 A layer-by-layer microcapsule immobilized with DNA templates encoding translationally activated riboswitches and RNA aptamers to successfully meet the appropriate biocompatibility and semipermeability. Reproduced with permission from ref. 82. Copyright 2020 American Chemical Society. | ||
Polyelectrolyte complex hydrogels, such as biopolymers, have shown promise as therapeutic agents and cell carriers.83 The nanoarchitectonics of these polyelectrolyte complex hydrogels in the form of microcapsules and others have been investigated for their potential application as drug and cell carriers. Duan and co-workers used sodium alginate and poly-L ornithine as polyanions and polycations, and tailored polyelectrolyte composite hydrogels based on their electrostatic interactions. In particular, poly-L-ornithine, a positively charged synthetic amino acid, promotes cell adhesion.84 It also improves the stability of alginate chelated with polyvalent cations. Poly-L-ornithine has a weaker immune response and better biocompatibility than other polycations, e.g., poly-L-lysine. Furthermore, they can efficiently encapsulate enzymes and nano-sized therapeutic agents. Based on this property, a sustained release of at least 21 days was achieved. Alginate/poly-L-ornithine fibers have suitable mechanical properties and self-healing behavior. They are a favorable medium for cell growth and differentiation. The nanoarchitectonics of these bio-polyelectrolyte composite hydrogels is expected to make a significant contribution to biomedical applications as therapeutic agents and cell carriers.
Various nanomaterials have been used both in vitro and in vivo to facilitate intracellular delivery of small interfering RNA (siRNA) to induce post-transcriptional gene silencing via RNA interference.85 However, in many cases silencing efficiency is hampered by poor intracellular and nuclear delivery. Ahlenstiel and co-workers have delivered siRNA into the nucleus of HIV-1 infected cells to silence HIV-1 transcribed genes by layer-by-layer capsules (Fig. 15).86 The siRNA was complexed with multilayer particles formed by a layer-by-layer assembly of poly(styrenesulfonate) and poly(arginine). siRNA was incubated with HIV-infected cell types to study its action. This layer-by-layer capsule system mediates the nuclear delivery of siPromA to gene promoter sites and induces a reduction in viral RNA and protein levels. Viral RNA and protein were measured and functional viral silencing by siRNA delivered with the particles was confirmed after 16 days of treatment. These results pave the way for future studies on particle-delivered siRNA for efficient transcriptional gene silencing of various diseases and infections, including HIV. It provides a non-viral particle platform for nuclear-targeted siRNA delivery as well. More importantly, it brought the fundamental knowledge for research to achieve epigenetic silencing of genes involved in disease.
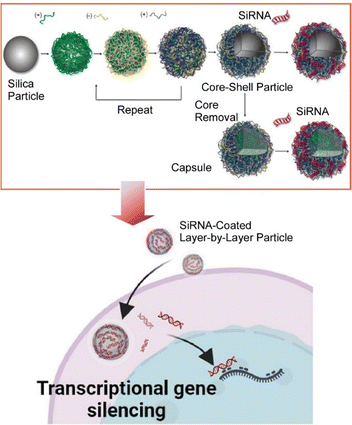 | ||
| Fig. 15 Delivery of siRNA into the nucleus of HIV-1 infected cells to silencing HIV-1 transcribed genes by layer-by-layer capsules of poly(styrenesulfonate) and poly(arginine). Reproduced with permission from ref. 86. Copyright 2023 American Chemical Society. | ||
Structural formation by layer-by-layer nanoarchitectonics is beneficial for organizing diverse functional units, including various bio-components, into the same system. In particular, biomedical applications require complex biological and chemical communications among many units, and layer-by-layer assemblies that can be adapted to a variety of situations can meet the demand. Layer-by-layer assembly can be effective in dealing with diseases that do not fit into a simple framework. It is useful for biomedical applications such as efficient drug delivery, gene delivery and gene silencing.
6. Short summary and perspectives
The concept of nanoarchitectonics universally applies to atoms, molecules, and nano-units for architecting functional material systems. Asymmetric structures and hierarchical organization, rather than mere assembly and organization of structures, and rational physical and chemical communications will lead to the development of more advanced functional materials. Layer-by-layer assembly can be a powerful tool for this purpose, as exemplified in this feature paper. Cleverly organized layer-by-layer structures produce various types of physical and chemical communications. They contribute to functions related to the control of thermal communication, optical communication, and chemical signal communication. Hierarchical and directed layer-by-layer structures also serve to control biological functions. Layer-by-layer nanofilm oriented structures can mimic the complex states found in real living organisms based on topographic cues and cell interactions. Alternatively, nanoarchitectonics of supramolecular assemblies that create communication between functional units not found in living organisms and co-assemble bacteriorhodopsin and ATP synthase in an artificial biomimetic system, which enables the conversion of light energy into chemical energy. Furthermore, layer-by-layer organization is useful for biomedical applications such as efficient drug delivery, gene delivery and gene silencing. On the other hand, the methodology of layer-by-layer assembly itself is still being pioneered. For example, highly oriented layer-by-layer membrane structures are being fabricated by spraying from low angles, and spontaneous multilayer creation using miscible and immiscible interfaces is also being investigated. New strategies such as multilayer metal-quinone networks that complement layer-by-layer structures have also been proposed. It can be concluded that material nanoarchitectonics based on the layer-by-layer method is an easy method to assemble asymmetric structures and hierarchical organization, and is a powerful technique for developing functions through physical and chemical communications.Finally, as a simple future perspective, we considered the development of functional materials from a broader perspective. The interaction of chemical substances dispersed in solution can be considered as the basic functional expression. On the other hand, the most developed system at the opposite end of the spectrum is a rational, hierarchically organized functional system, such as that found in biological systems. These systems are created through processes such as self-organization against the thermodynamic fate of increasing entropy. This artful organization, which living systems have achieved for billions of years, is what humanity is trying to achieve in a few decades of history. Much effort has been expended and much has been accomplished through self-assembly by supramolecular chemistry.87 Nanoarchitectonics, an integrated technology, goes beyond supramolecular chemistry to more deliberate and multifaceted construction of functional systems.88 The greatest functional construction is through the control of physical and chemical communications. Layer-by-layer assembly is a rational technology for building such structures. The greatest advantage of the layer-by-layer method is that it can be applied to a wide variety of materials. This characteristic is advantageous in assembling complex tissue systems such as biological systems, which are composed of many components. However, most current research examples tend to be individualistic functional studies using individual components. Contrary to such a tendency, it is possible to construct more advanced functional systems by assembling a large number of functional units in a layer-by-layer assembly. It may be difficult to construct such complex systems using conventional approaches. However, current layer-by-layer nanoarchitectonics does not seem to be fully utilizing its great potential.
Fortunately, mankind has developed new methodologies of machine learning89 and material informatics90 using artificial intelligence. The introduction of this concept may enable the development of organized structures composed of more complex components. Conceptual fusion of nanoarchitectonics and material informatics is proposed for the development of functional nanoporous materials.91 This conceptual fusion is worth introducing to layer-by-layer assembly, which has achieved diverse applications. If this can be done, it may be possible to artificially assemble the functional systems with rationally integrated organization as found in biological systems. Humans will be able to develop the functional systems that nature has achieved over billions of years of evolution within a short time span that we can experience.
Finally, we summarize the main messages of this review. In materials with organized functional components, physical and chemical communications of energy, electrons, signals, and molecules between unit structures is necessary for functional expression. This is essential for response behavior to external stimuli, as well as for energy accumulation, signal transduction, and other functions. For such communication, what is realistic and immediately achievable is nanoarchitectonics of controlled layered structures. The most useful approach for this is layer-by-layer assembly. This nanoarchitectonics strategy would develop highly functional systems like living organisms. To achieve this, it is essential to take into account factors such as operating out of equilibrium, metabolism, compartmentalization, and reciprocal interaction with the environment, which have been difficult in the conventional self-assembly approach. In this case, it will be necessary to further develop layer-by-layer nanoarchitectonics and go beyond it. It will also be necessary to integrate nanoarchitectonics with new concepts such as materials informatics.
Author contributions
Katsuhiko Ariga: conceptualization, writing – original draft writing – review & editing, funding acquisition. Jingwen Song: conceptualization, review & editing. Kohsaku Kawakami: project administration, review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was partially supported by the Japan Society for the Promotion of Science KAKENHI (Grant Numbers JP20H00392 and JP23H05459).Notes and references
- (a) C. Empel, C. Pei and R. M. Koenigs, Chem. Commun., 2022, 58, 2788–2798 RSC; (b) S. Kawamura, P. Barrio, S. Fustero, J. Escorihuela, J. A. Han, V. Soloshonok and M. Sodeoka, Adv. Synth. Catal., 2023, 365, 398–462 CrossRef CAS; (c) N. Tsubaki, Y. Wang, G. Yan and Y. He, Bull. Chem. Soc. Jpn., 2023, 96, 291–302 CrossRef CAS; (d) M. Toya, T. Omine, F. Ishiwari, A. Saeki, H. Ito and K. Itami, J. Am. Chem. Soc., 2023, 145, 11553–11565 CrossRef CAS PubMed; (e) S. Sugita, H. Ishitan and S. Kobayashi, Bull. Chem. Soc. Jpn., 2023, 96, 744–751 CrossRef CAS.
- (a) T. Yoshii, K. Chida, H. Nishihara and F. Tani, Chem. Commun., 2022, 58, 3578–3590 RSC; (b) Md. S. Islam, Y. Shudo and S. Hayami, Bull. Chem. Soc. Jpn., 2022, 95, 1–25 CrossRef; (c) S. Yoshino, T. Takayama, Y. Yamaguchi, A. Iwase and A. Kudo, Acc. Chem. Res., 2022, 55, 966–977 CrossRef CAS PubMed; (d) H. Tokoro, K. Nakabayashi, S. Nagashima, Q. Song, M. Yoshikiyo and S. Ohkosh, Bull. Chem. Soc. Jpn., 2022, 95, 538–552 CrossRef CAS; (e) K. Chen, J. Xiao, J. J. M. Vequizo, T. Hisatomi, Y. Ma, M. Nakabayashi, T. Takata, A. Yamakata, N. Shibata and K. Domen, J. Am. Chem. Soc., 2023, 145, 3839–3843 CrossRef CAS PubMed.
- (a) H. Lin, H. Bai, Z. Yang, Q. Shen, M. Li, Y. Huang, F. Lv and S. Wang, Chem. Commun., 2022, 58, 7232–7244 RSC; (b) D. Zhang, D. Liu, T. Ubukata and T. Seki, Bull. Chem. Soc. Jpn., 2022, 95, 138–162 CrossRef CAS; (c) H. Lai, C. Jin, J. Park, R. Ikura, Y. Takashima and M. Ouchi, Angew. Chem., Int. Ed., 2023, 62, e202218597 CrossRef CAS PubMed; (d) S. Hosokawa, A. Nagao, Y. Hashimoto, A. Matsune, T. Okazoe, C. Suzuki, H. Wada, T. Kakiuchi and A. Tsuda, Bull. Chem. Soc. Jpn., 2023, 96, 663–670 CrossRef CAS; (e) H. Watanabe and M. Kamigaito, J. Am. Chem. Soc., 2023, 145, 10948–10953 CrossRef CAS PubMed.
- (a) K. Hamada, D. Shimoyama, T. Hirao and T. Haino, Bull. Chem. Soc. Jpn., 2022, 95, 621–627 CrossRef CAS; (b) K. Diao, D. J. Whitaker, Z. Huang, H. Qian, D. Ren, L. Zhang, Z.-Y. Li, X.-Q. Sun, T. Xiao and L. Wang, Chem. Commun., 2022, 58, 2343–2346 RSC; (c) M. Y. Zhou, Z.-S. Yu, W. Deng, H.-L. Lu, X.-F. Niu, J. Tong, S.-Y. Yu and M. Fujita, Inorg. Chem., 2023, 62, 10193–10202 CrossRef CAS PubMed; (d) X. Han, S. Wang, M. Liu and L. Liu, Bull. Chem. Soc. Jpn., 2022, 95, 1445–1452 CrossRef CAS; (e) Y. Zhao, H. Kawano, H. Yamagishi, S. Otake, Y. Itoh, H. Huang, E. W. Meijer and T. Aida, J. Am. Chem. Soc., 2023, 145, 13920–13928 CrossRef CAS PubMed.
- (a) Y. Shan, G. Zhang, W. Yin, H. Pang and Q. Xu, Bull. Chem. Soc. Jpn., 2022, 95, 230–260 CrossRef CAS; (b) D. Zhao, X. Wang, L. Yue, Y. He and B. Chen, Chem. Commun., 2022, 58, 11059–11078 RSC; (c) Y. Domoto, M. Abe, G. R. Genov, Z. Yu and M. Fujita, Angew. Chem., Int. Ed., 2023, 62, e202303714 CrossRef CAS PubMed; (d) T. Ohata, K. Tachimoto, K. J. Takeno, A. Nomoto, T. Watanabe, I. Hirosawa and R. Makiura, Bull. Chem. Soc. Jpn., 2023, 96, 274–282 CrossRef CAS; (e) S. Horike, Bull. Chem. Soc. Jpn., 2023, 96, 887–898 CrossRef CAS.
- (a) R. Zeng, J. Xu, L. Lu, Q. Lin, X. Huang, L. Huang, M. Li and D. Tang, Chem. Commun., 2022, 58, 7562–7565 RSC; (b) T. Tamura, M. Inoue, Y. Yoshimitsu, I. Hashimoto, N. Ohashi, K. Tsumura, K. Suzuki, T. Watanabe and T. Hohsaka, Bull. Chem. Soc. Jpn., 2022, 95, 359–366 CrossRef CAS; (c) M. I. Setyawati, Q. Wang, N. Ni, J. K. Tee, K. Ariga, P. C. Ke, H. K. Ho, Y. Wang and D. T. Leong, Nat. Commun., 2021, 14, 4269 CrossRef PubMed; (d) T. Murata, K. Minami, T. Yamazaki, T. Sato, H. Koinuma, K. Ariga and N. Matsuki, Bull. Chem. Soc. Jpn., 2023, 96, 29–34 CrossRef CAS; (e) A. Amano, N. Sanjo, W. Araki, Y. Anraku, M. Nakakido, E. Matsubara, T. Tomiyama, T. Nagata, K. Tsumoto, K. Kataoka and T. Yokota, J. Nanobiotechnol., 2023, 21, 36 CrossRef CAS PubMed.
- (a) C. Liu, N. Morimoto, L. Jiang, S. Kawahara, T. Noritomi, T. H. Yokoyama, K. Mayumi and K. Ito, Science, 2021, 372, 1078–1081 CrossRef CAS PubMed; (b) O. S. Hammond and A.-V. Mudring, Chem. Commun., 2022, 58, 3865–3892 RSC; (c) K. Maeda, F. Takeiri, G. Kobayashi, S. Matsuishi, H. Ogino, S. Ida, T. Mori, Y. Uchimoto, S. Tanabe, T. Hasegawa, N. Imanaka and H. Kageyama, Bull. Chem. Soc. Jpn., 2022, 95, 26–37 CrossRef CAS; (d) M. Tenjimbayashi, S. Yamamoto and K. Uto, Adv. Mater., 2023, 35, 2300486 CrossRef CAS PubMed; (e) T. Adschiri, S. Takami, M. Umetsu, S. Ohara, T. Naka, K. Minami, D. Hojo, T. Togashi, T. Arita, M. Taguchi, M. Itoh, N. Aoki, G. Seong, T. Tomai and A. Yoko, Bull. Chem. Soc. Jpn., 2023, 96, 133–147 CrossRef CAS; (f) S. d K. Sundaram, Md. M. Hossain, M. Rezki, K. Ariga and S. Tsujimura, Biosensors, 2023, 13, 1018 CrossRef PubMed.
- (a) D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Science, 2016, 351, 361–365 CrossRef CAS PubMed; (b) K. Yamamoto, T. Imaoka, M. Tanabe and T. Kambe, Chem. Rev., 2020, 120, 1397–1437 CrossRef CAS PubMed; (c) H. Imahori, Bull. Chem. Soc. Jpn., 2023, 96, 339–352 CrossRef CAS; (d) M. Terazima, Bull. Chem. Soc. Jpn., 2023, 96, 852–871 CrossRef CAS.
- (a) R. P. Feynman, Eng. Sci., 1960, 23, 32–36 Search PubMed; (b) M. Roukes, Sci. Am., 2001, 285, 48–51 CrossRef CAS PubMed.
- (a) Y. Sugimoto, P. Pou, M. Abe, P. Jelinek, R. Pérez, S. Morita and Ó. Custance, Nature, 2007, 446, 64–67 CrossRef CAS PubMed; (b) K. Kimura, K. Miwa, H. Imada, M. Imai-Imada, S. Kawahara, J. Takeya, M. Kawai, M. Galperin and Y. Kim, Nature, 2019, 570, 210–213 CrossRef CAS PubMed; (c) J. M. Beach, M. Kryuchkova, R. Fakhrullin, K. Mazurova, A. Stavitskaya, B. J. Cheatham and R. Fakhrullin, Bull. Chem. Soc. Jpn., 2023, 96, 72–83 CrossRef CAS; (d) T. Ishikawa, F. Tanaka, K. Kurushima, A. Yasuhara, R. Sagawa, T. Fujita, R. Yonesaki, K. Iseki, T. Nakamuro, K. Harano and E. Nakamura, J. Am. Chem. Soc., 2023, 145, 12244–12254 CrossRef CAS PubMed; (e) K. Tada, Y. Hinuma, S. Ichikawa and S. Tanaka, Bull. Chem. Soc. Jpn., 2023, 96, 373–380 CrossRef CAS; (f) M. Ishii, Y. Yamashita, S. Watanabe, K. Ariga and J. Takeya, Nature, 2023, 622, 285–291 CrossRef CAS PubMed.
- K. Ariga, Nanoscale Horiz., 2021, 6, 364–378 RSC.
- (a) K. Ariga, Q. Ji, J. P. Hill, Y. Bando and M. Aono, NPG Asia Mater., 2012, 4, e17 CrossRef; (b) K. Ariga and M. Aono, Jpn. J. Appl. Phys., 2016, 55, 1102A6 CrossRef.
- (a) K. Ariga, Q. Ji, W. Nakanishi, J. P. Hill and M. Aono, Mater. Horiz., 2015, 2, 406–413 RSC; (b) K. Ariga, K. Minami, M. Ebara and J. Nakanishi, Polym. J., 2016, 48, 371–389 CrossRef CAS; (c) K. Ariga, Curr. Opin. Colloid Interface Sci., 2023, 63, 101656 CrossRef CAS.
- (a) K. Ariga, ChemNanoMat, 2023, 9, e202300120 CrossRef CAS; (b) K. Ariga, Beilstein J. Nanotechnol., 2023, 14, 434 CrossRef CAS PubMed.
- (a) K. Ariga, J. Li, J. Fei, Q. Ji and J. P. Hill, Adv. Mater., 2016, 28, 1251–1286 CrossRef CAS PubMed; (b) K. Ariga, Small Methods, 2022, 6, 2101577 CrossRef PubMed; (c) K. Ariga, Small, 2023, 2305636 CrossRef PubMed.
- (a) M. Ramanathan, L. K. Shrestha, T. Mori, Q. Ji, J. P. Hill and K. Ariga, Phys. Chem. Chem. Phys., 2013, 15, 10580–10611 RSC; (b) W. Nakanishi, K. Minami, L. K. Shrestha, Q. Ji, J. P. Hill and K. Ariga, Nano Today, 2014, 9, 378–394 CrossRef CAS; (c) K. Ariga, Trends Chem., 2020, 2, 779–782 CrossRef CAS; (d) L. Huang, J. Yang, Y. Asakura, Q. Shuai and Y. Yamauchi, ACS Nano, 2023, 17, 8918–8934 CrossRef CAS PubMed; (e) X. Guan, Z. Li, X. Geng, Z. Lei, A. Karakoti, T. Wu, P. Kumar, J. Yi and A. Vinu, Small, 2023, 19, 2207181 CrossRef CAS PubMed.
- (a) L. Zhang, T. Wang, Z. Shen and M. Liu, Adv. Mater., 2016, 28, 1044–1059 CrossRef CAS PubMed; (b) K. Ariga, T. Mori, T. Kitao and T. Uemura, Adv. Mater., 2020, 32, 1905657 CrossRef CAS PubMed; (c) K. Ariga and M. Shionoya, Bull. Chem. Soc. Jpn., 2021, 94, 839–859 CrossRef CAS; (d) L. Cao, Y. Huang, B. Parakhonskiy and A. G. Skirtach, Nanoscale, 2022, 14, 15964–16002 RSC; (e) S. Pahal, R. Boranna, A. Tripathy, V. S. Goudar, V. T. Veetil, R. Kurapati, G. R. Prashanth and P. K. Vemula, ChemNanoMat, 2023, 9, e202200462 CrossRef CAS.
- (a) A. Nayak, S. Unayama, S. Tai, T. Tsuruoka, R. Waser, M. Aono, I. Valov and T. Hasegawa, Adv. Mater., 2018, 30, 1703261 CrossRef PubMed; (b) M. Gharibshahi, F. Jamali-Sheini and R. Yousefi, Adv. Powder Technol., 2022, 33, 103517 CrossRef CAS; (c) M. Eguchi, A. S. Nugraha, A. E. Rowan, J. Shapter and Y. Yamauchi, Adv. Sci., 2021, 8, 2100539 CrossRef CAS PubMed; (d) Y. Qiu, X. Zhou, X. Tang, Q. Hao and M. Chen, Materials, 2023, 16, 2253 CrossRef CAS PubMed; (e) D. Deepak, N. Soin and S. S. Roy, Mater. Today Commun., 2023, 34, 105412 CrossRef CAS.
- (a) S. Howorka, Langmuir, 2013, 29, 7344–7353 CrossRef CAS PubMed; (b) M. Komiyama, K. Yoshimoto, M. Sisido and K. Ariga, Bull. Chem. Soc. Jpn., 2017, 90, 967–1004 CrossRef; (c) X. Shen, J. Song, C. Sevencan and D. T. Leong, Sci. Technol. Adv. Mater., 2023, 23, 199–224 CrossRef PubMed; (d) R. Chang, L. Zhao, R. Xing, J. Li and X. Yan, Chem. Soc. Rev., 2023, 52, 2688–2712 RSC; (e) X. Jia, J. Chen, W. Lv, H. Li and K. Ariga, Cell Rep. Phys. Sci., 2023, 4, 101251 CrossRef CAS.
- (a) M. Kim, K. L. Firestein, J. F. S. Fernando, X. Xu, H. Lim, D. V. Golberg, J. Na, J. Kim, H. Nara, J. Tang and Y. Yamauchi, Chem. Sci., 2022, 13, 10836–10845 RSC; (b) A. Kumar, P. Choudhary, T. Chhabra, H. Kaur, A. Kumar, M. Qamar and V. Krishnan, Mater. Chem. Front., 2023, 7, 1197–1247 RSC; (c) G. Chen, S. K. Singh, K. Takeyasu, J. P. Hill, J. Nakamura and K. Ariga, Sci. Technol. Adv. Mater., 2023, 23, 413–423 CrossRef PubMed; (d) D. Sharma, P. Choudhary, S. Kumar and V. Krishnan, Small, 2023, 19, 2207053 CrossRef CAS PubMed; (e) X. Zhang, K. Matras-Postolek, P. Yang and S. P. Jiang, J. Colloid Interface Sci., 2023, 636, 646–656 CrossRef CAS PubMed.
- (a) S. Ishihara, J. Labuta, W. V. Rossom, D. Ishikawa, K. Minami, J. P. Hill and K. Ariga, Phys. Chem. Chem. Phys., 2014, 16, 9713–9746 RSC; (b) M. Komiyama, T. Mori and K. Ariga, Bull. Chem. Soc. Jpn., 2018, 91, 1075–1111 CrossRef CAS; (c) J. Liu, H. Zhou, W. Yang and K. Ariga, Acc. Chem. Res., 2020, 53, 644–653 CrossRef CAS PubMed; (d) S. Kim, S. Baek, R. Sluyter, K. Konstantinov, J. H. Kim, S. Kim and Y. H. Kim, EcoMat, 2023, 5, e12356 CrossRef; (e) J. V. Vaghasiya, C. C. Mayorga-Martinez and M. Pumera, npj Flexible Electron., 2023, 7, 26 CrossRef PubMed.
- (a) K. Ariga, Q. Ji, T. Mori, M. Naito, Y. Yamauchi, H. Abe and J. P. Hill, Chem. Soc. Rev., 2013, 42, 6322–6345 RSC; (b) J. M. Giussi, M. L. Cortez, W. A. Marmisollé and O. Azzaroni, Chem. Soc. Rev., 2019, 48, 814–849 RSC; (c) T. Tsuchiya, T. Nakayama and K. Ariga, Appl. Phys. Express, 2022, 15, 100101 CrossRef; (d) S. Baek, S. Kim, S. A. Han, Y. H. Kim, S. Kim and J. H. Kim, ChemNanoMat, 2023, 9, e202300104 CrossRef CAS; (e) O. Azzaroni, E. Piccinini, G. Fenoy, W. Marmisollé and K. Ariga, Nanotechnology, 2023, 34, 472001 CrossRef PubMed.
- (a) A. H. Khan, S. Ghosh, B. Pradhan, A. Dalui, L. K. Shrestha, S. Acharya and K. Ariga, Bull. Chem. Soc. Jpn., 2017, 90, 627–648 CrossRef; (b) J. Kim, J. H. Kim and K. Ariga, Joule, 2017, 1, 739–768 CrossRef CAS; (c) J.-C. Feng and H. Xia, Beilstein J. Nanotechnol., 2022, 13, 1185–1200 CrossRef CAS PubMed; (d) X. Zhang and P. Yang, ChemNanoMat, 2023, 9, e202300041 CrossRef CAS; (e) A. Dalui, K. Ariga and S. Acharya, Chem. Commun., 2023, 59, 10835–10865 RSC.
- (a) K. Ariga, S. Ishihara, H. Abe, M. Li and J. P. Hill, J. Mater. Chem., 2012, 22, 2369–2377 RSC; (b) H. Yu, Y. Pan, Y. Zhang, L. Gong, Z. Lou, W. Shan and Y. Xiong, J. Taiwan Inst. Chem. Eng., 2023, 143, 104716 CrossRef CAS; (c) K. K. R. Datta, ChemNanoMat, 2023, 9, e202300135 CrossRef CAS; (d) D. Barreca and C. Maccato, CrystEngComm, 2023, 25, 3968–3987 RSC; (e) B. N. Bhadra, L. K. Shrestha and K. Ariga, J. Inorg. Organomet. Polym. Mater., 2023, 33, 637–662 CrossRef CAS.
- (a) B. Li, Y. Huang and Q. Zou, ChemBioChem, 2023, 24, e202300002 CrossRef CAS PubMed; (b) W. Hu, J. Shi, W. Lv, X. Jia and K. Ariga, Sci. Technol. Adv. Mater., 2023, 23, 393 CrossRef PubMed; (c) J. Song, K. Kawakami and K. Ariga, Curr. Opin. Colloid Interface Sci., 2023, 65, 101702 CrossRef CAS; (d) P. Jayachandran, S. Ilango, V. Suseela, R. Nirmaladevi, M. R. Shaik, M. Khan, M. Khan and B. Shaik, Biomedicines, 2023, 11, 217 CrossRef CAS PubMed; (e) L. Sutrisno and K. Ariga, NPG Asia Mater., 2023, 15, 21 CrossRef CAS.
- R. B. Laughlin and D. Pines, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 28–31 CrossRef CAS PubMed.
- (a) K. Ariga and R. Fakhrullin, Bull. Chem. Soc. Jpn., 2022, 95, 774–795 CrossRef CAS; (b) K. Ariga, Beilstein J. Nanotechnol., 2023, 14, 738–740 CrossRef CAS PubMed; (c) K. Ariga, Bull. Chem. Soc. Jpn., 2024, 97, uoad001 CrossRef.
- K. Ariga, X. Jia, J. Song, J. P. Hill, D. T. Leong, Y. Jia and J. Li, Angew. Chem., Int. Ed., 2020, 59, 15424–15446 CrossRef CAS PubMed.
- M. Aono and K. Ariga, Adv. Mater., 2016, 28, 989–992 CrossRef CAS PubMed.
- K. Ariga, Mater. Chem. Front., 2017, 1, 208–211 RSC.
- (a) K. Ariga and Y. Yamauchi, Chem. – Asian J., 2020, 15, 718–728 CrossRef CAS PubMed; (b) J. Song, X. Jia and K. Ariga, Small Methods, 2020, 4, 2000500 CrossRef CAS.
- (a) I. R. Vetter and A. Wittinghofer, Science, 2001, 294, 1299–1304 CrossRef CAS PubMed; (b) K. N. Ferreira, T. M. Iverson, K. Maghlaoui, J. Barber and S. Iwata, Science, 2004, 303, 1831–1838 CrossRef CAS PubMed; (c) D. A. Bryant and D. P. Canniffe, J. Phys. B: At., Mol. Opt. Phys., 2018, 51, 033001 CrossRef.
- (a) K. Ariga, Y. Yamauchi, T. Mori and J. P. Hill, Adv. Mater., 2013, 25, 6477–6512 CrossRef CAS PubMed; (b) K. Ariga, Langmuir, 2020, 36, 7158–7180 CrossRef CAS PubMed; (c) M. Ito, Y. Yamashita, T. Mori, M. Chiba, T. Futae, J. Takeya, S. Watanabe and K. Ariga, Langmuir, 2022, 38, 5237–5247 CrossRef CAS PubMed; (d) S. Negi, M. Hamori, Y. Kubo, H. Kitagishi and K. Kano, Bull. Chem. Soc. Jpn., 2023, 96, 48–56 CrossRef CAS.
- (a) G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS; (b) K. Ariga, J. P. Hill and Q. Ji, Phys. Chem. Chem. Phys., 2007, 9, 2319–2340 RSC; (c) K. Ariga, Y. Lvov and G. Decher, Phys. Chem. Chem. Phys., 2022, 24, 4097–4115 RSC.
- (a) G. Rydzek, Q. Ji, M. Li, P. Schaaf, J. P. Hill, F. Boulmedais and K. Ariga, Nano Today, 2015, 10, 138–167 CrossRef CAS; (b) K. Ariga, T. Mori and J. Li, Langmuir, 2019, 35, 3585–3599 CrossRef CAS PubMed; (c) K. Ariga, Nanoscale, 2022, 14, 10610–10621 RSC; (d) K. Ariga, Chem. Mater., 2023, 35, 5233–5254 CrossRef CAS.
- (a) M. Ito, Y. Yamashita, Y. Tsuneda, T. Mori, J. Takeya, S. Watanabe and K. Ariga, ACS Appl. Mater. Interfaces, 2020, 12(50), 56522–56529 CrossRef PubMed; (b) O. N. Oliveira Jr., L. Caseli and K. Ariga, Chem. Rev., 2022, 122, 6459–6513 CrossRef CAS PubMed; (c) K. Ariga, Acc. Mater. Res., 2022, 3, 404–410 CrossRef CAS; (d) J. Adachi, M. Naito, S. Sugiura, N. H.-T. Le, S. Nishimura, S. Huang, S. Suzuki, S. Kawamorita, N. Komiya, J. P. Hill, K. Ariga, T. Naota and T. Mori, Bull. Chem. Soc. Jpn., 2022, 95, 889–897 CrossRef CAS.
- (a) G. Decher, M. Eckle, J. Schmitt and B. Struth, Curr. Opin. Colloid Interface Sci., 1998, 3, 32–39 CrossRef CAS; (b) J. J. Richardson, M. Björnmalm and F. Caruso, Science, 2015, 348, aaa2491 CrossRef PubMed; (c) Z. Zhang, J. Zeng, J. Groll and M. Matsusaki, Biomater. Sci., 2022, 10, 4077–4409 RSC.
- (a) W. B. Stockton and M. F. Rubner, Macromolecules, 1997, 30, 2717–2725 CrossRef CAS; (b) M. Dolmat, V. Kozlovskaya, D. Inman, C. Thomas and E. Kharlampieva, Polym. Sci., 2023, 61, 1052–1064 CrossRef CAS.
- (a) H. Lee, L. J. Kepley, H. G. Hong and T. E. Mallouk, J. Am. Chem. Soc., 1988, 110, 618–620 CrossRef CAS; (b) M. Altman, A. D. Shukla, T. Zubkov, G. Evmenenko, P. Dutta and M. E. van der Boom, J. Am. Chem. Soc., 2006, 128, 7374–7382 CrossRef CAS PubMed; (c) G. L. Lee, T. Chan, J. M. Palasz and C. P. Kubiak, J. Phys. Chem. C, 2022, 126, 16522–16528 CrossRef CAS.
- (a) Y. Shimazaki, M. Mitsuishi, S. Ito and M. Yamamoto, Langmuir, 1997, 13, 1385–1387 CrossRef CAS; (b) H. Fujita and T. Michinobu, Soft Matter, 2018, 14, 9055–9060 RSC.
- (a) T. Serizawa, K. Hamada, T. Kitayama, N. Fujimoto, K. Hatada and M. Akashi, J. Am. Chem. Soc., 2000, 122, 1891–1899 CrossRef CAS; (b) T. Serizawa, K. Hamada and M. Akashi, Nature, 2004, 429, 52–55 CrossRef CAS PubMed.
- A. Ikeda, T. Hatano, S. Shinkai, T. Akiyama and S. Yamada, J. Am. Chem. Soc., 2001, 123, 4855–4856 CrossRef CAS PubMed.
- (a) Y. Lvov, K. Ariga, I. Ichinose and T. Kunitake, J. Chem. Soc., Chem. Commun., 1995, 2313–2314 RSC; (b) J. Anzai, H. Takeshita, Y. Kobayashi, T. Osa and T. Hoshi, Anal. Chem., 1998, 70, 811–817 CrossRef CAS PubMed.
- (a) I. Ichinose, H. Senzu and T. Kunitake, Chem. Mater., 1997, 9, 1296–1298 CrossRef CAS; (b) S. Amigoni, E. Taffin de Givenchy, M. Dufay and F. Guittard, Langmuir, 2009, 25, 11073–11077 CrossRef CAS PubMed; (c) M. Li, S. Ishihara, M. Akada, M. Liao, L. Sang, J. P. Hill, V. Krishnan, Y. Ma and K. Ariga, J. Am. Chem. Soc., 2011, 133, 7348–7351 CrossRef CAS PubMed.
- (a) G. Decher, J. D. Hong and J. Schmitt, Thin Solid Films, 1992, 210–211, 831–835 CrossRef CAS; (b) S. S. Shiratori and M. F. Rubner, Macromolecules, 2000, 33, 4213–4219 CrossRef CAS.
- (a) O. Kulakovich, N. Strekal, A. Yaroshevich, S. Maskevich, S. Gaponenko, I. Nabiev, U. Woggon and M. Artemyev, Nano Lett., 2002, 2, 1449–1452 CrossRef CAS; (b) M. G. Kim, N.-K. Cho, J. H. Ma, M. H. Park, J. H. Park, W. Jeon and S. J. Kang, ACS Appl. Nano Mater., 2023, 6, 14114–14126 CrossRef CAS.
- (a) N. A. Kotov, I. Dekany and J. H. Fendler, J. Phys. Chem., 1995, 99, 13065–13069 CrossRef CAS; (b) Y. Lvov, K. Ariga, M. Onda, I. Ichinose and T. Kunitake, Langmuir, 1997, 13, 6195–6203 CrossRef CAS.
- (a) Y. Lvov, K. Ariga, I. Ichinose and T. Kunitake, Langmuir, 1996, 12, 3038–3044 CrossRef CAS; (b) T. Sasaki, Y. Ebina, T. Tanaka, M. Harada, M. Watanabe and G. Decher, Chem. Mater., 2001, 13, 4661–4667 CrossRef CAS.
- (a) Y. Lvov, K. Ariga and T. Kunitake, Chem. Lett., 1994, 2323–2326 CrossRef CAS; (b) Y. Lvov, K. Ariga, I. Ichinose and T. Kunitake, J. Am. Chem. Soc., 1995, 117, 6117–6123 CrossRef CAS.
- Y. Lvov, H. Haas, G. Decher, H. Moehwald, A. Mikhailov, B. Mtchedlishvily, E. Morgunova and B. Vainshtein, Langmuir, 1994, 10, 4232–4236 CrossRef CAS.
- (a) G. Decher and J. D. Hong, Makromol. Chem., Macromol. Symp., 1991, 46, 321–327 CrossRef CAS; (b) S. S. Shiratori and M. Yamada, Polym. Adv. Technol., 2000, 11, 810–814 CrossRef CAS.
- (a) S.-S. Lee, J.-D. Hong, C. H. Kim, K. Kim, J. P. Koo and K.-B. Lee, Macromolecules, 2001, 34, 5358–5360 CrossRef CAS; (b) C. Jiang, S. Markutsya and V. Tsukruk, Adv. Mater., 2004, 16, 157–161 CrossRef CAS.
- (a) J. B. Schlenoff, S. T. Dubas and T. Farhat, Langmuir, 2000, 16(26), 9968–9969 CrossRef CAS; (b) A. Izquierdo, S. S. Ono, J.-C. Voegel, P. Schaaf and G. Decher, Langmuir, 2005, 21, 7558–7567 CrossRef CAS PubMed.
- (a) E. Donath, G. B. Sukhorukov, F. Caruso, S. A. Davis and H. Möhwald, Angew. Chem, Int. Ed., 1998, 37, 2202–2205 CrossRef CAS; (b) F. Caruso, R. A. Caruso and H. Möhwald, Science, 1998, 282, 1111–1114 CrossRef CAS PubMed; (c) Y. Lvov, A. A. Antipov, A. Mamedov, H. Möhwald and G. B. Sukhorukov, Nano Lett., 2001, 1, 125–128 CrossRef CAS.
- Q. Ji, M. Miyahara, J. P. Hill, S. Acharya, A. Vinu, S. B. Yoon, J.-S. Yu, K. Sakamoto and K. Ariga, J. Am. Chem. Soc., 2008, 130, 2376–2377 CrossRef CAS PubMed.
- Q. Ji, S. B. Yoon, J. P. Hill, A. Vinu, J.-S. Yu and K. Ariga, J. Am. Chem. Soc., 2009, 131, 4220–4221 CrossRef CAS PubMed.
- Q. Ji, S. Acharya, J. P. Hill, A. Vinu, S. B. Yoon, J.-S. Yu, K. Sakamoto and K. Ariga, Adv. Funct. Mater., 2009, 19, 1792–1799 CrossRef CAS.
- P. Gutfreund, C. Higy, G. Fragneto, M. Tschopp, O. Felix and G. Decher, Nat. Commun., 2023, 14, 4076 CrossRef CAS PubMed.
- R. Blell, X. Lin, T. Lindström, M. Ankerfors, M. Pauly, O. Felix and G. Decher, ACS Nano, 2017, 11, 84–94 CrossRef CAS PubMed.
- (a) Z. Tang, N. A. Kotov, S. Magonov and B. Ozturk, Nat. Mater., 2003, 2, 413–418 CrossRef CAS PubMed; (b) U. G. K. Wegst, H. Bai, E. Saiz, A. P. Tomsia and R. O. Ritchie, Nat. Mater., 2015, 14, 23–36 CrossRef CAS PubMed.
- C. Zhao, P. Zhang, J. Zhou, S. Qi, Y. Yamauchi, R. Shi, R. Fang, Y. Ishida, S. Wang, A. P. Tomsia, M. Liu and L. Jiang, Nature, 2020, 580, 210–215 CrossRef CAS PubMed.
- S. Tang, J. Gong, Y. Shi, S. Wen and Q. Zhao, Nat. Commun., 2022, 13, 3227 CrossRef PubMed.
- (a) M. Bieri, M. Treier, J. Cai, K. Aït-Mansour, P. Ruffieux, O. Gröning, P. Gröning, M. Kastler, R. Rieger, X. Feng, K. Müllen and R. Fasel, Chem. Commun., 2009, 6919–6921 RSC; (b) D. N. Bunck and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 14952–14955 CrossRef CAS PubMed; (c) W. Liu, X. Luo, Y. Bao, Y. P. Liu, G.-H. Ning, I. Abdelwahab, L. Li, C. T. Nai, Z. G. Hu, D. Zhao, B. Liu, S. Y. Quek and K. P. Loh, Nat. Chem., 2017, 9, 563–570 CrossRef CAS PubMed.
- K. Liu, H. Qi, R. Dong, R. Shivhare, M. Addicoat, T. Zhang, H. Sahabudeen, T. Heine, S. Mannsfeld, U. Kaiser, Z. Zheng and X. Feng, Nat. Chem., 2019, 11, 994–1000 CrossRef CAS PubMed.
- A. Yurtsever, L. Sun, K. Hirata, T. Fukuma, S. Rath, H. Zareie, S. Watanabe and M. Sarikaya, ACS Nano, 2023, 17, 7311–7325 CrossRef CAS PubMed.
- L. Liu, X. Shan, X. Hu, W. Lv and J. Wang, ACS Nano, 2021, 15(12), 19771–19782 CrossRef CAS PubMed.
- H. K. Choi, A. Lee, M. Park, D. S. Lee, S. Bae, S.-K. Lee, S. H. Lee, T. Lee and T.-W. Kim, ACS Nano, 2021, 15, 829–839 CrossRef CAS PubMed.
- N. Lama, J. Wilhite, Y. Lvov, S. Konnova and R. Fakhrullin, ChemNanoMat, 2023, 9, e202300106 CrossRef CAS.
- W. Wu, Y. Battie, C. Genet, T. W. Ebbesen, G. Decher and M. Pauly, Adv. Opt. Mater., 2023, 11, 2202831 CrossRef CAS.
- (a) Z. Guo, A. K. Jena, G. M. Kim and T. Miyasaka, Energy Environ. Sci., 2022, 15, 3171–3222 RSC; (b) H. Mori, Y. Yamada, Y. Minagawa, N. Hasegawa and Y. Nishihara, Bull. Chem. Soc. Jpn., 2022, 95, 942–952 CrossRef CAS; (c) C.-Y. Lin and T. Michinobu, Beilstein J. Nanotechnol., 2023, 14, 454–466 CrossRef CAS PubMed; (d) Y. Tsuchii, T. Menda, S. Hwang and T. Yasuda, Bull. Chem. Soc. Jpn., 2023, 96, 90–94 CrossRef CAS; (e) W. Peng, K. Mao, F. Cai, H. Meng, Z. Zhu, T. Li, S. Yuan, Z. Xu, X. Feng, J. Xu, M. D. McGehee and J. Xu, Science, 2023, 379, 683–690 CrossRef CAS PubMed.
- R. Sun, Q. Wu, J. Guo, T. Wang, Y. Wu, B. Qiu, Z. Luo, W. Yang, Z. Hu, J. Guo, M. Shi, C. Yang, F. Huang, Y. Li and J. Min, Joule, 2020, 4, 407–419 CrossRef CAS.
- (a) F. Caruso, Nanoengineering of Particle Surfaces, Adv. Mater., 2001, 13, 11–22 CrossRef CAS; (b) Y. Wang, A. S. Angelatos and F. Caruso, Chem. Mater., 2008, 20, 848–858 CrossRef CAS; (c) T. Kruk, K. Chojnacka-Górka, M. Kolasińska-Sojka and S. Zapotoczny, Adv. Colloid Interface Sci., 2022, 310, 102773 CrossRef CAS PubMed.
- Q.-X. Zhong, J. J. Richardson, Y. Tian, H. Tian, J. Cui, S. Mann and F. Caruso, Angew. Chem., Int. Ed., 2023, 62, e202218021 CrossRef CAS PubMed.
- M. H. Iqbal, F. J. R. Revana, E. Pradel, V. Gribova, K. Mamchaoui, C. Coirault, F. Meyer and F. Boulmedais, ACS Nano, 2022, 16, 20034–20043 CrossRef CAS PubMed.
- Y. Zhang, J. L. Y. Wu, J. Lazarovits and W. C. W. Chan, J. Am. Chem. Soc., 2020, 142, 8827–8836 CrossRef PubMed.
- (a) Y. Lin, H. Zhang, Y. Zou, K. Lu, L. Li, Y. Wu, J. Cheng, Y. Zhang, H. Chen and Q. Yu, J. Mater. Sci. Technol., 2023, 132, 18–26 CrossRef CAS; (b) M. Usui, Y. Yoshii, S. Thiriet-Rupert, J.-M. Ghigo and C. Beloin, Commun. Biol., 2023, 6, 275 CrossRef CAS PubMed; (c) Y. Zhan, X. Hu, Y. Li, Y. Wang, H. Chen, C. A. Omolo, T. Govender, H. Li, F. Huang, L. Shi, X. Hu and Y. Liu, Adv. Funct. Mater., 2023, 33, 2214299 CrossRef CAS.
- E. Deiss-Yehiely, G. Cárcamo-Oyarce, A. G. Berger, K. Ribbeck and P. T. Hammond, ACS Biomater. Sci. Eng., 2023, 9, 4794–4804 CrossRef CAS PubMed.
- Z. Li, X. Xu, F. Yu, J. Fei, Q. Li, M. Dong and J. Li, Angew. Chem., Int. Ed., 2022, 61, e202116220 CrossRef CAS PubMed.
- (a) C. E. Mora-Huertas, H. Fessi and A. Elaissari, Int. J. Pharm., 2010, 385, 113–142 CrossRef CAS PubMed; (b) M. Delcea, H. Möhwald and A. G. Skirtach, Adv. Drug Delivery Rev., 2011, 63, 730–747 CrossRef CAS PubMed; (c) J. Li, B. V. Parakhonskiy and A. G. Skirtach, Chem. Commun., 2023, 59, 807–835 RSC.
- S. Correa, N. Boehnke, E. Deiss-Yehiely and P. T. Hammond, ACS Nano, 2019, 13, 5623–5634 CrossRef CAS PubMed.
- (a) W. C. Mak, K. Y. Cheung and D. Trau, Adv. Funct. Mater., 2008, 18, 2930–2937 CrossRef CAS; (b) A. S. Timin, A. R. Muslimov, K. V. Lepik, O. S. Epifanovskaya, A. I. Shakirova, U. Mock, K. Riecken, M. V. Okilova, V. Sergeev, B. V. Afanasyev, B. Fehse and G. B. Sukhorukov, Nanomedicine, 2018, 14, 97–108 CrossRef CAS PubMed.
- I. Drachuk, S. Harbaugh, J. L. Chávez and N. Kelley-Loughnane, ACS Appl. Mater. Interfaces, 2020, 12, 48329–48339 CrossRef CAS PubMed.
- (a) D. Jiao, Q. L. Zhu, C. Y. Li, Q. Zheng and Z. L. Wu, Acc. Chem. Res., 2022, 55, 1533–1545 CrossRef CAS PubMed; (b) D. Aycan, F. Karaca, A. Koca and N. Alemdar, Int. J. Biol. Macromol., 2023, 231, 123297 CrossRef CAS PubMed.
- W. Xue, B. Liu, H. Zhang, S. Ryu, M. Kuss, D. Shukla, G. Hu, W. Shi, X. Jiang, Y. Lei and B. Duan, Acta Biomater., 2022, 138, 182–192 CrossRef CAS PubMed.
- (a) G. M. Traber and A.-M. Yu, J. Pharmacol. Exp. Ther., 2023, 384, 133–154 CrossRef CAS PubMed; (b) M. Ito, Y. Miyata and M. Okada, Transl. Oncol., 2023, 31, 101634 CrossRef CAS PubMed.
- E. Czuba-Wojnilowicz, V. Klemm, C. Cortez-Jugo, S. Turville, A. Aggarwal, F. Caruso, A. D. Kelleher and C. L. Ahlenstiel, Mol. Pharmaceutics, 2023, 20, 2039–2052 CrossRef CAS PubMed.
- (a) S. Datta, Y. Kato, S. Higashiharaguchi, K. Aratsu, A. Isobe, T. Saito, D. D. Prabhu, Y. Kitamoto, M. J. Hollamby, A. J. Smith, R. Dalgliesh, N. Mahmoudi, L. Pesce, C. Perego, G. M. Pavan and S. Yagai, Nature, 2020, 583, 400–405 CrossRef CAS PubMed; (b) Y. Takeuchi, K. Ohkura and Y. Nishina, Bull. Chem. Soc. Jpn., 2023, 96, 113–119 CrossRef CAS; (c) N. Uchida and T. Muraoka, Chem. Commun., 2023, 59, 9687–9697 RSC; (d) T. Matsuno and H. Isobe, Bull. Chem. Soc. Jpn., 2023, 96, 406–419 CrossRef CAS.
- (a) K. Ariga, V. Malgras, Q. Ji, M. B. Zakaria and Y. Yamauchi, Coord. Chem. Rev., 2016, 320–321, 139–152 CrossRef CAS; (b) Y. Sang and M. Liu, Mol. Syst. Des. Eng., 2019, 4, 11–28 RSC; (c) K. Ariga, M. Nishikawa, T. Mori, J. Takeya, L. K. Shrestha and J. P. Hill, Sci. Technol. Adv. Mater., 2020, 20, 51–95 CrossRef PubMed.
- (a) W. Ming, P. Sun, Z. Zhang, W. Qiu, J. Du, X. Li, Y. Zhang, G. Zhang, K. Liu, Y. Wang and X. Guo, Int. J. Hydrogen Energy, 2023, 48, 5197–5228 CrossRef CAS; (b) Y. Yamamoto, S. Nakano and Y. Shigeta, Bull. Chem. Soc. Jpn., 2023, 96, 42–47 CrossRef CAS; (c) T. Yasuda, S. Ookawara, S. Yoshikawa and H. Matsumoto, Chem. Eng. J., 2023, 453, 139540 CrossRef CAS; (d) N. Saito, A. Nawachi, Y. Kondo, J. Choi, H. Morimoto and T. Ohshima, Bull. Chem. Soc. Jpn., 2023, 96, 465–474 CrossRef CAS.
- (a) R. Ramprasad, R. Batra, G. Pilania, A. Mannodi-Kanakkithodi and C. Kim, npj Comput. Mater., 2017, 3, 54 CrossRef; (b) K. Takahashi and L. Takahashi, J. Phys. Chem. Lett., 2023, 14(20), 4726–4733 CrossRef CAS PubMed; (c) M. Hayakawa, K. Sakano, R. Kumada, H. Tobita, Y. Igarashi, D. Citterio, Y. Oaki and Y. Hiruta, Polym. Chem., 2023, 14, 2383–2389 RSC.
- W. Chaikittisilp, Y. Yamauchi and K. Ariga, Adv. Mater., 2022, 34, 2107212 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |