 Open Access Article
Open Access ArticleChitosan-based multifunctional oxygenating antibiotic hydrogel dressings for managing chronic infection in diabetic wounds†
Shahrzad
Abri
a,
Hannah
Durr
b,
Hazel A.
Barton
c,
Kayla
Adkins-Travis
d,
Leah P.
Shriver
def,
Dipak D.
Pukale
a,
Judith A.
Fulton
gh and
Nic D.
Leipzig
 *ab
*ab
aDepartment of Chemical, Biomolecular, and Corrosion Engineering, The University of Akron, Akron, Ohio 44325, USA. E-mail: nl21@uakron.edu
bIntegrated Biosciences Program, Department of Biology, The University of Akron, Akron, Ohio 44325, USA
cDepartment of Geological Sciences, The University of Alabama, Tuscaloosa, Alabama 35487, USA
dDepartment of Chemistry, Washington University in Saint Louis, Saint Louis, MO 63130, USA
eCenter for Proteomics, Metabolomics, and Isotope Tracing, Washington University in Saint Louis, Saint Louis, MO 63130, USA
fDepartment of Medicine, Washington University in Saint Louis, Saint Louis, MO 63130, USA
gSumma Health System-Translational Research Center Akron, Akron, Ohio 44304, USA
hNortheast Ohio Medical University-REDIzone, Rootstown, Ohio 44272, USA
First published on 28th May 2024
Abstract
Current treatment strategies for infection of chronic wounds often result in compromised healing and necrosis due to antibiotic toxicity, and underlying biomarkers affected by treatments are not fully known. Here, a multifunctional dressing was developed leveraging the unique wound-healing properties of chitosan, a natural polysaccharide known for its numerous benefits in wound care. The dressing consists of an oxygenating perfluorocarbon functionalized methacrylic chitosan (MACF) hydrogel incorporated with antibacterial polyhexamethylene biguanide (PHMB). A non-healing diabetic infected wound model with emerging metabolomics tools was used to explore the anti-infective and wound healing properties of the resultant multifunctional dressing. Direct bacterial bioburden assessment demonstrated superior antibacterial properties of hydrogels over a commercial dressing. However, wound tissue quality analyses confirmed that sustained PHMB for 21 days resulted in tissue necrosis and disturbed healing. Therefore, a follow-up comparative study investigated the best treatment course for antiseptic application ranging from 7 to 21 days, followed by the oxygenating chitosan-based MACF treatment for the remainder of the 21 days. Bacterial counts, tissue assessments, and lipidomics studies showed that 14 days of application of MACF-PHMB dressings followed by 7 days of MACF dressings provides a promising treatment for managing infected non-healing diabetic skin ulcers.
Introduction
Non-healing chronic wounds associated with diabetes mellitus present a major challenge to patients and the healthcare system.1,2 Bacterial infection in these wounds is common, often leading to progressive systemic infection and limb amputation.3 This challenge can be further exacerbated by diabetic angiopathy and ischemia, which limit oxygen and supply of nutrients to the site of injury, leading to inflammation and increased risk of infection.4,5 Combating diabetic wound infections is often achieved via systemic and/or local antibiotic therapy to treat or prevent infections.6Considering the growing problem of multidrug resistance in bacteria, selecting an appropriate antibacterial treatment for infected non-healing wounds is challenging. This issue is highlighted for pathogenic bacterial strains such as Pseudomonas aeruginosa, due to their intrinsic resistance to antibiotics.7 Any long-term antibacterial treatment strategy must also consider that prolonged use of certain antibiotics can cause host tissue necrosis and inflammation, leading to chronic complications and further delayed wound healing.8 Antiseptics, such as PHMB or silver compounds, are effective against a wide spectrum of bacteria, with a reduced likelihood of triggering resistance.9 Nonetheless, use of antiseptics can be limited by their potential host toxicity towards cells essential to wound healing processes.8 Topical delivery systems can alleviate this problem by controlling the delivery of antiseptics to the skin, preventing the drug from entering systemic circulation in large doses and, ultimately, limiting tissue necrosis.10 As a result, the effectiveness of antiseptics for wound healing can be enhanced by encapsulating them into a carrier.
Many researchers, including our group,11–18 have previously utilized oxygen-delivering platforms to improve wound healing leading to enhancement of oxidative wound healing. However, to date there is limited research on wound oxygen therapies and their role in anti-infective strategies, especially in the form of easy-to-use oxygenating wound dressings. Oxygen availability is known to be important for host-driven antimicrobial responses for fuelling the production of ROS, while providing oxidative energy to upregulate the innate immune processes to combat infection.19,20 It has also been reported that supplemental oxygen can synergistically improve the antimicrobial effects of certain antibiotics by enhancing their functionality and increasing drug susceptibility of pathogens through stimulating aerobic respiration19,21 leading to an increased bacterial drug uptake and death.21,22
Therefore, with this motivation we designed an oxygenating controlled-release antibacterial platform via encapsulating PHMB antiseptic within our oxygenating perfluorocarbon methacrylamide chitosan (MACF) hydrogel sheet dressings. Chitosan, a naturally abundant carbohydrate, is the key component of our MACF-based wound dressing, imparting unique properties, such as antimicrobial activity, haemostatic properties, and ability to promote tissue regeneration and wound closure,23,24 that contribute to the efficacy and wound healing benefits of our dressings.19,20 For this study, we began with the hypothesis that chitosan would serve as a foundation for creating oxygenating MACF hydrogel sheet dressings against Pseudomonas aeruginosa infection and enhance wound healing processes in chronic diabetic dermal wounds. A main objective was to demonstrate that a chitosan-based oxygenating MACF dressing, encapsulating PHMB, provides superior anti-infective properties, accelerated wound closure, and reduced adverse effects compared to a commercial wound dressing (Kendall-PHMB). To achieve this, we created an infected chronic wound model challenged by P. aeruginosa in transgenic diabetic mice to evaluate the wound healing and anti-infective properties of our oxygenating and antibacterial dressings. Through comprehensive assessments, including bacterial bioburden measurements, lipidomic evaluations, and wound quality assessments, we demonstrated the potential of our innovative wound dressing to address the multifaceted challenges of infected non-healing diabetic ulcers. Lastly, upon discovering the detrimental effects of continuous PHMB antiseptic usage for 21 days, we aimed to enhance wound healing outcomes by optimizing the duration of PHMB application on our wound models and showed the advantages of a 14-day application of PHMB via MACF hydrogels through a series of wound quality assessments, lipidomic evaluations, and bacterial bioburden measurements.
Experimental
Materials and methods
To make the hydrogels, the corresponding polymer was dissolved in ultrapure water (3 wt%, 18 MΩ, Millipore, Billerica, MA, USA) and sterilized by autoclaving. Photo-crosslinker was prepared by dissolving hydroxycyclohexyl phenyl ketone in 1-vinyl-2-pyrolidone (both Sigma Aldrich) and added to polymer solution under sterile conditions (Final ratio: 0.9 mg photo-crosslinker per gram of polymer solution) and mixed via speed mixing (DAC 150 FVZ, Hauschild Engineering, Hamm, Germany) at 3000 rpm for 2 minutes. The polymer-photo initiator mixture was moulded in a six well plate (CELLSTAR, Greiner) to a liquid depth of 4 mm and UV-crosslinked for 4 minutes (using a UV-light source at 365 nm, Uvitron, West Springfield, MA). Hydrogels were then cut using a sterile 6 mm biopsy punch for use as wound dressings. Similar procedures were followed to make MAC-PHMB and MACF-PHMB dressings, except that PHMB (Musechem, Fairfield, NJ) was added to the polymer solution (0.5 wt%) before crosslinking. To perform the study, sterile MACF dressings (either with or without PHMB) were saturated with medical grade oxygen and kept inside a closed hypoxia chamber (Billups-Rothenberg, Inc., San Diego, CA) flushed with oxygen overnight. Oxygenation was repeated an hour before the dressing changes to ensure maximum oxygen saturation.
 | (1) |
Deuterated trifluoroacetic acid (d-TFA, Sigma Aldrich) was used as an internal standard (Cd-TFA = 1 nM) to calculate the concentration of fluorines (CF) in MACF using quantitative 19F NMR, based on the number of nuclei (N) for fluorine, as we have previously reported.14,29
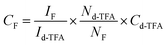 | (2) |
To study the mechanical properties, circular hydrogels (diameter: 8 mm, thickness: 3–5 mm) were placed at the centre of the plate on a rheometer (TA Instruments ARES-G2 Rotational Rheometer). The experiment was conducted in linear mode, with a strain of 1% and a shear rate ranging from 1 to 100 rad s−1. The frequency was increased by 5 rad s−1 and recorded using TRIOS software.
To test the swelling properties, hydrogels (with same dimensions as rheological analyses) were freeze-dried (Labconco, Freezone 4.5 Liter Freeze Dry System) for 24 hours and weighed to record their dry mass (MD). The freeze-dried hydrogels were re-hydrated in 3 mL of 1× PBS (pH 7.4) and incubated at 37 °C to initiate the swelling process. At specific time-points, gels were placed in a 40 μm cell strainer (Fisher Scientific, cat# 08-771-1) on a centrifuge tube, and briefly centrifuged at 1500 rpm for 60 seconds to remove any remaining surface PBS. The mass of the gels after swelling (MS) was then recorded using an analytical balance (Sartorius ME235S). The swelling ratio (QM) was calculated as:
 | (3) |
To evaluate cell viability responses after direct contact with hydrogels (with or without PHMB), HDFs were seeded at a density of 2500 cells per cm2 in 12 well plates. The cells were cultured in DMEM (high glucose, Sigma Aldrich), supplemented with 10% FBS (ThermoFisher Scientific) and 1% streptomycin/penicillin (ThermoFisher Scientific). Hydrogels including PHMB at concentrations of 0, 5, 10, 20, and 50 μg mL−1 were co-incubated with cells once the culture confluency reached approximately 75%. At specific time points, gels were removed and PrestoBlue™ Cell Viability Reagent (ThermoFisher Scientific) was added to the cells following the manufacturer's protocol. The fluorescence intensity of the cell supernatant was then recorded using a microplate reader (Tecan Infinite M200) with excitation and emission wavelengths of 560 nm and 590 nm, respectively. The same methodology was used to study the effect of direct contact with PHMB on the viability of HDFs.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, Santa Cruz Biotechnology, Dallas, TX) was used to label neutrophils. A primary mouse CD68 (KP1) Alexa Fluor 546 antibody (1
200, Santa Cruz Biotechnology, Dallas, TX) was used to label neutrophils. A primary mouse CD68 (KP1) Alexa Fluor 546 antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, Santa Cruz Biotechnology) was concurrently used to stain macrophages. Deparaffinized sections were subjected to heat antigen retrieval via tris-EDTA and 0.05% tween 20 for 8 minutes, followed by a rapid cooling on ice. Next, permeabilization was achieved using 0.1% Triton X-100, blocking with 10% goat serum for 1 hour, and incubation with the conjugated primary antibodies overnight at 4 °C. Staining of nuclei was performed using DAPI (10 μM for 7 min, Invitrogen, Waltham, MA).32 After washing, all slides were mounted and preserved using ProLong Gold Antifade Mount (Invitrogen) before imaging via a confocal microscope (FV1000, Olympus, Tokyo, Japan) with FluoView software. ImageJ was used to quantify the ratio of area positive for each antibody relative to the area covered by DAPI.
200, Santa Cruz Biotechnology) was concurrently used to stain macrophages. Deparaffinized sections were subjected to heat antigen retrieval via tris-EDTA and 0.05% tween 20 for 8 minutes, followed by a rapid cooling on ice. Next, permeabilization was achieved using 0.1% Triton X-100, blocking with 10% goat serum for 1 hour, and incubation with the conjugated primary antibodies overnight at 4 °C. Staining of nuclei was performed using DAPI (10 μM for 7 min, Invitrogen, Waltham, MA).32 After washing, all slides were mounted and preserved using ProLong Gold Antifade Mount (Invitrogen) before imaging via a confocal microscope (FV1000, Olympus, Tokyo, Japan) with FluoView software. ImageJ was used to quantify the ratio of area positive for each antibody relative to the area covered by DAPI.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH
1 MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA (Fisher Chemical and Sigma Aldrich) and mixed and stored in 4 °C overnight. Samples were run in negative and positive mode on an Agilent 1290 Infinity II LC and 6545 MS System via a Waters Acquity UPLC HSS T3 column (1.8 μm, 2.1 × 150 mm) with attached guard column. MS/MS fragmentation of a pooled sample was performed via DDA acquisition at 10 V and 20 V for identification. The LC method consisted of a flow rate of 0.25 mL min−1 and a gradient of 0–2 min: 30% B; 17 min: 75% B; 20 min: 85% B; 23 min: 100% B; 26 min: 100% B; 27 min: 30% B. Solvent A: 60% acetonitrile, 40% water, 10 mM ammonium formate, 0.1% formic acid, 2.5 μM medronic acid. Solvent B: 90% isopropanol, 10% acetonitrile, 10 mM ammonium formate, 0.1% formic acid, 2.5 μM medronic acid. Identification was performed via Lipid Annotator to match MS/MS spectra. [M + H] or [M − H] adducts of all available lipid classes were analyzed, with a maximum 10 ppm mass error and minimum 90% total score. Features with multiple constituents were reported as the dominant species if relative abundance >10%. Peak areas were assigned using Skyline software.
IPA (Fisher Chemical and Sigma Aldrich) and mixed and stored in 4 °C overnight. Samples were run in negative and positive mode on an Agilent 1290 Infinity II LC and 6545 MS System via a Waters Acquity UPLC HSS T3 column (1.8 μm, 2.1 × 150 mm) with attached guard column. MS/MS fragmentation of a pooled sample was performed via DDA acquisition at 10 V and 20 V for identification. The LC method consisted of a flow rate of 0.25 mL min−1 and a gradient of 0–2 min: 30% B; 17 min: 75% B; 20 min: 85% B; 23 min: 100% B; 26 min: 100% B; 27 min: 30% B. Solvent A: 60% acetonitrile, 40% water, 10 mM ammonium formate, 0.1% formic acid, 2.5 μM medronic acid. Solvent B: 90% isopropanol, 10% acetonitrile, 10 mM ammonium formate, 0.1% formic acid, 2.5 μM medronic acid. Identification was performed via Lipid Annotator to match MS/MS spectra. [M + H] or [M − H] adducts of all available lipid classes were analyzed, with a maximum 10 ppm mass error and minimum 90% total score. Features with multiple constituents were reported as the dominant species if relative abundance >10%. Peak areas were assigned using Skyline software.
Results and discussion
Hydrogel synthesis and characterization
MACF synthesis used a methacrylation reaction on chitosan (to make MAC) followed by fluorination of MAC. Fig. S1† details this reaction as well as the quantitative proton and fluorine NMR results to determine the degree of methacrylation (DM%, Fig. S1B†) and fluorine concentration (CF, Fig. S1C†) within MACF (26.28 ± 1%, and 90 ± 5 μM, respectively). These results indicated the successful incorporation of methacrylate and perfluorocarbon groups to the chitosan backbone, the latter being critical for oxygen transport enhancement properties. The appearance of the gels (Fig. 1A) shows changes in MAC's clear appearance changing to more of an opaque white colour in MACF.Prior to beginning animal studies, we aimed to formulate the chitosan-based MACF/MAC hydrogel treatments with similar release kinetics of PHMB to the commercial Kendall-PHMB to ensure all treatments delivered similar PHMB to wounds. The Kendall dressing is FDA approved for topical treatment of infected wounds, thus Kendall's 0.5 wt% PHMB is generally considered safe for this purpose. Moreover, we have previously confirmed that this concentration of PHMB shows acceptable toxicity responses in HDFs in culture.30,33 The results of this comparison demonstrated similar patterns of PHMB release for all three dressing types over the 72 hour course of study (Fig. 1B and C). The similar initial burst release for all dressings in the first 30 minutes of the release study is ideal for our application to allow early and rapid local accumulation of the antiseptic at the heavily infected wounds to eliminate bacteria. The steady release profile for the next 28 hours also aligned well with clinically recommended dressing change timepoints during our animal studies, which should be performed every 48–72 hours.34,35
Next, rheological properties of MAC and MACF hydrogels were studied to ensure dressings’ ability to maintain structural integrity while providing optimal conformability to wound contours for improved wound healing. The complex shear modulus G* was calculated as the arithmetic mean of the storage modulus (G′) and loss modulus (G′′) allowing for stiffness comparisons of hydrogels (a higher G* value corresponds to a stiffer gel).36 As shown in Fig. 1D, MACF hydrogels had lower G* values as compared to MAC. However, the slightly lowered stiffness did not affect MACF's handing efficiency when used as a wound dressing. Interestingly, PHMB incorporation did increase mechanical properties slightly for both MACF and MAC (p < 0.0001).
Liquid absorption is another critical factor to consider for wound dressings, especially when dealing with highly exudating wounds, such as infected wounds.34,35 Swelling studies of MAC and MACF over 48 hours showed the ability of all tested hydrogel types to retain liquids 11–15 times greater than their initial dry state (Fig. 1E). There was no detected significance in the liquid absorption properties of the hydrogels with or without PHMB (p > 0.05).
Finally, in vitro cytotoxicity screening was performed on HDFs to study toxicity responses to the MAC and MACF base hydrogels, as well as the impact of adding PHMB at increasing concentrations. We used PrestoBlue colorimetric assay to quantitatively measure and compare cell viability within our different treatments.37,38 Results of the PrestoBlue assay at equilibrium (72 hours of direct contact with both hydrogels) showed that soluble PHMB alone severely impacted viability, even at the lowest concentration (5 μg mL−1). However, MACF treatment reversed toxicity with over 85% viability at the highest concentration of PHMB (50 μg mL−1) in MACF (p < 0.0001 vs. soluble PHMB, Fig. 1F), while maintaining healthy cell morphologies (Fig. 1G). Cytotoxic impact of released PHMB was also minimized with application of MAC compared to free PHMB in media (p < 0.005), but significantly lower than MACF (p < 0.0001).
Considering the direct contact of our hydrogels with the wound for a minimum of 48 hours, it was crucial to evaluate their impact on skin cell viability. Our in vitro cytotoxicity assays with direct PHMB contact to HDFs, showed that MACF effectively reduced cytotoxic impacts of PHMB on cell viability (Fig. 1F and G), likely due to the impacts of oxygen on increasing metabolic processes of fibroblasts.39 Although our tested concentrations were lower than the encapsulated concentrations for in vivo application, we chose to incorporate the same initial concentration of PHMB (0.5 wt%; Fig. 1C) as in the FDA-approved Kendall-PHMB dressing, since previous reports suggested acceptable host cell toxicity in multiple similar formulations, including PHMB foam and silicone dressings.40 Although incorporating 0.5% PHMB in our hydrogels hypothetically means that the total amount of PHMB released could exceed 10 mg mL−1 locally, the actual bioavailable concentration of PHMB is much lower at every timepoint in vivo, which further supports our in vitro model. Also, wounds exudates, fluid circulation, drug degradation by host cells and bacteria, and the controlled release properties of our hydrogels, constantly dilute and inactivate a large portion of PHMB from the wound.41–43
Swelling properties of hydrogels were assessed due to their importance in maintaining wound moisture and enhancing healing processes.44 Results (Fig. 1E) suggested that dressings utilizing MACF or MAC were able to absorb high levels of liquids which implies their ability to draw bacteria away from the wounds by entrapping wound fluids and bacteria. Interestingly, incorporation of PHMB only slightly decreased MAC and MACF swelling properties, likely due to PHMB interactions with the crosslinked chitosan backbones.45 Swelling results lead to a recommendation for replacing MAC and MACF dressings every 48 hours in vivo to maintain exudate levels.
Wound treatments, size, morphology and histological evaluation
To assess the effectiveness of our treatments on wound healing in diabetic mice, we compared wound closure status on day 21 as a first readout (Fig. 2A, B and Fig. S6A, B† for MAC treatments). Our splinted wound model ensured that healing was achieved primarily through re-epithelialization and not contraction.15–17 Successful establishment of infection in the groups inoculated with P. aeruginosa was observed, (Fig. 1C and Fig. S6†), and is supported by the large wound areas on day 21 in all infected groups (Fig. 2, p < 0.03) accompanied by visible bacterial accumulation marked by the green colour visible due to production of pyocyanin by P. aeruginosa.46Interestingly, among all the groups that included PHMB (infected and non-infected), the commercial Kendall-PHMB dressing showed significantly larger wound areas as compared to hydrogel-PHMB groups (i.e., MACF-PHMB and MAC-PHMB) (p = 0.018 Kendall vs. Tegaderm-only infected control) (Fig. 2C, D and Fig. S6†). Kendall dressings resulted in the largest wound areas on day 21 (247% increase as compared to the original wound). Furthermore, infected wounds treated with PHMB (regardless of dressing) on day 21 had a larger average area compared to the original wound area (day 0). Wound morphology and closure trends from day 7 and day 14 of the study shown in Fig. S2† support the trends seen at day 21 with uninfected MACF hydrogel treatments showing the fastest wound closure responses. Wound areas in the hydrogel groups were similar to the infected vehicle control (p > 0.05). Only the non-infected wounds treated with MACF, MAC, and Tegaderm successfully closed by day 21 of the study. These wound measurements revealed that the presence of bacteria, PHMB, or both prevented the wound from closing at this timepoint in all dressing treatments as well as the infected vehicle control (Tegaderm (infected), Fig. 2D). We did not observe any significant difference between the infected groups treated with MAC vs. MACF hydrogels (p > 0.05), suggesting no impact of additional oxygen via MACF hydrogels. Regardless of the type of treatment or infection status, all animals maintained their diabetic state throughout the experimental period as shown in Fig. S4.†
As part of this study, we aimed to examine potential sex differences in infected wound healing, as evidence from the literature suggests that host sex can potentially modulate wound healing responses,39,45 where estrogen has been shown to speed up and regulate cutaneous healing.39 To achieve this, we randomly selected from the pool of treatments (infected and non-infected) and controls shown in Fig. S4† and assigned them to male and female mice. Our results using wound area analysis demonstrated no significant differences in overall wound sizes in male and female diabetic db/db mice, regardless of the infection status or type of treatment used (Fig. S5,†p > 0.05). Thus, for the remainder of animal work we utilized male mice.
Next, histological assessments were performed in endpoint samples, which revealed tissue necrosis responses (as indicated by bright red areas over the wound bed in Masson's trichrome images) and disruption of the underlying collagen layer in all groups that were treated continuously with PHMB, including the commercial Kendall dressing treated wounds (Fig. 3 and Fig. S6C, D† for MAC treatments). We observed significantly longer epithelial tongues in the non-infected MACF-treated wounds as compared to the Tegaderm (non-infected) control (5.95 ± 0.54 mm vs. 2.67 ± 0.17 mm, respectively; p = 0.0003), suggesting improvement due to supplemental local oxygen from MACF; however, when infection and/or PHMB were present, no significant differences were observed independent of the type of treatment used (p > 0.05).
From the representative histology, and in agreement with our wound closure analyses, the presence of P. aeruginosa and PHMB substantially disrupted the wound healing process leading to necrosis responses (Fig. 3A). The infected wound groups all showed greatly expanded wound cross-sectional areas, with a disturbed or lacking granulation tissue (Fig. 2B). Interestingly, we did not observe any significant differences in the morphological and histological examinations resulting from the additional oxygenation delivered via MACF treatments compared to MAC (Fig. 2C and D, p > 0.05). This likely can be attributed to the low levels of additional local oxygen supplied by MACF treatments, as a material that continuously boosts local biological oxygen tensions by ∼5–10 mmHg.14 This additional oxygenation may not be sufficient to upregulate host antibacterial responses, such as respiratory burst by circulating immune cells.47 Conversely, the positive impacts of wound oxygenation on infection control have been reported previously when using systemic hyperbaric oxygen therapy (HBOT), which boost systemic oxygenation by over an atmosphere of pressure, leading to upregulated immune responses to infection.19,21,48,49 Despite these observations there are other beneficial wound healing processes supplemental oxygenation for MACF could encourage as tied to oxidative cellular metabolic processes.4,5,50
Bacterial localization and quantification within the tissue
The degree of bacterial bioburden within wound tissues impacts wound healing responses, particularly in chronic wounds. Fig. 4 shows two analysis employed to better understand bacterial presence and load in endpoint samples. First, modified Gram staining of wound tissues harvested on day 21 revealed clusters of Gram-negative bacteria within the granulation tissue of the infected wounds, indicating deep-seated infection at the study endpoint. Despite the necrosis observed as associated with PHMB treatments, MACF-PHMB and MAC-PHMB groups exhibited remarkable reductions in bacterial loads compared to the Tegaderm (infected) control (Fig. 4A). In contrast, the group treated with the commercial Kendall-PHMB dressings showed uniformly dispersed populations of bacteria on day 21, indicating limited antibacterial action. Chitosan-based hydrogels, especially MACF-PHMB, performed better than the porous Kendall foam dressings in segregating bacteria. The depth of bacterial penetration in the MACF-PHMB and MAC-PHMB treated groups was improved, as evidenced by fewer bacteria in the deeper tissue layers compared to the Kendall dressing (Fig. 4A). During the study attempts to collect wound exudates via swabbing for conventional CFU counts proved to be challenging and unreliable due to the large variety and variability of natural flora on the wound. Thus, to confirm Gram-staining results and P. aeruginosa identity, whole wound tissues were subjected to CFU referenced qPCR assays (Fig. 4B). Infected Tegaderm controls showed the highest P. aeruginosa CFU values, however, treatment with PHMB, either via MACF hydrogels or the commercial Kendall dressing, significantly reduced P. aeruginosa numbers (p < 0.0027) in day 21 samples. Importantly, MACF-PHMB hydrogel treatment rendered P. aeruginosa undetectable in both infected and non-infected groups.P. aeruginosa localization deep in wounds in Gram-stained sections (Fig. 4A) of some infected treatments coincided with the lack of sufficient barrier function and the necrosis we observed in all infected groups at day 21 (Fig. 2B and 3A, B). Gjødsbøl et al.,51 also reported P. aeruginosa localization in deeper wound regions associated with enlarged wound size and delayed wound healing. Conversely, our chitosan-based hydrogel-treated groups resulted more shallow bacterial penetration. This aligns with previous findings and emphasizes the impact of dressing choice on infection control. The confirmation of applied P. aeruginosa identity through qPCR at a higher titre than host wound flora further supports the effectiveness of PHMB in reducing loads of the strain within our treatment models (Fig. 4B). Undetectable levels of P. aeruginosa in MACF-PHMB group (infected and non-infected) definitively highlight its potential as a robust therapeutic option.
This initial animal study revealed a key finding that tissue necrosis and damage were caused by a combination of bacterial infection alongside continuous PHMB antiseptic treatment, regardless of dressing material. Antiseptics can elicit cytotoxic and inflammatory responses on host cellular contributors in wound healing process, such as keratinocytes, fibroblasts, and immune cells.9 Consistently, a group52 using in vitro scratch wound models showed a substantial reduction in fibroblast proliferation and migration when wounds were treated with hydrogen peroxide and povidone-iodine antiseptics. Notably, in their research, among all tested antiseptics, chlorhexidine (with similar structure to PHMB) was found to be one of the least toxic to fibroblasts.
Leukocyte infiltration in wound tissues
For proper wound healing progression, neutrophils and their debris should exit the wound area to prevent chronic inflammation.40 Thus, we examined the persistence of leukocytes at the wound site to better understand the state of wound healing. Via IHC and microscopy we observed the highest accumulation of Ly6G+ cells in infected wounds that were treated with the commercial Kendall dressing (p = 0.0004 vs. Tegaderm (infected)) (Fig. 5 and Fig. S9† for MAC treatments). The infiltration of neutrophils in the Kendall dressing group appeared to be more substantial through the epidermis layer and similar to the Tegaderm (infected) group. Other experimental groups, including Tegaderm (non-infected) control, MACF hydrogel, and MACF-PHMB hydrogel showed more uniform neutrophil populations throughout the tissue sections.Inflammatory responses, such as infection and necrosis, are known to delay the process of neutrophil exit from wound sites.53 When neutrophils persist in the wound site, they undergo apoptosis and release damaging metabolites causing further inflammation.54 Accordingly, we observed significantly less neutrophils in infected wounds treated with MACF-PHMB groups as compared to MAC-PHMB treated infected wounds (p = 0.02) on day 21 (Fig. 5A and B). Supporting these results, a previous study reported decreased neutrophil adhesion to endothelial cells under elevated oxygen partial pressure conditions via HBOT in vitro.49 This was correlated to reduced expression of endothelial intercellular adhesion molecule-1 as well as vascular cell adhesion molecule-1 mediated by S-nitrosation under HBOT. Similarly, another study observed a drastic decrease in adhesion-inducing cytokine β2-integrin expression of neutrophils under HBOT in patients with chronic non-healing wounds, confirming an anti-inflammatory role of oxygen.50
During wound healing, macrophages assist in the later stages of post-inflammation wound healing, playing important roles including phagocyting the remaining debris from the wound bed.55 We did not observe any significant differences (p > 0.05) in macrophage distributions associated with any treatment used; however, a trend was observed in Kendall-PHMB treated infected group with the highest number of CD68+ (Fig. 5 and Fig. S9†) and CD11b+ cells (Fig. S10†). Our observations could be explained by the fact that the infected diabetic wounds remain trapped in an inflammatory phase, suggesting a non-healing state of the open wounds on day 21 could be tied to the extended presence of neutrophils and inflammation in the wound bed,55 leading to a non-healing state in this wound model.
Effect of oxygenating antibacterial hydrogels on wound lipid profiles
Untargeted lipidomics provided a high throughput assessment of all experimental groups by showing that PHMB treatment, infection, and wound dressing altered levels of two key lipid classes, phosphatidylglycerols (PGs) and sphingomyelins (SMs) (Fig. 6), which are important for homeostatic skin physiology.56,57 Treatment with MACF or MAC alone reduced levels of PG species when compared to all infected groups (Fig. 6 and Fig. S11† for MAC treatments). In contrast, PHMB delivery via MAC or MACF increased PG lipids within the treated wounds, in contrast to treatment via Kendall Dressing. Several SM species, including monounsaturated and polyunsaturated species, were increased by treatment with PHMB encapsulated in MACF and MAC, compared to both untreated, infected samples and those treated with Kendall dressings (Fig. 6 and Fig. S11†). While infection generally decreased the levels of lipids such as PGs and SMs, treatment with PHMB increased these levels. Interestingly, when PHMB was combined with MACF hydrogels, the infection-induced decrease was completely rescued.Here, we were interested in understanding the potential for lipidomics approaches to better understand wound healing responses in our infected diabetic wound model. Remarkably, MACF treatment stimulated increased levels of dioleoyl–PG (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/18
1/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and dilinoleoyl–PG (18
1) and dilinoleoyl–PG (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/18
2/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), both of which play a role in increasing keratinocyte proliferation (Fig. 6B). PGs are also known to modulate skin cell proliferation and promote anti-inflammatory responses.56–58 Therefore, MACF-PHMB-mediated the regulation of the wound microenvironment and may optimize cell division in favour of wound closure. We also observed increased SM levels for the wounds treated with MACF-PHMB hydrogels (Fig. 6). These lipids play a critical role in maintaining the skin barrier and serve as precursors for signalling molecules, such as sphingosine-1-phosphate to induce keratinocyte differentiation and anti-apoptotic properties.59,60 Wakita et al.61 showed that sphingosylphosphorylcholine enhanced wound repair by directly stimulating keratinocyte migration and proliferation in diabetic mouse wound models. Overall, the initial lipid profiling results suggested that our hydrogel dressings can upregulate levels of SM and PG lipids despite the necrotic responses we observed via other analyses and enhance keratinocyte migration, proliferation, and differentiation, and inflammatory responses to encourage the restoration of dermal barrier functions. Apart from these two lipid types that showed most significant differences within our treatment groups, the entire raw data profile for all recognized lipids is available as ESI.†
2), both of which play a role in increasing keratinocyte proliferation (Fig. 6B). PGs are also known to modulate skin cell proliferation and promote anti-inflammatory responses.56–58 Therefore, MACF-PHMB-mediated the regulation of the wound microenvironment and may optimize cell division in favour of wound closure. We also observed increased SM levels for the wounds treated with MACF-PHMB hydrogels (Fig. 6). These lipids play a critical role in maintaining the skin barrier and serve as precursors for signalling molecules, such as sphingosine-1-phosphate to induce keratinocyte differentiation and anti-apoptotic properties.59,60 Wakita et al.61 showed that sphingosylphosphorylcholine enhanced wound repair by directly stimulating keratinocyte migration and proliferation in diabetic mouse wound models. Overall, the initial lipid profiling results suggested that our hydrogel dressings can upregulate levels of SM and PG lipids despite the necrotic responses we observed via other analyses and enhance keratinocyte migration, proliferation, and differentiation, and inflammatory responses to encourage the restoration of dermal barrier functions. Apart from these two lipid types that showed most significant differences within our treatment groups, the entire raw data profile for all recognized lipids is available as ESI.†
Wound healing outcomes with reduced duration of PHMB application
Despite the positive effects of PHMB in clearing wound infection with our hydrogel treatments, the cumulative data (Fig. 1–5) suggested that the continuous application of this antiseptic over the 21-day study prolonged necrosis, inhibited the formation of epithelial and granulation layers, and may have led to chronic inflammation. Thus, we designed an additional study to determine if reducing the total length of PHMB exposure could improve wound re-epithelialization outcomes, while maintaining antibacterial actions. We divided animals into three groups: A. Animals infected with P. aeruginosa and treated with MACF-PHMB for 7 days then switched to plain oxygenating MACF hydrogels on day 7 to day 21 (no PHMB beyond D7); B. Animals with 14 days of MACF-PHMB treatment switched to plain oxygenating MACF hydrogels on day 14 to day 21 (no PHMB beyond D14); C. Animals given continuous PHMB-MACF treatment for 21 days. Control data from the earlier studies were included for key comparisons (Fig. 7A). Gram-stained tissues at the 21-day endpoint showed deeper permeation and bacterial load in infected wounds treated with PHMB for only 7 days, as compared to 14 or 21 days (Fig. 7B). The qPCR data confirmed this finding, as infected wounds treated with PHMB for only 7 days showed greater load of P. aeruginosa, while treatment with PHMB for 14 and 21 days on infected wounds showed no measurable P. aeruginosa increase (Fig. 7C). Compared to data from our first animal study (Fig. 4), untreated infection controls showed higher loads of bacteria. Continuous Kendall PHMB treatment concerningly showed persistence of P. aeruginosa in wounds (Fig. 7C). We then compared the wound areas and epithelialization state of the two new PHMB MACF treatment groups versus the results from the continuous application of PHMB for 21 days. Histological observations revealed denser granulation tissue structures and improved epithelial tonguing in no PHMB beyond day 7, MACF oxygenated treatment for 14 days prior, especially in the non-infected PHMB treatment group (Fig. 7D and Fig. S12†). Wound area measurements in our non-infected groups revealed the positive impacts when PHMB was stopped at an earlier timepoint, since there were significant size differences of non-infected wounds (p < 0.05, Fig. S1†). However, the deleterious impact of bacteria on the infected wounds inhibited the complete wound healing process even when PHMB treatment concluded at an earlier timepoint (Fig. S10C† (infected groups)). The lipidomics studies (Fig. 7E) confirmed that wounds that experienced PHMB delivery through MACF showed overall upregulated SM and PG levels, with the highest levels observed on day 7. This observation, plus the lowest level of these lipids in the continuous PHMB treatment group, confirms the detrimental effects of prolonged tissue contact with PHMB in tissue repair processes, and supports the need to limit exposure of PHMB for treating wound infections.The absence of P. aeruginosa in the infected wounds treated with MACF-PHMB hydrogels beyond day 14, confirmed that an optimal strategy to overcome infection and enhance healing outcomes involves applying PHMB for 14 days via our hydrogels, followed by switching to a plain MACF dressing to support tissue regeneration (Fig. 7B and C). Furthermore, as shown in Fig. 7D and S12,† along with the initiation of granulation tissue on day 14 with MACF-PHMB hydrogel (Fig. 7D and Fig. S12†), indicated that infected diabetic wounds treated with PHMB required an initial 14 days for PHMB to effectively clear the infection prior to switching to a regenerative treatment like MACF (Fig. 7C). Upon the clearance of bacterial infection on day 14 the wound healing process initiates, suggesting that these wounds likely need a period longer than 21 days total to fully close.15–17 Furthermore, these studies show that more than 7 days are needed to achieve closure using similar non infected wounding models.
Conclusions
In this study, we developed and evaluated oxygenating chitosan-based antibacterial hydrogel dressings for infected diabetic wound healing and compared the outcomes to a commercial foam antiseptic dressing (Kendall-PHMB). As the platform for our study, we created a wound model in db/db mice and infected them with P. aeruginosa to obtain a chronic bacterial infection in non-healing wounds. Despite the matched PHMB release kinetics of dressing treatments, our synergistic dual functioning MACF hydrogel dressings containing PHMB antiseptic showed enhanced wound closure outcomes compared to the commercial Kendall-PHMB dressing. Persistence of neutrophils in the wound bed demonstrated that hydrogel dressings with supplemental oxygen via MACF attenuated inflammatory responses compared to their non-oxygenating (MAC) and foam dressing (Kendall) counterparts. We also examined lipid profile regulation in different treatment groups and discovered that our hydrogel dressings upregulate the levels of two important lipids classes of phosphatidylglycerols and sphingomyelins, which are known improve keratinocyte differentiation, reduce inflammatory responses, and restore skin barrier functions. Our oxygenating antibacterial chitosan-based hydrogel dressing, MACF-PHMB, consistently outperformed commercial Kendall foam dressings in our studies. This highlights the crucial role of dressing material choice in supporting host tissue regeneration and minimizing toxicity from the antiseptic PHMB. Despite these positive results, we observed problematic tissue necrosis with prolonged PHMB application. To address this, we refined our treatment strategy to apply PHMB only until the infection ceased, focusing on wound healing thereafter. Thus, our proposed healing strategy involves a 14-day PHMB application via our hydrogels, followed by plain MACF hydrogel treatment to achieve full wound closure and to encourage granulation tissue maturation and regeneration. We suggest future studies with endpoints longer than 21 days to capture the wound closure timepoints for each treatment group, once the infection is completely resolved. While recognizing the significance of directly measuring local wound oxygen levels to validate our hydrogel treatments and assess their impact on wound healing and anti-infective processes, it is crucial to acknowledge that existing oxygen sensors lack the sensitivity and resolution needed for precise topical oxygen measurements in vivo. Challenges include the need to anesthetize animals to ensure motionlessness during measurements, which can influence tissue oxygen dynamics and potentially skew results. Future efforts should prioritize development of new oxygen monitoring technologies for real-time monitoring of wound oxygenation states.Overall, our designed antibacterial oxygenating hydrogels show strong promise for future translation as a multifunctional dressing for managing infected non-healing diabetic ulcers.
Abbreviations
| AAALAC | Association for assessment and accreditation of laboratory animal care |
| AMP | Antimicrobial peptide |
| ANOVA | Analysis of variance |
| ATCC | American type culture collection |
| CFU | Colony-forming unit |
| d-TFA | Deuterated trifluoroacetic acid |
| D2O | Deuterium oxide |
| DMEM | Dulbecco's modified Eagle's medium |
| DNA | Deoxyribonucleic acid |
| E. coli | Escherichia coli |
| ECM | Extracellular matrix |
| FBS | Foetal bovine serum |
| HDF | Human dermal fibroblasts |
| H&E | Hematoxylin and eosin |
| HBOT | Hyperbaric oxygen therapy |
| HEPA | High efficiency particulate air |
| HPLC | High-performance liquid chromatography |
| IACUC | Institutional animal care and use committee |
| IHC | Immunohistochemistry |
| IPA | Isopropyl alcohol |
| KDa | Kilodaltons |
| MAC | Methacrylamide chitosan |
| MACF | Fluorinated methacrylamide chitosan |
| MIC | Minimum inhibitory concentration |
| MS | Mass spectrometry |
| MMP | Matrix metalloproteinase |
| MWCO | Molecular weight cutoff |
| NIH | National institute of health |
| NMR | Nuclear magnetic resonance |
| OD | Optical density |
| OLAW | Office of laboratory animal welfare |
| P. aeruginosa | Pseudomonas aeruginosa |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| PFOC | Pentadecafluorooctanoyl chloride |
| PG | Phosphatidylglycerol |
| PHMB | Polyhexamethylene biguanide |
| qPCR | Quantitative polymerase chain reaction |
| ROS | Reactive oxygen species |
| RPMI | Roswell park memorial institute medium |
| S. aureus | Staphylococcus aureus |
| S.D | Standard deviation |
| SM | Sphingomyelin |
| TPA | 12-O-Tetradecanoylphorbol-13-acetate |
| UV | Ultraviolet |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support for this research provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS, R21AR074743). The content of this publication reflects the views of the authors only and not NIAMS or NIH.References
- Z. Lin, T. Wu, W. Wang, B. Li, M. Wang, L. Chen, H. Xia and T. Zhang, Int. J. Biol. Macromol., 2019, 140, 330–342 CrossRef CAS PubMed.
- T. Tokatlian, C. Cam and T. Segura, Adv. Healthc. Mater., 2015, 4, 1084–1091 CrossRef CAS PubMed.
- J. He, Y. Qiao, H. Zhang, J. Zhao, W. Li, T. Xie, D. Zhong, Q. Wei, S. Hua, Y. Yu, K. Yao, H. A. Santos and M. Zhou, Biomaterials, 2020, 234, 119763 CrossRef CAS PubMed.
- V. Skrypko, J. Pharm. Innovation, 2018, 7, 1–2 CrossRef CAS.
- T. O. Kharchenko, O. K. Melekhovets, I. V. Melekhovets, A. S. Radko, N. V. Kalashnyk and R. S. N. Shu, Problems of Endocrine Pathology, 2020, 72, 74–80 CrossRef.
- B. A. Lipsky and C. Hoey, Clin. Infect. Dis, 2009, 49, 1541–1549 CrossRef PubMed.
- R. E. Hancock and D. P. Speert, Drug Resistance Updates, 2000, 3, 247–255 CrossRef CAS PubMed.
- A. Punjataewakupt, S. Napavichayanun and P. Aramwit, Eur. J. Clin. Microbiol. Infect. Dis., 2019, 38, 39–54 CrossRef PubMed.
- B. S. Atiyeh, S. A. Dibo and S. N. Hayek, Int. Wound J., 2009, 6, 420–430 CrossRef PubMed.
- R. Smith, J. Russo, J. Fiegel and N. Brogden, Antibiotics, 2020, 9, 56 CrossRef CAS PubMed.
- H. Chen, Y. Cheng, J. Tian, P. Yang, X. Zhang, Y. Chen, Y. Hu and J. Wu, Sci. Adv., 2020, 6, eaba4311 CrossRef CAS PubMed.
- E. Blackman, C. Moore, J. Hyatt, R. Railton and C. Frye, Ostomy Wound Manage., 2010, 56, 24–31 Search PubMed.
- J. P. Jee, R. Pangeni, S. K. Jha, Y. Byun and J. W. Park, Int. J. Nanomed., 2019, 14, 5449–5475 CrossRef CAS PubMed.
- P. S. Patil, M. Mansouri and N. D. Leipzig, Adv. Biosyst., 2020, 4, e1900250 CrossRef PubMed.
- P. S. Patil, M. M. Evancho-Chapman, H. Li, H. Huang, R. L. George, L. P. Shriver and N. D. Leipzig, PLoS One, 2018, 13, e0203371 CrossRef PubMed.
- P. S. Patil, N. Fountas-Davis, H. Huang, M. Evancho-Chapman, J. A. Fulton, L. P. Shriver and N. D. Leipzig, Acta Biomater., 2016, 36, 164–174 CrossRef CAS PubMed.
- P. S. Patil, S. Fathollahipour, A. Inmann, A. Pant, R. Amini, L. P. Shriver and N. D. Leipzig, Adv. Wound Care, 2019, 8, 374–385 CrossRef PubMed.
- P. A. Shiekh, A. Singh and A. Kumar, Biomaterials, 2020, 249, 120020 CrossRef CAS PubMed.
- M. Y. Memar, M. Yekani, N. Alizadeh and H. B. Baghi, Biomed. Pharmacother., 2019, 109, 440–447 CrossRef CAS PubMed.
- A. A. Tandara and T. A. Mustoe, World J. Surg., 2004, 28, 294–300 CrossRef PubMed.
- C. J. Lerche, L. J. Christophersen, M. Kolpen, P. R. Nielsen, H. Trøstrup, K. Thomsen, O. Hyldegaard, H. Bundgaard, P. Jensen, N. Høiby and C. Moser, Int. J. Antimicrob. Agents, 2017, 50, 406–412 CrossRef CAS PubMed.
- M. A. Kohanski, D. J. Dwyer, B. Hayete, C. A. Lawrence and J. J. Collins, Cell, 2007, 130, 797–810 CrossRef CAS PubMed.
- A. Moeini, P. Pedram, P. Makvandi, M. Malinconico and G. Gomez d’Ayala, Carbohydr. Polym., 2020, 233, 115839 CrossRef CAS PubMed.
- S. Torkaman, H. Rahmani, A. Ashori and S. H. M. Najafi, Carbohydr. Polym., 2021, 258, 117675 CrossRef CAS PubMed.
- P. S. Patil and N. D. Leipzig, J. Biomed. Mater. Res., Part A, 2017, 105, 2368–2374 CrossRef CAS PubMed.
- S. Fathollahipour, P. S. Patil and N. D. Leipzig, Cells Tissues Organs, 2018, 205, 350–371 CrossRef CAS PubMed.
- T. R. Ham, D. D. Pukale, M. Hamrangsekachaee and N. D. Leipzig, Mater. Sci. Eng., C, 2020, 110, 110656 CrossRef CAS PubMed.
- M. Hamrangsekachaee, H. J. Baumann, D. D. Pukale, L. P. Shriver and N. D. Leipzig, ACS Appl. Bio Mater., 2022, 5, 2176–2184 CrossRef CAS PubMed.
- U. Holzgrabe, Prog. Nucl. Magn. Reson. Spectrosc., 2010, 57, 229–240 CrossRef CAS PubMed.
- S. Abri, A. A. Ghatpande, J. Ress, H. A. Barton and N. D. Leipzig, ACS Appl. Bio Mater., 2019, 2, 5848–5858 CrossRef CAS PubMed.
- S. C. Becerra, D. C. Roy, C. J. Sanchez, R. J. Christy and D. M. Burmeister, BMC Res. Notes, 2016, 9, 216 CrossRef PubMed.
- D. D. Pukale, M. Farrag and N. D. Leipzig, PLoS One, 2021, 16, e0252559 CrossRef CAS PubMed.
- J. Andrade Del Olmo, J. M. Alonso, V. Sáez-Martínez, S. Benito-Cid, I. Moreno-Benítez, M. Bengoa-Larrauri, R. Pérez-González, J. L. Vilas-Vilela and L. Pérez-Álvarez, Biomater. Adv., 2022, 139, 212992 CrossRef CAS PubMed.
- E. W. Group, Int. Wound J., 2008, 5, iii-19 Search PubMed.
- A. Novak, W. S. Khan and J. Palmer, Open Orthop. J, 2014, 8, 168–177 CrossRef PubMed.
- C. H. Wang, J. H. Cherng, C. C. Liu, T. J. Fang, Z. J. Hong, S. J. Chang, G. Y. Fan and S. D. Hsu, Int. J. Mol. Sci., 2021, 13, 7067 CrossRef PubMed.
- P. F. Hsieh, C. C. Yu, P. M. Chu and P. L. Hsieh, Antioxidants, 2021, 10, 1445 CrossRef CAS PubMed.
- N. Sianipar, Y. Hadisaputri, K. Assidqi, P. Simanjuntak and R. Purnamaningsih, Rasayan J. Chem., 2020, 13, 1992–1998 CrossRef CAS.
- M. S. Nakazawa, B. Keith and M. C. Simon, Nat. Rev. Cancer, 2016, 16, 663–673 CrossRef CAS PubMed.
- C. B. Shah, H. P. Swaniker, B. J. Dowd, B. Brandon and D. A. Hibbitt, Covidien, 2009, 10, 1–8 Search PubMed.
- W. Jin, P. Song, Y. Wu, Y. Tao, K. Yang, L. Gui, W. Zhang and F. Ge, ACS Biomater. Sci. Eng., 2022, 8, 4274–4288 CrossRef CAS PubMed.
- M. Burnet, D. G. Metcalf, S. Milo, C. Gamerith, A. Heinzle, E. Sigl, K. Eitel, M. Haalboom and P. G. Bowler, Diagnostics, 2022, 12, 2408 CrossRef CAS PubMed.
- N. Pajares-Chamorro, Y. Wagley, N. Hammer, K. Hankenson and X. Chatzistavrou, J. Am. Ceram. Soc., 2022, 105, 1778–1789 CrossRef CAS.
- V. Brumberg, T. Astrelina, T. Malivanova and A. Samoilov, Biomedicines, 2021, 9, 1235 CrossRef CAS PubMed.
- L. Pan, C. Li, Z. Wang, L. Yang and L. Zhang, Biochem. Eng. J., 2022, 187, 108626 CrossRef CAS.
- L. Allen, D. H. Dockrell, T. Pattery, D. G. Lee, P. Cornelis, P. G. Hellewell and M. K. Whyte, J. Immunol., 2005, 174, 3643–3649 CrossRef CAS PubMed.
- A. Ohta, Int. Immunol., 2018, 30, 335–343 CrossRef CAS PubMed.
- A. J. Almzaiel, R. Billington, G. Smerdon and A. J. Moody, Life Sci., 2013, 93, 125–131 CrossRef CAS PubMed.
- A. C. Kendall, J. L. Whatmore, P. G. Winyard, G. R. Smerdon and P. Eggleton, Wound Repair Regener., 2013, 21, 860–868 CrossRef PubMed.
- M. Baiula, R. Greco, L. Ferrazzano, A. Caligiana, K. Hoxha, D. Bandini, P. Longobardi, S. Spampinato and A. Tolomelli, PLoS One, 2020, 15, e0237746 CrossRef CAS PubMed.
- K. Gjødsbøl, J. J. Christensen, T. Karlsmark, B. Jørgensen, B. M. Klein and K. A. Krogfelt, Int. Wound J., 2006, 3, 225–231 CrossRef PubMed.
- G. W. Thomas, L. T. Rael, R. Bar-Or, R. Shimonkevitz, C. W. Mains, D. S. Slone, M. L. Craun and D. Bar-Or, J. Trauma, 2009, 66, 82–90 CAS ; discussion 90-81.
- A. R. Witter, B. M. Okunnu and R. E. Berg, J. Immunol., 2016, 197, 1557–1565 CrossRef CAS PubMed.
- J. Wang, Cell Tissue Res., 2018, 371, 531–539 CrossRef CAS PubMed.
- C. Murdoch, M. Muthana and C. E. Lewis, J. Immunol., 2005, 175, 6257–6263 CrossRef CAS PubMed.
- D. Xie, M. Seremwe, J. G. Edwards, R. Podolsky and W. B. Bollag, PLoS One, 2014, 9, e107119 CrossRef PubMed.
- W. M. Holleran, Y. Takagi and Y. Uchida, FEBS Lett., 2006, 580, 5456–5466 CrossRef CAS PubMed.
- V. Choudhary, R. Uaratanawong, R. R. Patel, H. Patel, W. Bao, B. Hartney, E. Cohen, X. Chen, Q. Zhong, C. M. Isales and W. B. Bollag, J. Invest. Dermatol., 2019, 139, 868–877 CrossRef CAS PubMed.
- L. Japtok, W. Bäumer and B. Kleuser, Allergo J. Int., 2014, 23, 54–59 CrossRef PubMed.
- S. Borodzicz, L. Rudnicka, D. Mirowska-Guzel and A. Cudnoch-Jedrzejewska, Lipids Health Dis., 2016, 15, 1–9 CrossRef PubMed.
- H. Wakita, Y. Tokura, H. Yagi, K. Nishimura, F. Furukawa and M. Takigawa, Arch. Dermatol. Res., 1994, 286, 350–354 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4bm00355a |
| This journal is © The Royal Society of Chemistry 2024 |







