Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoactivatable substrates show diverse phenotypes of leader cells in collective migration when moving along different extracellular matrix proteins†
Shimaa A.
Abdellatef
*a,
Francesca
Bard
ab and
Jun
Nakanishi
 *acd
*acd
aMechanobiology group, Research Centre for Macromolecules and Biomaterials, National Institute for Materials Science (NIMS), Tsukuba, Japan. E-mail: nims.email.Shimaa@gmail.com; NAKANISHI.Jun@nims.go.jp
bDepartment of Material Science and Engineering, Cornell University, Ithaca, NY, USA
cWaseda University Graduate School of Advanced Science and Engineering Department of Nanoscience and Engineering, Tokyo, Japan
dTokyo University of Science, advanced Graduate School of Engineering Materials Innovation Engineering, Japan
First published on 30th May 2024
Abstract
In cancer metastasis, collectively migrating clusters are discriminated into leader and follower cells that move through extracellular matrices (ECMs) with different characteristics. The impact of changes in ECM protein types on leader cells and migrating clusters is unknown. To address this, we investigated the response of leader cells and migrating clusters upon moving from one ECM protein to another using a photoactivatable substrate bearing photocleavable PEG (PCP), whose surface changes from protein-repellent to protein-adhesive in response to light. We chose laminin and collagen I for our study since they are abundant in two distinct regions in living tissues, namely basement membrane and connective tissue. Using the photoactivatable substrates, the precise deposition of the first ECM protein in the irradiated areas was achieved, followed by creating well-defined cellular confinements. Secondary irradiation enabled the deposition of the second ECM protein in the new irradiated regions, resulting in region-selective heterogeneous and homogenous ECM protein-coated surfaces. Different tendencies in leader cell formation from laminin into laminin compared to those migrating from laminin into collagen were observed. The formation of focal adhesion and actin structures for cells within the same cluster in the ECM proteins responded according to the underlying ECM protein type. Finally, integrin β1 was crucial for the appearance of leader cells for clusters migrating from laminin into collagen. However, when it came to laminin into laminin, integrin β1 was not responsible. This highlights the correlation between leader cells in collective migration and the biochemical signals that arise from underlying extracellular matrix proteins.
1. Introduction
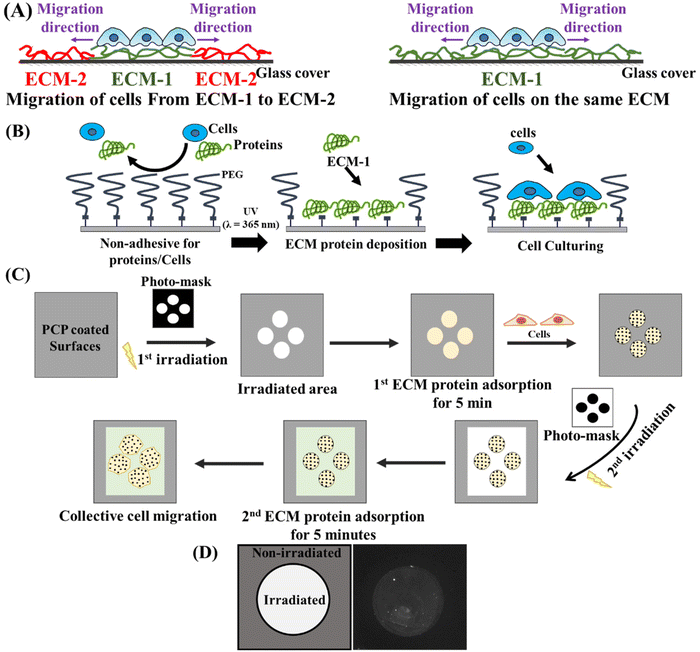
Collective cell migration is an intriguing process that plays a crucial role in several biological processes, such as morphogenesis, tissue repair, and cancer metastasis. It demonstrates the remarkable coordination of cells as they move together. The coordination of movement ranges from multicellular streaming until it reaches tightly connected cells that migrate as a cohesive unit, known as supracellular migration.1 Based on the topology of migrating populations and tissue-scale kinetics, the “leader-follower” model has been proposed2 with other models3 to understand the persistent directional collective movement. The “leader-follower” model is based on the idea that cell populations are discriminated into two distinct cell populations. These two groups are distinguished by their position within the moving cell group. Leader cells are specialized cells located at the front of the collective. On the other hand, follower cells constitute the majority of the collective and are located in the cell reservoir. The leader-follower formation is associated with various cellular changes, including genetic,4 epigenetic,5 biochemical,6,7 and mechanical factors.8 Leader cells are distinguishable by their position at the front of the migrating group, actin-rich lamellipodia, higher metabolic demands,7 and ability to guide migration direction and speed.9,10 They are also more sensitive to chemoattractants than follower cells.11,12 Follower cells that migrate after the leaders can also contribute passively or actively to collective migration.13 They can push, pull, or even compete with leaders for their positions. Leader cells play a significant role in cancer metastasis, where cells migrate from one organ to another distant organ. During this journey, leader cells, followed by other cells, move through the extracellular matrices with tremendously different characteristics. Leader cells pave the way in front of migrating clusters by biochemically and physically altering the surrounding environment. This is achieved through matrix deposition, physical remodeling, and proteolysis.14 However, before this can happen, it is not known how leader cells first sense and respond to the immediate changes in their environment. In recent years, advanced material fabrication has been used to mimic the different chemical and mechanical cues of the extracellular matrix (ECM) to understand how these cues affect the formation of leader cells and collective migration. However, most previous studies have compared collective cell migration and leader cell formation using different substrates with different ECM characteristics, assuming that cells migrate in the same ECM characteristics and record their behaviours in these different substrates. In reality, migrating clusters and leader cells face changes in the ECM characteristics and respond to them accordingly. Therefore, it is crucial to develop substrates that enable us to have different ECM characteristics while the cells are migrating. By doing so, we can gain a more comprehensive understanding of how leader cells respond to changes in their environment and develop more effective strategies to harness their migratory potential.To tackle this challenge, in this study, we used a photoactivatable surface that changes from protein-repellent to protein-adhesive in response to light.15 Several types of photoactivatable substrates based on photocleavable poly(ethylene glycol) (PCP) have been previously developed not only on microscopically friendly glass coverslip16 but also on stiffness-defined hydrogels17 to demonstrate cell culturing in specific patterns18 or the development and coculture systems.19 The use of photoactivatable surfaces in this study can greatly aid our research in creating surfaces coated with various ECM proteins. This enables us to study the collective migration of cells from one ECM to another and compare it to their migration on the same ECM (Fig. 1A). Additionally, as we demonstrated previously, by exposing the surface to UV light through a photomask, we can establish cellular constraints with preferred geometries20 to standardize cell morphology6 before the onset of migration, even in an inverted configuration.20 This remote controllability of cell migration induction would provide more reliable and cell-friendly assessments by eliminating the limitations associated with traditional tests that often impede the collective migration process due to damaged cells at the boundaries.
This study aims to examine the response of leader cells when moving across different types of extracellular matrix (ECM). We chose laminin and collagen I as simple examples of different ECM types since it is widely recognized that a combination of different ECM proteins forms the distinct structure of ECM in various organs and tissues.21 Therefore, it can really mimic what the leader cells could face during cancer metastasis. In detail, laminins (LM) are crucial components of basement membranes and play a vital role in the healing process of wounds. They are heterodimeric glycoproteins. Several isoforms of laminins have been identified.22 Laminin isoform LM332 promotes the persistent migration of keratinocytes,23 a vital step in wound re-epithelialization. Knocking down laminin LM-511, another isoform of laminin, inhibits the directional migration of MDCK cells.24 While collagen I is the most abundant ECM protein in connective tissue and bone,18 this protein comprises a long continuous triple helix that assembles into highly organized fibrils that mainly determine the ECM mechanics. Upregulation of collagen I is an early event in renal fibrosis.25 We have chosen these two proteins for our study since they are abundant in two distinct regions in living tissues: basement membrane and connective tissue. This will allow us to gain valuable insights into the role and function of alterations of ECM proteins in leader cell appearances in collective migration.
2. Results and discussion
2.1. Photoactivatable substrates as a testing platform to study collective cell migration on different ECMs
We have utilized glass surfaces functionalized with a photocleavable monolayer of PEG, as previously reported.26 These PEG groups were covalently linked to the glass, which made it resistant to cell adhesion and protein adsorption (Fig. 1B). We used a photocleavable 2-nitrobenzyl (2-NB) group to link PEG and glass surfaces, which can be easily cut by irradiation the surface with UV light (λ = 365 nm). Under this process, glass surfaces that were once passivated can now allow protein deposition and cell adhesion (Fig. 1B). Next, the stepwise deposition of ECM proteins and the subsequent cellular culture is a facile method to examine the effect of various ECM proteins on collective cell migration compared to conventional methods such as wound scratch assays. Fig. 1C presents a visual representation of the experimental procedure. Circular clusters with a diameter of 266 μm were created on PEG-coated glass surfaces using photomasks and UV light. The first ECM protein, such as collagen or laminin, was deposited for 5 minutes. Following that, cells were seeded to the circular patterns coated with the specific ECM protein in serum-free medium to reduce the risk of non-specific adhesion of serum proteins during cellular attachment. We seeded the cells for a brief period of 3–4 hours before the second irradiation. We chose this short incubation time because our group has previously observed that shorter culture time is positively associated with the higher appearance of leader cells and cellular expansion.6,20 A second irradiation was performed using the opposite photomask to shield the cells from UV hazards. Next, the second ECM protein (collagen or laminin) is deposited in the irradiated area for another 5 minutes. Finally, the expansion and migration of cell sheets were observed using live imaging. We have confirmed the adsorption of ECM proteins to glass surfaces after photoirradiation using Alexa Fluor-labelled antibodies (Fig. 1D). The patterned substrates were immersed in ECM protein for 5 minutes, forming a circular pattern of fluorescently labelled protein surrounded by non-irradiated areas (Fig. 1D). The photoactivatable surfaces not only allow for the creation of well-defined cellular confinements but also allow for the precise deposition of ECM in these patterns before the cellular seeding. Conventional methods like scratch wound healing assays for performing these sequential steps of extracellular matrix (ECM) deposition and cellular patterning are limited. However, photoactivatable surfaces provide a more practical and straightforward platform to explore collective cell migration on several ECM proteins. This emphasizes the potential of our system for further investigating the relationship between collective cell migration and other extracellular matrix cues.2.2. The leader cell appearance and expansion area are correlated to the underlying ECM proteins
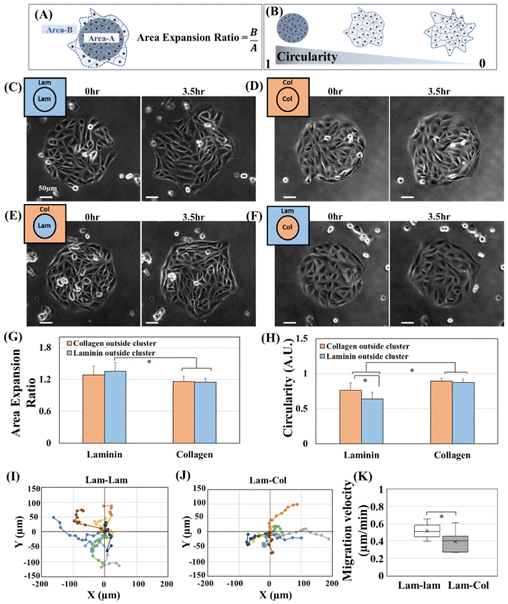
Next, we examined the migratory characteristics of MDCK cells migrating collectively on different ECM proteins. MDCK cells are normal epithelial cells and are a widely studied model of collective migration;25,26 they naturally differentiate between leaders and followers.27,28 Our study defines leader cells by their position and unique characteristics (Fig. S1†). These leader cells are located at the front of the migrating clusters; they are larger in size relative to their follower cells, possess a fan-shaped morphology, and show active ruffled lamellipodia. Leader cells could lead the migration for several hours or be replaced with another leader. After a longer duration of cell migration, we observe a similar cellular arrangement as the finger-like structures reported in several studies.28,29Our study used two indicators to measure cellular migration velocity and leader cell appearances. Firstly, we utilized the cluster area expansion ratio to indicate cellular migration velocity (Fig. 2A). Secondly, we used cluster circularity to indicate leader cell appearances. Since we started with circular clusters due to the utilization of photoactivatable surfaces, we were able to easily determine the appearance of leader cells by observing the decrease in cluster circularity (Fig. 2B). Movie 1† shows the expansion and collective migration of MDCK on various ECM proteins. Fig. 2C–F shows the cellular cluster expansion from 0 h compared to 3.5 h. We chose four conditions for cell migration. Our first condition involves the movement of cells from laminin-coated areas underneath cell clusters to the irradiated regions outside the clusters, which are also coated with laminin (Fig. 2C). In the second condition, we focus on cells migrating from collagen-coated areas underneath the clusters to irradiated areas coated with collagen (Fig. 2D). The third condition involves observing the migration of cells from laminin-coated areas into collagen-coated regions (Fig. 2E). Finally, in the fourth condition, we examined the migration of cells from collagen-coated areas into laminin-coated regions (Fig. 2F). For convenience, these four conditions will be mentioned in the rest of the study as Lam–Lam, Col–Col, Lam–Col, and Col–Lam, respectively. The circular clusters that moved on Lam–Lam show a higher expansion ratio than those that moved on Col–Col (Fig. 2G, laminin blue vs. collagen orange). This highlights the importance of the types of ECM proteins as chemical cues in determining the expansion of clusters and migration velocity. When we compared the Lam–Lam to the Lam–Col, we found no significant difference in the migration expansion area (Fig. 2G, laminin blue vs. laminin orange). In the alternative case, when we compare Col–Col and Col–Lam, we could not find also any significant difference in the migration expansion area (Fig. 2G, collagen blue vs. collagen orange). This suggests that the secondary ECM, whether collagen or laminin, did not play any role in the cluster's expansion ratio or migration velocity; rather, the primary ECM underneath the clusters represents the starting point for cluster expansion and migration. In other word, our results suggest the role of the follower cells to steer the migration from behind based on their underlying ECM proteins. Several studies have shown that the contribution of the follower cells to the migrating clusters goes beyond simply being dragged along passively.2,13,30 During the migration of zebrafish polster cells, follower cells guide their migration by pulling the leader cells from behind through E-cadherin/a-catenin mechanotransduction.31 Another work shows that followers push the leader cells during the migration of posterior lateral line primordium (PLLp) migrating within the zebrafish embryo.32 Therefore, the migration of cells is driven by biochemical signals originating from their interactions with primary extracellular matrix (ECM-1), rather than by the new chemical environment (ECM-2) to which they are migrating. After that, when we compared the change in circularity upon the cluster migration, we observed exciting results; first of all, when we compared the Lam–Lam and Col–Col circularity, we observed that cells migrating on Lam–Lam showed a decrease of circularity compared to cells migrating collagen Col–Col (Fig. 2H, laminin blue vs. collagen orange). This suggests that there are more leader cells for Lam–Lam compared to Col–Col. We concluded that the type of ECM proteins can be utilized as an ECM cue to modify the appearance of leader cells. Previously, several ECM cues have been shown to impact the appearance of leader cells, including chemical cues such as ECM concentration,6 geometrical cues,20,33,34 and mechanical cues such as compressive stress,8 substrate stiffness35 and, gravity vector.36 Interestingly, we found that the circularity of cells migrating on Lam–Lam was significantly lower than those migrating from Lam–Col (Fig. 2H, laminin blue vs. laminin orange). Despite having the same expansion ratio (Fig. 2G, laminin blue vs. laminin orange). On the other hand, the circularity of cells migrating from collagen to collagen or laminin remained equal and larger (Fig. 2H, collagen blue vs. collagen orange), with a lower expansion ratio (Fig. 2G, collagen blue vs. collagen orange). Based on these results, we can conclude that the secondary ECM is important in determining the appearance of leader cell formation in highly migrating cells such as Lam–Lam and Lam–Col. However, for slow-migrating cells, this difference was not significantly observed. In order to distinguish between the phenotypes of leader cells, we monitored the trajectories and velocity of highly migrating cells, specifically Lam–Lam and Lam–Col (Fig. 2I–K). Our observations revealed that the leader cells migrating on Lam–Lam exhibited a greater number of directional changes when compared to those migrating on Lam–Col (Fig. 2I and J). Moreover, the velocity of the leader cells was faster on laminin and secondary ECM compared to collagen (Fig. 2K). Despite these differences, when we think about the movement of epithelial cells, it is important to understand that this motion results from a global tug-of-war within clusters. This tug-of-war integrates each local force generated by leaders and submarginal followers, working together to produce the required tensile stress.37 It is worth noting that leaders guide migrating follower cells during migration and do not drag them along. Therefore, when comparing Lam–Lam and Lam–Col during cluster expansion, it is expected that the overall force generated would remain unchanged since the average cellular expansion was identical for both migrating clusters. This could be due to the fact that most of the follower cells remained in laminin during our experiments. However, it was observed that the number of leader cells, which act as track-generating cells, decreased for cells migrating to collagen compared to those migrating to laminin. This suggests that migration into collagen hinders the transformation of external cells into leader cells. This suggests that the expansion of cellular clusters and the generation of leader cells are completely different processes controlled by different biochemical signals and factors.
2.3. The variations in the formation of cytoskeletal and focal adhesion structures when cells migrate in different types of extracellular matrix proteins
Since we have identified that the tendency of leader cell appearance depends on the type of underlying ECM proteins, we planned to describe the cytoskeletal structures and formation of focal adhesion variations in these different ECM proteins. Our initial observations of single cells cultured on irradiated glass substrates coated with laminin and collagen revealed the presence of altered focal adhesions and actin structures formed for each ECM protein (Fig. S2†). Vinculin staining was used to visualize the shape and size of formed focal adhesions, and phalloidin staining was used to visualize the actin structures. In laminin, single cells exhibited smaller sizes and fewer vinculin and actin filaments compared to single cells on collagen, which exhibited larger sizes and higher numbers of vinculin and actin filaments (Fig. S2†). The same trend in the formation of focal adhesions was observed across cells of different origins, such as cervical human cancer (HeLa) and human breast cancer (MDA MB-468), when cultured on laminin and collagen (Fig. S3†). By utilizing the patterned photoactivatable surface, we could observe the formation of focal adhesions (FA) and actin structures for cells within the same cluster that exists in the heterogenous or homogenous ECM proteins (Fig. 3). For the Lam–Lam substrate, we observed the formation of small and scattered FA in both leader cells and cells within initial circular clusters coated with laminin. Fewer actin stress fibers were observed for both leading cells (migrating on laminin), and those follower cells remained in laminin (Fig. 3A). On the other hand, in cells migrating in Col–Col, we observed the formation of larger FAs and higher numbers, as visualized by vinculin staining by both leading cells (migrating on collagen) and the follower cells that remained on collagen (Fig. 3B). Additionally, both types of cells formed a higher number of stress fibers (Fig. 3B). Afterward, we examined the focal adhesion (FA) and actin structures of cells migrating in heterogeneous ECM proteins such as Lam–Col (Fig. 3C) and Col–Lam (Fig. 3D). We observed that each cell type maintained its original FA and actin stress fiber type based on the underlying ECM protein type. For instance, in the case of Lam–Col, leader cells that migrate on collagen showed the formation of larger FA and a lot of stress fibers, while the follower cells inside the clusters migrating on laminin displayed the formation of small FA and less actin stress fibers (Fig. 3C). Conversely, for cells on Col–Lam, where leader cells that migrate on laminin showed the formation of small FA and less actin stress fibers. On the other hand, the follower cells that remained in collagen showed the formation of larger FA and a lot of stress fibers. This result demonstrates the feasibility of our system to analyze changes in ECM protein types and how leader and follower cells respond to underneath biochemical signal alterations. Moreover, despite the observed differences in the size of focal adhesions, it's not possible to establish a direct correlation between the size of focal adhesions and the observed migratory characteristics. This is because there is a biphasic relationship between the size of focal adhesions and migration speed. Initially, the migration speed increases as the size of focal adhesions increases until a certain threshold, after which the migration speed decreases.38 However, our findings indicate that leader cells and followers that emerge during collective cell migration display distinct traits based on the extracellular matrix (ECM) protein type present. This implies that biochemical signals in the ECM could be linked to the development of different subtypes of leader cells, each with unique structural and morphological features. Finally, the utilization of photoactivatable substrates to monitor collective cell migration on various extracellular matrix (ECM) proteins could be incredibly valuable in understanding the impact of altered ECM proteins or other cues in scenarios like cancer metastasis or morphogenesis.2.4. Leader cell subtypes are different for cells migrating from laminin into laminin or collagen
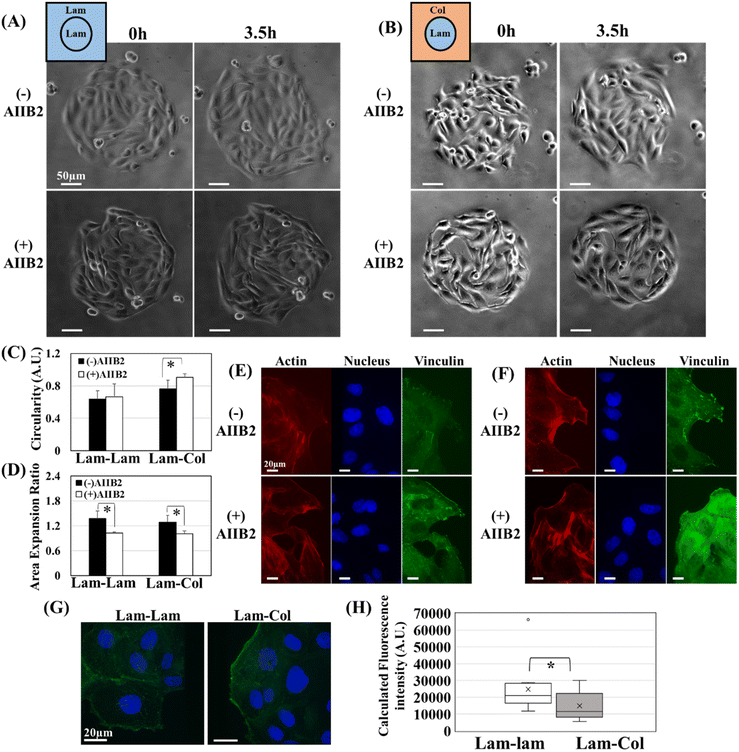
In the previous section, we demonstrated that the leader cells exhibit distinct appearance, structural, and morphological characteristics when they migrate from laminin to collagen compared to when they migrate from laminin to laminin. This observation suggests that the biochemical signals transmitted to the cells are different. Previous reports discuss the expression of integrin β1 specifically to the leading edges of migrating clusters,39,40 another report indicated that integrin β1 is upregulated in leader cells that migrate in collagen gel.28 This upregulation plays a crucial role in activating Rac, which is essential for driving collective migration and leader cell appearance.28 Therefore, if the signals that cause leader cells to appear are different between laminin and collagen, and we know for certain that integrin β1 is involved in the appearance of leader cells for collagen,28 then blocking integrin β1 on migrating clusters would result in different tendencies of leader cell appearance. To test this hypothesis, we used integrin β1 blocking antibodies (AIIB2) during the migration process (Movie 2†). In Fig. 4A and B, the phase contrast images of two types of cell clusters, Lam–Lam and Lam–Col, both with and without blocking antibodies, at 0 and 3.5 hours of migration were presented. We observed the change in cluster circularity and found that for the migrating clusters, Lam–Lam did not show any significant change with or without blocking antibodies (Fig. 4B). This indicates that integrin β1 does not play a role in the appearance of leader cells when the cells migrate within laminin. However, for Lam–Col substrates, we observed a significant increase in the cluster circularity, which indicates a decrease in the formation of leader cells in the presence of blocking antibodies (Fig. 4C). Additionally, the area expansion ratio decreased for both migrating clusters after treatment with antibodies (Fig. 4D), which likely suggests that different biochemical mechanisms control the expansion ratio and leader cell formation. This indicates that while integrin β1 contributed to the cluster expansion, it is not involved in leader cell formation for cells migrating in Lam–Lam substrates. However, it is engaged in both migration and leader cell formation in the other case (Lam–Col). In general, cells can express many integrin heterodimers that interact with partially overlapping sets of ECM molecules. MDCK cells, for instance, express a range of integrins, including β1, β3, β5, b6, β8, α5, and αV-integrins which alters their adhesion with different ECM proteins41 and associated functionalities.42 Although integrin β1 has an affinity to laminin as well as collagen I, other integrins are involved in the case of laminin. It was previously reported that the knockdown integrin β1 contributes to the spreading of MDCK on collagen and laminin 115, yet to a different extent.41 This result supports our finding since the expansion rate is delayed in both migrating clusters (Lam–Col) and (Lam–Lam), which could represent the contribution of integrin β1 in spreading, while leader cell formation requires the contribution of another type of integrins for those migrating on laminin. Our study has not identified the specific integrins involved in leader cell formation for laminin; however, based on previous literature, integrin α4β6 may be a promising candidate, as it is known to bind to laminin.43 Additionally, another study finds that the knockdown of α4β6 impedes collective migration, while the forced expression of integrin α4β6 in leading edge cells has been observed to restore this activity.44 Another interesting observation from this experiment is that even though the follower cells were found in the laminin-coated areas in both cases, this did not maintain the appearances of leaders for Lam–Col substrates, it seems that the primary factor determining external cell discrimination into leaders is the external cells themselves and their underlying environment. This is particularly true since the expansion rate is impeded for both cases, and backward followers could not push migrating clusters forward in either case. After that, we stained and characterized vinculin and actin for leader cells, with and without the blocking antibodies. In the formed leaders, we did not observe any significant difference in the vinculin formation for Lam–Lam surfaces with and without AIIB2 treatment (Fig. 4E). On the other hand, we observed a decrease in the localization of vinculin in the focal adhesions for Lam–Col surfaces after AIIB2 treatment, and it seemed to be distributed in the cytoplasm (Fig. 4F). This is in line with previous reports that suggest a defective localization of vinculin to FA upon the knockdown of integrin β1 in MDCK cultured in collagen I-coated surfaces.41 After demonstrating that the appearance of leader cells in extracellular matrix (ECM) proteins originates from different integrin subtypes, we investigated how this affects FAK phosphorylation. By staining the migrating clusters, we analyzed the phosphorylation levels of FAK at two phosphorylation sites, autophosphorylation at Tyr-397 (Fig. S4A†) and the second one at Tyr-861 (Fig. 4G). The results showed that the level of p-FAK-861 was higher in the leader cells migrating in Lam–Lam compared to those migrating in Lam–Col(Fig. 4H), while the FAK-397 levels remained the same (Fig. S4B†). These findings support our hypothesis that the leader cell formation is controlled by the underlying ECM protein composition, which is associated with different integrin subtypes and levels of FAK-861 phosphorylation. FAK-861 plays a vital role in the acquisition of metastatic potentials for a lot of cancer cells.45,46 Thus, we showed that integrin β1 is crucial for the focal adhesions in leader cells that are migrating into collagen I. However, when it comes to laminin, integrin β1 is not responsible for this function which was associated with different FAK activation. Finally, our study has achieved a significant milestone in understanding the link between leader cells and the different of ECM proteins.2.5. The leader cells’ appearance and ECM compositions
Our research aimed to provide a better understanding of the role of ECM composition as a biochemical cue in shaping the behavior of leader cells. Using photoactivatable surfaces have shed light on how leader cells respond during migration on heterogeneous ECM proteins compared to homogenous types (Fig. 5). Our findings also have highlighted the pivotal role of integrin β1 in the formation of leader cells in MDCK cells that migrate into collagen I. Furthermore, we have opened up exciting avenues for further research into the formation of leader cells in MDCK cells that migrate into laminin and other ECM proteins, what specific integrins are involved in this process, how FAK-861 involved, what are the underlying biochemical signals that regulate it. Understanding these differences will give us insights into the relationship between collective migration, extracellular matrix, and cancer metastasis. Especially when cancer metastasis is triggered by the modification of the ECM and the creation of very complex structures around the tumor.47 This involves altering the density, organization, composition, and structure of different ECM proteins and macromolecules, which in turn modify the chemical and mechanical properties of the ECM. This can be achieved by depositing ECM proteins, chemically modifying existing proteins through post-translational modifications, or using remodeling enzymes like proteases to release bioactive proteins. For example, basement membranes usually comprise laminin and collagen IV, and their remodeling is associated with tumor progression.48 Melanoma cells are involved in matrix remodeling by expression of specific MMP type, disposition, and cleavage of laminin.49 Collagen I is a substrate that hinders the collective migration of cancer cells that originate from epithelial tissue. However, the presence of fibroblasts can initiate the movement of these cancer cells by remodeling the matrix and creating a pathway for their movement.50 If we compare these results with our findings, we can observe a similar trend; MDCK, as epithelial cells, experience inhibited movement due to the presence of collagen I, while its presence in a heterogenous ECM has different outcomes. These unique results were obtained by utilizing photoactivatable systems. If these systems continue to be used, there are tremendous choices and combinations of proteins/peptides that could be explored. | ||
| Fig. 5 Cartoon depicting how switching from laminin to collagen in external ECM protein alters leader cell formation and integrin involved. | ||
From another perspective, changes in the composition of extracellular matrix (ECM) in nature are often linked to changes in its mechanical properties. For example, an increase in the ratio of collagen I to collagen III leads to stiffening of heart muscles.51,52 However, in our research, we used physically adsorbed collagen I/laminin on stiff glass surfaces to eliminate the effect of alteration of mechanical properties of ECM proteins. Our findings revealed the distinct formation of FAs, rearrangement of the cytoskeleton, migratory phenotypes, and leader cell formation, highlighting the importance of biochemical signals in ECM composition. Nonetheless, it raises an interesting question about cellular behavior on compliant surfaces with different ECM compositions. Would we observe similar cellular behavior associated with compliant surfaces? Could the extensively studied mechanisms for mechanosensing be altered, especially those involving the YAP/TAZ53 and integrin clustering54? To explore this area, further development of photoactivatable functionalization technology is needed to better understand the mechanisms for mechanosensing concerning the underlying ECM chemical as well as mechanical cues.
3. Conclusions
We have used photoactivatable substrates to investigate the importance of different type of extracellular matrix (ECM) proteins in collective cell migration and leader cell formation. Traditional methods, such as wound scratch assays, are ineffective in inducing ECM protein switching. In our research, we have focused on using Laminin and Collagen I as our primary EM proteins. We found that the secondary ECM plays a crucial role in determining the formation of leader cells in highly migrating cells, such as laminin to laminin substrate and laminin to collagen substrate. However, this difference was not significantly observed in slow-migrating cells such as collagen to collagen substrate and collagen to laminin substrate. Additionally, cells maintained their original type of focal adhesion (FA) and actin stress fiber based on the underlying ECM protein type. We also found that integrin β1 contributes to cluster expansion but is not involved in leader cell formation for cells migrating from laminin to laminin. However, it is engaged in both migration and leader cell formation in the other case such as laminin to collagen. Our findings highlight the promising potential of utilizing photoactivatable surfaces as a more efficient approach to explore collective cell migration across different and heterogeneous ECM proteins.4. Experimental
4.1. Photopatterning and ECM adsorption
Photoactivatable substrate functionalized with PCP was prepared according to the procedure reported previously.55 Photoirradiation occurred as previously reported with a slight modification;6,16 shortly, glass surfaces in PBS were exposed to a dose of 30 J cm−2 with a wavelength of 365 nm with a photomask in the field diaphragm of Olympus microscope (IF81-PAFM, Olympus, Tokyo, Japan) using HBO mercury arc lamp (Olympus) and focusing UV light through an objective (UPlanSApo 10×/0.4, Olympus). After cleaving the PEG12-NH, the primary extracellular matrix protein such as Laminin (from Engelbreth-Holm-Swarm murine sarcoma basement membrane, Sigma-Aldrich, MO, USA) or Collagen Type I (Rat tail, Discovery Labware, MA, USA) was diluted in PBS and deposited on irradiated surfaces (2 μg cm−2) for 5 minutes at 37 °C. Then, the surfaces were washed 3–4 times with PBS to remove non-adsorbed proteins. After that, cells were seeded on these patterned substrates at a density of 75 × 103 cells per cm2 in serum-free medium. After cellular attachment to irradiated clusters (∼1 h), the medium was changed into fresh medium (+) FBS. The second irradiation was done using an Axiovert 200 microscope (Zeiss, Oberkochen, Germany) equipped with a xenon lamp and a mercury arc lamp through an objective (Plan-APOCHROMAT 10×/0.45, Zeiss). Next, the medium was changed into a PBS solution with ECM proteins at the same concentration for 5 minutes at 37 °C. The surfaces were washed 3–4 times with PBS to remove non-adsorbed proteins. Finally, fresh medium (+) FBS was added to start the time-lapse observation using the same microscope. The image was captured using a Cool SNAP MYO camera (Photometrics, Tucson, AZ, USA). All systems were controlled using Metamorph software (Molecular Devices, Sunnyvale, CA, USA); captured images were processed using Image J.4.2. Cell culture and immunostaining
MDCK cells (RCB0995, RIKEN cell bank) were cultured in MEM (Sigma, St Louis, MO, USA) containing 10% FBS (heat-inactivated FBS; BioWest, EU origin), 100 units per mL penicillin and 100 ug mL−1 streptomycin (Nacalai, Japan), 1% MEM-nonessential amino acids (Nacalai, Japan), 1% sodium pyruvate (Nacalai, Japan), and 1% L-glutamine (Nacalai, Japan) at 37 °C in a humidified atmosphere containing 5% CO2 at 75% confluency of cell subculture. Cells were collected by trypsin/EDTA (Wako, Japan) and seeded on photo-irradiated glass. The cells were initially seeded in a medium without FBS for one hour, after which the medium was changed to a complete medium for the rest of the experiments. HeLa and MDA-MB468 cells were obtained from American Type culture collection (ATCC, Manassa, VA, USA). HeLa was maintained in a state of continuous growth in MEM containing 10% FBS, 100 units per mL penicillin, and 100 μg mL−1 streptomycin at 37 °C in a humidified atmosphere containing 5% CO2 and subcultured every 2 or 3 days. MDA MB-468 was maintained in a state of continuous growth in DMEM-high glucose containing 10% FBS, 1% glutamine, 100 units per mL penicillin, and 100 μg mL−1 streptomycin at 37 °C in a humidified atmosphere containing 5% CO2 and subcultured every 2 or 3 days. Confocal images were obtained by Olympus microscope (IF81-PAFM, Olympus, Tokyo, Japan) using a disk-scan unit (CSU-10, Yokogawa, Tokyo, Japan) and Andro CCD camera (SONA 4BV6U, UK), captured images were processed using Fiji (Image J, USA). For leader cell trajectory tracking and velocity calculations, each leader cell was manually outlined every 30 minutes, and then the centroid was determined using Fiji. In the immunofluorescence staining for ECM-coated surfaces, Fibronectin diluted in PBS with deposited (2 μg cm−2) in the irradiated glass for 5 min, followed by fixation with 4% paraformaldehyde (Nacalai, Japan), blocked with bovine serum albumin (Wako, Japan) for 30 min, then incubation with fibronectin-Alex Fluor-488 Antibody (A1918, Santa Cruz biotechnology). For cell staining, Fixation was done with 4% paraformaldehyde (Nacalai, Japan) for 15 min, quenched with 5% glycine (Wako, Japan) in PBS for 5 min, permeabilized with 0.5% Triton X-100 for 5 min, and blocked with bovine serum albumin (Wako, Japan) for 30 min; cells were then incubated with Alexa Fluor™ 565 Phalloidin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ThermoFisher Scientific, USA), mouse anti-vinculin (1
1000, ThermoFisher Scientific, USA), mouse anti-vinculin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, Sigma), anti-mouse IgG Alexa Fluor 488 (1
400, Sigma), anti-mouse IgG Alexa Fluor 488 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ThermoFisher Scientific, USA), and Hoechst 33342 (1
1000, ThermoFisher Scientific, USA), and Hoechst 33342 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Life Technologies, Eugene, OR, USA), Rabbit p-FAK 397 (1
1000, Life Technologies, Eugene, OR, USA), Rabbit p-FAK 397 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, Invitrogen, USA) and Rabbit p-FAK 861 (1
400, Invitrogen, USA) and Rabbit p-FAK 861 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, Abcam) anti-rabbit IgG Alexa Fluor 488 (1
400, Abcam) anti-rabbit IgG Alexa Fluor 488 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ThermoFisher Scientific, USA). To calculate the fluorescence intensities for p-FAK 861 and 397, each cell was manually outlined, and then the integrated density was calculated by selecting the integrated density option under the measurement function in Fiji. After that, the background was subtracted, where the background represents the cell area multiplied by the mean grey value of the non-fluorescent background.56 Integrin β1 (AIIB2) antibody (University of Iowa, Hybridoma bank, USA) was used in concentration (1.5 μg ml−1) for the integrin β1 blocking experiment. The statistical analyses were performed for the presented data using a student t-test.
1000, ThermoFisher Scientific, USA). To calculate the fluorescence intensities for p-FAK 861 and 397, each cell was manually outlined, and then the integrated density was calculated by selecting the integrated density option under the measurement function in Fiji. After that, the background was subtracted, where the background represents the cell area multiplied by the mean grey value of the non-fluorescent background.56 Integrin β1 (AIIB2) antibody (University of Iowa, Hybridoma bank, USA) was used in concentration (1.5 μg ml−1) for the integrin β1 blocking experiment. The statistical analyses were performed for the presented data using a student t-test.
Author contributions
Shimaa A. Abdellatef: conceptualization, data acquisition, formal analysis, investigation, methodology, funding acquisition, writing – original draft, writing – review & editing. Francesca Bard: data acquisition, formal analysis. Jun Nakanishi: project administration, supervision, methodology, validation, funding acquisition, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was partly supported by the Japan Society for the Promotion of Science, KAKENHI (22H00596, 23K17481, 21J40229), and the KAO-Crescent Award for women researchers. The authors are grateful to Mrs Elham Elmasry for her help in experiments and data analysis.References
- P. Rørth, Fellow travellers: emergent properties of collective cell migration, EMBO Rep., 2012, 13(11), 984–991 CrossRef PubMed.
- L. Qin, D. Yang, W. Yi, H. Cao and G. Xiao, Roles of leader and follower cells in collective cell migration, Mol. Biol. Cell, 2021, 32, 1267–1272 CrossRef CAS PubMed.
- P. Friedl and D. Gilmour, Collective cell migration in morphogenesis, regeneration and cancer, Nat. Rev. Mol. Cell Biol., 2009, 10(7), 445–457, DOI:10.1038/nrm2720.
- E. L. Zoeller, B. Pedro, J. Konen, B. Dwivedi, M. Rupji and N. Sundararaman, et al., Genetic heterogeneity within collective invasion packs drives leader and follower cell phenotypes, J. Cell Sci., 2019, 132, jcs231514 CrossRef CAS PubMed.
- J. M. Westcott, Y. Xie, G. W. Pearson, J. M. Westcott, A. M. Prechtl and E. A. Maine, et al., An epigenetically distinct breast cancer cell subpopulation promotes collective invasion, J. Clin. Invest., 2015, 125(5), 1927–1943 CrossRef PubMed.
- S. A. Abdellatef and J. Nakanishi, Photoactivatable substrates for systematic study of the impact of an extracellular matrix ligand on appearance of leader cells in collective cell migration, Biomaterials, 2018, 169, 72–84 CrossRef CAS PubMed.
- D. A. Chapnick and X. Liu, Leader cell positioning drives wound-directed collective migration in TGFβ-stimulated epithelial sheets, Mol. Biol. Cell, 2014, 25(10), 1586–1593 CrossRef PubMed.
- J. M. Tse, G. Cheng, J. A. Tyrrell, S. A. Wilcox-Adelman, Y. Boucher and R. K. Jain, et al., Mechanical compression drives cancer cells toward invasive phenotype, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(3), 911–916 CrossRef CAS PubMed.
- R. Mayor and S. Etienne-manneville, The front and rear of collective cell migration, Nat. Cell Biol., 2016, 17(2), 97–109 CrossRef CAS PubMed.
- J. Richardson, A. Gauert, L. B. Montecinos, A. Kabla, S. Ha and Z. M. Alhashem, Leader Cells Define Directionality of Trunk, but Not Cranial, Neural Crest Cell Migration, Cell Rep., 2016, 15, 2076–2088 CrossRef CAS PubMed.
- A. Hopkins and B. A. Camley, Leader cells in collective chemotaxis: Optimality and trade-offs, Phys Rev E, 2019, 100, 032417 CrossRef CAS PubMed.
- B. A. Camley, Collective gradient sensing and chemotaxis : modeling and recent developments, J. Phys.: Condens.Matter, 2018, 30, 223001 CrossRef PubMed.
- J. P. Campanale and D. J. Montell, Who ‘ s really in charge : Diverse follower cell behaviors in collective cell migration, Curr. Opin. Cell Biol., 2023, 81, 102160, DOI:10.1016/j.ceb.2023.102160.
- S. A. V. Mercedes, F. Bocci and H. Levine, Decoding leader cells in collective cancer invasion, Nat. Rev. Cancer, 2021, 21, 592–604 CrossRef PubMed.
- J. Nakanishi, Photoactivatable Substrates: A Material-Based Approach for Dissecting Cell Migration, Chem. Rec., 2017, 17(6), 611–621 CrossRef CAS PubMed.
- Y. Shimizu, M. Kamimura, S. Yamamoto, S. A. Abdellatef, K. Yamaguchi and J. Nakanishi, Facile preparation of photoactivatable surfaces with tuned substrate adhesiveness, Anal. Sci., 2016, 32(11), 1183–1188 CrossRef CAS PubMed.
- M. Kamimura, M. Sugawara, S. Yamamoto, K. Yamaguchi and J. Nakanishi, Dynamic control of cell adhesion on a stiffness-tunable substrate for analyzing the mechanobiology of collective cell migration, Biomater. Sci., 2016, 4(6), 933–937 RSC.
- Y. H. Gong, J. Yang, F. Y. Cao, J. Zhang, H. Cheng and R. X. Zhuo, et al., Photoresponsive smart template for reversible cell micropatterning, J. Mater. Chem. B, 2013, 1(15), 2013–2017 RSC.
- K. Kikuchi, K. Sumaru, J. I. Edahiro, Y. Ooshima, S. Sugiura and T. Takagi, et al., Stepwise assembly of micropatterned co-cultures using photoresponsive culture surfaces and its application to hepatic tissue arrays, Biotechnol. Bioeng., 2009, 103(3), 552–561 CrossRef CAS PubMed.
- C. G. Rolli, H. Nakayama, K. Yamaguchi, J. P. Spatz, R. Kemkemer and J. Nakanishi, Switchable adhesive substrates: Revealing geometry dependence in collective cell behavior, Biomaterials, 2012, 33(8), 2409–2418 CrossRef CAS PubMed.
- N. K. Karamanos, A. D. Theocharis, Z. Piperigkou, D. Manou, L. Duca and M. Durbeej, A guide to the composition and functions of the extracellular matrix, FEBS J., 2021, 288, 6850–6912 CrossRef CAS PubMed.
- V. Iorio, L. D. Troughton and K. J. Hamill, Laminins : Roles and Utility in Wound Repair, Adv. Wound Care, 2014, 1–14 Search PubMed.
- Z. Zhang, G. Chometon, T. Wen, H. Qu, C. Mauch and T. Krieg, et al., Migration of epithelial cells on laminins: RhoA antagonizes directionally persistent migration, Eur. J. Cell Biol., 2011, 90(1), 1–12 CrossRef CAS PubMed.
- P. G. Greciano, V. J. Moyano, M. M. Buschmann, J. Tang, Y. Lu, J. Rudnicki, A. Manninen and K. S. Matlin, Laminin 511 partners with laminin 332 to mediate directional migration of Madin-Darby canine kidney epithelial cells, Mol. Biol. Cell, 2012, 23(1), 121–136 CrossRef CAS PubMed.
- R. D. Bülow and P. Boor, Extracellular Matrix in Kidney Fibrosis : More Than Just a Scaffold, J. Histochem. Cytochem., 2019, 67(9), 643–661 CrossRef PubMed.
- Y. Kikuchi, J. Nakanishi, H. Nakayama, T. Shimizu, Y. Yoshino and K. Yamaguchi, et al., Grafting poly(ethylene glycol) to a glass surface via a photocleavable linker for light-induced cell micropatterning and cell proliferation control, Chem. Lett., 2008, 37(10), 1062–1063 CrossRef CAS.
- M. Reffay, L. Petitjean, S. Coscoy, E. Grasland-Mongrain, F. Amblard and A. Buguin, et al., Orientation and polarity in collectively migrating cell structures: Statics and dynamics, Biophys. J., 2011, 100(11), 2566–2575, DOI:10.1016/j.bpj.2011.04.047.
- N. Yamaguchi, T. Mizutani, K. Kawabata and H. Haga, Leader cells regulate collective cell migration via Rac activation in the downstream signaling of integrin β1 and PI3K, Sci. Rep., 2015, 5, 7656 CrossRef CAS PubMed.
- M. Reffay, M. C. Parrini, O. Cochet-Escartin, B. Ladoux, A. Buguin and S. Coscoy, et al., Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells, Nat. Cell Biol., 2014, 16(3), 217–223 CrossRef CAS PubMed.
- X. C. Wang, Y. L. Tang and X. H. Liang, Tumour follower cells : A novel driver of leader cells in collective invasion (Review), Int. J. Oncol., 2023, 63, 115 CrossRef CAS PubMed.
- A. Boutillon, S. Escot, L. Brusch, N. B. David, D. Jahn and L. Brusch, et al., Guidance by followers ensures long-range coordination of cell migration through a -catenin mechanoperception ll ll Article Guidance by followers ensures long-range coordination of cell migration through a -catenin mechanoperception, Dev. Cell, 2022, 57, 1529–1544 CrossRef CAS PubMed.
- N. Yamaguchi, Z. Zhang, T. Schneider, B. Wang, D. Panozzo and H. Knaut, Rear traction forces drive adherent tissue migration in vivo, Nat. Cell Biol., 2022, 24(2), 194–204, DOI:10.1038/s41556-022-00844-9.
- S. Rausch, T. Das, J. R. D. Soiné, T. W. Hofmann, C. H. J. Boehm and U. S. Schwarz, et al., Polarizing cytoskeletal tension to induce leader cell formation during collective cell migration, Biointerphases, 2013, 8(1), 1–11 CrossRef PubMed.
- T. Chen, A. Callan-Jones, E. Fedorov, A. Ravasio, A. Brugués and H. T. Ong, et al., Large-scale curvature sensing by directional actin flow drives cellular migration mode switching, Nat. Phys., 2019, 15(4), 393–402 Search PubMed.
- S. Yamamoto, K. Okada, N. Sasaki, A. C. Chang, K. Yamaguchi and J. Nakanishi, Photoactivatable Hydrogel Interfaces for Resolving the Interplay of Chemical, Mechanical, and Geometrical Regulation of Collective Cell Migration, Langmuir, 2019, 35(23), 7459–7468 CrossRef CAS PubMed.
- S. Sakakibara, S. A. Abdellatef, S. Yamamoto, M. Kamimura and J. Nakanishi, Photoactivatable surfaces resolve the impact of gravity vector on collective cell migratory characteristics, Sci. Technol. Adv. Mater., 2023, 24(1) DOI:10.1080/14686996.2023.2206525.
- X. Trepat, M. R. Wasserman, T. E. Angelini, E. Millet, D. A. Weitz and J. P. Butler, et al., Physical forces during collective cell migration, Nat. Phys., 2009, 5(6), 426–430 Search PubMed.
- D. Kim and D. Wirtz, Focal adhesion size uniquely predicts cell migration, FASEB J., 2013, 27, 1351–1361 CrossRef CAS PubMed.
- Y. Hegerfeldt, M. Tusch, E.-B. Bröcker and P. Friedl, Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies, Cancer Res., 2002, 62(7), 2125–2130 CAS.
- J. An, A. Enomoto, L. Weng, T. Kato, A. Iwakoshi and K. Ushida, et al., Significance of cancer-associated fibroblasts in the regulation of gene expression in the leading cells of invasive lung cancer, J. Cancer Res. Clin. Oncol., 2013 Mr, 139(3), 379–388 Search PubMed.
- T. P. Teräväinen, S. M. Myllymäki, J. Friedrichs, N. Strohmeyer, V. J. Moyano and C. Wu, et al., αV-integrins are required for mechanotransduction in MDCK epithelial cells, PLoS One, 2013, 8(8), e71485 CrossRef PubMed.
- R. J. De, A. Kerstens, G. Danuser, M. A. Schwartz, C. M. Waterman-Storer and J. De Rooij, et al., Integrin-dependent actomyosin contraction regulates epithelial cell scattering, J. Cell Biol., 2005 Ot, 171(1), 153–164 Search PubMed.
- C. S. Stipp, Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets, Expert Rev. Mol. Med., 2010, 12, 1–24 CrossRef PubMed.
- Z. T. Colburn and J. C. R. Jones, α6β4 integrin regulates the collective migration of epithelial cells, Am. J. Respir. Cell Mol. Biol., 2017, 56(4), 443–452 CrossRef CAS PubMed.
- Y. Lim, I. Han, J. Jeon, H. Park, Y. Y. Bahk and E. S. Oh, Phosphorylation of focal adhesion kinase at tyrosine 861 is crucial for ras transformation of fibroblasts, J. Biol. Chem, 2004, 279(28), 29060–29065, DOI:10.1074/jbc.M401183200.
- H. H. Chuang, Y. Y. Zhen, Y. C. Tsai, C. H. Chuang, M. Hsiao and M. S. Huang, et al., FAK in Cancer: From Mechanisms to Therapeutic Strategies, Int. J. Mol. Sci., 2022, 23(3), 1–28 Search PubMed.
- J. Winkler, A. Abisoye-Ogunniyan, K. J. Metcalf and Z. Werb, Concepts of extracellular matrix remodelling in tumour progression and metastasis, Nat. Commun., 2020, 11(1), 1–19, DOI:10.1038/s41467-020-18794-x.
- K. Kessenbrock, V. Plaks and Z. Werb, Matrix Metalloproteinases: Regulators of the Tumor Microenvironment, Cell, 2010, 141(1), 52–67 CrossRef CAS PubMed.
- R. E. B. Seftor, E. A. Seftor, N. Koshikawa, P. S. Meltzer, L. M. G. Gardner and M. Bilban, et al., Cooperative interactions of laminin 5 γ2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma, Cancer Res., 2001, 61(17), 6322–6327 CAS.
- C. Gaggioli, S. Hooper, C. Hidalgo-Carcedo, R. Grosse, J. F. Marshall and K. Harrington, et al., Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells, Nat. Cell Biol., 2007, 9(12), 1392–1400 CrossRef CAS PubMed.
- M. M. H. Marijianowski, P. Teeling, J. Mann and A. E. Becker, Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: A quantitative assessment, J. Am. Coll. Cardiol., 1995, 25(6), 1263–1272 CrossRef CAS PubMed.
- J. Beam, A. Botta, J. Ye, H. Soliman, B. J. Matier and M. Forrest, et al., Excess linoleic acid increases collagen I/III ratio, “stiffens” the heart muscle following high fat diets, J. Biol. Chem, 2015, 290(38), 23371–23384, DOI:10.1074/jbc.M115.682195.
- B. Cheng, M. Li, W. Wan, H. Guo, G. M. Genin and M. Lin, et al., Predicting YAP/TAZ nuclear translocation in response to ECM mechanosensing, Biophys. J., 2023, 122(1), 43–53, DOI:10.1016/j.bpj.2022.11.2943.
- B. Cheng, W. Wan, G. Huang, Y. Li, G. M. Genin and M. R. K. Mofrad, et al., Nanoscale integrin cluster dynamics controls cellular mechanosensing via FAKY397 phosphorylation, Sci. Adv., 2020, 6(1), eaax1909 CrossRef CAS PubMed.
- Y. Kikuchi, J. Nakanishi, H. Nakayama, T. Shimizu, Y. Yoshino and K. Yamaguchi, et al., Grafting Poly (ethylene glycol) to a Glass Surface via a Photocleavable Linker for Light-induced Cell Micropatterning and Cell Proliferation Control, Chem. Lett., 2008, 37(10), 10–11 CrossRef.
- S. Marlar, S. A. Abdellatef and J. Nakanishi, Reduced adhesive ligand density in engineered extracellular matrices induces an epithelial-mesenchymal-like transition, Acta Biomater., 2016, 39, 106–113 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4bm00225c |
| This journal is © The Royal Society of Chemistry 2024 |