 Open Access Article
Open Access ArticleCurrent trends in colorimetric biosensors using nanozymes for detecting biotoxins (bacterial food toxins, mycotoxins, and marine toxins)
Li
Feng
 *,
Mingcheng
Zhang
and
Zhiyi
Fan
*,
Mingcheng
Zhang
and
Zhiyi
Fan
Jiyang College, Zhejiang A&F University, Zhuji, Zhejiang 311800, China. E-mail: lifeng@zafu.edu.cn
First published on 9th September 2024
Abstract
Biotoxins, predominantly bacterial food toxins, mycotoxins, and marine toxins, have emerged as major threats in the fields of seafood, other foods, feeds, and medicine. They have potential teratogenic, mutagenic, and carcinogenic effects on humans, occasionally triggering high morbidity and mortality. One of the apparent concerns relates to the increasing consumption of fast food resulting in the demand for processed food without adequate consideration of the toxins they may contain. Therefore, developing improved methods for detecting biotoxins is of paramount significance. Nanozymes, a type of nanomaterials exhibiting enzyme-like activity, are increasingly being recognized as viable alternatives to natural enzymes owing to their benefits, such as customizable design, controlled catalytic performance, excellent biocompatibility, and superior stability. The remarkable catalytic activity of nanozymes has led to their broad utilization in the development of colorimetric biosensors. This has emerged as a potent and efficient approach for rapid detection, enabling the creation of innovative colorimetric sensing methodologies through the integration of nanozymes with colorimetric sensors. In this review, recent development in nanozyme research and their application in colorimetric biosensing of biotoxins are examined with an emphasis on their characteristics and performance. The study particularly focuses on the peroxidase (POD) activity, oxidase (OXD) activity, superoxide dismutase (SOD), and catalase (CAT) activity of nanozymes in colorimetric biosensors. Ultimately, the challenges and future prospects of these assays are explored.
1. Introduction
Nowadays, there is an emerging concern regarding the rapid monitoring of biotoxin presence in food products.1,2 Biotoxins are frequently found in bacteria, viruses, fungi, protozoa, rickettsiae, and infectious substances polluting feed, food, condiments, seafood, and so forth. Biotoxins pose a risk to public health through the food chain by inducing both chronic and acute toxicity, as well as exhibiting carcinogenic, teratogenic, and mutagenic impacts on human health.3–5 Certain biotoxin carriers and producers threaten human health and pollute the environment; they can lead to crop failures and diminish the quality of agricultural products. Marine toxins are categorized based on their carriers such as as ciguatoxins, shellfish toxins, mytilotoxin, tetrodotoxins, and others. There exists a wide range of marine toxins, exceeding 1000 different types, with several dozen having been effectively characterized. These toxins have the potential to infiltrate the food chain, leading to human toxicosis and potentially fatal outcomes. For instance, an estimated 750–7500 individuals die globally because of shellfish poisoning annually.6 Bacterial toxins represent a distinct category of biotoxins capable of inducing foodborne illnesses through the inhibition of protein synthesis, leading to neurotoxic effects. The diseases are linked to the impact of bacterial toxins on tissues and are associated with distinct clinical symptoms. The most lethal bacterial toxin associated with food consumption is botulinum toxin, which is created by Clostridium botulinum. It has been reported that 100 ng of this toxin can be fatal to humans.7 Mycotoxins are an important class of biotoxins that are produced as secondary metabolites by fungi, like Fusarium, Aspergillus, Penicillium, Claviceps, Alternaria, Trichoderma, Stachybotrys, Verticimonosporium, Chaetomium, and so forth, under favorable environmental conditions.8 The contamination can happen throughout processing, harvesting, transportation, and storage. The International Agency for Cancer Research has classified mycotoxins into distinct categories according to their capacity to trigger cancer in humans. These classifications encompass Group 1, Group 2A, Group 2B, Group 3, and Group 4 carcinogens. For example, AFB1 (Group 1 carcinogen aflatoxin B1) is the most toxic and abundant group of mycotoxins. It has been shown to trigger lung carcinoma, hepatocellular carcinoma, colon carcinoma, and gallbladder carcinoma in humans. Liver cancer is caused via AFB1 in around 28.2% of individuals.9In recent times, biosensors have emerged as a novel method of detection with a broad spectrum of applications for the timely quantitative/qualitative assessment of various biotoxins present in food products.10 A biosensor is a diagnostic tool that integrates three components: a biorecognition unit (e.g., aptamer, enzyme, antibody, phage, cell, etc.), a transducer and a signal conversion element.11,12 The biorecognition unit is employed to specifically identify the target molecule. The interaction between the target molecule and the recognition unit initiates a series of physicochemical interactions. This reaction is characterized by changes such as light absorption and electrical signals, which form the basis for further signal transduction processes. The transducer, being the most crucial component of the biosensor, is capable of converting the above physicochemical alterations into quantifiable signals, thereby facilitating signal transduction. There exist various types of sensors that can be classified into four main groups based on distinct signal transduction methods: optical sensors, electrochemical sensors, magnetoelectric sensors, and piezoelectric sensors.13,14 Among them, the optical sensor plays a crucial role in analyzing the optical signal produced during the integration of the target and the recognition unit, and then through the real-time conversion and signal amplification of the transducer, it will be converted into readable data to realize the quantitative sensing of the target substance. Colorimetric sensing technology is a quantitative optical method that relies on the correlation between the color change of a solution and the concentration of the target analyte. Compared with other optical sensors, the colorimetric sensor has the benefits of visualization, low cost, and simple operation, and could be coupled with a portable substrate, so the practical use is more extensive. This method provides naked-eye sensing abilities (qualitative) and could be incorporated with smartphone imaging (quantitative), making them well-suited for biotoxin presence detection in various foodstuffs.15,16
Enzymes are regarded as one of the earliest and most frequently utilized biometric components in biosensors. They have a dual role in recognizing the target substance and promoting electron transfer between the substrate, thereby catalyzing the chemical reaction of the specific substrate to induce corresponding signal alterations.17 Enzymes have the capacity to act as indicators for the identification of particular substances, facilitating the transformation quantities of the target substance into detectable signals for analytical objectives.18 As a result, the effectiveness of biosensor detection is somewhat dependent on the particular enzymes employed and their individual characteristics. The disadvantages of natural enzymes like difficulty in preservation, high cost, and poor stability limit their greater use in biosensors. The rapid advance of enzyme-like nanomaterials (also known as nanozymes) affords an innovative horizon for the choice of suitable signal markers for analyte identification. Nanozymes demonstrate catalytic properties like natural enzymes, allowing them to catalyze effectively even under extreme situations. This attribute expands the potential uses of nanozymes in biosensing applications.19,20 The exceptional catalytic efficiency of nanozymes allows them to enhance the color change of platforms, resulting in the generation of light signals upon the colorimetric platform introduction. The colorimetric biosensor that utilizes nanozymes primarily depends on the catalytic capabilities of the nanozymes to imitate the natural enzymes' catalytic functions. This mechanism entails the conversion of a colorless substrate into a colored product through oxidation, enabling visual detection. In contrast to colorimetric biosensors lacking nanozymes, the integration of nanozymes with colorimetric biosensors could serve as a means of signal amplification to a certain extent, thereby enhancing the determination capabilities of biosensors.21 Nanozymes have the potential to serve as recognition units for target analytes in biosensing systems, and their specific surface area offers more active sites to bind more targets, which could further enhance the sensitivity of colorimetric sensors. Furthermore, nanozymes do not possess inherent light-absorbing properties in colorimetric biosensors. However, upon the addition of a colorimetric substrate, nanozymes exhibit various mimetic enzyme activities that facilitate the catalysis of the substrate reaction, thereby initiating a light signal. This process contributes to signal amplification in colorimetric sensors.22 Also, the surface properties and specific structures of nanozymes enable them to function as adsorbents for selectively adsorbing target molecules. This capability can enhance the selectivity of the colorimetric sensor.
By now, nanozyme-enabled colorimetric biosensors have been broadly employed in food safety analysis owing to the advantages of naked-eye visibility, high sensitivity, easy operation, and portability. However, the applications of nanozyme-enabled colorimetric biosensors in the field of biotoxin analysis have not been specially summarized. Therefore, in this review, we focus on the classification of nanozymes according to the different nanomaterials and offer a detailed description of each type of nanozyme. Moreover, we tried to discuss the construction approach of nanozymes in colorimetric biosensors in terms of catalytic activity, and afford a comprehensive review of the recent research progress of nanozymes in the colorimetric biosensing of various biotoxins (mycotoxins, marine toxins, and bacterial food toxins) in food products. Finally, the main challenges and prospects of nanozyme-enabled colorimetric biosensors are also deliberated.
2. Classification of nanozymes
Based on their functional activities, nanozymes can be classified into two primary groups: the oxidoreductase family and the hydrolase family. The oxidoreductases predominantly participate in redox reactions and exhibit various activities, including catalase (CAT), oxidase (OXD), superoxide dismutase (SOD), and peroxidase (POD) like functions. Hydrolases play a crucial role in catalyzing hydrolysis reactions and exhibit functions that are analogous to those of proteases, nucleases, phosphatases, etc.23. The constituents of nanozymes primarily consist of metals, metal oxides, sulfides, salts, metal–organic frameworks (MOFs), carbon materials, and metal–carbon hybrid nanocomposites.24–26 Recently, studies on nanozymes have increasingly concentrated on nanomaterials that possess intrinsic catalytic properties, rather than enzymes or catalysts immobilized on nanomaterials. Currently, the majority of published research regarding the classification of nanozymes has focused on categorizing them according to their catalytic activity. In order to offer further classification methods for nanozymes, this study concentrates on categorizing them using different nanomaterials and analyzes the fabrication approaches of colorimetric assays based on the various catalytic nanozyme activities.2.1. Carbon-based nanozymes
Carbon-derived nanozymes, as a significant constituent of the nanozyme category, refer to carbon-based nanomaterials having enzyme-like catalytic functions. Owing to the benefits of environmental friendliness, low cost, good biocompatibility, and easy modification, carbon-based nanozymes are broadly employed in fields like biosensing, environmental protection, food safety, and disease diagnosis. Nevertheless, unlike other kinds of nanozymes, the synthesis of carbon-based nanozymes mainly relies on a trial-and-error approach. This methodology results in a notable level of uncontrollability in the modulation of the catalytic activity of carbon-based nanozymes. Furthermore, carbon-based nanozymes exhibit a lower degree of substrate specificity compared to other types of nanozymes, primarily due to the absence of substrate binding pockets. Consequently, there is a necessity to advance rational design approaches aimed at addressing this limitation. Extensive study into the characteristics of carbon-based nanomaterials has revealed their significant potential for uses related to catalytic features. The concept of carbon-based nanozymes was simultaneously introduced. The majority of carbon-based nanozymes are documented to exhibit POD-like or OXD-like function. Additionally, to enhance the catalytic properties of these carbon-based nanomaterials, the incorporation of other elemental dopants is often necessary. For instance, N-doped nanocarbon may show exceptional catalytic performance.27 Nevertheless, the synthesis of N-doped carbon nanozymes with a high nitrogen content presents challenges due to the instability of the N element at high temperatures. In 2019, Wei and colleagues utilized polyethyleneimine (PEI) as a source of carbon and nitrogen, alongside montmorillonite (MMT) as a template, to synthesize a carbon-based nanozyme characterized by a high nitrogen content.28 MMT was dispersed in an aqueous solution, after which the supernatant was subjected to incubation with a polyethyleneimine (PEI) solution. The obtained solution underwent freeze-drying to yield the assembled powder (MP), which was subsequently subjected to carbonization and etching processes to produce nitrogen-doped carbon nanomaterials. This study identified the critical factor, specifically the N doping content, which influences the catalytic performance of carbon-based nanozymes, and thus was of great importance for the development of carbon-based nanozymes with enhanced catalytic performance. Regardless of the significant progress, most of the existing carbon-based nanozymes exhibit only a singular enzymatic activity. Consequently, the development of methodologies to impart dual or multiple enzymatic activities to carbon-based nanozymes is of considerable interest. In light of this, Gao's team used Pluronic F127 as a soft template, and phenol, formalin and melamine as raw materials, to prepare a polymer, which consequently was pyrolyzed to acquire N-doped carbon.29 The N-doped carbon N-PCNSs3, prepared under optimal conditions, demonstrated a porous nanosheet morphology, thus enabling the substrates to diffuse into the pores to increase reaction performance. Furthermore, the nitrogen content in N-PCNSs3 is significantly greater than that of other comparable materials, which enhances its multi-enzymatic activities and overall catalytic efficiency. As estimated, N-PCNSs3 had POD-, OXD-, SOD-, and CAT-like activities. It is evident that the catalytic function of carbon-based nanozymes depends on the precursor. Consequently, altering the precursor may represent a viable approach for the development of high-performance carbon-based nanozymes. In 2019, Choi and colleagues identified the photo-responsive glucose oxidase (GOx) and POD-like activities of carbon nitride by carbonizing melamine in the presence of KCl and KOH.30Graphene, a subclass of carbon materials, features high conductivity, large surface area, strong thermal stability, and excellent transparency and thus, is considered a favorable candidate for preparing carbon-based nanozymes.31 In 2015, Qu's team found POD-like activity in graphene quantum dots (GQDs) containing hydroxyl, carboxylic, and carbonyl elements, and thoroughly explored the catalytic mechanism. Following the activity test, it was determined that the carbonyl group serves as the active site and is responsible for determining catalytic activity. The carboxylic group interacts with the substrate, while the hydroxyl group does not.32 To the best of our knowledge, this study explained the influence of surface functional elements on the catalytic functions of carbon-based nanozymes for the first time, and thereby will lead future researchers to progress the study of the preparation and design of high-performance carbon-based nanozymes. Nevertheless, graphene alone demonstrates minimal catalytic function. Graphene, in isolation, demonstrates minimal catalytic activity.33 To enhance its activity, it is necessary to employ a doping strategy involving other elements such as N, P, B and S. Furthermore, it is essential to optimize the doping methodology to achieve the most effective coordination effects. In 2019, Lee and his colleagues introduced a series of N- and B-doped reduced graphene oxides, like BN-rGO, N-rGO, B-rGO (reaction between melamine and B-rGO), NB-rGO (reaction between H3BO3 and N-rGO), and h-BN-rGO (reaction between H3BO3, melamine, and rGO).34 As expected, the synthesized rGO derivatives exhibited a nanosheet morphology, with the doped elements being uniformly distributed throughout the rGO matrix. This phenomenon may be attributed to the synergistic interaction between N and B, which notably enhanced the electron transmission rate during POD-assisted reactions.
2.2. Metal-based nanozymes
Metal-based nanozymes are one of the most typical nanozymes owing to their easy synthesis and stable structure. In general, metal-based nanozymes can be categorized into two primary types: single-metal nanozymes and metal alloy nanozymes, which consist of multiple metal constituents. Furthermore, various metal-doped materials, including metal core/shell nanostructures, have been successfully synthesized.35 These nanozymes show diverse shapes, like nanowires, nanoflowers, nanoparticles (NPs), nanosheets, and nanospheres. Various shapes can exhibit distinct catalytic characteristics. Among them, precious metal NPs like gold, silver, platinum, and palladium are commonly exploited in the determination of bacterial food toxins, mycotoxins, and marine toxins. The synthesis techniques of metal-based nanozymes comprise photochemical, high-temperature reduction, and mediated growth methods. In the experiments concerning nanozyme-assisted analyte sensing, the reduction technique is primarily utilized. For instance, PtNPs were produced using polyvinylpyrrolidone (PVP) as a stabilizing agent, and sodium citrate as a reducing agent, whereas AgNPs were prepared using potassium hydroxide and L-tyrosine as raw materials, which all have excellent peroxidase function.36,37 In addition to exhibiting single-enzyme-like activity, certain metal-based nanozymes may also demonstrate multi-enzyme-like activity. For instance, peroxidase-like and oxidase-like Ir NPs were prepared based on PVP and IrCl3–3H2O as raw materials through an alcohol reduction technique.38,39 The combined influence of these two activities enhanced the sensitivity and selectivity of the nanozyme. Due to the synergistic interactions among each component, bimetallic nano-alloys frequently exhibit enhanced catalytic behavior. Recently, bimetallic nanocomposites received significant attention owing to their distinctive synergistic effect and multiple functions, and are extensively exploited in the catalytic field. The Au–Pt nanozyme demonstrated superior peroxidase-like activity compared to the Au nanozyme alone and exhibited greater stability over time than horseradish peroxidase (HRP). This phenomenon can be attributed to the disparity in electronegativity between Pt and Au, which facilitates the migration of electrons from Pt to Au. This transfer enhances the surface electron density of Au, thereby augmenting its catalytic activity.40 To mitigate costs, copper (Cu) as an element was employed in the synthesis of bimetallic nanocomposites. Ramanathan et al. investigated the process of electroless deposition to facilitate the in situ growth of Cu NPs on the surface of cotton fabrics.41 The obtained cotton fabrics were then submerged in an aqueous solution containing AgNO3 or HAuCl4 or PdCl2 or H2PtCl6 to prepare bimetallic nanoparticles, like Cu–Ag, Cu–Au, Cu–Pd or Cu–Pt. Cu–Pt NPs displayed excellent POD-like function and a remarkable catalytic rate. Despite the extensive research conducted on metal-based nanozymes, the tendency to aggregate and the toxicity associated with certain heavy metals pose significant limitations to their potential applications.422.3. Metal oxide-based nanozymes
Similar to metal-based nanozymes, recently, metal oxide/sulfide/salt-derived nanozymes have gathered considerable attention because they enjoy the merits of simple preparation steps, low cost, and distinctive magnetic/dielectric/optimal features.43,44 Considering our understanding, the frequently applied techniques mainly include sol–gel, hydrothermal reaction, atomic layer deposition and air pyrolysis. Through adjusting the surface groups, structures, and categories, the catalytic behavior of nanozymes along with functionality and stability can be readily controlled. The pioneering research on nanozymes was documented via Yan in 2007. This study revealed that Fe3O4 NPs exhibited POD-like activity for the first time.45 Employing TMB, OPD and diaminobenzidine (DAB) as substrates, Fe3O4 NPs catalyzed their oxidation producing blue, brown and orange colors, on the basis of which Fe3O4 NPs revealed POD-like function.In addition to Fe3O4 NPs, V2O5 nanocomposites were also typically used to synthesize nanozymes, and to date, V2O5 enjoys OXD-, POD-, and dual-enzyme (GOx and POD)-like functions with 1D and 2D morphologies. The primary instance of V2O5 with POD-like performance was documented by Tremel's research team.46 Initially, KBrO3 and VOSO4 were combined, after which HNO3 was slowly added to achieve a pH of 2.0. Following this, thermal treatment was performed at 180 °C for 24 h to synthesize V2O5 nanocomposites. In another study, Doong et al. employed bulk V2O5 powder as the raw material, and used DMF to separate bulk V2O5 (ref. 47). The V2O5 nanosheets demonstrated significant oxidative activity, effectively catalyzing the oxidation of TMB to oxTMB. In 2023, Li et al. utilized the reaction between KBrO3 and VOSO4 to synthesize 2D V2O5 by optimizing the reaction conditions.48 The resulting 2D V2O5 exhibited superior POD-like activity compared to V2O5 with other morphologies.
MnO2 was also typically used to develop nanozymes with CAT-, POD-, OXD-, GOx- and SOD-like activities. For instance, Han and colleagues used BSA as a soft template to controllably produce a 2D MnO2 nanozyme.49 Actually BSA with rich NH2 and –COOH groups could efficiently fix Mn2+ in its molecular structure to generate Mn2+@BSA. Upon the addition of NaOH, Mn2+ present in the Mn2+@BSA complex was initially converted into MnO(OH). This intermediate subsequently underwent oxidation by O2, ultimately resulting in the formation of MnO2. As anticipated, the synthesized MnO2 under alternative conditions exhibited an irregular flocculent morphology, highlighting the significant influence of BSA.
To sum up, metal oxide-based nanozymes, such as peroxidase-like V2O5, GeO2, TiO2, Fe3O4, and oxidase-like MnO2, are also employed in the determination of various toxins. Nevertheless, unmodified metal oxide-derived nanozymes might display poor stability and other challenges.50 Consequently, further research is needed to alter specific components within the metal oxide to enhance its detection efficacy.
2.4. MOF-based nanozymes
MOFs are crystalline materials characterized by a periodic network structure that arises from the self-assembly of organic components, typically organic ligands such as pyridine or carboxylic acids, in conjunction with metal clusters or metal ions, predominantly comprising transition metal ions like Fe2+, Zn2+, and Cu2+.51,52 MOFs are a class of nanomaterials that have garnered significant interest in recent years due to their advantageous characteristics, including a high specific surface area, a porous network structure, and tunable chemical properties.53 Poor selectivity is prevalent among numerous nanozymes; therefore, it is essential to conduct a thorough investigation into the factors contributing to the high selectivity observed in natural enzymes and to incorporate these insights into the development of nanozymes. Similarly, when utilizing MOF-based nanozymes in vivo, it is imperative to assess their cytotoxicity and biosafety to confirm that they do not pose any harm to the organism. According to their synthesis approaches, MOF-based nanozymes can be divided into chemically modified MOFs, pristine MOFs, MOF derivatives and MOF-based composites.54The coordination binding sites of the metal centers within MOFs are often obstructed by the organic moieties, leading to diminished catalytic activity. Therefore, it is imperative to explore novel strategies to address this issue and enhance the catalytic performance of the unmodified MOFs. Strategies have been established to augment their catalytic performance, like assembly of metal nanoparticles, surface modification of MOFs, metal oxides, and other constituents in MOFs.55 Fe3O4@MIL-100(Fe) composites were effectively synthesized using an in situ growth technique, which involved the incorporation of Fe3O4 NPs within the MIL-100 framework. The catalytic potential of these MOF composites for photo-Fenton reactions was subsequently investigated.56 Research has also been conducted on the synthesis of AuNPs@MIL-101 composites through the hydrothermal deposition of AuNPs onto the MIL-101 MOF. This composite material exhibits catalytic properties for the oxidation of ascorbic acid, which can be utilized in conjunction with an electrochemical sensor for the detection of microcystin-LR in water samples.57 Both rGO and TiO2 have been revealed to couple with MIL MOFs to improve their catalytic function.58,59 In addition, MOFs could also be applied as templates or precursors to provide a series of MOF derivatives, for example carbon materials, metal oxides, metals, and so on, owing to their modifiable structures. For instance, by calcining the precursors of Fe-ZIF-67 and Fe-ZIF-8 at high temperatures, an Fe–N–C nanozyme with POD-like function was achieved, which exhibited good performance.60
3. Colorimetric biosensors using nanozyme activity
As is well known, colorimetric biosensing relies on the correlation between the extent of color alteration in the platform and the concentration of the analyte substance to enable quantitative determination. The alteration in substrate color is dependent on the catalytic function of the enzyme. Nanozymes typically facilitate the conversion of a colorless substrate into a colored one through their inherent enzyme-like properties. For instance, nanozymes exhibit the ability to induce color changes in various substrates, including OPD (o-phenylenediamine), TMB, ABTS, and other chromogenic substrates, enabling colorimetric recognition. Recently, most of the research endeavors concerning the catalytic capabilities of nanozymes have primarily concentrated on imitating oxidoreductase characteristics, specifically those associated with OXD, POD, CAT, and SOD functionalities.61 Some previous studies have explored hydrolytic enzyme mimics, however, the focus has shifted towards the oxidoreductase-imitating capabilities of nanozymes, particularly in the context of colorimetric sensing. This section primarily examines the development strategies and advancements in the employment of colorimetric nanoprobes that leverage the oxidoreductase-like properties of nanozymes. In recent years, there has been a growing interest in the integration of biosensors with innovative nanomaterials, and nanozymes are broadly exploited in colorimetric recognizing platforms owing to their superior stability and exceptional catalytic characteristics. From one perspective, nanozymes have the potential to serve as biorecognition units for analytes in biosensing applications, as they offer a large specific surface area that can render numerous active sites for binding multiple targets, which in turn can effectively boost the sensitivity of colorimetric sensors.62,63 Furthermore, nanozymes lack inherent light-absorbing characteristics in colorimetric nanoprobes. However, upon the addition of a colorimetric platform, nanozymes exhibit diverse simulated enzyme performances that facilitate the catalysis of the surface reaction, thereby initiating a light signal. This mechanism enables nanozymes to contribute to signal enhancement within colorimetric probes.22 Additionally, the distinct structure and interface characteristics of the nanozymes enable them to function as adsorbents for selectively capturing the desired molecules. This capability could enhance the colorimetric assay selectivity. More importantly, nanozymes play a crucial role in inducing a color change in the substrate as part of the catalytic process. This transformation enables the conversion of the concentration or amount of the desired molecules into visible hue indicators, facilitating the naked eye detection of signal variations through colorimetric sensors.3.1. POD-like activity
Peroxidase could catalyze reactions in the presence of peroxides such as ROOH, acting as a H2O2 redox substrate and electron acceptor, converting them into oxidation products and H2O products.64 The nanozymes' POD mimetic system exhibits a ping-pong mechanism and Michaelis–Menten kinetics similar to those observed in the catalytic behavior of natural peroxidase enzymes.61 The ping-pong mechanism is defined by the enzyme's alternating transition between its initial conformation and an altered state. This process involves the enzyme releasing the initial product upon binding with the initial substrate in its base state, transitioning to the altered form, and reverting to the original conformation upon binding with the second substrate to release the subsequent product.65 In contrast to the OXD mimetic activity, the POD mimetic system requires the presence of H2O2. H2O2 serves as a natural scaffold toward POD, and the nanozymes facilitate the breakdown of the O–O bond in H2O2 into ˙OH with a significant oxidation potential. The resulting ˙OH species can subsequently oxidize various colorimetric substrates, including OPD, ABTS, TMB, and other similar compounds.22 The production of ˙OH in processes mimicking peroxidase enzyme activity has been documented through two pathways. One pathway involves the Fenton reaction, where Fe2+ reacts with H2O2 to produce ˙OH and hydroxides (OH−). The Haber–Weiss reaction as a second pathway can catalyze superoxide (˙O2−) via iron ions and H2O2 leading to the generation of ˙OH.66The emerging trend in analytical chemistry involves utilizing nanozymes with inherent peroxidase-like activity in conjunction with colorimetric biosensors for the purpose of detecting specific target substances. This approach uses the catalytic color development concept through nanozymes. Graphite-like carbon nitride (g-C3N4) nanosheets exhibit properties similar to POD enzymes with notable stability. Notably, the functionalization of g-C3N4 with heteroatoms has been shown to substantially improve its POD-like activity. In this regard, Fu's group activated g-C3N4 with Pd NPs for the improvement of its enzyme-catalyzed performance and loaded the heteroatom-activated g-C3N4 with Fe3O4.67 A magnetic platform consisting of Fe3O4/Pd NPs supported on g-C3N4 was developed to enhance POD-like activity and utilized for immobilizing different natural enzymes, aiming to achieve material reusability and mitigate optical nanozyme interference caused by scattering in colorimetric sensors. Using Fe3O4/Pd NPs/g-C3N4/GOx, a colorimetric probe was developed for the determination of glucose. There was no detectable evidence at 652 nm when TMB, glucose, and Fe3O4/Pd NPs/g-C3N4 without GOx were present, indicating that Fe3O4/Pd NPs/g-C3N4 does not exhibit an inherent GOx-like function. The alteration in TMB hue was observed only when Fe3O4/Pd NPs/g-C3N4/GOx, TMB, and the target were co-present.
In another study, Li et al.68 produced S- and N-doped carbon-loaded POD-like FeCoZn triatomic catalysts that stemmed from ZIF-8 by taking benefit of two properties, specifically, the efficiency of MOFs in mitigating the issue of single-atom catalyst agglomeration and the potential of dispersing multiple metal atoms to boost the efficiency of single-atom nanozymes. A colorimetric nanozyme using a dual-channel assay was developed by integrating FeCoZn-TAC/SNC with a sensor array, aimed at differentiating food preservatives (Fig. 1). The FeCoZn-TAC/SNC nanozymes exhibit POD-like activity, facilitating the color development reactions of TMB and OPD to yield green and yellow products, respectively. The food preservatives can adhere to the nanozyme surface via hydrogen bonding and π–π stacking interactions. The diverse levels of interaction noted between FeCoZn-TAC/SNC and different food preservatives led to a reduction in the catalytic efficiency of the nanozymes. Consequently, this occurrence resulted in varying degrees of color signals, which serves as the fundamental mechanism for discriminating the preservatives. According to this principle, the colorimetric reaction profiles of various preservatives exhibit variations. Through linear discriminant analysis, a 100% accuracy rate was attained during the cross-validation of seven food preservatives. This result indicates that the sensor array adeptly distinguished seven varieties of food preservatives even at low concentrations.
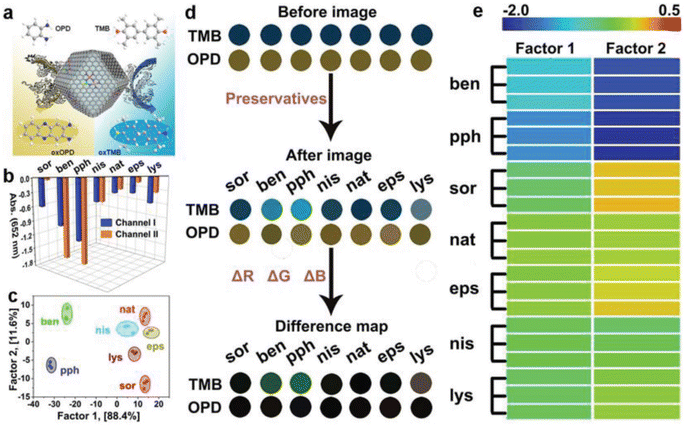 | ||
| Fig. 1 Schematic drawing of the fabrication of a colorimetric biosensor using a two-channel array on the basis of the POD-like function of nanozymes for discriminating food preservatives. Reprinted with permission from ref. 68. | ||
3.2. Oxidase-like activity
Oxidases have the ability to facilitate the oxidation of substrates (such as electron donors) in the presence of molecular oxygen or other oxidants (such as electron acceptors), leading to the formation of oxidation products along with H2O, H2O2, or O2−. Currently, a variety of nanozymes have been identified to exhibit oxidase-like activity. Massimiliano and colleagues discovered that gold nanoparticles (Au NPs), even when not loaded with protective agents, exhibited enhanced catalytic activity. Bare Au NPs exhibited the ability to catalyze the production of H2O2 and gluconate from glucose in the presence of O2. In contrast, colloidal particles of Ag, Cu, Pt, and Pd did not demonstrate comparable activity under identical conditions.69 Thereafter covalent organic frameworks, metal–organic frameworks, carbon-derived nanozymes, and other nanozymes have been identified to possess OXD-like activity.70–73 Colorimetric biosensors can be developed by utilizing the color change resulting from the catalytic activity of oxidases, where the in situ production of superoxide radicals and hydrogen peroxide oxidizes colorless substrates into colored products.74 To some extent, the OXD mimics are considered more appropriate for biochemical analysis compared to the POD mimics due to their ability to function without the need for H2O2 in the catalytic procedure. Additionally, the reaction conditions for the enzyme mimics are simpler and more direct. Nevertheless, Singh and colleagues found that nanozymes possessing OXD-like activity have the capability to trigger molecular oxygen conversion into reactive oxygen species like singlet oxygen, oxygen radicals, and hydroxyl radicals during the catalytic mechanism, which can be effectively utilized for the oxidation of diverse substrates.75–77 This results in a lack of specificity in the catalytic activity of the nanozymes. In order to address this issue, they developed a biomimetic approach utilizing MOF materials. Through the manipulation of MOF crystal growth in the Z direction, researchers successfully synthesized ultrathin nanosheets of Mn-UMOF using benzene dicarboxylic acid and triethylamine. The presence of the robust donor ligand triethylamine and the bridging ligand benzene dicarboxylic acid in Mn-UMOF leads to an increased density of active sites and enhanced substrate binding properties, especially for the case of electron unsaturation, when compared to the bulk Mn-BMOF. Additionally, Mn-UMOF could better catalyze the oxidation of substrates such as amplex red (AR), ABTS, and TMB without any external oxide addition.78In another study, Zhang's team established an innovative technique that can selectively identify thiophanate-methyl.79 A Cu-doped carbon nanozyme, denoted as Cu@NC, was developed with Cu serving as the active center site. The thiocarbamide-like and ethylenediamine-like building blocks in the target analyte, thiophanate-methyl, exhibit a robust affinity for metal ions. This property enables thiophanate-methyl to selectively interact with Cu@NC in the presence of other pesticides, leading to a significant reduction in the catalytic performance of the nanozyme and facilitating colorimetric detection (Fig. 2). The researchers also examined the specificity of the colorimetric sensor and revealed that thiophanate-methyl had a direct and specific inhibitory effect on the OXD performance of Cu@NC nanozymes. To examine the mechanism through which the nanozyme activity is inhibited, experiments were carried out. These experiments revealed a reduction in the nanozyme catalytic activity after pre-incubating the target molecule, thiophanate-methyl, with Cu@NC. Subsequently, thiophanate-methyl was introduced to the chromogenic substrate. Upon the introduction of thiophanate-methyl into the conventional system consisting of TMB and Cu@NC, it was observed that the catalytic activity remained unaffected. This suggests that the decrease in Cu@NC activity caused by thiophanate-methyl is a result of its direct interaction with Cu@NC, rather than from enhancing the reduction of oxTMB. Additional inhibitory elements were subsequently examined, and it was finally found that TM can be fixed onto the Cu@NC interface via π–π stacking interactions and attached to its metal sites to suppress its catalytic function.
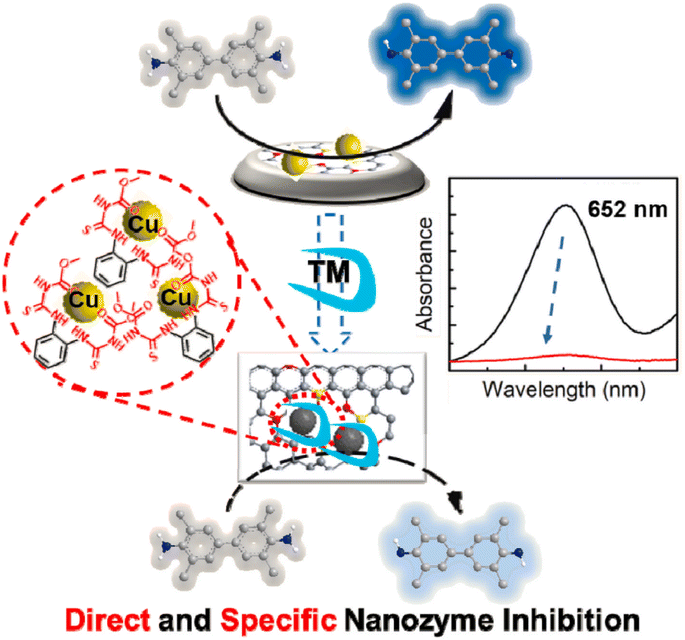 | ||
| Fig. 2 Diagram illustrating the development of a colorimetric nanoprobe utilizing the OXD-like properties of nanozymes for the precise identification of thiophanate-methyl. Reprinted with permission from ref. 79. | ||
CeO2 nanoparticles demonstrate remarkable mimetic activity similar to oxidase enzymes, enabling the catalysis of TMB color progress in the absence of hydrogen peroxide. In this regard, Jia's group developed a multifunctional portable colorimetric detecting substrate for the determination of bisphenol A.80 As depicted in Fig. 3, the experimental configuration utilized the CeO2@ZIF-8/Apt nanoprobe as both the signal generation unit and recognition component. Sodium alginate hydrogel tubes were employed to contain the TMB and catalytic reaction buffer as the reaction medium. The hydrogel containing the TMB platform was attached to the top of the tube, while the signal probe was placed at the base of the tube. Following the introduction of the sample and subsequent mixing with the signal probe, the Apt molecules on the signal probe dissociated and attached to the target, which resulted in the triggering of the OXD activity of the CeO2@ZIF-8. Upon completion of the reaction process, photographs of the reaction solution were taken using a smartphone, and were subsequently uploaded to a color analyzer based on the RGB app. This method was employed to quantitatively evaluate the target object.
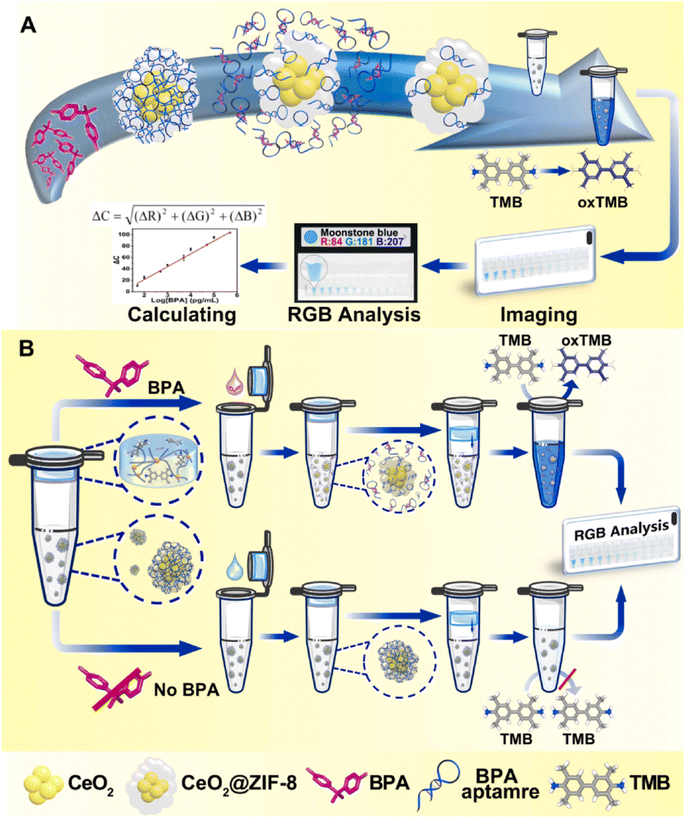 | ||
| Fig. 3 (A) Diagramatic description of the fabrication and the OXD-like properties of CeO2@ZIF-8/Apt nanoprobes. (B) The sensing mechanism of a one-pot portable testing substrate for bisphenol A determination. Reprinted with permission from ref. 80. | ||
3.3. Catalase-like activity
The catalase enzyme facilitates the degradation of hydrogen peroxide into oxygen and water. The catalase-like properties of NPs were first reported in amine-terminated PAMAM dendrimers that encapsulated gold nanoclusters, which were observed under both physiological and acidic conditions.81 Likewise, several compounds with a nanozyme-like activity such as manganese oxide (Mn3O4) nanoparticles, platinum nanoparticles, and cerium oxide nanoparticles are designed to display a catalase-like activity. The molecular-level investigation of the catalase-like behavior of nanozymes explores mechanisms involving bi-hydrogen peroxide association, base-like dissociation, or acid-like dissociation.82 The bi-hydrogen peroxide mechanism has been identified as the most appropriate explanation for the catalase-like activity in nanozymes, particularly in the case of cobalt (II, III) oxide (Co3O4) nanoparticles. The catalase-like activity of cerium oxide nanoparticles involves the oxidation of hydrogen peroxide on the nanoparticles' surface to form oxygen. This process leads to the reduction of cerium oxide to H2-ceric oxide. Subsequently, H2-ceric oxide reacts with another hydrogen peroxide molecule, resulting in the production of water.83 Furthermore, Zhang et al. illustrated the catalase-like function of iron-based single atom nanozymes (Fe-SANzymes) via obviously exposed edge-hosted defective Fe/N4 atomic sites.84 The mechanistic investigation demonstrates that defects facilitate a substantial charge transfer from the Fe atom to the carbon matrix. This process activates the central Fe atom, enhancing its interaction with hydrogen peroxide and simultaneously weakening the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.
O bond.
3.4. Multi-enzyme-like activity
Nanozymes with a CAT-like function are frequently employed to eliminate the extra-naturally occurring reactive oxygen hydrogen peroxide, which is somewhat close to POD enzymes. Nevertheless, nanozymes typically lack the ability to oxidize substrates to enhance hue. Consequently, it is less frequent to depend solely on nanozymes in terms of the CAT activity for the fabrication of colorimetric nanoprobes. Superoxide dismutase is a metalloenzyme with antioxidant properties found in various organisms, capable of enabling the dismutation of reactive superoxide anion radicals into O2 and H2O2. SOD enzymes like CAT enzymes were frequently employed to neutralize surplus reactive oxygen classes, playing a pivotal role in the body's oxidation and antioxidant equilibrium. The catalytic mechanism of SOD enzymes primarily entails the protonation of a superoxide anion (O2˙−), which is in turn protonated by water to produce OH− and HO2˙ radicals. Nanozymes with O2˙− scavenging ability are considered to be favorable substitutes to natural SOD enzymes. If nanozymes demonstrate multiple simulated enzyme functions simultaneously, like SOD, OXD, and POD, the dominant activity is expected to be the SOD enzyme activity. This activity is influenced by factors like environmental pH, surface ions, and nanomaterial structure.85 Currently, the majority of reported nanozyme activities focus on POD and OXD activities, with limited research on mimicking SOD activity. Moreover, given that the primary purpose of the SOD enzyme is to preserve redox equilibrium in cells and mitigate oxidative stress, a significant portion of research endeavors focusing on nanozymes with SOD-mimetic characteristics are primarily directed towards mitigating inflammation. In contrast, there is a conspicuous lack of research investigating the employment of SOD nanozymes in colorimetric recognizing methodologies. Like CAT enzymes, the utilization of SOD-like nanozymes in colorimetric probes typically depends on the multifunctional enzyme properties of the nanozymes.4. Applications of nanozymes based on the colorimetric biosensor for biotoxin detection
4.1. Mycotoxin detection
Mycotoxins, as one of the most alarming food and feed contaminants, are carcinogenic and highly toxic secondary metabolites produced by specific fungi, predominantly molds, which have the potential to contaminate a broad spectrum of crops (including nuts, grains, and legumes) and this, in turn, can be transferred to various food products. These naturally occurring toxins pose a substantial risk to humans, with exposure capable of inducing numerous adverse effects such as acute poisoning, chronic ailments, and potentially cancerous consequences. Mycotoxins are typically prevalent in cereals, grains, nuts, spices, coffee, cocoa, dried fruits, and animal-sourced products such as milk and meat. Prominent and extensively researched mycotoxins comprise aflatoxins, ochratoxins, fumonisins, trichothecenes, and zearalenone.86,87 Therefore, the implementation of effective and innovate mycotoxin analytical detection methods, and alongside that, novel nanomaterials, has become pivotal for safeguarding humans against health dangers and risks. This section has been conducted to review the detection of major mycotoxins, including aflatoxin B1 (AFB1) and ochratoxin A (OTA), based on nanozymes.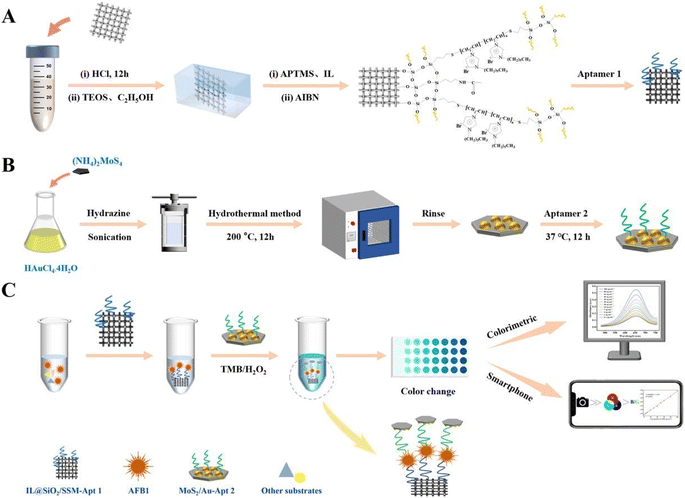 | ||
| Fig. 4 (A–C) Representation of the colorimetric aptasensor based on MoS2/Au for AFB1 determination. Reproduced with permission from ref. 94 Copyright Elsevier Science, 2024. | ||
Another excellent example of this concept was implemented in a colorimetric and photothermal dual-mode immunoassay using the peroxidase-like activity of Pt supported on nitrogen-doped carbon for AFB1 quantification.95 As shown in Fig. 5, glucose oxidase (GOx)-labeled AFB1-bovine serum albumin (BSA) competes immuno-competitively with AFB1, thereby releasing GOx to catalyze the glucose production of H2O2. Under normal conditions, the colorimetric signal was produced due to the oxidation of TMB to TMBOx. Along with the colorimetric signal, a thermal signal was achieved when TMBOx underwent photothermal conversion under 808 nm laser irradiation. The fabricated biosensor was able to detect AFB1 with an LOD of 0.22 and 0.76 pg mL−1.
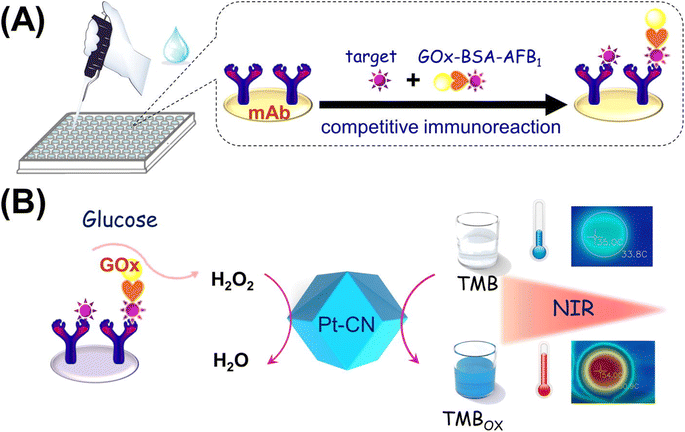 | ||
| Fig. 5 (A) Competitive immunosensor exploiting the GOx-labeled AFB1-BSA conjugate as the tag. (B) Illustration of colorimetric and photothermal measurement based on H2O2-responsive peroxidase-like activity of Pt–CN. Reproduced with permission from ref. 95. Copyright Elsevier Science, 2019. | ||
The application of multimodal biosensors based on nanozymes in AFB1 determination can introduce different signals, extending the linear range of quantification. In addition, these signals can be mutually verified to improve the accuracy of biosensing approaches.96,97 Another dual-mode approach for AFB1 quantification based on the Ag@Au IP6 bifunctional nanozyme, with peroxidase-like activity and SERS effect, was reported by Tan and colleagues.98 For this purpose, the surface of magnetic particles was decorated with AFB1 aptamers along with a trigger probe. In the presence of the target, the conjugation of targets with specific aptamers led to the trigger probe and this, in turn, initiated a hybridization chain reaction (HCR) which introduced alkaline phosphatase (ALP), catalyzing the self-assembly of the Ag@Au IP6 nanozyme. The constructed nanozyme revealed increased peroxidase-like activity, improving the oxidation of TMB to the blue-colored TMBOx; additionally, its core–shell structure also enabled strong SERS enhancement of the TMBOx signal.
Particularly, from an economic perspective, substituting precious metals that contribute to the activity of nanozymes, with more affordable transition metals, in the structure of nanozymes can markedly reduce the expenditure associated with nanozymes. For instance, Cai et al.99 designed a novel colorimetric nanozyme-based lateral flow assay (LFA) based on MnO2 nanosheets (MnO2 NSs) as an oxidase mimic and catalytic label for AFB1 determination. As shown in Fig. 6A, the test (T) line of the strip was decorated with anti-AFB1 antibody-conjugated MnO2 NSs for capturing AFB1. Impressively, thanks to the properties of MnO2 NSs in catalyzing the oxidation of TMB, the TMB solution was added onto the T-line. When the target was added to the immunosensing device, the oxidation could provide a visual color signal which was inversely proportional to the AFB1 concentration in the sample. The reported nanozyme-strip bioassay revealed an LOD of 15 pg mL−1 for AFB1, over 100-fold lower than the maximum limit set by the European Union. On the other hand, both the instability of the colloidal nature of nanozymes and their complicated interactions with bioreceptors can limit nanozyme exploitation in LFA. In this light, it is crucial to rationally design nanozyme-based signal labels with features that facilitate easy functionalization, good dispersibility, distinctly visible color, and enzymatic activity.101 These features are pivotal for increasing the applicability of nanozymes in LFA applications. A good example of this concept was prepared in another transition metal, CuCo, which was coated by polydopamine (PDA) with excellent biocompatibility, good adhesion, and rich functional groups (chinone, imine, and amine), leading to good hydrophilicity and binding ability with biomolecules.100 In this protocol, the carboxyl-functionalized aptamers were conjugated with the CuCo@PDA nanozyme via amide condensation reactions. The fabricated probe was used on the surface of the T line and the difference of color with/without the presence of the target was the principle of detection (Fig. 6B). Furthermore, the ability of the nanozyme to catalyze the oxidation of TMB-H2O2 could amplify the color change on the T-line. To illustrate this concept, the visual LOD was reduced to 0.1 ng mL−1 by TMB-H2O2 catalytic amplification. In 2024, Fan and colleagues102 designed another functionalized nanozyme, flower-like L-cysteine-functionalized FeNi bimetallic nanoparticles (L-Cys-FeNiNPs), with excellent peroxidase-like catalytic activity in a colorimetric aptasensor for the detection of AFB1. In the reported nanozyme, the peroxidase-like activity was attributed to the generation of superoxide radicals (˙O2−) and holes (h+) that were produced through the catalysis, and alongside that, the high selectivity of the probe was described by the conjugation of the specific aptamer with L-Cys-FeNiNPs via EDC/NHS chemistry and streptavidin–biotin interaction. Indeed, like the previous modification, the decoration of FeNiNPs with L-Cys can not only help increase the dispersibility and stability of the FeNiNPs, but also the biocompatibility and functionalization capability of nanozyme significantly improved. In the presence of the target, the reduction of the active sites of the L-Cys-FeNiNPs suppressed the TMB oxidation and reduced the color signal.
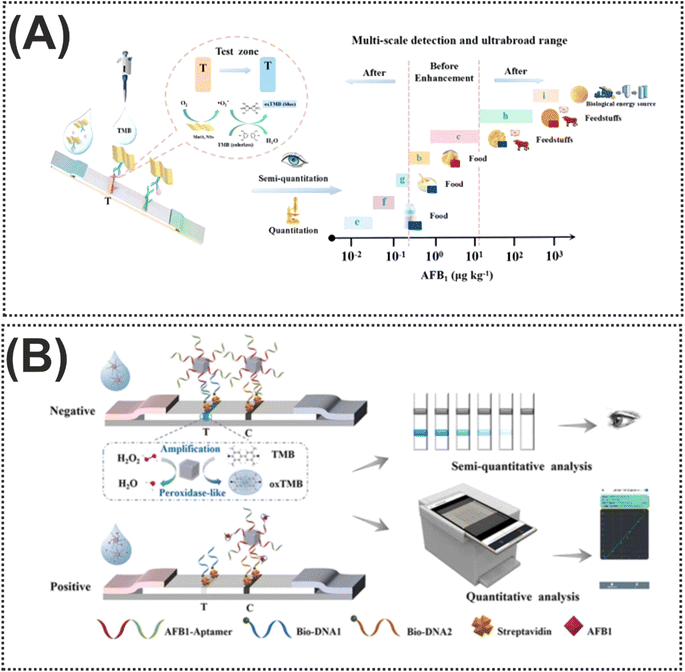 | ||
| Fig. 6 (A) Schematic of the nanozyme-strip bioassay based on MnO2 NSs for AFB1 detection. Reproduced with permission from ref. 99. Copyright Elsevier Science, 2022. (B) Illustration of the use of CuCo@PDA in the structure of LFA for AFB1 quantification. Reproduced with permission from ref. 100. Copyright Elsevier Science, 2024. | ||
Along with metal-based nanozymes, the application of metal–organic framework (MOF) based nanozymes can be considered to have high potential for the quantification of AFB1. Among different MOFs, porphyrin (PCN)-based organic linkers were employed for self-assembly with metal nodes, leading to the development of MOFs with different structures and functions. On the other hand, accessing the active sites within MOFs remains challenging. One of the efficient solutions is based on hybrid nanomaterials, which involves the use of platinum nanoparticles, Pt NPs, on two-dimensional support substrates.103,104 As an example, Zhang et al.105 reported a novel colorimetric approach exploiting the Pt@PCN-222 nanozyme, with oxygen vacancies, for AFB1 detection. The detection principle of this study was based on the oxidation of the 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) substrate to produce a blue-green colored product. In the presence of the target, the binding of Pt@PCN-222 with the target caused inhibition which decreased the number of active Pt@PCN-222 conjugates available for the ABTS oxidation reaction. Another technique for addressing accessibility to the active sites of MOFs is based on the synthesis of NanoMOFs, which can accelerate substrate diffusion in catalytic MOF materials and this, in turn, provides greater external surface area and lower diffusion barriers. For instance, Peng and co-workers106 developed a sensitive, reproducible, and accurate colorimetric immunoassay based on NanoPCN-223(Fe) with high peroxidase-like activity and excellent dispersion for AFB1 determination (Fig. 7). In the structure of the colorimetric technique, NanoPCN-223(Fe) produce color by catalyzing the oxidation of the colorless substrate TMB in the presence of H2O2. To illustrate this, through the catalysis process, the nanozyme generated hydroxyl radicals (˙OH) from H2O2, which oxidize the TMB substrate, converting the colorless TMB to the blue ox-TMB. Under normal conditions, the conjugation of the nanozyme and the target inhibited the catalyzed oxidation of TMB which reduced the intensity of the color.
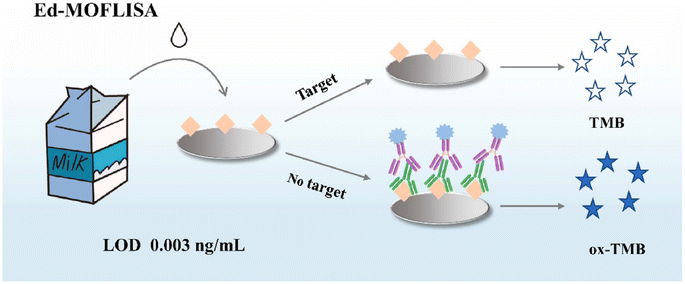 | ||
| Fig. 7 Illustration of the application of NanoMOFs for colorimetric detection of AFB1. Reproduced with permission from ref. 106. Copyright Elsevier Science, 2022. | ||
All things considered, the application of nanozymes based on metal nanoparticles and MOFs can be considered promising for aflatoxin detection. The investigation of these materials is based on two metal varieties, bimetallic and affordable materials, and alongside that, the amplification techniques can broaden our horizon on the performance of nanozymes in colorimetric approaches for the detection of aflatoxins.
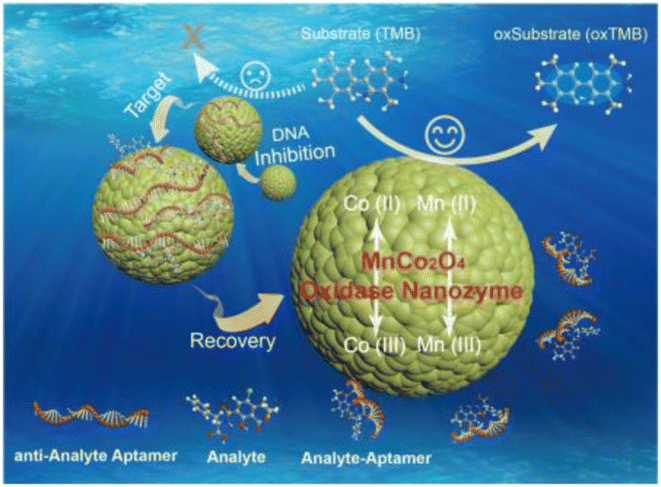 | ||
| Fig. 8 Schematic of using MnCo2O4 in the structure of the colorimetric aptasensor for OTA determination. Reproduced with permission from ref. 111. Copyright Elsevier Science, 2018. | ||
The distribution of cations among the octahedral and tetrahedral sites in the crystal structure can result in an inverse spinel structure, with a formula of (AB)4, which demonstrates different electronic and magnetic features compared to the spinel structure.112 Most recently, Liu and colleagues113 improved the performance of an anti-spinel structure by using Au and Pt in the structure of Fe3O4, through an ionic liquid (IL) as the cross-linker, for capturing the synergistic interaction between the alloy atoms. In this work, the surface of the designed nanozyme was modified with complementary DNA for conjugating with a stainless steel mesh-aptamer. In the presence of the OTA, the binding of the target with the aptamer caused separation of the signaling probe (AuPt@IL@Fe3O4) and this, in turn, was added to a tube containing H2O2 and TMB for observing the color change. All in all, the inverse spinel and spinel structures introduce effective bioreceptor immobilization and high catalytic activity, increasing the performance of biosensors. However, the limited surface area and non-optimal electronic properties of spinel structures can restrict their application. In terms of inverse spinels, although magnetic features and high catalytic activity improve their performance, complex synthesis processes can limit their nanozyme-based application.
Along with these structures, tetragonal crystals and nanocube structures are other structures used in the development of nanozymes for OTA detection. To illustrate this, tetragonal crystal systems have been widely exploited in biosensors based on nanozymes owing to several advantages such as high surface area to volume ratio, stability, electronic features, high catalytic activity, and versatile functionalization.114 Currently, one of the excellent examples of using nanozyme-based tetragonal crystal systems for detection of OTA was developed by Tian and co-workers.115 In this protocol, the principle of this study was measuring the oxidase-mimicking activity of MnO2 nanosheets. For this purpose, the decoration specific aptamer on the surface was exploited for capturing OTA, leading to the production of alkaline phosphatase-modified complementary DNA and the cascade reaction is triggered by the product (ascorbic acid) of alkaline phosphatase catalysis. The ascorbic acid reduced the oxidase-mimicking activity of MnO2 nanosheets, leading to a pale color of the enzyme catalytic substrate. Another structure is a layered structure, with unique benefits, which can provide efficient nanozymes in the quantification of OTA. In 2020, Zhu et al.116 fabricated a novel colorimetric immunosensor based on cobalt hydroxide nanoparticle Co(OH)2 nanocages, with specific properties such as high surface area and high catalytic activity, for OTA quantification (Fig. 9). In detail, the microwell plate decorated with dopamine was modified with OTA and ab1 and, followed, Co(OH)2-ab2 bounded to the prepared substrate. Under optimal circumstances, the difference of the color changes of TMB from the Co(OH)2 nanocage in the absence of H2O2 could present an efficient biosensing platform with a linear range and detection limit of 0.5 ng L−1 to 5 μg L−1 and 0.26 ng L−1, respectively. Despite the high surface area and catalytic activity of the nanocube structure, the stability issue under some conditions and aggregation of cubes can be considered important disadvantages of this structure. In addition, tetragonal crystal systems suffer from limited surface area.
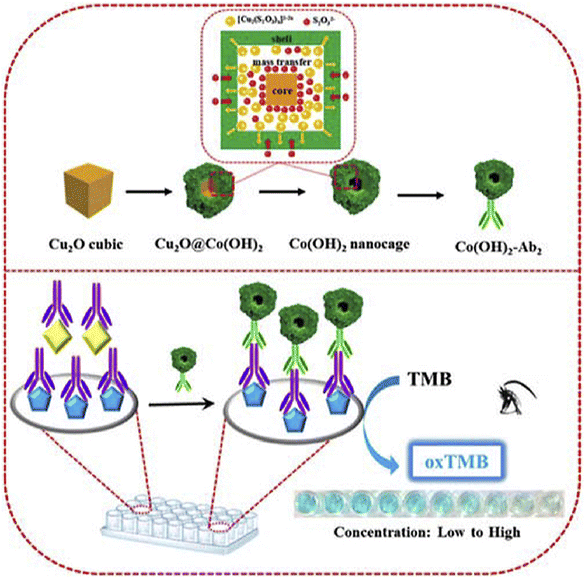 | ||
| Fig. 9 Illustration of the colorimetric immunoassay based on Cu2O nanocubes for the detection of OTA. Reproduced with permission from ref. 116. Copyright Elsevier Science, 2020. | ||
4.2. Marine toxin detection
Marine toxins, as poisonous substances, can be considered as natural metabolites which are produced by numerous organisms such as bacteria, algae, and many marine invertebrates. Among them, algal toxins (including ciguatera toxin, domoic acid, and saxitoxin), invertebrate toxins, and some bacterial toxins may get concentrated in various organisms through the food web. The negative consequences of these toxins in both humans and animals are undeniable.117,118 Importantly, various bioreceptors/receptors can improve the performance of nanozyme-based colorimetric biosensors for the determination of marine toxins. Antibodies, as one of the important bioreceptors, have been used in antibody–antigen interaction for presenting sensitivity and specificity detection approaches of marine toxins. Interestingly, the integration of nanozymes in the structures of colorimetric immunosensors can improve the analytical signal owing to the catalysis of the enzymatic reaction by nanozyme, which increases the color intensity. For example, Hendrickson and co-workers,119 prepared a conjugated Au@Pt nanozyme with an antibody for enhancing the okadaic acid quantitative labeling. The tendency of Au@Pt nanozyme to conjugate with anti-mouse antibodies, rather than anti-okadaic acid antibodies, could provide excellent conditions for an indirect competitive immunoassay format. This phenomenon led to unproductive immune binding without signal change, resulting in improving the sensitivity of the immunoassay. When the target was added to the system, the okadaic acid competed with the okadaic acid on the conjugate pad for binding with anti-okadaic acid antibodies and were immobilized on the T line of LFA.The lack of chemical and thermal stability of antibodies, under harsh environmental conditions, can make them sensitive to degradation and denaturation. Furthermore, the high cost of production and purification of these bioreceptors can be considered another significant issue. In this regard, a competitive colorimetric biosensor was implanted in aptasensors based on AuNP nanozymes for sensitive and selective quantification of saxitoxin.120 In this study, the competition of saxitoxin in samples with immobilized saxitoxin was the principle of the developed aptassay. To illustrate this, in the presence of the target, the separation of the specific aptamer from magnetic particles was conducted and this, in turn, led to triggering the hybridization chain reaction. The colorimetric signal was improved by amplifying the catalytic activity of the AuNP nanozymes. Similarly, in many colorimetric aptasensor studies, the adsorption of the aptamer could promote the enzyme-like activity of AuNPs for saxitoxin determination.121,122 Elaborately, this phenomenon could enhance the surface negativity of nanozymes which increased the adsorption and diffusion of positively charged substrates such as TMB, improving the catalytic efficiency. In 2022, Li and colleagues123 reported a novel aptasensor exploiting AuNPs@Fe2+ for multiple diarrhetic shellfish poison detection (Fig. 10). Indeed, the performance of the nanozyme, in terms of chemical stability and peroxidase-like activity, was improved by using Fe2+ in the structure of AuNPs. Furthermore, the high affinity of terminal-fixed aptamer (TF-DSP) was used in this study for the simultaneous detection of three diarrhetic shellfish poisons. Under normal circumstances, the catalysis of TMB/H2O2/acetic acid due to the excellent peroxidase-like activity and the brilliant selectivity of the aptamer could introduce a biosensing platform with a linear range and LOD of 0.4688–7.5 nM and 86.28 pM, respectively.
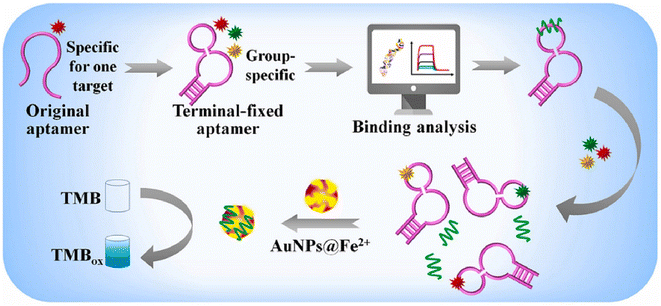 | ||
| Fig. 10 Schematic of the colorimetric aptassay using AuNPs decorated with Fe2+ for multiple diarrhetic shellfish poison detection. Reproduced with permission from ref. 123. Copyright Elsevier Science, 2022. | ||
The complex immobilization process on the surface of nanozymes and the side effect of potential interferences, in real samples, can be considered the most important limitation of using aptamers in the nanozyme structure. In addition, in terms of overcoming the stability issues of combining antibodies with nanozymes, molecularly imprinted polymers (MIPs), as artificial antibody and antigen systems, have attracted considerable attention for marine toxin detection.124,125 Most recently, Wu et al.126 integrated an MIP with Au–Pt nanoparticles modified Fe3O4 magnetic nanozymes for introducing an efficient biosensor of saxitoxin. For this purpose, Au–Pt nanoparticles were loaded into Fe3O4 magnetic particles and, subsequently, thanks to the hydrolysis polymerization reaction, the decorated Fe3O4 magnetic nanozymes with MIPs were prepared. In the presence of the target, the catalyzed oxidation of TMB enabled the developed biosensing approach for detection of saxitoxin to achieve a detection limit of 3.1 nM. Potential interference in signal transduction, and durability and stability issues can be considered the most important limitations of MIPs. In order to address these limitations, scholars must pay special attention to the fabrication of optimized and compatible MIP-nanozyme platforms. For instance, Cho and colleagues127 used peptides (as both the imprinting template and the signal peptide), instead of an antigen or aptamer, for competitive colorimetric quantification of saxitoxin. In this light, the exploitation of specific peptides of saxitoxin could overcome the difficulty of aptamer and antibody removal in biosensors based on the MIP-nanozyme. In addition, the integration of MIP with the specific peptide of saxitoxin was measured with AuNP/Co3O4@Mg/Al. In the presence of the target, the less specific peptide of saxitoxin was conjugated with MIP, leading to an intense color change.
4.3. Bacterial food toxins
Nowadays, bacterial food toxins, which are macromolecules mainly of protein origin, are one of the main issues in the realm of food safety. These microorganisms can produce toxins in food or once the pathogen has colonized the digestive tract. These types of toxins damage a specific organ of the host. To illustrate this, such toxins cause foodborne diseases including vomiting, nausea, abdominal cramps, and diarrhea.128,129 Despite the fact that the field of research on the detection of bacterial toxins is very wide, researchers attempted the use of different nanomaterials in the structures of nanozymes for amplification of detection signals. Various forms of Au-based nanomaterials such as AuNPs and gold nanostars (AuNSs) have been exploited as one of the important nanomaterials to amplify the signal due to several benefits such as excellent stability, repeatability, and accuracy. These nanomaterials gained great attention due to their ability to couple and integrate with different bioreceptors and nanomaterials for acting as nanozymes through chemical bonds and electrostatic adsorption. As for labelling nanomaterials, Ren et al.130 used the seed growth method for assembling AuNSs in the structure of Mn/Fe-MIL(53) for introducing an efficient nanozyme in the colorimetric and SERS detection of Shiga toxin type II. As shown in Fig. 11A, in terms of construction of signal probes which could oxidize colorless TMB into the blue color oxTMB, the surface of Mn/Fe-MIL(53) was decorated with AuNSs for providing an excellent substrate for immobilization of SH-complementary DNA, through Au–S bonding. In addition, to obtain the capturing probe, streptavidin-labeled magnetic beads were modified with a specific biotin-labeled aptamer. In the presence of the target, the conjugation of the aptamer with the target facilitates release of signal probes from the complex resulting in an enhancement of SERS and colorimetric signals. In the reported dual-mode, the integration of SERS and colorimetric approaches introduced complementary and validated results, improving the reliability of the Shiga toxin type II detection. In another example of Au-based nanozymes, Liang and co-workers131 designed a novel dual-signal probe (AuPt nanoparticle-loaded single atom nanocomposite, AuPt@Fe–N–C) for Staphylococcus aureus enterotoxin B quantification (Fig. 11B). For this purpose, ab1 was immobilized on the surface of AuPt@Fe–N–C and this was used for the sandwich structure. In addition to the target, AB1-Staphylococcus aureus enterotoxin B-ab2, which oxidises TMB from colorless to blue, had a detection limit of 0.066 7 pg mL−1 in 96-well plates. Along with Au-based nanozymes, the unique properties and structure of rhodium (Rh), a non-toxic transition metal, were exploited for staphylococcal enterotoxin B determination in milk samples.132 The implementation of Rh, with peroxidase-like catalytic activity, in the sensing zone of LFA could enable detection of staphylococcal enterotoxin B with a detection limit as low as 1.2 pg mL−1.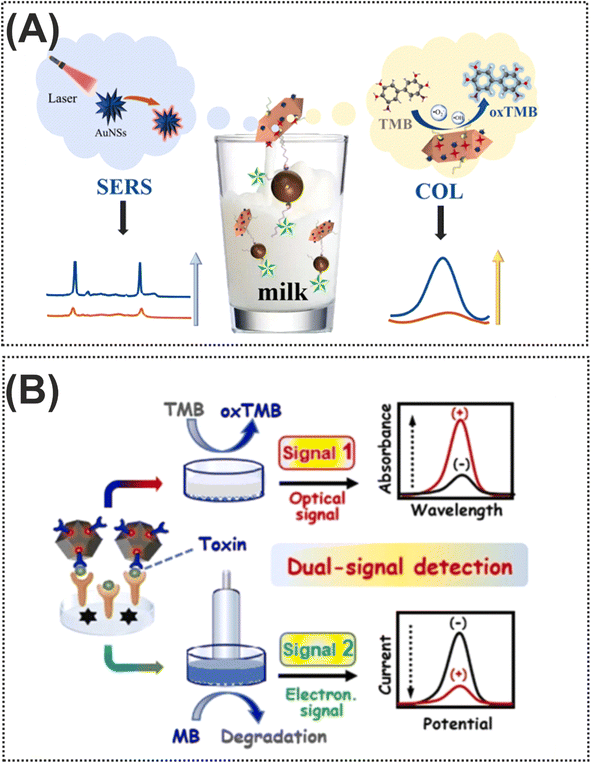 | ||
| Fig. 11 (A) Representation of the dual-mode colorimetric and SERS biosensor based on Mn/Fe-MIL(53)@AuNSs for Shiga toxin type II detection. Reproduced with permission from ref. 130. (B) Illustration of dual-signal electrochemical and colorimetric detection of Staphylococcus aureus enterotoxin B.131 Copyright Elsevier Science, 2024. | ||
5. Theranostic applications of nanozymes for biotoxins
Nanozymes' catalytic theranostics introduces an innovative and novel strategy for theranostic platforms, integrating detection and treatment in a single system.133 The importance of these platforms is highlighted in preventing the spread of biotoxins in contamination outbreaks by intervention to neutralize biotoxins and monitoring them.134 For the detection of biotoxins, catalytic color changing reactions are conducted for achieving sensitive colorimetric sensors, Table 1. In terms of neutralization and degradation of biotoxins, the oxidation and hydrolysis of biotoxins to break these toxic by-products down into non-toxic components by using nanozymes is an efficient strategy for rendering them harmless.135,136 Numerous techniques are exploited for biotoxin degradation such as the UV method for marine biotoxins demonstrating resistance to photodegradation. On the other hand, high performance degradation with UV/S2O82− and UV/H2O2 was achieved.137,138 In addition, biodegrading ATX-a into a nontoxic byproduct by Bacillus strains such as Bacillus flexus SSZ01 and Bacillus strain AMRI-03 can lead to a high performance method for water treatment like rapid degradation of saxitoxins, which is directly and indirectly associated with food safety.139 Furthermore, MOF-derived nanozymes were used as high potential materials in the neutralization process.140 Future research should be focused on the theranostic application of nanozymes for biotoxins due to a lack of research studies in this field. In other words, this field can advance biosensors and detoxification systems, managing biotoxin threats in different environments and food matrices.| Nanozymes | Enzyme activity | Substrate | Target | Linear range | LOD | Ref. |
|---|---|---|---|---|---|---|
| Au@HgNPs | OXD | TMB | AFB1 | 0.125 to 87.5 μg L−1 | 0.08 μg L−1 | 91 |
| CHNPs | OXD | TMB | AFB1 | 1 pg mL−1 to 20 ng mL−1 | 0.73 pg mL−1 | 92 |
| Au–Pt | OXD | TMB | AFB1 | 0.1 to 500 ng mL−1 | 5 pg mL−1 | 93 |
| MoS2/Au | POD | TMB | AFB1 | 1 to 100 ng mL−1 | 0.25 ng mL−1 | 141 |
| Pt–CN | POD | TMB | AFB1 | 1.0 pg mL−1 to 10 ng mL−1 | 0.22 pg mL−1 | 95 |
| Ag@Au IP6 | POD | TMB | AFB1 | 2 pg L−1 to 200 pg L−1 | 0.58 pg L−1 | 98 |
| MnO2 NSs | OXD | TMB | AFB1 | 0.01 to 150 ng mL−1 | 0.015 ng mL−1 | 99 |
| CuCo@PDA | POD | TMB | AFB1 | 0.01 to 50 ng mL−1 | 2.2 pg mL−1 | 100 |
| FeNiNPs | POD | TMB | AFB1 | 0.12 to 2 μg mL−1 | 36.57 ng mL−1 | 102 |
| Pt@PCN-222 | OXD | ABTS | AFB1 | 0.1 to 10 ng mL−1 | 0.074 μg L−1 | 142 |
| NanoPCN-223 | POD | TMB | AFB1 | 0.05 to 10 ng mL−1 | 0.003 ng mL−1 | 106 |
| MnCo2O4 | OXD | TMB | OTA | 0.1 to 10 ng mL−1 | 0.08 ng mL−1 | 111 |
| Fe3O4 | POD | TMB | OTA | 5 to 100 ng mL−1 | 0.078 ng mL−1 | 113 |
| MnO2 NSs | OXD | TMB | OTA | 1.25 to 250 nM | 0.069 nM | 115 |
| Cu2O nanocubes | OXD | TMB | OTA | 0.5 ng L−1 to 5 mg L−1 | 0.26 ng L−1 | 116 |
| Au@Pt | POD | TMB | Okadaic acid | 0.8 to 6.8 ng L−1 | 0.5 ng L−1 | 119 |
| AuNPs | POD | TMB | Saxitoxin | 78.13 to 2500 pM | 42.46 pM | 120 |
| AuNPs@Fe2+ | POD | TMB | Okadaic acid, dinophysistoxin-1, and dinophysistoxin-2 | 4688 to 7.5 nM | 86.28 pM | 123 |
| Fe3O4@Au–Pt | OXD | TMB | Saxitoxin | 0.01 to 100 μM | 3.1 nM | 126 |
| AuNP/Co3O4@Mg/Al cLDH | POD | TMB | Saxitoxin | 0 to1000 ng mL−1 | 3.17 ng mL−1 | 127 |
| Mn/Fe-MIL(53)@AuNSs | POD | TMB | Shiga toxin type II | 0.05 to 500 ng mL−1 | 26 pg mL−1 | 130 |
| AuPt@Fe–N–C | POD | TMB | Staphylococcus aureus enterotoxin B | 0.0002 to 10.000 ng mL−1 | 0667 pg mL−1 | 131 |
| Rh | POD | TMB | Staphylococcal enterotoxin B | 0 to 2 ng mL−1 | 1.2 pg mL−1 | 132 |
6. Conclusions and future perspectives
Numerous attempts have been undertaken to control biotoxins, however, their contamination remains largely inevitable. Therefore, there is an urgent need for research to explore biosensors for the rapid, sensitive, and convenient detection of biotoxins, given the detrimental impact of biotoxins on human health and food safety. So, as described in this literature update, owing to their favorable catalytic activity, excellent stability, and low cost, nanozymes and nanozyme-based biosensors have been extensively exploited to identify different biotoxins in food and environmental samples. In particular, the combination of colorimetric biosensors and nanozymes to construct an innovative biosensing scaffold provides a promising outlook in convenient, sensitive, and rapid quantification of various biotoxins. Herein, we have discussed the production, characteristics, and application of nanozymes in colorimetric biosensors, focusing on their diverse catalytic activities. Furthermore, a comprehensive overview of the research conducted on the utilization of nanozyme-based colorimetric biosensors for the identification of biotoxins is provided. Through deliberating the sensing approaches employed using nanozyme-based colorimetric biosensors, it becomes evident that these biosensors play crucial roles in the rapid diagnosis of biotoxins. Besides, we highlight the recent advancements in portable technologies, including hydrogels and paper-based platforms, which can be integrated with smartphones to enable on-site detection. Although nanozyme-based colorimetric biosensors possess the features of fast response, simple operation, high sensitivity, and low cost, they still have some challenges in several aspects.(i) Due to the inferior catalytic efficiency of nanozymes in comparison with natural enzymes, the application of nanozymes with multiple catalytic and high activity can be considered as one of the efficient strategies. Indeed, multiple catalytic activities including peroxidase-like, oxidase-like, and catalase-like activities can improve the performance of nanozymes. Furthermore, the concept of highly activated nanozymes is achieved by the integration of nanozymes with different nanomaterials. Also, the lower selectivity of nanozymes towards targets restricts specific recognition in the detection process. Consequently, the development of novel nanozymes with enhanced specificity could involve the integration of biomimetic recognition elements, such as MIPs, and exploring additional technical approaches holds promise for future advancements in this area.
(ii) Currently, an increasing number of studies are focusing on nanozymes exhibiting multiple enzyme activities. However, the utilization of nanozymes with multiple enzyme performances in colorimetric biosensors predominantly depends on their POD and OXD-like activities. Regulating the predominant activity of nanozymes with multiple enzyme-like functions simultaneously poses a significant challenge. This task necessitates a more precise comprehension of the catalytic mechanisms underlying various enzyme activities, along with a thorough understanding of the primary factors influencing these activities.
(iii) The catalytic reactions occur in specific regions on the nanozyme's surface which are considered as active places. Generally, these active places are operated in a similar manner to the active sites in natural enzymes. However, their function and structure can differ owing to the basic differences between biological macromolecules and nanomaterials.143 Elaborately, the presence of a small number of amino acids can allow natural enzymes to directly interact with the substrate. Hydrophobic interactions and hydrogen bonding are the most important of these interactions, resulting in high efficiency and specificity of the substrate. On the other hand, specific atoms or clusters of atoms on the surface of nanozymes act as active places. In detail, specific functional groups or metal ions can mimic the catalytic functions of natural enzymes. In addition, the chemical composition, size, and shape of nanozymes can impact their catalytic activity.144
To sum up, the investigation of nanozymes and nanozyme-based colorimetric biosensors represents merely the initial phase of a vast field of study. Nevertheless, it is evident that these biosensors exhibit significant promise in applications and merit further investigation. As research progresses and nanozyme-based colorimetric biosensors continue to evolve, it is anticipated that more cutting-edge technologies and portable devices will be developed and extensively employed to safeguard food and environmental integrity.
Data availability
No data were used for the research described in the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Jiyang College of Zhejiang A&F University (RQ1911F12) and Scientific Research Project of Education Department of Zhejiang Province (Y202352649).References
- M. Mahmoudpour, J. Ezzati Nazhad Dolatabadi, M. Torbati, A. Pirpour Tazehkand, A. Homayouni-Rad and M. de la Guardia, Biosens. Bioelectron., 2019, 143, 111603 CrossRef CAS PubMed.
- M. Mahmoudpour, J. Ezzati Nazhad Dolatabadi, M. Torbati and A. Homayouni-Rad, Biosens. Bioelectron., 2019, 127, 72–84 CrossRef CAS PubMed.
- J. Nicolas, R. L. Hoogenboom, P. J. Hendriksen, M. Bodero, T. F. Bovee, I. M. Rietjens and A. Gerssen, Global Food Secur., 2017, 15, 11–21 CrossRef.
- M.-L. Liu, X.-M. Liang, M.-Y. Jin, H.-W. Huang, L. Luo, H. Wang, X. Shen and Z.-L. Xu, J. Agric. Food Chem., 2024, 72, 10753–10771 CrossRef CAS PubMed.
- F. Javaheri-Ghezeldizaj, M. Mahmoudpour, R. Yekta and J. Ezzati Nazhad Dolatabadi, J. Mol. Liq., 2020, 310, 113259 CrossRef CAS.
- C. Lin, Z.-S. Liu, C.-Y. Tan, Y.-P. Guo, L. Li, H.-L. Ren, Y.-S. Li, P. Hu, S. Gong and Y. Zhou, Environ. Sci. Pollut. Res., 2015, 22, 1545–1553 CrossRef CAS PubMed.
- D. Liu, in Molecular Medical Microbiology, Elsevier, 2024, pp. 933–944 Search PubMed.
- P. Sadeghi, H. Sohrabi, M. R. Majidi, A. Eftekhari, F. Zargari, M. de la Guardia and A. A. Mokhtarzadeh, Trac. Trends Anal. Chem., 2024, 117722 CrossRef CAS.
- Y. Liu and F. Wu, Environ. Health Perspect., 2010, 118, 818–824 CrossRef CAS PubMed.
- S. Siva, J.-O. Jin, I. Choi and M. Kim, Biosens. Bioelectron., 2023, 219, 114845 CrossRef CAS PubMed.
- M. Mahmoudpour, S. Ding, Z. Lyu, G. Ebrahimi, D. Du, J. Ezzati Nazhad Dolatabadi, M. Torbati and Y. Lin, Nano Today, 2021, 39, 101177 CrossRef CAS.
- Z. Karimzadeh, M. Mahmoudpour, E. Rahimpour and A. Jouyban, Adv. Colloid Interface Sci., 2022, 305, 102705 CrossRef CAS PubMed.
- T. A. Rocha-Santos, Trac. Trends Anal. Chem., 2014, 62, 28–36 CrossRef CAS.
- Z. Golsanamlou, M. Mahmoudpour, J. Soleymani and A. Jouyban, Crit. Rev. Anal. Chem., 2023, 53, 1116–1131 CrossRef CAS PubMed.
- Z. Khoshbin, M. Moeenfard, K. Abnous and S. M. Taghdisi, Food Chem., 2024, 433, 137355 CrossRef CAS PubMed.
- L. Lu, R. Yu and L. Zhang, Food Chem., 2023, 421, 136205 CrossRef CAS PubMed.
- R. L. F. Melo, F. S. Neto, D. N. Dari, B. C. C. Fernandes, T. M. Freire, P. B. A. Fechine, J. M. Soares and J. C. S. Dos Santos, Int. J. Biol. Macromol., 2024, 130817 CrossRef CAS PubMed.
- T. Vyas, V. Singh, P. Kodgire and A. Joshi, Crit. Rev. Biotechnol., 2023, 43, 521–539 CrossRef CAS PubMed.
- J. Qin, N. Guo, J. Yang and J. Wei, Food Chem., 2024, 139019 CrossRef CAS PubMed.
- A. Baranwal, R. Shukla and V. Bansal, Trac. Trends Anal. Chem., 2024, 117573 CrossRef CAS.
- L. Yang, X. Xu, Y. Song, J. Huang and H. Xu, Chem. Eng. J., 2024, 487, 150612 CrossRef CAS.
- Z. Chi, Q. Wang and J. Gu, Analyst, 2023, 148, 487–506 Search PubMed.
- B. Unnikrishnan, C.-W. Lien, H.-W. Chu and C.-C. Huang, J. Hazard. Mater., 2021, 401, 123397 Search PubMed.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS PubMed.
- H. Wei, L. Gao, K. Fan, J. Liu, J. He, X. Qu, S. Dong, E. Wang and X. Yan, Nano Today, 2021, 40, 101269 Search PubMed.
- L. Zhang, H. Wang and X. Qu, Adv. Mater., 2024, 36, 2211147 CrossRef CAS PubMed.
- Z. Li, W. Liu, P. Ni, C. Zhang, B. Wang, G. Duan, C. Chen, Y. Jiang and Y. Lu, Chem. Eng. J., 2022, 428, 131396 Search PubMed.
- Z. Lou, S. Zhao, Q. Wang and H. Wei, Anal. Chem., 2019, 91, 15267–15274 Search PubMed.
- K. Fan, J. Xi, L. Fan, P. Wang, C. Zhu, Y. Tang, X. Xu, M. Liang, B. Jiang, X. Yan and L. Gao, Nat. Commun., 2018, 9, 1440 CrossRef PubMed.
- P. Zhang, D. Sun, A. Cho, S. Weon, S. Lee, J. Lee, J. W. Han, D.-P. Kim and W. Choi, Nat. Commun., 2019, 10, 940 CrossRef PubMed.
- S. Pandit and M. De, Nanoscale Adv., 2021, 3, 5102–5110 RSC.
- H. Sun, A. Zhao, N. Gao, K. Li, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2015, 54, 7176–7180 CrossRef CAS PubMed.
- M. Mahmoudpour, J. E. N. Dolatabadi, M. Hasanzadeh, A. H. Rad, M. Torbati and F. Seidi, RSC Adv., 2022, 12, 29602–29612 RSC.
- M. S. Kim, S. Cho, S. H. Joo, J. Lee, S. K. Kwak, M. I. Kim and J. Lee, ACS Nano, 2019, 13, 4312–4321 Search PubMed.
- Z. Wang, Z. Xu, X. Xu, J. Xi, J. Han, L. Fan and R. Guo, Colloids Surf., B, 2022, 217, 112671 CrossRef CAS PubMed.
- F. Li, J. Jiang, H. Peng, C. Li, B. Li and J. He, Sens. Actuators, B, 2022, 369, 132334 CrossRef CAS.
- L. Zhu, W. Zeng, Y. Li, Y. Han, J. Wei and L. Wu, Sci. Total Environ., 2024, 921, 171236 CrossRef CAS PubMed.
- X. Liang, X. Wang, Y. Zhang, B. Huang and L. Han, J. Agric. Food Chem., 2022, 70, 3898–3906 CrossRef CAS PubMed.
- X. Zhang, Y. Xu, X. Wang, T. Chen, Q. Yao, S. Chang, X. Guo, X. Liu, H. Wu and Y. Cui, Food Chem., 2024, 140710 CrossRef CAS PubMed.
- C. Lu, L. Tang, F. Gao, Y. Li, J. Liu and J. Zheng, Biosens. Bioelectron., 2021, 187, 113327 Search PubMed.
- S. Naveen Prasad, P. Weerathunge, M. N. Karim, S. Anderson, S. Hashmi, P. D. Mariathomas, V. Bansal and R. Ramanathan, Anal. Bioanal. Chem., 2021, 413, 1279–1291 Search PubMed.
- D. Li, D. Dai, G. Xiong, S. Lan and C. Zhang, Small, 2023, 19, 2205870 CrossRef CAS PubMed.
- P. T. Nguyen, J. Lee, A. Cho, M. S. Kim, D. Choi, J. W. Han, M. I. Kim and J. Lee, Adv. Funct. Mater., 2022, 32, 2112428 CrossRef CAS.
- Y. Lin, C. Xu, J. Ren and X. Qu, Angew. Chem., 2012, 51, 12579–12583 CrossRef CAS PubMed.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- R. André, F. Natálio, M. Humanes, J. Leppin, K. Heinze, R. Wever, H. C. Schröder, W. E. Müller and W. Tremel, Adv. Funct. Mater., 2011, 21, 501–509 CrossRef.
- A. B. Ganganboina and R.-a. Doong, Sens. Actuators, B, 2018, 273, 1179–1186 CrossRef CAS.
- H. Li, S. Zhao, Z. Wang and F. Li, Small, 2023, 19, 2206465 CrossRef CAS PubMed.
- L. Han, H. Zhang, D. Chen and F. Li, Adv. Funct. Mater., 2018, 28, 1800018 CrossRef.
- Q. Liu, A. Zhang, R. Wang, Q. Zhang and D. Cui, Nano-Micro Lett., 2021, 13, 1–53 CrossRef PubMed.
- Z. Karimzadeh, M. Mahmoudpour, M. d. l. Guardia, J. Ezzati Nazhad Dolatabadi and A. Jouyban, Trac. Trends Anal. Chem., 2022, 152, 116622 CrossRef CAS.
- Z. Karimzadeh, M. Mahmoudpour, E. Rahimpour and A. Jouyban, RSC Adv., 2024, 14, 9571–9586 RSC.
- Z. Karimzadeh, A. Jouyban, A. Ostadi, A. Gharakhani and E. Rahimpour, Anal. Chim. Acta, 2022, 1227, 340252 CrossRef CAS PubMed.
- X. Zhang, G. Li, D. Wu, X. Li, N. Hu, J. Chen, G. Chen and Y. Wu, Biosens. Bioelectron., 2019, 137, 178–198 CrossRef CAS PubMed.
- X. Huang, S. Zhang, Y. Tang, X. Zhang, Y. Bai and H. Pang, Coord. Chem. Rev., 2021, 449, 214216 CrossRef CAS.
- W. He, Z. Li, S. Lv, M. Niu, W. Zhou, J. Li, R. Lu, H. Gao, C. Pan and S. Zhang, Chem. Eng. J., 2021, 409, 128274 CrossRef CAS.
- K. Zhang, K. Dai, R. Bai, Y. Ma, Y. Deng, D. Li, X. Zhang, R. Hu and Y. Yang, Chin. Chem. Lett., 2019, 30, 664–667 CrossRef CAS.
- Z. Sun, M. Wang, J. Fan, R. Feng, Y. Zhou and L. Zhang, Adv. Compos. Hybrid Mater., 2021, 4, 1322–1329 CrossRef CAS.
- Y. Su, M. Lu, R. Su, W. Zhou, X. Xu and Q. Li, Chin. Chem. Lett., 2022, 33, 2573–2578 CrossRef CAS.
- L. Zheng, F. Wang, C. Jiang, S. Ye, J. Tong, P. Dramou and H. He, Coord. Chem. Rev., 2022, 471, 214760 CrossRef CAS.
- X. Niu, B. Liu, P. Hu, H. Zhu and M. Wang, Biosensors, 2022, 12, 251 CrossRef CAS PubMed.
- H. Deng, Y. Zhang, X. Cai, Z. Yin, Y. Yang, Q. Dong, Y. Qiu and Z. Chen, Small, 2024, 20, 2306155 CrossRef CAS PubMed.
- Z. Wang, M. Li, H. Bu, D. S. Zia, P. Dai and J. Liu, Mater. Chem. Front., 2023, 7, 3625–3640 RSC.
- M.-L. Ye, Y. Zhu, Y. Lu, L. Gan, Y. Zhang and Y.-G. Zhao, Talanta, 2021, 230, 122299 CrossRef CAS PubMed.
- Y. Song, K. Qu, C. Zhao, J. Ren and X. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- Z. Lyu, J. Zhou, S. Ding, D. Du, J. Wang, Y. Liu and Y. Lin, Trac. Trends Anal. Chem., 2023, 117280 CrossRef CAS.
- X. Zhang, C. Sun, R. Li, X. Jin, Y. Wu and F. Fu, Anal. Chem., 2023, 95, 5024–5033 CrossRef CAS PubMed.
- H. Li, M. Sun, H. Gu, J. Huang, G. Wang, R. Tan, R. Wu, X. Zhang, S. Liu and L. Zheng, Small, 2023, 19, 2207036 CrossRef CAS PubMed.
- M. Comotti, C. Della Pina, R. Matarrese and M. Rossi, Angew. Chem., Int. Ed., 2004, 43, 5812–5815 CrossRef CAS PubMed.
- Y. Peng, M. Huang, L. Chen, C. Gong, N. Li, Y. Huang and C. Cheng, Nano Res., 2022, 15, 8783–8790 CrossRef CAS.
- M. Ren, Y. Zhang, L. Yu, L. Qu, Z. Li and L. Zhang, Talanta, 2023, 255, 124219 CrossRef CAS PubMed.
- X. Zhou, M. Wang, J. Chen and X. Su, Talanta, 2022, 245, 123451 CrossRef CAS PubMed.
- S. Khajir, Z. Karimzadeh, M. Khoubnasabjafari, V. Jouyban-Gharamaleki, E. Rahimpour and A. Jouyban, J. Pharm. Biomed. Anal., 2023, 223, 115141 CrossRef CAS PubMed.
- S. Singh, Front. Chem., 2019, 7, 46 CrossRef CAS PubMed.
- S. Singh, Int. J. Biol. Macromol., 2024, 129374 CrossRef CAS PubMed.
- D. Mehta, P. Sharma and S. Singh, Colloids Surf., B, 2023, 231, 113531 CrossRef CAS PubMed.
- M. S. Lord, J. F. Berret, S. Singh, A. Vinu and A. S. Karakoti, Small, 2021, 17, 2102342 CrossRef CAS PubMed.
- A. K. Singh, K. Bijalwan, N. Kaushal, A. Kumari, A. Saha and A. Indra, ACS Appl. Nano Mater., 2023, 6, 8036–8045 CrossRef CAS.
- M. Zhang, Y. Wang, N. Li, D. Zhu and F. Li, Biosens. Bioelectron., 2023, 237, 115554 CrossRef CAS PubMed.
- M. Jia, F. Xu, F. Zhai, X. Yu and M. Du, J. Colloid Interface Sci., 2024, 653, 1805–1816 CrossRef CAS PubMed.
- C. P. Liu, T. H. Wu, C. Y. Liu, K. C. Chen, Y. X. Chen, G. S. Chen and S. Y. Lin, Small, 2017, 13, 1700278 CrossRef PubMed.
- S. Guo, Y. Han and L. Guo, Catal. Surv. Asia, 2020, 24, 70–85 CrossRef CAS.
- Z. Wang, X. Shen, X. Gao and Y. Zhao, Nanoscale, 2019, 11, 13289–13299 RSC.
- R. Zhang, B. Xue, Y. Tao, H. Zhao, Z. Zhang, X. Wang, X. Zhou, B. Jiang, Z. Yang and X. Yan, Adv. Mater., 2022, 34, 2205324 CrossRef CAS PubMed.
- W. Yang, X. Yang, L. Zhu, H. Chu, X. Li and W. Xu, Coord. Chem. Rev., 2021, 448, 214170 CrossRef CAS.
- A. Casu, M. Camardo Leggieri, P. Toscano and P. Battilani, Compr. Rev. Food Sci. Food Saf., 2024, 23, e13323 CrossRef PubMed.
- M.-H. Moosavy, M. Hasanzadeh, J. Soleymani and A. Mokhtarzadeh, Anal. Methods, 2019, 11, 3910–3919 RSC.
- Y. Zhang, X. Chen, X. Xie, D. Li, Y. Fan, B. Huang and X. Yang, Curr. Anal. Chem., 2024, 20, 242–254 CrossRef.
- Z. Xue, Y. Zhang, W. Yu, J. Zhang, J. Wang, F. Wan, Y. Kim, Y. Liu and X. Kou, Anal. Chim. Acta, 2019, 1069, 1–27 CrossRef CAS PubMed.
- C. Peng, R. Pang, J. Li and E. Wang, Adv. Mater., 2024, 36, 2211724 CrossRef CAS PubMed.
- X. Zhao, Q. Li, H. Li, Y. Wang, F. Xiao, D. Yang, Q. Xia and Y. Yang, Food Chem., 2023, 424, 136443 CrossRef CAS PubMed.
- W. Lai, J. Guo, Y. Wang, Y. Lin, S. Ye, J. Zhuang and D. Tang, Talanta, 2022, 247, 123546 CrossRef CAS PubMed.
- L. Wu, M. Zhou, Y. Wang and J. Liu, J. Hazard Mater., 2020, 399, 123154 CrossRef CAS PubMed.
- W. Jiang, Q. Yang, H. Duo, W. Wu and X. Hou, Food Chem., 2024, 138917 CrossRef CAS PubMed.
- S. Huang, W. Lai, B. Liu, M. Xu, J. Zhuang, D. Tang and Y. Lin, Spectrochim. Acta, Part A, 2023, 284, 121782 CrossRef CAS PubMed.
- N. Ullah, T. A. Bruce-Tagoe, G. A. Asamoah and M. K. Danquah, Int. J. Mol. Sci., 2024, 25, 5959 CrossRef CAS PubMed.
- S. Ganguly and S. Margel, Talanta, 2023, 100243 CrossRef.
- X. Tan, K. Kang, R. Zhang, J. Dong, W. Wang and W. Kang, Sens. Actuators, B, 2024, 412, 135854 CrossRef CAS.
- X. Cai, M. Liang, F. Ma, Z. Zhang, X. Tang, J. Jiang, C. Guo, S. R. Mohamed, A. A. Goda and D. H. Dawood, Food Chem., 2022, 377, 131965 CrossRef CAS PubMed.
- X. Zhu, J. Tang, X. Ouyang, Y. Liao, H. Feng, J. Yu, L. Chen, Y. Lu, Y. Yi and L. Tang, J. Hazard. Mater., 2024, 465, 133178 CrossRef CAS PubMed.
- T. Bu, P. Jia, X. Sun, Y. Liu, Q. Wang and L. Wang, Sens. Actuators, B, 2020, 320, 128440 CrossRef CAS.
- Y. Fan, D. Li, X. Xie, Y. Zhang, L. Jiang, B. Huang and X. Yang, Microchem. J., 2024, 197, 109842 CrossRef CAS.
- X. Zhang, M. C. Wasson, M. Shayan, E. K. Berdichevsky, J. Ricardo-Noordberg, Z. Singh, E. K. Papazyan, A. J. Castro, P. Marino and Z. Ajoyan, Coord. Chem. Rev., 2021, 429, 213615 CrossRef CAS PubMed.
- P. Li, R. C. Klet, S.-Y. Moon, T. C. Wang, P. Deria, A. W. Peters, B. M. Klahr, H.-J. Park, S. S. Al-Juaid and J. T. Hupp, Chem. Commun., 2015, 51, 10925–10928 RSC.
- S. Zhang, H. Li, Q. Xia, D. Yang and Y. Yang, J. Food Sci., 2024, 89, 3618–3628 CrossRef CAS PubMed.
- S. Peng, K. Li, Y.-x. Wang, L. Li, Y.-H. Cheng and Z. Xu, Anal. Biochem., 2022, 655, 114829 CrossRef CAS PubMed.
- C. Jiang, L. Lan, Y. Yao, F. Zhao and J. Ping, Trac. Trends Anal. Chem., 2018, 102, 236–249 CrossRef CAS.
- Y. Alhamoud, D. Yang, S. S. F. Kenston, G. Liu, L. Liu, H. Zhou, F. Ahmed and J. Zhao, Biosens. Bioelectron., 2019, 141, 111418 CrossRef CAS PubMed.
- Q. Zhao, Z. Yan, C. Chen and J. Chen, Chem. Rev., 2017, 117, 10121–10211 CrossRef CAS PubMed.
- L. Gao, K. Fan and X. Yan, Nanozymology: Connecting Biology and Nanotechnology, 2020, pp. 105–140 Search PubMed.
- L. Huang, K. Chen, W. Zhang, W. Zhu, X. Liu, J. Wang, R. Wang, N. Hu, Y. Suo and J. Wang, Sens. Actuators, B, 2018, 269, 79–87 CrossRef CAS.
- J. M. Gonçalves, L. V. de Faria, A. B. Nascimento, R. L. Germscheidt, S. Patra, L. P. Hernández-Saravia, J. A. Bonacin, R. A. Munoz and L. Angnes, Anal. Chim. Acta, 2022, 1233, 340362 CrossRef PubMed.
- Q. Liu, S. Xin, X. Tan, Q. Yang and X. Hou, Microchim. Acta, 2023, 190, 364 CrossRef CAS PubMed.
- M. K. Masud, J. Kim, M. M. Billah, K. Wood, M. J. Shiddiky, N.-T. Nguyen, R. K. Parsapur, Y. V. Kaneti, A. A. Alshehri and Y. G. Alghamidi, J. Mater. Chem. B, 2019, 7, 5412–5422 RSC.
- F. Tian, J. Zhou, B. Jiao and Y. He, Nanoscale, 2019, 11, 9547–9555 RSC.
- H. Zhu, Z. Quan, H. Hou, Y. Cai, W. Liu and Y. Liu, Anal. Chim. Acta, 2020, 1132, 101–109 CrossRef CAS PubMed.
- L. Zhao, H. Guo, H. Chen, B. Zou, C. Yang, X. Zhang, Y. Gao, M. Sun and L. Wang, Bioengineering, 2022, 9, 684 CrossRef CAS PubMed.
- H. kholafazad Kordasht, S. Hassanpour, B. Baradaran, R. Nosrati, M. Hashemzaei, A. Mokhtarzadeh and M. de la Guardia, Biosens. Bioelectron., 2020, 165, 112403 CrossRef PubMed.
- O. D. Hendrickson, E. A. Zvereva, V. G. Panferov, O. N. Solopova, A. V. Zherdev, P. G. Sveshnikov and B. B. Dzantiev, Biosensors, 2022, 12, 1137 CrossRef CAS PubMed.
- Y. Zhao, L. Li, R. Ma, L. Wang, X. Yan, X. Qi, S. Wang and X. Mao, Anal. Chim. Acta, 2021, 1173, 338710 CrossRef CAS PubMed.
- X. Qi, L. Li, X. Yan, Y. Zhao, L. Wang, R. Ma, S. Wang and X. Mao, J. Ocean Univ. China, 2022, 21, 1343–1350 CrossRef CAS.
- S. Wang, Y. Zhao, R. Ma, W. Wang, L. Zhang, J. Li, J. Sun and X. Mao, Food Chem., 2023, 401, 134053 CrossRef CAS PubMed.
- L. Li, R. Ma, Y. Zhao, L. Wang, S. Wang and X. Mao, Talanta, 2022, 246, 123534 CrossRef CAS PubMed.
- F. Cui, Z. Zhou and H. S. Zhou, Sensors, 2020, 20, 996 CrossRef CAS PubMed.
- J. Marfà, R. Pupin, M. Sotomayor and M. Pividori, Anal. Bioanal. Chem., 2021, 413, 6141–6157 CrossRef PubMed.
- L. Wu, Y. Li, Y. Han, X. Liu, B. Han, H. Mao and Q. Chen, J. Food Compos. Anal., 2024, 130, 106190 CrossRef CAS.
- C. H. Cho, J. H. Kim, N. S. Padalkar, Y. V. M. Reddy, T. J. Park, J. Park and J. P. Park, Biosens. Bioelectron., 2024, 255, 116269 CrossRef CAS PubMed.
- C. Hernández-Cortez, I. Palma-Martínez, L. U. Gonzalez-Avila, A. Guerrero-Mandujano, R. C. Solís and G. Castro-Escarpulli, Poisoning: from Specific Toxic Agents to Novel Rapid and Simplified Techniques for Analysis, 2017, vol. 33 Search PubMed.
- R. Gupta, N. Raza, S. K. Bhardwaj, K. Vikrant, K.-H. Kim and N. Bhardwaj, J. Hazard Mater., 2021, 401, 123379 CrossRef CAS PubMed.
- K. Ren, M. Duan, T. Su, D. Ying, S. Wu, Z. Wang and N. Duan, Talanta, 2024, 270, 125636 CrossRef CAS PubMed.
- H. Liang, H. Liu, H. Lin, G. Ning, X. Lu, S. Ma, F. Liu, H. Zhao and C. Li, Food Sci. Hum. Wellness, 2024, 13, 2025–2035 CrossRef CAS.
- X. Cai, Y. Luo, C. Zhu, D. Huang and Y. Song, Sens. Actuators, B, 2022, 367, 132066 CrossRef CAS.
- Y. Xing, F. Yasinjan, S. Sun, J. Yang, Y. Du, H. Zhang, Y. Liang, H. Geng, Y. Wang and J. Sun, Nano Today, 2024, 57, 102386 CrossRef CAS.
- D. Li, T. Fan and X. Mei, Nanoscale, 2023, 15, 15885–15905 RSC.
- Q. Lu, Q. Li, Y. An, X. Duan, R. Zhao, D. Zhao and S. An, J. Clean. Prod., 2022, 376, 134117 CrossRef CAS.
- M. Tudi, H. Daniel Ruan, L. Wang, J. Lyu, R. Sadler, D. Connell, C. Chu and D. T. Phung, Int. J. Environ. Res. Publ. Health, 2021, 18, 1112 CrossRef CAS PubMed.
- M. Song, Improving Potable Reuse Water Quality by Understanding Bulk Organic Matter Present during Advanced Oxidation and N-nitrosodimethylamine Precursors, University of Nevada, Reno, 2022 Search PubMed.
- N. K. V. Leitner, in Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications, IWA Publishing, 2017, pp. 429–460 Search PubMed.
- Z. A. Mohamed, Y. Mostafa, S. Alamri, M. Hashem and S. Alrumman, Arch. Microbiol., 2022, 205, 63 CrossRef PubMed.
- X. Yang, J. Pan, J. Hu, S. Zhao and K. Cheng, Chem. Eng. J., 2023, 467, 143381 CrossRef CAS.
- W. Jiang, Q. Yang, H. Duo, W. Wu and X. Hou, Food Chem., 2024, 447, 138917 CrossRef CAS PubMed.
- S. Zhang, H. Li, Q. Xia, D. Yang and Y. Yang, J. Food Sci., 2024, 89, 3618–3628 CrossRef CAS PubMed.
- R. Zhang, X. Yan and K. Fan, Acc. Mater. Res., 2021, 2, 534–547 CrossRef CAS.
- Y. Chong, Q. Liu and C. Ge, Nano Today, 2021, 37, 101076 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |
