Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improving the analysis of phase-separated bio-fuel samples with slice-selective total correlation NMR spectroscopy†
Jaskamal Singh
Khangura
a,
Bridget
Tang
 a,
Katie
Chong
a,
Katie
Chong
 b and
Robert
Evans
b and
Robert
Evans
 *a
*a
aChemical Engineering and Applied Chemistry, College of Engineering and Physical Sciences, Aston University, Birmingham, B4 7ET, UK. E-mail: r.evans2@aston.ac.uk
bEnergy and Bioproducts Research Institute, Aston University, Aston Triangle, Birmingham B4 7ET, UK
First published on 7th August 2024
Abstract
Separated samples are a particular challenge for NMR experiments. The boundary is severely detrimental to high-resolution spectra and normal NMR experiments simply add the two spectra of the two layers together. Pyrolysis bio-oils represent an increasingly important alternative fuel resource yet readily separate, whether due to naturally high water content or due to blending, a common practice for producing a more viable fuel. Slice-selective NMR, where the NMR spectrum of only a thin slice of the total sample is acquired, is extended here and improved, with slice-selective two-dimensional correlation experiments used to resolve the distinct chemical spectra of the various components of the phase-separated blended fuel mixtures. Analysis of how the components of any blended biofuel samples partition between the two layers is an important step towards understanding the separation process and may provide insight into mitigating the problem.
Introduction
Nuclear magnetic resonance (NMR) is a powerful and nearly ubiquitous spectroscopic technique. Typically, solution-state NMR requires a homogeneous liquid and the spectra are acquired from a relatively large volume at the centre of the sample. Heterogeneous, or separated, samples are avoided. The presence of a boundary between two phases detrimentally affects the resolution and quality of the spectrum, due to the differences in magnetic susceptibility of the two phases. Furthermore, standard 1D NMR measurements of the separated sample will simply sum the signals of the two phases together.These issues can be avoided by using slice-selective, or spatially-resolved, NMR.1 In these NMR experiments, excitation of a given slice of the sample is accomplished by applying a long, low-power, radiofrequency pulse in the presence of a pulsed magnetic field gradient. NMR spectrometers are now fitted with such pulsed magnetic field gradients as standard. On most standard NMR probes, these gradients are applied along the direction of the magnetic field or z-axis. When a linear field gradient, Gz, is applied to a sample, the magnetic field strength, B(z), depends on the position along the axial direction of the NMR tube, z, such that B(z) = B0 + zGz. Therefore, all resonance frequencies experience an offset, Ω, that depends on the vertical deviation (z) from the center of the gradient coil, see eqn (1), where γ is the gyromagnetic ratio of the spins being measured.
| Ω = γGzz/2π | (1) |
A soft pulse, with a bandwidth ΔB, employed at this offset will selectively excite a horizontal slice of the sample, centered at z, with a thickness Δz obtained from eqn (2).
| Δz = (2π/γGz)ΔB | (2) |
Only this thin horizontal slice of the sample will be excited by the soft radiofrequency pulse. Before acquisition of the NMR data, the field gradient is switched off and the spectrum of the slice is acquired as in a normal experiment. Slices can be easily moved by changing the offset of the selective pulse. Thinner slices can be acquired, at the expense of reduced signal intensities. The method is not limited to superconducting magnets; so long as the spectrometer has pulsed field gradients in an appropriate geometry, the method described here is transferable.
The use of slice-selective NMR in 1-dimensional chemical applications is a growing field and has been demonstrated in a number of studies, including idealized systems, such as benzene floating on water2 or water and olive oil mixtures,3 and the diffusion of small molecules in non-equilibrium systems, such as the mutual diffusion of small molecules,4 CO2 in ionic liquids,5 and small molecules through gels.6–8 Slice-selective NMR spectroscopy has more recently been utilized in increasingly complex analyses, such as hydrophilic/hydrophobic metabolites,9 crude oil emulsions,10 and separated biofuels.11 Its use is not limited to observing 1H, with application to the study of 7Li ions in polymer gels12,13 and in systems intended to resemble Li-ion batteries.14
Pyrolysis oils, or bio-oils, are an important example of samples where analysis is often hindered by phase-separation of the samples, yet NMR techniques can give important insight into the nature of the mixture. Pyrolysis is a thermochemical conversion process, involving irreversible heat-driven decomposition of materials, such as lignocellulosic biomass, in the absence of oxygen.15 The pyrolysis products typically contain char, gases, and an oil. The oil is a potential fuel, but typically cannot be used directly in unmodified engines as it contains too much water and various other oxygen-containing species present16 render it too acidic. There are several methods by which the utility of a pyrolysis product can be improved,17–19 such as by blending with other products.20–22 Such multiple component blends are typically opaque and can readily separate into a multiple-phase solution.23–25 Once separated, the blends are highly unsuitable as fuel products and could cause significant damage to an engine if used. A key challenge to the successful blending of these fuel products is the analysis, understanding and mitigation of this phase separation. The NMR analysis of pyrolysis oils, also known as bio-oils, is well-established and comprehensive reviews are available.26,27 However, any analysis of these oils, blended or otherwise, is complicated by the large number of species present and the range of functional groups that may be present.
Here, the improved performance of slice-selective NMR analysis of complex phase-separated samples, such as pyrolysis bio-oils and their blends, is demonstrated by combining slice-selective methods with two-dimensional NMR techniques. Total correlation spectroscopy (TOCSY) is used here, as the final spectra produced can be phased to give pure absorption mode peaks.
Experimental
Methods and materials
Six blended biofuel samples containing differing amounts of the bio-oil, marine gas oil, fatty acid methyl ester, and butanol were analyzed.| Sample | Component composition (%) | |||
|---|---|---|---|---|
| Bio-oil | Butanol | FAME | Marine gas oil | |
| A | 20 | 40 | 40 | 0 |
| B | 30 | 40 | 0 | 30 |
| C | 30 | 20 | 50 | 0 |
| D | 10 | 80 | 10 | 0 |
| E | 20 | 50 | 7.5 | 22.5 |
| F | 30 | 40 | 15 | 15 |
NMR experiments
All 1H NMR measurements were performed on a 300 MHz Bruker Avance spectrometer at 298 K, using a 5 mm BBO probe equipped with a z gradient coil producing a maximum gradient strength of 0.55 T m−1. For the slice-selective NMR experiments, a G4 cascade29 was used for the selective pulse, with a 5000 Hz bandwidth and applied at offsets of + and −5000 Hz, corresponding to the upper and lower layers respectively. A gradient of 5% of the maximum gradient strength was applied concurrently with the selective pulse. This corresponds to a slice 4.3 mm in width, exciting a slice centred 4.3 mm from the centre of the Gz coils. No deuterated solvents were added to the samples. All NMR experiments were acquired without the use of the lock and shimming was achieved using the area of the acquired FID. The data presented here were all acquired with a slice-selective 2D TOCSY experiment with 256 increments, 8 scans and 16 dummy scans, for an experimental duration of 2 hours and 30 minutes. 1D 1H experiments of all blended samples were also acquired, using 64 scans, for a duration of 16 seconds. All data were processed using TopSpin. The NMR analysis of the blended oils was performed blind. The identities and compositions of the oils were only revealed to the NMR spectroscopists after the analysis of the NMR spectra was completed.Results and discussion
2D 1H TOCSY of un-separated bio-oil blends
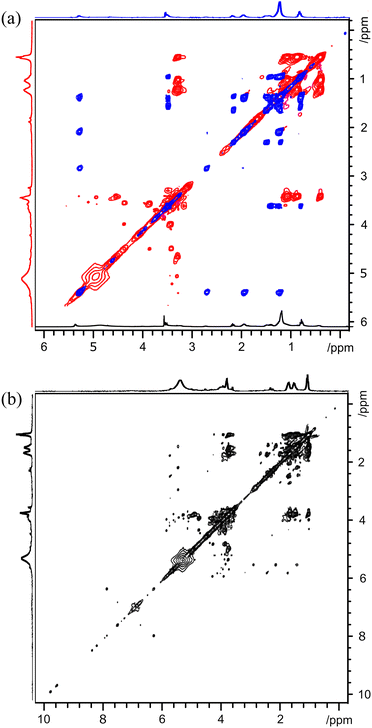
To first demonstrate the general utility of 2D TOCSY experiments in the analysis of biofuels, Fig. 1 depicts a spectrum acquired from unseparated, three-component, samples (sample A). The contour levels in Fig. 1 have been adjusted to capture the smaller peaks present in the sample. The butanol peaks dominate the 1D 1H spectrum and, as expected, the TOCSY spectrum contains all expected cross peaks from butanol, the major component of sample A. | ||
| Fig. 1 2D 1H TOCSY spectra of three-component unseparated sample A. Gray inset details peaks along horizontal row at ca. 5.3 ppm. | ||
However, the TOCSY spectrum reveals additional components of the mixture, both expected and unexpected. The fatty acid methyl ester can also be observed, as a row of resonances horizontally or vertically along 5.4 ppm. With the long alkyl chain on the fatty acid methyl ester, a suitably long spin-lock is needed to couple together the most distant protons on the chain. The signals highlighted in the inset confirm that the spin-lock period selected is appropriate for the sample. While the intensity of the methyl peak is low, particularly compared with the more plentiful methylene signals, it does appear along the same horizontal line as the other FAME signals. Additional peaks, belonging to neither butanol nor fatty acid methyl ester are observed as cross peaks between ca. 1.5 ppm and ca. 2 ppm. This spectrum shows the advantages of the TOCSY pulse sequence. The complete NMR spectrum of individual components can be readily resolved.
Two-dimensional TOCSY spectra of an additional unseparated, three-component and two unseparated, four-component, samples, samples D, E and F, are presented in the ESI as Fig. S2–S4.† While these all contain different amounts of bio-oil, butanol, marine gas oil and FAME, their spectra share many of the features of sample A revealed in Fig. 1.
Slice-selective 2D 1H TOCSY of separated samples
To demonstrate the effectiveness of slice-selective 2D TOCSY experiments in analyzing bio-oil samples, two phase-separated, three-component, samples were studied in this work. The NMR spectra of these separated samples were also acquired using both standard 1D 1H and slice-selective 1H NMR pulse sequences. Fig. 2 depicts the upper (left) and lower (right) layers of a separated three component blended bio-oil sample (sample B). The sample contains the bio-oil, butanol and marine gas oil. The proton spectra indicate the key differences between the samples, with the upper layer consisting mainly of the marine gas oil and an aqueous lower layer. The butanol is partitioned between the two layers, with ca. 93% found in the lower, aqueous layer. The similarity in methyl and methylene chemical shifts render the full TOCSY spectra rather similar, particularly between 1 and 4 ppm. Less intense cross-peaks do appear in the spectra of both layers. In the upper layer, there is a cross peak indicating coupling between aromatic species and alkyl groups. In the lower layer, the cross peak indicating coupling between species at 3.5 and ca. 5 ppm suggests the presence of both polar species and olefinic groups.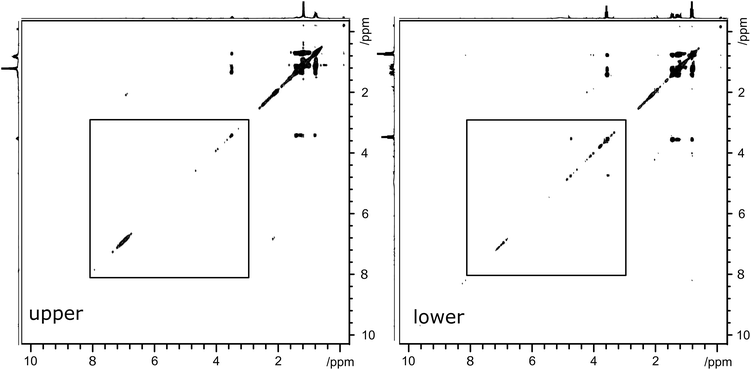 | ||
| Fig. 2 2D 1H TOCSY spectra of three-component separated sample B. Left-hand spectrum depicts the upper layer and right-hand spectrum depicts the lower layer. Spectral regions indicated by boxes are reproduced, enlarged, in Fig. 3. | ||
In order to make a more detailed comparison between the two layers, the regions from 3 to 8 ppm, indicated by boxes in Fig. 2, are magnified and overlaid. This comparison is depicted in Fig. 3, with the upper layer in blue and the lower layer in red. This presentation of the two-dimensional TOCSY spectra makes the differences between the two layers easily visible. The cross peaks between ca. 7 ppm and ca. 2 ppm in the upper layer spectrum confirms the presence aromatic species with alkyl substituents. The broad nature of these peaks indicates a wide range of species, likely polymeric or fused ring systems. As these species are found in the upper, oil, layer, they are likely to be heavier fractions of the marine gas oil. On the other hand, the cross peaks in the lower layer are both smaller in area and are clustered around 3.5 and 5.5 ppm, indicating a large number of distinct, smaller compounds with both polar functional groups and unsaturated, olefinic, chains. With pyrolysis bio-oil being produced from lignocellulosic biomass, the presence of compounds with structures based on monolignols or the constituent units of lignin is expected.
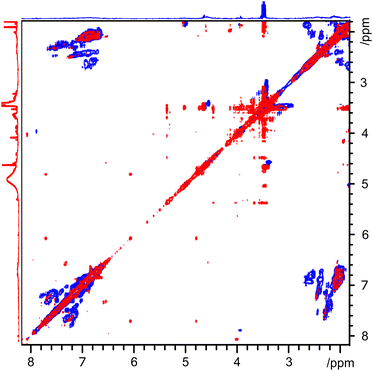 | ||
| Fig. 3 2D 1H TOCSY spectrum between 2 and 8 ppm (region indicated by box in Fig. 2) of three-component separated sample B. Blue spectrum indicates upper layer while red spectrum indicates lower layer. The individual slice-selective 2D TOCSY spectra of each layer are reproduced in ESI as Fig. S5 and S6.† | ||
Fig. 4(a) depicts overlaid 2D TOCSY spectra of both the upper layer, in blue, and the lower layer, in red, of a final separated, three component sample (sample C). In this figure, a large range of chemical shifts with a broad dynamic range is depicted and the contour levels of the 2D plots have been adjusted to show as full a range of smaller, less intense, peaks as possible. These peaks are particularly evident in the lower layer, with a large number of small, sharp cross peaks between 3 and 5 ppm. These indicates that, practically, all of the bio-oil components are found in the aqueous layer.
Butanol is again partitioned between the upper and lower layers, more evenly than in the previous example, with ca. 50% in each layer. Identification and measurement of the butanol peaks is made easier by the improved resolution of the spectrum. This sample contains no marine gas oil. These observations are confirmed in Fig. 4(b), which depicts the 2D TOCSY of only the lower slice of the final separated sample for an expanded chemical shift range.
As with every 2D spectrum of the lower, aqueous, layer, there are many sharp peaks, with numerous cross peaks observed between 3 and 5 ppm. What are likely to be small chain alcohols can be observed the shadow of the intense butanol peaks at ca. 4 ppm. In addition, the horizontal lines along ca. 5.5 ppm suggests the presence of olefinic groups. Each distinct cross peak in the two-dimensional spectrum corresponds to coupling between two distinct proton environments within the same spin system. Further analysis and identification of individual components in the bio-oil component of the mixture could be achieved through use of machine learning tools applied to this large set of NMR data.
Conclusions
In this paper, the extension of slice-selective NMR using two-dimensional techniques has been successfully demonstrated. This extension should not end here. One of the main advantages of NMR spectrometry is the range of experiments and nuclei that can be studied. For a start, NMR techniques are certainly not limited to studying protons. One-dimensional carbon experiments, such as the DEPT family, both improve spectral resolution and can provide additional information about the different species present in the samples.30 Two-dimensional NMR techniques, of which TOCSY is only one example, have only been rarely used in the study of bio-fuels, bio-oils and related samples.31 Recently, a slice-selective 1H–13C HSQC experiment has been successfully demonstrated for metabolomics in highly heterogeneous samples.32 NMR techniques can also be used to study physical parameters of systems. Diffusion NMR produces information on the sizes of species present33 and the viscosity of the sample.34 The relaxation times of water peaks have been shown to be related to the pH of some micellar systems35 and could be used in such a manner in these bio-oil-based fuel samples. Both diffusion and relaxation techniques can act as filters, to remove unwanted signals belonging to either small36 or large37 species.This paper demonstrates the improved analysis of phase-separated samples by the successful implementation of slice-selective two-dimensional TOCSY NMR. Blended bio-oil samples are often unstable, separating into two distinct, often opaque, phases. By extending the spectra into a second dimension, the resolution of individual peaks are significantly improved and it is easier to identify specific species in the different layers of the sample. In addition, coupling information is now revealed, allowing for identification of more components in the mixtures and tentative assignments of compounds present. Improved analysis of how the components of any blended biofuel samples partition between the two layers is an important step towards understanding the separation processes and may provide insight into mitigating the problem. With the wider use of NMR techniques in studying biofuel samples as well as other separated or heterogeneous samples, slice-selective NMR techniques offer a powerful, additional analytical tool.
Data availability
Data supporting this article have also been included as part of the ESI.† All NMR data can be found at DOI: https://doi.org/10.17632/8rp2cvk7kn.2.Author contributions
JSK: investigation, visualisation, writing original draft. BT: supervision, writing and editing. KC: writing and editing. RE: conceptualisation, supervision, visualisation, resources, funding acquisition, writing, and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Jaskamal Singh Khangura received funding from the Royal Society of Chemistry Analytical Trust Fund (ACSS 23/0007). Bridget Tang was funded through an EPSRC DTP (EP/T518128/1).References
- J. N. Dumez, Prog. Nucl. Magn. Reson. Spectrosc., 2018, 109, 101 CrossRef CAS PubMed.
- J. Lambert, R. Hergenroder, D. Suter and V. Deckert, Angew. Chem., Int. Ed., 2009, 48, 6343 CrossRef CAS PubMed.
- C. Mantel, P.-A. Bayle, S. Hediger, C. Berthon and M. Bardet, Magn. Reson. Chem., 2010, 48, 600 CrossRef CAS PubMed.
- C. F. Pantoja, J. A. Bolanos, A. Bernal and J. Wist, Magn. Reson. Chem., 2017, 55, 519 CrossRef CAS PubMed.
- J. Allen and K. Damodaran, Magn. Reson. Chem., 2015, 53, 200 CrossRef CAS PubMed.
- C. Garcia-Aparicio, I. Quijada-Garrido and L. Garrido, J. Colloid Interface Sci., 2012, 368, 14 CrossRef CAS PubMed.
- Y. Mitrev, S. Simova and D. Jeannerat, Chem. Commun., 2016, 52, 5418 RSC.
- M. A. Wisniewska and J. G. Seland, J. Colloid Interface Sci., 2019, 533, 671 CrossRef CAS PubMed.
- R. Evans, A. Sandhu, A. V. Bridgwater and K. Chong, Energy Fuels, 2017, 31, 4135 CrossRef CAS.
- T. N. Hjartnes, G. H. Sørland, S. Simon and J. Sjöblom, Ind. Eng. Chem. Res., 2019, 58, 2310 CrossRef CAS.
- K. Seeger, Anal. Chem., 2021, 93, 1451 CrossRef CAS PubMed.
- A. C. Poppler, S. Frischkorn, D. Stalke and M. John, ChemPhysChem, 2013, 14, 3103 CrossRef PubMed.
- T. Niklas, D. Stalk and M. John, Chem. Commun., 2015, 51, 1275 RSC.
- S. A. Krachkovskiy, A. D. Pauric, I. C. Halalay and G. R. Goward, J. Phys. Chem. Lett., 2013, 4, 3940 CrossRef CAS.
- A. V. Bridgwater, Biomass Bioenergy, 2012, 38, 68 CrossRef CAS.
- D. Mohan, C. U. Pittman Jr and P. H. Steele, Energy Fuels, 2006, 20, 848 CrossRef CAS.
- E. Butler, G. Devlin, D. Meier and K. A. McDonnell, Renewable Sustainable Energy Rev., 2011, 15, 4171 CrossRef CAS.
- P. M. Mortensen, J. D. Grunwaldt, P. A. Jensen, K. G. Knudsen and A. D. Jensen, Appl. Catal., A, 2011, 407, 1 CrossRef CAS.
- A. H. Zacher, M. V. Olarte, D. M. Santosa, D. C. Elliott and S. B. Jones, Green Chem., 2014, 16, 491 RSC.
- M. Garcia-Perez, J. Shen, X. S. Wang and C. Z. Li, Fuel Process. Technol., 2010, 91, 296 CrossRef CAS.
- T. Y. Kim, S. Lee and K. Kang, Energy, 2015, 93, 2241 CrossRef CAS.
- M. Zhang and H. Wu, Energy Fuels, 2014, 28, 4650 CrossRef CAS.
- A. Krutof and K. Hawboldt, Renewable Sustainable Energy Rev., 2016, 59, 406 CrossRef CAS.
- K. J. Chong and A. V. Bridgwater, Environ. Prog. Sustainable Energy, 2017, 36, 677 CrossRef CAS.
- A. Alcala and A. V. Bridgwater, Fuel, 2013, 109, 417 CrossRef CAS.
- C. A. Mullen, G. D. Strahan and A. A. Boateng, Energy Fuels, 2009, 23, 2707 CrossRef CAS.
- N. Hao, H. Ben, C. G. Yoo, S. Adhikari and A. J. Ragauskas, Energy Fuels, 2016, 30, 6863 CrossRef CAS.
- C.-Y. Lin, Energies, 2013, 6, 4945 CrossRef.
- L. Emsley and G. Bodenhausen, Chem. Phys. Lett., 1990, 165, 469 CrossRef CAS.
- D. M. Doddrell, D. T. Pegg and M. R. Bendall, J. Magn. Reson., 1982, 48, 323 CAS.
- J. B. Nielsen, A. Jensen, C. B. Schandel, C. Felby and A. D. Jensen, Sustainable Energy Fuels, 2017, 1, 2006 RSC.
- D. H. Lysak, W. Bermel, V. Moxley-Paquette, C. Michal, R. Ghosh-Biswas, R. Soong, B. Nashman, A. Lacerda and A. J. Simpson, Anal. Chem., 2023, 95, 14392 CrossRef CAS PubMed.
- R. Evans, Z. Deng, A. K. Rogerson, A. S. McLachlan, J. J. Richards, M. Nilsson and G. A. Morris, Angew. Chem., Int. Ed., 2013, 52, 3199 CrossRef CAS PubMed.
- W. Li, G. Kagan, R. Hopson and P. G. Williard, J. Chem. Educ., 2011, 88, 1331 CrossRef CAS.
- N. A. Halliday, A. C. Peet and M. M. Britton, J. Phys. Chem. B, 2010, 114, 13745 CrossRef CAS PubMed.
- N. Esturau and J. F. Espinosa, J. Org. Chem., 2006, 71, 4103 CrossRef CAS PubMed.
- J. A. Aguilar, M. Nilsson, G. Bodenhausen and G. A. Morris, Chem. Commun., 2012, 48, 811 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Pulse sequences used and additional NMR spectra. See DOI: https://doi.org/10.1039/d4ay01006j |
| This journal is © The Royal Society of Chemistry 2024 |