 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly selective solid–liquid extraction of microplastic mixtures as a pre-preparation tool for quantitative nuclear magnetic resonance spectroscopy studies†
Marcel
Günther
 and
Wolfgang
Imhof
and
Wolfgang
Imhof
 *
*
Institute of Integrated Natural Sciences, University Koblenz, Universitätsstr. 1, D-56070 Koblenz, Germany. E-mail: imhof@uni-koblenz.de
First published on 27th September 2024
Abstract
Despite various developments in the application of quantitative nuclear magnetic resonance (qNMR) spectroscopy toward microplastics in recent years, this method still lacks suitable sample preparation and fractionation procedures. As this poses a crucial obstacle for its utilisation on environmental samples, which contain various mixtures of polymers along with other matrix substances, this research aims to address this missing link by presenting an easy-to-apply procedure based on common laboratory equipment. The process selectively separates microplastics from inorganic constituents while performing the necessary fractionation of different types of microplastics prior to qNMR analysis. It allows subsequent quantification of polystyrene (PS), polybutadiene rubber (BR), polymethylmethacrylate (PMMA), polyvinylchloride (PVC), polyethylene terephthalate (PET) and polyamide (PA) from a single sample, establishing recovery rates greater than 88% for all tested polymer types. Additionally, we extended our previous qNMR protocol to include two common polymer types: polymethylmethacrylate (PMMA) and polyacrylonitrile (PAN), achieving limits of detection down to 1.76 μg ml−1 and 12.53 μg ml−1 as well as limits of quantification down to 5.88 μg ml−1 and 41.78 μg ml−1, respectively. Thus, the qNMR method presented herein is now applicable to eight abundant polymer types, allowing the quantification of up to three different types simultaneously.
Introduction
Over the past decades, research on microplastic (MP) pollution has evolved into one of the major topics in environmental analytics. Alone, the number of publications on the term itself had an exponential increase from 2015 to 2022, by a factor of 19. Similar trends can be observed for various aspects related to MP as well.1 Despite this emerging interest, there is still no common definition of the term microplastic. Often, particle sizes between 5 mm and 1 μm are presented alongside physical and chemical aspects such as polymeric structure and biological persistence.2,3 However, depending on the applied methods and targets, these definitions can be significantly altered.Among other things, these variations emerge from the fast dynamics of MP-related analysis. The most commonly applied methods include particle-based techniques like infrared (IR) or Raman spectroscopy on one hand and mass-based methods like pyrolysis gas chromatography coupled with mass spectrometry (pyr-GC/MS) or thermoextraction and desorption (TED)-GC/MS on the other hand.4–6 Unfortunately, there is no method available yet that encompasses all possible types of MP. Thus, increasing numbers of methods other than the common ones or additional tools for them are being developed to overcome certain obstacles.5
In recent years, nuclear magnetic resonance (NMR) spectroscopy, although initially utilised mainly for qualitative studies, has also been considered for the quantitative determination of MP. The first applications were published by Peez et al. in 2019, followed by further optimisation and implementation of various tools in subsequent years by other groups like Nelson et al. and Papini et al.7–9 By now, so-called qNMR procedures have been published for various polymers including polystyrene (PS), polyamide (PA), polyethylene terephthalate (PET), acrylonitrile–butadiene–styrene copolymer (ABS), low-density polyethylene (LDPE), polyvinylchloride (PVC), styrene–butadiene copolymer (SBR) and polybutylene adipate terephthalate (PBAT).7–12 These studies were performed using high-field as well as low-field devices (benchtop NMR), covering deuterated and non-deuterated solvents (NoD), and have been combined with chemical digestion protocols and peak fitting calculations. Hence, a highly versatile toolbox is available.
Despite these developments, and although details of the first applications in environmental studies have been published, qNMR still lacks sufficient and well-defined sample preparation techniques for MP analysis.7,8 Most available publications describe testing of only a few polymer types and do not include further fractionation or enhanced purification processes. Both aspects are necessary when applied to mixtures of different polymer types, which also contain other organic and inorganic matter, as is typical of environmental samples.
In this study, we aim to focus on the fractionation of polymer mixtures prior to qNMR analysis. Numerous techniques have already been developed for other analytical methods like IR or pyr-GC/MS. The simplest and therefore the most common procedure is density separation. In a review by Bellasi et al., about 73% of the studies considered therein use this kind of MP separation.13 In this method, samples are stirred in saline solutions of various densities. Thus, lighter particles agglomerate at the surface.14 There are even specifically designed devices to perform such separations.15 Despite easy handling and application, this procedure suffers from various drawbacks. As many different salts can be used for separation, no standardised protocol has been established yet. In addition, common sodium chloride solutions do not achieve densities sufficient for heavier polymers like polyethylene terephthalate (PET). Instead, either hazardous or expensive salts, such as zinc chloride or sodium iodide, are often required. Finally, the density of MP can change due to weathering and fouling, either disabling such protocols completely or necessitating additional treatment. All these aspects lead to highly varying results between different studies.13,14,16
Similar to density separation, oil-based procedures have also been proposed recently. Different from saline solutions, the hydrophobic properties of most polymers are utilised for separation instead of their density.17 Unfortunately, despite avoiding toxic chemicals and being cheaper than heavy saline solutions, additional detergents, alcohols or related additives are often still required. So, according to He et al., most procedures require further development.18
Another upcoming separation method, following promising research by Fuller and Gautam, is called pressurised liquid extraction (PLE).19 This method is a variant of solid–liquid extractions, in which elevated pressures and temperatures lead to complete or partial solubilisation or emulsification of particles. This way, extraction rates can be enhanced as well as extraction times reduced. Although this is increasingly considered a more suitable method of MP extraction from environmental matrices based on the literature and our preliminary testing, the technique is less selective than extractions under atmospheric conditions.19–21 As we also faced difficulties like subsequent crosslinking under such harsh conditions, we concluded that PLE does not allow sufficient fractionation of MP for qNMR.
Still, as polymers need to be dissolved to be measured by qNMR, considering the aforementioned methods, solid–liquid extraction emerges as the most suitable concept. However, in differing from PLE, harsh conditions must be avoided to allow NMR-specific fractionation and subsequent reconstitution before measurements. Such a procedure will take slightly more time but, at the same time, will enhance the specific dissolution properties of the applied solvents.
A similar extraction method, although with a different intention, has already been proposed by Castelvetro et al. in 2021 using Soxhlet extraction.22 They utilised DCM and xylene for the extraction of PS, PET and LDPE, combined with depolymerisation of PET prior to analysis. However, the protocol was developed as a sample preparation method for size exclusion chromatography (SEC) analysis and is thus not suitable for qNMR.
In our recent paper, we combined multiple polymer types in one shared solvent, allowing simultaneous quantification of PS, butadiene rubber (BR) and PVC as well as PET and PA.23 In the current study, we extend our qNMR method to include two additional polymer types, polymethylmethacrylate (PMMA) and polyacrylonitrile (PAN), incorporating them into suitable fractions. Furthermore, we present a protocol that is able to sufficiently fractionate six out of eight tested polymer types using a conceptional solid–liquid extraction process avoiding elevated pressures and using moderate temperatures. Finally, various issues related to the remaining polymers and how these might be overcome in future research are discussed.
Experimental
Materials
Eight types of polymers are investigated: polystyrene (PS) as expanded beads with sizes of 0.5–1 mm, provided by Kissenwelt, Germany; polybutadiene rubber (BR) with a MW of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–300
000–300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, cut from bulk mass and purchased from Sigma-Aldrich Chemie GmbH, Germany; PMMA as a powder with grain sizes of 55–85 μm provided by Voxeljet AG, Germany; polyvinylchloride (PVC) as a powder with particle sizes <250 μm provided by Werth-Metall, Germany; and polyethylene terephthalate (PET) as fibres with length 500 μm and diameters of 10–20 μm, polyamide (PA) as fibres with length of 500 μm and diameters of 27–30 μm, low-density polyethylene (LDPE) as fibres with lengths of 0.5–0.8 mm and width of 40 μm and polyacrylonitrile (PAN) as fibres with length of 2 mm and width of 18 μm, all provided by Schwarzwälder Textil-Werke Heinrich Kautzmann GmbH, Germany. All listed polymers were not produced to serve as reference materials and are therefore of technical quality.
000 g mol−1, cut from bulk mass and purchased from Sigma-Aldrich Chemie GmbH, Germany; PMMA as a powder with grain sizes of 55–85 μm provided by Voxeljet AG, Germany; polyvinylchloride (PVC) as a powder with particle sizes <250 μm provided by Werth-Metall, Germany; and polyethylene terephthalate (PET) as fibres with length 500 μm and diameters of 10–20 μm, polyamide (PA) as fibres with length of 500 μm and diameters of 27–30 μm, low-density polyethylene (LDPE) as fibres with lengths of 0.5–0.8 mm and width of 40 μm and polyacrylonitrile (PAN) as fibres with length of 2 mm and width of 18 μm, all provided by Schwarzwälder Textil-Werke Heinrich Kautzmann GmbH, Germany. All listed polymers were not produced to serve as reference materials and are therefore of technical quality.
As for solvents, tetrahydrofuran (THF) ≥99.5%, trifluoroacetic acid (TFA) ≥99.9%, trifluoroethanol ≥99.8%, chloroform (CHCl3) ≥99.5% and xylene (isomers) ≥98% were purchased from Carl Roth GmbH & Co. KG, Germany; formic acid (FA) 98% was purchased from AppliChem GmbH, Germany; hexamethyl disiloxane (HMDSO) 99.7% (NMR grade) was purchased from Thermo Fisher Scientific Inc., Germany; deuterated chloroform (CDCl3) ≥99.8% over Ag was purchased from Deutero GmbH, Germany.
Annealed sea sand (approximately 0.1–0.3 mm; cleaned with acid) was purchased from Köhler GmbH, Germany.
Extraction setup and general operation
A glass frit (porous 4; 10–16 μm), which functions as an extraction vessel, is placed into a narrowly encasing beaker of 50 ml volume. Into that frit, a stirring bar, almost the same length as the diameter of the glass frit, is added for agitation. To exclude any contamination, the beaker is sealed with a watch glass or aluminium foil whenever possible. Samples are filled within the glass frit, while the whole beaker is placed in a water or oil bath during operation to allow heating. Solvents are added manually into the glass frit. After each extraction cycle, lifting the frit allows the liquid to run into the beaker, where it can easily be collected. Extracts are collected in separate vials. In addition, the frit and thus the sample are rinsed once with approximately 5 ml of solvent after each cycle, and this liquid is added to the extract. Afterwards, the frit can be lowered back into the beaker to perform another extraction cycle. Before changing the extracting solvents, the setup is dried at room temperature for at least one hour. After each experiment, the setup is thoroughly cleaned by rinsing with solvents able to dissolve the applied polymers, as well as using mechanical tools. An example of the setup is depicted in Fig. S1 in the ESI.† Preliminary experiments were partially performed using the automated extraction system “EDGE” by CEM, as stated.Extraction of samples
The polymers PS, BR, PMMA, PVC, PET, PA, PAN and LDPE are tested for extraction using THF, TFA in chloroform (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80), FA in chloroform (40
80), FA in chloroform (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) and xylene as extraction solvents. 6 g of annealed sea sand, acting as a model for inorganic constituents, is mixed with each polymer sample and placed into a glass frit.
60) and xylene as extraction solvents. 6 g of annealed sea sand, acting as a model for inorganic constituents, is mixed with each polymer sample and placed into a glass frit.
We did not investigate the removal of organic matter as part of this publication. The content and composition of organic matter can vary significantly depending on the type of sample and the sampling site. Freshwater sampled from a river or lake will depict much lower organic matter content than a wastewater sample from a treatment plant. Thus, such removal protocols need to be adjusted accordingly. The aim of this paper is to establish a general fractionation method as preparation for subsequent qNMR analysis, which is meant to be applied after an appropriate digestion protocol. An overview of possible protocols suitable for this purpose is given by Pfeiffer and Fischer24 as well as Thomas et al.25
First, individual extractions are performed by adding only one polymer type at a time and extracting it with just a single type of solvent. Hence, 10 to 15 mg of each polymer is weighed into the frit. In total, three repetitions are performed for each tested polymer and solvent. Additionally, three blanks are analysed for each solvent, without any polymer. Following this, 15 mixed samples are analysed by adding all eight polymer types in one shared frit. Again, 10 to 15 mg of each polymer is added, preparing three sets of five repetitions as well as three blanks not containing any polymer. The mixed samples are subsequently extracted using THF first, followed by TFA/CHCl3, FA/CHCl3 and finally xylene for the first 10 samples (Md1–Md10), and for the last five samples (Mp11–Mp15), THF is used first, followed by pure FA and finally TFA/CHCl3.
For each solvent, two cycles are conducted, each heated at 50 °C for 30 min. In the case of xylene, the setup was heated to 130 °C. 15 ml of solvent is added in each extraction cycle together with 5 ml of solvent for rinsing afterwards. Thus, in total 40 ml of extract are collected for each solvent and sample.
NMR preparation
For a detailed description of the calibration and quantification method, please refer to our previous paper.23 Calibration data for PS, BR, PET, PA and PVC are given in the ESI.† As NMR solvents, TFA/TFE (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) is used for PET, PA and PAN, CDCl3 is used for PS, BR and PMMA, and non-deuterated THF is used for PVC. For the mixed samples, PS and BR are partially measured in non-deuterated THF, as described below. In all cases, 0.1 vol% HMDSO is added to each NMR solvent as an internal standard. The solvent from each extract is evaporated at 60 °C under a gentle continuous stream of air for about one hour. Afterwards, the residues, except for those from the mixed samples extracted with THF, are reconstituted in at least 1 ml of the corresponding NMR solvent. Samples with concentrations expected to exceed the calibrated concentration range are further diluted, respectively. Dried THF extracts from mixed samples are instead first dissolved in 2 ml of THF before being split into 2 fractions of 1 ml each. These are dried as previously described and treated similarly to the other samples. One fraction is measured in THF, whereas the other one is measured in CDCl3. For NMR measurements, 0.7 ml of these samples is filled into NMR tubes and sealed with PTFE caps.
20) is used for PET, PA and PAN, CDCl3 is used for PS, BR and PMMA, and non-deuterated THF is used for PVC. For the mixed samples, PS and BR are partially measured in non-deuterated THF, as described below. In all cases, 0.1 vol% HMDSO is added to each NMR solvent as an internal standard. The solvent from each extract is evaporated at 60 °C under a gentle continuous stream of air for about one hour. Afterwards, the residues, except for those from the mixed samples extracted with THF, are reconstituted in at least 1 ml of the corresponding NMR solvent. Samples with concentrations expected to exceed the calibrated concentration range are further diluted, respectively. Dried THF extracts from mixed samples are instead first dissolved in 2 ml of THF before being split into 2 fractions of 1 ml each. These are dried as previously described and treated similarly to the other samples. One fraction is measured in THF, whereas the other one is measured in CDCl3. For NMR measurements, 0.7 ml of these samples is filled into NMR tubes and sealed with PTFE caps.
NMR measurements
Measurements were performed at room temperature using a JEOL® 500 spectrometer with a 500 MHz 5 mm TH ATM probe head. Standard proton sequences were selected for measurements in CDCl3, while measurements in THF and TFA/TFE were performed using automated 1H WET sequences provided by JEOL. The detailed parameters are as follows — CDCl3: standard 1H sequence, scans: 25, acquisition time: 4.36767 s, relaxation delay: 15 s, angle: 90°, receiver gain: 56, set off: 5 ppm, spectral width: 15 ppm, acquired size: 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 768; TFA/TFE: 1D WET (automated JEOL sequence) [target: TFA, signals: 1], scans: 25, acquisition time: 4.36767 s, relaxation delay: 15 s, receiver gain: 36, set off: 5 ppm, spectral width: 15 ppm, acquired size: 32
768; TFA/TFE: 1D WET (automated JEOL sequence) [target: TFA, signals: 1], scans: 25, acquisition time: 4.36767 s, relaxation delay: 15 s, receiver gain: 36, set off: 5 ppm, spectral width: 15 ppm, acquired size: 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 768; THF: 1D WET (automated JEOL sequence) [target: THF, signals: 2], scans: 25, acquisition time: 4.36767 s, relaxation delay: 15 s, receiver gain: 46, set off: 5 ppm, spectral width: 15 ppm, acquired size: 32
768; THF: 1D WET (automated JEOL sequence) [target: THF, signals: 2], scans: 25, acquisition time: 4.36767 s, relaxation delay: 15 s, receiver gain: 46, set off: 5 ppm, spectral width: 15 ppm, acquired size: 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 768.
768.
Data analysis
NMR spectra are processed using the software “MestReNova”.26 Baseline correction and phase correction were performed manually. Apodisation was set to 0.5 Hz. The following integration ranges were selected — in CDCl3: HMDSO = −0.25–0.35 ppm, PMMA = 3.50–3.70 ppm, BR = 5.05–5.70 ppm, PS = 6.10–6.84 ppm [referenced to CDCl3 at 7.25 ppm]; in THF: HMDSO = 0.00–0.10 ppm, PVC = 4.28–4.70 ppm, BR = 5.05–5.70 ppm, PS = 6.33–6.78 ppm [referenced to HMDSO at 0.07 ppm]; in TFA/TFE: HMDSO = −0.25–0.25 ppm, PAN = 2.75–3.115 ppm and 1.71–2.11 ppm, PA = 3.115–3.25 ppm and 2.20–2.50 ppm, PET = 7.70–7.90 ppm [referenced to HMDSO at 0.08 ppm]. Further calculations were performed according to our previous paper.23Calculations were performed in “Excel”. Mean values were calculated as arithmetic averages, and standard deviations are based on repetitions. Recovery rates were calculated as the mass quantified after extraction divided by the initial mass weighed into the frit.
Results and discussion
qNMR protocol extension to PMMA and PAN
In our previous publication, we validated a qNMR method for the analysis of PS, BR, PVC, PET and PA.23 Here, we will add another two important types of microplastic polymers to this method. Hence, the following part will focus on the validation of qNMR methods for the quantification of PMMA and PAN (Fig. 1, Tables 1 and 2). For detailed information on PS, BR, PVC, PET and PA, please refer to the ESI.† | ||
| Fig. 1 Calibration curve (––) of PMMA (left) and PAN (right), including confidence intervals (---), calibration samples (×) and model samples (○). | ||
| Polymer | PMMA | PAN |
|---|---|---|
| Solvent | CDCl3 | TFA/TFE (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
| Calibration range [mg mL−1] | 0.5 to 2.5 | 0.5 to 2.5 |
| Linearity r2 | 0.99965 | 0.99974 |
| RMSD | 0.00667 | 0.00005 |
| LOQ [μg mL−1] | 5.88 | 41.78 |
| LOD [μg mL−1] | 1.76 | 12.53 |
| Model sample | m true [mg] | M calc. [mg] | Bias [%] | RSD [%] |
|---|---|---|---|---|
| PMMA1 | 2.15 | 2.06 | 95.9 | 99.9 |
| PMMA2 | 0.87 | 0.85 | 98.2 | 99.8 |
| PMMA3 | 1.47 | 1.39 | 94.6 | 99.9 |
| PAN1 | 0.96 | 0.94 | 98.1 | 99.7 |
| PAN2 | 1.66 | 1.64 | 98.4 | 99.9 |
| PAN3 | 1.84 | 1.74 | 94.6 | 99.4 |
PMMA exhibits two signals within the NMR spectrum (Fig. S2†), both arising from methyl groups either attached directly to the polymer backbone at 2.07 ppm or bound to an ester group at 3.59 ppm. As these are singlet-shaped signals representing three protons, sharp and narrow peaks are established enabling high sensitivity and good baseline separation in further calibrations. Other signals within the spectra originating from PS, BR and HMDSO do not overlap, BR being the closest one at 5.37 ppm. However, despite offering two well-separated signals, only the second one at 3.59 ppm should be used for quantifications. The methyl ester group is a functional group indicative of PMMA, whereas the methyl group attached to the backbone is also found in various other polymers.10 Furthermore, not only polymer material but also organic matter is likely to create signals in the region of 2 to 3 ppm, thus posing a risk of potential signal overlap and miscalculations.12 Unfortunately, as recently published in our previous study, PMMA, despite being soluble in THF, could not be included in the mixture of PS, BR and PVC in THF, because dominant THF signals within the spectra would completely cover both PMMA signals.23 Hence, quantification would not be possible in such a mixture.
Similar to PMMA, PAN depicts two signals in its spectrum (Fig. S3†), alternating with various signals arising from PA. Whereas the first signal at 1.89 ppm originates from a CH2 group, which is also part of various other polymers, as already explained for PMMA, the second signal at 3.06 ppm is caused by a single proton directly neighbouring the polymer-specific nitrile group. Nevertheless, due to the close PA signals and its rather broad shape of signals, quantifications of PAN should be treated with care in mixtures including PA. High concentrations of PA will lead to less distinct baseline separation and thus can cause minor miscalculations. As an alternative, PAN can be quantified in the absence of PET and PA, when measured in DMSO-d6 (Fig. S5†), although this increases the number of necessary measurements. Initially, the mix of PET and PA was measured in a solution containing TFA and FA at concentrations of 80% and 20%. However, although highly concentrated TFA dissolves PAN and would thus allow for potential combination of PET, PA and PAN, the presence of FA causes PAN to precipitate, as preliminary experiments have shown. To circumvent this issue, FA was replaced by TFE, while keeping a similar concentration of 80% TFA and 20% TFE. Analogous to FA, TFE, even at small concentrations, is able to dissolve PA in such a mixture, but does not cause precipitation of PAN. Hence, a solvent combination of TFA/TFE (80/20) allows the simultaneous quantification of PET, PA and PAN.
PMMA as well as PAN could be added to existing mixtures of common microplastics, broadening the spectrum of polymer types available for qNMR analysis. The precision and accuracy of detecting both polymers are in the range of 93% to 100%. For accuracy, similar values of 95.6–110% were obtained for PVC, PA and ABS by Peez et al.10 The precision data in their publication, on the other hand, were only evaluated by replicate data analyses instead of separate measurements. Thus, we can only compare the data depicted here with that in our previous publication, as very few reports of research data on MP measured by qNMR have been published so far. For PS, BR and PVC, we established precision values of 99.1–99.9% within replicates of the same day.23 The precision values of PAN and PMMA also fall within this range.
The LOD and LOQ of PMMA are comparable to those of PET, with both polymers showing values below 10 μg ml−1 (Table 1 and S3†), whereas PAN is comparable to PVC at values between 10 and 100 μg ml−1 (Table 1 and S5†). The difference can be attributed to the sharp signals of PMMA representing a higher number of protons and rather broad signals of PAN constituted by only one proton. Both properties are similar in the case of PET and PVC and directly impact the SNR, which is used to determine these values. Consequently, the number of scans must be increased if higher SNR values and thus lower LOD and LOQ values are required. Peez et al., for example, established, for the same number of scans, LOD and LOQ values in the range of 10–300 μg ml−1 for most of their examined polymer types, with only PET resulting in limits below 10 μg ml−1.10 Nelson et al., on the other hand, used 128 scans – around five times the number of scans – to establish LOD and LOQ values between 1 and 5 μg ml−1 for PBAT.8 Nevertheless, even higher LOD and LOQ values such as those for PAN are expected to be within the range of most environmental concentrations.27,28 At the same time, since we used rather fewer scans for our validation, a further reduction of these values should be possible, if required.
Compared to other analytical methods, in general, most techniques report the number of MP rather than quantifying their overall masses. Within this context, pyr-GC/MS represents the dominant technique for MP quantification.4,5 So far, only a limited number of studies have reported on the LOQ for PAN with values down to 0.4 μg.29 In the case of PMMA, even lower LOQ values in the range of 0.8–0.005 μg have been reported.30–32 Although these represent much lower limits compared to qNMR, two additional considerations must be taken into account. First, MP is quantified in terms of concentrations in NMR; thus, considering the least required volume for making a measurement (approximately 0.5 ml), the reported LOD and LOQ can already be halved in terms of absolute masses. Second, the SNR and thus LOD and LOQ can be adjusted by the number of scans. Depending on the applied sequence, only around 5 minutes of measurement time is required for the qNMR protocols reported here, much shorter than the time required for most comparable pyr-GC/MS methods. Therefore, with similar measurement times, comparable LOQ and LOD values might be achievable.
Nevertheless, the methods presented herein in combination with those described in our previous publications now allow the quantification of eight different types of polymers by qNMR, most of which can already be measured using only two fractions, specifically, PET, PA and PAN can be analysed in TFA/TFE and PS and BR, either in combination with PVC in THF or in combination with PMMA in CDCl3. Only for LDPE are individual measurements necessary, as described by Peez et al.9
Extraction method
As already explained in the introduction, the idea of our extraction procedure is to avoid harsh conditions such as in PLE to enhance selectivity and thus suitability for qNMR analysis. At the same time, handling, extraction times and economic aspects should be considered as well. We performed several preliminary experiments to determine the necessary requirements that must be fulfilled by an analytical workflow for the quantification of MP:• Resistance to acidic conditions (as solvents like TFA and FA are necessary for polymers like PET and PA).
• Agitation during extraction (as extraction times would be dependent on diffusion).
• Controlled heating (to accelerate dissolution without undesired side effects).
• Quantitative separation of liquid and solid phases.
• Minimisation of extraction volume.
• Easy solvent exchange (to avoid unnecessary sample transfers).
To our knowledge, the currently available automatic extraction devices most often only fulfil a limited number of the above-listed requirements. PLE devices, for example, are often severely damaged when acids like TFA and FA are used and lack agitation tools since extraction time is reduced by elevated pressure instead.19,20 The Soxhlet apparatus, on the other hand, also does not allow agitation of the sample itself, but even more, requires the solvent to be evaporated during the process.22 This can cause certain side effects, as will be explained later. Hence, we developed a manual setup based on common laboratory equipment, which satisfies the mentioned requirements and can thus prove the overall concept of our process.
Solvents for the extractions were chosen based on our knowledge from qNMR analysis as well as several other considerations. In general, availability, costs, selectivity for certain polymers, sufficient dissolution capacity and subsequent removal were the most dominant aspects to be considered. In addition, solvents should not alter any polymer, whether extracted or not, during the process, to allow step-by-step fractionation.
After extensive testing, THF, xylene as well as TFA and FA (both diluted with chloroform) were selected for extraction. THF has already been shown to have beneficial dissolution properties for PS, BR, PVC and PMMA during our preliminary NMR experiments. Its low boiling point allows easy removal by evaporation after the extraction without the need for elevated temperatures. Unfortunately, PMMA cannot be measured directly in THF. Later reconstitution in CDCl3 or splitting of extracts into several subsamples still enables analysis of PMMA and PVC in parallel. THF usually contains butylated hydroxytoluene (BHT) as a stabiliser, which might be concentrated during the process, as its boiling point is inherently higher. However, BHT signals do not overlap with any polymer signals in our described method except for those of PS.33 Proper selection of integration ranges allows quantification without interference. Alternatively, non-stabilised THF can be applied.
For the polymers PET and PA, diluted TFA and FA (either pure or well diluted with chloroform) were used as extraction solvents. Unfortunately, preliminary tests have shown that combining TFA and FA does not lead to sufficient extraction of the targeted polymers. In our tests, only mixtures of TFA and FA (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) without any additional organic solvent were able to dissolve both PA and PET at the same time. The addition of chloroform or dichloromethane, used to dilute pure TFA or FA in the case when both polymers are measured individually, caused PA to precipitate.9,10 A pure mixture, however, is not considered for extractions due to the high cost of the required volume of solvent. Thus, respective extractions have to be performed in two separate steps. TFA concentrations down to 20% and FA concentrations down to 40% can be used while still retaining sufficient dissolution properties for either PAN or PA. To minimise environmental hazards, larger volumes of TFA and chloroform were reclaimed by distillation. Low boiling points of chloroform and TFA allow easy evaporation of residual solvent afterwards. FA on the other hand, despite having a rather high boiling point, can be easily converted into a volatile ester by the addition of methanol.34
20) without any additional organic solvent were able to dissolve both PA and PET at the same time. The addition of chloroform or dichloromethane, used to dilute pure TFA or FA in the case when both polymers are measured individually, caused PA to precipitate.9,10 A pure mixture, however, is not considered for extractions due to the high cost of the required volume of solvent. Thus, respective extractions have to be performed in two separate steps. TFA concentrations down to 20% and FA concentrations down to 40% can be used while still retaining sufficient dissolution properties for either PAN or PA. To minimise environmental hazards, larger volumes of TFA and chloroform were reclaimed by distillation. Low boiling points of chloroform and TFA allow easy evaporation of residual solvent afterwards. FA on the other hand, despite having a rather high boiling point, can be easily converted into a volatile ester by the addition of methanol.34
Finally, xylene was considered a potential extraction solvent. It has already been used in several solubilisation protocols of mostly inert polymers like PE and PP.22,35 Other than for THF, TFA/CHCl3 and FA/CHCl3, higher temperatures were necessary, not only for accelerated dissolution but also to allow the dissolution of PE and PP in general. Likewise, these polymers precipitate after extraction when cooling down to room temperature.19 Removal of xylene requires distillation at reduced pressure, which can be easily accomplished using a rotational evaporator.
The extraction process can be primarily controlled by four to five parameters: agitation, temperature, volume of solvent and number of extraction cycles as well as extraction time. We used annealed sea sand to test the effects of inorganic matrices on our setup. Similar materials have already been used as reported in other publications on the extraction of MP for various reasons.19,20 In preliminary tests on the EDGE system (CEM), we observed low recovery rates, which we attributed primarily to insufficient agitation. Hence, in our case, sea sand was added to simulate insoluble material, which can be expected to be part of actual sediment samples. For optimal distribution, the agitation was set to 250 rpm in the manual setup. Higher agitation speed is not advised to prevent any overflow of solvent while stirring. By using a stirring bar, which covers almost the complete diameter of the frit, no dead zones were visible. Thus, high extraction rates as well as reproducibility could be achieved. Further details on our preliminary experiments are given in the ESI.†
Based on previous experiments, we set the temperature to 50 °C. At higher temperatures, dissolution is accelerated, but it can cause post-crosslinking of polymers, and some solvents, like FA, also carry the risk of decomposition.36,37 In particular for BR, we observed the formation of highly interconnected gels at elevated temperatures, which impaired later dissolution for NMR measurements. In initial tests on an “EDGE” system by CEM, these effects were visible for temperatures down to at least 80 °C. Precise measurements for these tests were not performed as the extracts could not be reconstituted after solvent evaporation. Furthermore, within a short time, the clogging of valves and tubes impaired further experiments using that device. Thus, we continued with the described conceptual manual setup. In addition, the applied temperature is limited by the boiling point of the extraction solvent, since evaporation during extraction should be avoided. Extraction at room temperature, however, would require longer extraction times.
The volume for each cycle was kept to a minimum since solvent removal is necessary. Furthermore, costs and environmental effects will be minimised that way. The exact volumes predominantly depend on the individual setup, as the solvent should at least cover the whole sample. For our experiments, 15 ml was sufficient in all cases. Moreover, for the presented polymer types, the maximum solubility is not expected to be exceeded, as within our experiments, stock solutions up to 0.1 g ml−1 were prepared without any issues, while typical environmental concentrations are much lower.27,28
Finally, the number of extraction cycles and the extraction time have to be adjusted. Therefore, we conducted several extraction experiments varying the number and duration of cycles taking BR as an example. BR was received as bulk material and showed the slowest dissolution properties out of all tested polymers. A brief illustration is provided in Fig. 2. A slight increase in recovery rates was observed between the first and second cycle. Further cycles, however, did not significantly alter the results. Therefore, we applied two extraction cycles of 30 minutes each in subsequent extractions.
 | ||
| Fig. 2 Compared recovery rates of BR extracted by THF using either one, two or three extraction cycles. | ||
We did not adjust the pressure during extraction for several reasons. First of all, applying different pressures would require a sealed apparatus, which is hard to design given standard laboratory equipment, while retaining easy handling as well as enabling heating and stirring at the same time. Second, we cannot exclude that the crosslinking effects on BR, which we observed during our experiments on the EDGE system, were at least partially caused by elevated pressures. Hence, we kept working at atmospheric pressure.
Individual extractions
As revealed by the results depicted in Table 3, THF gives almost perfect results regarding its application as an extraction solvent. Recovery rates of BR, PS, PVC and PMMA all exceed 90% while their standard deviations remain below 3%. Only for BR are slightly higher standard deviations of up to 8% observed, which still result in recovery rates greater than 90%. This minor uncertainty likely arises from the BR particles, made from bulk material, which are the most difficult to dissolve among all tested polymers due to their comparably small surface area. At the same time, partial post hoc crosslinking and therefore gel formation cannot be excluded during the process. While PS, BR, PVC and PMMA can be extracted in prominent amounts, other polymers, like PET and PA, remain unaffected by this extraction step. The NMR spectra after the extraction of all other polymers did show, if at all, only negligible traces of PET, PA, PAN or PE. This is important, as it allows further extraction steps to be applied, without impairing the recovery rates of the remaining polymers. As a result, THF can function as an extremely selective solvent for the extraction of PS, BR, PVC and PMMA.| MP | PS | BR | PMMA | PVC | PET | PA | PAN | LDPE |
|---|---|---|---|---|---|---|---|---|
| THF | ||||||||
| RRNMR,Ø [%] | 97.94 | 93.70 | 95.18 | 90.75 | <4.83 | <4.71 | <4.94 | n.d. |
| σ RR,NMR [%] | 2.71 | 7.41 | 1.02 | 1.73 | n.d. | n.d. | n.d. | n.d. |
| TFA/CHCl 3 (20/80) | ||||||||
| RRNMR,Ø [%] | 79.88 | 12.31 | 67.50 | <4.46 | 92.27 | <4.66 | <4.83 | n.d. |
| σ RR,NMR [%] | 17.50 | 7.40 | 8.28 | n.d. | 3.13 | n.d. | n.d. | n.d. |
| FA/CHCl 3 (40/60) | ||||||||
| RRNMR,Ø [%] | <4.51 | <4.27 | 99.16 | <4.55 | 12.59 | 83.35 | <4.74 | n.d. |
| σ RR,NMR [%] | n.d. | n.d. | 1.08 | n.d. | 2.36 | 11.05 | n.d. | n.d. |
| Xylene | ||||||||
| RRNMR,Ø [%] | 91.46 | 76.63 | 89.16 | 27.34 | <4.28 | <4.28 | <4.80 | n.d. |
| σ RR,NMR [%] | 2.94 | 16.60 | 1.55 | 0.47 | n.d. | n.d. | n.d. | n.d. |
TFA/CHCl3 was predominantly applied for the extraction of PET, but it was also used to study possible effects on other acid-sensitive polymers like PMMA, PA or PAN.24,38 First of all, PET was extracted with a 92.27% recovery rate and 3.13% standard deviation, as shown in Table 3. Therefore, not only excellent extraction rates but also high reproducibility could be achieved. These results are comparable to the previously discussed results from THF (Table 3). Unfortunately, similar to THF, no relevant amounts of PA and PAN could be extracted. Thus, although simultaneous measurement of these polymers would be possible based on their NMR properties, extraction with TFA does require further extractions using other solvents for their quantification. As TFA was diluted by CHCl3 to increase the operative extraction volume, PS, BR and PMMA were influenced by this extraction step. In all three cases, amounts between 12% and 80% were extracted. However, their extraction rates were most often very unpredictable, as shown by high standard deviations. Polymers like PS and BR seem to be affected by post hoc crosslinking under these acidic conditions as during reconstitution insoluble traces remain in their samples similar to those observed for BR at elevated temperatures. In the case of PMMA, cleavage of ester bonds could not be excluded.38 Overall, PET can be effectively extracted using TFA/CHCl3, without disturbing subsequent extractions of PA and PAN. For PS, BR and PMMA, on the other hand, severe influences can be observed; thus, these must be removed prior to TFA/CHCl3 application (Fig. 3).
Since only PS, BR, PVC, PMMA and PET were extracted using the previously mentioned solvents, FA/CHCl3, aimed for the extraction of PA, was also tested for its potential effects on the remaining PAN and PE. The recovery rates of PA are slightly worse than those for the previously described polymers, resulting in a mean value of only around 83%. At the same time, the standard deviation of 11% indicates somewhat higher deviations, resulting in decreased reproducibility. Similar to the use of TFA, several adverse effects were observed due to acidic conditions or partial dissolution in CHCl3. Thus, FA/CHCl3 should not be applied to samples containing PS, BR or PMMA. PAN, however, again was not detected. In contrast to PA in TFA/CHCl3, PET did show a slight response to extraction with FA/CHCl3. In the initial attempt, recovery rates of up to 15% were observed. Unfortunately, further optimisation of extraction conditions, such as varying the concentration of FA, increasing the temperature or extending the extraction time, did not lead to sufficient results for extraction purposes. Experiments in pure FA instead did not show any extraction of PET at all. Hence, PET and PA remain to be extracted in two separate steps.
Extractions using xylene were performed under comparably harsh thermal conditions of 130 °C. Preliminary tests indicated sufficient dissolution of polyethylene at this temperature. However, gravimetrical evaluations reveal rather uncertain recoveries on this basis. Respective results are provided in the ESI.† As for the other investigated polymers, quantifications by NMR reveal effective extractions for PS and PMMA as well as partial extractions for BR and PVC. Nevertheless, the extracted BR was affected by crosslinking, as discussed previously. PET, PA and PAN were not detected in relevant traces. Thus, although xylene might extract some polymers, other solvents like THF are more efficient for these types.
Polyethylene and polyacrylonitrile
Our initial attempt was aimed at designing a suitable extraction protocol, specifically tailored as a sample preparation method for subsequent qNMR analyses. Although we succeeded in this attempt for most of the selected polymer types, we faced various issues in our experiments with PAN and PE.Polymers like polyethylene and polypropylene have dissolution properties that allow liquid NMR measurements only within very strict boundaries. Usually, high temperatures are required to dissolve these polymers, which at the same time impair valid quantification by NMR in most cases due to solvent evaporation, causing altered concentrations as well as altered relaxation rates and difficulties in temperature control.39–41 Moreover, solvents with acceptable dissolution properties for PE and PP, like deuterated toluene or benzene, are rather expensive and not suitable for routine applications. On that account, NMR spectra of these polymers are mostly measured in the solid state or only qualitatively evaluated.42–44 Furthermore, they do not contain any specific functional groups that would support distinction from other polymers. Despite these facts, in 2019, Peez et al. published a method for the analysis of spiked polyethylene solutions by qNMR.9 However, this method was only tested on a specific type of low-density polyethylene and validated only using laboratory-based model samples, without any other polymer type being present. Most other PE materials will require higher dissolution temperatures, which then would not allow quantification at 60 °C. We therefore decided not to quantify PE by NMR. Instead, we performed gravimetric quantification after a final extraction step using xylene. Unfortunately, as the method was mainly designed for subsequent NMR analysis, not for gravimetric evaluations, these data – despite being based on measurements in THF, TFA/TFE and CDCl3 not containing any other polymers – are too inconsistent to allow valid quantification of PE (cf. the ESI†).
PAN on the other hand would allow potential analysis by qNMR as we demonstrated herein earlier. However, in this case, we were not able to establish a suitable extraction protocol. PAN is known for its high chemical resistance. Thus, PAN may only be dissolved in very specific solvents. Examples are dimethylformamide, dimethyl sulfoxide (DMSO) or dimethylacetamide.45 In initial tests, DMSO has been shown to readily dissolve PAN and would in principle also allow extraction of PA. However, the crucial issue arises in the later removal of these solvents. Their extremely high boiling points impair proper evaporation, and preliminary testing had shown that precipitation of PA caused by the addition of non-dissolving liquids did not lead to quantitative PA recovery. On the other hand, strong acids like TFA or concentrated nitric acid dissolve PAN as well, but their removal either leads to further concentration of these acids or is extremely expensive and hazardous. Hence, PAN had to be omitted from the extraction protocol as well.
Extraction of mixed samples
The results obtained from individual extractions already predefine a certain order of extraction steps. PS, BR, PVC, and PMMA were effectively extracted using THF with no other polymers being affected within this step. Other solvents like TFA or FA, however, caused severe side effects and did not lead to sufficient extraction rates. Thus, THF functions as an ideal starting point for the process. For the remaining polymers, PET, PA, PAN and PE, TFA/CHCl3 was initially selected for the second extraction step. This solvent can extract PET, keeping the other polymers unaffected. Ultimately, FA/CHCl3 was applied to the first ten samples (Md1–Md10) to extract the remaining PA. Within the initial five samples (Md1–Md5) in the case of extraction with FA/CHCl3 and TFA/CHCl3, some outliers were excluded from calculations due to recovery rates being far below 80%.Thus, another five samples (Md6–Md10) were extracted to verify the results obtained from the remaining samples. As FA/CHCl3 already extracts various amounts of PET, PS, PMMA and BR while causing side reactions similar to TFA/CHCl3, the idea was to apply this solvent after the separation of the respective polymer types. Unfortunately, as shown in Fig. 4, the extraction rates of PA drastically decreased even further in these additional five samples (Md6–Md10). PA is sensitive to strongly acidic conditions, which usually lead to depolymerisation.46 The previous application of TFA, although not extracting PA, has caused cleavage and perhaps further modifications that impair its subsequent dissolution in FA/CHCl3. Hence, in a final approach, we switched the order of TFA and FA, while using pure FA instead of a mixture diluted with CHCl3. Unlike in FA/CHCl3, PET was not affected by pure FA, making the extraction of PA prior to PET possible. Out of another five samples (Mp11–Mp15), sufficient recovery rates for PA and PET were established this time.
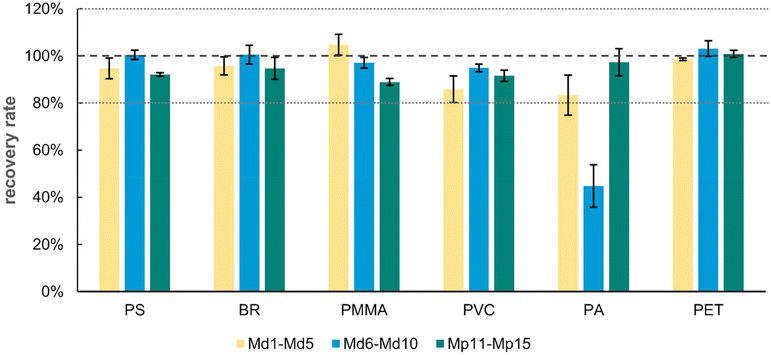 | ||
| Fig. 4 Recovery rates of PS, BR, PMMA, PVC, PA and PET after consecutive extractions with either THF, TFA/CHCl3, FA/CHCl3 and xylene (Md1–Md10) or THF, pure FA and TFA/CHCl3 (Mp11–Mp15). | ||
As explained previously, PAN and PE had to be omitted from the extraction protocol, as none of the tested solvents were able to extract relevant amounts of these polymers. The final order applied to the mixed samples, containing all eight described polymers, involves the subsequent extraction first by THF, followed by pure FA and completed by TFA/CHCl3 to yield a mixture of PS, BR, PVC and PMMA as the first fraction, a solution of PA in FA as the second fraction and a solution of PET in a TFA/CHCl3 mixture as the third fraction of the process.
Overall, the values shown in Table 4 are in agreement with the results obtained by previously conducted individual extractions. The only exception is the extraction of PA using FA/CHCl3. Among the individual extractions, PA already showed slightly lower recovery rates than the other polymers in their respective solvents. However, these results decreased even further in combination with other extraction steps. The final extraction order including pure FA before the application of TFA/CHCl3, on the other hand, again fits well with the individual extraction results.
| Sample | MP | PS | BR | PMMA | PVC | PETa | PA | PAN | LDPE |
|---|---|---|---|---|---|---|---|---|---|
| Solvent | THF | THF | THF | THF | TFA/CHCl3 | FA/CHCl3b | n.d. | Xylene | |
| a Samples Md2 and Md3 were removed as outliers, resulting in only three repetitions for PET. b FA was not diluted by chloroform for the extractions of samples Mp11–Mp15; instead pure FA was applied. | |||||||||
| Md1–5 | RRØ [%] | 94.67 | 95.78 | 104.71 | 85.85 | 98.54 | 83.32 | n.d. | n.d. |
| σ RR [%] | 4.36 | 3.85 | 4.42 | 5.65 | 0.50 | 8.48 | n.d. | n.d. | |
| Md6–10 | RRØ [%] | 100.44 | 100.50 | 97.07 | 94.87 | 103.09 | 44.78 | n.d. | n.d. |
| σ RR [%] | 1.98 | 4.00 | 2.26 | 1.60 | 3.33 | 9.00 | n.d. | n.d. | |
| Mp11–15 | RRØ [%] | 92.11 | 94.67 | 88.94 | 91.54 | 100.85 | 97.29 | n.d. | n.d. |
| σ RR [%] | 0.73 | 4.64 | 1.45 | 2.38 | 1.48 | 5.73 | n.d. | n.d. | |
Also, in comparison with other methods like Soxhlet and PLE, the results presented herein are fully competitive. Castelvetro et al. achieved recovery rates of 95.3–106.4% for PS and 96.4–98.2% for PET at quantities between 4.5 and 11 mg per polymer using a Soxhlet extraction of 3–6 h duration, whereas in our experiments PS results in slightly lower recoveries down to 92.1%, PET even exceeds their performance.22 A similar comparison can be drawn for the method of Fuller and Gautam.19 Within their PLE process, PE, PP, PS, PVC and PET were recovered at values between 101% and 111%, taking around 2 h for the extraction of 7–50 mg. Much lower polymer masses were extracted by Dierkes et al. using concentrations of 0.05–0.75 mg g−1.20 Hence, their recovery rates show stronger variations with 114–131% for PS, 85–95% for PP and 77–118% for PE.
In our extraction protocol, the final recovery rates for PS, BR, PVC, PMMA, PET and PA all exceed 88% while demonstrating high reproducibility. The process aimed to achieve recovery rates between 80% and 120%, which is a usual standard for trace analytical extractions and is completely fulfilled by our method.19 Additionally, no other adverse effects were observed from the application of multiple extraction steps involving various solvents, when following the aforementioned final order.
Conclusions
The fractionation of polymer types plays an important role for measuring MP by qNMR, as not every polymer is soluble in the same solvent. Thus, a suitable separation protocol is a key step in the measurement of environmental samples, where all different types of MP may be present simultaneously.Here, we presented a workflow that not only allows the respective separation but also can be adjusted depending on the polymer types present and which can easily compete with comparable extraction methods. The whole process can be performed in about 3–4 hours depending on whether the complete set of polymers needs to be analysed. Consequently, extraction times can be reduced even further. Compared to common Soxhlet and PLE processes, the extraction method presented here demonstrates similar or even faster performance, while separating up to six polymer types into three different fractions ready for qNMR analysis.22 Our method can make use of acidic solvents, required for selective PET and PA extraction. In comparable methods, these would either damage PLE extraction devices or pose a risk of solvent decomposition, when evaporation, like in Soxhlet extraction, takes place.37 The conditions applied during extraction do not differ from the ones applied in measurements. Thus, subsequent sample preparations are easy to handle, as only evaporation and later reconstitution by dissolution in NMR solvents are necessary. The method so far only covers a rather conceptual design; however, automation should be possible if required. At the same time, the use of commonly available laboratory equipment diminishes the need for expensive extraction devices and the procedure can be therefore readily applied in most laboratories without the need to invest in new laboratory devices. Finally, due to a stronger focus on selectivity, we are now in a position to target multiple polymers at once, which was not possible with qNMR.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- M. Davtalab, S. Byčenkienė and I. Uogintė, Environ. Sci. Pollut. Res., 2023, 30, 107403–107418 CrossRef PubMed.
- N. B. Hartmann, T. Hüffer, R. C. Thompson, M. Hassellöv, A. Verschoor, A. E. Daugaard, S. Rist, T. Karlsson, N. Brennholt, M. Cole, M. P. Herrling, M. C. Hess, N. P. Ivleva, A. L. Lusher and M. Wagner, Environ. Sci. Technol., 2019, 53, 1039–1047 CrossRef CAS PubMed.
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), EFSA J., 2016, 14, 1–30 Search PubMed.
- S. Belz, I. Bianchi, C. Cella, H. Emteborg, F. Fumagalli, O. Geiss, D. Gilliland, A. Held, U. Jakobsson, R. La Spina, D. Mėhn, Y. Ramaye, P. Robouch, J. Seghers, E. B. Sokull-Kluettgen, E. Stefaniak and J. Stroka, Current status of the quantification of microplastics in water Results of a JRC/BAM inter-laboratory comparison study on PET in water, Publications Office of the European Union, 2021 Search PubMed.
- S. Primpke, S. H. Christiansen, W. Cowger, H. De Frond, A. Deshpande, M. Fischer, E. B. Holland, M. Meyns, B. A. O'Donnell, B. E. Ossmann, M. Pittroff, G. Sarau, B. M. Scholz-Böttcher and K. J. Wiggin, Appl. Spectrosc., 2020, 74, 1012–1047 CrossRef CAS PubMed.
- D. He, Y. Luo, S. Lu, M. Liu, Y. Song and L. Lei, TrAC, Trends Anal. Chem., 2018, 109, 163–172 CrossRef CAS.
- G. Papini, G. Petrella, D. O. Cicero, C. Boglione and A. Rakaj, Mar. Pollut. Bull., 2024, 198, 115784 CrossRef CAS.
- T. F. Nelson, S. C. Remke, H.-P. E. Kohler, K. McNeill and M. Sander, Environ. Sci. Technol., 2020, 54, 266–275 CrossRef CAS.
- N. Peez, M. C. Janiska and W. Imhof, Anal. Bioanal. Chem., 2019, 411, 823–833 CrossRef CAS PubMed.
- N. Peez and W. Imhof, Analyst, 2020, 145, 5363–5371 RSC.
- N. Peez, T. Rinesch, J. Kolz and W. Imhof, Magn. Reson. Chem., 2022, 60, 172–183 CrossRef CAS.
- N. Peez, J. Becker, S. M. Ehlers, M. Fritz, C. B. Fischer, J. H. E. Koop, C. Winkelmann and W. Imhof, Anal. Bioanal. Chem., 2019, 411, 7409–7418 CrossRef CAS.
- A. Bellasi, G. Binda, A. Pozzi, G. Boldrocchi and R. Bettinetti, Chemosphere, 2021, 278, 130357 CrossRef CAS PubMed.
- M. A. Cashman, K. T. Ho, T. B. Boving, S. Russo, S. Robinson and R. M. Burgess, Mar. Pollut. Bull., 2020, 159, 111507 CrossRef CAS PubMed.
- H. K. Imhof, J. Schmid, R. Niessner, N. P. Ivleva and C. Laforsch, Limnol. Oceanogr.: Methods, 2012, 10, 524–537 CrossRef CAS.
- M. Constant, G. Billon, N. Breton and C. Alary, J. Hazard. Mater., 2021, 420, 126571 CrossRef CAS PubMed.
- E. M. Crichton, M. Noël, E. A. Gies and P. S. Ross, Anal. Methods, 2017, 9, 1419–1428 RSC.
- D. He, X. Zhang and J. Hu, J. Hazard. Mater., 2021, 409, 124640 CrossRef CAS.
- S. Fuller and A. Gautam, Environ. Sci. Technol., 2016, 50, 5774–5780 CrossRef CAS PubMed.
- G. Dierkes, T. Lauschke, S. Becher, H. Schumacher, C. Földi and T. Ternes, Anal. Bioanal. Chem., 2019, 411, 6959–6968 CrossRef CAS.
- J. Kamp, G. Dierkes, P. N. Schweyen, A. Wick and T. A. Ternes, Environ. Sci. Technol., 2023, 57, 4806–4812 CrossRef CAS.
- V. Castelvetro, A. Corti, S. Bianchi, G. Giacomelli, A. Manariti and V. Vinciguerra, Environ. Pollut., 2021, 273, 115792 CrossRef CAS.
- M. Günther and W. Imhof, Analyst, 2023, 148, 1151–1161 RSC.
- F. Pfeiffer and E. K. Fischer, Front. Environ. Sci., 2020, 8, 572424 CrossRef.
- D. Thomas, B. Schütze, W. M. Heinze and Z. Steinmetz, Sustainability, 2020, 12, 1–28 Search PubMed.
- MestReNova (14.1.1 – 24571) Mestrelab Research S.L., Santiago de Compostela, 2019.
- C. Dibke, M. Fischer and B. M. Scholz-Böttcher, Environ. Sci. Technol., 2021, 55, 2285–2295 CrossRef CAS PubMed.
- S. Lasee, J. Mauricio, W. A. Thompson, A. Karnjanapiboonwong, J. Kasumba, S. Subbiah, A. N. Morse and T. A. Anderson, Integr. Environ. Assess. Manage., 2017, 13, 528–532 CrossRef CAS PubMed.
- S. J. Lim, Y.-K. Park, H. Kim, J. Kwon, H. M. Moon, Y. Lee, A. Watanabe, N. Teramae, H. Ohtani and Y.-M. Kim, Chem. Eng. J., 2022, 434, 134653 CrossRef CAS.
- H. Li, Q. Wu, J. Ng, D. Yu, S. H. Chan and A. Li, Anal. Bioanal. Chem., 2022, 414, 6647–6656 CrossRef CAS PubMed.
- M. Fischer and B. M. Scholz-Böttcher, Anal. Methods, 2019, 11, 2489–2497 RSC.
- X.-X. Zhou, S. He, Y. Gao, H.-Y. Chi, D.-J. Wang, Z.-C. Li and B. Yan, Environ. Sci. Technol., 2021, 55, 3032–3040 CrossRef CAS.
- G. R. Fulmer, A. J. M. Miller, N. H. Sherden, H. E. Gottlieb, A. Nudelman, B. M. Stoltz, J. E. Bercaw and K. I. Goldberg, Organometallics, 2010, 29, 2176–2179 CrossRef CAS.
- D. Kaiser, L. Beckmann, J. Walter and M. Bertau, Catalysts, 2021, 11, 869 CrossRef CAS.
- H. Liu, B. Pan, J. Zhang, Z. Huang, P. Li and J. Shen, J. Sep. Sci., 2024, 47, 2300253 CrossRef CAS.
- O. Chiantore, M. P. L. di Cortemiglia, M. Guaita and G. Rendina, Makromol. Chem., 1989, 190, 3143–3152 CrossRef CAS.
- H. N. Barham and L. W. Clark, J. Am. Chem. Soc., 1951, 73, 4638–4640 CrossRef CAS.
- J. Semen and J. B. Lando, Macromolecules, 1969, 2, 570–575 CrossRef CAS.
- S. K. Bharti and R. Roy, TrAC, Trends Anal. Chem., 2012, 35, 5–26 CrossRef CAS.
- H. Pasch, L.-C. Heinz, T. Macko and W. Hiller, Pure Appl. Chem., 2008, 80, 1747–1762 CrossRef CAS.
- A. J. Hadi, G. F. Najmuldeen and K. Bin Yusoh, J. Polym. Eng., 2013, 33, 471–481 CAS.
- Å. G. Larsen, K. Olafsen and B. Alcock, Polym. Test., 2021, 96, 107058 CrossRef.
- Y. Teymouri, A. Adams and B. Blümich, Eur. Polym. J., 2016, 80, 48–57 CrossRef CAS.
- R. C. Ferguson, Macromolecules, 1971, 4, 324–329 CrossRef CAS.
- M. Hattori, H. Yamazaki, M. Saito, K. Hisatani and K. Okajima, Polym. J., 1996, 28, 594–600 CrossRef CAS.
- D. S. Achilias, L. Andriotis, I. A. Koutsidis, D. A. Louka, N. P. Nianias, P. Siafaka, I. Tsagkalias and G. Tsintzou, in Material Recycling - Trends and Perspectives, ed. D. S. Achilias, InTech, 2012 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Calibration data for PS and BR in CDCl3, for PS, BR and PVC in THF and for PA and PET in TFA/TFE; 1H-NMR spectra as examples for measurements in THF, CDCl3, TFA/TFE and DMSO-d6; extensive extraction data, including each repetition, and gravimetric data for each extraction. See DOI: https://doi.org/10.1039/d4an00991f |
| This journal is © The Royal Society of Chemistry 2024 |

