Open Access Article
Open Access ArticleProgress on 2D–2D heterostructured hybrid materials for efficient electrocatalysis
Xia
Long
 *a,
Lihua
Zhang
ab,
Zheng
Tan
a and
Bowei
Zhou
a
*a,
Lihua
Zhang
ab,
Zheng
Tan
a and
Bowei
Zhou
a
aChina-UK Low Carbon College, Shanghai Jiao Tong University, No. 3, Yinlian Road, Lingang, Shanghai 201306, China. E-mail: x.long@sjtu.edu.cn
bSchool of Mechanical Engineering, Shanghai Jiao Tong University, Shanghai, 200240, China
First published on 26th December 2022
Abstract
Two-dimensional (2D) materials are widely used in many energy conversion reactions, especially 2D–2D heterostructured hybrid materials that have been extensively investigated in recent years. In this review article, we provide a comprehensive review of the recent research progress on 2D–2D hybrid materials, starting from the introduction of these emerging hybrids. Then, the fabrication strategies are discussed with their pros and cons. Furthermore, the techniques, especially those for heterointerface characterization, are highlighted. Afterward, the applications of these 2D–2D heterostructured hybrid materials in energy conversion reactions with representative examples in the last five years are discussed and summarized. Finally, a summary and perspectives for realizing the rational design and synthesis of 2D–2D hybrids as advanced electrocatalysts towards active and stable energy conversion reactions are provided.
Introduction
Developing renewable green energy sources is one of the most critical topics in material- and energy-related fields nowadays, due to the growing demand for energy and increasing concerns regarding environmental pollution problems.1,2 Meanwhile, the generation and conversion efficiency of these renewable energy systems largely depend on the effectiveness of the catalysts. Among the developed functional materials for energy conversion reactions, such as hydrogen production via water splitting, carbon dioxide reduction to form valuable chemicals and nitrogen reduction to form ammonia, etc., two-dimensional (2D) materials offer unique advantages for these processes due to their large specific surface area, high charge migration rate, adjustable electronic properties, ultralight weight, assembly properties to form heterostructures, high mechanical flexibility, etc.3–8Actually, 2D materials have boomed since the successful fabrication of graphene from graphite in 2004 by Novoselvo and co-workers.9 Since then, many 2D materials have been developed such as MXenes,10,11 layered double hydroxides (LDHs),8,12,13 transition metal-based dichalcogenides (TMDs),14–17 black phosphorus,18–20 covalent-organic frameworks (COFs)21,22 metal–organic frameworks (MOFs),23–25 metallenes,26 borophene,27etc.28,29 The unique physical, electronic and chemical properties of these 2D materials mean that they can be expected to have rich and exciting structural and physiochemical properties, making them promising for numerous applications in electronics, sensing, photonics, catalysis, bioengineering, energy harvesting, flexible electronics, etc.3,7,8,30
Furthermore, based on the unique assembly properties of 2D materials, they can be easily combined with other functional materials to form heterostructured hybrids that would give rise to intriguing features, such as integrating the merits and overcoming the weaknesses of the individual counterparts and even generating new properties or functions.31–33
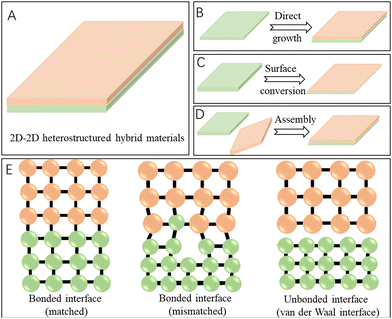
Generally, among all the 2D heterostructured hybrids, the 2D–2D heterostructures have the largest interfacial contact area and the strongest interactions between the two distinct components (Fig. 1A), when compared with the 0D–2D and 1D–2D heterostructures. So, the coupling of the heterointerfaces in the face to face stacking of 2D–2D hybrid materials would be intense, which would further benefit the rearrangement of the electronic structures of the active sites and facilitate the charge transfer during energy conversion reactions, thus contributing to improved catalytic performance.34,35 Owing to the above-mentioned merits (Table 1), the 2D–2D heterostructured hybrid materials exhibit vast application prospects and a large number of related works have been reported recently. Though there are many excellent review articles on 2D-based materials,7,33,36–39 a comprehensive review on 2D–2D hybrids with representative examples reported in the last five years and the emphasis on the characterization and effects of heterointerfaces would provide a timely understanding of progress of this promising research field and also promote the development of 2D–2D heterostructured hybrid materials for efficient electrocatalysis towards energy conversion reactions.
| Hybrids | Merits |
|---|---|
| 2D–2D | Large interfacial contact area |
| Strong heterointerface interaction | |
| Intense coupling effects | |
| Favourable rearrangement of electronic structure | |
| Facilitating better charge transfer |
This review focuses on the most recently published and instructive studies based on the 2D–2D hybrid catalysts for energy conversion reactions (Fig. 1). Firstly, the fabrication methods of 2D–2D hybrid materials and their unique physicochemical properties will be introduced. Next, the techniques for the characterization of heterointerfaces and their effects will be discussed. Furthermore, the application of the 2D–2D heterostructured hybrid materials in energy conversion catalysis, especially towards hydrogen production via water splitting, will be fully demonstrated. Finally, the challenges and perspectives in this promising research area will be expounded based on the current research status.
Fabrication of 2D–2D heterostructured hybrid materials
The performance of 2D–2D heterostructured materials in energy conversion catalysis not only depends on their chemical composition, crystal phase, microstructure, and exposed facets, but also on the distribution of each component and the quality of the heterointerface. Therefore, rational design and controlled synthesis methods are critically important for achieving advanced catalytic performance of the as-prepared 2D–2D hybrid materials. Generally, the construction of 2D–2D hybrid materials with heterointerfaces could be classified as growth and conversion processes and assembly processes that will be introduced in detail in this section.Growth and conversion processes
The growth of 2D–2D heterostructured hybrid materials can be generally described as the growth or formation of the second 2D material on the surface of the primarily synthesized 2D material (the substrate material) (Fig. 1B). Epitaxial growth is one of the most promising approaches for controlled synthesis of materials with heterointerfaces, which refers to a crystalline material grown on the well-defined surface of another crystalline material (the substrate) via chemical vapor deposition (CVD) or wet-chemical processes. The deposited crystalline material generally has the same orientation of crystal lattice as the substrate material. Graphene is one of the most widely used 2D substrates for epitaxial growth of 2D–2D hybrid materials such as graphene-TMDs40,41 and graphene-MoC2.42 In addition, h-BN has also been widely used as a 2D substrate material for epitaxial growth of graphene,43 MoS2,44 and WS245 to form the corresponding 2D–2D hybrids.In addition to carbon materials, transition metal-based 2D materials have also been applied as the substrate for the growth of the second 2D materials. For example, by altering the growth temperature, MoS2/WS2 and WS2/MoS2 hybrids were synthesized via a two-step CVD strategy (Fig. 2A).46 What is more, it was found that by carefully controlling the temperature (Fig. 2B), a one-step growth was enough for realizing the formation of both vertically stacked and in-plane interconnected heterostructured 2D–2D WS2/MoS2 hybrids (Fig. 2C).47 This synthesis method could be further modified to have scalability and patterning characters for producing large-area 2D–2D hybrids.48 Besides, many 2D heterostructured hybrids have been reported by using liquid phase epitaxial growth, such as CuS–TiS2, ZnS–TiS2, Ni3S2–TiS2, etc.49
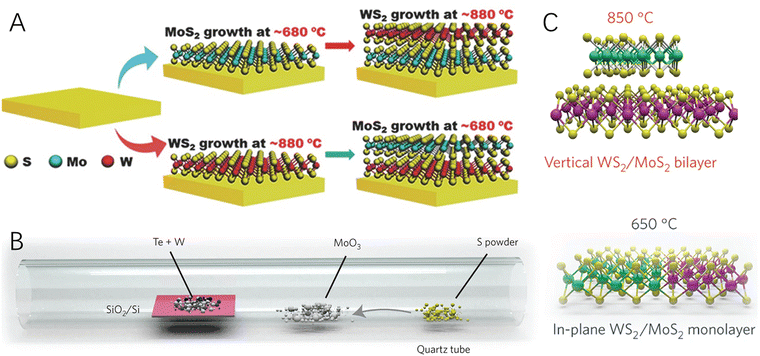 | ||
| Fig. 2 Schematic illustration of CVD processes for the formation of 2D–2D hybrids. (A) The tunable two-step CVD growth process for the fabrication of MoS2–WS2 and WS2–MoS2 2D–2D hybrids. Reproduced with permission from ref. 46, copyright Wiley. (B) Schematic of the synthesis process for vertical and in-plane structured WS2/MoS2 hybrids. (C) Schematic of the vertically stacked and in-plane WS2/MoS2 heterostructures at 850 °C and 650 °C, respectively. Reproduced with permission from ref. 47, copyright Springer Nature. | ||
Though many 2D–2D hybrids have been successfully fabricated by epitaxial growth, the selection of the two components is relatively limited, which should have similar lattice structures to lower the degradation of the quality of the interface resulting from the lattice mismatch. In this regard, functionalization of the surface of 2D substrate materials to make them more suitable for the growth of the second 2D material is necessary. Gao et al.50 fabricated Ti3C2 nanosheets with a negatively charged surface and then grew Bi2WO6 nanosheets on them and successfully constructed Ti3C2–Bi2WO6 hybrid nanosheets. By similar wet-chemical processes, Xia et al.51 also fabricated a MnO2–gC3N4 hybrid by adsorption of Mn2+ ions on the defect- and OH-rich g-C3N4 nanosheets.
Different from the growth of the second 2D component, the controlled topotactic conversion of the 2D substrate materials is also an efficient method for the fabrication of 2D–2D hybrids (Fig. 1C). Actually, morphology-retained topotactical conversion processes have been successfully applied to synthesize many functional materials to change their physiochemical properties and then catalytic performance.52–55 For 2D–2D hybrid construction, Qian et al. fabricated a Bi4Ti3O12/I-BiOCl heterostructured hybrid by adding HCl into a Bi4Ti3O12 nanosheet suspension. The surface of the Bi4Ti3O12 nanosheets was dissolved in the presence of HCl and the released Bi3+ ions near the surface hydrolyzed to BiO+, which was further transformed to IBiOCl, resulting in the formation of a Bi4Ti3O12/I-BiOCl 2D–2D hybrid.56 Recently, via an in situ topotactic hydrothermal transformation process, Shi et al. constructed a Cu2S/Zn0.67Cd0.33S hybrid nanosheet with a 2D–2D atomic level in-plane heterointerface by using 2D CuZnCdAl LDH as the precursor.57
Assembly processes
Distinct from the above-mentioned growth or conversion processes, the formation of 2D–2D hybrids was achieved via assembly processes generally through the following steps: first, the two 2D components were designed and synthesized separately and then these two pre-synthesized 2D materials were combined together through assembly processes (Fig. 1D). In contrast to the epitaxial growth of 2D–2D hybrids that have a lattice matching constraint, the 2D–2D heterostructures were made by assembly processes often attracted by much weaker electrostatic or van der Waals forces, enabling much more selection space for the construction of 2D–2D hybrids (Fig. 1E).58,59 Moreover, although the force is relatively weak, the coupling effects between the two 2D components around the interface are still strong enough to affect the re-organization of the electronic structure and then mediate the catalytic performance of the hybrid material for energy conversion catalysis.Considering that both the CrSe2 and Ti3C2 MXene are advanced energy storage materials with 2D layered structures, Raj et al. constructed a CrSe2/Ti3C2 MXene 2D/2D hybrid by a facile assembly process. The as-prepared hybrids showed a promising energy storage property owing to the increased number of active sites and improved electrochemical stability arising from the synergistic interplay effect triggered by fast ionic/electronic transportation, reduced agglomeration and volume expansion.60 Recently, Chen et al. reported an LDH-birnessite hybrid by a self-assembly of exfoliated LDH nanosheets and birnessite microplates, by taking advantage of the positively charged LDH and negatively charged birnessite and the 2D characters of these two components that readily formed a film.61 The coupling of the two components at the atomic level allows the formation of a built-in electric field in the hybrid, which contributed to lowering the overpotential of the OER catalysed by this heterostructured hybrid catalyst. Actually, a variety of advanced 2D–2D materials have been successfully synthesized by this route, such as WO3-ZnIn2S4,62 black phosphorus-Bi2WO6,63 Fe2O3/g-C3N4,64 metal-free phosphorene (FP)/graphitic carbon nitride,65etc.
In addition to the low temperature assembly process, 2D–2D hybrids were also prepared via the assembly of two components under hydrothermal conditions, to further enhance the coupling of the two materials.66,67 More recently, Ju et al. assembled an LDH with ppy to form an organic–inorganic 2D–2D hybrid of LDH-ppy via two steps. Firstly, the negatively charged pyrrole monomer was intercalated into the interlayer of the LDH nanosheets, where they were further polymerized to form a 2D ultrathin film by ultraviolet illumination,68 suggesting the universality of the assembly process for the fabrication of 2D–2D hybrid materials with various chemical compositions.
Besides wet-chemical assembly processes, the solid state sintering process has also been proven to be an effective approach for the construction of 2D–2D heterostructured materials. For example, Xu et al. mixed Bi4NbO8Cl and g-C3N4 nanosheets together by ball-milling and then annealed the mixture in air. The as-prepared Bi4NbO8C–g-C3N4 showed a clear heterointerface, suggesting the 2D–2D heterostructure of the hybrid.69 Similarly, a Bi4Ti3O12–Ni(OH)2 2D–2D hybrid was also reported by the solid state sintering process.70 Though there is no lattice matching constraint for the selection of the two 2D components to form the 2D–2D hybrid via solid state sintering methods, they should be chemically and structurally stable during the high temperature annealing procedures. What is more, the interface adhesion in these 2D–2D hybrids is relatively weak, which would probably result in the deterioration of the heterointerface during the catalytic performance evaluation. Therefore, rationally modifying the surface of the 2D materials to consolidate the heterointerface with electrostatic, van der Waals force or even covalent bonding would improve the stability of the 2D–2D hybrid catalysts.
Though the above-mentioned assembly method could produce a large quantity of 2D–2D hybrid materials, they cannot realize the fabrication of large-area 2D–2D hybrids that maintain the intrinsic properties of the two components, while producing pristine interlayer heterointerfaces. Therefore, Kang et al.59 developed a programmed vacuum stack process (PVS) that could generate wafer-scale 2D–2D hybrid films with a very high level of spatial uniformity and pristine interfaces (Fig. 3). The chemical composition and properties of these 2D–2D hybrid films could be designed at atomic scale using layer-by-layer assembly of the 2D building blocks under a vacuum. What is more, the as-prepared large-scale hybrid films were detachable, suspendable and compatible with water or other surfaces, enabling the integration of the prepared 2D–2D hybrid materials with advanced optical and mechanical systems.
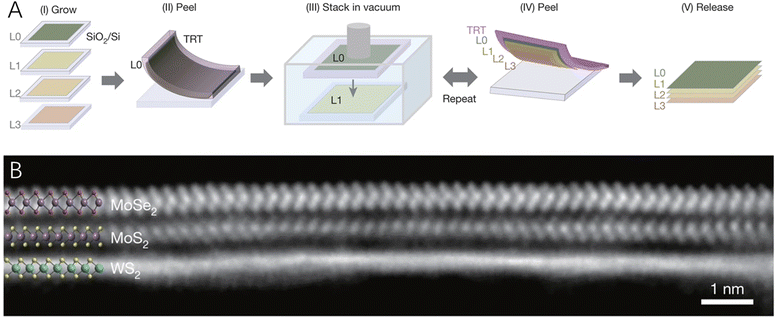 | ||
| Fig. 3 (A) A schematic of the programmed vacuum stack (PVS) process. (B) The cross-sectional STEM image of a MoSe2/MoS2/WS2 film with the electron beam aligned with the armchair axis of MoSe2 (top) and MoS2 (middle). Reproduced with permission from ref. 59, copyright Springer Nature. | ||
In summary, the growth and conversion methods for preparing 2D–2D hybrid materials start from the synthesis of the 2D substrate materials, and then the second 2D materials are in situ grown or formed via topotactic conversion. Therefore, this method would be scalable for constructing many different kinds of 2D–2D hybrid materials with relatively strong interface interaction. What is more, the 2D–2D hybrids constructed via the in situ CVD process generally have high quality heterointerfaces that are even suitable for applications in electronics, though expensive facilities are required, while the assembly process is the most universal method to fabricate 2D–2D hybrids by using the two 2D materials that have been synthesized separately as the building blocks. Therefore, the 2D–2D hybrids made by the assembly method feature relatively larger interlayer spacings and more defects, which could benefit the mass/charge transport and provide more active sites during the electrochemical processes. To further realize the uniform hybridization, and then enhance the catalytic performance of the 2D–2D hybrids via the assembly processes, the quality and the surface charge of the two 2D building blocks, and the assembly conditions such as temperature, stirring speed, dispersing agent, etc. should be well controlled.
Characterization and roles of heterointerfaces
The techniques for characterization of heterointerfaces are vital to guarantee their quality and figure out the role of the interface for catalysis and then promote the development of heterostructured hybrid materials with tailored interfacial structures and properties. Therefore, to fully understand the critical roles of the heterointerfaces and to further improve the overall performance of the hybrids, it is of great importance to obtain accurate information about the microscopic structures and properties of the heterointerfaces by applying reliable characterization techniques.Electron microscopy techniques such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are powerful and extensively used for visualization of the surface of materials at the nanometer scale. However, they could not provide the critical information of the heterointerface of hybrid materials. However, high resolution TEM (HRTEM) that could achieve atomic resolution, when coupled with the focused ion beam lithography (FIB) technique, is a widely used tool for getting the lattice alignment at the heterointerfaces of the 2D–2D hybrid materials. For example, the interface of Cu1.94S–CdS 2D–2D nanoplates has been well observed by HRTEM, which showed well-aligned lattice fringes of Cu1.94S (800) and CdS (100) planes.71 The boundary between TiS2–CuS heterostructured nanoplates was also characterized by HRTEM and SAED,49 suggesting the universality of this technique for investigating the interfaces of heterostructured materials.
X-ray photoelectron spectroscopy (XPS) is a useful surface sensitive spectroscopic technique to determine the elemental composition of materials. For the 2D–2D hybrids, the coupling effects arising from the close contact of two distinct materials at the heterointerface would change the electronic configuration of the material, which could be identified by XPS. What is more, the interaction between the two materials would change the surface potential of the hybrid, which could be well characterized by Kelvin probe force microcopy (KPFM). Specifically, KPFM gives precise information on the work function distribution of the materials and the interfacial interaction between the 2D materials. For example, MoS2/WS2 hetero-junction arrays have been electrically characterized by using conducting atomic force microscopy (CAFM) and the surface potentials of MoS2 and WS2 layers were measured by KPFM, which showed a built-in field at the interface to align the Fermi level.72 Recently, due to the structural compatibility of Bi2WO6 and Bi2O2S, both of which were 2D semiconductors sharing a similar structural unit of a [Bi2O2]2+, Xing et al.73 fabricated an ultrathin nanosheet with a tightly bonded 2D–2D heterojunction between the two components by facilely joining the [Bi2O2]2+ and [S]2− slices (Fig. 4A). The as-prepared Bi2WO6–Bi2O2S 2D–2D hybrid not only extended light absorption but also significantly enhanced photo(electro)chemical water splitting efficiencies due to the close bonding-promoted interfacial charge separation that has been observed by in situ KPFM (Fig. 4B and C).
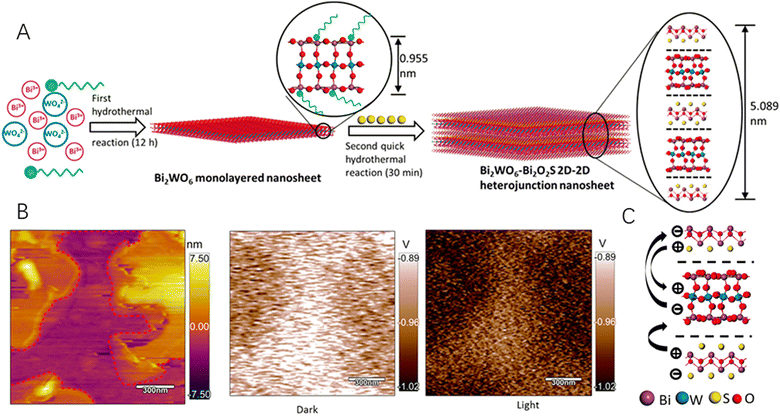 | ||
| Fig. 4 (A) Schematics of the hydrothermal synthesis process and the transformation process from the monolayered Bi2WO6 nanosheet to the Bi2WO6-Bi2O2S 2D–2D heterojunction nanosheet. (B) The AFM and KPFM images in the dark and under illumination of Bi2WO6–Bi2O2S nanosheets. (C) Schematics of the charge flow direction in a 2D–2D heterojunction. Reproduced with permission from ref. 73, copyright ACS. | ||
Though HRTEM gives lattice information and KPFM provides the work function distribution of hybrid materials, they could not determine the exact elemental location around the heterointerface, which is critically important for identifying the boundary of the heterointerface and then facilitating the investigation of the critical roles of the heterointerface for energy conversion catalysis. Electron tomography (CT), which could provide the three-dimensional structure of complex materials in nanometer resolution, is powerful for the characterization of heterostructured catalytic materials, especially the scanning transmission electron microscopy (STEM). STEM is one of the most widely used imaging modes that avoids the appearance of diffraction-related artifacts and is also highly sensitive to the chemical composition of the material. Therefore, it has been rapidly developed as a powerful technique for the characterization of heterostructured catalytic systems,74 especially the 2D–2D hybrid catalysts. For example, the heterointerface of Bi2Te3 with a six-fold symmetry and FeTe with a four-fold symmetry was clearly observed by spherical aberration-corrected STEM.75 Both the high-angle annular dark field (HAADF, Fig. 5A) and annular bright field (Fig. 5B) images have high contrast with significant sensitivity to the atomic number and thus provide robust visibility of both light and heavy atoms. From the high-magnification HAADF image (Fig. 5C), the Bi2Te3 and FeTe were found to be separated by a van der Waals gap with their own lattices undisrupted, confirming the van der Waals epitaxial growth of this 2D–2D hybrid material. Similarly, through atomic-resolution HAADF-STEM, a continuous lattice fringe without any grain boundary or misorientation in a lateral WSe2–MoS2 heterohybrid was also clearly observed76 and the distribution of Mo and W atoms around the heterointerface could be well determined due to the distinct weights of the two elements, suggesting that powerful STEM provides both the structural and chemical information of the heterointerface of 2D–2D hybrid materials.
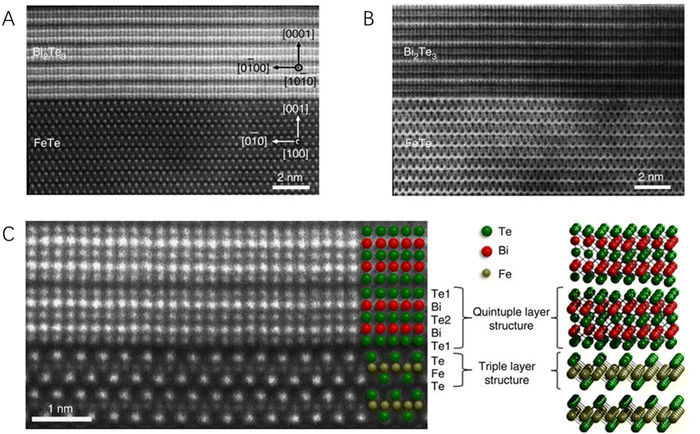 | ||
| Fig. 5 The scanning transmission electron microscopy micrographs of a Bi2Te3/FeTe heterostructure. (A) The HADDF image. (B) The annular bright field image. (C) The higher-magnification HADDF image showing the atomically sharp interface between Bi2Te3 and FeTe. Reproduced with permission from ref. 75, copyright Springer Nature. | ||
Though there are many powerful techniques that could provide some important information on the heterointerfaces and many in situ/operando techniques that could visualize the dynamic behaviours of the catalyst surface, there are few techniques that could track the real time change of heterointerfaces during the catalytic reaction process. What is more, the complexity of the 2D–2D hybrid catalysts would further make the revelation of the effects of the heterointerfaces difficult. Therefore, to determine the critical role of the heterointerfaces in the catalytic performance of the 2D–2D hybrid materials, the coupling of many advanced in situ characterization techniques should be developed and a facile synthesis approach for controlled fabrication of well-designed 2D–2D hybrid materials that have relatively simple structures and chemical compositions would also be critically important.
Application of 2D–2D hybrid materials for energy conversion reactions
As mentioned above, 2D materials offer unique merits to energy conversion catalysis due to their large specific surface area, high charge migration rate, tuneable electronic properties, ultralight weight, assembly properties and high mechanical flexibility. What is more, the 2D–2D hybrids with heterostructures and heterointerfaces could further overcome many drawbacks of the single component 2D materials. For energy conversion reaction catalysis, the coupling of the two components at atomic level was found to enhance the activity of the catalytic material,77 optimize the electronic structure of active ions and also accelerate the electron transport processes, all of which would contribute to the enhanced inherent catalytic activity of the 2D–2D hybrid materials.78 Actually, the advanced 2D–2D hybrids have been widely applied in diverse energy conversion reactions that will be fully discussed with typical examples reported in the last five years in this section.Hydrogen produced by water splitting is one of the most promising alternatives to replace the fossil fuels, owing to its high energy density (142 MJ kg−1), zero-carbon combustion production and eco-friendly synthesis route.79,80 For realizing efficient hydrogen production, effective catalysts are critically important and should possess a low overpotential, fast reaction kinetics and good long-term stability in practical applications. Early in 2014, Long et al.81 reported a reduced graphene oxide (rGO) intercalated NiFe LDH 2D–2D hybrid as an efficient OER catalyst in alkali solutions. The direct contact of the highly conductive rGO and OER active NiFe LDH at the atomic level greatly enhanced the charge transfer properties and accelerated the OER kinetics. Later, a superlattice of alternately stacked GO-LDH and rGO-LDH hybrids was further synthesized and applied as both the cathode and anode, which exhibited advanced full water splitting performance that could be driven by a commercial 1.5 V AA battery.82 What is more, many other 2D materials such as MXenes, TMDs, etc. coupled with 2D carbon (graphene, graphene oxides, and rGO) have also been reported, which showed much improved catalytic performance for hydrogen production via water splitting, due to the increased active sites and accelerated mass transfer arising from the support of 2D carbon that inhibited the self-stacking of the catalytic active 2D materials and the much enhanced conductivity that improved the charge transfer.83–88
In addition to carbon materials, transition metal-based 2D nanomaterials have also been coupled with 2D black phosphorus to construct efficient 2D–2D heterostructured catalysts for water splitting.89–91 Mei et al.92 reported heterostructured Ni(OH)2-black phosphorus (BP) hybrid nanosheets (BNHNSs) by combining the liquid-exfoliated and mildly oxidized BP with wet-chemical synthesized Ni(OH)2 nanosheets (Fig. 6A). According to the charge density difference (CDD) and electron localization function (ELF) maps (Fig. 6B), an interfacial charge transfer from the 2D Ni(OH)2 nanosheets to the 2D BP nanosheets at the heterointerface could be clearly observed, leading to a favourable electronic structure of the as-prepared BNHNSs. What is more, the adsorption properties of BNHNSs were also found to be desirable for intermediates of alkaline water splitting reactions. Therefore, when applied as an OER catalyst, the 2D–2D BNHNS heterostructured hybrid exhibited excellent catalytic performance with a low overpotential, fast reaction kinetics (Fig. 6C) and long-term stability (Fig. 6D).
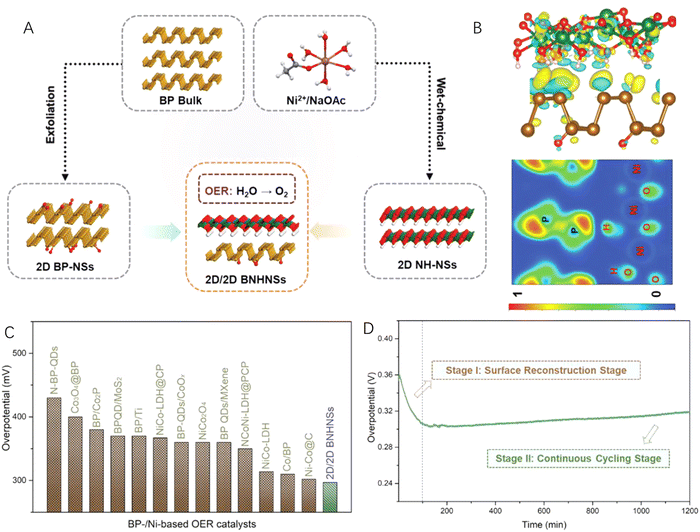 | ||
| Fig. 6 (A) Schematic illustration of the synthesis of 2D–2D heterostructured nanosheets (BNHNSs) by coupling 2D Ni(OH)2 nanosheets (NH-NSs) and 2D black phosphorus (BP) nanosheets (BP-NSs). (B) The charge density difference and electron localization function maps of 2D–2D BNHNSs. (C) Comparison of the overpotentials with those of the reported electrocatalysts at 10 mA cm−2. (D) Time-dependent overpotential curve of the 2D–2D BNHNSs catalyst in KOH for 1200 min. Reproduced with permission from ref. 92, copyright Wiley. | ||
The combination of two 2D materials, both of which have excellent redox characters that could work as electrocatalysts, have attracted much attention. For example, Shinde et al.93 developed a MoTe2/Ti3C2Tx 2D–2D hybrid that exhibited efficient HER activity in acidic solutions. The overpotential for attaining the current density of 10 mA cm−2 was as low as 293 mV and the Tafel slope was 65 mV dec−1, which was much lower than the two single component counterparts. Based on the analysis of the electronic density of states (DOS), charge transfer, and charge density plot, the authors found that an improvement in electronic density states near the Fermi energy for the hybrid system due to orbital hybridization increased the conductivity of the hybrid material. More recently, Chen et al.61 reported a LDH-birnessite hybrid that realized the direct contact of two OER active species of brucite layer of LDH and MnOx layer of birnessite at the atomic level (Fig. 7A). They found an electron transfer from Ni sites to both the in-plane Fe site and through-plane Mn sites, which not only greatly idealized the electronic structure of the three catalytic active sites (Fe and Ni in LDH and Mn in birnessite, Fig. 7B), but also created a built-in electric field (Fig. 7A) that accelerated the charge transfer during the OER process, hence greatly increasing the reaction kinetics of the alkali OER (Fig. 7C and D). The as-prepared 2D–2D hybrid solved the instability problem of LDH and the low activity of birnessite together, leading to an excellent OER performance with both high activity and long-term stability even under industrial alkali water splitting conditions (Fig. 7E).61 Similarly, transition metal-based LDH-TMDs with different chemical compositions,94 LDH-Mxenes,95–102 MXene-TM sulphides,103 and LDH-LDH104 have been successfully synthesized and all of them showed improved catalytic performance towards water splitting, due to the synergistic effect arising from the coupling of two components at the heterointerface.105,106 However, despite much progress on the fabrication of 2D–2D hybrid materials towards efficient water splitting and many of them even showing superior activity under industrial alkali water splitting conditions, the long-term stability and durability the 2D–2D hybrid catalysts should be further improved and evaluated to meet the requirements of practical applications.
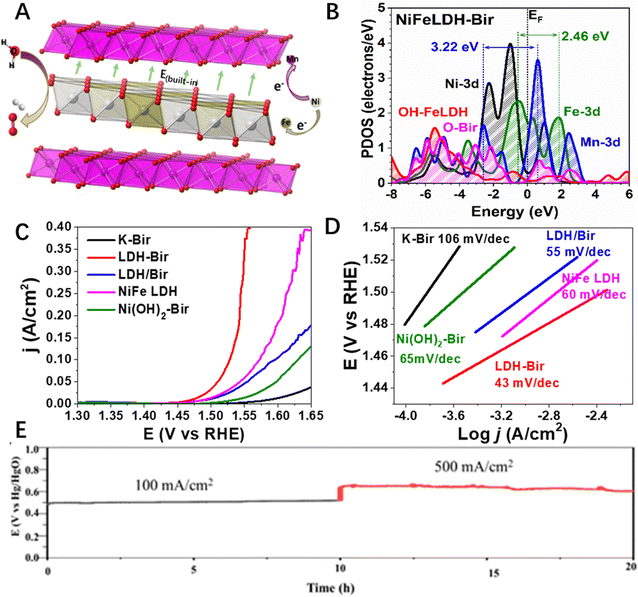 | ||
| Fig. 7 (A) Schematic illustration the built-in electric field in the LDH-Bir 2D–2D hybrid. (B) PDOS of LDH-Bir, (C) lsv and (D) Tafel plots of LDH-Bir and the control catalysts, and (E) long term stability of LDH-Bir under the industrial alkali water splitting conditions. Reproduced with permission from ref. 61, copyright Wiley. | ||
In addition to the production of green hydrogen via water splitting, efficient CO2 reduction (CO2R) could mitigate the environmental pollution problems, while generating valuable chemicals. However, efficient CO2R is still challenging for realizing high activity, selectivity and stability, in which, the advanced catalysts are critically important. Due to the above-mentioned structural merits, 2D–2D heterostructured hybrid materials have promising potential as advanced CO2RR catalysts.107 For example, Gu et al. reported an ultrathin conjugated metalloporphyrin COF-graphene van der Waals heterostructured hybrid material for electrocatalytic CO2 reduction. The strong electronic coupling between the van der Waals layers accelerated the dynamics of the CO2 conversion process, leading to advanced conversion efficiency with high CO selectivity.108
However, due to the strong reductive reaction conditions of the CO2RR, the transition metal-based active species would easily be reduced, which would result in the change of both the physiochemical properties and microstructures of the catalysts and hence deteriorate the catalytic performance of the materials. For example, Shen et al. found that the CuS–Bi2S3 heterojunction would be in situ reconstructed to Cu-doped bismuth (CDB) during the CO2 reduction reaction, though the as-formed CDB also exhibited CO2RR catalytic performance with an industrially compatible current density of −1.1 A cm−2 and high formate selectivity.109 Therefore, the stability of the 2D–2D hybrids under the CO2RR conditions should be paid more attention.
Actually, many strategies have been developed to stabilize the CO2RR catalysts under the highly reductive conditions such as doping and surface/interface engineering110–114 and rational construction of 2D–2D heterostructures is one of the promising methods. For example, Cai et al.115 reported metallic copper–CuxOF 2D hybrid nanoplates for the CO2RR (Fig. 8A–C). The authors found that the Cu/CuxOF 2D–2D hybrid surface showed a strong perturbation of the electronic distribution (Fig. 8D) and the Cu-3d orbitals were located at a position close to the Fermi level (Fig. 8E), suggesting a higher d-band centre and thus an improved electroactivity. When applied as the CO2RR catalysts, the prepared Cu/CuxOF 2D–2D hybrid exhibited excellent CO2RR activity with high selectivity for acetic acid formation (Fig. 8F). The authors proposed that the F-modified heterointerface could effectively tailor the exposed facets of the metallic Cu nanoparticles, stabilize the oxidized copper active species under the CO2RR conditions, and purposely induce the s, p–d coupling between the metal and hetero-anions, leading to the selective formation of acetate.
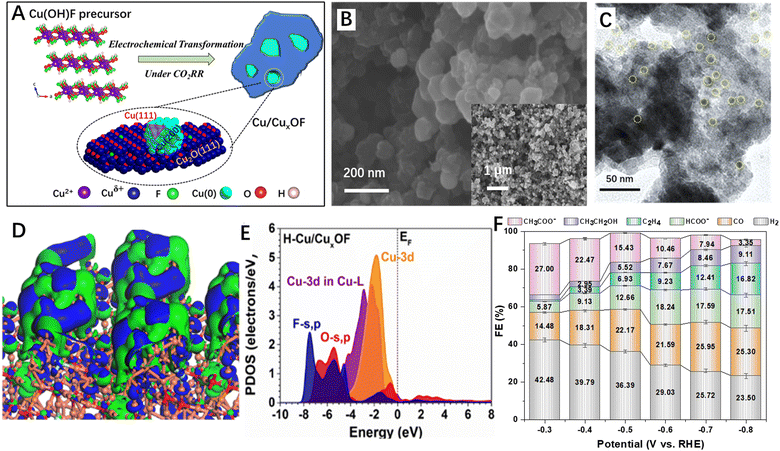 | ||
| Fig. 8 2D–2D hybrid materials for the CO2RR. (A) Schematic atomic structures of Cu(OH)F and the transformed Cu/CuxOF hybrid. (B) The SEM images with high and low (inset) magnifications of Cu/CuxOF 2D–2D nanosheets. (C) TEM images of Cu/CuxOF 2D–2D nanosheets. (D) 3D contour plot for electronic distribution and (E) PDOS of Cu/CuxOF. (F) FEs of all the products on Cu/CuxOF at various applied potentials. Reproduced with permission from ref. 115, copyright RSC. | ||
However, despite many successful applications of 2D–2D hybrids for efficient CO2RR and other energy conversion reactions such as nitrogen fixation,107,116–119 this promising research area is still in an initial stage that needs more efforts. Revealing the critical roles of heterointerface by using advanced techniques is of importance for directing the rational design of advanced 2D–2D materials and developing a general and controllable construction method to realize the fabrication of targeted 2D–2D heterostructured hybrid materials with particular chemical composition and interface properties is also critical for achieving efficient energy conversion with high activity, selectivity and stability.
Summary and perspectives
The 2D–2D heterostructured hybrid materials have the largest interfacial contact area and the strongest interactions between the two 2D components, resulting in strong coupling effects that would influence the electronic structure of the active sites and the charge transfer character during energy conversion reactions. In this review article, we focus on the most recently reported and instructive studies based on the 2D–2D hybrid catalysts for energy conversion reactions, including the fabrication methods, characterization techniques and applications in energy conversion reactions, particularly in water splitting (Table 2).| Catalysts | Performance | Ref. |
|---|---|---|
| Note: RT means room temperature. | ||
| LDH-rGO | Alkali OER: η = 210 mV@10 mA cm−2, 1 M KOH, and RT | 81 |
| MoS2–graphene | Acidic HER: η = 137 mV@10 mA cm−2, 0.5 M H2SO4, and RT | 85 |
| MXene/CN/rGO | Acidic HER: η = 147 mV@10 mA cm−2, 0.5 M H2SO4, and RT | 88 |
| Black P@NG | Full water splitting, cell voltage of 1.54 V at 10 mA cm−2, 1 M KOH, and RT | 89 |
| CoFeOx-black P | η(OER) = 266 mV@10 mA cm−2, η(HER) = 88 mV@10 mA cm−2, 1 M KOH, and RT | 91 |
| Black P-Ni(OH)2 | Alkali OER: η = 297 V @10 mA cm−2, 1 M KOH, and RT | 92 |
| MXene-LDH | Alkali OER: η = 298 V @10 mA cm−2, 1 M KOH, and RT | 96 |
| LDH-LDH | Full water splitting, cell voltage of 1.59 V at 10 mA cm−2, 1 M KOH, and RT | 104 |
| TMD-LDH | Full water splitting, cell voltage of 1.49 V at 10 mA cm−2, 0.1 M KOH, and RT | 106 |
| MoTe2/Ti3C2Tx | Acidic HER: η = 293 mV@10 mA cm−2, 0.5 M H2SO4, and RT | 93 |
| LDH-Bir | Industrial alkali OER: 0.6 V vs. Hg/HgO, 6 M KOH, 85 °C | 61 |
| Co-COF/graphene | CO2RR: 8.2 mA cm−2 in H cell, FE(CO) = 97%, 191 mA cm−2 in flow cell, FE(CO) = 99% | 108 |
| Cu-CuxOF | CO2RR: FE(acetic acid) = 27%, −0.3 V | 115 |
| MoS2/C3N4 | NRR: NH3 yield = 18.5 μg h−1 mg−1, FE (−0.3 V) = 17.8% | 117 |
| CuSe/g-C3N4 | CO2RR: FE(CO) = 85.28%, −1.2 V | 118 |
Despite the considerable achievements in the design and application of 2D–2D hybrid materials for energy conversion reactions, there are still many challenges ahead in this promising research area. (1) There is a lack of a facile and economic method for the preparation of large scale and high quality 2D–2D hybrid materials with controlled chemical compositions, sizes and microstructures. (2) The synergistic effects between the two 2D components are complicated; however, they are critically important for understanding the roles of the heterointerface in catalysis. So, advanced characterization techniques, especially the in situ/operando techniques that could provide the real time information on the structural and compositional evolution of 2D–2D heterostructured hybrid materials during the catalysis processes and rationally designed and precisely controlled 2D–2D hybrid systems need to be developed. (3) Theoretical simulations such as DFT with accurate models should be carried out for understanding the reaction mechanism, which could couple with high throughput screening and machine learning to promote a better construction of 2D–2D hybrid materials to meet the requirements of targeted reactions. (4) Fabrication methods of free-standing and large scale 2D–2D heterostructured hybrid material-based electrodes need to be developed to guarantee the high activity and increase the durability of the catalysis reactions under practical application conditions, and hence close the huge gap between the laboratory work and commercialization.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors appreciate financial support from Shanghai Jiao Tong University (No. WH220828001, ZXDF280001) and Shenzhen Science and Technology Innovation Commission (JCYJ20190808155413194).References
- S. Chu and A. Majumdar, Nature, 2012, 488, 294 CrossRef CAS PubMed.
- C. Zhao, Carbon Neutrality, 2022, 1, 2 CrossRef.
- D. H. Deng, K. S. Novoselov, Q. Fu, N. F. Zheng, Z. Q. Tian and X. H. Bao, Nat. Nanotechnol., 2016, 11, 218 CrossRef CAS PubMed.
- J. Di, C. Yan, A. D. Handoko, Z. W. Seh, H. M. Li and Z. Liu, Mater. Today, 2018, 21, 749 CrossRef CAS.
- M. Nasilowski, B. Mahler, E. Lhuillier, S. Ithurria and B. Dubertret, Chem. Rev., 2016, 116, 10934 CrossRef CAS PubMed.
- V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Science, 2013, 340, 1420 CrossRef CAS.
- C. L. Tan, X. H. Cao, X. J. Wu, Q. Y. He, J. Yang, X. Zhang, J. Z. Chen, W. Zhao, S. K. Han, G. H. Nam, M. Sindoro and H. Zhang, Chem. Rev., 2017, 117, 6225 CrossRef CAS PubMed.
- X. Long, Z. L. Wang, S. Xiao, Y. M. An and S. H. Yang, Mater. Today, 2016, 19, 213 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248 CrossRef CAS PubMed.
- V. M. H. Ng, H. Huang, K. Zhou, P. S. Lee, W. X. Que, J. Z. Xu and L. B. Kong, J. Mater. Chem. A, 2017, 5, 3039 RSC.
- D. J. Zhou, P. S. Li, X. Lin, A. McKinley, Y. Kuang, W. Liu, W. F. Lin, X. M. Sun and X. Duan, Chem. Soc. Rev., 2021, 50, 8790 RSC.
- M. Xu and M. Wei, Adv. Funct. Mater., 2018, 28, 1802943 CrossRef.
- H. Xia, Z. D. Shi, C. S. Gong and Y. M. He, J. Mater. Chem. A, 2022, 10, 19067 RSC.
- Q. Fu, J. C. Han, X. J. Wang, P. Xu, T. Yao, J. Zhong, W. W. Zhong, S. W. Liu, T. L. Gao, Z. H. Zhang, L. L. Xu and B. Song, Adv. Mater., 2021, 33, 1907818 CrossRef CAS PubMed.
- S. Manzeli, D. Ovchinnikov, D. Pasquier, O. V. Yazyev and A. Kis, Nat. Rev. Mater., 2017, 2, 17033 CrossRef CAS.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147 CrossRef CAS PubMed.
- L. K. Li, F. Y. Yang, G. J. Ye, Z. C. Zhang, Z. W. Zhu, W. K. Lou, X. Y. Zhou, L. Li, K. Watanabe, T. Taniguchi, K. Chang, Y. Y. Wang, X. H. Chen and Y. B. Zhang, Nat. Nanotechnol., 2016, 11, 592 Search PubMed.
- B. S. Li, C. Lai, G. M. Zeng, D. L. Huang, L. Qin, M. M. Zhang, M. Cheng, X. G. Liu, H. Yi, C. Y. Zhou, F. L. Huang, S. Y. Liu and Y. K. Fu, Small, 2019, 15, 1804565 CrossRef PubMed.
- H. Liu, A. T. Neal, Z. Zhu, Z. Luo, X. F. Xu, D. Tomanek and P. D. Ye, ACS Nano, 2014, 8, 4033 CrossRef CAS PubMed.
- J. W. Colson, A. R. Woll, A. Mukherjee, M. P. Levendorf, E. L. Spitler, V. B. Shields, M. G. Spencer, J. Park and W. R. Dichtel, Science, 2011, 332, 228 CrossRef CAS PubMed.
- K. Y. Geng, T. He, R. Y. Liu, S. Dalapati, K. T. Tan, Z. P. Li, S. S. Tao, Y. F. Gong, Q. H. Jiang and D. L. Jiang, Chem. Rev., 2020, 120, 8814 CrossRef CAS PubMed.
- Y. Peng, Y. S. Li, Y. J. Ban, H. Jin, W. M. Jiao, X. L. Liu and W. S. Yang, Science, 2014, 346, 1356 CrossRef CAS PubMed.
- T. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, F. X. L. I. Xamena and J. Gascon, Nat. Mater., 2015, 14, 48 CrossRef CAS PubMed.
- U. Khan, A. Nairan, J. K. Gao and Q. C. Zhang, Small Struct., 2022, 2200109, DOI:10.1002/sstr.202200109.
- P. Prabhu and J.-M. Lee, Chem. Soc. Rev., 2021, 50, 6700 RSC.
- Y. V. Kaneti, D. P. Benu, X. Xu, B. Yuliarto, Y. Yamauchi and D. Golberg, Chem. Rev., 2021, 122, 1000 CrossRef PubMed.
- X. Wu, S. Xiao, Y. Long, T. Ma, W. Shao, S. Cao, X. Xiang, L. Ma, L. Qiu, C. Cheng and C. Zhao, Small, 2022, 18, 2105831 CrossRef CAS PubMed.
- H. Jin, T. Song, U. Paik and S.-Z. Qiao, ACC. Mater. Res., 2021, 2, 559 CrossRef CAS.
- K. L. Huang, C. H. Li, H. Z. Li, G. M. Ren, L. Wang, W. T. Wang and X. C. Meng, ACS Appl. Nano Mater., 2020, 3, 9581 CrossRef CAS.
- C. L. Tan, J. Z. Chen, X. J. Wu and H. Zhang, Nat. Rev. Mater., 2018, 3, 17089 CrossRef CAS.
- Y. P. Liu, S. Y. Zhang, J. He, Z. M. M. Wang and Z. W. Liu, Nano-Micro Lett., 2019, 11, 13 CrossRef CAS PubMed.
- J. Mei, T. Liao and Z. Q. Sun, Energy Environ. Mater., 2022, 5, 115 CrossRef CAS.
- D. S. Wang, Chin. J. Catal., 2022, 43, 1380 CrossRef.
- H. L. Hou, X. K. Zeng and X. W. Zhang, Sci. China Mater., 2020, 63, 2119 CrossRef CAS.
- T. Li, T. Jing, D. Rao, S. Mourdikoudis, Y. Zuo and M. Wang, Inorg. Chem. Front., 2022, 9, 6008 RSC.
- S. Aftab, M. Z. Iqbal, M. W. Iqbal, M. Asghar and H. Ullah, J. Mater. Chem. C, 2022, 10, 14795 RSC.
- S. Chandrasekaran, D. T. Ma, Y. Q. Ge, L. B. Deng, C. Bowen, J. Roscow, Y. Zhang, Z. Q. Lin, R. D. K. Misra, J. Q. Li, P. X. Zhang and H. Zhang, Nano Energy, 2020, 77, 105080 CrossRef CAS.
- C. Chang, W. Chen, Y. Chen, Y. H. Chen, Y. Chen, F. Ding, C. H. Fan, H. J. Fan, Z. X. Fan, C. Gong, Y. J. Gong, Q. Y. He, X. Hong, S. Hu, W. D. Hu, W. Huang, Y. Huang, W. Ji, D. H. Li, L. J. Li, Q. Li, L. Lin, C. Y. Ling, M. H. Liu, N. Liu, Z. Liu, K. P. Loh, J. M. Ma, F. Miao, H. L. Peng, M. F. Shao, L. Song, S. Su, S. Sun, C. L. Tan, Z. Y. Tang, D. S. Wang, H. Wang, J. L. Wang, X. Wang, X. R. Wang, A. T. S. Wee, Z. M. Wei, Y. E. Wu, Z. S. Wu, J. Xiong, Q. H. Xiong, W. G. Xu, P. Yin, H. B. Zeng, Z. Y. Zeng, T. Y. Zhai, H. Zhang, H. Zhang, Q. C. Zhang, T. R. Zhang, X. Zhang, L. D. Zhao, M. T. Zhao, W. J. Zhao, Y. X. Zhao, K. G. Zhou, X. Zhou, Y. Zhou, H. W. Zhu, H. Zhang and Z. F. Liu, Acta Phys-Chim Sin, 2021, 37(12), 2108017 Search PubMed.
- H. Ago, S. Fukamachi, H. Endo, P. Solis-Fernandez, R. M. Yunus, Y. Uchida, V. Panchal, O. Kazakova and M. Tsuji, ACS Nano, 2016, 10, 3233 CrossRef CAS PubMed.
- X. L. Liu, I. Balla, H. Bergeron, G. P. Campbell, M. J. Bedzyk and M. C. Hersam, ACS Nano, 2016, 10, 1067 CrossRef CAS PubMed.
- C. Xu, S. A. Song, Z. B. Liu, L. Chen, L. B. Wang, D. X. Fan, N. Kang, X. L. Ma, H. M. Cheng and W. C. Ren, ACS Nano, 2017, 11, 5906 CrossRef CAS PubMed.
- W. Yang, G. R. Chen, Z. W. Shi, C. C. Liu, L. C. Zhang, G. B. Xie, M. Cheng, D. M. Wang, R. Yang, D. X. Shi, K. Watanabe, T. Taniguchi, Y. G. Yao, Y. B. Zhang and G. Y. Zhang, Nat. Mater., 2013, 12, 792 CrossRef CAS PubMed.
- D. Y. Fu, X. X. Zhao, Y. Y. Zhang, L. J. Li, H. Xu, A. R. Jang, S. I. Yoon, P. Song, S. M. Poh, T. H. Ren, Z. Ding, W. Fu, T. J. Shin, H. S. Shin, S. T. Pantelides, W. Zhou and K. P. Loh, J. Am. Chem. Soc., 2017, 139, 9392 CrossRef CAS PubMed.
- B. Wu, H. H. Zheng, S. F. Li, J. N. Ding, Y. J. Zeng, Z. W. Liu and Y. P. Liu, Nanoscale, 2022, 14, 12447 RSC.
- J. P. Shi, R. Tong, X. B. Zhou, Y. Gong, Z. P. Zhang, Q. Q. Ji, Y. Zhang, Q. Y. Fang, L. Gu, X. N. Wang, Z. F. Liu and Y. F. Zhang, Adv. Mater., 2016, 28, 10664 CrossRef CAS PubMed.
- Y. J. Gong, J. H. Lin, X. L. Wang, G. Shi, S. D. Lei, Z. Lin, X. L. Zou, G. L. Ye, R. Vajtai, B. I. Yakobson, H. Terrones, M. Terrones, B. K. Tay, J. Lou, S. T. Pantelides, Z. Liu, W. Zhou and P. M. Ajayan, Nat. Mater., 2014, 13, 1135 CrossRef CAS PubMed.
- J. M. Woods, Y. Jung, Y. J. Xie, W. Liu, Y. H. Liu, H. H. Wang and J. J. Cha, ACS Nano, 2016, 10, 2004 CrossRef CAS PubMed.
- C. L. Tan, Z. Y. Zeng, X. Huang, X. H. Rui, X. J. Wu, B. Li, Z. M. Luo, J. Z. Chen, B. Chen, Q. Y. Yan and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 1841 CrossRef CAS PubMed.
- S. W. Cao, B. J. Shen, T. Tong, J. W. Fu and J. G. Yu, Adv. Funct. Mater., 2018, 28, 1800136 CrossRef.
- P. F. Xia, B. C. Zhu, B. Cheng, J. G. Yu and J. S. Xu, ACS Sustainable Chem. Eng., 2018, 6, 965 CrossRef CAS.
- X. Long, Z. J. Ma, H. Yu, X. Y. Gao, X. Y. Pan, X. X. Chen, S. H. Yang and Z. G. Yi, J. Mater. Chem. A, 2016, 4, 14939 RSC.
- X. Long, H. Lin, D. Zhou, Y. M. An and S. H. Yang, ACS Energy Lett., 2018, 3, 290 CrossRef CAS.
- X. Long, G. X. Li, Z. L. Wang, H. Y. Zhu, T. Zhang, S. Xiao, W. Y. Guo and S. H. Yang, J. Am. Chem. Soc., 2015, 137, 11900 CrossRef CAS PubMed.
- Z. W. Chen, Z. Wang, R. M. Cai, Y. S. Xie, J. Yu, X. Long, B. Yang and S. H. Yang, Nanoscale, 2020, 12, 2472 RSC.
- K. Qian, L. Xia, Z. F. Jiang, W. Wei, L. L. Chen and J. M. Xie, Catal. Sci. Technol., 2017, 7, 3863 RSC.
- J. J. Shi, S. D. Li, F. M. Wang, L. N. Gao, Y. M. Li, X. R. Zhang and J. Lu, Dalton Trans., 2019, 48, 3327 RSC.
- Y. Liu, Y. Huang and X. F. Duan, Nature, 2019, 567, 323 CrossRef CAS PubMed.
- K. Kang, K. H. Lee, Y. M. Han, H. Gao, S. E. Xie, D. A. Muller and J. Park, Nature, 2017, 550, 229 CrossRef PubMed.
- K. A. S. Raj, N. Barman, K. Namsheer, R. Thapa and C. S. Rout, Sustainable Energy Fuels, 2022, 6, 5187 RSC.
- Z. W. Chen, M. Ju, M. Z. Sun, L. Jin, R. M. Cai, Z. Wang, L. Dong, L. M. Peng, X. Long, B. L. Huang and S. H. Yang, Angew. Chem., Int. Ed., 2021, 60, 9699 CrossRef CAS PubMed.
- P. F. Tan, A. Q. Zhu, L. L. Qiao, W. X. Zeng, Y. J. Ma, H. G. Dong, J. P. Xie and J. Pan, Inorg. Chem. Front., 2019, 6, 929 RSC.
- J. D. Hu, D. Y. Chen, Z. Mo, N. J. Li, Q. F. Xu, H. Li, J. H. He, H. Xu and J. M. Lu, Angew. Chem., Int. Ed., 2019, 58, 2073 CrossRef CAS PubMed.
- Q. Xu, B. Zhu, C. Jiang, B. Cheng and J. Yu, Solar RRL, 2018, 2, 1800006 CrossRef.
- J. R. Ran, W. W. Guo, H. L. Wang, B. C. Zhu, J. G. Yu and S. Z. Qiao, Adv. Mater., 2018, 30, 1800128 CrossRef PubMed.
- D. L. Jiang, T. Y. Wang, Q. Xu, D. Li, S. C. Meng and M. Chen, Appl Catal B Environ, 2017, 201, 617 CrossRef CAS.
- X. D. Ma, D. L. Jiang, P. Xiao, Y. Jin, S. C. Meng and M. Chen, Catal. Sci. Technol., 2017, 7, 3481 RSC.
- M. Ju, R. M. Cai, J. Z. Ren, J. X. Chen, L. M. Qi, X. Long and S. H. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 37063 CrossRef CAS PubMed.
- Y. Xu, Y. You, H. W. Huang, Y. X. Guo and Y. H. Zhang, J. Hazard. Mater., 2020, 381, 121159 CrossRef CAS PubMed.
- X. Q. Liu, Z. P. Zhou, D. L. Han, T. Wang, C. C. Ma, P. W. Huo and Y. S. Yan, J. Alloy Compd., 2020, 816, 152530 CrossRef CAS.
- M. D. Regulacio, C. Ye, S. H. Lim, M. Bosman, L. Polavarapu, W. L. Koh, J. Zhang, Q. H. Xu and M. Y. Han, J. Am. Chem. Soc., 2011, 133, 2052 CrossRef CAS PubMed.
- I. Sharma and B. R. Mehta, J. Alloy Compd., 2017, 723, 50 CrossRef CAS.
- Z. Xing, J. Hu, M. Ma, H. Lin, Y. M. An, Z. H. Liu, Y. Zhang, J. Y. Li and S. H. Yang, J. Am. Chem. Soc., 2019, 141, 19715 CrossRef CAS PubMed.
- A. B. Hungria, J. J. Calvino and J. C. Hernandez-Garrido, Top. Catal., 2019, 62, 808 CrossRef CAS.
- Q. L. He, H. C. Liu, M. Q. He, Y. H. Lai, H. T. He, G. Wang, K. T. Law, R. Lortz, J. N. Wang and I. K. Sou, Nat. Commun., 2014, 5, 4247 CrossRef CAS PubMed.
- M. Y. Li, Y. M. Shi, C. C. Cheng, L. S. Lu, Y. C. Lin, H. L. Tang, M. L. Tsai, C. W. Chu, K. H. Wei, J. H. He, W. H. Chang, K. Suenaga and L. J. Li, Science, 2015, 349, 524 CrossRef CAS PubMed.
- R. Y. Ge, J. J. Huo, M. J. Sun, M. Y. Zhu, Y. Li, S. L. Chou and W. X. Li, Small, 2021, 17, 1903380 CrossRef CAS PubMed.
- H. Wang, J. Qi, N. L. Yang, W. Cui, J. Y. Wang, Q. H. Li, Q. H. Zhang, X. Q. Yu, L. Gu, J. Li, R. B. Yu, K. K. Huang, S. Y. Song, S. H. Feng and D. Wang, Angew. Chem., Int. Ed., 2020, 59, 19691 CrossRef CAS PubMed.
- X. Li, L. L. Zhao, J. Y. Yu, X. Y. Liu, X. L. Zhang, H. Liu and W. J. Zhou, Nano-Micro Lett., 2020, 12, 131 CrossRef CAS PubMed.
- F. Dawood, M. Anda and G. M. Shafiullah, Int. J. Hydrogen Energy, 2020, 45, 3847 CrossRef CAS.
- X. Long, J. K. Li, S. Xiao, K. Y. Yan, Z. L. Wang, H. N. Chen and S. H. Yang, Angew. Chem., Int. Ed., 2014, 53, 7584 CrossRef CAS PubMed.
- W. Ma, R. Ma, C. Wang, J. Liang, X. Liu, K. Zhou and T. Sasaki, ACS Nano, 2015, 9, 1977 CrossRef CAS PubMed.
- Z. Zhang, P. Z. Liu, Y. H. Song, Y. Hou, B. S. Xu, T. Liao, H. X. Zhang, J. J. Guo and Z. Q. Sun, Adv. Sci., 2022, 2204297 CrossRef CAS PubMed.
- A. Rendon-Patino, A. Domenech-Carbo, A. Primo and H. Garcia, Nanomaterials, 2020, 10, 839 CrossRef CAS PubMed.
- P. Xiong, R. Z. Ma, N. Sakai, L. Nurdiwijayanto and T. Sasaki, ACS Energy Lett., 2018, 3, 997 CrossRef CAS.
- G. H. Wu, T. T. Li, Z. L. Wang, M. Z. Li, B. W. Wang and A. G. Dong, Angew. Chem., Int. Ed., 2020, 59, 20628 CrossRef CAS PubMed.
- C. Tang, L. Zhong, B. Zhang, H. F. Wang and Q. Zhang, Adv. Mater., 2018, 30, 1705110 CrossRef PubMed.
- H. He, Y. Chen, C. Yang, L. Yang, Q. Jiang and H. Huang, J. Energy Chem., 2022, 67, 483 CrossRef CAS.
- Z. K. Yuan, J. Li, M. J. Yang, Z. S. Fang, J. H. Jian, D. S. Yu, X. D. Chen and L. M. Dai, J. Am. Chem. Soc., 2019, 141, 4972 CrossRef CAS PubMed.
- X. Wang, R. K. M. Raghupathy, C. J. Querebillo, Z. Q. Liao, D. Q. Li, K. Lin, M. Hantusch, Z. Sofer, B. H. Li, E. Zschech, I. M. Weidinger, T. D. Kuhne, H. Mirhosseini, M. H. Yu and X. L. Feng, Adv. Mater., 2021, 33, 2008752 CrossRef CAS PubMed.
- X. Y. Li, L. P. Xiao, L. Zhou, Q. C. Xu, J. Weng, J. Xu and B. Liu, Angew. Chem., Int. Ed., 2020, 59, 21106 CrossRef CAS PubMed.
- J. Mei, J. Shang, T. W. He, D. C. Qi, L. Z. Kou, T. Liao, A. J. Du and Z. Q. Sun, Adv. Energy Mater., 2022, 12, 2201141 CrossRef CAS.
- P. V. Shinde, P. Mane, D. J. Late, B. Chakraborty and C. S. Rout, ACS Appl. Energy Mater., 2021, 4, 11886 CrossRef CAS.
- K. R. G. Lim, A. D. Handoko, S. K. Nemani, B. Wyatt, H.-Y. Jiang, J. Tang, B. Anasori and Z. W. Seh, ACS Nano, 2020, 14, 10834 CrossRef CAS PubMed.
- P. W. Xu, H. H. Wang, J. Liu, X. Z. Feng, W. J. Ji and C. T. Au, ACS Appl. Mater. Interfaces, 2021, 13, 34308 CrossRef CAS PubMed.
- M. Z. Yu, S. Zhou, Z. Y. Wang, J. J. Zhao and J. S. Qiu, Nano Energy, 2018, 44, 181 CrossRef CAS.
- C. Y. Li, D. D. Zhang, J. Cao, P. F. Yu, M. Okhawilai, J. Yi, J. Q. Qin and X. Y. Zhang, ACS Appl. Energy Mater., 2021, 4, 7821 CrossRef CAS.
- C. Y. Hao, Y. Wu, Y. J. An, B. H. Cui, J. N. Lin, X. N. Li, D. H. Wang, M. H. Jiang, Z. X. Cheng and S. Hu, Mater. Today Energy, 2019, 12, 453 CrossRef.
- C. F. Du, K. N. Dinh, Q. H. Liang, Y. Zheng, Y. B. Luo, J. L. Zhang and Q. Y. Yan, Adv. Energy Mater., 2018, 8, 1801127 CrossRef.
- M. Z. Yu, Z. Y. Wang, J. S. Liu, F. Sun, P. J. Yang and J. S. Qiu, Nano Energy, 2019, 63, 103880 CrossRef CAS.
- M. Yu, J. Zheng and M. Guo, J. Energy Chem., 2022, 70, 472 CrossRef CAS.
- Y. Wen, Z. Wei, J. Liu, R. Li, P. Wang, B. Zhou, X. Zhang, J. Li and Z. Li, J. Energy Chem., 2021, 52, 412 CrossRef CAS.
- G. Zuo, Y. Wang, W. L. Teo, A. Xie, Y. Guo, Y. Dai, W. Zhou, D. Jana, Q. Xian and W. Dong, Angew. Chem., 2020, 132, 11383 CrossRef.
- R. Yang, Y. M. Zhou, Y. Y. Xing, D. Li, D. L. Jiang, M. Chen, W. D. Shi and S. Q. Yuan, Appl. Catal., B, 2019, 253, 131 CrossRef CAS.
- H. Zhang, G. Q. Shen, X. Y. Liu, B. Ning, C. X. Shi, L. Pan, X. W. Zhang, Z. F. Huang and J. J. Zou, Chin. J. Catal., 2021, 42, 1732 CrossRef CAS.
- M. S. Islam, M. Kim, X. Y. Jin, S. M. Oh, N. S. Lee, H. Kim and S. J. Hwang, ACS Energy Lett., 2018, 3, 952 CrossRef CAS.
- M. A. Ahsan, T. W. He, J. C. Noveron, K. Reuter, A. R. Puente-Santiago and R. Luque, Chem. Soc. Rev., 2022, 51, 812 RSC.
- H. Gu, G. Shi, L. Zhong, L. Liu, H. Zhang, C. Yang, K. Yu, C. Zhu, J. Li, S. Zhang, C. Chen, Y. Han, S. Li and L. Zhang, J. Am. Chem. Soc., 2022, 144(47), 21502 CrossRef CAS PubMed.
- H. Shen, Y. Zhao, L. Zhang, Y. He, S. Yang, T. Wang, Y. Cao, Y. Guo, Q. Zhang and H. Zhang, Adv. Energy Mater., 2022, 13(1), 2202818 CrossRef.
- Y. S. Zhou, F. L. Che, M. Liu, C. Q. Zou, Z. Q. Liang, P. De Luna, H. F. Yuan, J. Li, Z. Q. Wang, H. P. Xie, H. M. Li, P. N. Chen, E. Bladt, R. Quintero-Bermudez, T. K. Sham, S. Bals, J. Hofkens, D. Sinton, G. Chen and E. H. Sargent, Nat. Chem., 2018, 10, 974 CrossRef CAS PubMed.
- D. Wakerley, S. Lamaison, F. Ozanam, N. Menguy, D. Mercier, P. Marcus, M. Fontecave and V. Mougel, Nat. Mater., 2019, 18, 1222 CrossRef CAS PubMed.
- W. C. Ma, S. J. Xie, T. T. Liu, Q. Y. Fan, J. Y. Ye, F. F. Sun, Z. Jiang, Q. H. Zhang, J. Cheng and Y. Wang, Nat. Catal., 2020, 3, 478 CrossRef CAS.
- H. F. Li, T. F. Liu, P. F. Wei, L. Lin, D. F. Gao, G. X. Wang and X. H. Bao, Angew. Chem., Int. Ed., 2021, 60, 14329 CrossRef CAS PubMed.
- Z. Y. Yin, C. Yu, Z. L. Zhao, X. F. Guo, M. Q. Shen, N. Li, M. Muzzio, J. R. Li, H. Liu, H. H. Lin, J. Yin, G. Lu, D. Su and S. H. Sun, Nano Lett., 2019, 19, 8658 CrossRef CAS PubMed.
- R. M. Cai, M. Z. Sun, J. Z. Ren, M. Ju, X. Long, B. L. Huang and S. H. Yang, Chem. Sci., 2021, 12, 15382 RSC.
- G. Z. S. Ling, S. F. Ng and W. J. Ong, Adv. Funct. Mater., 2022, 32, 2111875 CrossRef CAS.
- K. Chu, Y. P. Liu, Y. B. Li, Y. L. Guo and Y. Tian, ACS Appl. Mater. Interfaces, 2020, 12, 7081 CrossRef CAS PubMed.
- H. Zhang, T. W. Ouyang, J. M. Li, M. M. Mu and X. H. Yin, Electrochim. Acta, 2021, 390, 138766 CrossRef CAS.
- K. Wang, Q. P. Wang, K. J. Zhang, G. H. Wang and H. K. Wang, J. Mater. Sci. Technol., 2022, 124, 202 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |