Open Access Article
Open Access ArticleAdded value of the emissions fractions approach when assessing a chemical's potential for adverse effects as a result of long-range transport†
Knut
Breivik
 *ab,
Michael S.
McLachlan
*ab,
Michael S.
McLachlan
 c and
Frank
Wania
c and
Frank
Wania
 d
d
aNorwegian Institute for Air Research, P.O. Box 100, NO-2027 Kjeller, Norway. E-mail: kbr@nilu.no
bDepartment of Chemistry, University of Oslo, P.O. Box 1033, NO-0315 Oslo, Norway
cDepartment of Environmental Science, Stockholm University, SE-106 91 Stockholm, Sweden
dDepartment of Physical and Environmental Sciences, University of Toronto Scarborough, 1265 Military Trail, Toronto, Ontario M1C 1A4, Canada
First published on 29th August 2023
Abstract
It is of considerable interest to identify chemicals which may represent a hazard and risk to environmental and human health in remote areas. The OECD POV and LRTP Screening Tool (“The Tool”) for assessing chemicals for persistence (P) and long-range transport potential (LRTP) has been extensively used for combined P and LRTP assessments in various regulatory contexts, including the Stockholm Convention (SC) on Persistent Organic Pollutants (POPs). The approach in The Tool plots either the Characteristic Travel Distance (CTD, in km), a transport-oriented metric, or the Transfer Efficiency (TE, in %), which calculates the transfer from the atmosphere to surface compartments in a remote region, against overall persistence (POV). For a chemical to elicit adverse effects in remote areas, it not only needs to be transported and transferred to remote environmental surface media, it also needs to accumulate in these media. The current version of The Tool does not have a metric to quantify this process. We screened a list of >12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 high production volume chemicals (HPVs) for the potential to be dispersed, transferred, and accumulate in surface media in remote regions using the three corresponding LRTP metrics of the emission fractions approach (EFA; ϕ1, ϕ2, ϕ3), as implemented in a modified version of The Tool. Comparing the outcome of an assessment based on CTD/TE and POV with the EFA, we find that the latter classifies a larger number of HPVs as having the potential for accumulation in remote regions than is classified as POP-like by the existing approach. In particular, the EFA identifies chemicals capable of accumulating in remote regions without fulfilling the criterion for POV. The remote accumulation fraction of the EFA is the LRTP assessment metric most suited for the risk assessment stage in Annex E of the SC. Using simpler metrics (such as half-life criteria, POV, and LRTP–POV combinations) in a hazard-based assessment according to Annex D is problematic as it may prematurely screen out many of the chemicals with potential for adverse effects as a result of long-range transport.
000 high production volume chemicals (HPVs) for the potential to be dispersed, transferred, and accumulate in surface media in remote regions using the three corresponding LRTP metrics of the emission fractions approach (EFA; ϕ1, ϕ2, ϕ3), as implemented in a modified version of The Tool. Comparing the outcome of an assessment based on CTD/TE and POV with the EFA, we find that the latter classifies a larger number of HPVs as having the potential for accumulation in remote regions than is classified as POP-like by the existing approach. In particular, the EFA identifies chemicals capable of accumulating in remote regions without fulfilling the criterion for POV. The remote accumulation fraction of the EFA is the LRTP assessment metric most suited for the risk assessment stage in Annex E of the SC. Using simpler metrics (such as half-life criteria, POV, and LRTP–POV combinations) in a hazard-based assessment according to Annex D is problematic as it may prematurely screen out many of the chemicals with potential for adverse effects as a result of long-range transport.
Environmental significanceThe Stockholm Convention (SC) has motivated the development of various models and metrics to assess a chemical's potential for long-range environmental transport (LRT). Using a new set of metrics, referred to as the emission fractions approach (EFA), we explore whether the existing LRT metrics recommended by the OECD align with the needs of the SC. A comparison between the existing metrics and the EFA suggests that the utility of the existing approach is limited in terms of identifying whether a chemical is likely as a result of its LRT to elicit adverse effects. The screening approach explored herein should enable future model applications of regulatory and scientific interest that are not possible using the existing method recommended by the OECD. |
1. Introduction
The environmental occurrence of Persistent Organic Pollutants (POPs) and other organic chemicals of emerging concern (CECs) in remote areas such as the Arctic and the Antarctic has received considerable attention. A major concern has been the detection of POPs and CECs in biota, potentially leading to adverse effects on wildlife and human health.1,2 A prerequisite for significant adverse human health and/or environmental effects occurring as a result of long-range environmental transport (LRT) is the accumulation of a given chemical in surface media of the remote environment.The key regulatory framework for POPs is the Stockholm Convention (SC), which is a global agreement to protect human health and the environment from chemicals that are persistent (P), bioaccumulative (B), toxic (T) and have potential for long-range environmental transport (LRTP) to remote areas.3 The requirement for an organic CEC to be listed under the SC is that it “is likely as a result of its long-range environmental transport to lead to significant adverse human health and/or environmental effects”, i.e., the chemical must not only undergo LRT to remote regions, but it must also accumulate in environmental surface media to an extent sufficient to cause harm.
The number of chemicals which have been recognized as POPs has increased since the SC came into effect, as the SC includes provisions for parties to nominate new chemicals for potential amendment. A nomination process involves simple screening criteria such as an atmospheric half-life in air larger than 2 days for LRTP. The UNECE Convention on long-range transboundary air pollution additionally calls for evidence that the substance has a vapor pressure < 1000 Pa for LRATP.4 However, these criteria do not offer any information on whether and to what extent a chemical of interest has the potential for accumulation in surface media in a remote region following long-range atmospheric transport (LRAT).
The simplest criteria for P under the SC and other regulatory frameworks are based on half-lives in surface media, i.e., the time required for the concentration in an environmental medium of interest to be reduced by 50%. Under the SC, a chemical fulfils the P criterion if it has a half-life in water larger than 2 months, and in soil/sediment in excess of 6 months. However, single-media half-life criteria may not be relevant unless the chemical is likely to reside in the media under consideration. For example, the degradation half-life in surface media does not offer relevant information for a highly volatile and inert chemical which is emitted to air, and which does not deposit.
In response to the need for a more comprehensive assessment of LRTP and P that takes into account how chemicals distribute in the environment, different mathematical models have been developed, e.g.5–9 The OECD POV and LRTP Screening Tool for assessing chemicals for P and LRTP (“The Tool”)10 is an example of a model designed for screening the environmental hazard potential of chemicals. As an alternative to single-media half-lives, The Tool calculates an overall persistence (POV) which accounts for the distribution of a chemical across environmental compartments as influenced by the mode of entry into the environment.11 The two LRTP metrics in The Tool10 quantify the potential of a chemical for (i) transport from a source to a remote region (CTD – characteristic travel distance12) in air or water, and (ii) transfer from the atmosphere to surface media in a remote region (TE – transfer efficiency13). However, neither of these metrics explicitly addresses the potential for accumulation in surface media in a remote region.14 Rather, the main outputs of the model are two charts plotting CTD and TE, respectively, against POV, i.e., the assessment is based on the assumption that the combination of a LRTP metric and POV identifies chemicals potentially hazardous to remote regions.
The first version of The Tool became available in 2005, whereas the current version (2.2) was published in 2009.10 This software has been applied in the regulatory assessment of a large number of POP candidates under the SC (Annex E, risk profiles). Recent examples include UV-328,15 Dechlorane Plus,16 and medium chain chlorinated paraffins.17 It has also been used in scientific studies screening chemicals for LRTP.18,19 We have recently identified a number of limitations of the LRTP metrics in The Tool which could lead to false positive and negative categorisations in LRTP assessment: (1) Neither CTD nor TE allow for combined transport in water and air. (2) The TE does not account for transfer to a remote region via media other than air, nor does it consider reversible atmospheric deposition and the consequences thereof. (3) Neither CTD nor TE assess accumulation in environmental surface media. As an alternative to CTD and TE, we have proposed a set of alternative metrics, collectively referred to as the Emission Fractions Approach (EFA),20 which offers opportunities for more coherent LRTP assessments.
The objective of this study was to assess the implications of the choice of LRTP metrics when screening a large set of chemicals. Of specific interest was the extent of agreement between the outcome of LRTP assessments based on the current approach [plots of CTD or TE versus POV] versus an alternative EFA metric, which in addition to dispersion and transfer explicitly accounts for accumulation in surface media in a remote region.
2. Methods
We used a version of The Tool (version 2.2) that calculates the EFA metrics20 in addition to POV, CTD and TE.10 We refer to Wegmann et al.10 for a detailed description of The Tool and the existing metrics and offer only a brief description herein. The Tool is a consensus-based model reflecting the state-of-science at the time of its development.7,8,10,21,22 It is a steady-state (level 3), fugacity-based multimedia model23 with one air, one water and one soil compartment. It is not spatially resolved, and the parameters have been chosen to reflect the global environment.10 For example, the surface area of soil and water in the model reflect the area of the globe covered by land and ocean, respectively. The input parameters required to characterize each chemical are degradation half-lives in air, water and soil, as well as the logarithm of the equilibrium partitioning ratios between air and water (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW) and octanol and water (log
KAW) and octanol and water (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW). Each chemical is simulated three times, using three different emission scenarios (100% emissions to air, water and soil, respectively). The maximum values for POV, CTD and TE across these emission scenarios are highlighted in the tabulated outputs and are used in the CTD/TE vs. POV plots. Hence, the default output for POV, CTD and TE of a chemical may not necessarily reflect the same emission scenario.10 For example, the highest CTD is typically obtained for the model scenario with 100% emissions to air, whereas the highest value for POV for chemicals with a relatively high hydrophobicity (high log
KOW). Each chemical is simulated three times, using three different emission scenarios (100% emissions to air, water and soil, respectively). The maximum values for POV, CTD and TE across these emission scenarios are highlighted in the tabulated outputs and are used in the CTD/TE vs. POV plots. Hence, the default output for POV, CTD and TE of a chemical may not necessarily reflect the same emission scenario.10 For example, the highest CTD is typically obtained for the model scenario with 100% emissions to air, whereas the highest value for POV for chemicals with a relatively high hydrophobicity (high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW) is likely to be calculated in the scenario with 100% emissions to soil.
KOW) is likely to be calculated in the scenario with 100% emissions to soil.
The three metrics of the EFA approach are described in Breivik et al.20 and defined in Table 1. Because these three metrics are all normalized by the rate of global emissions, they are all intensive properties, i.e., are independent of the amount of chemical. The environmentally dispersed fraction ϕ1 expresses the relative potential of a chemical to undergo dispersion (i.e. long-range environmental transport) by air and water combined. The remotely transferred fraction ϕ2 expresses the relative extent to which a chemical is net transferred to surface compartments of a remote region, accounting for environmental dispersion in air and water (ϕ1). The remotely accumulated fraction ϕ3 expresses the relative extent to which a chemical is accumulating in surface compartments of a remote region, accounting for dispersion (ϕ1) and transfer (ϕ2) in air and water. Hence, CTD and ϕ1 are transport-oriented LRTP metrics, TE and ϕ2 are transfer-oriented LRTP metrics, whereas ϕ3 is a metric which explicitly targets remote accumulation, similar to the Arctic contamination potential.14 Neither CTD nor TE mirrors the scope of ϕ3. The model in The Tool can be used to calculate the three EFA metrics.
| Metric | Definition |
|---|---|
| ϕ 1 | The environmentally dispersed fraction (ϕ1) quantifies the relative extent to which a chemical can reach remote regions |
| ϕ 2 | The remotely transferred fraction (ϕ2) expresses to what relative extent a chemical can reach surface media in remote regions |
| ϕ 3 | The remotely accumulated fraction (ϕ3) assesses the fraction of chemicals emissions accumulating in surface media of remote regions |
While all environmental input parameters for our calculations were taken from Wegmann et al.,10 we modified the code to account for the intermittency of precipitation.20 While this will lead to different numerical results for chemicals subject to wet deposition compared to outputs from the existing version of The Tool, it ensures a consistent approach in the analysis presented herein.
For the analysis we chose a data set of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 HPVs curated by Arnot et al.,24 because it contained the required physical–chemical partitioning properties and degradation half-lives. The point of departure for this data set was a list of 15
615 HPVs curated by Arnot et al.,24 because it contained the required physical–chemical partitioning properties and degradation half-lives. The point of departure for this data set was a list of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 organic chemicals identified by their CAS numbers that included not only neutral chemicals, but also acids, bases, and salts. Structural information was used to remove dissociating chemicals from the dataset. The properties of the final set of discrete chemical structures span a wide range. Specifically, log
800 organic chemicals identified by their CAS numbers that included not only neutral chemicals, but also acids, bases, and salts. Structural information was used to remove dissociating chemicals from the dataset. The properties of the final set of discrete chemical structures span a wide range. Specifically, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW varies from −12 to 3 while log
KAW varies from −12 to 3 while log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW ranges from −4 to 9. Degradation half-lives in air, water and soil vary from <2 s to >150 years, 12 hours to >10 years, and 23 hours to 23 years, respectively.
KOW ranges from −4 to 9. Degradation half-lives in air, water and soil vary from <2 s to >150 years, 12 hours to >10 years, and 23 hours to 23 years, respectively.
The thresholds for the LRTP metrics and POV, shown in Table 2 were determined as the lowest values obtained for a set of 14 legacy POPs with any of the three default emission scenarios.20Table 2 also gives the number and percentage of chemicals out of the 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 exceeding each threshold. We have also identified the chemical which defines each threshold (i.e., the POP which has the lowest value of a metric).
615 exceeding each threshold. We have also identified the chemical which defines each threshold (i.e., the POP which has the lowest value of a metric).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 organic chemicals exceeding each of these thresholds are included
615 organic chemicals exceeding each of these thresholds are included
N = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 615 |
ϕ 1 | ϕ 2 | ϕ 3 | CTD | TE | P OV |
|---|---|---|---|---|---|---|
| Threshold | 7.7 × 10−4 | 8.4 × 10−5 | 8.2 × 10−6 | 1021 km | 0.32 | 480 days |
| Threshold (log) | −3.1 | −4.1 | −5.1 | 3.01 | −0.49 | 2.68 |
| Chemical defining the threshold | cis-Chlordane | trans-Chlordane | PCB-28 | cis-Chlordane | trans-Chlordane | HCB |
| N ≥ threshold | 3538 | 5986 | 2980 | 3312 | 5262 | 1511 |
| N ≥ threshold (%) | 28.0 | 47.5 | 23.6 | 26.3 | 41.7 | 12.0 |
3. Results
3.1. Existing metrics (CTD–POV and TE–POV plots)
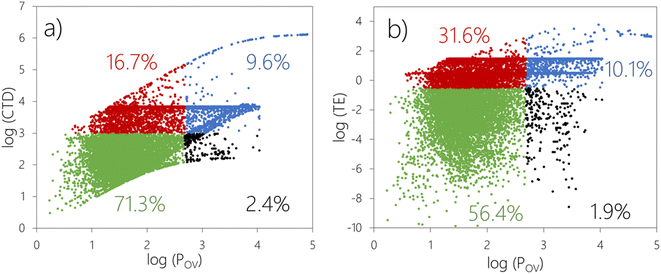
Fig. 1 displays the CTD–POV and TE–POV plots. Chemicals falling into each quadrant in these plots are highlighted with differently colored markers. The combined assessment based on CTD–POV (Fig. 1a) flags 8997 out of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 chemicals (71.3%) as non-POP like for both LRTP and POV (green markers). 306 chemicals (2.4%) are assessed as not having LRTP according to CTD (i.e., CTD below the threshold in Table 2) but POP-like POV (black markers). 2107 chemicals (16.7%) are assessed as having LRTP (CTD) but not POP-like POV, while 1205 chemicals (9.6%) are flagged with POP-like LRTP behavior and persistence.
615 chemicals (71.3%) as non-POP like for both LRTP and POV (green markers). 306 chemicals (2.4%) are assessed as not having LRTP according to CTD (i.e., CTD below the threshold in Table 2) but POP-like POV (black markers). 2107 chemicals (16.7%) are assessed as having LRTP (CTD) but not POP-like POV, while 1205 chemicals (9.6%) are flagged with POP-like LRTP behavior and persistence.
The assessment relying on TE–POV (Fig. 1b) predicts that (i) 7115 chemicals (56.4%) are not POP-like regarding both LRTP and POV, (ii) 238 chemicals (1.9%) do not exceed the threshold for TE, but exceed the threshold for POV, (iii) 3989 chemicals (31.6%) are POP-like in terms of TE, but not above the threshold for POV, and (iv) 1273 chemicals (10.1%) are POP-like according to both TE and POV.
1131 chemicals (9.0%) are flagged as POP-like by both TE–POV and CTD–POV, i.e., fall into the upper right quadrant in both the CTD–POV plot (Fig. 1a) and TE–POV plot (Fig. 1b). Another 216 chemicals exceed either the CTD–POV criteria or the TE–POV criteria, but not both.
3.2. CTD versus ϕ1 and TE versus ϕ2
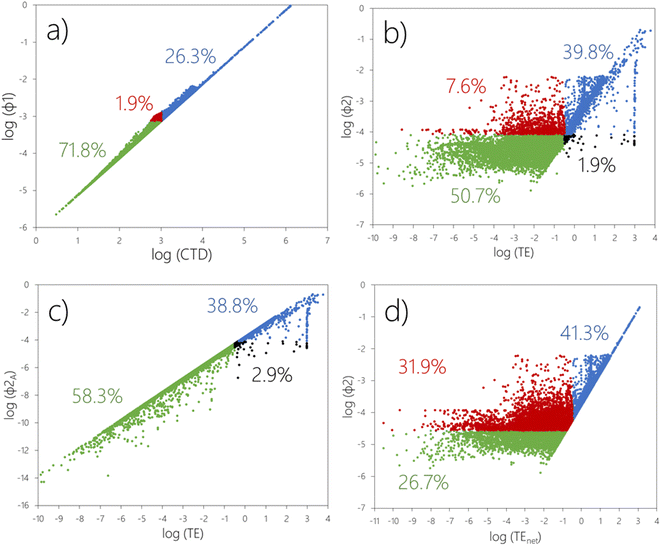
In Fig. 2a, we have plotted the existing as well as the alternative transport-oriented metrics against each other, i.e., CTD versus ϕ1. Please note that the CTD here is the larger of the CTD in air or water. The existing and alternative transfer-oriented metrics, i.e., TE versus ϕ2, are analyzed in Fig. 2b–d.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line. In Fig. 2a, 242 chemicals (1.9%) fall into the region highlighted with red markers. These chemicals are above the threshold for ϕ1A, but below the threshold for CTD (Table 2). In other words, these are chemicals which only will be classified as having POP-like LRTP if combined transport in air and water is considered. While the fraction of chemicals affected may appear small, it clearly indicates the potential for false negative LRTP classification, even if CTDA and CTDW are both taken into consideration. We note that two decades ago, Beyer and Matthies25 already used model simulations to show that “combined transport in coupled air–ocean systems can accelerate the overall transport into remote regions”, and later Stroebe et al.26 confirmed that combined transport may enhance the transport efficiency for some chemicals.
1 line. In Fig. 2a, 242 chemicals (1.9%) fall into the region highlighted with red markers. These chemicals are above the threshold for ϕ1A, but below the threshold for CTD (Table 2). In other words, these are chemicals which only will be classified as having POP-like LRTP if combined transport in air and water is considered. While the fraction of chemicals affected may appear small, it clearly indicates the potential for false negative LRTP classification, even if CTDA and CTDW are both taken into consideration. We note that two decades ago, Beyer and Matthies25 already used model simulations to show that “combined transport in coupled air–ocean systems can accelerate the overall transport into remote regions”, and later Stroebe et al.26 confirmed that combined transport may enhance the transport efficiency for some chemicals.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line in a plot of ϕ2A against TE (Fig. 2c). This is because the only difference between the two metrics is that ϕ2A considers reversibility of atmospheric deposition whereas TE does not. The TE therefore overestimates atmospheric inputs to surface media of the remote region for any chemical that undergoes repeated air–surface exchange,10,20 causing false positives for 374 chemicals in the screening data set (2.9%) (Fig. 2c, black markers).
1 line in a plot of ϕ2A against TE (Fig. 2c). This is because the only difference between the two metrics is that ϕ2A considers reversibility of atmospheric deposition whereas TE does not. The TE therefore overestimates atmospheric inputs to surface media of the remote region for any chemical that undergoes repeated air–surface exchange,10,20 causing false positives for 374 chemicals in the screening data set (2.9%) (Fig. 2c, black markers).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line in a plot of ϕ2 against TEnet in Fig. 2d. This is because ϕ2 allows for transport to the remote region with water whereas this pathway is not accounted for in the TEnet. In Fig. 2d, there also appears to be an upper boundary for log
1 line in a plot of ϕ2 against TEnet in Fig. 2d. This is because ϕ2 allows for transport to the remote region with water whereas this pathway is not accounted for in the TEnet. In Fig. 2d, there also appears to be an upper boundary for log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϕ2 occurring around −2.3 for chemicals with a log
ϕ2 occurring around −2.3 for chemicals with a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEnet < 1.5. This is because any involatile chemical that is emitted to air and undergoes LRAT has a predicted TE (and TEnet) of 0.5% and a predicted ϕ2A (and ϕ2) of 0.005. ϕ2A is also identical to ϕ1A because airborne involatiles readily deposit in the remote region. Note that similar mechanistic inferences cannot be made based on the existing transport- and transfer-oriented metrics (CTDs and TEs) as these are inconsistent with each other.
TEnet < 1.5. This is because any involatile chemical that is emitted to air and undergoes LRAT has a predicted TE (and TEnet) of 0.5% and a predicted ϕ2A (and ϕ2) of 0.005. ϕ2A is also identical to ϕ1A because airborne involatiles readily deposit in the remote region. Note that similar mechanistic inferences cannot be made based on the existing transport- and transfer-oriented metrics (CTDs and TEs) as these are inconsistent with each other.
In summary, the TE's failure to account for transport in water affects a far larger fraction of screened chemicals (31.9%, red markers in Fig. 2d) than TE's failure to account for reversible deposition (2.9%, black markers in Fig. 2c). However, the failure to account for reversible deposition often compensates for the failure to account for water transport, so overall TE misclassifies “only” 9.5% of screened chemicals (red plus black markers in Fig. 2b).
3.3. LRTP–POVversus accumulation (ϕ3)
The aim of Annex E of the SC is to evaluate whether the chemical is likely, as a result of its long-range environmental transport, to lead to significant adverse human health and/or environmental effects, such that global action is warranted.27 The existing approach to LRTP assessment does not have a metric that seeks to quantify the accumulation of a chemical in a remote region, even though such accumulation is arguably a prerequisite for significant adverse effects to occur. To assess whether an existing LRTP metric and a persistence metric in combination identify chemicals with the potential for accumulation in remote regions, we first compare the chemicals categorized as having POP-like LRTP based on a metric directly quantifying remote accumulation (ϕ3) versus CTD and TE without consideration of the POV (Fig. 3a), and next with the chemicals identified as POP-like in the CTD–POV and TE–POV plots in Section 3.1 above (Fig. 3b).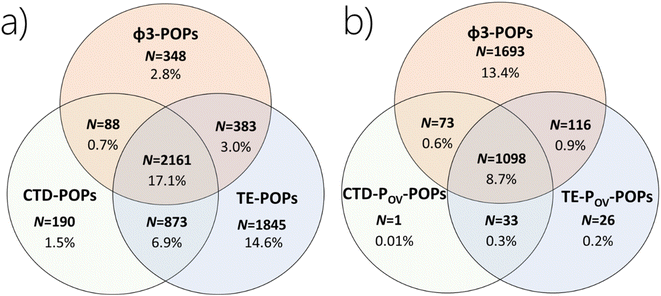 | ||
Fig. 3 Comparison of the number of chemicals in the screening data set which (a) exceed the criteria for CTD, TE and ϕ3, and (b) CTD–POV, TE–POV and ϕ3 (percentages of all 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 chemicals studied). 615 chemicals studied). | ||
From Table 2, we see that there are 3312, 5262 and 2980 chemicals which exceed the thresholds for CTD, TE, and ϕ3, respectively. Fig. 3a can be used to explore how successful CTD and TE would be at identifying chemicals that are judged by ϕ3 to have potential for accumulation in a remote environment. Of the 2980 chemicals with a ϕ3 above the threshold, 731 (25%) and 436 (15%) are not identified by CTD and TE, respectively. If both CTD and TE were used, there would be 819 (27%) false negatives among the 2980 chemicals with POP-like ϕ3, whereas the number of false negatives would be reduced to 348 chemicals (12%) if either CTD or TE was used. Thus, CTD and TE are moderately useful for screening for accumulation in remote regions.
In Fig. 3b we compare the overlap between categories of chemicals which exceed CTD–POV and/or TE–POV and/or ϕ3. There are 1693 chemicals (13.4% in the screening data set) which are not captured as POP-like according to ϕ3 using either CTD–POV or TE–POV. The number of chemicals which are POP-like using the existing approach (CTD–POV and TE–POV) is 1131 or 9.0%, whereas the number of chemicals which are either CTD–POV or TE–POV but not both is 216 (1.7%). In comparison, the total number of chemicals which exceeds the criterion for POP-like accumulation (ϕ3) is 2980 or 23.6% (Table 2), i.e., more than 2.6 times the number of chemicals fulfilling both the CTD–POV and the TE–POV criteria. Among the 2980 chemicals in the screening data set which exceed the criterion for POP-like accumulation (ϕ3), there are 1400 chemicals which fulfill the criterion for POV and 1580 chemicals which do not. In other words, there are many chemicals assessed to have the potential to accumulate in remote regions (based on ϕ3) without meeting the POV criterion. We conclude that using the CTD–POV and/or TE–POV combination to identify chemicals that have remote accumulation potential would be prone to many false negative decisions.
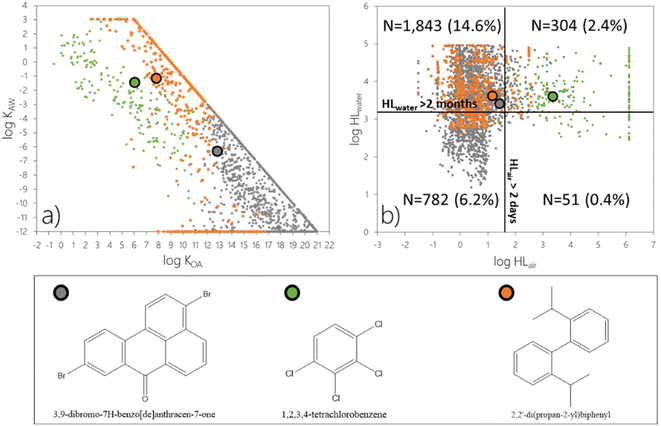
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA below or above 11, respectively. Orange markers are used to identify the rest of the chemicals which exceed the threshold for ϕ3 only if both LRAT and LRWT are considered. While the log
KOA below or above 11, respectively. Orange markers are used to identify the rest of the chemicals which exceed the threshold for ϕ3 only if both LRAT and LRWT are considered. While the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW and log
KOW and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW of the chemicals in the screening data set were capped at values of 9 and -12, respectively24 (as apparent in Fig. 4a), the model result is not sensitive to these partitioning properties, when they exceed these values. Fig. 4 additionally highlights model predictions for one chemical from each of these three categories. Data on physical–chemical properties and environmental degradation half-lives for individual chemicals discussed herein are included in Table S1.†
KAW of the chemicals in the screening data set were capped at values of 9 and -12, respectively24 (as apparent in Fig. 4a), the model result is not sensitive to these partitioning properties, when they exceed these values. Fig. 4 additionally highlights model predictions for one chemical from each of these three categories. Data on physical–chemical properties and environmental degradation half-lives for individual chemicals discussed herein are included in Table S1.†
Most chemicals which exceed the criterion for ϕ3 as result of LRAT have a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA above 11 (N = 2242 or 17.8%) and therefore are predominantly sorbed to atmospheric particles in The Tool. They can undergo LRAT even if their degradation half-life in air is relatively short (Fig. 4b; grey markers) because it is assumed that sorption to particles prevents them from undergoing atmospheric degradation reactions. An example is 3,9-dibromo-7H-benzo[de]anthracen-7-one with a degradation half-life in air of 28 h and an estimated log
KOA above 11 (N = 2242 or 17.8%) and therefore are predominantly sorbed to atmospheric particles in The Tool. They can undergo LRAT even if their degradation half-life in air is relatively short (Fig. 4b; grey markers) because it is assumed that sorption to particles prevents them from undergoing atmospheric degradation reactions. An example is 3,9-dibromo-7H-benzo[de]anthracen-7-one with a degradation half-life in air of 28 h and an estimated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA of 12.8 (Table S1 and Fig. S1a†). This chemical, if emitted to air, exceeds the threshold for ϕ3 (Table 2) by more than an order of magnitude (Table S2†). Under this emission scenario, 3,9-dibromo-7H-benzo[de]anthracen-7-one is furthermore prone to be dispersed in the atmosphere (ϕ1), transferred to water (72.6%) and soil (27.4%) (ϕ2), and to mainly accumulate in water (ϕ3) (bars in the lower part of Fig. S1a†). This chemical is also predicted to exceed the threshold for ϕ3 if it is emitted to water (Fig. S1a†). As 3,9-dibromo-7H-benzo[de]anthracen-7-one is not prone to evaporate from the water compartment if emitted to water, the predicted dispersion, transfer, and accumulation are all almost exclusively associated with the water compartment (Fig. S1a†).
KOA of 12.8 (Table S1 and Fig. S1a†). This chemical, if emitted to air, exceeds the threshold for ϕ3 (Table 2) by more than an order of magnitude (Table S2†). Under this emission scenario, 3,9-dibromo-7H-benzo[de]anthracen-7-one is furthermore prone to be dispersed in the atmosphere (ϕ1), transferred to water (72.6%) and soil (27.4%) (ϕ2), and to mainly accumulate in water (ϕ3) (bars in the lower part of Fig. S1a†). This chemical is also predicted to exceed the threshold for ϕ3 if it is emitted to water (Fig. S1a†). As 3,9-dibromo-7H-benzo[de]anthracen-7-one is not prone to evaporate from the water compartment if emitted to water, the predicted dispersion, transfer, and accumulation are all almost exclusively associated with the water compartment (Fig. S1a†).
On the other hand, chemicals which exceed the criterion for ϕ3 as a result of LRAT but predominantly occur in the atmospheric gas phase, i.e., have an estimated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA < 11 (N = 224 or 1.8%, green markers), need to be relatively persistent in air (HLair > 1 day) (Fig. 4b). 1,2,3,4-Tetrachlorobenzene with a log
KOA < 11 (N = 224 or 1.8%, green markers), need to be relatively persistent in air (HLair > 1 day) (Fig. 4b). 1,2,3,4-Tetrachlorobenzene with a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA of 6.07 and a degradation half-life in air of 98 days serves as an example (Table S1†). This chemical exceeds the thresholds for ϕ1, ϕ2, and ϕ3 irrespective of the mode of emissions (Fig. S1b†).
KOA of 6.07 and a degradation half-life in air of 98 days serves as an example (Table S1†). This chemical exceeds the thresholds for ϕ1, ϕ2, and ϕ3 irrespective of the mode of emissions (Fig. S1b†).
Not surprisingly, the 514 chemicals (4.1%) that only exceed the ϕ3 threshold when both LRAT and LRWT are accounted for are not readily degraded in water, and all have a HLwater > 17 days (orange markers in Fig. 4b). They also have partitioning properties that do not allow for significant evaporation from water, either because they have a low log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW (approximately below −4) or because they are particle-bound in water (have a log
KAW (approximately below −4) or because they are particle-bound in water (have a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW > 6) (orange markers in Fig. 3a). 2,2′-Di(propan-2-yl)biphenyl qualifies for the latter criterion with a log
KOW > 6) (orange markers in Fig. 3a). 2,2′-Di(propan-2-yl)biphenyl qualifies for the latter criterion with a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW of 6.67. This chemical exceeds the criterion for accumulation (ϕ3) only if emitted to water (Fig. S1c†). While 2,2′-di(propan-2-yl)biphenyl is predicted to be dispersed from the source region in both air and water in equal amounts under this emission scenario, it is transferred to, and accumulates mostly in, water (Fig. S1c†). These results demonstrate that the combination of diagnostic plots (Fig. 4) in concert with the plots shown in Fig. S1† offer a powerful tool to assess the factors which dictate LRTP of chemicals in screening exercises.
KOW of 6.67. This chemical exceeds the criterion for accumulation (ϕ3) only if emitted to water (Fig. S1c†). While 2,2′-di(propan-2-yl)biphenyl is predicted to be dispersed from the source region in both air and water in equal amounts under this emission scenario, it is transferred to, and accumulates mostly in, water (Fig. S1c†). These results demonstrate that the combination of diagnostic plots (Fig. 4) in concert with the plots shown in Fig. S1† offer a powerful tool to assess the factors which dictate LRTP of chemicals in screening exercises.
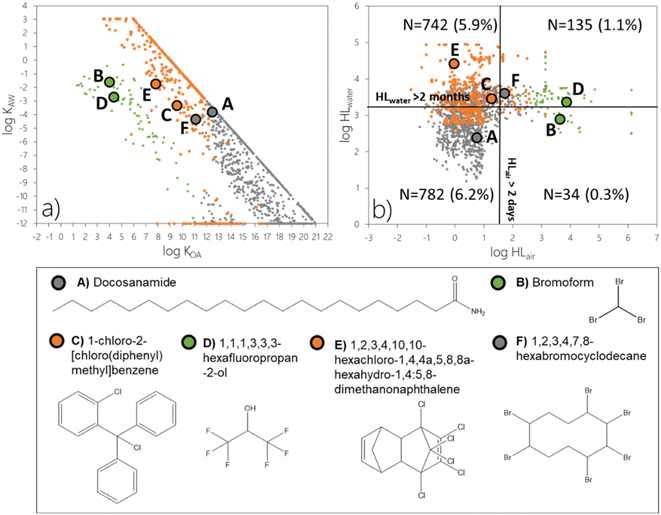
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA > 11 (N = 1204 out of 2242 or 9.5% of the screening data set, grey markers), close to half of the chemicals undergoing LRAT in the gas phase (N = 99 out of 224; 0.8%, green markers), and most of the chemicals subject to LRWT (N = 390 out of 514; 3.1%, orange markers). In terms of partitioning ratios, these 1693 chemicals span a very wide range. Log
KOA > 11 (N = 1204 out of 2242 or 9.5% of the screening data set, grey markers), close to half of the chemicals undergoing LRAT in the gas phase (N = 99 out of 224; 0.8%, green markers), and most of the chemicals subject to LRWT (N = 390 out of 514; 3.1%, orange markers). In terms of partitioning ratios, these 1693 chemicals span a very wide range. Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA varies by more than 17 orders of magnitude (3.5 to 21), log
KOA varies by more than 17 orders of magnitude (3.5 to 21), log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW varies by ∼15 orders of magnitude (−12 to 3), while log
KAW varies by ∼15 orders of magnitude (−12 to 3), while log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW varies by 13 orders of magnitude (−4 to 9) (Fig. 5a). Clearly, partitioning ratios are poor predictors to identify chemicals which are POP-like according to ϕ3 but not TE–POV and/or CTD–POV.
KOW varies by 13 orders of magnitude (−4 to 9) (Fig. 5a). Clearly, partitioning ratios are poor predictors to identify chemicals which are POP-like according to ϕ3 but not TE–POV and/or CTD–POV.
A comparison of the grey markers between Fig. 4b and 5b reveals that it is mostly the involatile chemicals that are highly persistent in surface media (with a log(HLwater/hour) > 4) that are recognized by the LRTP–POV approach as being subject to remote accumulation. This is in contrast to the EFA, which recognizes involatile chemicals as prone to remote accumulation above the threshold for ϕ3 in spite of relatively short degradation half-lives in surface media. Docosanamide and to some extent 1,2,3,4,7,8-hexabromocyclodecane serve as examples (Fig. 5; S2a and S2f†). While a high potential for remote accumulation of chemicals which have short degradation half-lives in surface media may appear counterintuitive, this is a result of the multiplicative feature of the EFA. Any involatile chemical is predicted to have high ϕ1 and ϕ2 if emitted to air. Hence, ϕ3 may exceed the threshold even if the fraction of deposited chemical that is retained in surface media is relatively limited (Fig. S2a and f†).
A comparison of Fig. 4 and 5 similarly suggests that it is the highly volatile chemicals (green markers in the upper left of Fig. 4a) and those that are extremely persistent in air (green markers on the right side of Fig. 4b) that the existing LRTP–POV approach will recognize as POP-like. However, the LRTP–POV approach does not recognize the potential for remote accumulation of many volatile chemicals which are persistent in air because they do not fulfill the criterion for POV. An example is 1,1,1,3,3,3-hexafluoropropan-2-ol (Fig. 5) with an atmospheric reaction half-life of about 7500 hours (Table S1†) and a POV of 174 days (Table S2†) which exceeds the criterion for ϕ3 for any emission scenario (Fig. S2d†). Bromoform represents another example of a chemical which is persistent in air but which neither exceeds the criteria for overall persistence (Table S2†) nor does it have a degradation half-life in water in excess of 2 months (Table S1†).
1,2,3,4,10,10-Hexachloro-1,4,4a,5,8,8a-hexahydro-1,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5,8-dimethanonaphthalene and 1-chloro-2-[chloro(diphenyl)methyl]benzene offer examples of chemicals which do not meet the criterion for the existing LRTP–POV approaches but exceed the criterion for ϕ3 when transport in both air and water is accounted for (Fig. 5) if emitted to water (Fig. S2e and c†). For this emission scenario, remote transfer of these two chemicals mainly occurs in water, which is not accounted for in The Tool.
5,8-dimethanonaphthalene and 1-chloro-2-[chloro(diphenyl)methyl]benzene offer examples of chemicals which do not meet the criterion for the existing LRTP–POV approaches but exceed the criterion for ϕ3 when transport in both air and water is accounted for (Fig. 5) if emitted to water (Fig. S2e and c†). For this emission scenario, remote transfer of these two chemicals mainly occurs in water, which is not accounted for in The Tool.
The use of either CTD–POV or TE–POV flags many of the same substances in the screening data set (Fig. 3b). Plots showing the compounds not identified by TE–POV look rather similar to the plots showing the compounds not identified by CTD–POV in Fig. 5 and are therefore not discussed. There are 60 chemicals which exceed the thresholds for either CTD–POV and/or TE–POV but not the threshold for ϕ3 (Fig. 3b). 59 out of these 60 chemicals exceed the threshold for TE–POV. Most chemicals are among the most volatile chemicals in the screening data and very persistent in air (Fig. S3†). Some involatile chemicals which exceed the persistence criteria in water with a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW of ∼4 are also suggested to be POP-like according to TE–POV but not ϕ3.
KOW of ∼4 are also suggested to be POP-like according to TE–POV but not ϕ3.
It is clear from this analysis that the existing POP-like criteria involving a threshold for POV (CTD–POV and/or TE–POV) do not identify more than half of the chemicals that are subject to dispersion, transfer and accumulation in surface media of the remote region according to the EFA. Fig. 5 illustrates that the explanation for this is not simple. The failure to account for combined air and water transport is one of the reasons. Another is that some chemicals have the potential for remote accumulation without meeting the POV persistence criterion.
3.4. Implications for LRTP assessments
The listing of a new POP in the Stockholm Convention involves two steps: The proposal, outlined in Annex D, should provide evidence that the nominated chemical is persistent, bioaccumulative, has the potential for long range transport and elicits adverse effects. A subsequent risk profile, described in Annex E, “further elaborates on, and evaluates, the information referred to in Annex D” with the purpose of establishing in the assessment “whether the chemical is likely, as a result of its long-range environmental transport, to lead to significant adverse human health and/or environmental effects, such that global action is warranted” (paragraph 7a of article 8). This procedure is built on the premise that a chemical needs to fulfill the four criteria in Annex D in order to fulfill the ultimate listing requirement in Annex E, in other words it did not foresee the possibility that a chemical can lead to significant adverse effects in remote regions without being persistent and bioaccumulative. However, clearly the main criterion of Annex E should supersede the criteria of Annex D. The Stockholm Convention is not targeting chemicals that meet formal criteria of P, B, LRTP, and T. These criteria rather are meant to aid in the task of identifying chemicals that meet the definition in paragraph 7a of article 8.The results above demonstrate that accumulation in remote regions cannot be approximated by the intersection of POV and TE/CTD and that a metric such as ϕ3 is better suited to provide insight for assessment within the context of Annex E of the SC. This is because ϕ3 screens for accumulation in the remote environment, while the CTD–POV or TE–POV combination screens for LRTP and persistence relevant for Annex D. The question arises whether the existing approach (LRTP–POV) and the EFA (ϕ3) are consistent with each other when the former is applied for Annex D and the latter for Annex E? The answer is no because any assessment based on predictions of LRTP–POV by the existing version of The Tool alone may eliminate many chemicals with a potential to accumulate in remote regions at the screening stage (N = 1693 or 13.4%, Fig. 3b). One may then ask whether the consistency of the EFA approach also ensures consistency across the two Annexes of the SC? In other words, that all chemicals identified as having a potential for remote accumulation in the context of Annex E would have been flagged during the screening stage if CTD/TE are replaced with ϕ2? The answer is no because a chemical may fall below the threshold for ϕ2 yet remain above the threshold for ϕ3 (Fig. S4†). Secondly, it is not necessarily the same emission scenario which leads to the highest ϕ1, ϕ2 and ϕ3. It may then be prudent to assess whether all 2980 chemicals which fulfill the criterion for ϕ3 will be captured during the screening stage based on degradation half-lives in air (LRATP criteria) and water (persistence criteria)? Our results suggest that this is not the case as only 304 chemicals among the 2980 chemicals which exceed the threshold for ϕ3 will be identified in this manner (Fig. 4b, upper right quadrant and Fig. S5†). Even if the existing approach (LRTP–POV) is applied in addition to these half-life criteria, there will still be 1558 chemicals left out (Fig. 5b, upper left, lower left and lower right quadrants). Note that the number of chemicals falling into the lower left quadrant of Fig. 4b and 5b is identical (N = 782). Hence, 12.4% of the chemicals in the screening data set will not be identified as having a potential for accumulation in the remote region in the context of Annex E, even if LRTP–POV, degradation half-life in air, as well as degradation half-life in water are applied under Annex D. Among the reasons for this are (i) the assumption that involatile chemicals do not degrade in air (grey markers in Fig. 4a), (ii) the possibility of chemicals undergoing LRT when both LRAT and LRWT are accounted for (orange markers in Fig. 4a), and (iii) the possibility of chemicals undergoing LRT without exceeding the half-life criteria (Fig. 4b). This suggests that a screening step relying on simple LRTP–POV criteria may wrongly screen out most of the chemicals with a potential to accumulate in the remote region according to ϕ3.
This study demonstrates that there are many chemicals in the screening data set which have the potential to accumulate in a remote region without meeting the screening criteria under Annex D. This highlights that the screening for LRTP under Annex D leads to possible false negatives in the context of Annex E, which suggests that the tiered screening under the SC does not work very well. In other words, the possible LRTP screening using degradation half-lives in air or water, transport- or transfer-metrics may lead to false negatives if it eliminates chemicals that may qualify as POP-like if ϕ3 is the endpoint of interest. There may be two approaches to overcome the issue of inconsistency of metrics. The simple approach would be to use ϕ3 in the context of both Annex D and Annex E. The other metrics can then be disregarded. The other approach would be to define thresholds for the other metrics that are consistent with ϕ3. This could be done by identifying the lowest value of CTD, TE, ϕ1, ϕ2, POV, HLair, and HLwater among the chemicals that exceed the threshold for ϕ3. This implies, however, that the numerical thresholds would be dependent on the selection of chemicals used.
Following the original approach in The Tool, thresholds for LRTP and POV were derived by using the lowest value of a metric obtained for a select subset of 14 POPs (Table 2). We established these thresholds to be able to explore our main question on how the choice of metric affects the outcome for a non-regulatory screening of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 chemicals. The use of these thresholds in this study does not mean that regulatory decision should be based on thresholds derived from this particular subset of POPs, or even that thresholds based on reference or benchmark chemicals should be used at all.
615 chemicals. The use of these thresholds in this study does not mean that regulatory decision should be based on thresholds derived from this particular subset of POPs, or even that thresholds based on reference or benchmark chemicals should be used at all.
Obviously, the choice of reference POPs can influence the threshold values, and in a regulatory context this choice would have to be well justified. While it may be tempting to suggest that the set of benchmark POPs should comprise all chemicals listed in the Annexes of the Stockholm Convention, this would result in fairly low thresholds because The Tool calculates low LRTP metric values for several POPs. For example, if methoxychlor, chlorpyrifos and UV-328 were added to the group of 14 reference POPs (Table S3†), the threshold for CTD would be defined by the value for UV-328 (CTD = 228 km) instead of cis-chlordane (CTD = 1021 km; Table 2). The number of chemicals exceeding the threshold for CTD would increase from ∼3300 (26.3%) to ∼7300 (57.9%). An approach that classifies a huge fraction of assessed chemicals as having LRTP will cease to be useful in discriminating between chemicals in need of global regulatory action and those that can be regulated within national or regional jurisdictions. While thresholds were defined here to enable the screening of thousands of chemicals, the primary interest in other contexts may be the LRTP assessment of a single chemical. Alternative model-based strategies could then become feasible, such as comparative approaches that rank a chemical along with all regulated POPs.
3.5. Cautionary notes on simplified model assumptions
The reader should furthermore keep in mind that The Tool is a relatively simple evaluative model for non-ionizing substances. The chemical fate processes in the multimedia model within The Tool are described relying on a number of simplifying assumptions10 which affect the outcome of the screening. Examples explicitly mentioned by Wegmann et al.10 are the use of an average aerosol size, an average deposition velocity and a constant rain fall rate. While the latter simplification has been addressed by implementing a description of intermittent precipitation in this study, another simplification which deserves to be highlighted is that involatile substances that are sorbed to particles in the atmosphere (i.e., have a high KOA) are assumed to be completely persistent in air. Chemicals which undergo reactions in the atmospheric particle phase28 may be incorrectly classified as undergoing LRAT.20 We have shown that the EFA flags many high-KOA chemicals emitted to air as having a ϕ3 above the threshold even if they are not particularly persistent in surface media (Fig. 4).The parameterization of the sinking velocity of chemicals that are sorbed to solids in water (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW > ∼6) furthermore becomes an important consideration when accounting for the combined transport in air and water. The sinking of hydrophobic chemicals sorbed to solids is expected to be subject to considerable environmental variability.29,30 Further in-depth LRTP assessments may therefore be warranted, using more sophisticated models that account for spatial and temporal variability. A major advantage of the EFA is that the calculation of emissions fractions is not tied to a particular, simple model but can be implemented in higher-tier models which in turn can be evaluated against observations. Secondly, because the EFA is better suited than the existing LRTP metrics to identify which processes dictate LRTP for a particular chemical, it could additionally help guide LRTP assessments using higher-tier models.
KOW > ∼6) furthermore becomes an important consideration when accounting for the combined transport in air and water. The sinking of hydrophobic chemicals sorbed to solids is expected to be subject to considerable environmental variability.29,30 Further in-depth LRTP assessments may therefore be warranted, using more sophisticated models that account for spatial and temporal variability. A major advantage of the EFA is that the calculation of emissions fractions is not tied to a particular, simple model but can be implemented in higher-tier models which in turn can be evaluated against observations. Secondly, because the EFA is better suited than the existing LRTP metrics to identify which processes dictate LRTP for a particular chemical, it could additionally help guide LRTP assessments using higher-tier models.
4. Conclusions
While The Tool was only developed to screen for two hazard criteria10 as relevant for Annex D, it has nonetheless been extensively applied in the Risk Profiles of various chemicals under Annex E. It is important to note that the existing approach leads to a risk of false positive/negative decisions not only in the context of Annex D, but also in the context of Annex E.Here, we demonstrate that the existing and alternative LRTP metrics do not convey the same information and do not lead to similar outcomes in LRTP assessments. We propose that the coherent set of EFA metrics represents a more mechanistically sound approach to LRTP assessment than the existing metrics. Specifically,
• Assessing dispersion potential with CTD instead of ϕ1 does not account for the possibility of chemicals undergoing combined LRT in air and water.
• Assessing potential for transfer to a remote region with TE instead of ϕ2 does not account for (i) reversible atmospheric deposition, and (ii) the possibility of chemicals being transferred to the remote environment in water.
• Neither the CTD–POV combination nor the TE–POV combination quantify the potential for accumulation in a remote region.
• Any assessment of the potential for accumulation in a remote region with the CTD–POV combination instead of ϕ3 will not capture chemicals that accumulate in remote regions (i) without meeting persistence criteria and (ii) due to transport in air and water combined.
• Any assessment of the potential for accumulation in a remote region with the TE–POV combination instead of ϕ3 will not account for chemicals that accumulate in remote regions (i) without meeting persistence criteria and (ii) due to transport in water.
By integrating LRT, persistence and partitioning, ϕ3 may serve as an indicator of accumulation in remote environments in the context of the Stockholm Convention and beyond.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The study was financed by the Long-range Research Initiative of the European Chemicals Industry Council (project ECO-53). KB received additional support from the Research Council of Norway (#343110) and an internal grant from NILU.References
- R. Dietz, R. J. Letcher, J. P. Desforges, I. Eulaers, C. Sonne, S. Wilson, E. Andersen-Ranberg, N. Basu, B. D. Barst, J. O. Bustnes, J. Bytingsvik, T. M. Ciesielski, P. E. Drevnick, G. Gabrielsen, A. Haarr, K. Hylland, B. M. Jenssen, M. Levin, M. A. McKinney, R. D. Norregaard, K. E. Pedersen, J. Provencher, B. Styrishave, S. Tartu, J. Aars, J. T. Ackerman, A. Rosing-Asvid, R. Barrett, A. Bignert, E. W. Borns, M. Branigan, B. Braune, C. E. Bryan, M. Dam, C. A. Eagles-Smith, M. Evans, T. J. Evans, A. T. Fisk, M. Gamberg, K. Gustavson, C. A. Hartman, B. Helander, M. P. Herzog, P. F. Hoekstra, M. Houde, K. Hoydal, A. K. Jackson, J. Kucklick, E. Lie, L. Loseto, M. L. Mallory, C. Miljeteig, A. Mosbech, D. C. G. Muir, S. T. Nielsen, E. Peacock, S. Pedro, S. H. Peterson, A. Polder, F. F. Riget, P. Roach, H. Saunes, M. H. S. Sinding, J. U. Skaare, J. Sondergaard, G. Stenson, G. Stern, G. Treu, S. S. Schuur and G. Vikingsson, Current state of knowledge on biological effects from contaminants on Arctic wildlife and fish, Sci. Total Environ., 2019, 696, 133792 CrossRef CAS.
- F. Riget, A. Bignert, B. Braune, M. Dam, R. Dietz, M. Evans, N. Green, H. Gunnlaugsdottir, K. S. Hoydal, J. Kucklick, R. Letcher, D. Muir, S. Schuur, C. Sonne, G. Stern, G. Tomy, K. Vorkamp and S. Wilson, Temporal trends of persistent organic pollutants in Arctic marine and freshwater biota, Sci. Total Environ., 2019, 649, 99–110 CrossRef CAS PubMed.
- UNEP, The Stockholm Convention on Persistent Organic Pollutants (POPs), 2001 Search PubMed.
- UNECE, Executive body decision 1998/2 on information to be submitted and the procedure for adding substances to Annexes I, II or III to the POPs Protocol (EB.AIR/WG.5/52, Annex II), United Nations Economic Commission for Europe, 1998 Search PubMed.
- M. Scheringer, Persistence and spatial range as endpoints of an exposure-based assessment of organic chemicals, Environ. Sci. Technol., 1996, 30(5), 1652–1659 CrossRef CAS.
- A. Beyer, D. Mackay, M. Matthies, F. Wania and E. Webster, Assessing long-range transport potential of persistent organic pollutants, Environ. Sci. Technol., 2000, 34(4), 699–703 CrossRef CAS.
- K. Fenner, M. Scheringer, M. MacLeod, M. Matthies, T. McKone, M. Stroebe, A. Beyer, M. Bonnell, A. C. Le Gall, J. Klasmeier, D. Mackay, D. Van De Meent, D. Pennington, B. Scharenberg, N. Suzuki and F. Wania, Comparing estimates of persistence and long-range transport potential among multimedia models, Environ. Sci. Technol., 2005, 39(7), 1932–1942 CrossRef CAS PubMed.
- J. Klasmeier, M. Matthies, M. Macleod, K. Fenner, M. Scheringer, M. Stroebe, A. C. Le Gall, T. McKone, D. Van De Meent and F. Wania, Application of multimedia models for screening assessment of long-range transport potential and overall persistence, Environ. Sci. Technol., 2006, 40(1), 53–60 CrossRef CAS PubMed.
- M. Scheringer, Long-range transport of organic chemicals in the environment, Environ. Toxicol. Chem., 2009, 28(4), 677–690 CrossRef CAS PubMed.
- F. Wegmann, L. Cavin, M. MacLeod, M. Scheringer and K. Hungerbühler, The OECD software tool for screening chemicals for persistence and long-range transport potential, Environ. Model. Softw., 2009, 24(2), 228–237 CrossRef.
- E. Webster, D. Mackay and F. Wania, Evaluating environmental persistence, Environ. Toxicol. Chem., 1998, 17(11), 2148–2158 CrossRef CAS.
- D. H. Bennett, T. E. McKone, M. Matthies and W. E. Kastenberg, General formulation of characteristic travel distance for semivolatile organic chemicals in a multimedia environment, Environ. Sci. Technol., 1998, 32(24), 4023–4030 CrossRef CAS.
- M. MacLeod and D. Mackay, Modeling transport and deposition of contaminants to ecosystems of concern: a case study for the Laurentian Great Lakes, Environ. Pollut., 2004, 128(1–2), 241–250 CrossRef CAS PubMed.
- F. Wania, Assessing the potential of persistent organic chemicals for long-range transport and accumulation in polar regions, Environ. Sci. Technol., 2003, 37(7), 1344–1351 CrossRef CAS.
- UNEP, UV 328 Risk Profile. UNEP/POPS/POPRC.17/13/Add.3, 2021 Search PubMed.
- UNEP, Dechlorane Plus Risk Profile. UNEP/POPS/POPRC.17/13/Add.2, 2022 Search PubMed.
- UNEP, Chlorinated paraffins with carbon chain lengths in the range C14-17 and chlorination levels at or exceeding 45 per cent chlorine by weight. Risk Profile. In UNEP/POPS/POPRC.18/11/Add.3, 2023 Search PubMed.
- E. Reppas-Chrysovitsinos, A. Sobek and M. MacLeod, In Silico Screening-Level Prioritization of 8468 Chemicals Produced in OECD Countries to Identify Potential Planetary Boundary Threats, Bull. Environ. Contam. Toxicol., 2018, 100(1), 134–146 CrossRef CAS PubMed.
- X. Zhang, X. F. Sun, R. F. Jiang, E. Y. Zeng, E. M. Sunderland and D. C. G. Muir, Screening New Persistent and Bioaccumulative Organics in China’s Inventory of Industrial Chemicals, Environ. Sci. Technol., 2020, 54(12), 7398–7408 CrossRef CAS PubMed.
- K. Breivik, M. S. McLachlan and F. Wania, The Emissions Fractions Approach to Assessing the Long-Range Transport Potential of Organic Chemicals, Environ. Sci. Technol., 2022, 56(17), 11983–11990 CrossRef CAS PubMed.
- A. Hollander, M. Scheringer, V. Shatalov, E. Mantseva, A. Sweetman, M. Roemer, A. Baart, N. Suzuki, F. Wegmann and D. van de Meent, Estimating overall persistence and long-range transport potential of persistent organic pollutants: a comparison of seven multimedia mass balance models and atmospheric transport models, J. Environ. Monit., 2008, 10(10), 1139–1147 RSC.
- OECD, Guidance Document on the Use of Multimedia Models for Estimating Overall Environmental Persistence and Long-Range Transport. Series on Testing and Assessment 45, OECD Environment, Health and Safety Publications. Paris, France, 2004 Search PubMed.
- D. Mackay, Multimedia Environmental Models: the Fugacity Approach, 2 edn, CRC Press, Boca Raton, FL, 2001, p. 272 Search PubMed.
- J. A. Arnot, T. N. Brown, F. Wania, K. Breivik and M. S. McLachlan, Prioritizing Chemicals and Data Requirements for Screening-Level Exposure and Risk Assessment, Environ. Health Perspect., 2012, 120(11), 1565–1570 CrossRef PubMed.
- A. Beyer and M. Matthies, Long-range transport potential of semivolatile organic chemicals in coupled air-water systems, Environ. Sci. Pollut. Res., 2001, 8(3), 173–179 CrossRef CAS PubMed.
- M. Stroebe, M. Scheringer, H. Held and K. Hungerbühler, Inter-comparison of multimedia modeling approaches: modes of transport, measures of long range transport potential and the spatial remote state, Sci. Total Environ., 2004, 321(1–3), 1–20 CrossRef CAS PubMed.
- Z. Y. Wang, S. Adu-Kumi, M. L. Diamond, R. Guardans, T. Harner, A. Harte, N. Kajiwara, J. Klanova, J. G. Liu, E. G. Moreira, D. C. G. Muir, N. Suzuki, V. Pinas, T. Seppala, R. Weber and B. Yuan, Enhancing Scientific Support for the Stockholm Convention's Implementation: An Analysis of Policy Needs for Scientific Evidence, Environ. Sci. Technol., 2022, 56(5), 2936–2949 CrossRef CAS PubMed.
- S. Zhou, B. C. H. Hwang, P. S. J. Lakey, A. Zuend, J. P. D. Abbatt and M. Shiraiwa, Multiphase reactivity of polycyclic aromatic hydrocarbons is driven by phase separation and diffusion limitations, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(24), 11658–11663 CrossRef CAS PubMed.
- F. T. Xie, Z. Tao, X. Zhou, T. T. Lv and J. Wang, Spatial and Temporal Variations of Particulate Organic Carbon Sinking Flux in Global Ocean from 2003 to 2018, Remote Sens., 2019, 11(24), 2941 CrossRef.
- M. J. Lutz, K. Caldeira, R. B. Dunbar and M. J. Behrenfeld, Seasonal rhythms of net primary production and particulate organic carbon flux to depth describe the efficiency of biological pump in the global ocean, J. Geophys. Res.: Oceans, 2007, 112(C10), C10011 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3va00189j |
| This journal is © The Royal Society of Chemistry 2023 |