Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel highly efficient Bi3+-activated phosphors for warm WLEDs
Xiudi
Wu
ab,
Xibao
Zhang
ab,
Yonghui
Xu
ab,
Shuwen
Yin
a,
Chuansheng
Zhong
ab,
Liang
Zhou
 *ab and
Hongpeng
You
*ab and
Hongpeng
You
 *abc
*abc
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: zhoul@ciac.ac.cn; hpyou@ciac.ac.cn; Fax: +86 431 85698041
bUniversity of Science and Technology of China, Hefei 230026, P. R. China
cGanjiang Innovation Academy, Chinese Academy of Sciences, Ganzhou 341000, China
First published on 19th May 2023
Abstract
In this paper, novel Bi3+-activated Ca2LuZrScAl2GeO12 (CLZSAG) phosphors with a garnet structure were prepared by a high temperature solid-state method. There are two kinds of luminescent centers in the CLZSAG:Bi3+ phosphors, which exhibit a blue emission under 365 nm excitation and a rare near-ultraviolet emission with a narrow band (FWHM = 34 nm) under 290 nm excitation. The combination of the structure and luminescence reveals that the Bi3+ ions occupy the sites of the LuO8 and ScO6 polyhedra with different local coordination environments, leading to two different luminescence behaviors. In addition, the prepared phosphors also have excellent thermostability (I423K/298K = 80%), and high quantum efficiencies of 72% and 60% under 290 and 365 nm excitation, respectively. Since the absorption band of the phosphor in the UV-visible region can match well with the commercial 365 nm chips, a high-performance WLED device with a high color rendering index (Ra = 98.5) and a low correlated color temperature (CCT = 4455 K) was obtained by combining commercial phosphors Lu-YAG:Ce3+ and CaAlSiN3:Eu2+ and samples of the CLZSAG:Bi3+ phosphor with 365 nm chips. Our results demonstrate that CLZSAG:Bi3+ has great potential application in WLED devices.
Introduction
Since mankind lit the world's first fire, the pursuit of more environmentally friendly and efficient light sources has never stopped. With the improvement of people's living standards and energy utilization efficiency, the application of various artificial lighting devices, from incandescent lamps to halogen lamps, xenon lamps, fluorescent lamps, and white light emitting diodes (WLEDs), is continuously driving the development of lighting technology.1–9 Compared with traditional light sources, phosphor-converted WLED (pc-WLED) technology has become the dominant light source because of its advantages of high efficiency, environmental protection, fast response time, and small volume.10–17 Considering the application needs of various fields, there is still great room for the progress of pc-WLED technology. The color rendering index (CRI) is an important parameter to evaluate the quality of pc-WLEDs, and the higher the CRI, the better the color reproduction.18–21 Currently, the commercially available fabrication technique for pc-WLEDs is based on the combination of the blue LED chip (440–480 nm), CaAlSiN3:Eu2+ red phosphor and Lu/Ga-YAG:Ce3+ yellow-green phosphor. However, the prepared LED devices are limited in their applicability due to their low CRI (∼82) and high CCT (>4500 K) values.7,22–26 Therefore, other production techniques for pc-WLEDs have occasionally been developed, involving the use of near-UV LED chips and trichromatic (red, green and blue) phosphors. Surprisingly, near-UV excited pc-WLEDs can easily achieve warm white light with a high CRI. High CRI values (>90) can satisfy the demands of certain spaces with high color requirements, such as libraries, cinemas, art galleries, jewellery, surgeries and cosmetic sales counters, making high CRI values an important standard in lighting displays.3,27,28As one of the most crucial components of pc-WLEDs, blue light-emitting phosphors greatly affect the performance of lighting equipment. Previous studies on blue phosphors were focused on rare earth metal ions (Ce3+ and Eu2+) due to the controlled luminescence color and high quantum yields, which resulted from the 4f–5d electronic transitions.29 Previous reports on Ce3+ and Eu2+ activated blue light-emitting phosphors are common, such as BaGa2Si2O8:Ce3+ and Na3KMg7(PO4)6:Eu2+.30,31 However, the emission spectrum of the Eu2+-doped phosphor is not sufficiently wide to adequately fill the cyan gap. Some Ce3+-doped phosphors still have drawbacks such as low quantum efficiency and poor thermal stability, such as BaCa2Y6O12:Ce3+ (I423K/I298K = 4.8%),32 Sr3Sc4O9:Ce3+ (I423K/I298K = 19.1%),33 BaLa2Si2S8:Ce3+ (IQE: 36.07%),34 and YScSi4N6C:Ce3+ (IQE: 30.3%).35 In addition, blue phosphors should have a strong absorption in the UV-visible region to ensure a match with the near-UV-excited chip. Excellent thermal stability and internal photoluminescence (PL) quantum efficiency are the most critical factors related to the overall electro-optical conversion efficiency of packaged white LEDs.36–38 Consequently, it is still a considerable challenge to seek excellent blue phosphors with near-UV absorption.
Herein, we designed and synthesized new Ca2LuZrScAl2GeO12:Bi3+ (CLZSAG:Bi3+) phosphors with a garnet structure. The crystal structure, quantum efficiency, luminescence properties, optimal doping concentration, lifetime and temperature-dependent spectra were systematically investigated. There are two kinds of luminescent centers in the CLZSAG:Bi3+ phosphor, which gives blue and near-ultraviolet emissions with a narrow band (FWHM = 34 nm) under different excitations. These luminescence behaviors can be ascribed to the site occupation of the Bi3+ ions in LuO8 and ScO6 polyhedrons with different local coordination environments. Furthermore, a high-performance warm WLED device with a high color rendering index (Ra = 98.5) and a low correlated color temperature (CCT = 4455 K) was fabricated by combining commercial yellow-green phosphor Lu-YAG:Ce3+, prepared blue phosphor CLZSAG:0.14Bi3+ and red phosphor CaAlSiN3:Eu2+ with 365 nm near-UV LED chips.
Experimental section
Materials and synthesis
A series of samples of CLZSAG:xBi3+ (x = 0–0.25) were prepared using the conventional solid-state reaction route. In the first step, the raw materials CaCO3 (99.9%), Lu2O3 (99.99%), Sc2O3 (99.9%), ZrO2 (99.9%), Al2O3 (99.9%), GeO2 (99.99%), and Bi2O3 (99.999%) were weighed according to the stoichiometric ratio. After 30 minutes of grinding, the raw materials were transferred to the alumina crucible and heated in a muffle furnace at 1500 °C for 4 hours under air atmosphere. After the reaction, the final samples were ground into fine powder and collected for further characterization.Characterization
X-ray diffraction (XRD) patterns, scanning electron microscopy (SEM), excitation and emission spectrum measurements, temperature-dependent emission spectrum determination, fluorescence lifetime and quantum efficiency tests were described in detail in our team's previous work.39,40 The photoelectric performances of pc-LEDs were measured using Starspec SSP6612 instruments.LED fabrication
The prepared blue phosphor CLZSAG:0.14Bi3+, commercial yellow-green phosphor Lu-YAG:Ce3+ and red phosphor CaAlSiN3:Eu2+ were mixed with epoxy resin (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B = 1
B = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 40 min to form a composite solution. The pc-wLED was fabricated by uniformly coating the composite solution on the 365 nm chip and drying it at 353 K. The optical properties of the fabricated WLED lamp were measured at an operating current of 20–120 mA.
1) for 40 min to form a composite solution. The pc-wLED was fabricated by uniformly coating the composite solution on the 365 nm chip and drying it at 353 K. The optical properties of the fabricated WLED lamp were measured at an operating current of 20–120 mA.
Results and discussion
Structure and morphology
In order to investigate the luminescence properties of the Bi3+ ions in the host materials, we prepared a set of CLZSAG:xBi3+ phosphors. As shown in Fig. 1a, all diffraction peaks match well with the data on the standard card, and no impurity phase has been detected, indicating that the Bi3+ ions were successfully introduced into the CLZSAG host without altering the crystal structure.41 The structure is refined by XRD Rietveld refinement, where the structure of CLZSAG is used as the initial model of refinement, as shown in Fig. 1b. The Rietveld refinement results show that the sample is isomorphic with CLZSAG and no impurity phase is detected. The detailed structural parameters and crystallographic data of CLZSAG and CLZSAG:0.04Bi3+ components are given in Table 1. The low R factor value shows that the Rietveld refining result is reliable (Table 1).| Compound | Ca2LuZrScAl2GeO12:0.04Bi3+ | Ca2LuZrScAl2GeO12 |
|---|---|---|
| Space group |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d (230) d (230) |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d (230) d (230) |
| Symmetry | Cubic | Cubic |
| a = b = c/Å | 12.460 | 12.440 |
| V Å−3 | 1934.561 | 1930.017 |
| α = β = γ | 90° | 90° |
| Z | 8 | 8 |
| R wp, % | 7.17 | 8.28 |
| R p, % | 4.97 | 6.12 |
| χ2 | 4.99 | 7.17 |
Fig. 1c shows that the CLZSAG:0.14Bi3+ phosphor possesses a garnet structure with a Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d (230) space group. As a typical non-rare earth activator ion, Bi3+ ions can emit in the range of ultraviolet to near-infrared due to the influence of the coordination environment in the host materials. Ca2+/Lu3+, Sc3+/Zr4+, and Al3+/Ge4+ cations are coordinated by eight, six and four surrounding O2− to form a dodecahedron, an octahedron and a tetrahedron, respectively. In addition, the [(Ca/Lu)O8] dodecahedron are connected to the [(Al/Ge)O4] tetrahedron and the [(Sc/Zr)O6] octahedron by sharing edges. Namely, Zr4+/Sc3+ or Al3+/Ge4+ can affect the coordination environment of the dodecahedron site, thus affecting the luminescence of Bi3+ ions. According to the previously reported CLZSAG crystal, there are six types of cations.41 Wherein, the ionic radius of Ca2+ is 1.12 Å (CN = 8, CN: coordination number), Lu3+ is 0.977 Å (CN = 8), Zr4+ is 0.72 Å (CN = 6), Sc3+ is 0.745 Å (CN = 6), Al3+ is 0.39 Å (CN = 4), Ge4+ is 0.73 Å (CN = 4), and Bi3+ are 1.03 Å (CN = 6) and 1.17 Å (CN = 8), respectively. Considering the similar ionic radius and the same charge, the Bi3+ ions are most likely to occupy the Ca2+, Lu3+ or Sc3+ sites. It is well known that there should be less than 30% ionic radius between the host ion and the dopant ion. As a result, it can be calculated using the equation below:
d (230) space group. As a typical non-rare earth activator ion, Bi3+ ions can emit in the range of ultraviolet to near-infrared due to the influence of the coordination environment in the host materials. Ca2+/Lu3+, Sc3+/Zr4+, and Al3+/Ge4+ cations are coordinated by eight, six and four surrounding O2− to form a dodecahedron, an octahedron and a tetrahedron, respectively. In addition, the [(Ca/Lu)O8] dodecahedron are connected to the [(Al/Ge)O4] tetrahedron and the [(Sc/Zr)O6] octahedron by sharing edges. Namely, Zr4+/Sc3+ or Al3+/Ge4+ can affect the coordination environment of the dodecahedron site, thus affecting the luminescence of Bi3+ ions. According to the previously reported CLZSAG crystal, there are six types of cations.41 Wherein, the ionic radius of Ca2+ is 1.12 Å (CN = 8, CN: coordination number), Lu3+ is 0.977 Å (CN = 8), Zr4+ is 0.72 Å (CN = 6), Sc3+ is 0.745 Å (CN = 6), Al3+ is 0.39 Å (CN = 4), Ge4+ is 0.73 Å (CN = 4), and Bi3+ are 1.03 Å (CN = 6) and 1.17 Å (CN = 8), respectively. Considering the similar ionic radius and the same charge, the Bi3+ ions are most likely to occupy the Ca2+, Lu3+ or Sc3+ sites. It is well known that there should be less than 30% ionic radius between the host ion and the dopant ion. As a result, it can be calculated using the equation below:
| Dr = (Rm (CN) − Rd (CN))/(Rm (CN)) |
where Dr is the percentage of the radius difference, and Rm (CN) and Rd (CN) are the radii of the host ion and the doped ion, respectively. The calculated percentages of the Bi3+ ions occupying the sites of host cations Ca2+, Lu3+and Sc3+ are less than 30%. Considering the charge effects, Sc3+, Lu3+ and Bi3+ have the same charge and Ca2+ does not match with the Bi3+ charge, and Bi3+ should prefer to occupy the Sc3+ and Lu3+ sites.
The scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM) images of the representative CLZSAG:0.14Bi3+ phosphor is shown in Fig. 1d. It can be seen from the SEM that the sample is composed of several micrometers, with irregular shapes but a smooth surface. The average particle size is estimated to be about 10 μm. By analyzing the element mapping image and energy dispersive X-ray spectroscopy (EDS) spectra, Ca, Lu, Sc, Zr, Al, Ge, O and Bi elements are uniformly distributed in the particles, which also further proves that the Bi3+ ions were successful incorporated into host materials. As shown in Fig. 1e, sharp diffraction spots can be found in the electron diffraction pattern of the selected region, and the corresponding interplanar distances of (211) and (321) are 0.614 and 0.318 nm, respectively.
Luminescence properties
Fig. 2 shows the diffuse reflection (DR) spectra of CLZSAG and CLZSAG:0.14Bi3+ samples. There is an obvious broadband absorption band in the range of 200–400 nm, which corresponds to the 1S0→3P1 (300–400 nm) and 1S0→1P1 (200–300 nm) transitions of the Bi3+ ions. The optical band gap can be calculated using the formula:41 | (1) |
| [F(R)hv]n = D(hv − Eg) | (2) |
 | ||
| Fig. 2 DR spectra of the CLZSAG host and CLZSAG:0.14Bi3+ phosphors (the inset shows the calculated optical band gap values of the CLZSAG host based on the DR spectra). | ||
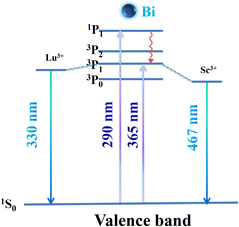
It is widely known that Bi3+ has an electronic configuration of [Xe] 4f145d106s2, and the free Bi3+ ions are very sensitive to the crystal field and are susceptible to the influence of the matrix material to emit different colors.42–44 As shown in Fig. 3, different bands are composed in the spectrum by transitions from the ground state to the excited states as the 3P0, 3P1, 3P2 and 1P1 states (in order of increasing energy).45 Among them, the transitions of 1S0→3P0 and 1S0→3P2 are spin forbidden and crystal field forbidden transition, so the absorption band in the 200–400 nm range can only be produced by the 1S0→3P1 and 1S0→1P1 transition.46,47 The excited electrons in the 1P1 energy level are transferred to the 3P1 energy level by the nonradioactive transition. The matrix environment affects Bi3+ to induce crystal field splitting, and the stronger crystal field causes a lower 3P1 energy level, resulting in a redshift in the emission band. Finally, the excited electrons return to the ground state via radiative transitions from the 3P1 energy level, while releasing light.48,49
The photoluminescence emission (PL) and excitation (PLE) spectra of the prepared CLZSAG:0.14Bi3+ at room temperature are shown in Fig. 4a. When monitored at 467 nm, the two excitation bands of CLZSAG:0.14Bi3+ are observed at ∼290 and ∼365 nm, attributed to the 1S0→1P1 and 1S0→3P1 transition of Bi3+. Under 290 nm excitation, two narrow emission band peaks at 330 (FWHM = 33 nm) and 467 nm (FWHM = 67 nm) were observed, which can be attributed to the 3P1→1S0 transition of the Bi3+ ions. Under the excitation of 365 nm, a blue light emission band at 467 nm displays in the visible region of 400–700 nm. Furthermore, the excitation of the prepared blue-emitting luminescent material CLZSAG:0.14Bi3+ matched well with the emission of the commercial near-UV 365 nm chips and can be used to develop high-performance WLEDs.
In order to investigate the local crystal environment of the Bi3+ emitting centers in the CLZSAG host, it is crucial to determine the crystal field strength of the Lu/Sc sites. The following equation can be used to calculate the crystal field strength (Dq):50
 | (3) |
The longer the bond length, the weaker the crystal field and lead to a shorter emission wavelength.5 The average bond lengths of Sc–O and Lu–O are 2.09 Å and 2.52 Å, respectively. Therefore, the crystal field strength of the Lu3+ site is lower than that of the Sc3+ site. On the basis of the preceding discussion, the 330 nm emission band comes from the Bi3+ ions occupying the Lu3+ sites and the 467 nm emission band results from the Bi3+ ions in the Sc3+ sites. The FWHM of emission spectra at 7 K became narrower (Fig. 4c), due to the decrease in electron–phonon interactions at lower temperatures.
The naked 6s and 6p electrons of Bi3+ are very sensitive to the local environment.43,51 [LuO8] is surrounded by [(Al/Ge)O4] tetrahedra and [(Sc/Zr)O6] octahedra, making the Bi3+ activators in a very symmetric and compact microenvironment, which effectively limits the broadening of the emission band.52 Thus, CLZSAG:Bi3+ shows a relatively narrow band (FWHM = 34 nm) at 330 nm, which is significantly narrower than most other Bi3+-doped phosphors such as K2ZrSi2O7 (125 nm) and Ca3Ga4O9 (96 nm).53,54 Intriguingly, the Stokes shift of CLZSAG:Bi3+ was calculated to be about 4179 cm−1 based on the excitation band at 290 nm and the emission band at 330 nm, which is smaller than those reported for most Bi3+-activated phosphors.42,55 Such a small Stokes shift indicates that it may have high efficiency and narrow emission bands.
We also investigated the relationship between the Bi3+ concentration and the PL intensity to determine the optimal doping amount. Normally, Bi3+-activated phosphors exhibit a low quenching concentration. As shown in Fig. 1c, it can be seen that the distances between adjacent Lu3+ sites and Sc3+ sites are 3.81 and 6.22 Å, respectively. The small distance between the Bi3+ and Lu3+ sites facilitates the concentration quenching effect, corresponding to a lower critical quenching concentration of 6%. While the longer distance between the Bi3+ and Sc3+ sites can effectively suppress this effect and obtain a higher critical quenching concentration of 14%. As shown in Fig. 4b and d, the PL emission intensity reaches a maximum at x = 0.06 with an increasing Bi3+ doping concentration under the excitation of 290 nm. And the optimal Bi3+ doping concentration is 14% under 365 nm light excitation. The emission intensity starts to decrease with the further increase of the Bi3+ doping concentration due to the concentration quenching effect. Moreover, the absorption of CLZSAG:0.14Bi3+ matches well with the 365 nm near-UV chips, indicating that it can be used to develop high-performance WLED devices.
Quantum efficiency is one of the important criteria to evaluate the performance of phosphors. The narrow emission band intensity of 330 nm is much stronger than that of 467 nm, and the Stokes shift is also relatively smaller, indicating a relatively higher quantum efficiency. As shown in Fig. 5, the CLZSAG:Bi3+ phosphor exhibited excellent quantum efficiencies, where the quantum efficiencies were 72% under 290 nm light excitation and 60% under 360 nm excitation. Compared with blue phosphors RbNa3(Li3SiO4)4:Eu2+ (IQE = 53%, EQE = 13%),56 the CLZSAG:Bi3+ phosphors exhibit a higher quantum efficiency, revealing its great potential application for WLEDs.
For practical applications, the decay behavior in the host material is a critical component. The decay curves of CLZSAG:xBi3+ were measured at room temperature (Fig. 5c). The average decay time τ are calculated according to the following equation:
 | (4) |
Therefore, the τ values of Bi3+ are estimated to be 1.75, 1.73, 1.67, 1.66, and 1.57 μs respectively, corresponding to the concentrations x of 0.02, 0.06, 0.08, 0.10, and 0.14. The decay time decreases with the increase of the CLZSAG:xBi3+ concentration due to the energy transfer between Bi3+ ions.
Temperature dependence of Bi3+ luminescence
Thermal stability is an essential feature for phosphors used in high-power LEDs, because the operating temperature can reach up to 423 K due to the long-term operation. Fig. 6a and b depict the temperature dependent spectra of the CLZSAG:0.14Bi3+ phosphor, and the PL emission intensity (λex = 365 nm) gradually decreases with the increment of temperature due to the quenching effect, but the intensity still maintains 80% at 423 K, compared with the emission intensity at room temperature. CLZSAG:0.14Bi3+ exhibits excellent thermal stability which is better than many other reported blue-emitting phosphors, such as MgAl2Si4O6N4:Eu2+ (I423K/I298K = 65%),57 ScCaOBO3:Ce3+ (I423K/I298K = 21%),58 BaLa2Si2S8:Ce3+ (I423K/I298K = 20%)34 and Ca4HfGe3O12:Bi3+ (I423K/I298K = 51%).59 As the temperature increases, the interaction between electrons and phonons is enhanced. The excited electrons gain phonon energy through nonradiative relaxation to overcome the energy barrier, leading to thermal quenching. The activation energy (ΔE) is what is meant by the term “energy barrier”, and the higher the activation energy, the weaker the thermal quenching is. We calculate the activation energy (ΔE) using the Arrhenius formula:49,60 | (5) |
Moreover, the temperature-dependent spectra of CLZSAG:Bi3+ have been measured and investigated. As shown in Fig. 6a and d, it can be seen that there is no obvious change of the emission peak with the increase of temperature, while the FWHM of the emission spectrum is slightly broadened which can be due to the electron–phonon interaction. These results demonstrate that the obtained sample has great thermal stability, which can meet the requirement of high-power WLEDs.
Application in pc-WLEDs
To explore the application potential of the as-prepared CLZSAG:Bi3+ phosphors, we prepared WLED devices using 365 nm near-UV chips in combination with commercial yellow-green powder LuYAG:Ce3+, red powder CaAlSiN3:Eu2+ and as-prepared CLZSAG:Bi3+ samples. Fig. 7 shows the electroluminescence spectrum and the Commission International deI’ Eclairage (CIE) coordinate is (0.3531, 0.3694). The inset shows an image of the working WLED device taken in the dark. As can be observed in Fig. 7c, the emission intensity begins to increase as the current increases, peaking at 60 mA, due to the increases in the intensity of the stimulated light. As the current increases further (>60 mA), the emission intensity decreases, which may come from the thermal quenching of the phosphor. Table 2 lists the CIE color coordinates, CCT, and CRI dependence curves for WLED devices with respect to operating currents. It is remarkable that the WLED has an ultra-high color rendering index (Ra = 98.5) and a low correlation color temperature (CCT = 4455 K) and the luminous efficiency is 24.5 lm W−1 at 10 mA driving current. The CRI index increases from 82 to 98.5 compared with the WLED device fabricated by LuYAG:Ce3+, CaAlSiN3:Eu2+ and a blue chip mentioned in the reference (Ra > 80, CCT > 4500 K).22 This result indicates that the prepared CLZSAG:Bi3+ phosphors have great potential applications in high-performance warm WLEDs.| Type | Current (mA) | CIE coordinates | CCT (K) | CRI |
|---|---|---|---|---|
| pc-WLED | 10 | 0.3531, 0.3694 | 4455 K | 98.5 |
| 20 | 0.3505, 0.3680 | 4872 K | 96.0 | |
| 40 | 0.3452, 0.3614 | 5030 K | 93.8 | |
| 60 | 0.3436, 0.3576 | 5077 K | 92.1 | |
| 80 | 0.3422, 0.3547 | 5119 K | 90.9 | |
| 100 | 0.3420, 0.3531 | 5122 K | 90.0 | |
| 120 | 0.3412, 0.3511 | 5152 K | 89.4 |
Conclusions
In this study, a novel series of CLZSAG:Bi3+ phosphors with a garnet structure were synthesized by using a high-temperature solid-state reaction. CLZSAG:Bi3+ phosphors exhibit blue light emission under 365 nm excitation and a rare ultra-narrow UV-emission band (FWHM = 34 nm) under 290 nm excitation. The CLZSAG:Bi3+ phosphors also have excellent thermostability (I423K/298K = 80%) and a high quantum efficiency of 60% under 365 nm excitation. The CLZSAG:Bi3+ phosphors emit bright light and can be used as a component of blue emission phosphors. The pc-WLED device was created by fusing blue CLZSAG:Bi3+, LuYAG:Ce3+, and CaAlSiN3:Eu2+ with a 365 nm near-UV chip with a high color rendering index (Ra = 98.5) and a low correlated color temperature (CCT = 4455 K). These characteristics demonstrate that the CLZSAG:Bi3+ phosphors have potential applications for WLED technology stimulated by near-UV light.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant No. 52072363), the National Key Research and Development Program (Grant No. 2022YFC2905201), the Key Program of the Frontier Science of the Chinese Academy of Sciences (Grant No. YZDY-SSW-JSC018), and the Research Projects of Ganjiang Innovation Academy, Chinese Academy of Sciences (E255C001).References
- X. Pan, L. Mei, Y. Zhuang, T. Seto, Y. Wang, M. Plyaskin, W. Xi, C. Li, Q. Guo and L. Liao, Chem. Eng. J., 2022, 434, 134652 CrossRef CAS.
- B. Shao, J. Huo and H. You, Adv. Opt. Mater., 2019, 7, 1900319 CrossRef.
- H. Yuan, F. Massuyeau, N. Gautier, A. B. Kama, E. Faulques, F. Chen, Q. Shen, L. Zhang, M. Paris and R. Gautier, Angew. Chem., Int. Ed., 2020, 59, 2802–2807 CrossRef CAS PubMed.
- C. C. Lin, Y.-T. Tsai, H. E. Johnston, M.-H. Fang, F. Yu, W. Zhou, P. Whitfield, Y. Li, J. Wang, R.-S. Liu and J. P. Attfield, J. Am. Chem. Soc., 2017, 139, 11766–11770 CrossRef CAS PubMed.
- L. Dong, L. Zhang, Y. Jia, B. Shao, W. Lü, S. Zhao and H. You, ACS Sustainable Chem. Eng., 2020, 8, 3357–3366 CrossRef CAS.
- P. Pust, P. J. Schmidt and W. Schnick, Nat. Mater., 2015, 14, 454–458 CrossRef CAS PubMed.
- J. Qiao, L. Ning, M. S. Molokeev, Y. C. Chuang, Q. Zhang, K. R. Poeppelmeier and Z. Xia, Angew. Chem., Int. Ed., 2019, 58, 11521–11526 CrossRef CAS PubMed.
- M. Peng, X. Yin, P. A. Tanner, M. G. Brik and P. Li, Chem. Mater., 2015, 27, 2938–2945 CrossRef CAS.
- S. Liang, M. Shang, H. Lian, K. Li, Y. Zhang and J. Lin, J. Mater. Chem. C, 2016, 4, 6409–6416 RSC.
- J. Wang, L. Dong, Z. Lyu, D. Sun, T. Tan and H. You, Adv. Funct. Mater., 2023, 2214611, DOI:10.1002/adfm.202214611.
- L. Dong, L. Zhang, Y. Jia, B. Shao, W. Lu, S. Zhao and H. You, ACS Appl. Mater. Interfaces, 2020, 12, 7334–7344 CrossRef CAS PubMed.
- S. Li, L. Wang, N. Hirosaki and R. J. Xie, Laser Photonics Rev., 2018, 12, 1800173 CrossRef.
- Y. Wang, J. Ding, Y. Wang, X. Zhou, Y. Cao, B. Ma, J. Li, X. Wang, T. Seto and Z. Zhao, J. Mater. Chem. C, 2019, 7, 1792–1820 RSC.
- X. Ma and Y. Wang, Adv. Opt. Mater., 2023, 2202765, DOI:10.1002/adom.202202765.
- R. Shi, X. Zhang, Z. Qiu, J. Zhang, S. Liao, W. Zhou, X. Xu, L. Yu and S. Lian, Inorg. Chem., 2021, 60, 19393–19401 CrossRef CAS PubMed.
- J. Han, L. Li, M. Peng, B. Huang, F. Pan, F. Kang, L. Li, J. Wang and B. Lei, Chem. Mater., 2017, 29, 8412–8424 CrossRef CAS.
- Z. Xia and Q. Liu, Prog. Mater. Sci., 2016, 84, 59–117 CrossRef CAS.
- L. Sun, B. Devakumar, J. Liang, S. Wang, Q. Sun and X. Huang, J. Mater. Chem. C, 2020, 8, 1095–1103 RSC.
- J. Hye Oh, S. Ji Yang and Y. Rag Do, Light: Sci. Appl., 2014, 3, e141–e141 CrossRef.
- P. Dang, Q. Zhang, D. Liu, G. Li, H. Lian, M. Shang and J. Lin, Chem. Eng. J., 2021, 420, 127640 CrossRef CAS.
- J. Liang, B. Devakumar, L. Sun, S. Wang, Q. Sun and X. Huang, J. Mater. Chem. C, 2020, 8, 4934–4943 RSC.
- C. Zhong, L. Zhang, Y. Xu, X. Wu, S. Yin, X. Zhang and H. You, Mater. Today Chem., 2022, 26, 101233 CrossRef CAS.
- Q. Wei, J. Ding, H. Chen, Q. Zhang and Y. Wang, Chem. Eng. J., 2020, 385, 123392 CrossRef CAS.
- Q. Zhang, X. Wang and Y. Wang, Inorg. Chem. Front., 2020, 7, 1034–1045 RSC.
- J. Han, F. Pan, M. S. Molokeev, J. Dai, M. Peng, W. Zhou and J. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 13660–13668 CrossRef CAS PubMed.
- Y. Li, S. Qi, P. Li and Z. Wang, RSC Adv., 2017, 7, 38318–38334 RSC.
- X. Huang, J. Liang, S. Rtimi, B. Devakumar and Z. Zhang, Chem. Eng. J., 2021, 405, 126950 CrossRef CAS.
- G. E. Wang, G. Xu, M. S. Wang, L. Z. Cai, W. H. Li and G. C. Guo, Chem. Sci., 2015, 6, 7222–7226 RSC.
- G. Li, Y. Tian, Y. Zhao and J. Lin, Chem. Soc. Rev., 2015, 44, 8688–8713 RSC.
- Y. Xu, L. Zhang, S. Yin, X. Wu and H. You, J. Alloys Compd., 2022, 911, 165149 CrossRef CAS.
- Z. Leng, H. Bai, Q. Qing, H. He, J. Hou, B. Li, Z. Tang, F. Song and H. Wu, ACS Sustainable Chem. Eng., 2022, 10, 10966–10977 CrossRef.
- T. Hasegawa, M. Iwaki, S. W. Kim, T. Ueda, K. Uematsu, K. Toda and M. Sato, J. Alloys Compd., 2019, 797, 1181–1189 CrossRef CAS.
- T. Hasegawa, S. W. Kim, T. Ueda, T. Ishigaki, K. Uematsu, H. Takaba, K. Toda and M. Sato, J. Mater. Chem. C, 2017, 5, 9472–9478 RSC.
- S. P. Lee, C. H. Huang, T. S. Chan and T. M. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 7260–7267 CrossRef CAS PubMed.
- C. Yan, Z. Liu, W. Zhuang, R. Liu, X. Xing, Y. Liu, G. Chen, Y. Li and X. Ma, Inorg. Chem., 2017, 56, 11087–11095 CrossRef CAS PubMed.
- Y.-C. Lin, M. Bettinelli and M. Karlsson, Chem. Mater., 2019, 31, 3851–3862 CrossRef CAS.
- P. Dang, W. Wang, H. Lian, G. Li and J. Lin, Adv. Opt. Mater., 2022, 10, 2102287 CrossRef CAS.
- J. Xue, Z. Yu, H. M. Noh, B. R. Lee, B. C. Choi, S. H. Park, J. H. Jeong, P. Du and M. Song, Chem. Eng. J., 2021, 415, 128977 CrossRef CAS.
- X. Zhang, L. Dong, L. Zhang, Y. Xu, X. Wu, S. Yin, C. Zhong and H. You, J. Mater. Chem. C, 2022, 10, 16857–16864 RSC.
- X. Zhang, L. Zhang, Y. Xu, X. Wu, S. Yin, C. Zhong and H. You, Inorg. Chem., 2022, 61, 7597–7607 CrossRef CAS PubMed.
- Y. Zheng, W. Zhuang, X. Xing, J. Zhong, R. Liu, Y. Li, Y. Liu and Y. Hu, RSC Adv., 2016, 6, 68852–68859 RSC.
- Y. Tang, M. Deng, M. Wang, X. Liu, Z. Zhou, J. Wang and Q. Liu, Adv. Opt. Mater., 2022, 11, 2201827 CrossRef.
- D. Liu, X. Yun, P. Dang, H. Lian, M. Shang, G. Li and J. Lin, Chem. Mater., 2020, 32, 3065–3077 CrossRef CAS.
- Y. Wei, Z. Gao, X. Yun, H. Yang, Y. Liu and G. Li, Chem. Mater., 2020, 32, 8747–8753 CrossRef CAS.
- Q. Wu, Y. Li, Y. Wang, H. Liu, S. Ye, L. Zhao, J. Ding and J. Zhou, Chem. Eng. J., 2020, 401, 126130 CrossRef CAS.
- P. Dang, D. Liu, G. Li, A. A. Al Kheraif and J. Lin, Adv. Opt. Mater., 2020, 8, 1901993 CrossRef CAS.
- Z. Zhou, X. Wang, X. Yi, H. Ming, Z. Ma and M. Peng, Chem. Eng. J., 2021, 421, 127820 CrossRef CAS.
- Y. Wei, W. Wang, Z. Wang, H. Yang, X. You, Y. Zhao, P. Dang, H. Lian, J. Hao, G. Li and J. Lin, Adv. Funct. Mater., 2022, 33, 2205829 CrossRef.
- S. Wu, P. Xiong, X. Liu, Y. Fu, Q. Liu, M. Peng, Y. Chen and Z. Ma, J. Mater. Chem. C, 2020, 8, 16584–16592 RSC.
- P. Dang, S. Liang, G. Li, Y. Wei, Z. Cheng, H. Lian, M. Shang, S. J. Ho and J. Lin, J. Mater. Chem. C, 2018, 6, 6449–6459 RSC.
- H. Li, R. Pang, Y. Luo, H. Wu, S. Zhang, L. Jiang, D. Li, C. Li and H. Zhang, ACS Appl. Electron. Mater., 2019, 1, 229–237 CrossRef CAS.
- S. Wang, H. Wu, Y. Fan, Q. Wang, T. Tan, R. Pang, S. Zhang, D. Li, L. Jiang, C. Li and H. Zhang, Chem. Eng. J., 2022, 432, 134265 CrossRef CAS.
- D.-Y. Wang, Z.-B. Tang, W. U. Khan and Y. Wang, Chem. Eng. J., 2017, 313, 1082–1087 CrossRef CAS.
- D. Liu, X. Yun, G. Li, P. Dang, M. S. Molokeev, H. Lian, M. Shang and J. Lin, Adv. Opt. Mater., 2020, 8, 2001037 CrossRef CAS.
- P. Boutinaud, Inorg. Chem., 2013, 52, 6028–6038 CrossRef CAS PubMed.
- H. Liao, M. Zhao, M. S. Molokeev, Q. Liu and Z. Xia, Angew. Chem., Int. Ed., 2018, 57, 11728–11731 CrossRef CAS PubMed.
- Y. Liu, Y. Liu, M. Wu, C. He, Q. Liu, X. Huang, X. Min, Z. Huang, H. Wang and R. Mi, Dalton Trans., 2022, 51, 16639–16647 RSC.
- W. Wang, T. Tan, S. Wang, S. Zhang, R. Pang, D. Li, L. Jiang, H. Li, C. Li and H. Zhang, Mater. Today Chem., 2022, 26, 101030 CrossRef CAS.
- S. Piao, Y. Wang, G. Zhu, J. Zhang, X. Zhang, D. Wu, Y. Cao, X. Li and B. Chen, J. Mater. Chem. C, 2021, 9, 14777–14787 RSC.
- P. Xiong, M. Peng, K. Qin, F. Xu and X. Xu, Adv. Opt. Mater., 2019, 7, 1901107 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |