Solar cells sensitized by donor-linked concerted companion dyes†
Jiaxin
Luo
a,
Yuqing
Wang
a,
Shaojin
Shi
a,
Yuankun
Wu
a,
Taochun
Ma
a,
Leyao
Wang
a,
Glib
Baryshnikov
 b,
Xinyan
Wu
b,
Xinyan
Wu
 *a,
Chengjie
Li
*a,
Chengjie
Li
 *a and
Yongshu
Xie
*a and
Yongshu
Xie
 *a
*a
aKey Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, Institute of Fine Chemicals, School of Chemistry and Molecular Engineering, East China University of Science & Technology, Shanghai 200237, China. E-mail: yshxie@ecust.edu.cn; chengjie.li@ecust.edu.cn; xinyanwu@ecust.edu.cn
bDepartment of Science and Technology, Laboratory of Organic Electronics, Linköping University, Norrköping SE-60174, Sweden
First published on 12th April 2023
Abstract
Recently, concerted companion (CC) dyes have been developed by covalently linking the acceptors of organic and porphyrin dye units. Herein, a new class of CC dyes XW85 and XW86 have been designed by linking the donors of porphyrin and organic dye units with C6H12 and C12H24 chains, respectively. The DSSCs of XW85 based on the I3−/I− electrolyte show significant JSC (17.20 mA cm−2) and PCE (8.96%), and XW86 exhibits higher JSC (18.55 mA cm−2) and PCE (9.76%), which are also higher than those of the corresponding cosensitization systems. However, the PCEs for XW85 and XW86 are lower than that of the acceptor-linked reference dye XW76 despite the obviously larger dye adsorption amounts. Desorption studies reveal that the CC dyes may be either double-anchored or single-anchored, with the double/single anchoring ratios lying in a sequence of XW85 (1.31) < XW86 (1.88) < XW76 (6.34), consistent with that of increasing PCE. These observations indicate that the non-adsorbed sub-dye unit cannot effectively contribute to electron injection, and thus relatively large mono-anchoring proportions for XW85 and XW86 result in their relatively low JSC, and the difference between XW85 and XW86 indicates that a longer linking chain is beneficial for partially alleviating the unfavorable single anchoring, resulting in superior performance. The results indicate that the photovoltaic behavior for the CC dyes may be further enhanced by avoiding the unfavorable alignment of the two carboxyl groups in opposite directions and thus simultaneously anchoring the two carboxyl groups, which may be realized through more rational molecular design.
Introduction
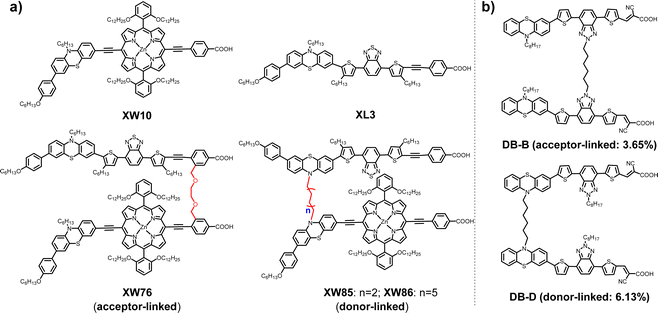
The conversion and utilization of solar energy have attracted extensive attention because of the demand for energy consumption, carbon emission reduction, and environmental protection.1 As a representative technique in the field of photoelectric conversion, dye-sensitized solar cells (DSSCs) have been considerably developed since the first report by Grätzel and O’Regan in 1991.2–5 As an essential component of DSSCs, light absorbing sensitizers harvest sunlight and transfer the photogenerated electrons into the semiconductor (TiO2). Various types of sensitizers, such as ruthenium-based complexes,6,7 metal-free organic dyes,8–14 and porphyrin dyes,15–21 have been designed with the purpose of improving the photovoltaic performance.22–25Although high power conversion efficiencies (PCEs) have been achieved for the DSSCs based on porphyrin dyes due to their excellent light-harvesting properties,3,4,15,26–28 one of the main drawbacks for porphyrin sensitizers is the weak absorption in the green color region between the Soret and Q bands, which limits sunlight harvesting and the PCE.29–32 To address this problem, a cosensitization strategy has been developed and extensively employed by using a porphyrin dye and an organic dye with complementary absorption to achieve panchromatic absorption and PCE improvement.33–36 Despite the success in this respect, the optimization process of the cosensitization approach is somewhat complicated and it is challenging to control the distribution of the two dyes on the semiconductor interface.37,38 Recently, we have developed a class of concerted companion (CC) dyes by covalently linking the organic dye and porphyrin dye units through flexible chains at the acceptor moieties.15,39–41 For example, XW76 has been constructed by linking the acceptors of two sub-dye units corresponding to XW10 and XL3 (Fig. 1a).26,40 Notably, these dyes exhibit panchromatic absorption in the visible region, resulting in high efficiencies and long-term photostability because of the double anchoring effect.
It has been reported that the capability of suppressing charge recombination may be improved by linking two branches of organic dye units at the donor parts, and thus the photovoltaic performance may be enhanced (Fig. 1b).42–46 Inspired by these results, the sub-dye units identical to those used for XW76 have been employed to construct two novel CC dyes XW85 and XW86 by linking the phenothiazine donors with flexible chains of C6H12 and C12H24, respectively (Fig. 1a).
As a result, XW86 exhibits higher incident photon-to-current conversion efficiency (IPCE) plateau, larger JSC, and higher PCEs (9.76% for I3−/I− and 8.78% for [Co(bpy)3]3+/2+) compared to those of XW85. Notably, the PCE of XW86 is also higher than those obtained by the cosensitizations of XW10 and XL3. However, the PCE obtained for XW86 is lower than that of the reference acceptor-linked CC dye XW76. The desorption behavior measurements have revealed that considerable amounts of dyes are singly anchored in the devices of XW85 and XW86, which lowers the PCE of the cells. Compared with XW85, XW86 with the longer linking chain affords an enhanced double/single anchoring ratio (1.88 for XW86vs. 1.31 for XW85), resulting in superior performance for XW86. The results indicate that the photovoltaic performance for CC dyes may be further improved by enhancing the double-anchoring proportion through elaborate molecular design in the future.
Results and discussion
Syntheses of the CC dyes
The synthetic procedures for the donor-linked CC dyes XW85 and XW86 are outlined in Scheme 1. Initially, the Suzuki coupling of diphenothiazine tetrabromides PTZ-a and PTZ-b47 with 4-(hexyloxy)phenylboronic acid yielded the donor-attached dibromides 1a and 1b, respectively. Subsequent Pd-catalyzed Sonogashira coupling reactions with porphyrin alkynyl intermediate ZnP-A48 produced 2a and 2b, respectively. Thereafter, Pd-catalyzed Miyaura coupling reactions of 2a and 2b with bis(pinacolato)diboron followed by Suzuki coupling with the organic dye acceptor unit BT-BTD-A49 provided esters 3a and 3b, respectively, and then hydrolysis of the ester groups afforded XW85 and XW86. The structures of all the synthesized compounds have been characterized by 1H NMR, 13C NMR, mass spectra, IR spectra and melting point (see the Experimental section and the ESI†).Optical properties
The absorption spectra of the dyes in THF and anchored on thin TiO2 films are presented in Fig. 2, with the corresponding data summarized in Table S1 (ESI†). Due to the structural similarity with the reported CC dye XW76, the donor-linked CC dyes XW85 and XW86 exhibit panchromatic absorption in the visible region similar to that of XW76.40 The similar absorption characteristics of the three CC dyes indicate that the linking chain positions and lengths hardly perturb their light-harvesting capability. Upon anchoring onto the TiO2 films, all the dyes show broadened bands (Fig. 2b), which can facilitate light-harvesting in the DSSCs.To explore the possibility of energy transfer between the sub-dye units of the CC dyes, the emission spectra of the dyes have been measured. The porphyrin dye XW10 and the organic dye XL3 exhibit emission at 675 nm and 629 nm, respectively (Fig. S1a, ESI†), and the CC dyes XW85, XW86, and XW76 demonstrate a strong emission peak at ca. 675 nm corresponding to the porphyrin unit (Fig. S1a and b, ESI†), with the peak at ca. 629 nm contributed by the organic dye unit considerably weakened, irrespective of the excitation wavelength (Fig. S1c, ESI†). These results are indicative of effective energy transfer from the organic dye unit to the porphyrin unit, which may partially compensate for the photocurrent loss for the singly anchored CC dyes via the porphyrin unit (vide infra).
Electrochemical properties
The electrochemical behavior of the CC dyes was investigated by cyclic voltammetry (CV) and differential pulse voltammetry (DPV) (Fig. S2, ESI†) to estimate the thermodynamic feasibility of the charge transfer processes. The energy level diagrams for the sensitizers, electrolyte redox potential, together with the conduction band edge of TiO2 are shown in Fig. 3. The oxidation potentials (Eox) of XW85 and XW86, corresponding to their highest occupied molecular orbital (HOMO) levels, were found to be 0.83 and 0.80 V (vs. NHE), respectively, which are lower than the I3−/I− and [Co(bpy)3]3+/2+ redox potentials (∼0.4 V and 0.56 V vs. NHE, respectively) indicative of enough driving force for the reduction of the dye cations.50 The optical band gaps (E0–0) of XW85 and XW86 were both estimated to be 1.91 eV from the intersection of the normalized absorption and fluorescence spectra in THF. From the difference between the E0–0 and Eox values, the excited state potentials were calculated to be −1.08 and −1.11 V (vs. NHE), considerably higher than that of the conduction band of TiO2 (−0.5 V vs. NHE) suggesting that both of the sensitizers provide sufficient driving force for electron injection. | ||
| Fig. 3 Energy level diagrams of the sensitizers with respect to the conduction band of TiO2 and the redox potentials of I3−/I− and [Co(bpy)3]3+/2+. | ||
Photovoltaic performance
To evaluate the photovoltaic behavior of the donor-bridged CC dyes, the corresponding DSSCs have been fabricated using the I3−/I− and [Co(bpy)3]3+/2+ electrolytes (see the ESI† for details). The photocurrent density–voltage (J–V) curves (Fig. 4a and c) and IPCE spectra (Fig. 4b and d) were measured under simulated sunlight (AM 1.5) and the corresponding photovoltaic parameters are collected in Table 1. For the DSSCs based on the I3−/I− electrolyte, XW85 exhibits VOC, JSC, and PCE of 767 mV, 17.20 mA cm−2, and 8.96%, respectively. Compared with XW85, XW86 exhibits a slightly decreased VOC (755 mV) but an elevated JSC (18.55 mA cm−2), and the resulting PCE (9.76%) is higher than that of XW85. Compared to the corresponding component dyes XL3 (PCE of 8.69%)40 and XW10 (PCE of 8.6%),26XW85 and XW86 show moderately improved photovoltaic performance. Furthermore, the efficiency achieved for XW86 is higher than that of 9.28% obtained for the device cosensitized with XW10 and XL3, which exhibits a considerably lower JSC as a result of the considerably lowered adsorption amount of the porphyrin dye accompanied with the coadsorption of the organic dye (Table 1 and Table S2, S3, ESI†). Compared with the iodine-based DSSCs, the devices based on the cobalt electrolyte exhibit improved VOC attributable to the higher potential of the cobalt redox mediator.50 Despite the improved VOC, the JSC values are considerably lowered because of the mass transport limitation of the bulky cobalt complex.51 As a result, XW85 and XW86 exhibit lowered efficiencies of 7.80% and 8.78%, respectively. | ||
| Fig. 4 J–V characteristic curves of the DSSCs using (a) iodine-based and (c) cobalt-based redox mediators, and IPCE spectra of the DSSCs using (b) iodine-based and (d) cobalt-based redox mediators. | ||
| Dyes | Electrolyte | V OC [mV] | J SC [mA cm−2] | J IPCE [mA cm−2]c | FF [%] | PCE [%] | Dye loading [× 10−8 mol cm−2] |
|---|---|---|---|---|---|---|---|
| a The data were collected from three parallel cells (average values and errors). b The TiO2 electrode was dipped in a mixed dye solution (XW10/XL3, 1/2) in a mixture of chloroform and ethanol (1/1, v/v) for 10 h (device M3 in the ESI). c The JIPCE values were obtained by integrating the IPCE spectra, and the deviations from the corresponding JSC values obtained from the J–V tests are listed in the parentheses. | |||||||
| XW85 | I3−/I− | 767 ± 2 | 17.20 ± 0.27 | 15.91 (8.1%) | 67.92 ± 0.52 | 8.96 ± 0.08 | 12.6 |
| XW86 | I3−/I− | 755 ± 2 | 18.55 ± 0.32 | 16.83 (10.2%) | 69.66 ± 0.59 | 9.76 ± 0.16 | 11.7 |
| XW10 + XL3b | I3−/I− | 794 ± 4 | 16.60 ± 0.26 | 14.78 (12.3%) | 70.40 ± 0.23 | 9.28 ± 0.15 | 0.42 (XW10)/23.5(XL3) |
| XL3 40 | I3−/I− | 810 ± 3 | 16.31 ± 0.16 | 14.84 (9.9%) | 65.81 ± 0.24 | 8.69 ± 0.07 | 25.0 |
| XW10 26 | I3−/I− | 711 ± 5 | 17.90 ± 0.04 | 16.08 (11.3%) | 68.4 ± 0.5 | 8.6 ± 0.1 | 9.4 |
| XW76 40 | I3−/I− | 765 ± 5 | 19.94 ± 0.18 | 18.28 (9.1%) | 70.70 ± 0.66 | 10.78 ± 0.11 | 8.2 |
| XW85 | [Co(bpy)3]3+/2+ | 923 ± 2 | 11.79 ± 0.14 | 11.72 (0.6%) | 71.65 ± 0.55 | 7.80 ± 0.12 | 7.4 |
| XW86 | [Co(bpy)3]3+/2+ | 911 ± 2 | 13.33 ± 0.29 | 13.02 (2.4%) | 72.29 ± 0.80 | 8.78 ± 0.11 | 6.9 |
| XW10 + XL3b | [Co(bpy)3]3+/2+ | 951 ± 1 | 11.27 ± 0.15 | 11.11 (1.4%) | 71.59 ± 0.69 | 7.68 ± 0.17 | 0.2 (XW10)/17.2(XL3) |
| XL3 | [Co(bpy)3]3+/2+ | 975 ± 1 | 10.79 ± 0.11 | 10.58 (2.0%) | 72.10 ± 1.15 | 7.59 ± 0.15 | 17.6 |
| XW10 | [Co(bpy)3]3+/2+ | 891 ± 2 | 12.09 ± 0.21 | 11.91 (1.5%) | 72.78 ± 0.49 | 7.84 ± 0.16 | 5.1 |
| XW76 | [Co(bpy)3]3+/2+ | 919 ± 2 | 14.63 ± 0.22 | 14.55 (0.5%) | 72.12 ± 1.00 | 9.69 ± 0.13 | 4.2 |
As illustrated in Fig. 4b and d, the IPCE spectra of XW85 and XW86 exhibit a panchromatic response over the visible region, similar to that of XW76. By integrating the IPCE data, JIPCE values could be obtained, and they were found to be lower than the corresponding JSC values obtained from the J–V tests by 0.5%–12.3% (Table 1 and Fig. S4, ESI†). These results may be rationalized by the fact that the full irradiation applied in the J–V test generates more heat and electrons, resulting in higher charge transport and collecting efficiencies, and thus higher JSC values were obtained in the J–V tests.30 Compared with the IPCE of XW85, XW86 presents an elevated plateau, consistent with its higher JSC, indicating that the longer linking chain in XW86 is favorable for improving the JSC. Compared with XW85 and XW86, the traditional cosensitization approach affords inferior IPCE values around the Q-band region of the porphyrin dyes within 600–750 nm, which may be related to the very small amount of adsorbed porphyrin dye XW10 (0.42 × 10−8 mol cm−2 for I3−/I− and 0.2 × 10−8 mol cm−2 for Co3+/2+) relative to the organic dye XL3 (23.5 × 10−8 mol cm−2 for I3−/I− and 17.2 × 10−8 mol cm−2 for Co3+/2+) (Table 1). As a result, relatively small JSC values were obtained for the cosensitized devices. These results indicate that the donor-linked CC dyes XW85 and XW86 are indeed effective in enhancing the spectral response and the JSC of the DSSCs because of their panchromatic absorption. However, the IPCE plateau and the JSC for XW85 and XW86 are still obviously lower than those of the acceptor-linked CC dye XW76, despite their similar absorption spectra. As a result, XW85 and XW86 exhibit lowered PCE values relative to XW76 (Table 1).
Considering that internal factors like electron injection efficiency (ηinj) and dye regeneration efficiency (ηreg) may induce the difference in the IPCE values between the two types of CC dyes,52,53 time-resolved fluorescence spectra of the dyes adsorbed on Al2O3 and TiO2 films were checked to evaluate the electron injection dynamics. Compared to those of the dyes on Al2O3, the emission for all the dyes on the TiO2 films are strongly quenched, indicating that the electrons in the excited singlet states of all the adsorbed dyes can effectively inject into TiO2, showing high ηinj values exceeding 85% (Fig. S5 and Table S4, ESI†). The transient absorption measurements were further used to study the regeneration kinetics of the dyes with inert electrolyte and active redox electrolytes. The decay signals for the dye-sensitized TiO2 films with inert electrolyte originate from the recombination between the dye cations and the injected electrons, with the recombination lifetimes (τrec) lying within 79–99 μs (Fig. S6 and Table S5, ESI†). Upon contact with the redox electrolytes, the electron transfer from the I− or Co2+ species to the dye cations accelerates the decay processes, showing regeneration lifetimes of less than 10 μs and ηreg exceeding 90%. The relatively high ηinj and ηreg values for all the dyes ensure smooth electron injection and dye regeneration processes. Although ηinj and ηreg roughly lie in the sequence of XW85 < XW86 < XW76, identical with that of gradually improved IPCE and JSC, the relatively small differences for the ηinj and ηreg values of these dyes cannot fully account for the considerable IPCE and JSC differences. Another possible factor affecting IPCE and JSC is the adsorption amounts of the dyes. In contrast to the fact that XW85 and XW86 exhibit lowered JSC values relative to XW76, the adsorption amounts of XW85 and XW86 are obviously higher than that of XW76 (Table 1). This disagreement indicates that the anchoring states for the CC dyes linked at different positions may be quite different. Hence, we continued to check their anchoring behavior.
Desorption behavior
The anchoring behavior was explored by checking the dye desorption processes. Thus, the TiO2 films adsorbed with the dyes were soaked in 0.01 M NaOH solution (THF/H2O), and the plots of the remaining dye percentages versus the soaking time are shown in Fig. 5. As a result, XL3 and XW10 are completely desorbed within 14 minutes, indicating that the desorption processes are rather fast for the mono-anchored dyes. In contrast, the desorption behavior of the CC dyes is considerably different. A fast desorption process in the initial 14 minutes affords 55%, 63% and 84% of the remaining adsorption amounts for XW85, XW86 and XW76, respectively. Subsequently, a much slower desorption process was observed. When the TiO2 films were soaked for a total of 1 h, the remaining adsorption amounts were found to be 46%, 55%, and 82%, respectively. The two desorption processes imply that the CC dyes may adsorb on the TiO2 nanoparticles in two distinct modes, i.e., single-anchoring or double-anchoring. Thus, the single-anchoring mode corresponds to the fast desorption in the first 14 minutes, while the double-anchoring relates to the subsequent slow desorption process. Judging from the desorption ratios, the acceptor-bridged CC dye XW76 exhibits a higher ratio of double anchoring on TiO2, compared with those of XW85 and XW86. Thus, the slow desorption process for each CC dye was linearly fitted and extrapolated to evaluate the single-anchored dye amount (Fig. S7 and Table S6, ESI†). As a result, the double/single anchoring ratios were obtained to be 1.31, 1.88 and 6.34 for XW85, XW86 and XW76, respectively. These data indicate that extending the linking chain between the donor units is favorable for enhancing the double-anchoring ratio, and the double/single anchoring ratios for the donor-linked CC dyes (XW85 and XW86) are considerably lower than that for the acceptor-linked one (XW76). These desorption results are consistent with the theoretical calculations (vide infra). | ||
| Fig. 5 Plots of the decrease in dye adsorption amounts versus the soaking time in 0.01 M NaOH solution (THF/H2O, 1/1). | ||
To further monitor the anchoring states of the dyes, the ATR-FTIR spectra of the dyes have been collected. All the dyes show a characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band of the carboxyl group at ∼1690 cm−1 (Fig. S8, ESI†). Upon anchoring onto the TiO2 films, this peak disappears for XL3, XW10, and XW76, while the peak at ∼1390 cm−1 attributable to symmetric stretching vibrations (νsym) of COO− is considerably intensified, indicating the nearly complete deprotonation and adsorption of the dyes on the TiO2 surface. In contrast, the corresponding spectra for the adsorbed dyes of XW85 and XW86 still exhibit a weak peak at 1689 cm−1, indicating the presence of considerable amounts of non-adsorbed carboxyl groups, which is consistent with the existence of mono-anchored dyes as revealed by the desorption study (vide supra). Similar results have also been reported for other multi-anchoring dyes.54,55 In addition, the desorption processes for the dyes were also monitored by ATR-FTIR. Unfortunately, no obvious peak could be observed at ∼1690 cm−1 corresponding to free carboxyl groups after the initial fast desorption stage (2 min) for XW85 and XW86, although the single-anchored dye has not been completely desorbed at this stage. This result may be attributed to the formation of carboxylate salts under strongly basic conditions. Further prolonged desorption time (14 and 60 min) afforded similar IR characters.
O stretching band of the carboxyl group at ∼1690 cm−1 (Fig. S8, ESI†). Upon anchoring onto the TiO2 films, this peak disappears for XL3, XW10, and XW76, while the peak at ∼1390 cm−1 attributable to symmetric stretching vibrations (νsym) of COO− is considerably intensified, indicating the nearly complete deprotonation and adsorption of the dyes on the TiO2 surface. In contrast, the corresponding spectra for the adsorbed dyes of XW85 and XW86 still exhibit a weak peak at 1689 cm−1, indicating the presence of considerable amounts of non-adsorbed carboxyl groups, which is consistent with the existence of mono-anchored dyes as revealed by the desorption study (vide supra). Similar results have also been reported for other multi-anchoring dyes.54,55 In addition, the desorption processes for the dyes were also monitored by ATR-FTIR. Unfortunately, no obvious peak could be observed at ∼1690 cm−1 corresponding to free carboxyl groups after the initial fast desorption stage (2 min) for XW85 and XW86, although the single-anchored dye has not been completely desorbed at this stage. This result may be attributed to the formation of carboxylate salts under strongly basic conditions. Further prolonged desorption time (14 and 60 min) afforded similar IR characters.
Accompanying the trend of decreasing double/single anchoring ratios, decreasing JSC values were obtained for the CC dyes XW76, XW86, and XW85, despite the increasing adsorption amounts (Table 1). These observations indicate that each singly anchored CC dye molecule may contain a sub-dye unit hanging away from TiO2, and thus it occupies a smaller area on the TiO2 film. As a result, more dye molecules may be adsorbed, leading to an increase in the adsorption amounts, which may partially compensate for the JSC loss induced by the single anchoring effect. In addition, the effective energy transfer from the organic dye unit to the porphyrin dye unit may also partially compensate for the JSC loss. As a consequence, the lowered JSC values obtained for XW85 and XW86 (17.20 and 18.55 mA cm−2) are still comparable to that of XW76 (19.94 mA cm−2) despite the unfavorable single anchoring effect.
Theoretical calculations
To further understand the anchoring behavior of the CC dyes, theoretical calculations were performed (Fig. S9, ESI†).56,57 The binding energies (Ebe) were calculated by subtracting the energy of the total system from the sum of the respective energies of the dye and TiO2: Ebe = (Edye + ETiO2) − Edye/TiO2. The calculated Ebe values for the double-anchored dyes lie in the order of XW85 (23.8 kcal mol−1) < XW86 (26.0 kcal mol−1) < XW76 (38.4 kcal mol−1) (Table S7, ESI†), consistent with the much stronger adsorption of XW76 than XW85 and XW86 revealed by the desorption study. Furthermore, the Ebe values for the two single-anchoring modes of XW85 and XW86 were calculated to be much smaller, lying within the range of 1.7–11.0 kcal mol−1, implying that the adsorption of the mono-anchored dyes may be weaker and easier to desorb from the film, which is fully consistent with the desorption studies (vide supra). As mentioned above, the porphyrin dye XW10 adsorbs much less efficiently than the organic dye XL3 in the cosensitized systems. However, theoretical calculations reveal that there is no obvious difference between their Ebe values, indicating that the preferable adsorption of XL3 in the cosensitization systems may be related to its smaller molecular size. In contrast, the single-anchored CC dyes XW85 and XW86 exhibit quite different adsorption behavior. The adsorption via the porphyrin unit may be preferable, as evidenced by the higher Ebe values (8.9 and 11.0 kcal mol−1) relative to those of adsorption via the organic unit (1.7 and 6.1 kcal mol−1) (Table S7, ESI†). As a result, the energy transfer from the organic dye unit to the porphyrin unit may indeed partially compensate for the JSC loss induced by the single anchoring of XW85 and XW86 (vide supra).Electrochemical impedance spectroscopy
The electrochemical impedance spectroscopy (EIS) data for the DSSCs were measured in the dark to explore the effect of electrode interface properties on the VOC. Theoretically, for a certain redox couple, the VOC is mainly dominated by the semiconductor conduction band energy level (ECB) and the free electron density,51 and the ECB for TiO2 is affected by the density of states (DOS),58 which is proportional to the capacitance (Cμ) level. As a result, the DSSCs based on the I3−/I− electrolyte exhibit slightly different DOS values for all the dyes at a fixed bias voltage (Fig. 6a), indicative of a relatively weak effect of the conduction band shift on the VOC. On the other hand, the free electron density is mainly governed by the charge recombination resistance (Rrec) values, which increase in the order of XW10 (60.0 Ω cm2) < XW86 (81.8 Ω cm2) < XW76 (171.6 Ω cm2) < XW85 (199.9 Ω cm2) < XW10 + XL3 (698.6 Ω cm2) < XL3 (784.1 Ω cm2) at the fixed bias voltage of −0.75 V (Fig. 6b), in a trend the same as that of increasing VOC values. On this basis, the electron lifetimes (τ) were calculated from the equation of τ = Cμ × Rrec and plotted against DOS (Fig. 6c). At the same DOS, the order of increasing τ values is roughly consistent with those of increasing Rrec and VOC values. These results indicate that the densities of the free electrons in the TiO2 films dominate the VOC of the devices. Similar EIS properties were also observed for the cobalt-based DSSCs (Fig. S10 and Table S8, ESI†).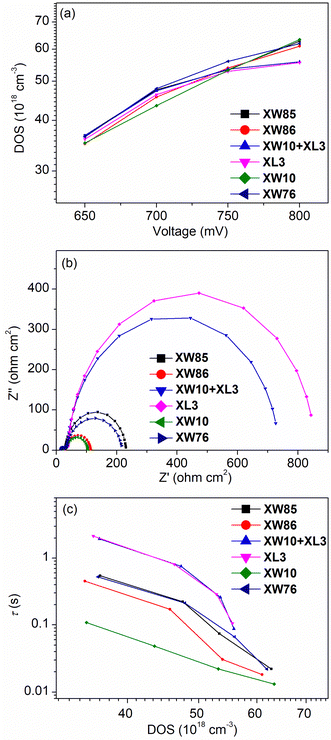 | ||
| Fig. 6 (a) Plots of DOS versus the bias voltages, (b) complex-plane plots at applied voltage of −0.75 V, and (c) τ versus DOS of the DSSCs using I3−/I− electrolyte measured in the dark. | ||
The similar Cμ values obtained for XW85 and XW86 indicate that the linking chain lengths only slightly affect the conduction band positions. On the other hand, ethylene glycol units can usually trap Li+, shift the CB upward and thus enhance the VOC.59 However, XW85 exhibits a Cμ close to that of XW76. The slightly higher VOC obtained for XW85 may be related to the fact that the adsorption amount for XW85 is obviously higher than that of XW76 and thus a more compact dye layer is formed for XW85, which suppresses the charge recombination process as thus a higher Rrec is observed for XW85. Compared with XW86, XW85 possesses a shorter linkage and it shows higher Rrec and VOC values (Table 1), indicating that the presence of a more non-anchored sub-dye unit may block the penetration of oxidized species in the electrolyte to approach TiO2, and thus suppress charge recombination. Based on the EIS data, the electron collection efficiencies (ηcol) were also calculated, which lie in the ranges of 85.8–98.1% and 83.8–88.4% for I3−/I− (Vbias = −0.75 V) and Co3+/2+ (Vbias = −0.85 V), respectively (Table S8, ESI†). The lower ηcol values obtained for the cobalt electrolyte are consistent with the lower JSC and IPCE platforms observed for the corresponding DSSCs (Fig. 4b and d).
Durability
The PCE of the DSSCs soaked in long-term visible light was monitored to evaluate the photostability, which is a crucial factor for practical applications of DSSCs.60 The cells sensitized by XW85 and XW86 with I3−/I− redox shuttle provided PCEs of 8.05% and 8.86% after 500 h of visible light soaking, which are 89.8% and 90.8% of the initial efficiencies, respectively (Fig. 7), lying between those of 88.8% and 93.1% observed for XW10 and XW76, respectively. The sequence agrees well with that of the double/single anchoring ratios, i.e., the singly anchored dye XW10 exhibits the worst photostability. Whereas, XW85, XW86 and XW76 exhibit increasing photostability with the double/single anchoring ratios gradually improving from 1.31 to 6.34. These results indicate that enhancing the double anchoring proportion will improve the photostability, which is favorable for real applications.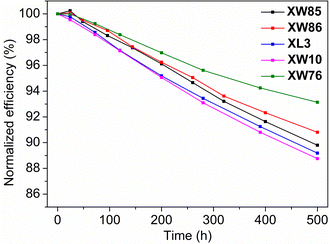 | ||
| Fig. 7 Plots of the changes in the PCEs vs. the illumination time for the solar cells sensitized by XW85, XW86, XL3, XW10 and XW76, respectively. | ||
Conclusions
In summary, two novel CC dyes XW85 and XW86 have been constructed by linking the donors of two sub-dye units corresponding to XL3 and XW10 with flexible chains of C6H12 and C12H24, respectively. Compared to XW85, XW86 exhibits higher JSC values of 18.55 and 13.33 mA cm−2 and PCEs of 9.76% and 8.78% for I3−/I− and [Co(bpy)3]3+/2+ electrolytes, respectively, which are also higher than those of the cosensitized DSSCs based on XL3 and XW10. However, the PCE of XW86 does not outperform that of the acceptor-linked reference CC dye XW76. The desorption study revealed that the CC dyes may adsorb either in the double anchoring mode or the single anchoring mode, and the double/single anchoring ratios lie in the increasing sequence of XW85 (1.31) < XW86 (1.88) < XW76 (6.34), consistent with the sequence of increasing PCE. These results indicate that the lower photovoltaic performance of XW85 and XW86 relative to XW76 may be related to the fact that the non-adsorbed sub-dye unit in the mono-anchored dye molecules may not effectively contribute to electron injection, and thus large portions of mono-anchoring observed for XW85 and XW86 result in their relatively poor JSC and PCE. Compared with XW85, the larger double/single anchoring ratio observed for XW86 indicates that elongation of the linking chain is favorable for double anchoring, leading to an enhanced JSC of XW86, despite its smaller adsorption amount than that of XW85.The adverse single-anchoring problem of the CC dyes revealed in this work indicates that the photovoltaic behavior may be further enhanced by addressing this problem through more rational molecular design in the future. For example, both the donors and the acceptors of the respective sub-dye units may be linked with flexible chains to rule out the possibility of opposite alignment of the two carboxyl groups between sub-dye units and thus simultaneously anchor the carboxyl groups, resulting in enhanced JSC and PCE.
Experimental
Synthesis of the CC dyes XW85–XW86
Compounds PTZ-a, PTZ-b, BT-BTD-A, and ZnP-A were synthesized according to the literature.47–49 The synthetic routes for dyes XW85 and XW86 are shown in Scheme 1 with the details described as follows:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(v: v), ppm): δ 9.56 – 9.49 (m, 4H), 8.72 (d, J = 4.6 Hz, 2H), 8.69 (d, J = 4.5 Hz, 2H), 8.17 (s, 2H), 8.15 (s, 2H), 8.05 (d, J = 8.4 Hz, 2H), 8.01–7.97 (m, 3H), 7.92 (s, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 7.7 Hz, 1H), 7.76 (d, J = 8.8 Hz, 1H), 7.71–7.66 (m, 2H), 7.66 (d, J = 1.8 Hz, 1H), 7.57 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 7.37 (d, J = 8.5 Hz, 2H), 7.33 (d, J = 8.1 Hz, 1H), 7.30–7.27 (m, 2H), 7.24 (d, J = 8.0 Hz, 1H), 7.21 (d, J = 1.8 Hz, 1H), 7.03 (d, J = 8.5 Hz, 4H), 6.94 (d, J = 8.5 Hz, 2H), 6.90–6.85 (m, 3H), 6.81 (d, J = 8.5 Hz, 2H), 3.91 (t, J = 6.5 Hz, 4H), 3.83 (t, J = 6.6 Hz, 12H), 2.80–2.75 (m, 2H), 2.71 (t, J = 1.9 Hz, 2H), 1.74–1.64 (m, 8H), 1.44–1.39 (m, 8H), 1.33–1.27 (m, 18H), 1.23–1.18 (m, 12H), 1.16–1.09 (m, 8H), 1.07–1.00 (m, 16H), 0.97–0.91 (m, 14H), 0.90–0.82 (m, 20H), 0.79–0.72 (m, 18H), 0.59–0.50 (m, 14H), 0.40–0.31 (m, 8H). MS (MALDI-TOF, m/z): [M]+ calcd for C180H212N8O10S5Zn, 2869.4; found, 2869.3. FT-IR (ATR, cm−1): 2920 (s), 2848 (s), 2187 (m), 1689 (s), 1585 (s), 1500 (w), 1454 (s), 1390 (m), 1242 (s), 1205 (m), 1095 (s), 997 (m), 879 (w), 791 (m), 768 (m), 710 (m). mp 121–123 °C.
1(v: v), ppm): δ 9.56 – 9.49 (m, 4H), 8.72 (d, J = 4.6 Hz, 2H), 8.69 (d, J = 4.5 Hz, 2H), 8.17 (s, 2H), 8.15 (s, 2H), 8.05 (d, J = 8.4 Hz, 2H), 8.01–7.97 (m, 3H), 7.92 (s, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 7.7 Hz, 1H), 7.76 (d, J = 8.8 Hz, 1H), 7.71–7.66 (m, 2H), 7.66 (d, J = 1.8 Hz, 1H), 7.57 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 7.37 (d, J = 8.5 Hz, 2H), 7.33 (d, J = 8.1 Hz, 1H), 7.30–7.27 (m, 2H), 7.24 (d, J = 8.0 Hz, 1H), 7.21 (d, J = 1.8 Hz, 1H), 7.03 (d, J = 8.5 Hz, 4H), 6.94 (d, J = 8.5 Hz, 2H), 6.90–6.85 (m, 3H), 6.81 (d, J = 8.5 Hz, 2H), 3.91 (t, J = 6.5 Hz, 4H), 3.83 (t, J = 6.6 Hz, 12H), 2.80–2.75 (m, 2H), 2.71 (t, J = 1.9 Hz, 2H), 1.74–1.64 (m, 8H), 1.44–1.39 (m, 8H), 1.33–1.27 (m, 18H), 1.23–1.18 (m, 12H), 1.16–1.09 (m, 8H), 1.07–1.00 (m, 16H), 0.97–0.91 (m, 14H), 0.90–0.82 (m, 20H), 0.79–0.72 (m, 18H), 0.59–0.50 (m, 14H), 0.40–0.31 (m, 8H). MS (MALDI-TOF, m/z): [M]+ calcd for C180H212N8O10S5Zn, 2869.4; found, 2869.3. FT-IR (ATR, cm−1): 2920 (s), 2848 (s), 2187 (m), 1689 (s), 1585 (s), 1500 (w), 1454 (s), 1390 (m), 1242 (s), 1205 (m), 1095 (s), 997 (m), 879 (w), 791 (m), 768 (m), 710 (m). mp 121–123 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v: v), ppm): δ 9.56–9.49 (m, 4H), 8.72 (d, J = 4.7 Hz, 2H), 8.69 (d, J = 4.5 Hz, 2H), 8.16 (s, 2H), 8.15 (s, 2H), 8.05 (d, J = 8.4 Hz, 2H), 8.02–7.96 (m, 3H), 7.91 (s, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.79 (d, J = 7.5 Hz, 1H), 7.75 (d, J = 8.6 Hz, 1H), 7.71–7.66 (m, 2H), 7.66 (d, J = 1.7 Hz, 1H), 7.58 (d, J = 8.1 Hz, 2H), 7.43 (d, J = 8.3 Hz, 2H), 7.37 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 8.8 Hz, 1H), 7.30–7.26 (m, 2H), 7.24 (d, J = 8.4 Hz, 1H), 7.21 (s, 1H), 7.03 (d, J = 8.5 Hz, 4H), 6.94 (d, J = 8.3 Hz, 2H), 6.88 (d, J = 7.7 Hz, 3H), 6.81 (d, J = 8.4 Hz, 2H), 3.91 (t, J = 6.5 Hz, 4H), 3.84 (t, J = 6.5 Hz, 12H), 2.80–2.75 (m, 2H), 2.68–2.62 (m, 2H), 1.77–1.60 (m, 12H), 1.45–1.38 (m, 8H), 1.35–1.29 (m, 18H), 1.27–1.23 (m, 24H), 1.16–1.10 (m, 8H), 1.08–1.00 (m, 12H), 0.96–0.82 (m, 36H), 0.79–0.72 (m, 16H), 0.59–0.49 (m, 14H), 0.41–0.29 (m, 8H). MS (MALDI-TOF, m/z): [M]+ calcd for C186H224N8O10S5Zn, 2953.5; found, 2953.4. FT-IR (ATR, cm−1): 2920 (s), 2848 (s), 2185 (m), 1689 (s), 1600 (s), 1585 (s), 1497 (w), 1456 (s), 1392 (m), 1240 (s), 1203 (m), 1095 (s), 995 (m), 878 (w), 791 (m), 768 (m), 714 (m). mp 108–110 °C.
1 (v: v), ppm): δ 9.56–9.49 (m, 4H), 8.72 (d, J = 4.7 Hz, 2H), 8.69 (d, J = 4.5 Hz, 2H), 8.16 (s, 2H), 8.15 (s, 2H), 8.05 (d, J = 8.4 Hz, 2H), 8.02–7.96 (m, 3H), 7.91 (s, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.79 (d, J = 7.5 Hz, 1H), 7.75 (d, J = 8.6 Hz, 1H), 7.71–7.66 (m, 2H), 7.66 (d, J = 1.7 Hz, 1H), 7.58 (d, J = 8.1 Hz, 2H), 7.43 (d, J = 8.3 Hz, 2H), 7.37 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 8.8 Hz, 1H), 7.30–7.26 (m, 2H), 7.24 (d, J = 8.4 Hz, 1H), 7.21 (s, 1H), 7.03 (d, J = 8.5 Hz, 4H), 6.94 (d, J = 8.3 Hz, 2H), 6.88 (d, J = 7.7 Hz, 3H), 6.81 (d, J = 8.4 Hz, 2H), 3.91 (t, J = 6.5 Hz, 4H), 3.84 (t, J = 6.5 Hz, 12H), 2.80–2.75 (m, 2H), 2.68–2.62 (m, 2H), 1.77–1.60 (m, 12H), 1.45–1.38 (m, 8H), 1.35–1.29 (m, 18H), 1.27–1.23 (m, 24H), 1.16–1.10 (m, 8H), 1.08–1.00 (m, 12H), 0.96–0.82 (m, 36H), 0.79–0.72 (m, 16H), 0.59–0.49 (m, 14H), 0.41–0.29 (m, 8H). MS (MALDI-TOF, m/z): [M]+ calcd for C186H224N8O10S5Zn, 2953.5; found, 2953.4. FT-IR (ATR, cm−1): 2920 (s), 2848 (s), 2185 (m), 1689 (s), 1600 (s), 1585 (s), 1497 (w), 1456 (s), 1392 (m), 1240 (s), 1203 (m), 1095 (s), 995 (m), 878 (w), 791 (m), 768 (m), 714 (m). mp 108–110 °C.
Further details about characterization figures, fabrication of DSSCs, and photovoltaic behavior measurements can be found in the ESI.†
Author contributions
Jiaxin Luo: formal analysis, investigation, methodology, visualization, and writing – original draft. Yuqing Wang and Glib Baryshnikov: formal analysis. Shaojin Shi, Yuankun Wu, Taochun Ma, and Leyao Wang: investigation. Xinyan Wu, Chengjie Li and Yongshu Xie: conceptualization, formal analysis, investigation, methodology, visualization, writing – review & editing, funding acquisition, and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 22131005, 21971063, 22201074, and 22075077), the Program of Shanghai Academic Research Leader (No. 20XD1401400), the Natural Science Foundation of Shanghai (No. 20ZR1414100, 22ZR1416100), and the Fundamental Research Funds for the Central Universities (No. 222201717003).Notes and references
- N. Yan, C. Zhao, S. You, Y. Zhang and W. Li, Chin. Chem. Lett., 2020, 31, 643–653 CrossRef CAS.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629–634 CrossRef CAS PubMed.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- Y. Ren, D. Zhang, J. Suo, Y. Cao, F. T. Eickemeyer, N. Vlachopoulos, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Nature, 2023, 613, 60–65 CrossRef CAS PubMed.
- Q. Yu, Y. Wang, Z. Yi, N. Zu, J. Zhang, M. Zhang and P. Wang, ACS Nano, 2010, 4, 6032–6038 CrossRef CAS PubMed.
- C. E. Housecroft and E. C. Constable, Chem. Sci., 2022, 13, 1225–1262 RSC.
- H. Jiang, Y. Ren, W. Zhang, Y. Wu, E. C. Socie, B. I. Carlsen, J.-E. Moser, H. Tian, S. M. Zakeeruddin, W.-H. Zhu and M. Grätzel, Angew. Chem., Int. Ed., 2020, 59, 9324–9329 CrossRef CAS PubMed.
- C. Liao, K. Zeng, H. Wu, Q. Zeng, H. Tang, L. Wang, H. Meier, Y. Xie and D. Cao, Cell Rep. Phys. Sci., 2021, 2, 100326 CrossRef CAS.
- D. Zhang, M. Stojanovic, Y. Ren, Y. Cao, F. T. Eickemeyer, E. Socie, N. Vlachopoulos, J.-E. Moser, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Nat. Commun., 2021, 12, 1777 CrossRef CAS PubMed.
- L. Zhang, X. Yang, W. Wang, G. G. Gurzadyan, J. Li, X. Li, J. An, Z. Yu, H. Wang, B. Cai, A. Hagfeldt and L. Sun, ACS Energy Lett., 2019, 4, 943–951 CrossRef CAS.
- Y. K. Eom, I. T. Choi, S. H. Kang, J. Lee, J. Kim, M. J. Ju and H. K. Kim, Adv. Energy Mater., 2015, 5, 1500300 CrossRef.
- Z. Chai, J. Wang, Y. Xie, P. Lin, H. Li, K. Chang, T. Xu, A. Mei, Q. Peng, M. Wang, H. Han, Q. Li and Z. Li, ACS Appl. Mater. Interfaces, 2019, 11, 27648–27657 CrossRef CAS PubMed.
- Z. Chai, M. Wu, M. Fang, S. Wan, T. Xu, R. Tang, Y. Xie, A. Mei, H. Han, Q. Li and Z. Li, Adv. Energy Mater., 2015, 5, 1500846 CrossRef.
- K. Zeng, Y. Chen, W.-H. Zhu, H. Tian and Y. Xie, J. Am. Chem. Soc., 2020, 142, 5154–5161 CrossRef CAS PubMed.
- K. Zeng, Y. Lu, W. Tang, S. Zhao, Q. Liu, W. Zhu, H. Tian and Y. Xie, Chem. Sci., 2019, 10, 2186–2192 RSC.
- S. H. Kang, M. J. Jeong, Y. K. Eom, I. T. Choi, S. M. Kwon, Y. Yoo, J. Kim, J. Kwon, J. H. Park and H. K. Kim, Adv. Energy Mater., 2017, 7, 1602117 CrossRef.
- Y. Kurumisawa, T. Higashino, S. Nimura, Y. Tsuji, H. Iiyama and H. Imahori, J. Am. Chem. Soc., 2019, 141, 9910–9919 CrossRef CAS PubMed.
- T. Higashino, Y. Kurumisawa, A. B. Alemayehu, R. F. Einrem, D. Sahu, D. Packwood, K. Kato, A. Yamakata, A. Ghosh and H. Imahori, ACS Appl. Energy Mater., 2020, 3, 12460–12467 CrossRef CAS.
- Y. Hu, A. Alsaleh, O. Trinh, F. D'Souza and H. Wang, J. Mater. Chem. A, 2021, 9, 27692–27700 RSC.
- Y. Hu, W. A. Webre, M. B. Thomas, A. Moss, S. N. Hancock, J. Schaffner, F. D'Souza and H. Wang, J. Mater. Chem. A, 2019, 7, 10712–10722 RSC.
- H. Song, Q. Liu and Y. Xie, Chem. Commun., 2018, 54, 1811–1824 RSC.
- K. Zeng, Z. Tong, L. Ma, W.-H. Zhu, W. Wu and Y. Xie, Energy Environ. Sci., 2020, 13, 1617–1657 RSC.
- T. Higashino and H. Imahori, ACS Energy Lett., 2022, 7, 1926–1938 CrossRef CAS.
- M. Aftabuzzaman, S. Sarker, C. Lu and H. K. Kim, J. Mater. Chem. A, 2021, 9, 24830–24848 RSC.
- Y. Xie, Y. Tang, W. Wu, Y. Wang, J. Liu, X. Li, H. Tian and W.-H. Zhu, J. Am. Chem. Soc., 2015, 137, 14055–14058 CrossRef CAS PubMed.
- J.-M. Ji, H. Zhou, Y. K. Eom, C. H. Kim and H. K. Kim, Adv. Energy Mater., 2020, 10, 2000124 CrossRef CAS.
- A. Mahmood, J.-Y. Hu, B. Xiao, A. Tang, X. Wang and E. Zhou, J. Mater. Chem. A, 2018, 6, 16769–16797 RSC.
- C.-C. Chen, J.-S. Chen, V. S. Nguyen, T.-C. Wei and C.-Y. Yeh, Angew. Chem., Int. Ed., 2021, 60, 4886–4893 CrossRef CAS PubMed.
- H. Song, J. Zhang, J. Jin, H. Wang and Y. Xie, J. Mater. Chem. C, 2018, 6, 3927–3936 RSC.
- Y. Zhang, K. Ren, L. Wang, L. Wang and Z. Fan, Chin. Chem. Lett., 2022, 33, 33–60 CrossRef CAS.
- Y. Tang, X. Liu, Y. Wang, Q. Liu, X. Li, C. Li, X. Shen and Y. Xie, Chin. Chem. Lett., 2020, 31, 1927–1930 CrossRef CAS.
- C.-L. Wang, J.-Y. Hu, C.-H. Wu, H.-H. Kuo, Y.-C. Chang, Z.-J. Lan, H.-P. Wu, E. Wei-Guang Diau and C.-Y. Lin, Energy Environ. Sci., 2014, 7, 1392–1396 RSC.
- S. Chang, H. Wang, Y. Hua, Q. Li, X. Xiao, W.-K. Wong, W. Y. Wong, X. Zhu and T. Chen, J. Mater. Chem. A, 2013, 1, 11553–11558 RSC.
- T. Hua, K. Zhang, Z.-S. Huang, L. Wang, H. Tang, H. Meier and D. Cao, J. Mater. Chem. C, 2019, 7, 10379–10388 RSC.
- J. M. Cole, G. Pepe, O. K. Al Bahri and C. B. Cooper, Chem. Rev., 2019, 119, 7279–7327 CrossRef CAS PubMed.
- Y. Lu, Q. Liu, J. Luo, B. Wang, T. Feng, X. Zhou, X. Liu and Y. Xie, ChemSusChem, 2019, 12, 2802–2809 CrossRef CAS PubMed.
- J. Zou, Y. Tang, G. Baryshnikov, Z. Yang, R. Mao, W. Feng, J. Guan, C. Li and Y. Xie, J. Mater. Chem. A, 2022, 10, 1320–1328 RSC.
- Y. Chen, Y. Tang, J. Zou, K. Zeng, G. Baryshnikov, C. Li and Y. Xie, ACS Appl. Mater. Interfaces, 2021, 13, 49828–49839 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. Zou, X. Wu, X. Gong, C. Li and Y. Xie, Chin. Chem. Lett., 2022, 33, 4313–4316 CrossRef CAS.
- J. Zou, Y. Wang, G. Baryshnikov, J. Luo, X. Wang, H. Ågren, C. Li and Y. Xie, ACS Appl. Mater. Interfaces, 2022, 14, 33274–33284 CrossRef CAS PubMed.
- S. Jiang, S. Fan, X. Lu, G. Zhou and Z.-S. Wang, J. Mater. Chem. A, 2014, 2, 17153–17164 RSC.
- Z.-S. Huang, C. Cai, X.-F. Zang, Z. Iqbal, H. Zeng, D.-B. Kuang, L. Wang, H. Meier and D. Cao, J. Mater. Chem. A, 2015, 3, 1333–1344 RSC.
- C.-Y. Lo, D. Kumar, S.-H. Chou, C.-H. Chen, C.-H. Tsai, S.-H. Liu, P.-T. Chou and K.-T. Wong, ACS Appl. Mater. Interfaces, 2016, 8, 27832–27842 CrossRef CAS PubMed.
- H. Meier, Z.-S. Huang and D. Cao, J. Mater. Chem. C, 2017, 5, 9828–9837 RSC.
- Z. Wang, Q. Chen, Y. Zou, J. Chen, Y. Luo, Y. Liu, S. Ding, P. Cai, J. Yuan and M. Liang, Dyes Pigm., 2021, 187, 109134 CrossRef CAS.
- Y. H. Lee, R. K. Chitumalla, B. Y. Jang, J. Jang, S. Thogiti and J. H. Kim, Dyes Pigm., 2016, 133, 161–172 CrossRef CAS.
- K. Zeng, W. Tang, C. Li, Y. Chen, S. Zhao, Q. Liu and Y. Xie, J. Mater. Chem. A, 2019, 7, 20854–20860 RSC.
- J. An, X. Yang, B. Cai, L. Zhang, K. Yang, Z. Yu, X. Wang, A. Hagfeldt and L. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 46397–46405 CrossRef CAS PubMed.
- S. M. Feldt, E. A. Gibson, E. Gabrielsson, L. Sun, G. Boschloo and A. Hagfeldt, J. Am. Chem. Soc., 2010, 132, 16714–16724 CrossRef CAS PubMed.
- S. C. Pradhan, A. Hagfeldt and S. Soman, J. Mater. Chem. A, 2018, 6, 22204–22214 RSC.
- M. Pazoki, U. B. Cappel, E. M. J. Johansson, A. Hagfeldt and G. Boschloo, Energy Environ. Sci., 2017, 10, 672–709 RSC.
- Y. Wang, L. Yang, M. Xu, M. Zhang, Y. Cai, R. Li and P. Wang, J. Phys. Chem. C, 2014, 118, 16441–16446 CrossRef CAS.
- R. Ambre, K.-B. Chen, C.-F. Yao, L. Luo, E. W.-G. Diau and C.-H. Hung, J. Phys. Chem. C, 2012, 116, 11907–11916 CrossRef CAS.
- W. Zhang, X. Dai, X. Liao, X. Zang, H. Zhang, X. Yin, C. Yu, C. Ke and Y. Hong, Sol. Energy, 2020, 212, 220–230 CrossRef CAS.
- J. J. P. Stewart, J. Mol. Model., 2007, 13, 1173–1213 CrossRef CAS PubMed.
- M. Korth, M. Pitoňák, J. Řezáč and P. Hobza, J. Chem. Theory Comput., 2010, 6, 344–352 CrossRef CAS PubMed.
- J. Bisquert, F. Fabregat-Santiago, I. Mora-Seró, G. Garcia-Belmonte and S. Giménez, J. Phys. Chem. C, 2009, 113, 17278–17290 CrossRef CAS.
- S. Vijaya, G. Landi, H.-C. Neitzert and S. Anandan, Phys. Chem. Chem. Phys., 2020, 22, 18183–18191 RSC.
- M. Kokkonen, P. Talebi, J. Zhou, S. Asgari, S. A. Soomro, F. Elsehrawy, J. Halme, S. Ahmad, A. Hagfeldt and S. G. Hashmi, J. Mater. Chem. A, 2021, 9, 10527–10545 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc05167b |
| This journal is © The Royal Society of Chemistry 2023 |