Enhancing the stability of the polymeric Lewis-base-assisted dual-phase 3D CsPbBr3–Cs4PbBr6 perovskite by molecular engineering and self-passivation†
Fang-Cheng
Liang‡
 a,
Zhen-Li
Yan‡
ab,
Dhana Lakshmi
Busipalli
c,
Jean-Sebastien
Benas
a,
Zhi-Xuan
Zhang
a,
Su-Ting
Han
a,
Zhen-Li
Yan‡
ab,
Dhana Lakshmi
Busipalli
c,
Jean-Sebastien
Benas
a,
Zhi-Xuan
Zhang
a,
Su-Ting
Han
 d,
Ye
Zhou
d,
Ye
Zhou
 *e,
Jyh-Chiang
Jiang
*e,
Jyh-Chiang
Jiang
 *c and
Chi-Ching
Kuo
*c and
Chi-Ching
Kuo
 *a
*a
aInstitute of Organic and Polymeric Materials, Research and Development Center of Smart Textile Technology, National Taipei University of Technology, Taipei, 10608, Taiwan. E-mail: kuocc@mail.ntut.edu.tw; Fax: +886-2-27317174; Tel: +886-2-27712171 (ext. 2407)
bInstitute of Polymer Science and Engineering and Advanced Research Center for Green Materials Science and Technology, National Taiwan University, Taipei 10617, Taiwan
cDepartment of Chemical Engineering, National Taiwan University of Science and Technology, Taipei, 10608, Taiwan. E-mail: jcjiang@mail.ntust.edu.tw
dInstitute of Microscale Optoelectronics, Shenzhen University, Shenzhen 518060, P. R. China
eInstitute for Advanced Study Shenzhen University, Shenzhen 518060, P. R. China. E-mail: yezhou@szu.edu.cn
First published on 28th November 2022
Abstract
Inorganic metal halide perovskites have attracted attention for use in next-generation perovskite light-emitting diodes (PeLEDs) due to their excellent optical performance. However, the performance of most PeLEDs is influenced by surface defects and carrier diffusion properties. Herein, we present a facile and effective approach to form a self-ordered macromolecular intermediate phase by incorporating high molecular weight Lewis base polyvinylpyrrolidone (HM-PVP) within perovskite films. The synergistic effect of thermodynamically controlled perovskite grain growth and grain boundary passivation enables the formation of a highly cross-linked and bridged long-range-ordered polymer–perovskite composite. Furthermore, theoretical density functional theory calculations confirmed that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in HM-PVP induce a shift of the electronic cloud toward the Pb2+ ions, resulting in a decrease in the perovskite surface energy and favoring thermodynamically modulated perovskite growth. Significantly, silver nanoparticle incorporation into the hole transport layer improves carrier transmission efficiency in HM-7% PVP bulk 3D perovskites and quasi-2D perovskite composite devices, exhibiting luminances of 12
O groups in HM-PVP induce a shift of the electronic cloud toward the Pb2+ ions, resulting in a decrease in the perovskite surface energy and favoring thermodynamically modulated perovskite growth. Significantly, silver nanoparticle incorporation into the hole transport layer improves carrier transmission efficiency in HM-7% PVP bulk 3D perovskites and quasi-2D perovskite composite devices, exhibiting luminances of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 and 9500 cd m−2 and current efficiencies of 11.5 cd A−1 and 15.4 cd A−1, respectively. Our results demonstrate that employing a polymeric passivating agent as a Lewis base adduct thermodynamically modulates perovskite growth and improves the perovskite film's quality for achieving highly stable PeLEDs.
000 cd m−2 and 9500 cd m−2 and current efficiencies of 11.5 cd A−1 and 15.4 cd A−1, respectively. Our results demonstrate that employing a polymeric passivating agent as a Lewis base adduct thermodynamically modulates perovskite growth and improves the perovskite film's quality for achieving highly stable PeLEDs.
Introduction
Metal halide perovskites have emerged as a promising candidate for use in optoelectronic devices including photovoltaics,1–3 light-emitting devices,4–6 and photodetectors7,8 owing to their low cost and excellent optoelectronic properties. Development of perovskite light-emitting diodes (PeLEDs) has been mainly based on the hybrid organic–inorganic perovskite (OIP) system because of its high luminance, high external quantum efficiency (EQE), and tunable optical band gap.9–13 Despite great progress in the performance of OIP-based PeLEDs, the intrinsic moisture and thermal instability originating from the organic component remain major concerns that restrict their practical mass production. By contrast, all-inorganic halide perovskites (CsPbX3, where X = Cl, Br, or I) have been tested and attracted considerable attention for their application in LEDs due to their high stability and optoelectronic properties, such as high thermal and chemical stability (∼580 °C for CsPbBr3, compared with ∼220 °C for MAPbBr3),14,15 narrow emission wavelength (17 ≤ full width at half maximum [FWHM] ≤ 19 nm), high photoluminescence quantum yield (PLQY),16,17 and low exciton binding energy (40 meV).18–20 Given these advantages, CsPbBr3, which has high stability and efficiency, has been extensively investigated.21–25 All-inorganic halide perovskites are highly stable against humidity and under continuous operation and are thus promising building blocks for the development of sustainable applications.Tailoring the structurally defective grain boundaries of perovskite films is essential for improving their optoelectronic properties and device stability.26–30 Studies have demonstrated that pinholes and discontinuous surface coverage in perovskite films provide pathways for electrical short circuits, which hinder the PeLED performance.29 Furthermore, most defects, such as dangling bonds and vacancies, provide charge accumulation pathways at grain boundaries and trigger degradation of charge carrier transport and device performance.31–35 Theoretical simulations and experimental studies have demonstrated that disordered octahedral structures can form defect states and are associated with electronic trap states and nonradiative recombination centers; they thus limit the practical application of PeLEDs. Hence, high-quality perovskite films for highly efficient optoelectronic devices must fulfil several crucial criteria, including (1) modulation of perovskite crystallization kinetics to obtain a perovskite film without pinhole defects to suppress internal structural defects, (2) spontaneous passivation of surface defects at grain boundaries, and (3) intergrain cross-linking to synergistically control perovskite growth and provide a solid foundation for enhanced stability against degradation due to a hostile environment. The inter-grain polymer cross-linking of perovskite grains reduces the number of trap sites and thermodynamically regulates the grain growth resulting in lower electrical decoupling. Yang Yang et al.27,28 reported a polymer possessing numerous bases interacting with a perovskite precursor via Lewis adduct formation to obtain several benefits. Lewis adduct formation induces electronic cloud displacement toward the perovskite. (4) Intrinsic perovskite defect suppression can be carried out through strict screening effect management of carrier and defect coulombic interaction. Comprehensive discussion of the electro–phonon coupling with charge carriers and defect behaviors within perovskites remains scarce and needs to be addressed urgently. So far, reorientation of organic dipoles and ionic lattice-induced polaronic relaxation have resulted in a screening effect that induces increased control of carrier coulombic interaction as well as defect management.36–38 Therefore, our PVP with N functions as an extrinsic surface passivator to provide numerous and strong Lewis adduct formation sites, thus playing two keys roles: intergrain bridging and efficient grain passivation as well as a screening effect enhancer. The correlation between the screening effect and defect passivation results in suppressed electrical decoupling, a larger charge transport rate, and lower trap site density.
Defect passivation engineering has been exploited to produce highly stable and efficient PeLEDs.39–41 Numerous reports have indicated that Lewis base additive passivation is widely considered an effective method of passivation for PeLEDs. The functional groups in Lewis base additives, such as sulfonic groups, carboxyls, and amines, have been employed to passivate halide vacancies to form Lewis acid–base adducts through coordination with undercoordinated Pb2+ defects.3,26–30,42–46 This approach suppresses nonradiative recombination, simultaneously improves the perovskite's phase stability, and enhances the device performance.31–35,47 Noel et al. proposed that organic Lewis bases containing pyridine and thiophene groups modulate perovskite nucleation and crystallization. The nitrogen donor of pyridine, with a strong interaction effect between N atoms and Pb atoms, helps improve the quality of perovskite crystals, whereas the sulfur donor of thiophene, with weak electronegativity difference between S atoms and Pb atoms, helps manipulate perovskite's crystallization kinetics.3 Lee et al. reported that when the oxygen donor of urea was used to slow the perovskite formation process by establishing an intermediate phase between urea and undercoordinated Pb ions, nonradiative recombination traps at grain boundaries were suppressed.26 Han et al. reported that the introduction of amino-functionalized 1,4,8,11-tetraazacyclotetradecane molecules in CsPbBr3-based perovskites facilitated defect site passivation and improved PeLED performance.45 Despite reports highlighting the suitability of small-molecule species for stabilizing the perovskite crystal structure, practical use of small molecules has been limited by the molecules’ high volatility and diffusion coefficients; thus, their application in a device would cause degradation upon exposure to a harsh environment. By contrast, the incorporation of polymeric additives into perovskite films endows the films with a stable macromolecular intermediate phase. This unique strategy provides strong cross-linking passivation agents to bridge the perovskite grains, minimizes intergrain electrical decoupling, and greatly improves device performance.26–30
Hence, we exploit the synergistic effect of Lewis base additives including low molecular weight N-vinyl-2-pyrrolidone (SM-VP) and high molecular weight polyvinylpyrrolidone (HM-PVP) to engineer perovskite grains with Cs4PbBr6-induced charge carrier and exciton binding energy engineering. Compared with SM-VP, HM-PVP possesses both a highly cross-linked structure and numerous repeating units, bridging perovskite grains and resulting in a pinhole-free perovskite film. The formation of a macromolecular intermediate is facilitated and the perovskite crystallization kinetics is controlled via Lewis base adducts (Fig. 1(a)). The coordination mechanism is confirmed by density functional theory (DFT) calculations; the HM-PVP oxygen donor coordinates with undercoordinated Pb2+, thereby decreasing perovskite surface energy and efficiently passivating grain boundary defect sites. The synergistic effect of HM-PVP and Cs4PbBr6 leads to the formation of strong excitons with a lifetime as high as 28.84 ns through suppression of intergrain electrical decoupling and high environmental stability against degradation sources. As a proof of concept, we fabricated HM-7% PVP bulk 3D perovskite and HM-7% PVP quasi-2D perovskite composite devices. Champion device luminances of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 and 9500 cd m−2 and high current efficiencies peaking at 11.5 cd A−1 and 15.4 cd A−1 could be achieved for HM-7% PVP bulk 3D perovskites and HM-7% PVP quasi-2D perovskites, respectively, by silver nanoparticle (AgNP) incorporation into the hole transport layer which effectively improved carrier transmission efficiency. All of these constitute substantial improvements over bulk 3D CsPbBr3–Cs4PbBr6 PeLEDs.11 Our investigation provides insight into the thermodynamically controlled perovskite grain growth with strengthened binding energy for fabrication of reliable PeLEDs.
000 cd m−2 and 9500 cd m−2 and high current efficiencies peaking at 11.5 cd A−1 and 15.4 cd A−1 could be achieved for HM-7% PVP bulk 3D perovskites and HM-7% PVP quasi-2D perovskites, respectively, by silver nanoparticle (AgNP) incorporation into the hole transport layer which effectively improved carrier transmission efficiency. All of these constitute substantial improvements over bulk 3D CsPbBr3–Cs4PbBr6 PeLEDs.11 Our investigation provides insight into the thermodynamically controlled perovskite grain growth with strengthened binding energy for fabrication of reliable PeLEDs.
Experimental section
Materials
A pre-patterned indium-tin-oxide (ITO) substrate (5 Ω) was purchased from Lumtic Ltd. Polyvinylpyrrolidone (PVP, Mw = 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), N-vinyl-2-pyrrolidone (NVP), cesium bromide (CsBr, 99.95%), lead bromide (PbBr2, 99.999%), tris-[1-phenyl-1H-benzimidazole] (TPBi), lithium fluoride (LiF, 99.995%), phenethylammonium bromide (PEABr, 99.9%), silver nitrate (AgNO3, ≥99.0%), and dimethyl sulfoxide (DMSO, anhydrous, 99.8%) were purchased from Sigma-Aldrich and used as received. Hydrogen peroxide (H2O2, 35 wt%, Acros Organics), trisodium-citrate dihydrate (Na3CA·H2O, 99.0%, Showa Chemical Co., Ltd), and sodium borohydride (NaBH4, >98%, Acros Organics) were purchased. Poly(3,4-ethylenedioxythi ophene):poly-(styrenesulfonate) (PEDOT:PSS, AI4803) was purchased from Clevious. Poly(4-butylphenyl-diphenyl-amine) (poly-TPD) was purchased from American Dye Source and was used as received.
000), N-vinyl-2-pyrrolidone (NVP), cesium bromide (CsBr, 99.95%), lead bromide (PbBr2, 99.999%), tris-[1-phenyl-1H-benzimidazole] (TPBi), lithium fluoride (LiF, 99.995%), phenethylammonium bromide (PEABr, 99.9%), silver nitrate (AgNO3, ≥99.0%), and dimethyl sulfoxide (DMSO, anhydrous, 99.8%) were purchased from Sigma-Aldrich and used as received. Hydrogen peroxide (H2O2, 35 wt%, Acros Organics), trisodium-citrate dihydrate (Na3CA·H2O, 99.0%, Showa Chemical Co., Ltd), and sodium borohydride (NaBH4, >98%, Acros Organics) were purchased. Poly(3,4-ethylenedioxythi ophene):poly-(styrenesulfonate) (PEDOT:PSS, AI4803) was purchased from Clevious. Poly(4-butylphenyl-diphenyl-amine) (poly-TPD) was purchased from American Dye Source and was used as received.
Synthesis of quasi-two dimensional perovskites and polymer-based perovskite film preparation
The CsPbBr3 precursor solution (145 mg mL−1) was prepared by mixing CsBr and PbBr2 with a desired molar ratio of 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in anhydrous DMSO. The Lewis based VP monomers and PVP polymers with an appropriate 0–7 wt% were mixed with the CsPbBr3 precursor solution and stirred at 50 °C until all components were dissolved. For the preparation of quasi two-dimensional (Q-2D) perovskites, the PEA2Csn−1PbnBr3n+1 Q-2D perovskite was prepared by mixing CsBr, PbBr2, and PEABr with a desired molar ratio of 1.2
1 in anhydrous DMSO. The Lewis based VP monomers and PVP polymers with an appropriate 0–7 wt% were mixed with the CsPbBr3 precursor solution and stirred at 50 °C until all components were dissolved. For the preparation of quasi two-dimensional (Q-2D) perovskites, the PEA2Csn−1PbnBr3n+1 Q-2D perovskite was prepared by mixing CsBr, PbBr2, and PEABr with a desired molar ratio of 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 in anhydrous DMSO. Afterward, the 7% PVP polymer was mixed with the PEA2Csn−1PbnBr3n+1 precursor solution and stirred at 50 °C until all components were dissolved. For perovskite film fabrication, the precursor solution was prepared by spin-coating at 2000 rpm for 60 s followed by annealing at 80 °C for 10 min in a nitrogen-filled glovebox.
0.5 in anhydrous DMSO. Afterward, the 7% PVP polymer was mixed with the PEA2Csn−1PbnBr3n+1 precursor solution and stirred at 50 °C until all components were dissolved. For perovskite film fabrication, the precursor solution was prepared by spin-coating at 2000 rpm for 60 s followed by annealing at 80 °C for 10 min in a nitrogen-filled glovebox.
Synthesis of AgNPs
AgNPS were synthesized according to a previously published method.6 First, an aqueous solution of polyvinylpyrrolidone (PVP, Mw = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) with trisodium citrate (Na3CA·H2O 75 mM, 2 mL), H2O2 (30 wt%, 120 μL), ethanol (99.8%, 400 μL) and silver nitrate (AgNO3, 0.05 M, 200 μL) was stirred at room-temperature. In order to perform silver reduction, sodium borohydride (NaBH4, 100 mM) was swiftly added to the solution which resulted in the appearance of a light-yellow solution. Ratio tuning between NaBH4 and H2O2 resulted in the formation of Ag NPs of various dimensions that was observed from the change in solution color from dark yellow to red.
000) with trisodium citrate (Na3CA·H2O 75 mM, 2 mL), H2O2 (30 wt%, 120 μL), ethanol (99.8%, 400 μL) and silver nitrate (AgNO3, 0.05 M, 200 μL) was stirred at room-temperature. In order to perform silver reduction, sodium borohydride (NaBH4, 100 mM) was swiftly added to the solution which resulted in the appearance of a light-yellow solution. Ratio tuning between NaBH4 and H2O2 resulted in the formation of Ag NPs of various dimensions that was observed from the change in solution color from dark yellow to red.
Perovskite LED device fabrication
Patterned ITO substrates were cleaned by using deionized water, acetone, and IPA for 20 min and dried under a flow of N2 gas. The substrate was sequentially treated with UV-ozone for 20 min before use. The hole injection layer (HTL) of PEDOT:PSS solutions was filtered by 0.45 μm poly(tetrafluoroethylene) syringe filters and spin-coated onto the ITO substrates at 3000 rpm for 60 s, followed by annealing at 150 °C for 10 min. A series of perovskite precursor solutions with different desired ratios of Lewis based polymers were spin-coated onto the HTL by using the method describe above. TPBI (15 nm), LiF (1 nm), and Ag (80 nm) anodes were deposited by thermal evaporation under high vacuum (∼4 × 10−6 Pa). Before taking out of the glovebox, the glass cover was used for device encapsulation by using epoxy glue. The device active area was 0.2 × 0.2 cm2 as defined using the overlapping area between the ITO and Ag electrodes.Characterization
1H NMR data were recorded at room temperature using a Bruker AM 300 (300 MHz) spectrometer and the residual proton resonance of deuterated chloroform. Transmission electron microscopy (TEM) images were recorded using a JEM-2100F field emission electron microscope equipped with a selected area electron diffraction (SAED) system operating at 200 kV. The TEM samples were prepared by dropping dilute toluene dispersions of high purity on Formvar carbon–copper grids. The morphologies of perovskites were characterized using scanning electron microscopy (SEM; Hitachi S-520) with EDX spectroscopy. Each of the samples was coated with platinum and further analysed and characterized at an acceleration rate of 15 kV. The ultraviolet-visible (UV-vis) absorption and photoluminescence (PL) spectra were recorded using a Shimadzu UV-vis spectrophotometer and a Fluorolog-3 spectrofluorometer (Horiba Jobin Yvon). The photoluminescence quantum yield (PLQY) was determined using a research grade spectrofluorometer system (FP-8500, JASCO). The Fourier transform infrared (FT-IR) spectra were recorded using a Bio-Rad 155 FT-IR spectrometer at ambient temperature in the range of 650–4000 cm−1. The XRD patterns of the prepared films were characterized using a PANalytical diffractometer (X’ Pert3 Powder). The grazing-incidence wide angle X-ray scattering (GIWAXS) patterns were recorded on a beamline BL23A1 at the National Synchrotron Radiation Research Center (NSRRC), Taiwan.The conversion from the scattering angle (θ) to the scattering vector (q) is based on the formula, Q = 4π/λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) sinθ, where λ is the X-ray wavelength. Ultraviolet photoelectron spectroscopy (UPS) was performed using valence band ultraviolet radiation (He I photon energy, 21.2 eV) under a high vacuum condition of ∼8 × 10−10 Pa. X-ray photoelectron spectroscopy (XPS) spectra were measured through a Thermo VG-Scientific spectrometer using a monochromatic Al KR radiation source (1486.6 eV). The electroluminescence spectra were characterized using a Hamamatsu PMA-11 photonic multichannel analyzer. The device performance was determined using a Keithley 2400 source meter and a Minolta CS200 luminance meter was used to record the current–voltage and luminance–voltage characteristics. TR-PL spectra were detected using a spectrometer (iHR320, HORIBA) coupled with a Hamamatsu C10910 streak camera and an M10913 slow single-sweep unit. (NSRRC).
sinθ, where λ is the X-ray wavelength. Ultraviolet photoelectron spectroscopy (UPS) was performed using valence band ultraviolet radiation (He I photon energy, 21.2 eV) under a high vacuum condition of ∼8 × 10−10 Pa. X-ray photoelectron spectroscopy (XPS) spectra were measured through a Thermo VG-Scientific spectrometer using a monochromatic Al KR radiation source (1486.6 eV). The electroluminescence spectra were characterized using a Hamamatsu PMA-11 photonic multichannel analyzer. The device performance was determined using a Keithley 2400 source meter and a Minolta CS200 luminance meter was used to record the current–voltage and luminance–voltage characteristics. TR-PL spectra were detected using a spectrometer (iHR320, HORIBA) coupled with a Hamamatsu C10910 streak camera and an M10913 slow single-sweep unit. (NSRRC).
Computational details
All the density functional theory (DFT) calculations were performed using the projector augmented wave (PAW) method,48 as implemented in the Vienna Ab initio Simulation Package (VASP).49–51 The Perdew–Burke–Ernzerhof functional (PBE) within the generalized gradient approximation (GGA) was used to describe the exchange–correlation effects.52 The optB86b-vdW functional was used to include the van der Waals interaction.52,53 The structural relaxation was performed until the forces were less than 0.02 eV Å−1 on each atom. The convergence criterion was set to a 10−4 eV change in the energy during geometry optimization. The energy cutoff of the plane-wave basis sets was 550 eV, and the Brillion zone sampled using 6 × 4 × 6 and 2 × 4 × 1 Monkhorst–Pack K-points was employed for bulk and surface calculations, respectively.54 One excess electron was added when electron polarons were studied, and one electron was removed from the system when hole polarons were investigated. The calculated lattice parameters for the bulk CsPbBr3 perovskite structure are a = 8.401 Å, b = 11.784 Å and c = 7.992 Å, which are in agreement with the experimental lattice parameters (a = 8.244 Å, b = 11.735 Å and c = 8.198 Å).55 In this study we selected the CsPbBr3(121) surface to study the interaction between the polymer and the perovskite surface based on the corresponding XRD patterns. All the calculations were carried out on the (121) surface using a (2 × 1) slab model, in which the slab consists of four molecular layers. We calculated the surface energy for each surface termination of the CsPbBr3(121) surface as shown in Fig. S1 (ESI†) and Table S1 (ESI†). Surface number 17 had a lower surface energy (0.056 eV Å−2) than that of other possible surface terminations, thereby this surface was chosen for a follow-up study. The most stable termination of the (121) surface has been confirmed to have the smallest surface energy. To avoid interaction between neighboring slabs, the vacuum region was set to be 20 Å. During geometry optimization, the bottom two molecular layers were fixed, and the top two layers were relaxed. Here, we compared the adsorption energy (Eads) of the PVP polymer at different sites and found the most favorable interaction site.Results and discussion
The newly designed macromolecular intermediate phases are constructed with intermolecular forces between the polymeric Lewis base and perovskite forming an intergrain cross-linking structure. The key advantages of this approach are the provision of important factors to sustain suitable interaction with the perovskite precursors and modulate the perovskite crystallization; this modulation is achieved because the polymers have greater molecular length and lower molecular chain mobility than the small-molecule additives (Fig. 1(a)). The Lewis base of PVP plays a dual role of a cross-linker and a passivation agent at the perovskite grain boundaries to enhance the moisture resistance of the perovskite film. First, PVP forms a strong Lewis acid–base adduct with the perovskite precursors, and this retards the perovskite formation process through the formation of cross-links between perovskite grains. Second, the oxygen donor of PVP contributes a lone pair electron to passivate halide vacancies and form a Lewis acid–base adduct through coordination with the undercoordinated Pb2+ defects.46 Hence, Lewis bases with different repeating units, SM-VP and HM-PVP, were employed to investigate the effect of Lewis acid–base adducts on the perovskite precursor behavior, as illustrated in Fig. 1(a). Various concentrations of SM-VP and HM-PVP in perovskite films were systematically employed, and the crystal growth and crystallographic orientation were determined from GIWAXS spectra. In the 1D GIWAXS profiles presented in Fig. 1(b), the diffraction peaks at the (101), (121), (202), (141), and (321) planes correspond to the orthorhombic (Pnma space group) CsPbBr3 phase (ICSD, No. 97851), whereas the new diffraction peaks at (012), (030), (124), and (132) correspond to the hexagonal (R3cH space group) Cs4PbBr6 phase (ICSD no. 25124); these findings were consistent with the XRD spectra (Fig. S2 (ESI†)) and confirmed that all planes were at preferable positions for dual-phase CsPbBr3–Cs4PbBr6 composites.56 Moreover, in comparison with the pristine perovskite and that containing other concentrations of SM-VP and HM-PVP (Fig. 1(c) and Fig. S3 (ESI†)), the incorporation of 7% PVP into the perovskite strengthened the corresponding ring intensity of CsPbBr3(121) and Cs4PbBr6(132) and resulted in a highly homogeneous crystallographic orientation and formation of well-ordered crystals. This observation verified that HM-PVP improved perovskite crystallization and orientation. Fig. 1(d) and (e) presents the optical absorption (UV) and PL of the dual-phase CsPbBr3–Cs4PbBr6 composites and perovskite films with various SM-VP and HM-PVP concentrations. The spectrum of the dual-phase CsPbBr3–Cs4PbBr6 composite has absorption peaks at 313 and 508 nm, respectively. The strong peak at 313 nm corresponds to Cs4PbBr6 and is associated with the Pb2+ centers of the isolated PbBr64− octahedra that result in 1S0 → 3P1 transition; by contrast, the weak peak at 508 nm is attributed to CsPbBr3, indicating that the two phases, namely, CsPbBr3 and Cs4PbBr6, coexist, as confirmed from the XRD and UV results (Fig. 1(b) and (e)). When VP and PVP were present within the perovskite films, a new characteristic peak was obtained at 362 nm, which was assigned to the n → π* transition of the carbonyl group of VP and PVP (Fig. 1(d)).57 The spectrum of the pristine perovskite and VP-perovskite with an FWHM of 22 nm has a wide emission peak centered at 523 nm. By contrast, the HM-PVP PL intensity is clearly increased and the FWHM is narrowed to 20 nm when the concentration is increased to 7% (Fig. 1(e)). Furthermore, Fig. 1(f) presents the corresponding 2D PL excitation pattern of HM-7% PVP and shows the narrow shape of the emission band over the excitation wavelength range of 300–450 nm; when compared with the concentrations of SM-VP and HM-PVP (Fig. S4 (ESI†)), it is evident that HM-PVP not only acts as an efficient Lewis acid–base adduct but also effectively passivates the perovskite defects. Moreover, the spectrum for HM-PVP has a band gap that is not clearly shifted compared with that for the pristine perovskite and SM-VP (Fig. S5 (ESI†)), revealing that the introduction of the polymeric additive of PVP had a strong stabilizing effect on the perovskite structure.Fig. 2 shows the morphological characterization of HM-7% PVP perovskite composites. High-resolution transmission electron microscopy (HRTEM) images showed two lattice fringes of 0.42 and 0.32 nm corresponding to the (121) and (132) crystal planes of the dual-phase CsPbBr3–Cs4PbBr6 composite, respectively (Fig. 2(a)); such findings are consistent with the XRD results presented in Fig. S2 (ESI†). Furthermore, the fast Fourier transform (FFT) pattern and selected-area electron diffraction (SAED) pattern both showed that the orthorhombic–hexagonal CsPbBr3–Cs4PbBr6 phases coexisted, as shown in the inset of Fig. 2(a) and (b). We conducted energy dispersive spectroscopy (EDS) analysis of the HM-7% PVP perovskite composite for elemental mapping, as shown in Fig. 2(c) and (g). The elemental mapping of Cs, Pb, and Br atoms was performed to confirm that the CsPbBr3–Cs4PbBr6 composites had a well-defined crystal structure (Fig. 2(c) and (f)). Notably, the O atomic distribution overlapped with the perovskite atomic element distribution and further expanded beyond the crystal boundaries (Fig. 2(g)), indicating that HM-7% PVP not only created a bridge and formed a robust intermediate and protective phase between perovskite grains but also efficiently preserved the material's structural integrity, as revealed in Fig. S6 (ESI†).
Fig. 2(h) and (i) and Fig. S7a–h (ESI†) show the morphology of the SM-VP and HM-PVP composites with various concentrations of VP and PVP in scanning electron microscopy (SEM) and atomic force microscopy (AFM) images. The pristine CsPbBr3–Cs4PbBr6, with large pinholes and agglomerations at the perovskite grain boundaries, resulted in the formation of a discontinuous perovskite film, as shown in Fig. S7a (ESI†), whereas the HM-7% PVP (Fig. 2(h)), with a pinhole-free morphology and compact structure, effectively inhibited aggregation and fissure formation, indicating that the high molecular Lewis bases dominated the smooth surface effects and resulted in uniform perovskite dispersal within the polymeric films. These results were verified from the AFM images presented in Fig. 2(i) and Fig. S7e–h (ESI†). The pristine CsPbBr3–Cs4PbBr6 had a surface with relatively high surface roughness, specifically 21 nm, which resulted in high pinhole defect density at the perovskite grain boundaries (Fig. S7e (ESI†)). By contrast, the HM-7% PVP (Fig. 2(i)), with relatively low surface roughness of 14 nm, further passivated the defects in the perovskite grain boundaries.
The mechanism of forming a stable dual-phase CsPbBr3–Cs4PbBr6 composite was based on introducing a long alkyl chain PVP with a steric hindrance effect and thus preventing aggregation of the perovskite crystal. The HM-PVP passivation mechanism was further investigated by determining the solubility parameter under saturated conditions of CsBr−HM-PVP and PbBr2−HM-PVP in DMSO solution in order to avoid the solvation effects (Fig. S8 (ESI†)). The CsBr−HM-PVP mixed solution was observed to be a turbid liquid, whereas HM-PVP−PbBr2 was a precipitate-free and homogeneous solution, thereby demonstrating strong interaction between HM-PVP and Pb atoms.45,46 The acylamino group of PVP donated oxygen and nitrogen lone-pair electrons and thus provided many coordination centers that could act as a Lewis base center. The resonance effect strengthened the basicity of the oxygen atom and increased its electronic cloud density, facilitating the Lewis acid–base adduct with a Pb2+ coordinating center.47,58 Moreover, the long-backbone PVP, with a substantially electronegative acylamino group structure, acted as an intermediate linker to attract more perovskite moieties to the coordination center, triggering the formation and growth of a dual-phase CsPbBr3–Cs4PbBr6 composite, as depicted in Fig. 3(a). To verify the potential coordination bonds between the Lewis bases (SM-VP and HM-PVP) and the perovskite, Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), and X-ray photoelectron spectroscopy (XPS) were performed (Fig. 3(c)–(f)). Fig. 3(e) shows absorption peaks at 1710 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1340–1508 cm−1 (C–H stretching), and 1240 cm−1 (C–N stretching) that corresponded to the acylamino groups of PVP. In comparison with the spectra of SM-3.5% VP and HM-3.5% PVP (Fig. S9 (ESI†) and Fig. 3(e)), the spectrum of HM-7% PVP has a large shift from 1710 to 1720 cm−1 corresponding to the C
O stretching), 1340–1508 cm−1 (C–H stretching), and 1240 cm−1 (C–N stretching) that corresponded to the acylamino groups of PVP. In comparison with the spectra of SM-3.5% VP and HM-3.5% PVP (Fig. S9 (ESI†) and Fig. 3(e)), the spectrum of HM-7% PVP has a large shift from 1710 to 1720 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration, which is ascribed to the HM-PVP having long-range-ordering polymer chains and thus providing a large degree of coordination for sharing the lone electron pair on the C
O vibration, which is ascribed to the HM-PVP having long-range-ordering polymer chains and thus providing a large degree of coordination for sharing the lone electron pair on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and further delocalizing to the empty orbital of Pb2+, resulting in proton-accepting hydrogen bond formation. Furthermore, the pristine PVP and HM-PVP perovskite were dissolved in deuterated DMSO-d6 solution, after which 1H and 13C NMR were performed. The characteristic resonances of C
O group and further delocalizing to the empty orbital of Pb2+, resulting in proton-accepting hydrogen bond formation. Furthermore, the pristine PVP and HM-PVP perovskite were dissolved in deuterated DMSO-d6 solution, after which 1H and 13C NMR were performed. The characteristic resonances of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (δ = 2.5) and N atoms (δ = 3.42) corresponded to the acylamino group of PVP. The chemical shift in the spectrum of HM-7% PVP was considerable (Δδ ≈ 0.3 ppm). Additionally, a resonance peak shifted up-field (Fig. 3(f)). Moreover, the corresponding 13C NMR spectra (Fig. S10 (ESI†)) revealed that HM-PVP caused an up-field shift of Δδ ≈ 1 ppm in the main chemical shift of 174 ppm (C
O groups (δ = 2.5) and N atoms (δ = 3.42) corresponded to the acylamino group of PVP. The chemical shift in the spectrum of HM-7% PVP was considerable (Δδ ≈ 0.3 ppm). Additionally, a resonance peak shifted up-field (Fig. 3(f)). Moreover, the corresponding 13C NMR spectra (Fig. S10 (ESI†)) revealed that HM-PVP caused an up-field shift of Δδ ≈ 1 ppm in the main chemical shift of 174 ppm (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group) compared with the pristine PVP, indicating that the long-chain molecules had more main functional groups (C
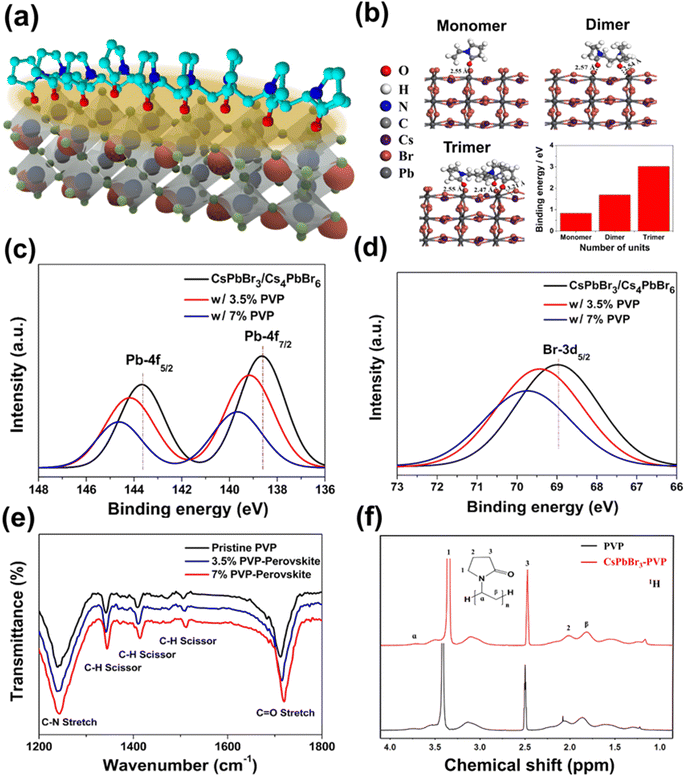
O group) compared with the pristine PVP, indicating that the long-chain molecules had more main functional groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) than the short-chain molecules, assisting the resonance-strengthening effects between the Pb cations of the perovskite and oxygen anions of PVP.47 The XPS spectra indicated the binding strength of Cs 3d, Pb 4f, and Br 3d orbital species and confirmed the incorporation of VP and PVP into the CsPbBr3–Cs4PbBr6 perovskite (Fig. 3(c), (d) and Fig. S11 (ESI†)). The spectrum of the pristine perovskite has two peaks located at 138.64 and 143.67 eV, corresponding to the Pb 4f7/2 and Pb 4f5/2 orbitals, respectively. The Br 3d5/2 peak at 68.98 eV corresponds to the Br+ cation region. Notably, when VP was present in the perovskite (Fig. S11 (ESI†)), the SM-7% VP spectrum showed a slight red-shift in the Pb 4f and Br 3d peak region. By contrast, for HM-7% PVP with a long-range order and well-ordered chain segments, the spectrum was shifted considerably to higher binding energy in the Pb 4f and Br 3d peak region, which resulted from an increase in the cationic charge of the Pb ions, indicating that the shifts in binding energy stabilized perovskite formation (Fig. 3(c) and (d)). We further used DFT calculation to investigate the interaction between the Lewis base additives (SM-VP and HM-PVP) and perovskites (Fig. 3(b)). The adsorption structures of VP monomer, dimer, and trimer forms were optimized. The adsorption energy (Eads) of VP adsorption on the CsPbBr3(121) perovskite surface was calculated:
O) than the short-chain molecules, assisting the resonance-strengthening effects between the Pb cations of the perovskite and oxygen anions of PVP.47 The XPS spectra indicated the binding strength of Cs 3d, Pb 4f, and Br 3d orbital species and confirmed the incorporation of VP and PVP into the CsPbBr3–Cs4PbBr6 perovskite (Fig. 3(c), (d) and Fig. S11 (ESI†)). The spectrum of the pristine perovskite has two peaks located at 138.64 and 143.67 eV, corresponding to the Pb 4f7/2 and Pb 4f5/2 orbitals, respectively. The Br 3d5/2 peak at 68.98 eV corresponds to the Br+ cation region. Notably, when VP was present in the perovskite (Fig. S11 (ESI†)), the SM-7% VP spectrum showed a slight red-shift in the Pb 4f and Br 3d peak region. By contrast, for HM-7% PVP with a long-range order and well-ordered chain segments, the spectrum was shifted considerably to higher binding energy in the Pb 4f and Br 3d peak region, which resulted from an increase in the cationic charge of the Pb ions, indicating that the shifts in binding energy stabilized perovskite formation (Fig. 3(c) and (d)). We further used DFT calculation to investigate the interaction between the Lewis base additives (SM-VP and HM-PVP) and perovskites (Fig. 3(b)). The adsorption structures of VP monomer, dimer, and trimer forms were optimized. The adsorption energy (Eads) of VP adsorption on the CsPbBr3(121) perovskite surface was calculated:
| Eads = Etotal − (Emolecule + Esurface) | (1) |
| Distance* | |||
|---|---|---|---|
| d O–Pb | d H–Br | d O–Cs | |
| Monomer | 2.55 | 2.83–3.97 (2) | — |
| Dimer | 2.54–2.57 (2) | 3.04–3.96 (6) | — |
| Trimer | 2.47–2.55 (2) | 2.67–3.83 (8) | 3.21–3.29 (2) |
To investigate the carrier's recombination pathways at the polymer–perovskite interface upon light irradiation, we first added or removed one electron from the neutral CsPbBr3(121) perovskite surface system to create electron and hole polarons.59–61 Bader charge analysis demonstrated that Pb atoms gained 0.52 electrons upon electron addition, whereas Br atoms lost 0.81 electrons upon removal of an electron; hence, electron and hole polarons were mainly localized on the Pb and Br atoms, respectively, indicating that electrons were transferred from Br atoms to Pb atoms when the perovskite was illuminated. We also optimized the adsorption structures of the VP monomer, dimer, and trimer on perovskite surfaces containing hole and electron polarons (Fig. S14 (ESI†)). Table S4 (ESI†) indicates that the adsorption energies of the VP monomer, dimer, and trimer on the surface with hole and electron polarons were −0.94, −2.23, and −3.09 eV, respectively. Similar to VP adsorption on the neutral surface, VP trimer adsorption on the surface with hole polarons was more stable than VP monomer adsorption. Furthermore, we observed that the strength of VP adsorption on the surface with hole polarons was greater than that on the neutral CsPbBr3(121) perovskite surface and CsPbBr3 surface with electron polarons. The hole polarons were close to the VP adsorption site, whereas the electron polarons were far away, resulting in enhanced stability of the charge-separated state. The detailed calculation of charge differences for VP adsorption on both neutral and charged systems is presented in Table S4 (ESI†). In addition, we investigated the impact on the exciton recombination dynamics of CsPbBr3–Cs4PbBr6, SM-VP, and HM-PVP by using time-resolved photoluminescence (TRPL). The PL decay was conducted through biexponential fitting by using the following equations:
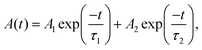 | (2) |
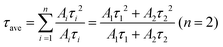 | (3) |
 | (4) |
CsPbBr3–Cs4PbBr6-based LEDs were prepared using various concentrations of SM-VP and HM-PVP, as shown in Fig. 5(a)–(c) and Table S6 (ESI†). The band structure of CsPbBr3–Cs4PbBr6, SM-VP, and HM-PVP was measured using ultraviolet photoelectron spectroscopy and optical absorption spectroscopy (Fig. S5 and S16, ESI†). The valence band and conduction band energy levels of HM-7% PVP were estimated to be −6 and −3.67 eV, respectively. The electroluminescence (EL) spectra of the CsPbBr3–Cs4PbBr6, SM-VP, and HM-PVP devices are shown in Fig. S17a (ESI†). The spectrum of HM-7% PVP has a narrow emission peak at 525 nm with a FWHM of 20 nm, thereby presenting stronger EL intensity than SM-VP and CsPbBr3–Cs4PbBr6; the trends are consistent with the PL spectra presented in Fig. 1(e) and (f). The dual synergistic effect of hole injection enhancement and luminescence quenching suppression results in formation of high performance devices. Notably, the underlying mechanism for insufficient hole injection and luminescence quenching originates from the mismatching of HIL stacking at the PEDOT:PSS anode. To overcome this drawback, we propose to embed PEDOT-PSS with AgNPs to improve band gap matching and carrier injection efficiency. We fabricate a LED device with the following architecture: ITO/PEDOT:PSS-AgNPs/HM-7%PVP-CsPbBr3–Cs4PbBr6/TPBi/LiF/Ag as the cathode (Fig. 5(a)). The current–voltage (I–V) curve of CsPbBr3–Cs4PbBr6 based on SM-VP and HM-PVP devices is displayed in Fig. 5(b). The HM-PVP device had a lower current density than that of the CsPbBr3–Cs4PbBr6 and SM-VP devices. The HM-7% PVP device effectively suppressed current leakage and charge injection imbalance because the perovskite structure had a uniform and compact morphology (Fig. 2(h)). The HM-7% PVP device (Fig. 5(b) and Fig. S17b, c (ESI†), and Table S6 (ESI†)) exhibited a maximum luminance of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, a current efficiency of 11.5 cd A−1 and an EQE value of 3.17%. This device performance reflected a 4.68-, 31.08-fold, and 25.36-fold improvement compared with that of the SM-7% VP device (2560 cd m−2, 0.37 cd A−1, and 0.125%, respectively) as well as 5.78-, 41.07-fold, and 29.35-fold improvement compared with that of the CsPbBr3–Cs4PbBr6 device (2075 cd m−2, 0.28 cd A−1, and 0.108%, respectively). These values are a substantial improvement from those of previously reported bulk 3D CsPbBr3–Cs4PbBr6 PeLEDs (EQE value of 2.4 × 10−5).11 Significantly, HM-7% PVP devices also show a longer operational lifetime (T50) of 5 min than that of the CsPbBr3–Cs4PbBr6 device (Fig. S17d (ESI†)). In addition, we further investigated the high concentrations of HM-PVP up to 14% through SEM morphology (Fig. S18a, ESI†) and device results (Fig. S18b–d (ESI†)). The HM-14% PVP device had a larger current density and provided substantial current leakage resulting in limiting the charge injection into PeLEDs (Fig. S18b, ESI†). These poor device results can be attributed to the non-uniform perovskite films. At higher concentrations and PVP/perovskite ratios, the pinholes and discontinuous surface coverage become much larger, providing pathways for electrical short circuits and increasing the charge injection imbalance compared to that in our optimized HM-7% PVP device (Fig. 5(b), (c), and Fig. S17b, c, S18b–d, ESI†). To analyze the molecular engineering effect between CsPbBr3–Cs4PbBr6, SM-VP and HM-PVP on the trap density of states, the current density–voltage curve of CsPbBr3–Cs4PbBr6 devices without and with SM-VP and HM-PVP composites are displayed in Fig. 5(d)–(f) and Fig. S18a, d (ESI†). According to the formula Nt = 2VTFLεε0/eL2, the trap density (Nt) was calculated from the trap-filled limit voltage (VTFL).69 The dark current characteristic of hole-only devices was conducted under applied bias. The CsPbBr3–Cs4PbBr6 devices with a high VTFL characteristic (0.91 V) exhibited large trap density (3.46 × 1017 cm−3). Upon addition of 3.5% HM-PVP and 7% HM-PVP into CsPbBr3–Cs4PbBr6 films, the VTFL of the optimized HM-7% PVP devices dramatically decreased from 0.91 to 0.13 and a lower trap density (4.95 × 1016 cm−3) was provided compared to that of pristine CsPbBr3–Cs4PbBr6 devices and SM-VP devices (Fig. 5(d)), Fig. S18a and d (ESI†), thereby demonstrating that the VTFL characteristic is associated with the VP monomer length results in effectively inhibited trap density (Fig. 3(b), Fig. S1 (ESI†) and Table S2, (ESI†)).
000 cd m−2, a current efficiency of 11.5 cd A−1 and an EQE value of 3.17%. This device performance reflected a 4.68-, 31.08-fold, and 25.36-fold improvement compared with that of the SM-7% VP device (2560 cd m−2, 0.37 cd A−1, and 0.125%, respectively) as well as 5.78-, 41.07-fold, and 29.35-fold improvement compared with that of the CsPbBr3–Cs4PbBr6 device (2075 cd m−2, 0.28 cd A−1, and 0.108%, respectively). These values are a substantial improvement from those of previously reported bulk 3D CsPbBr3–Cs4PbBr6 PeLEDs (EQE value of 2.4 × 10−5).11 Significantly, HM-7% PVP devices also show a longer operational lifetime (T50) of 5 min than that of the CsPbBr3–Cs4PbBr6 device (Fig. S17d (ESI†)). In addition, we further investigated the high concentrations of HM-PVP up to 14% through SEM morphology (Fig. S18a, ESI†) and device results (Fig. S18b–d (ESI†)). The HM-14% PVP device had a larger current density and provided substantial current leakage resulting in limiting the charge injection into PeLEDs (Fig. S18b, ESI†). These poor device results can be attributed to the non-uniform perovskite films. At higher concentrations and PVP/perovskite ratios, the pinholes and discontinuous surface coverage become much larger, providing pathways for electrical short circuits and increasing the charge injection imbalance compared to that in our optimized HM-7% PVP device (Fig. 5(b), (c), and Fig. S17b, c, S18b–d, ESI†). To analyze the molecular engineering effect between CsPbBr3–Cs4PbBr6, SM-VP and HM-PVP on the trap density of states, the current density–voltage curve of CsPbBr3–Cs4PbBr6 devices without and with SM-VP and HM-PVP composites are displayed in Fig. 5(d)–(f) and Fig. S18a, d (ESI†). According to the formula Nt = 2VTFLεε0/eL2, the trap density (Nt) was calculated from the trap-filled limit voltage (VTFL).69 The dark current characteristic of hole-only devices was conducted under applied bias. The CsPbBr3–Cs4PbBr6 devices with a high VTFL characteristic (0.91 V) exhibited large trap density (3.46 × 1017 cm−3). Upon addition of 3.5% HM-PVP and 7% HM-PVP into CsPbBr3–Cs4PbBr6 films, the VTFL of the optimized HM-7% PVP devices dramatically decreased from 0.91 to 0.13 and a lower trap density (4.95 × 1016 cm−3) was provided compared to that of pristine CsPbBr3–Cs4PbBr6 devices and SM-VP devices (Fig. 5(d)), Fig. S18a and d (ESI†), thereby demonstrating that the VTFL characteristic is associated with the VP monomer length results in effectively inhibited trap density (Fig. 3(b), Fig. S1 (ESI†) and Table S2, (ESI†)).
In comparison to bulk 3D perovskites, layered perovskites such as the quasi two-dimensional (2D) perovskite possesses highly confined excitons that prevent charge diffusion and trapping within the active layer trap site. The dimensionality reduction of the perovskite not only leads to improved stability but also enhanced charge confinement and reduced charge trapping. According to our previous studies,5 the polymeric Lewis base donating strength strongly impact the Lewis acid–base adduct formation with perovskites. The energy involved influences the perovskite crystallization kinetics through an increase in the crystal formation energy barrier. The quasi 2D perovskite is encapsulated with the polymer through Lewis acid–base interaction between Pb and O from polyethylene glycol.70 We hypothesize that the Lewis acid–base interaction occurs between Lewis acid Pb and Lewis base nitrogen atoms of PVP through adduct formation, thereby leading to perovskite grain passivation and improved quasi 2D PeLED performances.5 Based on the aforementioned hypothesis, the inclusion of Cs4PbBr6 into the HM-7%PVP-CsPbBr3 composite helps to regulate the quasi 2D perovskite layer lattice as confirmed from the UV spectra with a characteristic peak at 316 nm as shown in Fig. S19a (ESI†). We fabricated a LED device with the following architecture: ITO/PEDOT:PSS-AgNPs/poly-TPD/PVK/HM-7%PVP-CsPbBr3–Cs4PbBr6 Quasi-2D/TPBi/LiF/Ag as the cathode (Fig. 5(g)). Significantly, the luminance performance of HM-7%PVP-CsPbBr3–Cs4PbBr6 quasi-2D not only increased to 9500 cd m−2 (Fig. 5(h)) but also considerably improved the charge transport with a current efficiency and an EQE value as high as 15.4 cd A−1 and 5.13%, respectively (Fig. 5(i) and Fig. S20b (ESI†)). These current efficiency and EQE values display a 1.34-fold and 1.62-fold improvement in comparison to those of the pristine HM-7% bulk 3D CsPbBr3–Cs4PbBr6 device (11.5 cd A−1 and 3.17%) (Fig. 5(c), Fig. S17b, c, and S20b (ESI†)). The current work shows that the synergistic effect of Cs4PbBr6 with PVP not only induced an improved dielectric screening with higher exciton binding energy but also highly passivated perovskite grains through PVP-CsPbBr3 Lewis acid–base adduct chemistry. Finally, the dual effect induces a strengthened charge dielectric screening with strong dielectric confinement for enhanced perovskite device performances and lifetime.
Conclusions
In summary, we present an effective strategy to obtain an intergrain cross-linked network to improve the stability of perovskites and device efficiency by incorporating Lewis-base SM-VP and HM-PVP systems. The formation of a self-ordered macromolecular intermediate phase originates from perovskite precursor–Lewis base adduct bonds, thereby modulating the growth kinetics of polycrystalline perovskites. Through a nucleation energy barrier increase, perovskite precursor diffusion is strengthened and leads to fabrication of highly crystalline perovskite films. Furthermore, theoretical DFT calculations confirmed that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in PVP not only contributes to densification of the electronic cloud in the vicinity of the sp orbital of Pb2+ ions but also considerably reduces the perovskite surface energy. Grain passivation suppresses nonradiative recombination centers at perovskite grain boundaries and endows our HP-7% PVP with a long carrier lifetime (28.84 ns). Significantly, silver nanoparticle incorporation into the hole transport layer improves carrier transmission efficiency in bulk 3D CsPbBr3–Cs4PbBr6 and CsPbBr3–Cs4PbBr6 quasi-2D perovskites. As a result, our champion devices constructed using HM-7% PVP bulk 3D and HM-7% PVP quasi-2D perovskite composites exhibit luminances of 12
O group in PVP not only contributes to densification of the electronic cloud in the vicinity of the sp orbital of Pb2+ ions but also considerably reduces the perovskite surface energy. Grain passivation suppresses nonradiative recombination centers at perovskite grain boundaries and endows our HP-7% PVP with a long carrier lifetime (28.84 ns). Significantly, silver nanoparticle incorporation into the hole transport layer improves carrier transmission efficiency in bulk 3D CsPbBr3–Cs4PbBr6 and CsPbBr3–Cs4PbBr6 quasi-2D perovskites. As a result, our champion devices constructed using HM-7% PVP bulk 3D and HM-7% PVP quasi-2D perovskite composites exhibit luminances of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 and 9500 cd m−2, and current efficiencies of 11.5 cd A−1 and 15.4 cd A−1, respectively. Our study shows that in future, highly stable and reliable PeLEDs can be constructed using polymeric passivating agents as a Lewis base adduct to modulate perovskite growth and improve the perovskite film crystallinity.
000 cd m−2 and 9500 cd m−2, and current efficiencies of 11.5 cd A−1 and 15.4 cd A−1, respectively. Our study shows that in future, highly stable and reliable PeLEDs can be constructed using polymeric passivating agents as a Lewis base adduct to modulate perovskite growth and improve the perovskite film crystallinity.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
F.-C. Liang and Z.-L. Yan contributed equally to this work. F.-C. Liang acknowledges the financial support provided by the Ministry of Science and Technology of Taiwan (contracts: MOST 111-2222-E-027-001). C.-C. Kuo acknowledges the financial support provided by the Ministry of Science and Technology of Taiwan (contracts: MOST 109-2221-E-027-114-MY3) and the National Taipei University of Technology – Shenzhen University Joint Research Program (NTUT-SZU-111-01), NTUT-SZU Joint Research Program. The authors thank Ms C.-Y. Chien and S.-J. Ji of the Ministry of Science and Technology (National Taiwan University) for their assistance in FE-TEM and EDS.References
- A. Green, A. Ho-Baillie and H. J. Snaith, Nat. Photonics, 2014, 8, 506–514 CrossRef.
- J. You, L. Meng, T. B. Song, T. F. Guo, Y. M. Yang, W. H. Chang, Z. Hong, H. Chen, H. Zhou, Q. Chen, Y. Liu, N. De Marco and Y. Yang, Nat. Nanotechnol., 2016, 11, 75–81 CrossRef CAS PubMed.
- N. K. Noel, A. Abate, S. D. Stranks, S. P. Elizabeth, V. M. Burlakov, A. Goriely and H. J. Snaith, ACS Nano., 2014, 8, 9815–9821 CrossRef CAS PubMed.
- X. Yang, X. Zhang, J. Deng, Z. Chu, Q. Jiang, J. Meng, P. Wang, L. Zhang, Z. Yin and J. You, Nat. Commun., 2018, 9, 570 CrossRef PubMed.
- J.-S. Benas, F.-C. Liang, W.-C. Chen, C.-W. Hung, J.-Y. Chen, Y. Zhou, S.-T. Han, R. Borsali and C.-C. Kuo, Chem. Eng. J., 2022, 431, 133701 CrossRef CAS.
- W.-C. Chen, C.-W. Hung, C.-H. Chang, F.-C. Liang, J.-S. Benas, Z.-L. Yan, B.-H. Lin, J.-H. Lin and C.-C. Kuo, Chem. Eng. J., 2022, 443, 136496 CrossRef CAS.
- J. Song, L. Xu, J. Li, J. Xue, Y. Dong, X. Li and H. Zeng, Adv. Mater., 2016, 28, 4861–4869 CrossRef CAS PubMed.
- Z. Yang, M. Wang, H. Qiu, X. Yao, X. Lao, S. Xu, Z. Lin, L. Sun and J. Shao, Adv. Funct. Mater., 2018, 28, 1705908 CrossRef.
- L. N. Quan, R. Q. Bermudez, O. Voznyy, G. Walters, A. Jain, J. Z. Fan, X. Zheng, Z. Yang and E. H. Sargent, Adv. Mater., 2017, 29, 1605945 CrossRef PubMed.
- M. I. Saidaminov, O. F. Mohammed and O. M. Bakr, ACS Energy Lett., 2017, 2, 889–896 CrossRef CAS.
- J. Xu, W. Huang, P. Li, D. R. Onken, C. Dun, Y. Guo, K. B. Ucer, C. Lu, H. Wang, S. M. Geyer, R. T. Williams and D. L. Carroll, Adv. Mater., 2017, 29, 1703703 CrossRef PubMed.
- Q. Zhang, M. M. Tavakoli, L. Gu, D. Zhang, L. Tang, Y. Gao, J. Guo, Y. Lin, S. F. Leung, S. Poddar, Y. Fu and Z. Fan, Nat. Commun., 2019, 10, 727 CrossRef PubMed.
- Z.-L. Yan, F.-C. Liang, C.-Y. Yeh, D. Kurniawan, J.-S. Benas, W.-C. Chen, C.-J. Cho, W.-H. Chiang, R.-J. Jeng and C.-C. Kuo, Chem. Eng. J., 2022, 444, 136518 CrossRef CAS.
- T. Matsushima, F. Bencheikh, T. Komino, M. R. Leyden, A. S. D. Sandanayaka, C. Qin and C. Adachi, Nature, 2019, 572, 502–506 CrossRef CAS PubMed.
- S. Lee, J. H. Par, B. R. Lee, E. D. Jung, J. C. Yu, D. Di Nuzzo, R. H. Friend and M. H. Song, J. Phys. Chem. Lett., 2017, 8, 1784–1792 CrossRef CAS PubMed.
- M. Kulbak, S. Gupta, N. Kedem, I. Levine, T. Bendikov, G. Hodes and D. Cahen, J. Phys. Chem. Lett., 2016, 7, 167–172 CrossRef CAS PubMed.
- Q. Van Le, M. Park, W. Sohn, H. W. Jang and S. Y. Kim, Adv. Electron. Mater., 2017, 3, 1600448 CrossRef.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- A. Swarnkar, R. Chulliyil, V. K. Ravi, M. Irfanullah, A. Chowdhury and A. Nag, Angew. Chem., Int. Ed., 2015, 54, 15424–15428 CrossRef CAS PubMed.
- M. Kulbak, D. Cahen and G. Hodes, J. Phys. Chem. Lett., 2015, 6, 2452–2456 CrossRef CAS PubMed.
- F.-C. Liang, F.-C. Jhuang, Y.-H. Fang, J.-S. Benas, W.-C. Chen, Z.-L. Yan, W.-C. Lin, C.-J. Su, Y. Sato, T. Chiba, J. Kido and C.-C. Kuo, Adv. Mater., 2022 DOI:10.1002/adma.202207617.
- J. Song, T. Fang, J. Li, L. Xu, F. Zhang, B. Han, Q. Shan and H. Zeng, Adv. Mater., 2018, 30, 1805409 CrossRef PubMed.
- C.-L. He, F.-C. Liang, L. Veeramuthua, C.-J. Cho, J.-S. Benas, Y.-R. Tzeng, Y.-L. Tseng, W.-C. Chen, A. Rwei and C.-C. Kuo, Adv. Sci., 2021, 8, 2102275 CrossRef CAS PubMed.
- K. Lin, J. Xing, L. N. Quan, F. P. G. de Arquer, X. Gong, J. Lu, L. Xie, W. Zhao, D. Zhang, C. Yan, W. Li, X. Liu, Y. Lu, J. Kirman, E. H. Sargent, Q. Xiong and Z. Wei, Nature, 2018, 562, 245–248 CrossRef CAS PubMed.
- Z.-L. Yan, J.-S. Benas, C.-C. Chueh, W.-C. Chen, F.-C. Liang, Z.-X. Zhang, B.-H. Lin, C.-J. Su, T. Chiba, J. Kido and C.-C. Kuo, Chem. Eng. J., 2021, 414, 128774 CrossRef CAS.
- J.-W. Lee, S.-H. Bae, Y.-T. Hsieh, N. De Marco, M. Wang, P. Sun and Y. Yang, Chem, 2017, 3, 290–302 CAS.
- L. Zuo, H. Guo, D. W. deQuilettes, S. Jariwala, N. De Marco, S. Dong, R. DeBlock, D. S. Ginger, B. Dunn, M. Wang and Y. Yang, Sci. Adv., 2017, 3, 1700106 CrossRef PubMed.
- T. H. Han, J. W. Lee, C. Choi, S. Tan, C. Lee, Y. Zhao, Z. Dai, N. De Marco, S. J. Lee, S. H. Bae, Y. Yuan, H. M. Lee, Y. Huang and Y. Yang, Nat. Commun., 2019, 10, 520 CrossRef CAS PubMed.
- Y. Ling, Y. Tian, X. Wang, J. C. Wang, J. M. Knox, F. Perez-Orive, Y. Du, L. Tan, K. Hanson, B. Ma and H. Gao, Adv. Mater., 2016, 28, 8983–8989 CrossRef CAS PubMed.
- Y. Zong, Y. Zhou, Y. Zhang, Z. Li, L. Zhang, M.-G. Ju, M. Chen, S. Pang, X. C. Zeng and N. P. Padture, Chem, 2018, 4, 1404–1415 CAS.
- D. W. de Quilettes, S. M. Vorpahl, S. D. Stranks, H. Nagaoka, G. E. Eperon, M. E. Ziffer, H. J. Snaith and D. S. Ginger, Science, 2015, 348, 683–686 CrossRef CAS PubMed.
- S. Y. Leblebici, L. Leppert, Y. Li, S. E. Reyes-Lillo, S. Wickenburg, E. Wong, J. Lee, M. Melli, D. Ziegler, D. K. Angell, D. F. Ogletree, P. D. Ashby, F. M. Toma, J. B. Neaton, I. D. Sharp and A. Weber-Bargioni, Nat. Energy, 2016, 1, 16093 CrossRef CAS.
- Y. Kutes, Y. Zhou, J. L. Bosse, J. Steffes, N. P. Padture and B. D. Huey, Nano Lett., 2016, 16, 3434–3441 CrossRef CAS PubMed.
- J. Wu, J. Shi, Y. Li, H. Wu, Y. Luo, D. Li and Q. Meng, Adv. Energy Mater., 2019, 9, 1901352 CrossRef CAS.
- L. Xu, S. Yuan, H. Zeng and J. Song, Mater. Today Nano, 2019, 6, 100036 CrossRef.
- H. Zhu, K. Miyata, Y. Fu, J. Wang, P. P. Joshi, D. Niesner, K. W. Williams, S. Jin and X.-Y. Zhu, Science, 2016, 353, 1409–1413 CrossRef CAS PubMed.
- I. Anusca, S. Balčiūnas, P. Gemeiner, S. Svirskas, M. Sanlialp, G. Lackner, C. Fettkenhauer, J. Belovickis, V. Samulionis, M. Ivanov, B. Dkhil, J. Banys, V. V. Shvartsman and D. C. Lupascu, Adv. Energy Mater., 2017, 7, 1700600 CrossRef.
- R. Su, Z. Xu, J. Wu, D. Luo, Q. Hu, W. Yang, X. Yang, R. Zhang, H. Yu, T. P. Russell, Q. Gong, W. Zhang and R. Zhu, Nat. Commun., 2021, 12, 2479 CrossRef CAS PubMed.
- W. Xu, Q. Hu, S. Bai, C. Bao, Y. Miao, Z. Yuan, T. Borzda, A. J. Barker, E. Tyukalova, Z. Hu, M. Kawecki, H. Wang, Z. Yan, X. Liu, X. Shi, K. Uvdal, M. Fahlman, W. Zhang, M. Duchamp, J.-M. Liu, A. Petrozza, J. Wang, L.-M. Liu, W. Huang and F. Gao, Nat. Photonics, 2019, 13, 418–424 CrossRef CAS.
- X. Liu, Z. Yu, T. Wang, K. L. Chiu, F. Lin, H. Gong, L. Ding and Y. Cheng, Adv. Energy. Mater., 2020, 10, 2001958 CrossRef CAS.
- L. Xu, J. Li, B. Cai, J. Song, F. Zhgang, T. Fang and H. Zeng, Nat. Commun., 2020, 11, 3902 CrossRef CAS PubMed.
- Y. Ke, N. Wang, D. Kong, Y. Cao, Y. He, L. Zhu, Y. Wang, C. Xue, Q. Peng, F. Gao, W. Huang and J. Wang, J. Phys. Chem. Lett., 2019, 10, 380–385 CrossRef.
- Y. Tian, C. Zhou, M. Worku, X. Wang, Y. Ling, H. Gao, Y. Zhou, Y. Miao, J. Guan and B. Ma, Adv. Mater., 2018, 30, 1707093 CrossRef PubMed.
- C. Xu, Z. Zhang, S. Zhang, H. Si, S. Ma, W. Fan, Z. Xiong, Q. Liao, A. Sattar, Z. Kang and Y. Zhang, Adv. Funct. Mater., 2021, 31, 2009425 CrossRef CAS.
- B. Han, S. Yuan, T. Fang, F. Zhang, Z. Shi and J. Song, ACS Appl. Mater. Interfaces, 2020, 12, 14224–14232 CrossRef CAS.
- S. Wang, A. Wang, X. Deng, L. Xie, A. Xiao, C. Li, Y. Xiang, T. Li, L. Ding and F. Hao, J. Mater. Chem. A, 2020, 8, 12201–12225 RSC.
- B. Li, Y. Zhang, L. Fu, T. Yu, S. Zhou, L. Zhang and L. Yin, Nat. Commun., 2018, 9, 1076 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- J. Klimeš, D. R. Bowler and A. Michaelides, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 195131 CrossRef.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188 CrossRef.
- C. C. Stoumpos, C. D. Malliakas, J. A. Peters, Z. Liu, M. Sebastian, J. Im, T. C. Chasapis, A. C. Wibowo, D. Y. Chung, A. J. Freeman, B. W. Wessels and M. G. Kanatzidis, Cryst. Growth Des., 2013, 13, 2722–2727 CrossRef CAS.
- M. D. Bastiani, I. Dursun, Y. Zhang, B. A. Alshankiti, X.-H. Miao, J. Yin, E. Yengel, E. Alarousu, B. Turedi, J. M. Almutlaq, M. I. Saidaminov, S. Mitra, I. Gereige, A. Alsaggaf, Y. Zhu, Y. Han, I. S. Roqan, J.-L. Bredas, O. F. Mohammed and O. M. Bakr, Chem. Mater., 2017, 29, 7108–7113 CrossRef.
- X. Li, Z. Wen, S. Ding, F. Fang, B. Xu, J. Sun, C. Liu, K. Wang and X. W. Sun, Adv. Opt. Mater., 2020, 8, 2000232 CrossRef CAS.
- C. Li, Z. Chen, H. Yi, Y. Cao, L. Du, Y. Hu, F. Kong, R. K. Campen, Y. Gao, C. Du, G. Yin, I. Y. Zhang and Y. Tong, Angew. Chem., Int. Ed., 2020, 59, 15902–15907 CrossRef CAS PubMed.
- W. P. D. Wong, J. Yin, B. Chaudhary, X. Y. Chin, D. Cortecchia, S.-Z. A. Lo, A. C. Grimsdale, O. F. Mohammed, G. Lanzani and C. Soci, ACS Mater. Lett., 2020, 2, 20–27 CrossRef CAS.
- L. Zhang and P. H.-L. Sit, et al. , J. Mater. Chem. A, 2017, 5, 9042–9049 RSC.
- C. Peng, J. Chen, H. Wang and P. Hu, J. Phys. Chem. C, 2018, 122, 27340–27349 CrossRef CAS.
- M. Liu, H. Zhang, D. Gedamu, P. Fourmont, H. Rekola, A. Hiltunen, S. G. Cloutier, R. Nechache, A. Priimagi and P. Vivo, Small, 2019, 15, 1900801 CrossRef PubMed.
- M. Lu, Y. Zhang, S. Wang, J. Guo, W. W. Yu and A. L. Rogach, Adv. Funct. Mater., 2019, 29, 1902008 CrossRef.
- S. Sun, M. Lu, X. Gao, Z. Shi, X. Bai, W. W. Yu and Y. Zhang, Adv. Sci., 2021, 8, 2102689 CrossRef PubMed.
- X. Wang, Y. Liu, N. Liu, R. Sun, W. Zheng, H. Liu and Y. Zhang, J. Mater. Chem. A, 2021, 9, 4658–4663 RSC.
- R. Sun, N. Liu, W. Zheng, J. Zhang, N. Li, H. Lian, H. Liu and Y. Zhang, Chem. Mater., 2021, 33, 3721–3728 CrossRef CAS.
- B. Kang and K. S. Koushik, J. Phys. Chem. Lett., 2018, 9, 830–836 CrossRef CAS PubMed.
- Y. Jiang, M. Cui, S. Li, C. Sun, Y. Huang, J. Wei, L. Zhang, M. Lv, C. Qin, Y. Liu and M. Yuan, Nat. Commun., 2021, 12, 336 CrossRef CAS PubMed.
- S. Lee, J. H. Park, Y. S. Nam, B. R. Lee, B. D. Zhao, D. Di. Nuzzo, E. D. Jung, H. Jeon, J. Y. Kim, H. Y. Jeong, R. H. Friend and M. H. Song, ACS Nano, 2018, 12, 3417–3423 CrossRef CAS PubMed.
- Y. H. Chao, J. C. Chen, D. L. Yang, Y. J. Tseng, C. H. Hsu and J. Y. Chen, Adv. Funct. Mater., 2022, 32, 2112521 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc03690h |
| ‡ F.-C. Liang and Z.-L. Yan contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |