Open Access Article
Open Access ArticleSurface modified materials for active capture of enzymes†
Dandan
Wang
 a,
William F.
Hartz
a,
William F.
Hartz
 b and
Mark G.
Moloney
b and
Mark G.
Moloney
 *ab
*ab
aOxford Suzhou Centre for Advanced Research, Building A, 388 Ruo Shui Road, Suzhou Industrial Park, Jiangsu 215123, P. R. China. E-mail: mark.moloney@chem.ox.ac.uk
bChemistry Research Laboratory, Department of Chemistry, University of Oxford, Oxford, OX1 3TA, UK
First published on 10th February 2023
Abstract
The insertion of bis(diarylcarbene)s onto a glass fiber (GF) membrane surface provided an active coating for the direct capture of protein – exemplified by the enzyme, cellulase – through a mild diazonium coupling process which does not require additional coupling agents. Successful cellulase attachment on the surface was demonstrated by the disappearance of diazonium and formation of azo functions in the N 1s high resolution spectra, the appearance of carboxyl group in C 1s spectra, both observed by XPS; the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrational bond observed by ATR-IR; as well as the observation of fluorescence. Further, five support materials (polystyrene XAD4 bead, polyacrylate MAC3 bead, glass wool, glass fiber membrane, polytetrafluoroethylene membrane) with different morphology and surface chemistry, were examined in detail as supports for cellulase immobilization using this common surface modification protocol. Of interest is that such covalently bound cellulase on modified GF membrane gave both the highest enzyme loading (∼23 mg cellulase per g support), and retained more than 90% of activity after 6 cycles of re-use, compared with substantial loss of enzyme activity for physiosorbed cellulase after 3 cycles. Optimization of the degree of surface grafting and the effectiveness of a spacer between surface and enzyme for enzyme loading and activity were conducted. This work shows that carbene surface modification is a viable strategy for introducing enzymes onto a surface under very mild conditions and retaining a meaningful level of activity, and particularly, using GF membrane as a novel support provides a potential platform for enzyme and protein immobilization.
O vibrational bond observed by ATR-IR; as well as the observation of fluorescence. Further, five support materials (polystyrene XAD4 bead, polyacrylate MAC3 bead, glass wool, glass fiber membrane, polytetrafluoroethylene membrane) with different morphology and surface chemistry, were examined in detail as supports for cellulase immobilization using this common surface modification protocol. Of interest is that such covalently bound cellulase on modified GF membrane gave both the highest enzyme loading (∼23 mg cellulase per g support), and retained more than 90% of activity after 6 cycles of re-use, compared with substantial loss of enzyme activity for physiosorbed cellulase after 3 cycles. Optimization of the degree of surface grafting and the effectiveness of a spacer between surface and enzyme for enzyme loading and activity were conducted. This work shows that carbene surface modification is a viable strategy for introducing enzymes onto a surface under very mild conditions and retaining a meaningful level of activity, and particularly, using GF membrane as a novel support provides a potential platform for enzyme and protein immobilization.
1. Introduction
Surface modification of diverse materials by insertion of carbenes provides access to a variety of functional materials with adjusted chromophore, wettability, biocompatibility, crosslinking and adhesion properties.1–6 Carbene insertion may arise by reaction at X–H (X = C, O, N, P, S, etc.) as well as carbon–carbon double and triple bonds, all of which lead to covalent (i.e. irreversible) attachment of new surface functionality.7 Although this approach gives a relatively low loading on the surface, nonetheless there is sufficient capacity for the detection of chromophores and the binding and release of small molecules.8 We have earlier shown that surface modification of a variety of substrates, i.e. polymeric beads and powder using diaryldiazomethane derivatives leads to surface loading values of 1013–1015 molecules per cm2,9 and of 1011–1013 molecules per cm2via using bisdiazo derivatives for filter paper and HybondTM membrane.10 Of interest to us was whether this process might be successfully applied to macromolecule (enzyme) capture, and whether this loading level would give measurable and useful activity sufficient for bioengineering applications. Such an approach would be unique since it would provide a surface modification platform based upon carbene insertion for binding biomolecules, and the opportunity to use materials which have not been considered for enzyme immobilization.Cellulase is a complex enzyme composed mainly of endoglucanase, exoglucanase and β-glucosidase, and is the third largest industrial enzyme by value, able to decompose cellulose and its derivatives into reducing sugar;11–13 it was therefore chosen as the model enzyme for our study. Cellulase immobilization has been recently focused on micro- and nanomaterials with higher surface area, as reviewed by Rajnish et al.14 These materials exhibited varied loading capacity, retained activity and reusability performance. The highest cellulase loading of 860.6 mg g−1 with a retained cellulase activity of 52% was achieved using Fe3O4/acid activated montmorillonite composite via physisorption,15 while the highest retained cellulase activity was 98% using MWCNT as support material via chemical bonding.16 As to the reusability studies of the immobilize cellulase, metal–organic frameworks like UiO-66-NH2 possessed an enzyme loading capacity of 290 mg g−1 support via physisorption and more than 70% retained activity after 10 cycles,17 while a loading of 126 mg g−1 support and 70% retained activity after 5 cycles was reported using UiO-66-NH2 for covalent binding of cellulase.18 However, all these reports were based on specifically designed bespoke supports with high surface area, but our interest was in using commercial material for enzyme capture via covalent bonding with a generic platform surface modification approach. We therefore sought to utilize bis(diarylcarbene)s derived from bis(diaryldiazomethane) derivatives to provide a common surface modification method, which would be suitable for modifying a range of different commercial materials.7
Our plan was to modify commercial materials with bis(diarylcarbene)s to functionalize the surface with aniline groups (S-carbene-NH2, Fig. 1), which could then be used to create a surface without (S-carbene-N2+, Fig. 1) or with spacer (S-carbene-spacer-N2+, Fig. 1). Here, of crucial importance, the terminal diazonium surface was capable of coupling directly with tyrosine or other electron rich amino acid residues on cellulase, without the requirement for additional coupling agents. Such active trapping of the enzyme would operate under particularly mild conditions, but it is not a strategy that has been widely used. For example, beta-galactosidase bound to nylon membranes via diazotization was explored and turned out to be less stable at high temperature and acidic conditions,19 a polystyrene-based diazonium salt was synthesized and used to immobilize beta-glucosidase on three polymeric films,20 and a rapid cellulase immobilization method was developed by functionalizing graphene oxide with diazonium termini.21 While detailed characterization of the surface immobilized enzyme was missing in those studies, such a diazonium coupling approach has recently been further developed for the selective modification of proteins containing strongly nucleophilic tyrosine residues.22–25 For instance, the conjugation of trastuzumab with diazonium salts gave an average loading of 1.0–3.8 azo compound per antibody with high selectivity for tyrosine residues,22 a catch-and-release approach was reported by linking diazonium salts to resin Affi-Gel 10 and coupling to protein with accessible tyrosine residues,23 and a radiolabelling diazonium salt could react with tyrosine residues on neurotensin in high yield.24 All of this literature precedent suggested that diazonium coupling could be a good bioconjugation strategy. Arenediazonium salts have also been studied for protein conjugation and labelling,25 and one of our previous studies covering diazonium couplings with aromatic amines in solution and on a polymer surface provides detailed mechanistic understanding.26 Thus, successful covalent binding of cellulase onto a chemically modified surface using mild diazonium capture would offer a unique and stable platform capable of general application for enzyme immobilization, and we report here the outcome of our work to develop this approach.
2. Materials and methods
2.1 Chemicals and materials
Glass Fiber (GF) membrane, composed of pure borosilicate glass, was purchased from Whatman (1820-047). Amberlite®XAD-4 (XAD4) and Dowex® MAC-3 hydrogen form (MAC3) were purchased from Sigma-Aldrich (Merck Ltd). Glass wool, polytetrafluoroethylene (PTFE) membrane, sodium citrate dihydrate, citric acid, sodium carboxymethyl cellulose, and sodium acetate were supplied by Adams, Tansol Co. Ltd. Cellulase, dinitrosalicylic acid (DNS) reagents were obtained from Shanghai Yuanye Bio-technology Co. Ltd. Coomassie (Bradford) protein assay kit, and glacial acetic acid were from Thermo Scientific. AR grade solvents like dichloromethane (DCM), acetone, and ethanol were from Sinopharm Chemical Co. Ltd (Shanghai, China). The other reagents were purchase from Alfa-Aesar (Thermo Scientific). All the chemicals were used as received.2.2 Synthesis and surface modification
The synthesis of bis(diaryldiazomethane) 1,3-bis(diazo(4-phenoxyphenyl)methyl)benzene and dianiline derivative 4,4′-(((ethane-1,2-diylbis(oxy))bis(ethane-2,1-diyl))bis(oxy))dianiline (the precursor for spacer) has been reported10,27,28 and its synthetic route can be found in the ESI† and Fig. S1–S2 with detailed procedures provided in previous literature.2For surface modification, the procedure comprises: first, bis(diaryldiazomethane) (concentration w = 5, 20, 50 mg mL−1 in DCM) was used to modify GF membrane to give GF-carbene-NH2 and then the surface was either converted to diazonium surface GF-carbene-N2+via reaction with sodium nitrite in acidic conditions, or the surface can be directly bound to a spacer terminated with a diazonium unit to generate GF-carbene-spacer-N2+ (Fig. 1). The two diazonium surfaces were used directly for cellulase immobilization to give GF-carbene-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase and GF-carbene-spacer-N
N-cellulase and GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase (Fig. 1). Four more materials (XAD4, MAC3, glass wool, and PTFE membrane) were modified using the same protocol and their immobilized enzyme activity was studied. Here, different concentrations of bis(diaryldiazomethane) (w = 5, 20 and 50 mg mL−1) on modified GF membrane are denoted as GF-carbene-NH2-w mg, GF-carbene-N 2+-w mg and GF-carbene-spacer-N2+-w mg. All the details for surface modification can be found in previous reports2,10 and in the ESI.†
N-cellulase (Fig. 1). Four more materials (XAD4, MAC3, glass wool, and PTFE membrane) were modified using the same protocol and their immobilized enzyme activity was studied. Here, different concentrations of bis(diaryldiazomethane) (w = 5, 20 and 50 mg mL−1) on modified GF membrane are denoted as GF-carbene-NH2-w mg, GF-carbene-N 2+-w mg and GF-carbene-spacer-N2+-w mg. All the details for surface modification can be found in previous reports2,10 and in the ESI.†
2.3 Surface characterization
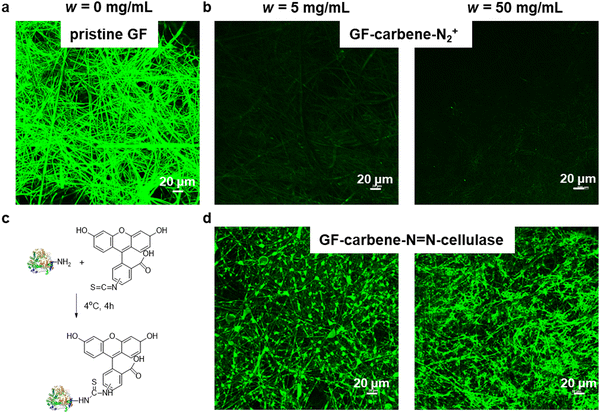
X-Ray Photoelectron Spectroscopy (XPS) was performed with an Al Kα X-ray source (hv = 1486.6 eV) at 10−9–10−10 Pa (ThermoScientific ESCALAB 250Xi). C 1s was calibrated to 284.8 eV using Thermo Avantage software. Attenuated total reflection-infrared (ATR-IR) spectra were measured from 4000 to 600 cm−1 using Shimadzu IRaffinity-1S spectrometer. Contact angle for water and diiodomethane was measured at three randomly selected areas with 2 μL drop size using DSA25E from Kruss Co. Scanning Electron Microscopy (SEM) photographs were taken on a Zeiss Sigma 300 (Zeiss Co., Germany) with all samples coated by ∼20 nm thickness of gold. Confocal laser scanning microscope (CLSM) was done using Olympus FV3000 (Olympus Co., Japan), the samples (5 mg) were treated with fluorescein isothiocyanate (FITC, 5 μg) at 4 °C for 4 h in 1 mL 70% ethanol solution, washed with extensive ethanol and water before bringing to CLSM for observation.2.4 General Procedure A for cellulase immobilization and loading test
Cellulase was immobilized onto the modified supports by diazo coupling, which formed an azo link between the supports and cellulase. Normally, the diazonium supports (S-carbene-N2+ or S-carbene-spacer-N2+) were shaken with 3 mg mL−1 cellulase in 5 mL citrate buffer (0.05 M, pH 4.8) at 150 rpm room temperature (25 °C) for 24 h.After completion of immobilization, the supernatant was collected, and the supports were washed with 5 mL citrate buffer for three times, the washed supernatant was combined with the initial supernatant, and the supports were stored at 4 °C until further use. The residual cellulase in the supernatant was used to determine the enzyme loading capacity using the Bradford method, with bovine serum albumin as a standard protein. The enzyme loading capacity was calculated using the following equation (eqn (1)):
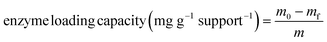 | (1) |
2.5 General Procedure B for determination of enzyme activity
The activity of free and immobilized cellulase was analyzed by hydrolysis of carboxymethylcellulose (CMC) according to previous reports.29 For free cellulase, cellulase (0.5 mL, 3 mg mL−1) was added into a conical flask followed by 5 mL 1% CMC in acetate buffer (pH 4.8, 0.05 M), and for immobilized cellulase on supports, 0.5 mL acetate buffer was added into the flask followed by 5 mL CMC solution. The reaction was carried out at 50 °C 200 rpm for 30 min, the produced glucose was determined by the 3,5-dinitrosalicylic acid (DNS) method:30 2 mL of the reaction mixture was mixed with 3 mL DNS and boiled for 5 min, and then, measurement was done with UV-Vis at 540 nm. One unit of cellulase is defined as the amount of enzyme that produces one micromole of glucose per min, the relative activities were calculated by comparing the immobilized enzyme activity with the activity of free enzyme.2.6 General Procedure C for enzyme reusability and thermal stability
For the reusability test, General Procedure C was used: immobilized cellulase on supports from the above activity test was washed with acetate buffer at least three times and CMC (5 mL, 1%) solution was added, and then shaken at 50 °C 200 rpm for 30 min. Similar to General Procedure B, 2 mL of the reaction mixture was mixed with 3 mL DNS and boiled for 5 min before measurement of absorption at 540 nm. This procedure was repeated for each cycle and in total 6 cycles were conducted including the initial activity test in the first cycle.Thermal stability of free and immobilized cellulase on GF membrane was assayed according to the following procedure: free and immobilized cellulase on GF membrane was added into CMC (10 mL, 1%) solution at 50 °C for 30, 60, 90, 120, 150, and 180 min respectively. 1 mL of reaction mixture was taken out after each time interval, mixed with 4 mL DNS and boiled for 5 min. After cooling, UV-Vis measurement was taken at 540 nm.
3. Results and discussion
3.1 Surface modification
We have reported the use of monocarbenes and dicarbenes for material surface modification,7 but in order to extend this to a strategy to permit protein capture at a modified surface, we wished to exploit the following sequence: first, carbene insertion onto a material surface using reaction of a diaryldiazo group as previously reported;2 second, conversion of the surface amine groups to diazonium salts which would be capable of active capture of protein, by coupling, for example, with electron rich tyrosine residues.19 This approach is of particular importance here because it is generally applicable to a wide range of surfaces, and it provides very mild conditions for surface protein conjugation, avoiding toxic agents like dialdehydes. Since a successful modification of bis(diarylcarbene) onto GF membrane was covered in our previous study,2 GF membrane was chosen as a substrate of interest and the modified GF membrane (GF-carbene-NH2) was initially investigated for capturing the cellulase.3.2 Characterization of cellulase on GF membrane
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase). A similar trend in color change has also been observed for supports with spacer (GF-carbene-spacer-N
N-cellulase). A similar trend in color change has also been observed for supports with spacer (GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase). The introduction of color onto colorless substrates is due to the attachment of a visible chromophore onto the surface, and moreover shows a good level of homogeneity. The lighter color of GF-carbene-spacer-N
N-cellulase). The introduction of color onto colorless substrates is due to the attachment of a visible chromophore onto the surface, and moreover shows a good level of homogeneity. The lighter color of GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase compared with GF-carbene-N
N-cellulase compared with GF-carbene-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase is mainly attributed to the break of conjugation by ethoxy chain from spacer.
N-cellulase is mainly attributed to the break of conjugation by ethoxy chain from spacer.
The surface wettability also varied significantly for supports before and after cellulase immobilization, (Fig. 2); for w = 0 mg mL−1, the water immediately penetrated the membrane, demonstrating the hydrophilicity and the high porosity of the fibers, while physiosorbed cellulase did not change the porosity of the membrane but kept the surface hydrophilic. The contact angle for the diazonium surface before immobilization was higher than 90° (as w increased from 5 to 50 mg mL−1, GF-carbene-N2+ changed from 136° to 130°, and GF-carbene-spacer-N2+ from 125° to 108°), with water not penetrating into the membrane.2 However, after enzyme immobilization, regardless of w values and spacer addition, all of the modified surfaces became hydrophilic and showed complete water penetration within 2 s. The surface energy decreased by half after cellulase immobilization on modified membrane compared with GF-cellulase (Table S1, ESI†). This significant change in wettability is due to the addition of hydrophilic molecules and/or cellulase on the surface.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase) shows that the Si element becomes buried after the GF membrane surface was coated with bis(diarylcarbene) and cellulase. The detection of the element S confirmed the presence of enzyme, although for GF-cellulase, no S was found probably due, firstly, to the lower enzyme loading for this surface, as expected, and secondly, to the lower sensitivity for S 2p. The N/C ratio is also evidence for enzyme on the surface, and for GF-cellulase, an increase in N/C compared with GF membrane was observed, attributed to physisorption of cellulase. Similar increase in N/C ratio is also observed for GF-carbene-N
N-cellulase) shows that the Si element becomes buried after the GF membrane surface was coated with bis(diarylcarbene) and cellulase. The detection of the element S confirmed the presence of enzyme, although for GF-cellulase, no S was found probably due, firstly, to the lower enzyme loading for this surface, as expected, and secondly, to the lower sensitivity for S 2p. The N/C ratio is also evidence for enzyme on the surface, and for GF-cellulase, an increase in N/C compared with GF membrane was observed, attributed to physisorption of cellulase. Similar increase in N/C ratio is also observed for GF-carbene-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase and GF-carbene-spacer-N
N-cellulase and GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase. These data imply the presence of cellulase on surface, but not its mode of attachment.
N-cellulase. These data imply the presence of cellulase on surface, but not its mode of attachment.
| Surface information | Elemental (atom%) | N/C | ||||
|---|---|---|---|---|---|---|
| C 1s | N 1s | O 2p | Si 2p | S 2p | ||
| GF membrane | 11.38 | 0.63 | 57.66 | 30.33 | 0 | 0.055 |
| GF-cellulase | 23.07 | 2.33 | 50.53 | 24.07 | 0 | 0.101 |
| GF-carbene-N2+ | 79.24 | 4.59 | 12.95 | 3.23 | 0 | 0.058 |
GF-carbene-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase N-cellulase
|
62.86 | 8.62 | 27.25 | 0.92 | 0.35 | 0.137 |
| GF-carbene-spacer-N2+ | 74.82 | 8.79 | 16.21 | 0.17 | 0 | 0.117 |
GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase N-cellulase
|
68.05 | 10.2 | 21.22 | 0.23 | 0.3 | 0.150 |
Further deconvolution of high-resolution spectra gives proof for covalent bonding of cellulase onto the modified surfaces. As shown in Fig. 3a, one single bonded nitrogen (–NH–) peak at 399.9 eV in GF-cellulase was from physisorbed cellulase, while for surfaces with diazonium (GF-carbene-N2+ and GF-carbene-spacer-N2+), the N 1s peak becomes broadened and showed two peaks at higher binding energy (401.1 eV and 402.9 eV) attributed to –N2+ and one peak at 399.8 eV ascribed to –NH–. With the covalent bonding of cellulase, disappearance of the diazonium peak and appearance of a weak –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– peak at 400.9 eV was observed for the covalently bonded cellulase surfaces (GF-carbene-N
N– peak at 400.9 eV was observed for the covalently bonded cellulase surfaces (GF-carbene-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase and GF-carbene-spacer-N
N-cellulase and GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase) but not for physiosorbed cellulase surface (GF-cellulase). This peak assignment is consistent with previous reports31–33 and firmly confirmed the bonding between surface and cellulase. In addition, the N 1s peak for cellulase immobilized on GF membrane with different w was deconvoluted as shown in Fig. 3b, and a –N
N-cellulase) but not for physiosorbed cellulase surface (GF-cellulase). This peak assignment is consistent with previous reports31–33 and firmly confirmed the bonding between surface and cellulase. In addition, the N 1s peak for cellulase immobilized on GF membrane with different w was deconvoluted as shown in Fig. 3b, and a –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– peak was found in all except for GF-cellulase, proving the peak around 400.9 eV was the –N
N– peak was found in all except for GF-cellulase, proving the peak around 400.9 eV was the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– peak formed by reaction of the diazonium unit with cellulase. For w = 20 mg mL−1, the highest –N
N– peak formed by reaction of the diazonium unit with cellulase. For w = 20 mg mL−1, the highest –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– peak area was found (Table S3, ESI†) showing that this concentration has the most chemically bonded cellulase.
N– peak area was found (Table S3, ESI†) showing that this concentration has the most chemically bonded cellulase.
The C 1s high resolution peak also gives informative results (Fig. 3c). C 1s spectra was deconvoluted into 2 or 3 peaks respectively, with 284.8 eV assigned to sp2/sp3 hybridized carbon (–C–H, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– or –C–C), ∼286 eV assigned to C–O/C–N, and 288 eV assigned to C
C– or –C–C), ∼286 eV assigned to C–O/C–N, and 288 eV assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and proportions of deconvoluted peaks and ratio between C–O/C–C are given in Table S2 (ESI†). The pristine GF membrane, used as control, showed a broad peak attributed to adventitious carbon on the surface, while an extra peak was observed for GF-cellulase at around 288 eV, arising from the C
O, and proportions of deconvoluted peaks and ratio between C–O/C–C are given in Table S2 (ESI†). The pristine GF membrane, used as control, showed a broad peak attributed to adventitious carbon on the surface, while an extra peak was observed for GF-cellulase at around 288 eV, arising from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the peptide (or amide) groups of cellulase. An increase of two times in peak area for C–O at ∼286 eV is observed for the surface with spacer (GF-carbene-spacer-N2+ and GF-carbene-spacer-N
O of the peptide (or amide) groups of cellulase. An increase of two times in peak area for C–O at ∼286 eV is observed for the surface with spacer (GF-carbene-spacer-N2+ and GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase), giving evidence for successful attachment of spacer (which contains repeating glycol units). The appearance of peaks at ∼288 eV gave conclusive proof of cellulase on surface, in line with the N 1s result.
N-cellulase), giving evidence for successful attachment of spacer (which contains repeating glycol units). The appearance of peaks at ∼288 eV gave conclusive proof of cellulase on surface, in line with the N 1s result.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration and around 1234 cm−1 contributed by C–O vibration were consistent with the increase in w. A weak peak was observed at 1659 cm−1 (Fig. 4a) as w changes from 5 mg mL−1 to 50 mg mL−1, attributed to the vibration of C
C vibration and around 1234 cm−1 contributed by C–O vibration were consistent with the increase in w. A weak peak was observed at 1659 cm−1 (Fig. 4a) as w changes from 5 mg mL−1 to 50 mg mL−1, attributed to the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and is evidence for the cellulase on the surface. For GF-cellulase, no obvious signal was observed at this wavelength, probably due to the lower loading of cellulase. Similarly, for surface with spacer, the signal was also too weak to be observed by ATR-IR due to the lower loading of cellulase.
O, and is evidence for the cellulase on the surface. For GF-cellulase, no obvious signal was observed at this wavelength, probably due to the lower loading of cellulase. Similarly, for surface with spacer, the signal was also too weak to be observed by ATR-IR due to the lower loading of cellulase.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase (Fig. 5d). This membrane becomes wettable after cellulase immobilization, and higher magnification (Fig. 5d, inset) indicates aggregated cellulase on the glass fiber. The morphology for GF-carbene-spacer-N2+ (Fig. 5e) shows irregular dots, due to the coupling of hydrophobic GF-carbene-NH2 surface with spacer which has a hydrophilic ethoxy group, leading to aggregation of spacer on surface. After cellulase immobilization reaction, the morphology for GF-carbene-spacer-N
N-cellulase (Fig. 5d). This membrane becomes wettable after cellulase immobilization, and higher magnification (Fig. 5d, inset) indicates aggregated cellulase on the glass fiber. The morphology for GF-carbene-spacer-N2+ (Fig. 5e) shows irregular dots, due to the coupling of hydrophobic GF-carbene-NH2 surface with spacer which has a hydrophilic ethoxy group, leading to aggregation of spacer on surface. After cellulase immobilization reaction, the morphology for GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase (Fig. 5f) was similar to GF-carbene-N
N-cellulase (Fig. 5f) was similar to GF-carbene-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase (Fig. 5d), with cross-linked fibers and both wettable by water, confirming the attachment of cellulase.
N-cellulase (Fig. 5d), with cross-linked fibers and both wettable by water, confirming the attachment of cellulase.
Since enzyme aggregates were observed in SEM, confocal microscopy was utilized to further visualize the distribution of enzyme over the membrane. FITC was used to label the enzyme and although pristine GF membrane was initially used as the control, it proved to be unsuitable because green fluorescence was observed across the entire surface due to physisorption (Fig. 6a). Therefore, GF-carbene-N2+ was chosen as an alternative control, and two concentrations w = 5 and 50 mg mL−1 of surface modification were chosen for evaluation. Very weak to no fluorescence was observed for GF-carbene-N2+ modified surface at both bisdiazo concentrations (Fig. 6b), but uniform green fluorescence (Fig. 6d) confirmed both the attachment of FITC to cellulase and the regular distribution of cellulase all over the membrane surface.
3.3 Cellulase immobilization study
For GF membrane, all the immobilized enzymes retained more than 40% enzyme activity compared to the free cellulase, with little difference in activity among the three GF membrane-based supports. For the relative enzyme activity on other supports (ESI,† Fig. S5), generally more than 20% of free enzyme activity was preserved for covalently bonded enzyme, while enzyme immobilized on supports containing spacer (S-carbene-spacer-N2+) showed the highest activity compared to other kinds of support (although PTFE membrane is an exception). This is probably because adding spacers onto the surface reduces undesired interactions between the enzyme and support, enhancing hydration and minimizing steric hindrance.36,37
The reusability for cellulase immobilized on GF membrane (Fig. 7c and four other supports in Fig. S5c–f, ESI†) was examined; it was found that for cellulase physisorbed on GF membrane, a large drop of 80% was observed over 3 cycles, presumable due to the ready leaching of cellulase from the support, while for cellulase covalently bonded to GF-carbene-N2+, 85% of the initial activity was retained after 6 cycles, and for cellulase immobilized onto GF-carbene-spacer-N2+, 73% of initial activity was retained after 6 cycles (Fig. 7c). Although the use of inorganic glass materials for enzyme immobilization has been investigated for decades, and various enzymes have been immobilized either via adsorption,38–40 entrapment,41 ionic binding42 or covalent via initial activation with silanes,43–45 a strategy using a bis(diarylcarbene) modification offers a different platform to the well-developed silyloxy linking strategy for glass, and a glass fiber surface for cellulase immobilization has never been before reported. Compared with the other surface-modified materials in our work, cellulase on modified GF membrane shows superior reusability, making it of particular interest for industrial application. The good reusability of cellulase immobilized on GF membrane probably arises from multiple factors. Firstly, fibers permit higher accessibility of substrate to enzyme compared to beads, for which enzymes can be entrapped in pores which limits diffusion through the substrate. Secondly, glass fibers in the micro- to nano-scale have a higher surface area compared to glass wool, affording 5 times higher enzyme loading. Also noteworthy is that supports with spacer (Fig. 1, GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase) do not actually improve the reusability of immobilized cellulase significantly compared to the supports without spacer (Fig. 1, GF-carbene-spacer-N
N-cellulase) do not actually improve the reusability of immobilized cellulase significantly compared to the supports without spacer (Fig. 1, GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase).
N-cellulase).
As compared to other studies of enzyme immobilization, Table S5 (ESI†) summarizes the main performance parameters of immobilized cellulase through covalent bonding. The highest enzyme loading is 575 mg g−1 support using magnetic nanoparticle (GO@Fe3O4@4arm-PEG-NH2),46 the highest enzyme activity retained was 93.5% using magnetic halloysite nanotubes,29 the highest enzyme reusability was 97% after 10 cycles using clay–poly(glycidyl methacrylate) composite,47 and all these three utilized glutaraldehyde for covalent bonding with cellulase. Though our approach does not have advantage against only one of these parameters (enzyme loading, retained activity and reusability), the immobilized enzyme has excellent recyclability and reusability on GF membrane support, which is commercially available, cost effective, easy to handle, and quick to modify with a mild diazonium coupling. Therefore, carbene modified GF membrane offers a viable alternative for immobilizing enzyme and realizing effective enzyme immobilization on a wide range of materials offers the opportunity for new approaches in bioengineering.
The effect of the density of surface grafting was investigated by varying the concentration of bis(diaryldiazomethane) reagent to modify the GF membrane, and both enzyme loading capacity and enzyme activity were measured (Fig. 7d). Since more covalent binding points for cellulase are created when bis(diaryldiazomethane) concentration w increases from 0 to 50 mg mL−1, cellulase loading increases and reaches the highest value at w = 20 mg mL−1 for GF-carbene-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase. This cellulase loading result was consistent with the XPS analysis for the formation of –N
N-cellulase. This cellulase loading result was consistent with the XPS analysis for the formation of –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– during cellulase immobilization, with the highest cellulase loading at w = 20 mg mL−1 corresponding to the highest peak area for deconvoluted –N
N– during cellulase immobilization, with the highest cellulase loading at w = 20 mg mL−1 corresponding to the highest peak area for deconvoluted –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (Table S3, ESI†). However, the highest enzyme loading, the lowest enzyme activity was obtained at w = 20 mg mL−1. The same trend goes for the surface with spacer (GF-carbene-spacer-N
N– (Table S3, ESI†). However, the highest enzyme loading, the lowest enzyme activity was obtained at w = 20 mg mL−1. The same trend goes for the surface with spacer (GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase), where the lowest cellulase loading at w = 50 mg mL−1 gave the highest relative enzyme activity (∼93%). This outcome clearly shows that higher enzyme loading can correlate with lower enzyme activity, which is consistent with literature18,47 and probably is mainly due to steric hinderance for both the immobilization process and the diffusion of substrate during enzyme activity measurement. On the other hand, the degree of surface grafting does not significantly influence enzyme reusability in both the absence and presence of spacer. The activity of physisorbed cellulase (GF-cellulase, w = 0) decreases significantly after 3 cycles, while the activity of covalently bonded cellulase is retained at more than 91% for surface without spacer (GF-carbene-N
N-cellulase), where the lowest cellulase loading at w = 50 mg mL−1 gave the highest relative enzyme activity (∼93%). This outcome clearly shows that higher enzyme loading can correlate with lower enzyme activity, which is consistent with literature18,47 and probably is mainly due to steric hinderance for both the immobilization process and the diffusion of substrate during enzyme activity measurement. On the other hand, the degree of surface grafting does not significantly influence enzyme reusability in both the absence and presence of spacer. The activity of physisorbed cellulase (GF-cellulase, w = 0) decreases significantly after 3 cycles, while the activity of covalently bonded cellulase is retained at more than 91% for surface without spacer (GF-carbene-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase), and more than 79% for surface with spacer (GF-carbene-spacer-N
N-cellulase), and more than 79% for surface with spacer (GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase) regardless of the value of w. In general, the lowest bis(diaryldiazomethane) concentration (w = 5 mg mL−1) for GF membrane modification and enzyme immobilization seems to be the optimal choice, which is particularly helpful for cost effectiveness of the surface modification process.
N-cellulase) regardless of the value of w. In general, the lowest bis(diaryldiazomethane) concentration (w = 5 mg mL−1) for GF membrane modification and enzyme immobilization seems to be the optimal choice, which is particularly helpful for cost effectiveness of the surface modification process.
The addition of a spacer to the support surface has been reported to increase the activity of immobilized enzyme,27,36,50 and we similarly found that the relative enzyme activity for the support with spacer (GF-carbene-spacer-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase) is 1.3 to 2.7 times higher than support without the spacer (GF-carbene-N
N-cellulase) is 1.3 to 2.7 times higher than support without the spacer (GF-carbene-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-cellulase) (Fig. 7d). Such an increase in activity likely arises by a decrease in steric hinderance between cellulase molecules at the surface and increase of the mobility and hydration of covalently bonded cellulase and its substrates, even though the glycol moieties of the spacer lead to a lower overall enzyme loading. On the other hand, interestingly, the retained activity during reusability testing for surface with spacer was always lower than for surface without spacer (Fig. 7e and f), and this is probably caused by the lower cellulase loading and higher enzyme activity which leads to denaturing and fouling during reuse of all the immobilized enzyme. Therefore, for cellulase immobilization on a bis(diaryldiazomethane) modified surface, the spacer does neither plays a crucial effect, nor is necessarily needed for improving immobilized cellulase properties. This is a major advantage of our protocol and simplifies the immobilization process.
N-cellulase) (Fig. 7d). Such an increase in activity likely arises by a decrease in steric hinderance between cellulase molecules at the surface and increase of the mobility and hydration of covalently bonded cellulase and its substrates, even though the glycol moieties of the spacer lead to a lower overall enzyme loading. On the other hand, interestingly, the retained activity during reusability testing for surface with spacer was always lower than for surface without spacer (Fig. 7e and f), and this is probably caused by the lower cellulase loading and higher enzyme activity which leads to denaturing and fouling during reuse of all the immobilized enzyme. Therefore, for cellulase immobilization on a bis(diaryldiazomethane) modified surface, the spacer does neither plays a crucial effect, nor is necessarily needed for improving immobilized cellulase properties. This is a major advantage of our protocol and simplifies the immobilization process.
4. Conclusion
In this study, it has been demonstrated that the surface modification using a reaction platform relying upon biscarbene insertion allows the covalent binding of enzyme on several substrates. The immobilization of cellulase on GF membrane particularly has been demonstrated by changes of wetting ability from hydrophobic for diazonium surface to hydrophilic for enzyme immobilized surface, along with fluorescence as observed by confocal microscopy. Moreover, high resolution XPS of N 1s proved the existence of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N formation during enzyme immobilization and C 1s showed the deconvoluted peak of C
N formation during enzyme immobilization and C 1s showed the deconvoluted peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from cellulase, proving that enzyme had been covalently attached at the surface. Importantly, using this approach, there are sufficient points of chemical attachment at the surface that binding of the enzyme, cellulase, is detectable and the method is sufficiently mild that enzyme activity is retained. Enzyme-modified GF membrane is robust and can be reused over more than 6 cycles, retaining high levels of activity. This work clearly shows that commercially available glass fiber membrane, among other readily available materials, and its surface modification via bis(diarycarbene), proves to be an effective platform for fundamental studies of enzyme immobilization and validates this approach for the direct immobilization of other enzyme and proteins with industrial application.
O from cellulase, proving that enzyme had been covalently attached at the surface. Importantly, using this approach, there are sufficient points of chemical attachment at the surface that binding of the enzyme, cellulase, is detectable and the method is sufficiently mild that enzyme activity is retained. Enzyme-modified GF membrane is robust and can be reused over more than 6 cycles, retaining high levels of activity. This work clearly shows that commercially available glass fiber membrane, among other readily available materials, and its surface modification via bis(diarycarbene), proves to be an effective platform for fundamental studies of enzyme immobilization and validates this approach for the direct immobilization of other enzyme and proteins with industrial application.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Weiwei Wang from Shiyanjia Lab (http://www.shiyanjia.com) for the XPS measurement.References
- R. Visbal and M. C. Gimeno, N-heterocyclic carbene metal complexes: photoluminescence and applications, Chem. Soc. Rev., 2014, 43(10), 3551–3574 RSC.
- D. Wang, W. Hartz, K. E. Christensen and M. G. Moloney, Surface Modification of Glass Fiber Membrane via insertion of a bis(diarylcarbene) assisted with polymerization and cross-linking reactions, Surf. Interfaces, 2022, 102155 CrossRef CAS.
- Z. Wang, N. V. Tzouras, S. P. Nolan and X. Bi, Silver N-heterocyclic carbenes: emerging powerful catalysts, Trends Chem., 2021, 3(8), 674–685 CrossRef CAS.
- S. Yang, S. Yi, J. Yun, N. Li, Y. Jiang, Z. Huang, C. Xu, C. He and X. Pan, Carbene-Mediated Polymer Cross-Linking with Diazo Compounds by C–H Activation and Insertion, Macromolecules, 2022, 55(9), 3423–3429 CrossRef CAS.
- L. Lepage Mathieu, C. Simhadri, C. Liu, M. Takaffoli, L. Bi, B. Crawford, S. Milani Abbas and E. Wulff Jeremy, A broadly applicable cross-linker for aliphatic polymers containing C–H bonds, Science, 2019, 366(6467), 875–878 CrossRef CAS PubMed.
- C. Simhadri, L. Bi, M. L. Lepage, M. Takaffoli, Z. Pei, S. F. Musolino, A. S. Milani, G. A. DiLabio and J. E. Wulff, Flexible polyfluorinated bis-diazirines as molecular adhesives, Chem. Sci., 2021, 12(11), 4147–4153 RSC.
- D. Wang, M. K. Khan and M. G. Moloney, Diazo and diazonium compounds for surface modification, Tetrahedron Lett., 2020, 61(14), 151672 CrossRef CAS.
- C. Choong, J. S. Foord, J.-P. Griffiths, E. M. Parker, L. Baiwen, M. Bora and M. G. Moloney, Post-polymerisation modification of surface chemical functionality and its effect on protein binding, New J. Chem., 2012, 36(5), 1187–1200 RSC.
- S. Iqbal, Y. Lui, J. G. Moloney, E. M. Parker, M. Suh, J. S. Foord and M. G. Moloney, A comparative study of diaryl carbene insertion reactions at polymer surfaces, Appl. Surf. Sci., 2019, 465, 754–762 CrossRef CAS.
- M. G. Moloney and P. Yang, Surface Modification of Polymers with Bis(arylcarbene)s from Bis(aryldiazomethane)s: Preparation, Dyeing and Characterization, RSC Adv., 2016, 6, 111276 RSC.
- S. L. Hirsh, M. M. Bilek, N. J. Nosworthy, A. Kondyurin, C. G. dos Remedios and D. R. McKenzie, A comparison of covalent immobilization and physical adsorption of a cellulase enzyme mixture, Langmuir, 2010, 26(17), 14380–14388 CrossRef CAS PubMed.
- T. C. Hung, C. C. Fu, C. H. Su, J. Y. Chen, W. T. Wu and Y. S. Lin, Immobilization of cellulase onto electrospun polyacrylonitrile (PAN) nanofibrous membranes and its application to the reducing sugar production from microalgae, Enzyme Microb. Technol., 2011, 49(1), 30–37 CrossRef CAS PubMed.
- R. Ahmad and S. K. Khare, Immobilization of Aspergillus niger cellulase on multiwall carbon nanotubes for cellulose hydrolysis, Bioresour. Technol., 2018, 252, 72–75 CrossRef CAS PubMed.
- K. N. Rajnish, M. S. Samuel, J. A. John, S. Datta, N. Chandrasekar, R. Balaji, S. Jose and E. Selvarajan, Immobilization of cellulase enzymes on nano and micro-materials for breakdown of cellulose for biofuel production-a narrative review, Int. J. Biol. Macromol., 2021, 182, 1793–1802 CrossRef CAS PubMed.
- L. M. Wu, Q. H. Zeng, R. Ding, P. P. Tu and M. Z. Xia, Fe3O4/Acid activated montmorillonite/cellulase composites: Preparation, structure, and enzyme activity, Appl. Clay Sci., 2019, 179, 105129 CrossRef CAS.
- M. Alkhatib, Statistical modelling optimisation of cellulase enzyme immobilisation on functionalised multi-walled carbon nanotubes for empty fruit bunches degradation, Aust. J. Basic Appl. Sci., 2012, 6, 30–38 CAS.
- I. N. Ahmed, X. L. Yang, A. A. Dubale, R. F. Li, Y. M. Ma, L. M. Wang, G. H. Hou, R. F. Guan and M. H. Xie, Hydrolysis of cellulose using cellulase physically immobilized on highly stable zirconium based metal-organic frameworks, Bioresour. Technol., 2018, 270, 377–382 CrossRef CAS PubMed.
- B. Qi, J. Luo and Y. Wan, Immobilization of cellulase on a core-shell structured metal-organic framework composites: Better inhibitors tolerance and easier recycling, Bioresour. Technol., 2018, 268, 577–582 CrossRef CAS PubMed.
- M. M. El-Masry, A. De Maio, P. L. Martelli, R. Casadio, A. B. Moustafa, S. Rossi and D. G. Mita, Influence of the immobilization process on the activity of β-galactosidase bound to Nylon membranes grafted with glycidyl methacrylate: Part 1. Isothermal behavior, J. Mol. Catal. B: Enzym., 2001, 16(3), 175–189 CrossRef CAS.
- X. Li, X. Wang, G. Ye, W. Xia and X. Wang, Polystyrene-based diazonium salt as adhesive: A new approach for enzyme immobilization on polymeric supports, Polymer, 2010, 51(4), 860–867 CrossRef CAS.
- J. Gao, C.-L. Lu, Y. Wang, S.-S. Wang, J.-J. Shen, J.-X. Zhang and Y.-W. Zhang, Rapid Immobilization of Cellulase onto Graphene Oxide with a Hydrophobic Spacer, Catalysts, 2018, 8(5), 180 CrossRef.
- N. Griebenow, S. Greven, M. Lobell, A. M. Dilmaç and S. Bräse, A study on the trastuzumab conjugation at tyrosine using diazonium salts, RSC Adv., 2015, 5(125), 103506–103511 RSC.
- C. Allan, M. Kosar, C. V. Burr, C. L. Mackay, R. R. Duncan and A. N. Hulme, A Catch-and-Release Approach to Selective Modification of Accessible Tyrosine Residues, ChemBioChem, 2018, 19(23), 2443–2447 CrossRef CAS PubMed.
- S. Leier, S. Richter, R. Bergmann, M. Wuest and F. Wuest, Radiometal-Containing Aryl Diazonium Salts for Chemoselective Bioconjugation of Tyrosine Residues, ACS Omega, 2019, 4(26), 22101–22107 CrossRef CAS PubMed.
- S. Sengupta and S. Chandrasekaran, Modifications of amino acids using arenediazonium salts, Org. Biomol. Chem., 2019, 17(36), 8308–8329 RSC.
- S. Chng, E. M. Parker, J. P. Griffiths, M. G. Moloney and L. Y. L. Wu, A study of diazonium couplings with aromatic nucleophiles both in solution and on a polymer surface, Appl. Surf. Sci., 2017, 401, 181–189 CrossRef CAS.
- Y. Wang and Y.-L. Hsieh, Enzyme immobilization to ultra-fine cellulose fibers via amphiphilic polyethylene glycol spacers, J. Polym. Sci., Part A: Polym. Chem., 2004, 42(17), 4289–4299 CrossRef CAS.
- K. W. Mahoney, J. N. Talbert and J. M. Goddard, Effect of polyethylene glycol tether size and chemistry on the attachment of lactase to polyethylene films, J. Appl. Polym. Sci., 2013, 127(2), 1203–1210 CrossRef CAS.
- D. Sillu and S. Agnihotri, Cellulase Immobilization onto Magnetic Halloysite Nanotubes: Enhanced Enzyme Activity and Stability with High Cellulose Saccharification, ACS Sustainable Chem. Eng., 2019, 8(2), 900–913 CrossRef.
- G. L. Miller, R. Blum, W. E. Glennon and A. L. Burton, Measurement of carboxymethylcellulase activity, Anal. Biochem., 1960, 1(2), 127–132 CrossRef CAS.
- L. J. Small, D. R. Wheeler and E. D. Spoerke, Nanoporous membranes with electrochemically switchable, chemically stabilized ionic selectivity, Nanoscale, 2015, 7(40), 16909–16920 RSC.
- S. Kesavan and S. A. John, A novel approach to fabricate stable graphene layers on electrode surfaces using simultaneous electroreduction of diazonium cations and graphene oxide, RSC Adv., 2016, 6(67), 62876–62883 RSC.
- S. Betelu, I. Tijunelyte, L. Boubekeur-Lecaque, I. Ignatiadis, J. Ibrahim, S. Gaboreau, C. Berho, T. Toury, E. Guenin, N. Lidgi-Guigui, N. Félidj, E. Rinnert and M. L. D. L. Chapelle, Evidence of the Grafting Mechanisms of Diazonium Salts on Gold Nanostructures, J. Phys. Chem. C, 2016, 120(32), 18158–18166 CrossRef CAS.
- K. Holmberg, F. Tiberg, M. Malmsten and C. Brink, Grafting with hydrophilic polymer chains to prepare protein-resistant surfaces, Colloids Surf., A, 1997, 123–124, 297–306 CrossRef CAS.
- I. Banerjee, R. C. Pangule and R. S. Kane, Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms, Adv. Mater., 2011, 23(6), 690–718 CrossRef CAS PubMed.
- J. Andre, D. Saleh, C. Syldatk and R. Hausmann, Effect of spacer modification on enzymatic synthetic and hydrolytic activities of immobilized trypsin, J. Mol. Catal. B: Enzym., 2016, 125, 88–96 CrossRef CAS.
- S. Yamamoto, A. Imamura, I. Susanti, K. Hori, Y. Tanji and H. Unno, Effect of Spacer Length on Beta-Lactoglobulin Hydrolysis by Trypsin Covalently Immobilized on a Cellulosic Support, Food Bioprod. Process., 2005, 83(1), 61–67 CrossRef CAS.
- M. Yoshimoto, T. Schweizer, M. Rathlef, T. Pleij and P. Walde, Immobilization of Carbonic Anhydrase in Glass Micropipettes and Glass Fiber Filters for Flow-Through Reactor Applications, ACS Omega, 2018, 3(8), 10391–10405 CrossRef CAS PubMed.
- A. Küchler, J. Adamcik, R. Mezzenga, A. D. Schlüter and P. Walde, Enzyme immobilization on silicate glass through simple adsorption of dendronized polymer–enzyme conjugates for localized enzymatic cascade reactions, RSC Adv., 2015, 5(55), 44530–44544 RSC.
- L. Ye, X. Liu, G. H. Shen, S. S. Li, Q. Y. Luo, H. J. Wu, A. J. Chen, X. Y. Liu, M. L. Li, B. Pu, W. Qin and Z. Q. Zhang, Properties comparison between free and immobilized wheat esterase using glass fiber film, Int. J. Biol. Macromol., 2019, 125, 87–91 CrossRef CAS PubMed.
- I. Gul, Q. Wang, Q. Jiang, R. Fang and L. Tang, Enzyme immobilization on glass fiber membrane for detection of halogenated compounds, Anal. Biochem., 2020, 609, 113971 CrossRef CAS PubMed.
- J. M. Bolivar and B. Nidetzky, Oriented and selective enzyme immobilization on functionalized silica carrier using the cationic binding module Z basic2: design of a heterogeneous D-amino acid oxidase catalyst on porous glass, Biotechnol. Bioeng., 2012, 109(6), 1490–1498 CrossRef CAS PubMed.
- E. Ruckenstein and W. Guo, Cellulose and Glass Fiber Affinity Membranes for the Chromatographic Separation of Biomolecules, Biotechnol. Prog., 2004, 20(1), 13–25 CrossRef CAS PubMed.
- J. Rogalski, J. Szczodrak, M. Pleszczyńska and J. Fiedurek, Immobilisation and kinetics of Penicillium notatum dextranase on controlled porous glass, J. Mol. Catal. B: Enzym., 1997, 3(6), 271–283 CrossRef CAS.
- R. H. Taylor, S. M. Fournier, B. L. Simons, H. Kaplan and M. A. Hefford, Covalent protein immobilization on glass surfaces: application to alkaline phosphatase, J. Biotechnol., 2005, 118(3), 265–269 CrossRef CAS PubMed.
- J. Han, P. Luo, Y. Wang, L. Wang, C. Li, W. Zhang, J. Dong and L. Ni, The development of nanobiocatalysis via the immobilization of cellulase on composite magnetic nanomaterial for enhanced loading capacity and catalytic activity, Int. J. Biol. Macromol., 2018, 119, 692–700 CrossRef CAS PubMed.
- G. Bayramoglu, B. F. Senkal and M. Y. Arica, Preparation of clay–poly(glycidyl methacrylate) composite support for immobilization of cellulase, Appl. Clay Sci., 2013, 85, 88–95 CrossRef CAS.
- J. Boudrant, J. M. Woodley and R. Fernandez-Lafuente, Parameters necessary to define an immobilized enzyme preparation, Process Biochem., 2020, 90, 66–80 CrossRef CAS.
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Improvement of enzyme activity, stability and selectivity via immobilization techniques, Enzyme Microb. Technol., 2007, 40(6), 1451–1463 CrossRef CAS.
- A. De Maio, M. M. El-Masry, M. Portaccio, N. Diano, S. Di Martino, A. Mattei, U. Bencivenga and D. G. Mita, Influence of the spacer length on the activity of enzymes immobilised on nylon/polyGMA membranes: Part 1. Isothermal conditions, J. Mol. Catal. B: Enzym., 2003, 21(4), 239–252 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tb02550g |
| This journal is © The Royal Society of Chemistry 2023 |