Zinc bromide: a general mediator for the ionothermal synthesis of microporous polymers via cyclotrimerization reactions†
Jaehwan
Kim‡
 a,
Minh H.
Le‡
a,
Minh H.
Le‡
 a,
Makayla C.
Spicer
a,
Casandra M.
Moisanu
a,
Suzi M.
Pugh
b and
Phillip J.
Milner
a,
Makayla C.
Spicer
a,
Casandra M.
Moisanu
a,
Suzi M.
Pugh
b and
Phillip J.
Milner
 *a
*a
aDepartment of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14850, USA. E-mail: pjm347@cornell.edu
bYusuf Hamied Department of Chemistry, University of Cambridge, Cambridge, CB2 1EW, UK
First published on 6th July 2023
Abstract
Conjugated microporous polymers (CMPs) are porous organic materials that display (semi)conducting behavior due to their highly π-conjugated structures, making them promising next-generation materials for applications requiring both electrical conductivity and porosity. Currently, most CMPs and related porous aromatic frameworks (PAFs) are prepared using expensive transition metals (e.g., Pd), significantly increasing the costs associated with their synthesis. Lewis acid-mediated cyclotrimerization reactions of methyl ketones and nitriles represent promising and green alternative methods for CMP and PAF synthesis. Herein, we demonstrate that the generality of the solvent-free cyclotrimerization reactions is significantly improved by using ZnBr2 instead of ZnCl2 as the ionothermal medium. Specifically, we show that 1,4-diacetylbenzene (DAB), 4,4′-diacetylbiphenyl (DABP), 2,7-diacetylfluorene (DAF), 1,3,5-triacetylbenzene (TAB), tetrakis(4-acetylphenyl)methane (TAPM), and 1,4-dicyanobenzene (DCNB) can be polymerized in molten ZnBr2 to produce highly conjugated and microporous materials, as confirmed by 77 K N2 adsorption measurements, IR, and solid-state NMR. These findings support that ZnBr2 is an excellent Lewis acid mediator and medium for the ionothermal synthesis of porous organic materials.
Introduction
Conjugated microporous polymers (CMPs) are intriguing amorphous organic materials that blend the porosity of porous organic polymers (POPs) with the extended π-conjugation of 2-dimensional materials.1–3 This combination makes them promising for applications that require guest-accessible pores in a (semi)conducting platform, such as heterogeneous electro/photocatalysis and supercapacitive charge storage.4,5 Relatedly, porous aromatic frameworks (PAFs) are ultra-stable, 3-dimensional POPs that are valuable for heterogeneous catalysis, toxic gas capture, and water purification.6 Both CMPs and PAFs are typically prepared under solvothermal conditions using Pd- or Ni-mediated reactions,7,8 contributing greatly to their synthesis costs on scale. Residual metal species can also contaminate the final insoluble polymers and alter their catalytic activities in ways that are difficult to predict.9,10 Although green approaches to preparing POPs have emerged in recent years,11 there remains an urgent need for general and sustainable methods amenable to the synthesis of CMPs and PAFs from simple monomers.Recently, cyclotrimerization reactions of methyl ketones12–29 and nitriles30–32 have emerged as promising approaches to prepare CMPs and PAFs without expensive transition metal catalysts and, in many cases, without additional organic solvents. In particular, the acid-catalyzed synthesis of POPs from polyacetylated monomers proceeds through dimerization via the aldol reaction to produce α,β-unsaturated ketones, followed by cyclotrimerization to produce 1,3,5-substituted benzene rings.20 Notably, only water is produced as a byproduct of this process, making it an attractive method for polymer synthesis.
With Brønsted acids such as methanesulfonic acid (MsOH) and p-toluenesulfonic acid (TsOH), the aldol reaction produces a mixture of α,β-unsaturated ketone and aromatic linkages due to incomplete cyclotrimerization, as evidenced by residual carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups detected by infrared (IR) spectroscopy and tunneling electron microscopy (TEM).13,14,18,19,25,26 These functionalities likely reduce the overall conjugation of the polymeric materials and change their fundamental optoelectronic properties as well.18 On the other hand, ionothermal Lewis acid mediators such as ZnCl2 drive cyclotrimerization reactions to higher conversions,12 but the requisite harsh reaction conditions lead to significant degradation and carbonization of the resulting polymers.33 As a result, this approach is currently only suitable for synthesizing a single CMP from 1,3,5-triacetylbenzene (TAB), referred to herein as TAB-CMP; all other tested monomers yielded non-porous or low surface area materials.12 The identification of superior Lewis acids that can function as both the mediator and reaction medium would enable a broader range of monomers to be converted into highly conjugated porous organic materials.
O) groups detected by infrared (IR) spectroscopy and tunneling electron microscopy (TEM).13,14,18,19,25,26 These functionalities likely reduce the overall conjugation of the polymeric materials and change their fundamental optoelectronic properties as well.18 On the other hand, ionothermal Lewis acid mediators such as ZnCl2 drive cyclotrimerization reactions to higher conversions,12 but the requisite harsh reaction conditions lead to significant degradation and carbonization of the resulting polymers.33 As a result, this approach is currently only suitable for synthesizing a single CMP from 1,3,5-triacetylbenzene (TAB), referred to herein as TAB-CMP; all other tested monomers yielded non-porous or low surface area materials.12 The identification of superior Lewis acids that can function as both the mediator and reaction medium would enable a broader range of monomers to be converted into highly conjugated porous organic materials.
Herein, we demonstrate that simply switching the ionothermal solvent from ZnCl2 to ZnBr2 greatly extends the scope of monomers compatible with the synthesis of CMPs and PAFs via ionothermal aldol cyclotrimerization. Using ZnBr2, five monomers could be effectively converted into microporous organic polymeric materials, with high conversion of the carbonyl groups into aromatic rings confirmed by Attenuated Total Reflectance IR spectroscopy (ATR-IR) and energy-dispersive X-ray spectroscopy (EDS). Notably, ZnBr2 could also be used to prepare a porous covalent triazine framework (CTF) under ionothermal conditions. Together, these results indicate that ZnBr2 is a potential replacement for ZnCl2 with improved monomer compatibility, paving the way for the sustainable synthesis of other POPs under solvent-free conditions.
Results and discussion
ZnBr2 as an alternative Lewis acid to ZnCl2
Because ZnCl2 is widely employed as a strong Lewis acid in both fine chemical and polymer synthesis,34 we hypothesized that the poor scope observed for ionothermal aldol cyclotrimerization reactions with ZnCl2 is likely due to its aggressive reactivity, which leads to significant monomer degradation and material carbonization.12,33 As such, switching to a milder Lewis acid should improve the monomer compatibility of this reaction. Previous work has shown that ZnBr2 enables delicate reactions that do not proceed well with stronger Lewis acids such as TiCl4.35,36 As an added benefit, switching from Cl− counteranions (∼1.75 Å) to larger Br− counteranions (∼1.85 Å) should help to better template forming micropores and prevent their collapse during the polymerization process.37–39 As such, we surmised that ZnBr2 might serve as a better ionothermal medium for POP synthesis. Indeed, our preliminary results using a molecular model system support that both ZnCl2 and ZnBr2 are competent mediators for aldol cyclotrimerization reactions under solvothermal conditions (ESI Section 5, Fig. S133–S135†).The ability of ZnBr2 to mediate POP synthesis from polyacetylated monomers was initially assessed using 1,4-diacetylbenzene (DAB) as a model monomer to produce a CMP termed CORN-CMP-2 (CORN = Cornell University) and referred to herein as DAB-CMP (Fig. 1, ESI Section 4†). Previous work has shown that Brønsted acids, such as MsOH (p-PPN, PPN = porous polymer network),14,19 TsOH (OFC-1B, OFC = porous organic framework by the cyclotrimerization reaction),22,26 and in situ generated HCl (OFC-1A)26 can be used to prepare porous polymeric materials from DAB. In contrast, the combination of DAB and ZnCl2 under ionothermal conditions at 300 °C or 400 °C produces non-porous solids (ESI Table S20†).12 Using a custom-built apparatus (ESI Fig. S7†), varying equivalents of anhydrous ZnBr2 (1.00 equiv., 5.00 equiv., and 10.0 equiv.) were combined with DAB under vacuum in flame-sealed tubes (see ESI Section 3† for details). The solid mixtures were transferred to a furnace and allowed to stand at 400 °C (ZnBr2 melting point = 394 °C) for either 24 h or 72 h. After cooling to room temperature, the resulting black solids were rinsed with aqueous HCl and water to remove residual Zn2+ salts and then with tetrahydrofuran (THF) and acetone to remove soluble organic species. The solids were finally dried under vacuum at 120 °C overnight prior to characterization by ATR-IR, surface area analysis, and powder X-ray diffraction (PXRD) (Fig. 2). All prepared materials were found to be amorphous by PXRD (ESI Fig. S19 and S20†) and scanning electron microscopy (SEM, ESI Fig. S9†). It should be noted that the black color of these solids is likely due to partial graphitization, a common challenge associated with high-temperature ionothermal synthesis.33 At this time, we cannot rule out that in situ generated HBr (resulting from the reaction of water produced from the polymerization with ZnBr2) plays a role under these conditions.
 | ||
| Fig. 1 Synthesis of DAB-CMP from DAB with varying equivalents of ZnBr2 at 400 °C for either 24 or 72 h. | ||
In contrast to the results obtained with Brønsted acids,19,22 only weak residual C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretches (∼1690 cm−1) were observed in the ATR-IR spectra of all DAB-CMP materials synthesized with ZnBr2 (Fig. 2a). The ATR-IR spectra of the samples prepared for 24 h, particularly that made with just 1.00 equiv. of ZnBr2, contain additional C
O stretches (∼1690 cm−1) were observed in the ATR-IR spectra of all DAB-CMP materials synthesized with ZnBr2 (Fig. 2a). The ATR-IR spectra of the samples prepared for 24 h, particularly that made with just 1.00 equiv. of ZnBr2, contain additional C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretches (∼1698 cm−1) that are absent from materials made for 72 h (ESI Fig. S17 and S18†), indicative of incomplete cyclotrimerization.22 The ATR-IR spectra of samples prepared with 5.00 or 10.0 equiv. of ZnBr2 for 24 h and 1.00 equiv. or 5.00 equiv. of ZnBr2 for 72 h were similar and dominated by a large stretch near 1570 cm−1, corresponding to the newly formed 1,3,5-substituted aromatic rings.12,22 The corresponding aromatic stretch in DAB is present as a shoulder near 1600 cm−1 that is masked by the dominant carbonyl C
O stretches (∼1698 cm−1) that are absent from materials made for 72 h (ESI Fig. S17 and S18†), indicative of incomplete cyclotrimerization.22 The ATR-IR spectra of samples prepared with 5.00 or 10.0 equiv. of ZnBr2 for 24 h and 1.00 equiv. or 5.00 equiv. of ZnBr2 for 72 h were similar and dominated by a large stretch near 1570 cm−1, corresponding to the newly formed 1,3,5-substituted aromatic rings.12,22 The corresponding aromatic stretch in DAB is present as a shoulder near 1600 cm−1 that is masked by the dominant carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch. Together, the ATR-IR spectra support that ZnBr2 is a competent mediator for aldol cyclotrimerization reactions and that ionothermal conditions that are too gentle or too harsh lead to sub-optimal results.
O stretch. Together, the ATR-IR spectra support that ZnBr2 is a competent mediator for aldol cyclotrimerization reactions and that ionothermal conditions that are too gentle or too harsh lead to sub-optimal results.
The porosity of prepared DAB-CMP samples was assessed using 77 K N2 adsorption/desorption isotherms (Fig. 2c and d). All six samples were found to be microporous, confirming that ZnBr2 enables the synthesis of much higher surface area materials than ZnCl2.12 The lowest Brunauer–Emmett–Teller (BET) surface areas were determined for DAB-CMP prepared with 1.00 equiv. of ZnBr2 for 24 h (399 ± 1 m2 g−1). The BET surface areas of the other samples were found to lie in a narrow range (∼600 m2 g−1), with the material prepared under intermediate conditions (DAB-CMP-5.00 equiv., 72 h) possessing the highest BET surface area (651 ± 2 m2 g−1) of all samples. The BET surface area of DAB-CMP-5.00 equiv., 72 h is slightly lower than those reported previously for the related materials OFC-1B (780–895 m2 g−1)22,26 and p-PPN (802–1054 m2 g−1),14,19 which possess a higher degree of approximately linear α,β-unsaturated ketone linkages that should decrease the density of the polymeric material. The density functional theory (DFT) calculated pore size distribution of DAB-CMP-5.00 equiv., 72 h assuming a carbon slit pore model revealed a maximum at 11.4 Å (ESI Fig. S35†), which is comparable to the pore diameter predicted for the idealized, non-interpenetrated structure (∼12 Å, ESI Fig. S156†).19 Though DAB-CMP-5.00 equiv., 72 h possesses the highest BET surface area of all DAB-CMP samples, it should be noted that high surface area material (584 m2 g−1) could be prepared using just 1.00 equiv. of ZnBr2, which is unusual for ionothermal POP synthesis.33 Last, the ionothermal synthesis of porous DAB-CMP was found to be reproducible (ESI Fig. S31†).
To support that ZnBr2 is a competent mediator for CMP synthesis, DAB-CMP-5.00 equiv., 72 h was further characterized using cross-polarized (CP) magic angle spinning (MAS) solid-state nuclear magnetic resonance spectroscopy (SSNMR, Fig. 2b, ESI Fig. S33 and S34†), Raman spectroscopy (ESI Fig. S37†), EDS (ESI Fig. S10 and Table S2†), combustion elemental analysis (ESI Table S3†), X-ray photoelectron spectroscopy (XPS, ESI Fig. S11–S16 and Table S4†), and diffuse reflectance UV-Vis spectroscopy (ESI Fig. S36†). The CP MAS 13C SSNMR spectrum of DAB-CMP-5.00 equiv., 72 h revealed a complex resonance localized near 127 ppm, which could be deconvoluted into signals corresponding to two different types of 13C(sp2)–H centers and one quaternary 13C(sp2) center (Fig. 2b). This spectrum is similar to those previously reported for DAB-CMP analogs prepared using Brønsted acid mediators.14,26 Notably, resonances corresponding to residual carbonyl groups (∼200 ppm) were not observed. The MAS 1H SSNMR of DAB-CMP-5.00 equiv., 72 h contained only a single broad resonance centered around 7 ppm, corresponding to aromatic C(sp2)–1H centers (ESI Fig. S33†). Consistent with the lack of residual carbonyl groups observed by ATR-IR and SSNMR spectroscopies, the EDS and XPS spectra of DAB-CMP-5.00 equiv., 72 h contained only trace O, along with small amounts of residual Zn, Cl, and Br (ESI Tables S2 and S4†). The presence of Cl in the washed and activated material is likely due to Br/Cl exchange with residual Zn salts upon soaking in HCl. Combustion elemental analysis (ESI Table S3†) confirmed the presence of trace Cl (1.55 wt%) in the sample, along with an observed H wt% (4.15%) close to the theoretical value (4.79%). Although Raman scattering lends evidence to some graphene-like character in the material, as expected of a highly conjugated material (ESI Fig. S37†), the detection of the expected amount of H in the sample supports that DAB-CMP was not completely carbonized under the ionothermal conditions. Last, the diffuse reflectance UV-Vis spectrum (ESI Fig. S36†) of DAB-CMP revealed broad absorbance over the visible regime, consistent with its black color and extended conjugated structure.12,26 Overall, the spectroscopic and gas sorption data herein support the successful synthesis of microporous DAB-CMP using ZnBr2 as an ionothermal mediator.
In order to assess whether ZnBr2 really is the optimal ionothermal mediator for aldol cyclotrimerization reactions, we evaluated sixteen other Lewis acid mediators, focusing on low-melting (≤400 °C) Zn2+, Sn2+, Al3+, Fe3+, Bi3+, Si4+, Ti4+, and Sb5+ salts (ESI Table S20, Section 6†). All Lewis acids were tested close to their melting point (or boiling point for salts that melt near room temperature) to minimize potential carbonization. The Lewis acids Al(NO3)3·9H2O, SbCl5, SiCl4, Zn(NO3)2·6H2O, and Zn(OAc)2·2H2O (48–250 °C) did not yield any isolable polymeric materials. Similar to the results obtained with ZnCl2 at 300 °C or 400 °C, the Lewis acids AlBr3, TiCl4, BiCl3, Zn(SO3CF3)2, and SnCl2, along with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 eutectic mixture of ZnCl2
1 eutectic mixture of ZnCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KCl
KCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaCl (100–310 °C), produced non-porous or low surface area materials, indicative of significant degradation. Only the Lewis acids AlCl3 (345 ± 2 m2 g−1), FeCl3 (302 ± 1 m2 g−1), FeBr3 (280 ± 2 m2 g−1), and TiBr4 (489 ± 7 m2 g−1) (200–310 °C) produced porous solids with BET surface areas close to ZnBr2 (651 m2 g−1), although the sample prepared using TiBr4 was contaminated with residual crystalline TiO2 (ESI Fig. S139†).40,41 Ten low-melting potential Brønsted acids were also investigated (ESI Table S21, Section 6†), but only TsOH led to high-surface area DAB-CMP, consistent with previous reports.22,26 Together, these results support that ZnBr2 is the optimal Lewis acid for POP synthesis via aldol cyclotrimerization.
NaCl (100–310 °C), produced non-porous or low surface area materials, indicative of significant degradation. Only the Lewis acids AlCl3 (345 ± 2 m2 g−1), FeCl3 (302 ± 1 m2 g−1), FeBr3 (280 ± 2 m2 g−1), and TiBr4 (489 ± 7 m2 g−1) (200–310 °C) produced porous solids with BET surface areas close to ZnBr2 (651 m2 g−1), although the sample prepared using TiBr4 was contaminated with residual crystalline TiO2 (ESI Fig. S139†).40,41 Ten low-melting potential Brønsted acids were also investigated (ESI Table S21, Section 6†), but only TsOH led to high-surface area DAB-CMP, consistent with previous reports.22,26 Together, these results support that ZnBr2 is the optimal Lewis acid for POP synthesis via aldol cyclotrimerization.
Cyclotrimerization monomer scope using ZnBr2
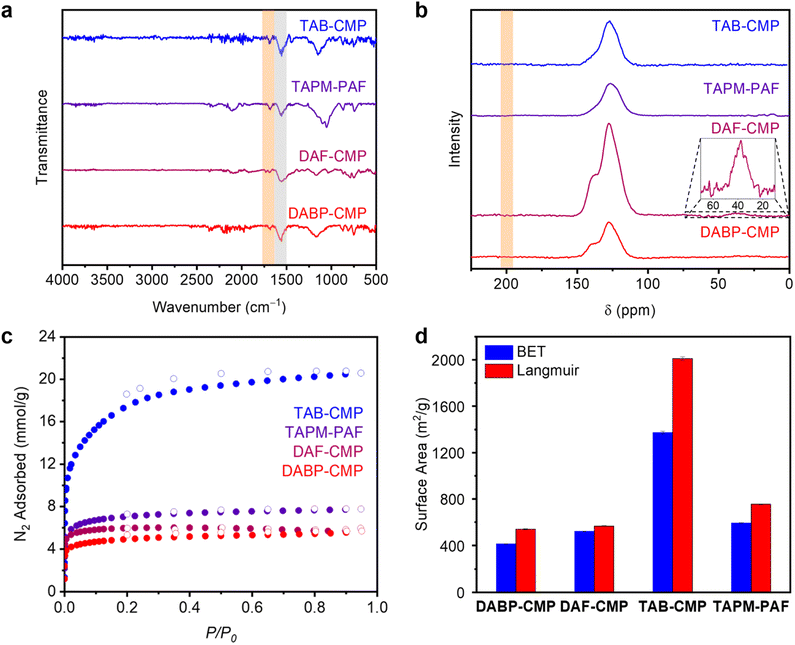
Given that ZnBr2 enables ionothermal polymerization with at least one monomer (DAB) that does not work with ZnCl2, its generality for POP synthesis was next examined using the monomers 1,3,5-triacetylbenzene (TAB), 4,4′-diacetylbiphenyl (DABP), 2,7-diacetylfluorene (DAF), and tetrakis(4-acetylphenyl)methane (TAPM) (Fig. 3). Among these, (porous) polymeric materials have been previously prepared from TAB,12,14,18DABP,18,22,23,26 and TAPM,25 whereas DAF represents a new monomer for this approach. As such, the optimal conditions for synthesizing DAB-CMP (5.00 equiv. ZnBr2, 400 °C, 72 h) were employed to prepare materials referred to herein as TAB-CMP (CORN-CMP-1), DABP-CMP (CORN-CMP-3), DAF-CMP (CORN-CMP-4), and TAPM-PAF (CORN-PAF-1) (see ESI Section 4† for synthetic details). In all cases, black amorphous polymeric materials were obtained in good yields (>90%).The four prepared materials were analyzed using the same techniques employed to characterize DAB-CMP-5.00 equiv., 72 h, including ATR-IR and CP MAS 13C SSNMR spectroscopies, 77 K N2 adsorption/desorption isotherms, EDS, and combustion analysis (Fig. 4, see ESI Section 4† for details). Consistent with the results obtained for DAB-CMP (Fig. 2a), ATR-IR spectroscopy confirmed the significant conversion of the carbonyl groups in the monomers (∼1665–1685 cm−1) into 1,3,5-substituted aromatic rings (∼1565 cm−1) in the polymeric materials in all cases (Fig. 4a; ESI Fig. S51, S69, S87, S105†). Consistently, the O wt% determined by EDS for DABP-CMP (2.54%, ESI Fig. S42 and Table S5†), DAF-CMP (2.18%, ESI Fig. S60 and Table S8†), TAB-CMP (1.76%, ESI Fig. S78 and Table S11†), and TAPM-PAF (2.25%, ESI Fig. S96 and Table S14†) are all low. All prepared materials also contained only trace amounts of Zn, Cl, and Br by EDS and XPS, supporting that washing with aqueous HCl and water is sufficient to remove the vast majority of residual Zn salts. Furthermore, across all four materials, CP MAS 13C SSNMR confirms the disappearance of the carbonyl groups and the appearance of chemical shifts at 125–130 ppm that correspond to aromatic carbons (Fig. 4b, ESI Fig. S50, S68, S86, and S104†). Notably, DAF-CMP retains a peak at 38.1 ppm, which corresponds to the sp3-hybridized methylene carbons (–CH2–) in the fluorene group (inset of Fig. 4b, ESI Fig. S68†). Unfortunately, the quaternary 13C resonance expected for TAPM-PAF (around 70 ppm) could not be observed, likely due to its inherently weak nature and partial graphitization reducing its relative intensity (ESI Fig. S104†).12
The porosity of all four polymeric materials was also assessed using 77 K N2 adsorption/desorption isotherms (Fig. 4c and d). Compared to samples of DABP-CMP prepared using Brønsted acid mediators, which display a broad range of BET surface areas (12–451 m2 g−1),22,23,25DABP-CMP has a relatively high surface area (415 ± 2 m2 g−1). The lower surface area of DABP-CMP compared to DAB-CMP – despite a nominally larger theoretical pore diameter (ESI Fig. S157 and 158†) – is likely due to interpenetration.19,22,26 Similarly, the BET surface area of the new material DAF-CMP (522 ± 2 m2 g−1) is slightly lower than that of DAB-CMP. The BET surface area of TAB-CMP (1373 ± 11 m2 g−1) is higher than that reported using ZnCl2 (929 ± 6 m2 g−1)12 and comparable to that for the optimal material prepared using MsOH (1235 m2 g−1),14 reflecting its good quality. The surface area of this material is consistently higher than those of other cyclotrimerized microporous polymers, which is likely due to a lack of interpenetration and its defective, non-planar structure (ESI Fig. S161†).14 Last, the BET surface area determined for TAPM-PAF (594 ± 3 m2 g−1) is only slightly lower than that reported for the material prepared using thionyl chloride as a mediator (832 m2 g−1).25 Critically, this surface area is much higher than that obtained using ZnCl2 under similar conditions (133 ± 1 m2 g−1),12 further reflecting the superiority of ZnBr2 as an ionothermal mediator. Combined with the spectroscopic results outlined above, these surface area data confirm that ZnBr2 is a general mediator for the ionothermal synthesis of porous materials via the aldol cyclotrimerization reaction.
Covalent triazine framework synthesis using ZnBr2
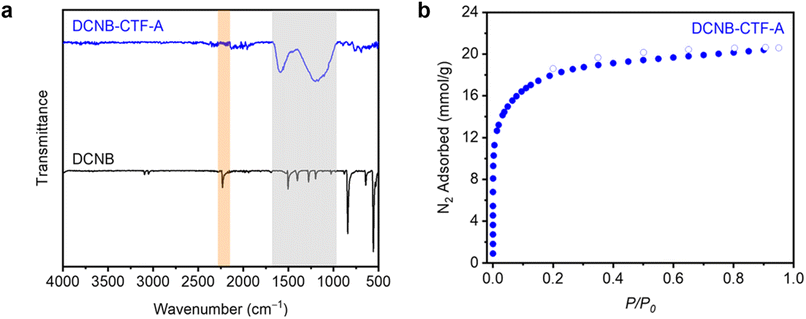
Beyond the aldol reaction, the Lewis acid-catalyzed conversion of nitrile groups into 1,3,5-triazines is a general strategy for preparing covalent triazine frameworks (CTFs), which can either be crystalline (C) or amorphous (A).30–32 Under ionothermal conditions, ZnCl2 mediates the cyclotrimerization of 1,4-dicyanobenzene (DCNB) into CTF-1, referred to herein as DCNB-CTF-A (amorphous) or DCNB-CTF-C (crystalline) (Fig. 5).32 Given the excellent performance of ZnBr2 as an ionothermal mediator, we hypothesized that it could serve as a replacement for ZnCl2 to enable the facile synthesis of CTFs as well. As such, we evaluated whether the combination of DCNB and ZnBr2 (5.00 equiv. ZnBr2, 400 °C, 40 h, time and temperature taken directly from literature for comparison32) can be used to prepare high-quality DCNB-CTF. After soaking in organic solvent and drying under vacuum, a shiny black solid was obtained in a good yield (90%, see ESI Section 4† for details). PXRD (ESI Fig. S125†) and SEM (ESI Fig. S113†) confirmed that the obtained material was amorphous, and thus it was assigned as DCNB-CTF-A.
DCNB-CTF-A was characterized by ATR-IR and CP MAS 13C SSNMR spectroscopies, 77 K N2 adsorption/desorption, EDS, XPS, and combustion analysis (Fig. 6, see ESI Section 4† for details). ATR-IR confirmed the complete loss of the nitrile C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretch in DCNB (2231 cm−1) and the appearance of triazine rings in the polymerized material (1170 and 1590 cm−1).32 Notably, the 13C SSNMR spectrum of DCNB-CTF-A is comparable to those reported for CTFs synthesized with ZnCl2 (ESI Fig. S123†),42,43 and the XPS spectrum of DCNB-CTF-A shows a noticeably broadened C 1s signal, which could be deconvoluted into carbons in a triazine environment (N–C
N stretch in DCNB (2231 cm−1) and the appearance of triazine rings in the polymerized material (1170 and 1590 cm−1).32 Notably, the 13C SSNMR spectrum of DCNB-CTF-A is comparable to those reported for CTFs synthesized with ZnCl2 (ESI Fig. S123†),42,43 and the XPS spectrum of DCNB-CTF-A shows a noticeably broadened C 1s signal, which could be deconvoluted into carbons in a triazine environment (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and benzene environment (C–C
N) and benzene environment (C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) (Fig. S116†). The porosity of DCNB-CTF-A was assessed using 77 K N2 adsorption/desorption measurements. Its BET surface area was determined to be 1446 ± 10 m2 g−1, which compares favorably to the reported surface areas for this material prepared at 400 °C with ZnCl2 (920–1123 m2 g−1).32,44 The DFT- calculated pore size distribution of DCNB-CTF-A assuming a carbon slit pore model also revealed a maximum at 11.4 Å (ESI Fig. S127†), similar to the pore diameter predicted for the idealized, non-interpenetrated structure (∼11 Å, ESI Fig. S165†). Notably, the synthesized DCNB-CTF-A did not contain detectable amounts of residual Zn or Br by EDS or XPS and contained only trace Cl (0.28 and 0.66 wt%, respectively). The observed C
C) (Fig. S116†). The porosity of DCNB-CTF-A was assessed using 77 K N2 adsorption/desorption measurements. Its BET surface area was determined to be 1446 ± 10 m2 g−1, which compares favorably to the reported surface areas for this material prepared at 400 °C with ZnCl2 (920–1123 m2 g−1).32,44 The DFT- calculated pore size distribution of DCNB-CTF-A assuming a carbon slit pore model also revealed a maximum at 11.4 Å (ESI Fig. S127†), similar to the pore diameter predicted for the idealized, non-interpenetrated structure (∼11 Å, ESI Fig. S165†). Notably, the synthesized DCNB-CTF-A did not contain detectable amounts of residual Zn or Br by EDS or XPS and contained only trace Cl (0.28 and 0.66 wt%, respectively). The observed C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio from combustion analysis (5.7
N ratio from combustion analysis (5.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is also comparable to those reported in the literature for samples prepared with ZnCl2 (3.8
1) is also comparable to those reported in the literature for samples prepared with ZnCl2 (3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5.1
1–5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).32,44 These values are all higher than the theoretical value (3.4
1).32,44 These values are all higher than the theoretical value (3.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), indicating that some graphitization occurs with both ionothermal mediators.33 Nonetheless, the data included here support the successful ionothermal synthesis of DCNB-CTF-A using ZnBr2.
1), indicating that some graphitization occurs with both ionothermal mediators.33 Nonetheless, the data included here support the successful ionothermal synthesis of DCNB-CTF-A using ZnBr2.
Conclusions
Herein, we have demonstrated that ZnBr2 is a general ionothermal mediator for synthesizing a range of microporous polymers via cyclotrimerization reactions. Six materials could be prepared from simple methyl ketone or nitrile monomers under identical solvent-free conditions. An evaluation of twenty-five other Brønsted and Lewis acid mediators supports the exceptional performance of ZnBr2. Our findings extend the scope of cyclotrimerized CMPs and PAFs that can be sustainably prepared, paving the way for their application as next-generation microporous materials for myriad applications. We hypothesize that ZnBr2 may serve as a viable alternative to ZnCl2 as an ionothermal mediator for the synthesis of other polymeric materials as well. Future work will focus on elucidating the mechanism of this ionothermal method, particularly on the potential role of in situ generated HBr.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The development of new porous materials was supported by the National Science Foundation under Grant No. CBET-2047627. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. This work made use of the Cornell Center for Materials Research (CCMR) Shared Facilities, which are supported through the NSF MRSEC program (DMR-1719875). 1H NMR data were collected on a Bruker INOVA 500 MHz spectrometer that was purchased with support from the NSF (CHE-1531632). We thank Cornell University for providing summer research fellowships to M. H. L. and C. M. M. and the CCMR REU program for supporting M. C. S. We also acknowledge support from a Camille Dreyfus Teacher-Scholar Award to P. J. M. (TC-23-048). We thank Dr. Alexander C. Forse (University of Cambridge) for helpful advice and financial support of S. M. P. through a UKRI Future Leaders Fellowship, a Leverhulme Trust Research Project Grant (RPG-2020-337), and a BP Next Generation Fellowship.Notes and references
- J.-S. M. Lee and A. I. Cooper, Chem. Rev., 2020, 120(4), 2171–2214 CrossRef CAS PubMed.
- S. Das, P. Heasman, T. Ben and S. Qiu, Chem. Rev., 2017, 117, 1515–1563 CrossRef CAS PubMed.
- N. Chaoui, M. Trunk, R. Dawson, J. Schmidt and A. Thomas, Chem. Soc. Rev., 2017, 46, 3302–3321 RSC.
- K. Amin, N. Ashraf, L. Mao, C. F. J. Faul and Z. Wei, Nano Energy, 2021, 85, 105958 CrossRef CAS.
- T. Zhang, G. Xing, W. Chen and L. Chen, Mater. Chem. Front., 2020, 4, 332–353 RSC.
- Y. Tian and G. Zhu, Chem. Rev., 2020, 120, 8934–8986 CrossRef CAS PubMed.
- T. Ben, H. Ren, S. Ma, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qiu and G. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457–9460 CrossRef CAS PubMed.
- J.-X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak and A. I. Cooper, Angew. Chem., Int. Ed., 2007, 46, 8574–8578 CrossRef CAS.
- J. Kosco, M. Sachs, R. Godin, M. Kirkus, L. Francas, M. Bidwell, M. Qureshi, D. Anjum, J. R. Durrant and I. McCulloch, Adv. Energy Mater., 2018, 8, 1802181 CrossRef.
- F. C. Krebs, R. B. Nyberg and M. Jørgensen, Chem. Mater., 2004, 16, 1313–1318 CrossRef CAS.
- T. L. Church, A. B. Jasso-Salcedo, F. Björnerbäck and N. Hedin, Sci. China: Chem., 2017, 60, 1033–1055 CrossRef CAS.
- J. Kim, C. M. Moisanu, C. N. Gannett, A. Halder, J. J. Fuentes-Rivera, S. H. Majer, K. M. Lancaster, A. C. Forse, H. D. Abruña and P. J. Milner, Chem. Mater., 2021, 33, 8334–8342 CrossRef CAS.
- S. Che, C. Li, C. Wang, W. Zaheer, X. Ji, B. Phillips, G. Gurbandurdyyev, J. Glynn, Z.-H. Guo, M. Al-Hashimi, H.-C. Zhou, S. Banerjee and L. Fang, Chem. Sci., 2021, 12, 8438–8444 RSC.
- C. Wang, C. Li, E. R. C. Rutledge, S. Che, J. Lee, A. J. Kalin, C. Zhang, H.-C. Zhou, Z.-H. Guo and L. Fang, J. Mater. Chem. A, 2020, 8, 15891–15899 RSC.
- C. Sreenivasulu, D. A. Thadathil, S. Pal and S. Gedu, Synth. Commun., 2020, 50, 112–122 CrossRef CAS.
- M.-Y. Wang, Q.-J. Zhang, Q.-Q. Shen, Q.-Y. Li and S.-J. Ren, Chin. J. Polym. Sci., 2020, 38, 151–157 CrossRef CAS.
- X. Yang and R. C. Smith, J. Polym. Sci. Part A: Polym. Chem., 2019, 57, 598–604 CrossRef CAS.
- S.-M. Jung, J. Park, D. Shin, H. Y. Jeong, D. Lee, I.-Y. Jeon, H. Cho, N. Park, J.-W. Yoo and J.-B. Baek, Angew. Chem., Int. Ed., 2019, 58, 11670–11675 CrossRef CAS PubMed.
- Z.-H. Guo, C. Wang, Q. Zhang, S. Che, H.-C. Zhou and L. Fang, Mater. Chem. Front., 2018, 2, 396–401 RSC.
- B. Yang, J. Björk, H. Lin, X. Zhang, H. Zhang, Y. Li, J. Fan, Q. Li and L. Chi, J. Am. Chem. Soc., 2015, 137, 4904–4907 CrossRef CAS PubMed.
- S. K. Samanta, E. Preis, C. W. Lehmann, R. Goddard, S. Bag, P. K. Maiti, G. Brunklaus and U. Scherf, Chem. Commun., 2015, 51, 9046–9049 RSC.
- F. M. Wisser, K. Eckhardt, D. Wisser, W. Böhlmann, J. Grothe, E. Brunner and S. Kaskel, Macromol, 2014, 47, 4210–4216 CrossRef CAS.
- X. Zhu, C. Tian, S. Chai, K. Nelson, K. S. Han, E. W. Hagaman, G. M. Veith, S. M. Mahurin, H. Liu and S. Dai, Adv. Mater., 2013, 25, 4152–4158 CrossRef CAS.
- T. İslamoğlu, M. Gulam Rabbani and H. M. El-Kaderi, J. Mater. Chem. A, 2013, 1, 10259 RSC.
- Y.-C. Zhao, D. Zhou, Q. Chen, X.-J. Zhang, N. Bian, A.-D. Qi and B.-H. Han, Macromol, 2011, 44, 6382–6388 CrossRef CAS.
- M. Rose, N. Klein, I. Senkovska, C. Schrage, P. Wollmann, W. Böhlmann, B. Böhringer, S. Fichtner and S. Kaskel, J. Mater. Chem., 2011, 21, 711–716 RSC.
- R. S. Sprick, A. Thomas and U. Scherf, Polym. Chem., 2010, 1, 283–285 RSC.
- Y. Shin, C. Wang, M. Englehard and G. E. Fryxell, Microporous Mesoporous Mater., 2009, 123, 345–348 CrossRef CAS.
- X.-Y. Cao, X.-H. Liu, X.-H. Zhou, Y. Zhang, Y. Jiang, Y. Cao, Y.-X. Cui and J. Pei, J. Org. Chem., 2004, 69, 6050–6058 CrossRef CAS PubMed.
- C. Krishnaraj, H. S. Jena, K. Leus and P. Van Der Voort, Green Chem., 2020, 22, 1038–1071 RSC.
- M. Liu, L. Guo, S. Jin and B. Tan, J. Mater. Chem. A, 2019, 7, 5153–5172 RSC.
- P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3450–3453 CrossRef CAS PubMed.
- K. Wang, L.-M. Yang, X. Wang, L. Guo, G. Cheng, C. Zhang, S. Jin, B. Tan and A. Cooper, Angew. Chem., Int. Ed., 2017, 56, 14149–14153 CrossRef CAS PubMed.
- D. M. M. Rohe and H. U. Wolf, in Ullmann's Encyclopedia of Industrial Chemistry, ed., Wiley-VCH Verlag GmbH & Co. KGaA, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000, pp. 747–752 Search PubMed.
- P. Erdmann and L. Greb, Angew. Chem., Int. Ed., 2022, 61, e202114550 CrossRef CAS PubMed.
- I. Paterson, Tetrahedron Lett., 1979, 20, 1519–1520 CrossRef.
- N. Fechler, T.-P. Fellinger and M. Antonietti, Adv. Mater., 2013, 25, 75–79 CrossRef CAS PubMed.
- J. S. Lee, X. Wang, H. Luo and S. Dai, Adv. Mater., 2010, 22, 1004–1007 CrossRef CAS PubMed.
- J. S. Lee, X. Wang, H. Luo, G. A. Baker and S. Dai, J. Am. Chem. Soc., 2009, 131, 4596–4597 CrossRef CAS PubMed.
- Ž. Antić, R. M. Krsmanović, M. G. Nikolić, M. Marinović-Cincović, M. Mitrić, S. Polizzi and M. D. Dramićanin, Mater. Chem. Phys., 2012, 135, 1064–1069 CrossRef.
- H. Ijadpanah-Saravi, S. Dehestaniathar, A. Khodadadi-Darban, M. Zolfaghari and S. Saeedzadeh, Desalin. Water Treat., 2016, 57, 20503–20510 CrossRef CAS.
- V. M. Rangaraj, K. S. K. Reddy and G. N. Karanikolos, Chem. Eng. J., 2022, 429, 132160 CrossRef CAS.
- J. Jia, Z. Chen, Y. Belmabkhout, K. Adil, P. M. Bhatt, V. A. Solovyeva, O. Shekhah and M. Eddaoudi, J. Mater. Chem. A, 2018, 6, 15564–15568 RSC.
- P. Kuhn, A. Forget, D. Su, A. Thomas and M. Antonietti, J. Am. Chem. Soc., 2008, 130, 13333–13337 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and electrochemical data. See DOI: https://doi.org/10.1039/d3ta01471a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |