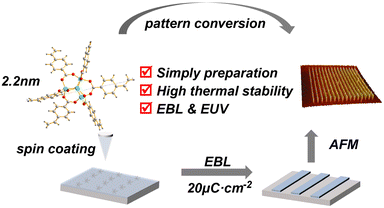
A novel stable zinc–oxo cluster for advanced lithography patterning†
Youming
Si
a,
Yingdong
Zhao
a,
Guangyue
Shi
d,
Danhong
Zhou
a,
Feng
Luo
d,
Pengzhong
Chen
 *ab,
Jiangli
Fan
*ab,
Jiangli
Fan
 *ac and
Xiaojun
Peng
*ac and
Xiaojun
Peng
 ac
ac
aState Key Laboratory of Fine Chemicals, Frontiers Science Center for Smart Materials Oriented Chemical Engineering, School of Chemical Engineering, Dalian University of Technology, Dalian 116024, China. E-mail: fanjl@dlut.edu.cn
bNingbo Institute of Dalian University of Technology, Ningbo 315016, China
cResearch Institute of Dalian University of Technology in Shenzhen, Shenzhen 518057, China
dSchool of Materials Science and Engineering, Nankai University, Tianjin 300350, China
First published on 11th February 2023
Abstract
Recently, the development of novel metal-containing resists has received much attention in extreme ultraviolet lithography (EUVL) owing to their smaller sizes and higher EUV absorptivity than traditional polymer resists. Herein, we report zinc (Zn)–VBA, a novel stable zinc–oxo cluster-based photoresist molecule with the [Zn4O]6+ inner core and six organic ligands based on 4-vinylbenzoic acid. Zn–VBA was simply prepared by the reactions between the zinc-oxide and 4-vinylbenzoic acid, exhibiting atomically precise structure and good batch stability. Based on single-crystal X-ray diffraction analysis, the size of Zn–VBA is 2.2 nm, which is in line with the high-resolution requirements for advanced lithography technology. Zn–VBA exhibits excellent thermal stability up to 400 °C, much better than the reported zinc-based clusters. Moreover, theoretical studies have revealed the roles of organic ligands in their high thermal stability. Spin-coating methods were employed to fabricate homogenous thin films of Zn–VBA, which showed a quite small roughness value in the absence of viscosifiers, as confirmed by atomic force microscopy images. More importantly, the Zn–VBA films performed well in the electron beam lithography (EBL) and EUVL tests without the addition of photoinitiators, giving prominent pattern lines. This work proves the structural advantages of benzoic-acid-based zinc–oxo clusters as promising EUV patterning materials, which inspire future exploration of metal–oxo clusters (MOCs) resist materials for advanced lithography.
Introduction
In the semiconductor industry, downscaling of device size in integrated circuits often pushes lithography technology1,2 to its resolution limits by continuously decreasing the wavelength of the light source. Therefore, it is important to develop photoresist materials that are compatible and have an optimized response to light irradiation.3,4 Because of EUV's ability to achieve sub-10 nm node patterns with high resolution, a commercial shift from deep ultraviolet (DUV, λ = 193 nm) lithography (DUVL) to shorter extreme ultraviolet (EUV, λ = 13.5 nm) lithography (EUVL) is underway.5–8 This shift overturned the traditional DUV lithography. Unlike DUVL, where UV light induces photoacid generation, EUV photons (92 eV) ionize most materials encountered. The role of the electron cascade produced by photoionization is still being investigated. In addition, when compared to DUV 193 nm lithography, the photon density of EUV was reduced to only 7% at a given exposure dose, resulting in appreciable statistical fluctuation. The drastic change in the photo energy, reduced photon density, and high-resolution patterns pose challenges regarding the chemically amplified resists (CARs), which are the workhorses of the DUVL. The large size and low EUV absorptivity of polymer-based CARs have become critical points, particularly in terms of resolution. Moreover, the stochastic distribution of chemical reactions easily occurs around the photon absorption area owing to the heterogeneities in the composition of CAR film. It may result in a high defect density in the patterns. Therefore, to circumvent the challenges posed by CARs, a new class of photoresist platforms are required to be developed. The most important design criteria for optimizing photoresist performance in EUV require research on materials with high EUV absorption elements, defined molecular structures, as well as small and homogeneous sizes.9–15Metal oxides16–18 that can incorporate high EUV absorption elements into the molecular frameworks exhibit promising application prospects in EUVL. In particular, among the various metal oxides, MOCs are hybrid inorganic–organic molecular compounds with a well-defined number of metallic elements and organic ligands. It is generally assumed that the central metallic atoms are able to absorb a large fraction of the EUV light and show high etch resistance, while the peripheral organic shells are responsible for processing and solubility switching properties. When exposed to EUV radiation, such materials usually undergo loss or decomposition of organic ligands, followed by crosslinking of metal and oxygen bonds to form a metal oxide network primarily acting as negative tone photoresists. Because of the wide range of metal centers and organic ligands available, the structures and properties of MOCs are highly variable and tunable.19,20 Among the transition metals with 3d electrons, zinc (Zn)-based MOCs have gained much attention. The absorption cross section21 of zinc–oxo clusters for 13.5 nm EUV22,23 is an order of magnitude higher than that of organic polymers. Moreover, zinc–oxo clusters are nontoxic and in line with the concept of green lithography.24,25 However, because of the uncertainty in synthesis, the structures of most reported zinc–oxo clusters are not well defined, resulting in undesirable purity and batch instability. Additionally, additives, such as photoinitiators or viscosifiers, are usually needed to initiate the chemical reaction of zinc–oxo clusters and improve the film formation for the lithography process.
Herein, we report a novel zinc–oxo cluster, i.e., Zn–VBA, containing 4-vinylbenzoic acid (VBA) as a component of the organic shell around the [Zn4O]6+ core. Compared to the reported zinc–oxo clusters, Zn–VBA exhibits advantages including simple preparation, good batch stability, and atomically precise structure. More importantly, Zn–VBA is a single-component photoresist material that is able to occur photolithographic reaction without photoinitiators. Single-crystal X-ray diffraction analysis revealed a Zn–VBA of 2.2 nm, which follows the high-resolution requirements for advanced resist compositions.16,26,27 (Scheme 1). Both experimental and theoretical methods were used to systematically study the thermal stability, solubility, and film-forming capability of Zn–VBA. By using cyclohexanone and propylene glycol monomethyl ether acetate (PGMEA) as spin-coating solvents, Zn–VBA formed a uniform thin film with extremely low roughness. Moreover, in the EBL and EUVL tests, Zn–VBA produced prominent pattern lines, exhibiting the potential as promising patterning materials for advanced lithography.
Results and discussion
Design and characterization
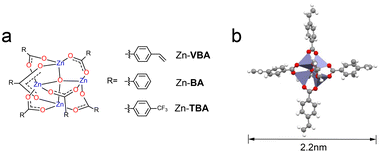
Due to the strong resonance towards EUV light of zinc, Zn-based clusters are expected to be promising patterning materials.7,16,17 Previous reported zinc–oxo clusters mainly focused on the [Zn4O]6+ type materials by using aliphatic acid ligands to constitute the organic shell, and the lithographic performance of the materials has been studied in detail. However, to the best of our knowledge, developing functional aromatic acid-containing zinc–oxo clusters as EUV patterning materials have yet to be reported. Since the aromatic ring is electron-rich and more rigid than the aliphatic series, it was anticipated that the introduction of phenyl subunits into the cluster would render the molecule more rigid without precluding their ability to act as effective metal complexants to provide good thermal stability, solubility and film-formation ability.28,29 Based on the above design criteria, 4-vinylbenzoic acid (VBA) was introduced as the organic ligand to construct the zinc–oxo cluster Zn–VBA. In addition, Zn–BA and Zn–TBA clusters containing benzoic acid (BA) and 4-trifluoromethyl benzoic acid (TBA) were also prepared as control molecules to study the electronic effects of para-substituted benzoic acids on the performance of zinc–oxo clusters (Fig. 1a, S1, and S2†). The electronic ESI† contains detailed synthetic procedures and characterization data of Zn–VBA, Zn–BA and Zn–TBA clusters.Single-crystal X-ray diffraction analysis was performed to identify the structural properties of Zn–VBA (Fig. 1b and Table S1†) and Zn–TBA (Fig. S3 and Table S2†), while the crystal structure of Zn–BA from the reported literature was used.30 Taking Zn–VBA as example, the crystal structure reveals that Zn–VBA comprises four Zn atoms bridged by one O atom (μ4-O) and six 4-vinylbenzoic acid ligands. The central oxygen is bonded to four zinc atoms, and each zinc atom exhibits a tetrahedral geometry coordinated to the other three oxygen atoms from carboxylate groups of the peripheral ligands, with Zn–O bond distances of about 1.941(9) Å. The binding carboxylate groups from six 4-vinylbenzoic acid ligands to zinc atoms display C–O bond lengths of 1.255(6) to 1.264(8) Å, and bond angles of 124.7(6)o, indicating the delocalization of the π-electrons within the bridging bidentate ligands. The whole molecule presents a spatially symmetric octahedral structure with a size of 2.2 nm, with each ligand orthogonally directed to the inner inorganic core. Only weak intermolecular CH–π interactions are observed between the phenyl rings of adjacent molecules, which inhibits compact molecular packing. Therefore, such organic–inorganic hybrid structural properties are expected to endow the zinc–oxo clusters with good solubility in spin-coating solvents, high sensitivity, and good thermal stability, all of which are crucial for the resolution targets in EUVL.
Thermal stability
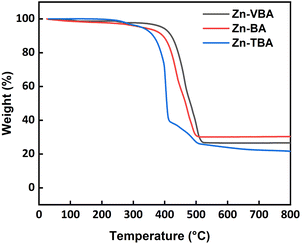
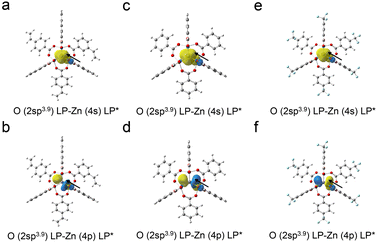
Thermal stability is a key factor that determines the performance of photoresist materials during the baking steps, which is important for film formation, reactions using light irradiation, and final pattern quality.31 Low thermal stability limits the application of photoresist materials in the photolithography process. Thermogravimetric analysis (TGA) was performed to investigate the thermal stability of the zinc–oxo clusters (Fig. 2). No obvious mass loss was observed at temperatures below 300 °C. The initial loss of 5 wt% for the three clusters occurred in the following order: Zn–VBA, 392 °C > Zn–BA, 327 °C > Zn–TBA, 326 °C. All of them exhibited much better thermal stability than the reported zinc–oxo clusters containing aliphatic acid ligands which showed T95 generally below 200 °C. Further increasing the temperature induced loss of organic ligands, which were completely decomposed at >500 °C and left the ZnO inner core. Moreover, the weight ratios of residues were determined to be 26.6%, 21.6%, and 30.4% for Zn–VBA, Zn–TBA, and Zn–BA, respectively, which corresponds to the calculated results.The natural bond orbitals (NBO)32 were calculated and analyzed (Table 1) to study the chemical bonding properties in the three clusters. It was revealed that Zn and outer O atoms are mainly bound by coordination bonds, whereas the others are bound by covalent bonds. Therefore, it can be assumed that the coordination bonds should be broken upon heating; that is, the thermal stability of the molecule depends on the stability of the coordination bond between Zn and O. The second-order perturbation stabilization energy ΔE(2) between the natural bond electron donor O atom and the electron acceptor Zn atom, which is used to evaluate their interaction intensities, was further analyzed. The larger the ΔE(2) value, the stronger the donor–acceptor interaction. Since the six peripheral ligands in the molecule have identical and symmetrical structures, only the analysis results of one of them are listed in Table 1. The schematic contours based on NBO analysis are shown in Fig. 3a–f.
| Compound | Donor NBOa | Occupancy | Acceptor NBO | Occupancy | ΔE(2) kcal mol−1 | |
|---|---|---|---|---|---|---|
| a NBO (natural bond orbital). b The bold data are the sum of ΔE(2). | ||||||
| Zn–TBA | O (2sp3.9) LP | 1.931 | Zn (4s) LP* | 0.296 | 29.24 | 50.1 |
| O (2sp3.9) LP | 1.931 | Zn (4p) LP* | 0.098 | 20.81 | ||
| Zn–BA | O (2sp3.9) LP | 1.838 | Zn (4s) LP* | 0.296 | 29.30 | 52.3 |
| O (2sp3.9) LP | 1.838 | Zn (4p) LP* | 0.121 | 23.01 | ||
| Zn–VBA | O (2sp3.9) LP | 1.838 | Zn (4s) LP* | 0.296 | 29.31 | 55.9 |
| O (2sp3.9) LP | 1.838 | Zn (4p) LP* | 0.121 | 26.56 | ||
Taking the Zn–TBA cluster as an example, the lone pair electrons of the approximate sp3 hybrid orbital of O are found to be transferred to the unoccupied 4s and 4p orbitals of Zn. The total stabilization energy for second-order perturbation is calculated to be 50.1 kcal mol−1. Similar coordination effects also exist in Zn–BA and Zn–VBA clusters. However, the stabilization energy for second-order perturbation is slightly higher than that of Zn–TBA, indicating stronger coordination effects. Because the O atom of the ligand participates in the π-conjugation of the benzene ring when a strong electron withdrawing group of CF3 locates in its para position, as shown in Zn–TBA, the electron donating capability of O is reduced, and thus the coordination bond weakens. In contrast, the presence of a contraposition vinyl group enhanced the electron donating capability of O in Zn–VBA. According to the above calculation results, the strength of the Zn–O coordination bond in three molecules decreases in the following order: Zn–VBA > Zn–BA > Zn–TBA. This result is consistent with the experimental observations of TGA.
Solubility and film-forming ability
Solubility is crucial for the performance of photoresist materials in the film-forming and developing processes of lithography. The solubility of Zn–VBA was tested in solvents with different parameters, such as dispersion, polarity, and hydrogen-bonding capability. As shown in Table S3,† Zn–VBA has good solubility in common organic solvents such as acetone, cyclohexanone, ethyl lactate, toluene, PGMEA, and propylene glycol monomethyl ether. On the other hand, Zn–VBA is almost insoluble in tert-butanol, ethanol, diethylene glycol, and water.The spin-coating method was then used to further study the film-forming ability of Zn–VBA in its “good” solvents, including PGMEA, cyclohexanone, and ethyl lactate. The morphology of the spin-coated films was initially evaluated by atomic force microscopy (AFM) (Fig. 4a–d and Table 2). Uniform films were formed at concentrations of 10–30 mg mL−1, and the thickness was determined to be 30–40 nm (Fig. 4a–d). The roughness (Rq), an important parameter to evaluate the film quality, was quantified as 0.20, 0.28, 0.24, and 0.33 nm for the four conditions, indicating good film-forming properties in these solvents. Furthermore, although Zn–VBA showed good solubility and film formation in PGMEA (Fig. 4c), it was found that the Rq value increased when the concentration of Zn–VBA in PGMEA solution was increased from 20 to 30 mg mL−1. High concentrations lead to the formation of precipitates, as reflected by the bright spot observed in the AFM image (Fig. 4d).
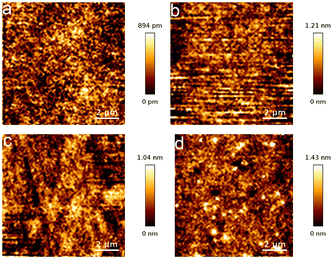 | ||
| Fig. 4 AFM images of Zn–VBA under the film-formation conditions a–d shown in Table 2. | ||
Storage stability
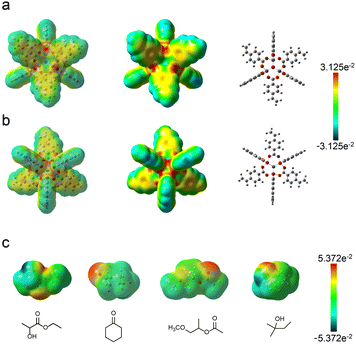
In general, polymer-based photoresist materials should be stored at optimal temperatures and protected from light illumination to avoid particle formation and photoactive compound loss, which would result in materials that were no longer capable of performing specific functions. The same requirements apply to inorganic–organic photoresist materials with particle features that tend to congregate into larger clusters. Such a conglomerate phenomenon is highly related to solvent properties. The storage stability of Zn–VBA was investigated in solvents such as cyclohexanone, ethyl lactate, and PGMEA. After one week of storage at 25 °C, a large number of insoluble substances precipitated in the ethyl lactate solution, whereas the other two solutions remained nearly transparent (Fig. S4†).The electrostatic interactions between Zn–VBA and solvent molecules, such as cyclohexanone, ethyl lactate, PGMEA, and tert-butanol, were investigated to elucidate the influence of solute–solvent interactions on the storage stability of Zn–VBA. The electrostatic potential (ESP) of Zn–VBA (Fig. 5a and b) and different solvent molecules (Fig. 5c) were calculated. The red area is negative and indicates nucleophilicity; the blue area is positive and represents electrophilicity; and the green area is neutral.
The ESP results revealed that the inner inorganic core showed nucleophilic activity. However, the inner core was unable to contact solvent molecules. Unlike the inner core, the peripheral organic ligands showed neutral and weak electrophilicity. Regarding the various solvents, the ESP results indicated that ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in PGMEA and cyclohexanone showed nucleophilic behavior, while tert-butanol showed electrophilic behavior owing to the presence of –OH. Ethyl lactate, in particular, showed dual electrostatic interaction because of the uniqueness of its structure (containing both
O in PGMEA and cyclohexanone showed nucleophilic behavior, while tert-butanol showed electrophilic behavior owing to the presence of –OH. Ethyl lactate, in particular, showed dual electrostatic interaction because of the uniqueness of its structure (containing both ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –OH). In addition, the hydroxyl groups in the two solvent molecules can form intermolecular hydrogen bonds between the solvent molecules, which weakened the electrostatic interaction with Zn–VBA and caused the Zn–VBA to precipitate.
O and –OH). In addition, the hydroxyl groups in the two solvent molecules can form intermolecular hydrogen bonds between the solvent molecules, which weakened the electrostatic interaction with Zn–VBA and caused the Zn–VBA to precipitate.
During the dissolution process, the solvent molecules (cyclohexanone, PGMEA, and ethyl lactate containing ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) are in constant contact with the solute molecules, exhibiting a dynamic behavior under violent stirring. Thus, the Zn–VBA dissolves under the driving force of electrostatic interaction. However, owing to the strong hydrogen bonds formed between the solvent molecules in ethyl lactate, solute molecules (Zn–VBA) precipitated after standing for a while, whereas the cyclohexanone and PGMEA solutions are relatively stable. It is because of the presence of –OH in ethyl lactate, which makes
O) are in constant contact with the solute molecules, exhibiting a dynamic behavior under violent stirring. Thus, the Zn–VBA dissolves under the driving force of electrostatic interaction. However, owing to the strong hydrogen bonds formed between the solvent molecules in ethyl lactate, solute molecules (Zn–VBA) precipitated after standing for a while, whereas the cyclohexanone and PGMEA solutions are relatively stable. It is because of the presence of –OH in ethyl lactate, which makes ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O a better hydrogen-bond acceptor. Hence, the broken hydrogen bonds were restored, as evidenced by the redissolution of Zn–VBA into ethyl lactate following heating or ultrasonic treatment. The electrostatic interaction also accounted for the poor solubility of Zn–VBA in tert-butanol owing to the presence of only –OH. Based on the dissolution and precipitation behaviours, the comparative storage stability of Zn–VBA in different solvents was as follows: cyclohexanone = PGMEA > ethyl lactate.
O a better hydrogen-bond acceptor. Hence, the broken hydrogen bonds were restored, as evidenced by the redissolution of Zn–VBA into ethyl lactate following heating or ultrasonic treatment. The electrostatic interaction also accounted for the poor solubility of Zn–VBA in tert-butanol owing to the presence of only –OH. Based on the dissolution and precipitation behaviours, the comparative storage stability of Zn–VBA in different solvents was as follows: cyclohexanone = PGMEA > ethyl lactate.
Lithography
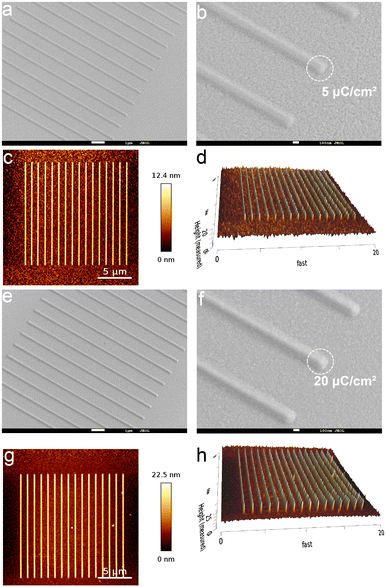
Based on the above analysis of the structure–property relationship of the obtained zinc–oxo cluster (Zn–VBA), we investigated its potential as resists for EBL and EUVL. The energy absorption processes between EUVL and EBL are different; however, both techniques generate low-energy secondary electrons in photoresists through ionization, which are responsible for chemical reactions.33–41 The detailed experimental setups of pre-expose preparation are provided in the ESI.†To evaluate the sensitivity of the Zn–VBA, the normalized resist thickness curve was measured in the form of step exposure by EBL because it can produce high-energy electrons during radiation to induce chemical reactions for lithography patterning (Fig. 6a). The exposure dose was varied from 0.2× to 8.0× with an increment of 0.2× at the initial dose of 5 μC cm−2, forming forty 1 μm square boxes on the silicon wafer because of the negative tone character of Zn–VBA photoresist (Fig. 6b–d). It was found that the contrast (γ) of Zn–VBA resist is 1.4. At the e-beam energy of 30 keV, the sensitivity was calculated to be 20 μC cm−2, corresponding to the required energy to pattern the resist.42
 | ||
| Fig. 6 (a) Sensitivity contrast curve of Zn–VBA resist at different exposure doses (1–40 μC cm−2) and (b) top view, (c) oblique view of AFM image, and (d) FE-SEM image of Zn–VBA patterns. | ||
The interaction between the beam cross section and the resist film demonstrated that the high-energy electron beam induced a large penetration depth into the substrate.43 Thus, 30 keV electron beam was chosen instead of the usual 5 keV or 10 keV for EBL test. A field-emission scanning electron microscope (FE-SEM) was used for surface morphological analysis of high-resolution patterned samples at the doses of 5 μC cm−2 (Fig. S5a, b,†7a and b) and 20 μC cm−2 (Fig. S5c, d,†7e and f). To better present the topographical information of the Zn–VBA resist, FE-SEM images were measured by a 45° turntable rotation in the Z-axis direction, as shown in Fig. 7a, b, e and f. Exposure doses of 5 and 20 μC cm−2 produced the same 200 nm L/4S resolution; however, remaining at the dose of 20 μC cm−2. The difference was clearly observed in the corresponding AFM images (Fig. 7c, d, g and h) and the section profiles of the AFM images (Fig. S6a and b†). Interestingly, the sensitivity of Zn–VBA is higher than that of most reported negative e-beam resists.
The ligands in the cluster largely determined the patterning properties. The patterning performance between Zn–VBA (with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and Zn–TBA (without C
C) and Zn–TBA (without C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) was evaluated by EBL to clarify the role of ligands during radiolysis (Fig. S7a and b†). The exposed patterns showed 100 nm L/2S resolution for both Zn–VBA and Zn–TBA with no bridging or buckling. However, the sensitivity of Zn–VBA (20 μC cm−2) is an order of magnitude higher than that of Zn–TBA (550 μC cm−2). Zn–VBA and Zn–TBA show different reaction mechanisms in the EBL test. Upon e-beam exposure, the zinc–oxo clusters are ionized, resulting in the emission of a photoelectron and the formation of a radical cation, which can further induce decarboxylation of the peripheral ligands. Then the resulting Zn-sites occur in oxidative/hydrolysis reaction and further aggregate with the neighboring clusters to form Zn–O analogs when the film is exposed to the ambient atmosphere after e-beam exposure. For Zn–VBA, in addition to the decarboxylation reaction, it undergoes an extra reaction where the terminal double bonds in the 4-vinylbenzoic acid ligands are polymerized to form a network due to the presence of highly reactive radical to initiate the polymerization reaction.27,44–48 This is the possible reason for the higher sensitivity of Zn–VBA than that of Zn–TBA. Because of the excellent e-beam performance of Zn–VBA, the lithographic performance under EUVL was further explored. The obtained Zn–VBA film was exposed to EUV and then developed with MIBK
C) was evaluated by EBL to clarify the role of ligands during radiolysis (Fig. S7a and b†). The exposed patterns showed 100 nm L/2S resolution for both Zn–VBA and Zn–TBA with no bridging or buckling. However, the sensitivity of Zn–VBA (20 μC cm−2) is an order of magnitude higher than that of Zn–TBA (550 μC cm−2). Zn–VBA and Zn–TBA show different reaction mechanisms in the EBL test. Upon e-beam exposure, the zinc–oxo clusters are ionized, resulting in the emission of a photoelectron and the formation of a radical cation, which can further induce decarboxylation of the peripheral ligands. Then the resulting Zn-sites occur in oxidative/hydrolysis reaction and further aggregate with the neighboring clusters to form Zn–O analogs when the film is exposed to the ambient atmosphere after e-beam exposure. For Zn–VBA, in addition to the decarboxylation reaction, it undergoes an extra reaction where the terminal double bonds in the 4-vinylbenzoic acid ligands are polymerized to form a network due to the presence of highly reactive radical to initiate the polymerization reaction.27,44–48 This is the possible reason for the higher sensitivity of Zn–VBA than that of Zn–TBA. Because of the excellent e-beam performance of Zn–VBA, the lithographic performance under EUVL was further explored. The obtained Zn–VBA film was exposed to EUV and then developed with MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA = 1
IPA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
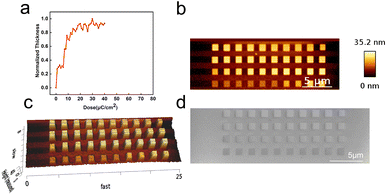
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The FE-SEM images showed excellently dense patterns at 92.5 mJ cm−2 with 37.5 nm 3L/5S resolution and no bridging or residues in the unexposed areas (Fig. 8a). However, the patterns showed bridging or buckling for other pitches (Fig. 8b and S8†). These results indicate that the Zn–VBA resist exhibits great potential for application in EUV and can be a strong candidate for the next generation of photolithographic materials.
3. The FE-SEM images showed excellently dense patterns at 92.5 mJ cm−2 with 37.5 nm 3L/5S resolution and no bridging or residues in the unexposed areas (Fig. 8a). However, the patterns showed bridging or buckling for other pitches (Fig. 8b and S8†). These results indicate that the Zn–VBA resist exhibits great potential for application in EUV and can be a strong candidate for the next generation of photolithographic materials.
 | ||
| Fig. 8 Zn–VBA resist patterned by EUV lithography (92.5 mJ cm−2) performed with different pitches: (a) 100 nm, (b) 75 nm. | ||
Conclusions
In this study, we presented a novel zinc–oxo cluster, Zn–VBA, which contains the [Zn4O]6+ as the inner core and six 4-vinylbenzoic acids as a component of the organic shell. Zn–VBA shows a size of 2.2 nm, meeting the high-resolution requirements for advanced lithography technology. It is proved that Zn–VBA solves the problem of low purity of zinc–oxo clusters used in photoresists while also being easy to synthesize with good batch stability. Zn–VBA also showed high thermal stability and good solubility in organic solvents such as cyclohexanone and PGMEA. Moreover, Zn–VBA was fabricated into a uniform thin film with extremely low roughness using the spin-coating method. The obtained Zn–VBA films performed well in the EBL and EUVL tests without additives, inspiring future exploration of MOCs resist materials for advanced lithography.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, 21925802, 22090011, 22008024), the National Key Research and Development Plan (2018AAA0100301), Key R&D Program of Shandong Province (2021CXGC010308), and the Fundamental Research Funds for China Central Universities (DUT22LAB601 and DUT21ZD407).Notes and references
- J. Liu, H. Guo, M. Li, C. Zhang, Y. Chu, L. Che, Z. Zhang, R. Li, J. Sun and Y. Lu, J. Mater. Chem. A, 2021, 9, 4262–4272 RSC.
- Z.-S. Wu, K. Parvez, X. Feng and K. Müllen, J. Mater. Chem. A, 2014, 2, 8288–8293 RSC.
- F. Luo, L. J. Heyderman, H. H. Solak, T. Thomson and M. E. Best, Appl. Phys. Lett., 2008, 92, 102505 CrossRef.
- A. Vaida, D. Christian, F. Patrick, A. G. Detlev, J. H. Laura, L. Feng, O. Sven, P. Celestino, S. Pratap, T. Tom, T. Audrey, D. Christian and H. S. Harun, J. Micro/Nanolithogr., MEMS, MOEMS, 2009, 8, 021204 CrossRef.
- Z. Wang, X. Yao, H. An, Y. Wang, J. Chen, S. Wang, X. Guo, T. Yu, Y. Zeng, G. Yang and Y. Li, J. Microelectron. Manuf., 2021, 4, 21040101 CAS.
- X. Guo, G. Yang and Y. Li, Laser Optoelectron. Prog., 2022, 59, 0922004 CrossRef.
- L. Li, X. Liu, S. Pal, S. Wang, C. K. Ober and E. P. Giannelis, Chem. Soc. Rev., 2017, 46, 4855–4866 RSC.
- T. Rath, C. Padeste, M. Vockenhuber, C. Fradler, M. Edler, A. Reichmann, I. Letofsky-Papst, F. Hofer, Y. Ekinci and T. Griesser, J. Mater. Chem. A, 2013, 1, 11135–11140 RSC.
- S. Hu, J. Chen, T. Yu, Y. Zeng, X. Guo, S. Wang, G. Yang and Y. Li, J. Photochem. Photobiol., A, 2023, 436, 114351 CrossRef CAS.
- S. Kawakami, T. Onitsuka, Y. A. Kamei, S. Shimura and C. Ha Park, Proc. SPIE, 2022, 12055, 120550N Search PubMed.
- S. Hu, J. Chen, T. Yu, Y. Zeng, S. Wang, X. Guo, G. Yang and Y. Li, J. Mater. Chem. C, 2022, 10, 9858–9866 RSC.
- Y. Wang, L. Chen, J. Yu, X. Guo, S. Wang and G. Yang, R. Soc. Open Sci., 2021, 8, 202132 CrossRef CAS PubMed.
- H.-T. Oh, K. Kim, B.-G. Park, S. Lee and J.-K. Lee, Proc. SPIE, 2020, 11326, 113260B Search PubMed.
- J. Chen, Q. Hao, S. Wang, S. Li, T. Yu, Y. Zeng, J. Zhao, S. Yang, Y. Wu, C. Xue, G. Yang and Y. Li, ACS Appl. Polym. Mater., 2019, 1, 526–534 CrossRef CAS.
- X. Peng, Y. Wang, J. Xu, H. Yuan, L. Wang, T. Zhang, X. Guo, S. Wang, Y. Li and G. Yang, Macromol. Mater. Eng., 2018, 303, 1700654 CrossRef.
- H. Xu, K. Sakai, K. Kasahara, V. Kosma, K. Yang, H. C. Herbol, J. Odent, P. Clancy, E. P. Giannelis and C. K. Ober, Chem. Mater., 2018, 30, 4124–4133 CrossRef CAS.
- N. Thakur, L.-T. Tseng, M. Vockenhuber, Y. Ekinci and S. Castellanos, J. Micro/Nanolithogr., MEMS, MOEMS, 2019, 18, 043504 CAS.
- B. Cardineau, R. Del Re, M. Marnell, H. Al-Mashat, M. Vockenhuber, Y. Ekinci, C. Sarma, D. A. Freedman and R. L. Brainard, Microelectron. Eng., 2014, 127, 44–50 CrossRef CAS.
- D. Wang, X. Yi and L. Zhang, Sci. China: Chem., 2022, 65, 114–119 CrossRef CAS.
- X. Yi, D. Wang, F. Li, J. Zhang and L. Zhang, Chem. Sci., 2021, 12, 14414–14419 RSC.
- Y. Ekinci, A. M. Brouwer, S. Castellanos, L. Wu, J. Haitjema and R. Fallica, J. Micro/Nanolithogr., MEMS, MOEMS, 2018, 17, 023505 Search PubMed.
- N. Thakur, R. Bliem, I. Mochi, M. Vockenhuber, Y. Ekinci and S. Castellanos, J. Mater. Chem. C, 2020, 8, 14499–14506 RSC.
- N. Thakur, M. Vockenhuber, Y. Ekinci, B. Watts, A. Giglia, N. Mahne, S. Nannarone, S. Castellanos and A. M. Brouwer, Proc. SPIE, 2019, 10957, 109570D Search PubMed.
- A. Grebenko, A. Bubis, K. Motovilov, V. Dremov, E. Korostylev, I. Kindiak, F. S. Fedorov, S. Luchkin, Y. Zhuikova, A. Trofimenko, G. Filkov, G. Sviridov, A. Ivanov, J. T. Dull, R. Mozhchil, A. Ionov, V. Varlamov, B. P. Rand, V. Podzorov and A. G. Nasibulin, Adv. Funct. Mater., 2021, 31, 2101533 CrossRef CAS.
- J. Gonzalez-Estrella, J. A. Field, C. K. Ober and R. Sierra-Alvarez, Green Mater., 2019, 7, 109–117 CrossRef.
- S. M. Lewis, M. S. Hunt, G. A. DeRose, H. R. Alty, J. Li, A. Wertheim, L. De Rose, G. A. Timco, A. Scherer, S. G. Yeates and R. E. P. Winpenny, Nano Lett., 2019, 19, 6043–6048 CrossRef CAS PubMed.
- R. Kumar, M. Chauhan, M. G. Moinuddin, S. K. Sharma and K. E. Gonsalves, ACS Appl. Mater. Interfaces, 2020, 12, 19616–19624 CrossRef CAS PubMed.
- K. E. Riley and P. Hobza, Acc. Chem. Res., 2013, 46, 927–936 CrossRef CAS PubMed.
- P. Jena and Q. Sun, Chem. Rev., 2018, 118, 5755–5870 CrossRef CAS PubMed.
- J. Lewinski, W. Bury, M. Dutkiewicz, M. Maurin, I. Justyniak and J. Lipkowski, Angew. Chem., Int. Ed., 2008, 47, 573–576 CrossRef CAS PubMed.
- W.-A. C. Bauer, C. Neuber, C. K. Ober and H.-W. Schmidt, Adv. Mater., 2011, 23, 5404–5408 CrossRef CAS PubMed.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- E. D. Martinez, A. Prado, M. Gonzalez, S. Anguiano, L. Tosi, L. Salazar Alarcon and H. Pastoriza, Front. Mater., 2021, 8, 629792 CrossRef.
- K.-d. Seong, J.-Y. Jung, J. Kang, D.-S. Kim, L. Lyu, S. Seo, J.-H. Kim and Y. Piao, J. Mater. Chem. A, 2020, 8, 25986–25994 RSC.
- N. R. Kiran, M. Chauhan, S. K. Sharma, S. Ghosh and K. E. Gonsalves, ACS Appl. Electron. Mater., 2020, 2, 3805–3817 CrossRef.
- A. S. Gangnaik, Y. M. Georgiev and J. D. Holmes, Chem. Mater., 2017, 29, 1898–1917 CrossRef CAS.
- S. Ghosh, C. P. Pradeep, S. K. Sharma, P. G. Reddy, S. P. Pal and K. E. Gonsalves, RSC Adv., 2016, 6, 74462–74481 RSC.
- L. Li, H. Lu and K. Deng, J. Mater. Chem. A, 2013, 1, 2089–2093 RSC.
- Y. Chen, Microelectron. Eng., 2015, 135, 57–72 CrossRef CAS.
- M. Tu, B. Xia, D. E. Kravchenko, M. L. Tietze, A. J. Cruz, I. Stassen, T. Hauffman, J. Teyssandier, S. De Feyter, Z. Wang, R. A. Fischer, B. Marmiroli, H. Amenitsch, A. Torvisco, M. d. J. Velasquez-Hernandez, P. Falcaro and R. Ameloot, Nat. Mater., 2021, 20, 93–99 CrossRef CAS PubMed.
- Y. Miao, D. T. Lee, M. D. de Mello, M. Ahmad, M. K. Abdel-Rahman, P. M. Eckhert, J. A. Boscoboinik, D. H. Fairbrother and M. Tsapatsis, Nat. Commun., 2022, 13, 420 CrossRef CAS PubMed.
- M. Yogesh, M. G. Moinuddin, M. Chauhan, S. K. Sharma, S. Ghosh and K. E. Gonsalves, ACS Appl. Electron. Mater., 2021, 3, 1996–2004 CrossRef CAS.
- Z. Cui, Nanofabrication: Principles, Capabilities and Limits, Springer, New York, 2008 Search PubMed.
- S. K. Sharma, R. Kumar, M. Chauhan, M. G. Moinuddin, J. Peter, S. Ghosh, C. P. Pradeep and K. E. Gonsalves, Proc. SPIE, 2020, 11326, 1132604 Search PubMed.
- S. K. Sharma, M. Chauhan, R. Kumar, K. Palit, S. Choudhary, K. E. Gonsalves, D. Guerrero and D. P. Sanders, Proc. SPIE, 2021, 11612, 1161208 Search PubMed.
- L. Wu, I. Bespalov, K. Witte, O. Lugier, J. Haitjema, M. Vockenhuber, Y. Ekinci, B. Watts, A. M. Brouwer and S. Castellanos, J. Mater. Chem. C, 2020, 8, 14757–14765 RSC.
- M. E. Krysak, J. M. Blackwell, S. E. Putna, M. J. Leeson, T. R. Younkin, S. Harlson, K. Frasure, F. Gstrein and S. Jose, Proc. SPIE, 2014, 9048, 904805 CrossRef.
- J. Passarelli, M. Murphy, R. Del Re, M. Sortland, L. Dousharm, M. Vockenhuber, Y. Ekinci, M. Neisser, D. A. Freedman and R. L. Brainard, Proc. SPIE, 2015, 9425, 94250T CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2215806 and 2225688. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ta00115f |
| This journal is © The Royal Society of Chemistry 2023 |