Development of rigidity-controlled terpolymer donors for high-performance and mechanically robust organic solar cells†
Jinseck
Kim‡
a,
Geon-U
Kim‡
a,
Dong Jun
Kim‡
b,
Seungjin
Lee
 a,
Dahyun
Jeong
a,
Dahyun
Jeong
 a,
Soodeok
Seo
a,
Seo-Jin
Ko
a,
Soodeok
Seo
a,
Seo-Jin
Ko
 *c,
Sung Cheol
Yoon
*c,
Sung Cheol
Yoon
 *c,
Taek-Soo
Kim
*c,
Taek-Soo
Kim
 *b and
Bumjoon J.
Kim
*b and
Bumjoon J.
Kim
 *a
*a
aDepartment of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea. E-mail: bumjoonkim@kaist.ac.kr
bDepartment of Mechanical Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea. E-mail: tskim1@kaist.ac.kr
cDivision of Advanced Materials, Korea Research Institute of Chemical Technology (KRICT), Daejeon, 34114, Republic of Korea. E-mail: yoonsch@krict.re.kr; sjko927@krict.re.kr
First published on 13th February 2023
Abstract
Organic solar cells (OSCs) are potential power sources for wearable electronic devices. However, the mechanical stretchability of active materials is not yet sufficient; one of the main reasons is the high rigidity of polymer donors (PDs) and the resultant excessive crystalline structures, which makes the active layer mechanically-fragile. In this study, we develop a series of PM6-based terpolymers (PM6-BX; X = 10–30, X indicates the mole percentage of the third component) in which a bulky electro-active third component, 7,8-bis(5-hexylthiophen-2-yl)-11H-benzo[4,5] imidazo[2,1-a]isoindol-11-one (BID), is introduced to reduce the tightness of their molecular packing. As a result, the neat PD film with 10 mol% BID (PM6-B10) exhibits significantly improved mechanical ductility (i.e., average crack onset strain (COSavg) of 23.8%) compared to that of the neat PM6 film (COSavg = 14.9%) without a BID unit. In addition, in terms of a blend film, the well-intermixed domains of PM6-B10 and a small molecule acceptor afford OSCs with a high power conversion efficiency (PCE) of 17.2% and mechanical stretchability (COSavg = 11.4%), outperforming the PM6 counterpart (PCE = 15.8%, COSavg = 2.0%). This study suggests important guidelines for the design of efficient PDs for high-performance, stretchable OSCs.
1. Introduction
Stretchable organic solar cells (OSCs) are considered potential power sources for next-generation, form factor–free, wearable devices because of their unique features, including light weight and intrinsic mechanical flexibility/stretchability.1–5 However, the two most important requirements for the stretchable OSCs, high photovoltaic performance and mechanical robustness, are difficult to achieve at the same time, because these two properties typically present a trade-off relationship.5–8 Especially, state-of-the-art polymer donors (PDs) have highly crystalline features with planar and rigid molecular conformation (e.g., PM6 with 4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene (FBDT) and 1,3-bis(2-ethylhexyl)-5,7-di(thiophen-2-yl)-4H,8H-benzo[1,2-c:4,5-c′]dithiophene-4,8-dione (DTBDD) units) to facilitate π–π intermolecular assembly and enhance electrical properties, however, their mechanical properties in films including tensile properties are poor.9–12In order to enhance the mechanical properties of PDs, the high molecular rigidity of polymer chains should be reduced by increasing the chain flexibility.12–17 A promising strategy to achieve this end is the introduction of flexible spacers through terpolymerization.12,18–20 For example, Thompson et al. reported that a terpolymer (10% T-10-T) with decyl spacers demonstrates an 8-fold increased crack onset strain (COS) than a corresponding polymer without decyl spacers.19 However, the presence of such electro-inactive spacers in PDs often impairs their electrical properties (i.e., charge mobility) due to inhibited intramolecular charge transfer.21,22 Therefore, it is necessary to explore electro-active (i.e., conjugated) spacers to tune the rigidity of polymer chains without compromising their electrical properties.
Accordingly, an effective molecular design strategy for PDs for stretchable OSCs involves the incorporation of bulky electro-active units into the polymer chains, thereby inducing sufficient steric hindrance to reduce the backbone rigidity and enhance the mechanical properties of the PDs.21,22 For example, Bao et al. developed a diketopyrrolopyrrole (DPP)-based terpolymer by incorporating a third component that contains a bulky naphthalene side chain, so that relative degree of crystallinity of the polymer significantly decreased from 1 to 0.37.22 As a result, the COS (∼15%) of the terpolymer considerably exceeded that (∼3%) of the corresponding polymer without the bulky third component. However, although the aforementioned molecular design strategy has been used to enhance the mechanical properties of films based on neat polymers, to the best of our knowledge, this strategy has not yet been explored to enhance the mechanical properties of films based on blends; especially, bulk-heterojunction (BHJ) blends of PDs and small molecule acceptors (SAs). While the mechanical properties of blend films are affected by the mechanical properties of each component (i.e., PD and SA), the morphological properties, which depend on the thermodynamic miscibility between PD and SA, also play an important role to determine both the electrical and mechanical properties of the blends.11,23–31 Thus, for achieving highly efficient and mechanically robust OSCs, it is important to design terpolymer donors containing appropriate bulky electro-active third components by considering their impact on the mechanical properties of the polymers as well as their blend morphology with SAs.
Herein, we report the development of a series of terpolymers (PM6-BX, X = 10–30) incorporating electro-active 7,8-bis(5-hexylthiophen-2-yl)-11H-benzo[4,5]imidazo[2,1-a]isoindol-11-one (BID) units for alleviating the excessive backbone rigidity of PM6. The neat PM6-B10 film shows a significantly higher average COS (COSavg) value of 23.8% than that of the neat PM6 film (14.9%), while showing a comparable hole mobility (μh) to that of PM6. Moreover, the PM6-B10:Y6-BO blend film contains a larger fraction of the intermixed domains than the PM6:Y6-BO blend film, leading to superior charge generation and mechanical properties. As a result, the PM6-B10:Y6-BO OSC achieves a high power conversion efficiency (PCE) of 17.2%, outperforming a PM6:Y6-BO OSC (PCE = 15.8%). In addition, the mechanical properties of the PM6-B10:Y6-BO blend film (COSavg = 11.4% and toughness = 4.1 MJ m−3) are superior to those of the PM6:Y6-BO blend film (COSavg = 2.0% and toughness = 0.3 MJ m−3). We carefully investigate the impact of the electro-active BID addition on the structural, optical, electrical, and mechanical properties of the PM6-BX terpolymers and study the polymer structure–property–device performance relationship in OSCs.
2. Results and discussion
2.1. Material design and terpolymer properties
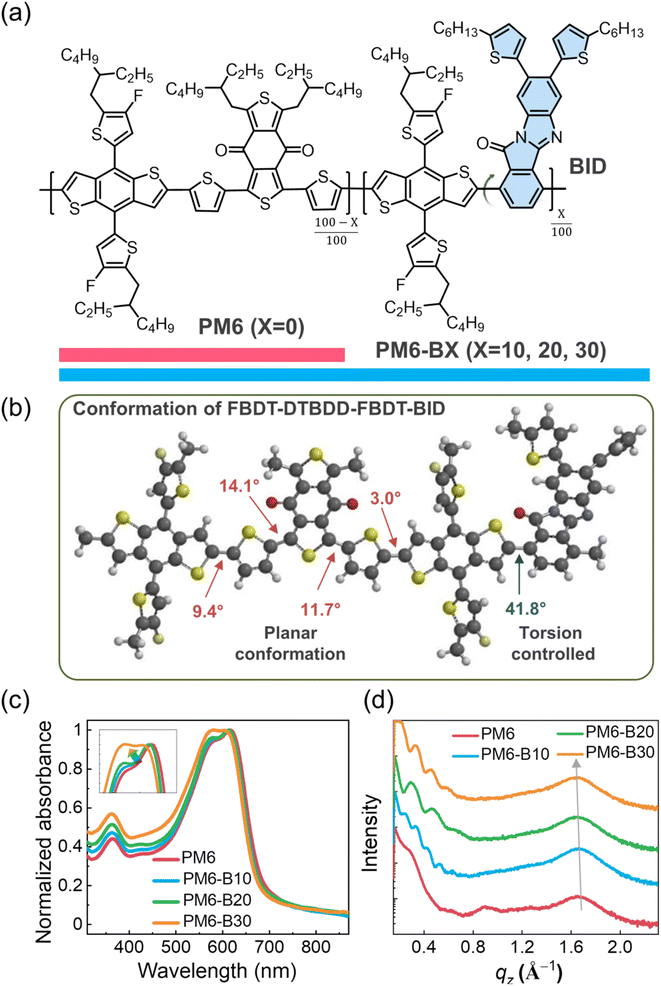
The molecular structures of the PDs featured in this study are described in Fig. 1a. PM6 was selected as a reference PD owing to its wide light absorption range, high absorption coefficient, and superior charge mobility, which enable the construction of highly efficient OSCs.32,33 However, the rigid, planar molecular conformation of PM6 (which consists of highly fused FBDT and DTBDD units) and its consequent strong/tight intermolecular assembly lead to poor stretchability in film.11,34 To reduce the high molecular rigidity of PM6, we employed a terpolymerization strategy, i.e., we incorporated a third component to tune the conformation of the polymer main chain and thus prevent its excessively tight packing.35,36 Here, to avoid any compromise of the electrical properties, we designed a new electro-active third component (BID unit) for constructing terpolymers. In general, bulky units have been shown to effectively adjust the torsion angle of polymers and induce higher steric hindrance than less bulky units. This steric hindrance manipulates the intermolecular packing of polymers, thereby enhancing the mechanical properties in film.22,37 In addition, the designed electro-active BID unit preserves good electrical and optical properties better than other reported flexible spacers.18,20,21,38–41 A series of terpolymers with different BID contents (10 − 30 mol%) was synthesized in this study to simultaneously optimize the electrical and mechanical properties of the terpolymers.The synthetic scheme and characterization data of the BID monomer (BID-Br) and PDs are provided in the ESI (Fig. S1–S4 and Table S1†). The BID monomer was synthesized via a condensation reaction between dibromophthalic anhydride and alkyl thiophene substituted o-phenylenediamine (Fig. S1†). The PDs were polymerized via a Stille-coupling reaction and purified by sequential Soxhlet extraction. The molecular structures of the PDs were verified by nuclear magnetic resonance (NMR) spectroscopy (Fig. S2†). The presence of the BID units in the terpolymers is confirmed by the characteristic peaks with a chemical shift of 2.7–2.8 ppm in the obtained NMR spectra, which correspond to the protons attached to the α-carbons of the thiophenes in BID. Although these peaks partially overlap with those corresponding to protons attached to the α-carbons of the side-chain thiophenes in FBDT, the increase in intensity of these characteristic peaks in the NMR spectra is consistent with the increasing BID contents of the terpolymers. The number-average molecular weights (Mns) of the PDs, estimated by gel permeation chromatography (GPC), are similar (127–144 kg mol−1); thus, the effect of molecular weight on the optoelectronic/physical properties of the PDs can be disregarded (Fig. S3† and Table 1). In addition, PM6 and the terpolymers are acceptably processable in chlorinated solvents, which are generally used to fabricate OSCs (Fig. S4 and Table S1†). The solubility test procedures are detailed in the ESI.†41
| PDs | M n (Đ)a [kg mol−1] | λ maxfilm [nm] | E optg [eV] | E HOMO [eV] | E LUMO [eV] | μ SCLCh (×10−4) [cm2 V−1 s−1] |
|---|---|---|---|---|---|---|
| a Estimated by GPC using 1,2,4-trichlorobenzene as eluent. b Determined from UV-Vis absorption spectra of the PDs in film. c Determined by cyclic voltammetry. d Calculated using the equation ELUMO = EHOMO + Eoptg. | ||||||
| PM6 | 144 (3.66) | 615 | 1.83 | −5.53 | −3.70 | 3.5 ± 0.6 |
| PM6-B10 | 137 (3.21) | 614 | 1.83 | −5.55 | −3.72 | 3.2 ± 0.4 |
| PM6-B20 | 133 (3.20) | 613 | 1.83 | −5.56 | −3.73 | 2.3 ± 0.7 |
| PM6-B30 | 127 (2.96) | 578 | 1.84 | −5.56 | −3.72 | 1.6 ± 0.7 |
Density functional theory (DFT) calculations, at the B3LYP/6-31G(d,p) level, were performed to investigate the structural conformations of the PDs (Fig. 1b and S5†). To simplify the DFT calculations, the length of each polymer backbone was shortened to the dimer level and the alkyl chains were represented by methyl groups. According to the calculations, the incorporation of BID in the PM6 backbone effectively modifies its molecular conformation; the largest torsional (or dihedral) angle of PM6 is ∼16° whereas that of the PM6-based terpolymers is ∼42°. Steric effects between the bulky BID units and the adjacent FBDT units in the terpolymers reduce their planarity. Consequently, excessively tight packing of PM6 is expected to be alleviated as the content of the BID unit increases in terpolymers. The suitable torsion and following mitigated polymer packing tend to afford films with enhanced ductility.22
The optical characteristics of the PDs were investigated (Fig. 1c, S6,† and Table 1). With increasing BID content, the UV-Vis absorption spectra of the PD films were blue-shifted and the ratios of the 0–0 transition peak intensity (located at ∼615 nm) to the 0–1 transition peak intensity (located at ∼575 nm) decreased (Fig. 1c).42 These results indicate that the molecular packing of the terpolymers was less tight than that of PM6, as expected based on the DFT calculations (Fig. 1b and S5†). To further examine the effect of the BID content on the aggregation behaviors of the PDs, we obtained their temperature-dependent UV-Vis absorption in solution (Fig. S6†). The pre-aggregation behaviors of PDs play an important role in determining the thin-film morphology and performance of the resulting OSCs.43,44 In the case of PM6, the 0–0 transition peak remained more prominent compared to the 0–1 transition peak across the entire temperature range, indicating strong pre-aggregation behaviors.43 Interestingly, the temperature-dependent pre-aggregation (TDA) behaviors of PM6-B10 and PM6-B20 were similar to that of PM6. These terpolymers may preserve short-range ordered aggregation in solution, which can facilitate charge carrier transport in thin film.43,45 In contrast, in the case of PM6-B30 with its relatively high BID content, the intensity of the 0–0 transition peaks in the UV-Vis absorption spectra gradually decreased with increasing solution temperature, suggesting relatively weak pre-aggregation behaviors.
The electrochemical properties of the PDs were investigated by cyclic voltammetry (CV) (Fig. S7†). The highest occupied molecular orbital (HOMO) energy levels of the terpolymers were slightly lower than that of PM6; i.e., the HOMO energy levels of PM6, PM6-B10, PM6-B20, and PM6-B30 were −5.53, −5.55, −5.56, and −5.56 eV, respectively. The substitution of the DTBDD units in PM6 with BID units reduces the number of electron-donating thiophenes, which may partly account for the lower HOMO energy levels of the terpolymers.46
The crystalline properties of the PDs in film were investigated by grazing incidence wide-angle X-ray scattering (GIXS) (Fig. 1d, S8, and Table S2†). The obtained GIXS linecut profiles in the in-plane (IP) and out-of-plane (OOP) directions are shown in Fig. 1d and S8,† respectively. Distinct (100) and (010) peaks in the GIXS linecut profiles in the IP and OOP directions, respectively, suggest that all the PDs have dominant face-on molecular packing orientation, which facilitates vertical charge transport.47,48 Meanwhile, the crystallinity of the PDs decreased with increasing BID content owing to an increase in the torsion of the polymer backbone, which is consistent with the DFT calculations and the results of UV-Vis absorption spectroscopy. For example, the coherence length (Lc(010)), estimated using the Scherrer equation, of the PDs decreased from 27.4 Å for PM6 to 24.8 Å for PM6-B30 (Table S2†).49 In addition, the π–π stacking distance (d(010)) of the PDs slightly increased from 3.82 Å for PM6 to 3.85 Å for PM6-B30 (Fig. 1d and Table S2†). To determine the correlation between the crystalline and electrical properties of the PDs, their μhs were measured using the space-charge limited current (SCLC) method (Table 1).50 The μh of PM6-B10 (3.2 × 10−4 cm2 V−1 s−1) was comparable to that of PM6 (3.5 × 10−4 cm2 V−1 s−1); however, that of PM6-B30 (1.6 × 10−4 cm2 V−1 s−1) was lower.
2.2. Photovoltaic and electrical properties
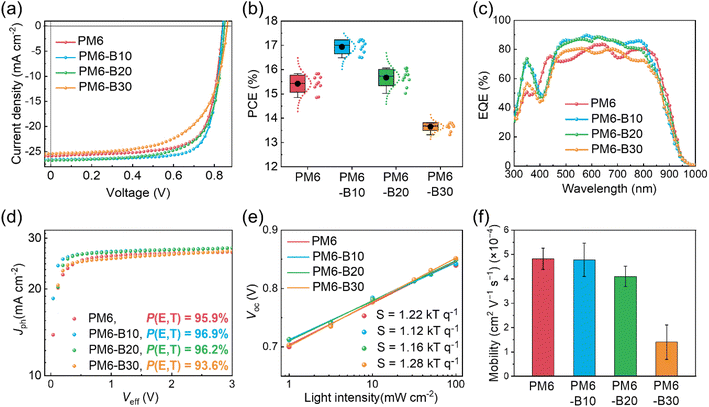
The photovoltaic performance of the PDs developed in this study was evaluated in normal-type OSCs with a configuration of indium tin oxide (ITO)/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS)/active layer/2,9-bis(3-((3-(dimethylamino)propyl)amino)propyl)anthra[2,1,9-def:6,5,10-d′e′f′]diisoquinoline-1,3,8,10-(2H,9H)-tetraone (PDINN)/Ag.51 The SA, 2,2′-((2Z,2′Z)-((12,13-bis(2-butyloctyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2′,3′:4′,5′]thieno[2′,3′:4,5]pyrrolo [3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (Y6-BO) was paired with the PDs in OSCs owing to its complementary light-absorption properties and appropriately aligned frontier orbital energy levels (Fig. S9†).52 The current density–voltage (J–V) curve, PCE distribution, and external quantum efficiency (EQE) spectrum of each OSC are displayed in Fig. 2a–c. The procedures for OSC fabrication and characterization are described in the ESI.† The photovoltaic parameters (open-circuit voltage (Voc), short-circuit current density (Jsc), fill factor (FF), and PCE) of the OSCs are listed in Table 2. Interestingly, the PCEs of PM6-B10:Y6-BO (Voc = 0.84 V, Jsc = 26.60 mA cm−2, FF = 0.77, and PCE = 17.23%) and PM6-B20:Y6-BO (Voc = 0.85 V, Jsc = 26.72 mA cm−2, FF = 0.71, and PCE = 16.06%) OSCs exceeded that of the PM6:Y6-BO OSC (Voc = 0.84 V, Jsc = 25.85 mA cm−2, FF = 0.73, and PCE = 15.83%; Table 2). In contrast, the PCE of PM6-B30:Y6-BO OSC (Voc = 0.86 V, Jsc = 25.42 mA cm−2, and FF = 0.63, PCE = 13.80%) was lower than that of the PM6:Y6-BO OSC. These results indicate that the incorporation of an optimal amount of BID in PM6 can afford OSCs with enhanced Jsc, FF, and PCE. And, the PM6-B10:Y6-BO and PM6-B20:Y6-BO OSCs showed enhanced EQE values in the absorption ranges of PD and SA (e.g., 300–400 and 500–800 nm) than those of the PM6:Y6-BO OSC (Fig. 2c). The Jsc calculated from the EQE spectrum of each OSC agreed well with its measured Jsc (within an error of 4%).| PDs | V oc [V] | J sc [mA cm−2] | Calc. Jsca [mA cm−2] | FF | PCEmax (PCEavg)b [%] |
|---|---|---|---|---|---|
| a Calculated from the EQE spectra. b Average values based on at least 10 devices. | |||||
| PM6 | 0.84 | 25.85 | 25.75 | 0.73 | 15.83 (15.42 ± 0.35) |
| PM6-B10 | 0.84 | 26.60 | 26.81 | 0.77 | 17.23 (16.94 ± 0.28) |
| PM6-B20 | 0.85 | 26.72 | 26.53 | 0.71 | 16.06 (15.67 ± 0.34) |
| PM6-B30 | 0.86 | 25.42 | 24.34 | 0.63 | 13.80 (13.65 ± 0.16) |
To demonstrate the advantage of employing the highly fused BID unit as the third component in our terpolymerization strategy, another PD (PM6-P10) was synthesized in which a less fused, more widely used phthalimide unit was incorporated as the third component into PM6. The mole percentage of the phthalimide unit in PM6-P10 was the same as that of BID in PM6-B10 (10 mol%), which exhibited the highest PCE among the BID-based terpolymers (Fig. S10†).8,53–55 For fair comparison, a batch with a molecular weight (Mn = 131 kg mol−1) similar to those of the other PDs was synthesized (Table S3†). The photovoltaic performance of PM6-P10:Y6-BO OSCs (Voc = 0.85 V, Jsc = 25.04 mA cm−2, FF = 0.65, and PCE = 13.77%) was significantly poorer than those of PM6:Y6-BO and PM6-B10:Y6-BO devices (Table S4†), which can be mainly attributed to the inferior μh of PM6-P10 (0.8 × 10−4 cm2 V−1 s−1).8,56 This result confirms the importance of introducing BID unit as the third component of terpolymer donors for achieving high-performance OSCs.
To elucidate the origin of the different photovoltaic performances depending on PDs, the charge generation and recombination properties of the OSCs were investigated (Fig. 2d, e and S11†). First, the exciton dissociation probability (P(E,T)) of each OSC was determined from its photocurrent density (Jph)–effective voltage (Veff) curve, calculated by dividing the Jph under short-circuit conditions by the saturated current density (Jsat) at Veff = 3 V.57 The P(E,T)s of all the OSCs were in the range of 94–97%, indicating efficient exciton dissociation and charge generation; the PM6-B10:Y6-BO OSC exhibited the highest P(E,T) of 97%, which contributed to its superior Jsc (Fig. 2d). The charge recombination properties of the OSCs were investigated by examining their light intensity (Plight)–dependent Voc and Jsc. In general, the slope (S) of Voc–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Plight plot, with unit of kBT/q (kB = Boltzmann constant, T = temperature in Kelvin, and q = elementary charge), and the slope (α) of log
Plight plot, with unit of kBT/q (kB = Boltzmann constant, T = temperature in Kelvin, and q = elementary charge), and the slope (α) of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Jsc–log
Jsc–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Plight plot indicate the degrees of monomolecular/trap-assisted and bimolecular recombination of OSCs, respectively.58–60 The S and α values associated with the PM6-B10:Y6-BO (S = 1.12 kBT/q, α = 0.91) and PM6-B20:Y6-BO (S = 1.16 kBT/q, α = 0.90) OSCs were nearer to unity (S = 1 kBT/q and α = 1) than those of the PM6:Y6-BO OSC (S = 1.22 kBT/q, α = 0.88), suggesting that the degrees of monomolecular/trap-assisted and bimolecular recombination of those OSCs are comparatively low (Fig. 2e and S11†). However, the PM6-B30:Y6-BO OSC showed more severe monomolecular/trap-assisted recombination behavior (S = 1.28 kBT/q) compared to the PM6:Y6-BO OSC, explaining its low Jsc and FF.
Plight plot indicate the degrees of monomolecular/trap-assisted and bimolecular recombination of OSCs, respectively.58–60 The S and α values associated with the PM6-B10:Y6-BO (S = 1.12 kBT/q, α = 0.91) and PM6-B20:Y6-BO (S = 1.16 kBT/q, α = 0.90) OSCs were nearer to unity (S = 1 kBT/q and α = 1) than those of the PM6:Y6-BO OSC (S = 1.22 kBT/q, α = 0.88), suggesting that the degrees of monomolecular/trap-assisted and bimolecular recombination of those OSCs are comparatively low (Fig. 2e and S11†). However, the PM6-B30:Y6-BO OSC showed more severe monomolecular/trap-assisted recombination behavior (S = 1.28 kBT/q) compared to the PM6:Y6-BO OSC, explaining its low Jsc and FF.
To compare the charge transport ability of each blend film, its μh and electron mobility (μe) were measured using the SCLC method (Fig. 2f and Table S5†). The μhs of the PM6:Y6-BO, PM6-B10:Y6-BO, PM6-B20:Y6-BO, and PM6-B30:Y6-BO blend films were (4.8 ± 0.4) × 10−4, (4.8 ± 0.7) × 10−4, (4.1 ± 0.4) × 10−4, and (1.4 ± 0.7) × 10−4 cm2 V−1 s−1, respectively. Interestingly, the μh of the PM6-B10:Y6-BO blend film was comparable to that of the PM6:Y6-BO blend film, showing a well-balanced electron–hole mobility (μe/μh = 1.2). These results indicate that the presence of the electro-active BID units in PM6-B10 does not compromise its electrical properties.61–63 The balanced electron–hole mobility (μe/μh) of the PM6-B10:Y6-BO blend film is consistent with the low degree of charge recombination and high FF in OSCs.
2.3. Morphological properties of blend films
To further elucidate the differences in the photovoltaic performances of the OSCs depending on the PDs, we investigated the morphological characteristics of the PD:Y6-BO blend films by GIXS, resonant soft X-ray scattering (RSoXS), differential scanning calorimetry (DSC), and contact angle measurements. First, we found that the PD:Y6-BO blend films showed similar crystalline properties (Fig. S12 and Table S6†). For example, the PM6 and PM6-BX (X = 10, 20, and 30)-based blend films showed dominantly face-on oriented packing structures, as confirmed by distinct (100) peaks along the IP direction and (010) peaks along the OOP direction in the GIXS linecut profiles. Also, the Lc values of the terpolymer-based blend films were relatively well-maintained (Lc(100) = 6.3 nm and Lc(010) = 2.4 nm for PM6:Y6-BO and Lc(100) = 5.2 nm and Lc(010) = 2.3 nm for PM6-B20:Y6-BO) (Table S6†). Interestingly, a significant difference in the morphological characteristics between PM6 and terpolymers was found in the RSoXS results in terms of domain purity and size of the blend films (Fig. 3a and Table 3).64 The RSoXS profiles were obtained with a beam energy of 285.2 eV to maximize the contrast between the PD and SA. The evaluation of the relative domain purity of the blend films is described in the ESI.†65 The RSoXS profile of the PM6:Y6-BO blend film exhibited a strong scattering peak at q value of 0.010 Å−1 (corresponding to domain spacing of 63 nm, domain spacing = 2π/qpeak). In contrast, the peaks were much less distinct in the RSoXS profiles of the PM6-B10:Y6-BO and PM6-B20:Y6-BO blend films; their relative domain purities were only 0.25 and 0.29, respectively, relative to the PM6:Y6-BO blend film (domain purity = 1.00). These results indicate that the incorporation of the BID unit into PM6 induced better-intermixed domains in the blend film. These results explain the higher P(E,T) values of the PM6-B10:Y6-BO and PM6-B20:Y6-BO OSCs.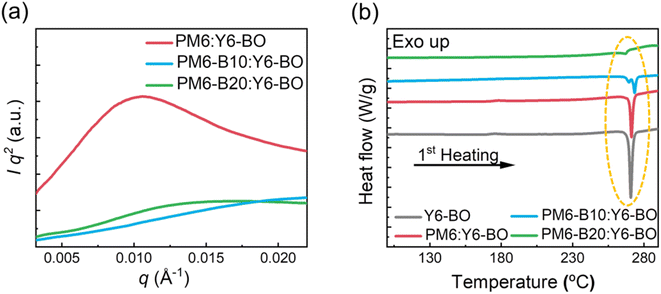 | ||
| Fig. 3 (a) RSoXS profiles of PD:Y6-BO blend films. (b) DSC thermograms of Y6-BO and PD:Y6-BO blend films obtained during the 1st heating cycle. | ||
To support the enhanced intermixing of the PD and SA domains in PM6-B10:Y6-BO and PM6-B20:Y6-BO blend films, DSC measurements were performed (Fig. 3b and Table 3). The blend samples for the DSC measurements were prepared using the same conditions as those used for the OSC fabrication. In detail, the blend solutions were spun-cast onto glass substrates and the films were collected in DSC pans. We then compared the DSC thermograms of the samples obtained during the first heating cycle. The DSC thermograms of all blend films exhibited distinct peaks at 265–275 °C, which are associated with the melting transition of Y6-BO.66,67 The melting enthalpy (ΔHm) of the blend films gradually decreased with increasing BID content of the PD. For example, the ΔHms of PM6:Y6-BO, PM6-B10:Y6-BO, and PM6-B20:Y6-BO blend films were 13.0, 7.9, and 1.5 J g−1, respectively. These results indicate effectively suppressed crystalline features of the PM6-B10:Y6-BO and PM6-B20:Y6-BO blend films compared to those of the PM6:Y6-BO blend films. The DSC data also support the formation of a larger fraction of intermixed PD/SA domains in the PM6-B10:Y6-BO and PM6-B20:Y6-BO blend films than in PM6:Y6-BO.68
To examine the origin of the well-intermixed PD and SA domains in the PM6-B10:Y6-BO and PM6-B20:Y6-BO blend films, we investigated the molecular compatibility of the PDs and Y6-BO by estimating their interfacial tension (γD–A) based on the Wu model (Fig. S13, Tables S7 and S8†).69–71 The water and glycerol contact angles of the PDs and Y6-BO were measured to determine the surface tensions of each material. The γD–A values of the PM6-B10:Y6-BO and PM6-B20:Y6-BO blend films were 0.46 and 0.43 mN m−1, respectively, which were lower than that of the PM6:Y6-BO blend film (0.93 mN m−1). A low γD–A value indicates better molecular compatibility between the PD and SA at their interface, which facilitates intermixing of the domains. Atomic force microscopy (AFM) measurements were also performed to understand the surface topology of each blend film (Fig. S14†). While similar root-mean-square (RMS) roughness (Rq) values of 2.2–2.6 nm were obtained for the PM6:Y6-BO, PM6-B10:Y6-BO, and PM6-B20:Y6-BO blend films, the PM6-B30:Y6-BO blend film exhibited very rough surface (Rq = 7.5 nm). This result was mainly due to large aggregates associated with the low solubility of PM6-B30. Accordingly, PM6-B30 was not further considered in the stretchability test below.
2.4. Mechanical properties of neat and blend films
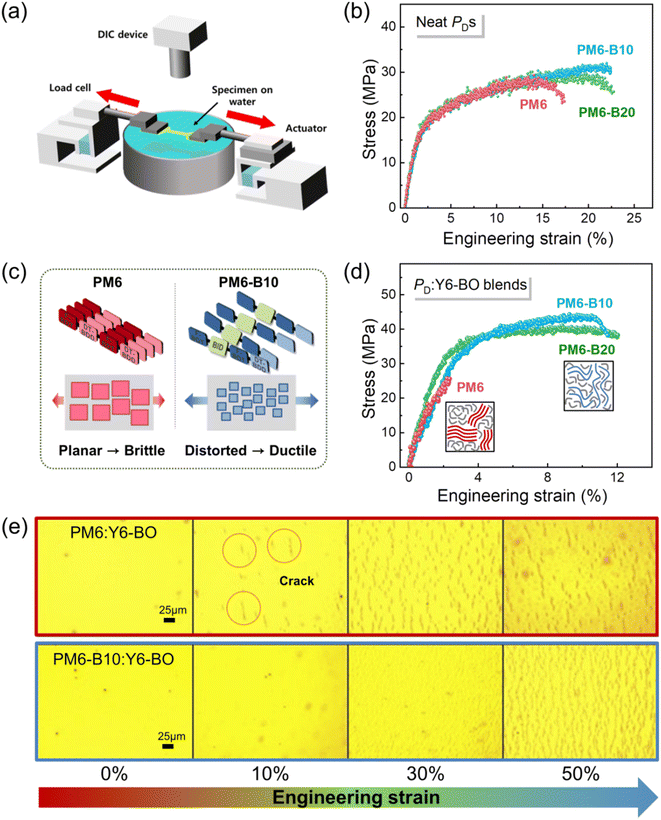
Considering that the molecular packing of PDs can have impact on the mechanical properties of their thin films, we investigated the tensile properties (i.e., COS and toughness) of the PDs in film using a pseudo free-standing tensile test (Fig. 4a).72,73 The pseudo free-standing method can minimize interference between the sample and substrate by floating thin films on frictionless water surface, enabling accurate evaluation of the tensile properties. The COS refers to the strain at which cracks first occur, and the toughness indicates the ability to absorb energy up to fracture. As shown in Fig. 4b, the tensile properties of PM6-B10 and PM6-B20 neat films were superior to those of the PM6 film. For example, the COSavg of PM6-B10 (23.8%) and PM6-B20 (22.7%) were significantly higher than that of PM6 (COSavg = 14.9%) (Fig. 4b and Table S9†). Also, the toughness of the neat films increased from 3.5 MJ m−3 (PM6) to 5.9 MJ m−3 (PM6-B10) and 5.5 MJ m−3 (PM6-B20) by 1.6–1.7 times. These results emphasize that the large plastic deformation of BID-incorporated PD films effectively dissipate a significant amount of strain energy (Fig. 4c). This energy dissipation leads to the prevention of crack generation/propagation in PD films.We also investigated the tensile properties of PM6:Y6-BO, PM6-B10:Y6-BO and PM6-B20:Y6-BO blend films (Fig. 4d and Table S9†), which are influenced by both the blend morphologies and tensile properties of the PD component. As a result, the tensile properties of PM6-B10:Y6-BO and PM6-B20:Y6-BO blend films (COSavg of 11.4 and 12.6%, respectively, and toughness of 4.1 and 4.8 MJ m−3, respectively) were superior to those of PM6:Y6-BO blend film (COSavg = 2.0% and toughness = 0.3 MJ m−3). In particular, the COSavg of the PM6-B10:Y6-BO blend film exceeded that of the PM6:Y6-BO blend film by a factor of five, which is much larger than the difference in the neat PD films. This result can be attributed to the fact that the mechanical properties of the blend films are determined by both the tensile properties of each constituent and their interfacial properties.12,20 For example, superior mechanical properties of PM6-B10:Y6-BO and PM6-B20:Y6-BO blend films are attributed to the synergistic effect of better tensile properties of their terpolymer donors and more developed intermixing of their PD and SA domains that suppresses crack formation/propagation.
Lastly, to demonstrate the potential of the BID-based PDs for stretchable OSC application, the crack formation behavior was monitored while stretching the PD:Y6-BO blend films mounted on the thermoplastic polyurethane (TPU) substrates (Fig. 4e). It is noteworthy that cracks in the PM6-B10:Y6-BO blend film were barely observed during stretching up to 30% strain, whereas the development of cracks was noticeable in the PM6:Y6-BO blend film at only 10% strain. Thus, this result was consistent with the pseudo free-standing tensile test that the PM6-B10:Y6-BO blend film exhibited superior resistance to crack propagation under mechanical stress. The high stretchability of the PM6-B10:Y6-BO blend film on the substrate demonstrates its high feasibility for the application in stretchable OSCs.
3. Conclusions
In this study, we developed a highly efficient and mechanically robust terpolymer donors featuring an electro-active third component (BID). The incorporation of 10 mol% BID in PM6 produced a PM6-B10 with optimized rigidity and crystallinity, leading to superior electrical and mechanical properties in film (μh = 3.2 × 10−4 cm2 V−1 s−1, COSavg = 23.8%, and toughness = 5.9 MJ m−3). Importantly, these beneficial features of PM6-B10 were reflected in its blend with Y6-BO. The PM6-B10:Y6-BO OSC demonstrated a higher PCE (17.2%) than the PM6:Y6-BO OSC (PCE = 15.8%), with improved charge generation and recombination properties. The mechanical properties of the PM6-B10:Y6-BO blend film (COSavg = 11.4% and toughness = 4.1 MJ m−3) were also superior to those of the PM6:Y6-BO blend film (COSavg = 2.0% and toughness = 0.3 MJ m−3) due to the combined contribution of the high ductility of PM6-B10 and enhanced intermixing of PD and SA domains. Therefore, the results of this study demonstrate the effectiveness of incorporating an optimal amount of a bulky electro-active third component into PD for high-performance and mechanically robust OSCs.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by the National Research Foundation of Korea (2017M3A7B8065584, 2020M3D1A2102869 and 2017M3D1A1039553). This research is additionally performed as a cooperation project of “Basic project”, supported by the Korea Research Institute of Chemical Technology (KRICT). The experiment at the Advanced Light Source was supported by a DOE office of Science User Facility under contract no. DE-AC02-05CH11231.References
- A. X. Chen, A. T. Kleinschmidt, K. Choudhary and D. J. Lipomi, Chem. Mater., 2020, 32, 7582–7601 CrossRef CAS.
- E. Dauzon, X. Sallenave, C. Plesse, F. Goubard, A. Amassian and T. D. Anthopoulos, Adv. Mater., 2021, 33, 2101469 CrossRef CAS PubMed.
- R. Ma, S.-Y. Chou, Y. Xie and Q. Pei, Chem. Soc. Rev., 2019, 48, 1741–1786 RSC.
- J. Qin, L. Lan, S. Chen, F. Huang, H. Shi, W. Chen, H. Xia, K. Sun and C. Yang, Adv. Funct. Mater., 2020, 30, 2002529 CrossRef CAS.
- J. S. Park, G.-U. Kim, S. Lee, J.-W. Lee, S. Li, J.-Y. Lee and B. J. Kim, Adv. Mater., 2022, 34, 2201623 CrossRef CAS PubMed.
- A. D. Printz and D. J. Lipomi, Appl. Phys. Rev., 2016, 3, 021302 Search PubMed.
- Z. Ding, D. Liu, K. Zhao and Y. Han, Macromolecules, 2021, 54, 3907–3926 CrossRef CAS.
- P. J. W. Sommerville, Y. Li, B. X. Dong, Y. Zhang, J. W. Onorato, W. K. Tatum, A. H. Balzer, N. Stingelin, S. N. Patel, P. F. Nealey and C. K. Luscombe, Macromolecules, 2020, 53, 7511–7518 CrossRef CAS.
- G. P. Kini, H. S. Park, S. J. Jeon, Y. W. Han and D. K. Moon, Sol. Energy, 2020, 207, 720–728 CrossRef CAS.
- B. Zheng, L. Huo and Y. Li, NPG Asia Mater., 2020, 12, 3 CrossRef CAS.
- K. Zhou, K. Xian, Q. Qi, M. Gao, Z. Peng, J. Liu, Y. Liu, S. Li, Y. Zhang, Y. Geng and L. Ye, Adv. Funct. Mater., 2022, 32, 2201781 CrossRef CAS.
- J.-W. Lee, C. Lim, S.-W. Lee, Y. Jeon, S. Lee, T.-S. Kim, J.-Y. Lee and B. J. Kim, Adv. Energy Mater., 2022, 2202224 CrossRef CAS.
- B. Roth, S. Savagatrup, N. V. de los Santos, O. Hagemann, J. E. Carlé, M. Helgesen, F. Livi, E. Bundgaard, R. R. Søndergaard, F. C. Krebs and D. J. Lipomi, Chem. Mater., 2016, 28, 2363–2373 CrossRef CAS.
- H. You, A. L. Jones, B. S. Ma, G.-U. Kim, S. Lee, J.-W. Lee, H. Kang, T.-S. Kim, J. R. Reynolds and B. J. Kim, J. Mater. Chem. A, 2021, 9, 2775–2783 RSC.
- M. U. Ocheje, M. Selivanova, S. Zhang, T. H. Van Nguyen, B. P. Charron, C.-H. Chuang, Y.-H. Cheng, B. Billet, S. Noori, Y.-C. Chiu, X. Gu and S. Rondeau-Gagné, Polym. Chem., 2018, 9, 5531–5542 RSC.
- Y.-W. Huang, Y.-C. Lin, H.-C. Yen, C.-K. Chen, W.-Y. Lee, W.-C. Chen and C.-C. Chueh, Chem. Mater., 2020, 32, 7370–7382 CrossRef CAS.
- F. Zhao, Y. Yuan, Y. Ding, Y. Wang, X. Wang, G. Zhang, X. Gu and L. Qiu, Macromolecules, 2021, 54, 5440–5450 CrossRef CAS.
- E. L. Melenbrink, K. M. Hilby, K. Choudhary, S. Samal, N. Kazerouni, J. L. McConn, D. J. Lipomi and B. C. Thompson, ACS Appl. Polym. Mater., 2019, 1, 1107–1117 CrossRef CAS.
- E. L. Melenbrink, K. M. Hilby, M. A. Alkhadra, S. Samal, D. J. Lipomi and B. C. Thompson, ACS Appl. Mater., 2018, 10, 32426–32434 CrossRef CAS PubMed.
- J.-W. Lee, D. Jeong, D. J. Kim, T. N.-L. Phan, J. S. Park, T.-S. Kim and B. J. Kim, Energy Environ. Sci., 2021, 14, 4067–4076 RSC.
- D. Liu, J. Mun, G. Chen, N. J. Schuster, W. Wang, Y. Zheng, S. Nikzad, J.-C. Lai, Y. Wu, D. Zhong, Y. Lin, Y. Lei, Y. Chen, S. Gam, J. W. Chung, Y. Yun, J. B. H. Tok and Z. Bao, J. Am. Chem. Soc., 2021, 143, 11679–11689 CrossRef CAS PubMed.
- D. Liu, Y. Lei, X. Ji, Y. Wu, Y. Lin, Y. Wang, S. Zhang, Y. Zheng, Y. Chen, J.-C. Lai, D. Zhong, H.-W. Cheng, J. A. Chiong, X. Gu, S. Gam, Y. Yun, J. B. H. Tok and Z. Bao, Adv. Funct. Mater., 2022, 2203527 CrossRef CAS.
- J.-W. Lee, C. Sun, S.-W. Lee, G.-U. Kim, S. Li, C. Wang, T.-S. Kim, Y.-H. Kim and B. J. Kim, Energy Environ. Sci., 2022, 15, 4672–4685 RSC.
- J.-W. Lee, C. Sun, B. S. Ma, H. J. Kim, C. Wang, J. M. Ryu, C. Lim, T.-S. Kim, Y.-H. Kim, S.-K. Kwon and B. J. Kim, Adv. Energy Mater., 2021, 11, 2003367 CrossRef CAS.
- J.-W. Lee, G.-U. Kim, D. J. Kim, Y. Jeon, S. Li, T.-S. Kim, J.-Y. Lee and B. J. Kim, Adv. Energy Mater., 2022, 12, 2200887 CrossRef CAS.
- Z. Peng, K. Jiang, Y. Qin, M. Li, N. Balar, B. T. O'Connor, H. Ade, L. Ye and Y. Geng, Adv. Energy Mater., 2021, 11, 2003506 CrossRef CAS.
- R. Ma, K. Zhou, Y. Sun, T. Liu, Y. Kan, Y. Xiao, T. A. Dela Peña, Y. Li, X. Zou, Z. Xing, Z. Luo, K. S. Wong, X. Lu, L. Ye, H. Yan and K. Gao, Matter, 2022, 5, 725–734 CrossRef CAS.
- J. Wang, C. Han, F. Bi, D. Huang, Y. Wu, Y. Li, S. Wen, L. Han, C. Yang, X. Bao and J. Chu, Energy Environ. Sci., 2021, 14, 5968–5978 RSC.
- L.-H. Chou, T. Mikie, M. Saito, C.-L. Liu and I. Osaka, ACS Appl. Mater., 2022, 14, 14400–14409 CrossRef CAS PubMed.
- J. Zhang, Q. Huang, K. Zhang, T. Jia, J. Jing, Y. Chen, Y. Li, Y. Chen, X. Lu, H. Wu, F. Huang and Y. Cao, Energy Environ. Sci., 2022, 15, 4561–4571 RSC.
- X. Yuan, Y. Zhao, D. Xie, L. Pan, X. Liu, C. Duan, F. Huang and Y. Cao, Joule, 2022, 6, 647–661 CrossRef CAS.
- M. Zhang, X. Guo, W. Ma, H. Ade and J. Hou, Adv. Mater., 2015, 27, 4655–4660 CrossRef CAS PubMed.
- Q. Guo, Q. Guo, Y. Geng, A. Tang, M. Zhang, M. Du, X. Sun and E. Zhou, Mater. Chem. Front., 2021, 5, 3257–3280 RSC.
- R. Yu, G. Wu and Z. Tan, J. Energy Chem., 2021, 61, 29–46 CrossRef CAS.
- Y. Cui, H. Yao, L. Hong, T. Zhang, Y. Xu, K. Xian, B. Gao, J. Qin, J. Zhang, Z. Wei and J. Hou, Adv. Mater., 2019, 31, 1808356 CrossRef PubMed.
- W. Peng, Y. Lin, S. Y. Jeong, Z. Genene, A. Magomedov, H. Y. Woo, C. Chen, W. Wahyudi, Q. Tao, J. Deng, Y. Han, V. Getautis, W. Zhu, T. D. Anthopoulos and E. Wang, Nano Energy, 2022, 92, 106681 CrossRef CAS.
- Z. Zhang, J. Miao, Z. Ding, B. Kan, B. Lin, X. Wan, W. Ma, Y. Chen, X. Long, C. Dou, J. Zhang, J. Liu and L. Wang, Nat. Commun., 2019, 10, 3271 CrossRef PubMed.
- J. Y. Oh, S. Rondeau-Gagné, Y.-C. Chiu, A. Chortos, F. Lissel, G.-J. N. Wang, B. C. Schroeder, T. Kurosawa, J. Lopez, T. Katsumata, J. Xu, C. Zhu, X. Gu, W.-G. Bae, Y. Kim, L. Jin, J. W. Chung, J. B. H. Tok and Z. Bao, Nature, 2016, 539, 411–415 CrossRef CAS PubMed.
- S. Savagatrup, X. Zhao, E. Chan, J. Mei and D. J. Lipomi, Macromol. Rapid Commun., 2016, 37, 1623–1628 CrossRef CAS PubMed.
- Y.-C. Lin, M. Matsuda, C.-K. Chen, W.-C. Yang, C.-C. Chueh, T. Higashihara and W.-C. Chen, Macromolecules, 2021, 54, 7388–7399 CrossRef CAS.
- Z. Genene, J.-W. Lee, S.-W. Lee, Q. Chen, Z. Tan, B. A. Abdulahi, D. Yu, T.-S. Kim, B. J. Kim and E. Wang, Adv. Mater., 2022, 34, 2107361 CrossRef CAS PubMed.
- J. Wu, G. Li, J. Fang, X. Guo, L. Zhu, B. Guo, Y. Wang, G. Zhang, L. Arunagiri, F. Liu, H. Yan, M. Zhang and Y. Li, Nat. Commun., 2020, 11, 4612 CrossRef CAS PubMed.
- S. Seo, J. Kim, H. Kang, J.-W. Lee, S. Lee, G.-U. Kim and B. J. Kim, Macromolecules, 2021, 54, 53–63 CrossRef CAS.
- H. Hu, P. C. Y. Chow, G. Zhang, T. Ma, J. Liu, G. Yang and H. Yan, Acc. Chem. Res., 2017, 50, 2519–2528 CrossRef CAS PubMed.
- J. Mun, Y. Ochiai, W. Wang, Y. Zheng, Y.-Q. Zheng, H.-C. Wu, N. Matsuhisa, T. Higashihara, J. B. H. Tok, Y. Yun and Z. Bao, Nat. Commun., 2021, 12, 3572 CrossRef CAS PubMed.
- C. J. Brabec, A. Cravino, D. Meissner, N. S. Sariciftci, T. Fromherz, M. T. Rispens, L. Sanchez and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 374–380 CrossRef CAS.
- Y. Li, M. Kim, Z. Wu, C. Lee, Y. W. Lee, J.-W. Lee, Y. J. Lee, E. Wang, B. J. Kim and H. Y. Woo, J. Mater. Chem. C, 2019, 7, 1681–1689 RSC.
- J. R. Tumbleston, B. A. Collins, L. Yang, A. C. Stuart, E. Gann, W. Ma, W. You and H. Ade, Nat. Photonics, 2014, 8, 385–391 CrossRef CAS.
- J. Rivnay, S. C. B. Mannsfeld, C. E. Miller, A. Salleo and M. F. Toney, Chem. Rev., 2012, 112, 5488–5519 CrossRef CAS PubMed.
- Z. Chiguvare and V. Dyakonov, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 235207 CrossRef.
- J. Yao, B. Qiu, Z.-G. Zhang, L. Xue, R. Wang, C. Zhang, S. Chen, Q. Zhou, C. Sun, C. Yang, M. Xiao, L. Meng and Y. Li, Nat. Commun., 2020, 11, 2726 CrossRef CAS PubMed.
- L. Hong, H. Yao, Z. Wu, Y. Cui, T. Zhang, Y. Xu, R. Yu, Q. Liao, B. Gao, K. Xian, H. Y. Woo, Z. Ge and J. Hou, Adv. Mater., 2019, 31, 1903441 CrossRef PubMed.
- Y. Li, W. K. Tatum, J. W. Onorato, Y. Zhang and C. K. Luscombe, Macromolecules, 2018, 51, 6352–6358 CrossRef CAS.
- Y. Li, W. K. Tatum, J. W. Onorato, S. D. Barajas, Y. Y. Yang and C. K. Luscombe, Polym. Chem., 2017, 8, 5185–5193 RSC.
- K.-J. Baeg, J. Kim, D. Khim, M. Caironi, D.-Y. Kim, I.-K. You, J. R. Quinn, A. Facchetti and Y.-Y. Noh, ACS Appl. Mater., 2011, 3, 3205–3214 CrossRef CAS PubMed.
- J. S. Park, G.-U. Kim, D. Lee, S. Lee, B. Ma, S. Cho and B. J. Kim, Adv. Funct. Mater., 2020, 30, 2005787 CrossRef CAS.
- V. Shrotriya, Y. Yao, G. Li and Y. Yang, Appl. Phys. Lett., 2006, 89, 063505 CrossRef.
- L. J. A. Koster, M. Kemerink, M. M. Wienk, K. Maturová and R. A. J. Janssen, Adv. Mater., 2011, 23, 1670–1674 CrossRef CAS PubMed.
- H. Jiang, C. Han, Y. Li, F. Bi, N. Zheng, J. Han, W. Shen, S. Wen, C. Yang, R. Yang and X. Bao, Adv. Funct. Mater., 2021, 31, 2007088 CrossRef CAS.
- G. A. H. Wetzelaer, M. Kuik, M. Lenes and P. W. M. Blom, Appl. Phys. Lett., 2011, 99, 153506 CrossRef.
- Y. Zheng, G.-J. N. Wang, J. Kang, M. Nikolka, H.-C. Wu, H. Tran, S. Zhang, H. Yan, H. Chen, P. Y. Yuen, J. Mun, R. H. Dauskardt, I. McCulloch, J. B. H. Tok, X. Gu and Z. Bao, Adv. Funct. Mater., 2019, 29, 1905340 CrossRef CAS.
- R. Noriega, J. Rivnay, K. Vandewal, F. P. V. Koch, N. Stingelin, P. Smith, M. F. Toney and A. Salleo, Nat. Mater., 2013, 12, 1038–1044 CrossRef CAS PubMed.
- S. Himmelberger and A. Salleo, MRS Commun., 2015, 5, 383–395 CrossRef CAS.
- S. Mukherjee, C. M. Proctor, J. R. Tumbleston, G. C. Bazan, T.-Q. Nguyen and H. Ade, Adv. Mater., 2015, 27, 1105–1111 CrossRef CAS PubMed.
- C. Lee, T. Giridhar, J. Choi, S. Kim, Y. Kim, T. Kim, W. Lee, H.-H. Cho, C. Wang, H. Ade and B. J. Kim, Chem. Mater., 2017, 29, 9407–9415 CrossRef CAS.
- Z. Abbas, S. U. Ryu, M. Haris, C. E. Song, H. K. Lee, S. K. Lee, W. S. Shin, T. Park and J.-C. Lee, Nano Energy, 2022, 101, 107574 CrossRef CAS.
- M. Zhang, L. Zhu, T. Hao, G. Zhou, C. Qiu, Z. Zhao, N. Hartmann, B. Xiao, Y. Zou, W. Feng, H. Zhu, M. Zhang, Y. Zhang, Y. Li, T. P. Russell and F. Liu, Adv. Mater., 2021, 33, 2007177 CrossRef CAS PubMed.
- L. Ye, H. Hu, M. Ghasemi, T. Wang, B. A. Collins, J.-H. Kim, K. Jiang, J. H. Carpenter, H. Li, Z. Li, T. McAfee, J. Zhao, X. Chen, J. L. Y. Lai, T. Ma, J.-L. Bredas, H. Yan and H. Ade, Nat. Mater., 2018, 17, 253–260 CrossRef CAS PubMed.
- J. Comyn, Int. J. Adhes. Adhes., 1992, 12, 145–149 CrossRef CAS.
- S. Wu, J. Polym. Sci., Part C: Polym. Symp., 1971, 34, 19–30 CrossRef.
- C. Han, J. Wang, S. Zhang, L. Chen, F. Bi, J. Wang, C. Yang, P. Wang, Y. Li and X. Bao, Adv. Mater., 2023, 2208986 CrossRef PubMed.
- J.-H. Kim, A. Nizami, Y. Hwangbo, B. Jang, H.-J. Lee, C.-S. Woo, S. Hyun and T.-S. Kim, Nat. Commun., 2013, 4, 2520 CrossRef PubMed.
- W. Kim, J. Choi, J.-H. Kim, T. Kim, C. Lee, S. Lee, M. Kim, B. J. Kim and T.-S. Kim, Chem. Mater., 2018, 30, 2102–2111 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta09990j |
| ‡ J. K., G.-U K. and D. J. K. equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2023 |