A multifunctional paper-based supercapacitor with excellent temperature adaptability, plasticity, tensile strength, self-healing, and high thermoelectric effects†
Chuanyin
Xiong
 *a,
Qi
Yang
a,
Weihua
Dang
a,
Qiusheng
Zhou
a,
Xue
Jiang
a,
Xuhui
Sun
b,
Zequn
Wang
b,
Meng
An
*b and
Yonghao
Ni
c
*a,
Qi
Yang
a,
Weihua
Dang
a,
Qiusheng
Zhou
a,
Xue
Jiang
a,
Xuhui
Sun
b,
Zequn
Wang
b,
Meng
An
*b and
Yonghao
Ni
c
aCollege of Bioresources Chemical & Materials Engineering, Shaanxi University of Science and Technology, Xi'an 710021, China. E-mail: xiongchuanyin@126.com; xiongchuanyin@sust.edu.cn
bCollege of Mechanical and Electrical Engineering, Shaanxi University of Science & Technology, Xi'an 710021, China. E-mail: anmeng@sust.edu.cn
cDepartment of Chemical and Biomedical Engineering, University of Maine, Orono, Maine 04469, USA
First published on 2nd February 2023
Abstract
In recent years, the development of multi-functional supercapacitors with high flexibility and strong environmental adaptability has gradually become a focus of attention. In this work, a multi-functional paper-based supercapacitor with excellent temperature adaptability, plasticity, tensile strength, strong adhesion, and self-healing properties is obtained by introducing PVA and ZnCl2 into carbonized paper-based materials (APC-PVA@ZnCl2). The as-obtained APC-PVA@ZnCl2 supercapacitor has excellent adaptability to the environment, even in a low-temperature environment below zero, still maintaining a high volumetric specific capacitance of 3438.45 mF cm−3 and high volumetric specific energy density of 44 mW h cm−3. Moreover, the APC-PVA@ZnCl2 supercapacitor can be stretched beyond 2400–2500% strain, indicating good tensile properties. In addition, the APC-PVA@ZnCl2 composite can also be used as a sensing material to detect current signals with different characteristics generated by the movement of different parts of the human body, showing excellent sensing performance. Besides, the APC-PVA@ZnCl2 composite also displays outstanding thermoelectric properties. It is hoped that this work can provide a reference for the development of green multi-functional supercapacitors.
1. Introduction
Energy and environmental issues have always been two key core issues that cannot be avoided in the development of human society.1–5 Especially with the continuous reduction of petrochemical resources and outstanding environmental problems caused by them, researchers have been focussing on the research and development of new and sustainable green electrochemical energy sources.6–10 Among them, supercapacitors, as electrochemical energy storage devices with high power density, long cycle life, good safety, and other advantages, have attracted extensive attention from researchers.11–16 Although the comprehensive electrochemical storage performance of supercapacitors has made great progress after decades of development, there are still some key problems to be studied further as follows: (1) as we all know, electrode materials, as one of the key factors affecting energy storage devices, have always been an important aspect of researchers' attention and improvement and various types of electrode materials are also available. However, electrode materials that can truly give green, sustainable, and high performance are still limited. (2) Recently, with the rapid development of flexible wearable devices, people have been increasingly having high requirements for a comprehensive performance of energy storage devices, such as good environmental adaptability, excellent flexibility, good mechanical properties, plasticity, and self-healing ability, not just limited to energy storage characteristics. Incorporating these excellent performances into integrated energy storage devices still has certain challenges.In recent years, as a green, sustainable, widely sourced, and naturally rich porous structural material, biomass materials are increasingly favored by researchers in the field of electrochemical energy storage,17–22 providing a further feasible idea to solve the above-mentioned key problems. Among them, paper-based materials, as a derivative product derived from biomass materials, not only have some advantages of biomass materials but also have good flexibility and integrity with potential as excellent energy storage materials.23–26 Many researchers have used paper-based materials for energy storage research and have also achieved a series of good research results. Currently, paper-based materials are used as flexible substrates in the field of energy storage.27–29 However, paper-based materials are derived from biomass materials and poor conductivity is still an unavoidable disadvantage. Therefore, the carbonization treatment of biomass materials is an effective strategy to improve its conductivity and has been widely adopted by researchers. It is true that carbonization treatment can effectively improve the conductivity of biomass materials, but the flexibility, mechanical strength, and environmental adaptability of carbonized materials are seriously affected.
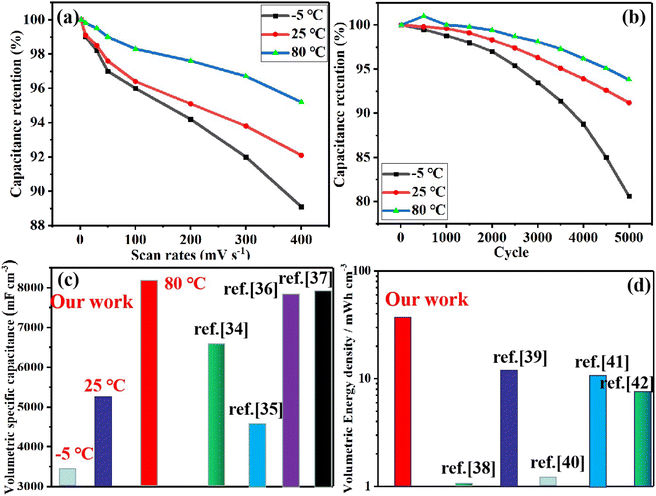
Based on these challenges mentioned above, in this research, for the first time, by introducing PVA and ZnCl2 into carbonized paper-based materials (APC-PVA@ZnCl2), using the cross-linked network structure formed by PVA, ZnCl2, and paper-based materials and the interaction between Zn2+, PVA, and paper-based fibers, a multi-functional paper-based supercapacitor with excellent temperature adaptability, plasticity, super-tensile, strong adhesion and self-healing properties is obtained. Furthermore, the interaction between PVA, ZnCl2, and paper fibers was simulated by molecular dynamics, which confirmed the experimental results. Finally, for the as-obtained APC-PVA@ZnCl2 supercapacitor, high specific capacitances of 3438.45 and 8176.94 mF cm−3 were still obtained at a scan rate of 50 mV s−1 at −5 °C and 80 °C at a scan rate of 50 mV s−1, respectively. The volumetric energy density is up to 44 mW h cm−3. These electrochemical properties prove its strong adaptability in different environments. Moreover, the APC-PVA@ZnCl2 supercapacitor can be stretched beyond 2400–2500% strain, proving they have good tensile properties. Besides, the APC-PVA@ZnCl2 multi-functional composite can also be used as a sensing material to detect current signals with different characteristics generated by the movement of different parts of the human body, showing excellent sensing performance. In addition, the APC-PVA@ZnCl2 multi-functional composite also shows a sparkling thermoelectric effect. Finally, molecular dynamics simulation and density functional theory are used to study the effect of material structures and functional groups on the diffusion of Zn2+. In a word, this research work provides new ideas and feasible references for the above-mentioned challenging problems in the field of energy storage.
2. Experiments section
Fig. 1 shows the schematic diagram of fabricating versatile integrated paper-based functional materials with good plasticity based on carbonized paper-based materials. The specific preparation details and relevant test methods are displayed in the ESI.† Here, TP represents tissue paper, and APC is carbonized activated paper carbon.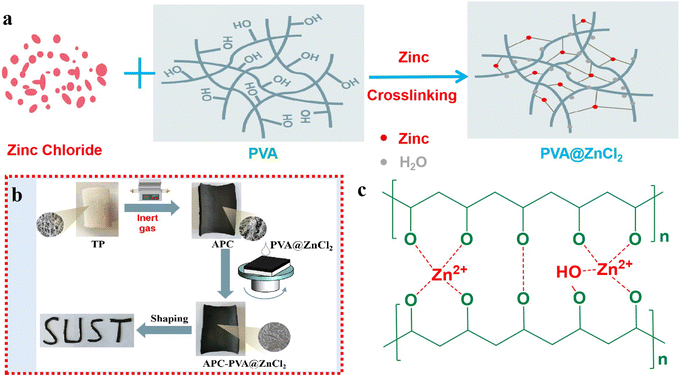 | ||
| Fig. 1 (a) Configuration mechanism of PVA@ZnCl2 solution. (b) The preparation process of APC-PVA@ZnCl2 with good plasticity. (c) The cross-linking mechanism between PVA and Zn2+. | ||
3. Results and discussion
3.1. Structure and morphology
For the carbonized paper-based materials, Fig. 2a–c show the SEM images of activated paper carbon (APC) under different magnifications. At higher magnification, it can be seen that the morphology of crude fibers still exists after the carbonization of paper-based material (Fig. 2a and b), at a lower magnification, it can be seen that the carbonized paper still retains the hierarchical network structure of the original paper (Fig. 2c). This not only increases the effective loading and stability of the active material but also improves the effective penetration depth of electrolyte into the paper-based active layer, thus significantly increasing the energy density of APC. Fig. 2d–f are SEM images of carbonized paper soaked in PVA solution (APC@PVA) under different magnifications. It can be seen that the PVA solution is tightly coated on the surface of the carbon fiber (Fig. 2d and e). Moreover, the layered porous structure of the paper base can provide more channels for electrolyte ions to transport, and the PVA solution can be well immersed into the layered porous structure of APC. Fig. 2g–i show the SEM images of carbonized paper soaked in PVA@ZnCl2 solution (APC-PVA@ZnCl2) under different magnifications. Compared with the SEM images of the APC@PVA composite, it can be found that the pore size of APC-PVA@ZnCl2 is smaller, and the cross-linking structure is complete, indicating that the PVA@ZnCl2 solution almost covers every carbon fiber.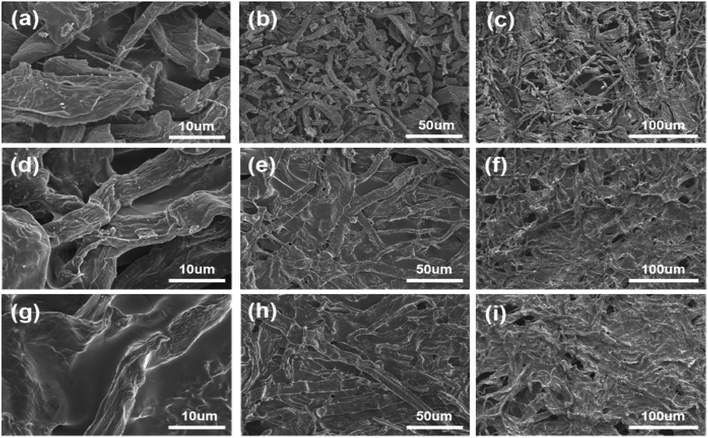 | ||
| Fig. 2 The SEM images of (a–c) APC, (d–f) APC@PVA, (g–i) APC-PVA@ZnCl2 under different magnifications. | ||
Furthermore, the composition of the paper-based material is further determined by infrared spectra, as shown in the Fig. S1 of the ESI.† Fig. S1† displays the infrared patterns of APC, APC@PVA, and APC-PVA@ZnCl2 samples. For the APC@PVA sample, the characteristic bands of 3388 cm−1 and 2928 cm−1 belong to the stretching vibration of the O–H and C–H groups, respectively. The peaks at 1636, 1380, 1075, 830, and 522 cm−1 are attributed to O–H bending vibration, symmetric –CH2– bending vibration, C–O tensile vibration, and C–C tensile vibration, respectively.30,31 For APC-PVA@ZnCl2 samples, there are similar bar graphs, but the positions are slightly different. Compared with APC@PVA, the vibration peak caused by O–H stretching and bending is significantly increased. Moreover, C–O and C–C group stretching vibrations not only increase the peak value but also have a more obvious red shift.26,32 This is because the coordination of Zn2+ and the reduction of water content promote the interaction between PVA chains in the amorphous region.33
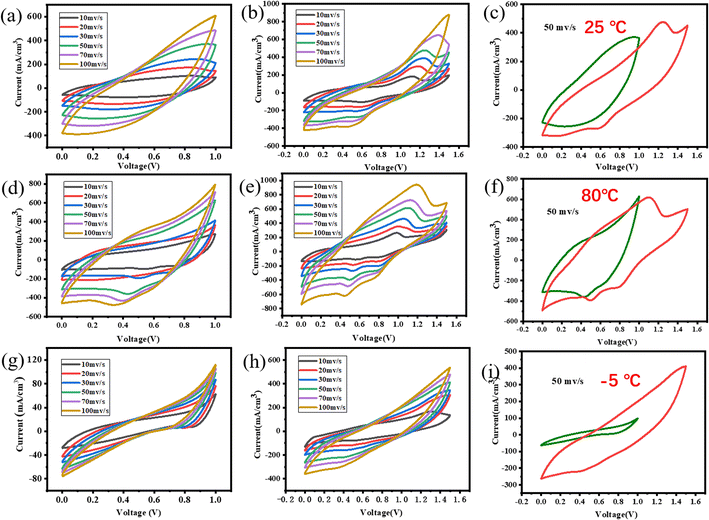
3.2. Electrochemical performance
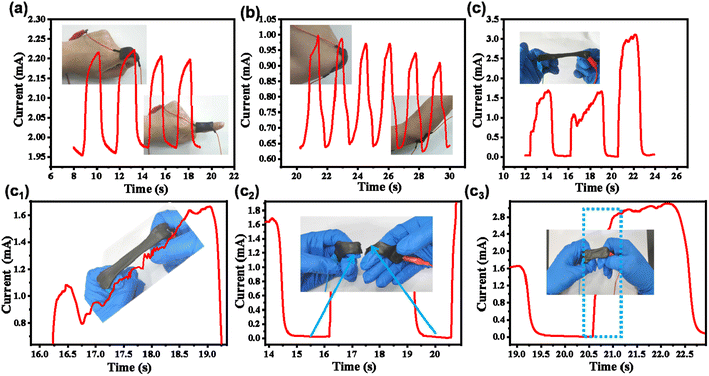
3.3. Integrated APC-PVA@ZnCl2 paper-based supercapacitor applied in sensor
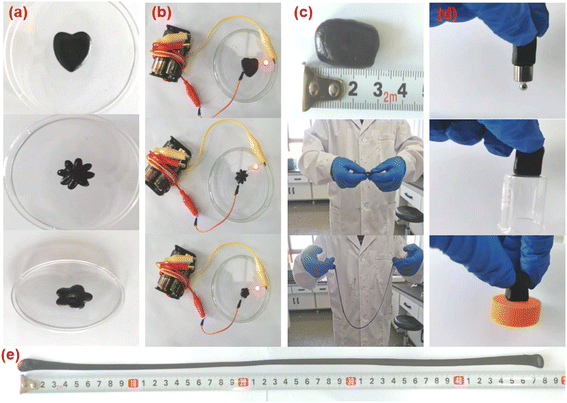 | ||
| Fig. 6 (a) Digital photograph of the plasticity of APC-PVA@ZnCl2 materials; (b) electrical conductivity; (c) stretchability; (d) adhesion; (e) tensile length. | ||
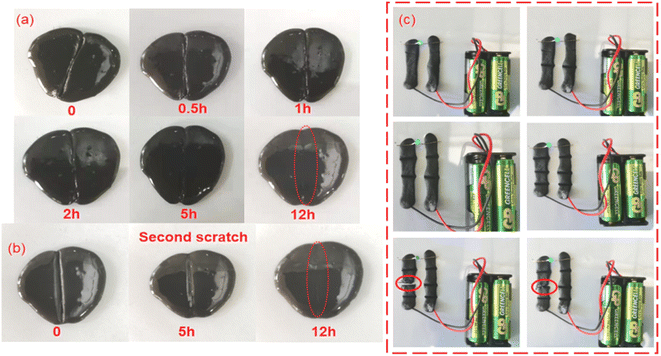
In addition, the APC-PVA@ZnCl2 composite material also has good self-healing characteristics, which is beneficial to realize the reuse of the material. To prove this, a deep incision was made to a piece of APC-PVA@ZnCl2 composite material was cut, and it was found that with the passage of time, the incision would heal slowly. Nearly half of the incision healed after 5 h, and after 12 h, the cut APC-PVA@ZnCl2 material was restored to its original state (Fig. 7a). Furthermore, the APC-PVA@ZnCl2 composite material was cut in the same incision again at the same position. After the same 12 h, the new incision healed again perfectly (Fig. 7b). The above phenomenon is mainly attributed to the fact that Zn2+ can hybridize with hydroxyl groups on PVA through sp3 to form coordination bonds through coordination, thus forming intermolecular forces. At the same time, PVA contains a large number of hydroxyl groups, with intramolecular forces increasing the mechanical properties of the material and making the prepared APC-PVA@ZnCl2 composites have excellent self-healing properties. To further verify the self-healing performance of APC-PVA@ZnCl2 composites, some bulb experiments under a closed loop were carried out. First, two strips of APC-PVA@ZnCl2 ribbon composite, 5 mm thick and 70 mm long, were used as part of the closed loop. It is obvious that the small bulb can be normally lit after the power is on, and then a knife is used to cut a part of the belt in the closed circuit. It is found that as long as the belt structure is not completely cut off, the closed circuit can supply small bulbs with normal power. When the belt is completely cut off, the closed circuit cannot work normally, and the small bulb goes out. However, due to the self-healing function of the APC-PVA@ZnCl2 composite, it was found that the incision began to heal after about 0.5 h. The result shows that once the incision began to contact, the small bulb would light again (Fig. 7b), which further proved that the APC-PVA@ZnCl2 composite has excellent self-healing property. To sum up, it is firmly believed that these outstanding characteristics of the APC-PVA@ZnCl2 composite material will further expand its application range.
3.4. Molecular dynamics simulation
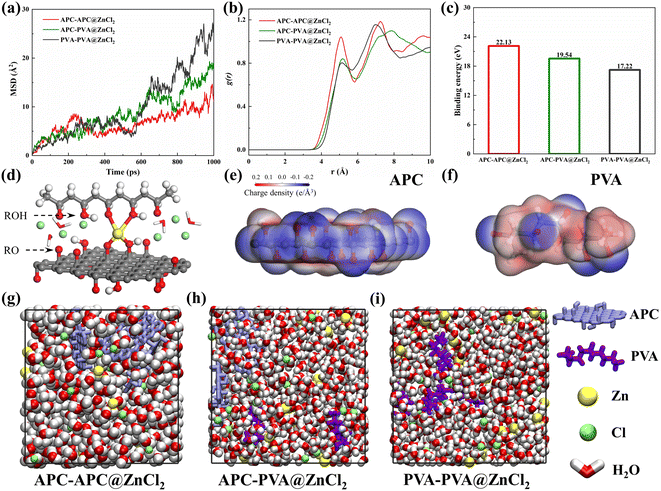
Molecular dynamics simulations were performed to gain insights into the interactions between coordinated ion and skeleton materials at the molecular level.47,48 Three structures were built: APC-APC@ZnCl2, APC-PVA@ZnCl2, and PVA-PVA@ZnCl2 system, respectively (Fig. 9g–i). Molecular simulation details are shown in ESI.† The mean square displacements (MSD) of Zn2+ in different systems are shown in Fig. 9a. It is found that the diffusion of Zn2+ in the APC-APC@ZnCl2 system is much larger than that in the PVA-PVA@ZnCl2 system. The diffusion of Zn2+ is enhanced when PVA is introduced and forms the APC-PVA@ZnCl2 system. The coordination strength of different systems to Zn2+ is quantified to exhibit the binding of Zn2+ through radial distribution functions (RDFs), defined as the probability of finding a Zn2+ at distance r around Ochain (Fig. 9b). The significant differences in the coordination number between APC-APC@ZnCl2 and PVA-PVA@ZnCl2 are shown in Fig. 9b. For PVA-PVA@ZnCl2, there is no obvious peak in RDFs. Correspondingly, the distinct peak of the APC-APC@ZnCl2 system indicates its stronger binding to Zn2+, which is favorable for the movement of Cl−. Moreover, the binding energies of Zn2+ in different systems are shown in Fig. 9c. With the mixing of APC chains and PVA chains, the binding energy of Zn2+ to its coordination environment is 19.54 eV, which is larger than the binding energy of Zn2+ in the PVA-PVA@ZnCl2 system, with a binding energy of 17.22 eV. It is true that for the APC-PVA@ZnCl2 system, the strong Zn–Ochain coordinated bonding results in higher coordination numbers of Zn2+ around the oxygen-containing functional groups of the chain. In the APC-PVA@ZnCl2 system, the Zn2+ can form multiple coordinations with rich oxygen functional groups (Fig. 9d), including hydroxyl (ROH), alkoxide (RO−), and hydroxyl of water molecules. The binding of Zn2+ by the oxygen functional group is a crucial factor for Zn2+ conductivity and transport. To clarify the effect of oxygen functional group on the binding of Zn2+, the charge density distribution of APC chain and PVA chain were calculated and plotted (Fig. 9e and f), and the presence of oxygen-containing functional groups makes the surface negatively charged (blue area). In other words, the blue area has strong electrostatically attractive interactions with Zn2+ while the red area shows a strong electrostatic rejection of Zn2+. It can be clearly observed that there are more and darker blue areas on the APC chain, which indicates its stronger binding effect on Zn2+. Now the theoretical calculations highlighted that the mix of APC chains and PVA chains not only enhances the toughness of the material through the strong coordination of Zn2+ in the APC-PVA@ZnCl2 system but also makes the conduction more stable by restricting the diffusion of Zn2+.4. Conclusion
In conclusion, a simple and effective method has been successfully designed to construct a kind of carbonized paper-based integrated multi-functional composites. Due to the light and porous properties of paper-based materials and the interaction between nano-fiber, PVA, and ZnCl2, the as-fabricated integrated paper-based supercapacitor exhibits the following merits: (1) due to the formation of coordination bonds and hydrogen bonds between PVA/ZnCl2 and nano-fiber, the integrated paper-based supercapacitor shows excellent comprehensive mechanical properties. (2) PVA/ZnCl2 acts as a gel electrolyte, endowing the integrated paper-based energy storage device with ultra-high specific capacitance, specific energy, and specific power density. (3) Due to the introduction of PVA/ZnCl2, the integrated paper-based supercapacitor shows excellent electrochemical energy storage characteristics at different ambient temperatures, such as high, normal, and low temperatures. (4) The constructed integrated paper-based supercapacitor shows excellent plasticity, tensile strength (elongation can reach 2400–2500%), adhesion, and self-healing. (5) The integrated paper-based supercapacitor can also act as a sensor, showing good stability and high sensitivity in monitoring human movements. (6) The constructed integrated paper-based supercapacitor shows a superior thermoelectric effect. Moreover, the results of molecular dynamics simulation further support the above results. In summary, this research work provides a new idea for the multi-functional and integrated high-efficiency development, application, and recycling of green biomass materials from the perspective of experiment and theory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by funds from the National Natural Science Foundation of China (22078184 and 52006130), China Postdoctoral Science Foundation (2019M653853XB), Natural science advance research foundation of Shaanxi University of Science and Technology (2018QNBJ-03), the Youth Science and Technology Nova Program of Shaanxi Province (2021KJXX-07), and the Joint Research Funds of Department of Science and Technology of Shaanxi Province and Northwestern Polytechnical University (2020GXLH-Z-025).References
- A. K. Ettinger, C. J. Chamberlain and E. M. Wolkovich, Nat. Clim. Change, 2022, 12(4), 305–307 CrossRef.
- A. G. Olabi and M. A. Abdelkareem, Renewable Sustainable Energy Rev., 2022, 158, 112111 CrossRef CAS.
- C. Xiong, T. Wang, Z. Zhao and Y. Ni, Smart Mater., 2023, 4(2), e1158 CAS.
- M. Wu, W. Zheng, X. Hu, F. Zhan, Q. He, H. Wang, Q. Zhang and L. Chen, Small, 2022, 18(50), 2205101 CrossRef CAS PubMed.
- C. Xiong, T. Wang, Y. Zhang, M. Zhu and Y. Ni, Nano Res., 2022, 15(8), 7506–7532 CrossRef.
- F. Wang, Y. Liu, H. Wei, T. Li, X. Xiong, S. Wei, F. Ren and A. A. Volinsky, Rare Met., 2021, 40, 448–470 CrossRef CAS.
- M. Peng, L. Wang, L. Li, Z. Peng, X. Tang, T. Hu, K. Yuan and Y. Chen, eScience, 2021, 1, 83–90 CrossRef.
- C. Xiong, T. Wang, Y. Zhang, B. Li, Q. Han, D. Li and Y. Ni, Int. J. Hydrogen Energy, 2022, 47(4), 2389–2398 CrossRef CAS.
- J. Chen, W. Xu, H. Wang, X. Ren, F. Zhan, Q. He, H. Wang and L. Chen, J. Mater. Chem. A, 2022, 10, 21197 RSC.
- C. Xiong, W. Dang, S. Nie, C. Qin, D. Li, L. Dai, M. Shen, Y. Xu and Y. Ni, Cellulose, 2021, 28(3), 1455–1468 CrossRef CAS.
- J. Huang, S. Han, J. Zhu, Q. Wu, H. Chen, A. Chen, J. Zhang, B. Huang, X. Yang and L. Guan, Adv. Funct. Mater., 2022, 32(35), 2205708 CrossRef CAS.
- C. Xiong, Y. Zhang and Y. Ni, J. Power Sources, 2023, 560, 232698 CrossRef CAS.
- L. Mao, X. Zhao, Y. Li and L. Chen, J. Colloid Interface Sci., 2022, 624, 482 CrossRef CAS PubMed.
- C. Xiong, B. Li, H. Liu, W. Zhao, C. Duan, H. Wu and Y. Ni, J. Mater. Chem. A, 2020, 8(21), 10898–10908 RSC.
- J. Chen, D. Shi, Z. Yang, W. Dong and M. Chen, J. Power Sources, 2022, 532, 231326 CrossRef CAS.
- M. Zhou, S. Yan, Q. Wang, M. Tan, D. Wang, Z. Yu, S. Luo, Y. Zhang and X. Liu, Rare Metals, 2022, 41(7), 2280–2291 CrossRef CAS.
- J. Guo, J. Xu, X. Xiao, L. Dai, C. Zhang and K. Huo, Green Chem., 2022, 24, 8827–8839 RSC.
- C. Xiong, C. Zheng, B. Li and Y. Ni, Ind. Crops Prod., 2022, 178, 114565 CrossRef CAS.
- P. B. Naik, P. Yadav, R. Nagaraj, R. Puttaswamy, H. K. Beere, U. N. Maiti, C. Mondal, N. S. Kotrappanavar and D. Ghosh, ACS Sustainable Chem. Eng., 2022, 10(4), 1471–1481 CrossRef CAS.
- C. Xiong, M. Li, Q. Han, W. Zhao, L. Dai and Y. Ni, J. Mater. Sci. Technol., 2022, 97, 190–200 CrossRef.
- W. Kang, L. Zeng, X. Liu, H. He, X. Li, W. Zhang, P. S. Lee, Q. Wang and C. Zhang, Electrochem. Energy Rev., 2022, 5(3), 8 CrossRef CAS.
- M. Shen, W. Hu, C. Duan, J. Li, S. Ding, L. Zhang, J. Zhu and Y. Ni, J. Colloid Interface Sci., 2023, 629, 778–785 CrossRef CAS PubMed.
- Y. Sun, Y. Yuan, X. Geng, C. Han, S. Lu, I. Mitrovic, L. Yang, P. Song and C. Zhao, Carbon, 2022, 199, 224–232 CrossRef CAS.
- I. Alali and R. Mokaya, Energy Environ. Sci., 2022, 15(11), 4710–4724 RSC.
- Z. Qu, X. Zhang, R. Huang, S. Wu, R. Chen, F. Wu and L. Li, Nano Lett., 2022, 22(10), 4115–4123 CrossRef CAS PubMed.
- C. Xiong, B. Li, C. Duan, L. Dai, S. Nie, C. Qin, Y. Xu and Y. Ni, Chem. Eng. J., 2021, 418, 129518 CrossRef CAS.
- D. Gogoi, P. Makkar, M. R. Das and N. N. Ghosh, ACS Appl. Electron. Mater., 2022, 4(2), 795–806 CrossRef CAS.
- S. Long, Y. Feng, F. He, J. Zhao, T. Bai, H. Lin, W. Cai, C. Mao, Y. Chen, L. Gan, J. Liu, M. Ye, X. Zeng and M. Long, Nano Energy, 2021, 85, 105973 CrossRef CAS.
- Y. Hu, Z. Chen, H. Zhuo, L. Zhong, X. Peng and R. Sun, Adv. Funct. Mater., 2019, 29(44), 1904472 CrossRef CAS.
- Q. Ding, X. Xu, Y. Yue, C. Mei, C. Huang, S. Jiang, Q. Wu and J. Han, ACS Appl. Mater. Interfaces, 2018, 10(33), 27987–28002 CrossRef CAS PubMed.
- J. Han, H. Wang, Y. Yue, C. Mei, J. Chen, C. Huang, Q. Wu and X. Xu, Carbon, 2019, 149, 1–18 CrossRef CAS.
- M. Darabi, A. Khosrozadeh, Y. Wang, N. Ashammakhi, H. Alem, A. Erdem, Q. Chang, K. Xu, Y. Liu, G. Luo, A. Khademhosseini and M. Xing, Adv. Sci., 2020, 7(21), 1902740 CrossRef CAS PubMed.
- B. Chen, Q. Chen, S. Xiao, J. Feng, X. Zhang and T. Wang, Sci. Adv., 2021, 7(48), eabi7233 CrossRef CAS PubMed.
- Y. Heo, J. Lee, S. Kim, S. Mun, S. Lee and S. Park, ACS Appl. Mater. Interfaces, 2022, 14(37), 42671–42682 CrossRef CAS PubMed.
- H. Peng, S. Huang, D. N. Tranca, F. Richard, W. Baaziz, X. Zhuang, P. Samori and A. Ciesielski, ACS Nano, 2022, 15(11), 18580–18589 CrossRef PubMed.
- D. Mudusu, K. R. Nandanapalli, G. D. Moon and S. Lee, Nano Energy, 2021, 88, 106274 CrossRef CAS.
- A. M. Patil, N. R. Chodankar, E. Jung, S. Roy, D. P. Dubal, G. Guan, Y. K. Han and S. C. Jun, J. Mater. Chem. A, 2021, 9(46), 26135–26148 RSC.
- F. Wang, J. Cheong, J. Lee, J. Ahn, G. Duan, H. Chen, Q. Zhang, I. D. Kim and S. Jiang, Adv. Funct. Mater., 2021, 31(31), 2101077 CrossRef CAS.
- Q. Liu, A. Zhao, X. He, Q. Li, J. Sun, Z. Lei and Z. Liu, Adv. Funct. Mater., 2021, 31(22), 2010944 CrossRef CAS.
- H. Shi, S. Chen, W. Shi, Z. Peng, J. Li, Z. Liu, G. Zhang and L. Liu, J. Mater. Chem. A, 2021, 9(31), 16852–16859 RSC.
- Z. Peng, J. Huang, Q. He, L. Tan and Y. Chen, J. Mater. Chem. A, 2021, 9(7), 4273–4280 RSC.
- C. Yu, H. Xu, Y. Gong, R. Chen, Z. Hui, X. Zhao, Y. Sun, Q. Chen, J. Zhou and W. Ji, Research, 2021, 2021, 6742715 CAS.
- J. Xie, M. Han, X. Zeng, D. Mao, H. Li, X. Zeng, R. Liu, L. Ren, R. Sun and J. Xu, Chem. Eng. J., 2022, 435(3), 135172 CrossRef CAS.
- S. Liu, Y. Yang, H. Huang, J. Zheng, G. Liu, T. H. To and B. Huang, Sci. Adv., 2022, 8(1), eabj3019 CrossRef CAS PubMed.
- E. H. Suh, M. K. Jeong, K. Lee, W. Jeong, Y. J. Jeong, I. H. Jung and J. Jang, Adv. Funct. Mater., 2022, 32(51), 2207886 CrossRef CAS.
- O. Zapata-Arteaga, S. Marina, G. Zuo, K. Xu, B. Dorling, L. A. Perez, J. S. Reparaz, J. Martin, M. Kemerink and M. Campoy-Quiles, Adv. Energy Mater., 2022, 12(19), 2104076 CrossRef CAS.
- C. Chi, M. An, X. Qi, Y. Li, R. Zhang, G. Liu, C. Lin, H. Huang, H. Dang and B. Demir, Nat. Commun., 2022, 13(1), 221 CrossRef CAS PubMed.
- W. Zhou, Y. Wu, H. Huang, M. Zhang, X. Sun, Z. Wang, F. Zhao, H. Zhang, T. Xie and M. An, Renewable Sustainable Energy Rev., 2022, 168, 112767 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta09654d |
| This journal is © The Royal Society of Chemistry 2023 |