Composite membranes consisting of acidic carboxyl-containing polyimide and basic polybenzimidazole for high-temperature proton exchange membrane fuel cells
Erli
Qu
a,
Geng
Cheng
a,
Min
Xiao
a,
Dongmei
Han
ab,
Sheng
Huang
a,
Zhiheng
Huang
 a,
Wei
Liu
a,
Shuanjin
Wang
*a and
Yuezhong
Meng
a,
Wei
Liu
a,
Shuanjin
Wang
*a and
Yuezhong
Meng
 *a
*a
aThe Key Laboratory of Low-carbon Chemistry & Energy Conservation of Guangdong Province, State Key Laboratory of Optoelectronic Materials and Technologies, School of Materials Science and Engineering, Sun Yat-sen University, Guangzhou 510275, China. E-mail: mengyzh@mail.sysu.edu.cn
bSchool of Chemical Engineering and Technology, Sun Yat-sen University, Zhuhai 519000, China
First published on 17th January 2023
Abstract
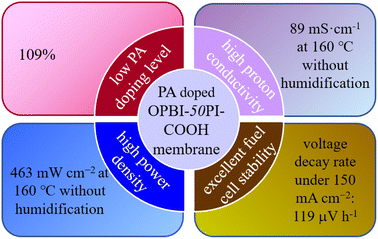
A novel acid-base composite membrane consisting of acidic carboxyl-containing polyimide (PI-COOH) and basic polybenzimidazole (OPBI) was fabricated and employed as a high-temperature proton exchange membrane (HT-PEM). PI-COOH was used to prepare HT-PEMs for the first time. The PBI-based acid-base composite membranes and ordinary composite membranes were investigated to elucidate the correlation between the polymer structure and membrane properties, especially in situations where phosphoric acid (PA) is used. The results show that OPBI-xPI-COOH composite membranes (x represents the weight percentage of PI-COOH in the entire membrane) deliver high proton conductivities and superior PA retention due to the continuous hydrogen bond network between OPBI and PI-COOH. The OPBI-50PI-COOH composite membrane revealed a proton conductivity of 89 mS cm−1 under low PA uptake (109%). Compared to the linear OPBI and OPBI-40PI composite membrane, a single cell with a low PA-doped OPBI-50PI-COOH composite membrane presented superior durability and power density (463 mW cm−2) at 160 °C and 0% relative humidity. In a short-term durability test of 369 h, the voltage attenuation rate was only 119 μV h−1. These outstanding outcomes indicate that the obtained acid-base composite membranes can be considered as capable candidates for HT-PEMs with enhanced performance.
10th Anniversary statementCongratulations on the 10th anniversary of Journal of Materials Chemistry A! For the past few decades, environmentally friendly energy materials and technologies have indeed revolutionized our lives, and they currently play a pivotal role in managing the energy-environment nexus, which is key to a sustainable future. In this connection, the Journal, with its high standards and well-known reputation, has been the primary communication platform for all scientists in the world. Benefiting from the platform, our article entitled “Polymer electrolytes for lithium polymer batteries” (Journal of Materials Chemistry A, 2016, 4, 10038–10069) has been cited over 800 times. We are interested in new types of sustainable energy materials and devices, improving the performance of green energy devices, and enabling new concepts for the conversion and storage of energy. In this regard, we contribute this article to celebrate this grand festival and hope our journal becomes even more popular. |
1 Introduction
Currently, proton exchange membrane fuel cells (PEMFCs) have been proven to be one of the most promising sustainable power generation technologies. In particular, high-temperature proton exchange membrane fuel cells (HT-PEMFCs) utilized at low humidity (or no humidity) and high temperature (>120 °C) are used for eco-friendly energy conversion in automobiles and fixed equipment, and they are capable of enhancing the kinetics of oxygen reduction reactions, increasing CO resistance of the catalyst, and simplifying water/heat management systems.1–5Considerable research has been conducted on proton exchange membranes (PEMs), which are the paramount unit of HT-PEMFCs.6–8 High-performance PEMs must engage in high proton conductivity, and possess excellent thermo-mechanical stability and durability at elevated temperatures and low humidity (or under anhydrous conditions). Based on these practical principles, phosphoric acid (PA)-adsorbed polybenzimidazole (PBI) is considered to be the most mature and prospective PEM among various candidate membrane materials.9–12 Nevertheless, higher PA doping amounts should be absorbed on behalf of acquiring higher proton conductivity, which often results in a severe decrease in mechanical strength due to the plasticizing effect of PA.13–15 Furthermore, this may bring about the loss of PA on account of its low molecular weight during the period of the long-term running of the cell and affect its overall performance. This problem may be conquered by generating interactions with PBI to promote proton conduction by means of substituting amphoteric polyacid for PA.16–20 Therefore, PA-doped PBI-type PEMs are still facing many challenges in the process of achieving commercialization, such as attaining higher proton conductivity, additional optimal mechanical properties, and PA retention ability with increased stability.
In the past few years, several strategies, such as organic-inorganic hybrids,21–23 chemically and physically cross-linked membranes,24–26 and acid-base composite membranes,27–30 have been investigated to enhance the overall performance of PBI as a PEM. Acid-base polymer blends containing an acidic polymer and a basic polymer have been found to be excellent candidates for acid-base blends. With the physical crosslinking between N-containing basic groups and acidic groups, hydrogen bonding was also envisaged, which can increase dimensional stability and proton conductivity by the construction of new proton transportation channels.
Over the last few years, different types of sulfonated aromatic polymer materials, including sulfonated polyarylene ether sulfone (SPAES),31,32 sulfonated polyimide (SPI),33 sulfonated polybenzimidazole (SPBI),34,35 sulfonated polyether ether ketone (SPEEK),36 sulfonated poly(fluorenyl ether ketone) (SPFEK),37 and sulfonated polyether sulfone (SPES),38 have been developed and applied in HT-PEMs due to their remarkable structural stability and mechanical strength. For instance, Ye et al.39 reported noncovalent cross-linked membranes using one uracil-terminated telechelic sulfonated polyimide (SPI-U) and another adenine-based crosslinking agent (SMA-A) to form bio-complementary hydrogen bonding, and the modified composite membranes presented improved proton conductivity and more optimal antioxidant performance. However, the common feature of these materials is that their comprehensive properties are strongly dependent on the degree of sulfonation, and when the degree of sulfonation increases, the chemical and mechanical properties will deteriorate.40
Polyimide (PI) is a high-performance polymer material with a trapezoidal structure that possesses high mechanical strength, and outstanding thermal and chemical stability. Thus, it has been widely applied in engineering plastics, advanced composite materials, fibers, films, proton transfer membranes, coatings, microelectronics, and in other fields.41–43 However, because many polyimides are insoluble in common organic solvents, it is difficult to directly process them for large-scale production. In this situation, it is necessary to first treat a polyamide acid, and then heat-treat it for imidization. On the contrary, excellent solubility in organic solvents has been observed for polyimides containing fluorinated or ether-bonded monomers, for example, 4,4'-(hexafluoroisopropylidene)diphthalic anhydride (6FDA) and 4,4′-oxydiphthalic anhydride (ODPA).44 Because of the above-mentioned properties, there is considerable demand for copolymerized PIs with satisfactory solubility in organic solvents.
In this work, PI with functional groups, i.e., carboxyl groups, was prepared to form a high-molecular-weight acidic polymer (PI-COOH), which was used as a dopant for OPBI to form an acid-base polymer blend PEM with the capability to retain the proton transfer ability of OPBI under high temperatures and low humidity. The hydrogen bond network generated between the carboxylic acid group (–COOH) in PI-COOH and the imidazole group in OPBI can further prevent the leaching of low-molecular-weight PA, so as to increase the proton transport of the acid-base composite membranes through a hopping mechanism. Compared to the pristine linear OPBI, the as-prepared OPBI-50PI-COOH membrane presented higher proton conductivity under much lower PA-doping amounts. Moreover, it delivered improved H2/O2 single cell performance and excellent fuel cell stability (Fig. 1).
As far as we know, such a composite membrane has not been reported in the relevant literature or patents, implying its great potential as a HT-PEM. As a comparison, the proton conductivity, mechanical performance, thermal and antioxidative stabilities, and electrochemical performance of acid-base composite membranes between unfunctionalized PI and OPBI (OPBI-40PI) were also prepared and characterized as the control in this study.
2 Experimental
2.1 Materials
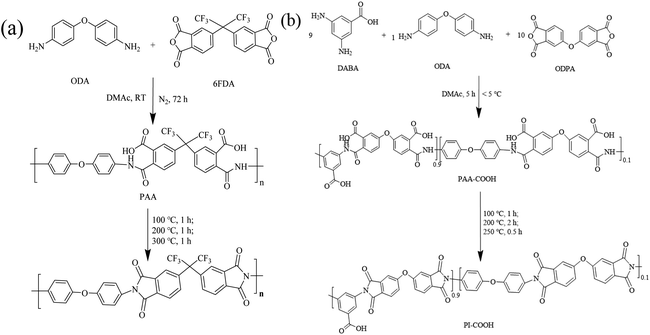
Poly[2,2′-(p-oxdiphenylene)-5,5′-benzimidazole] with a dynamic viscosity of 6000 Pa.S (OPBI) was supplied by Shanghai Shengjun Plastic Technology Co., Ltd. 3,5-Diaminobenzoic acid (DABA), 4,4'-(hexafluoroisopropylidene)diphthalic anhydride (6FDA), 4,4′-oxydianiline (ODA), 4,4′-oxydiphthalic anhydride (ODPA), phosphoric acid solution (85 wt%), and N,N-dimethylacetamide (DMAc) were supplied by Aladdin Chemical Co. Ltd.2.2 Synthesis of soluble polyimide (PI) and acidic carboxyl-containing polyimide (PI-COOH)
A typical synthetic route for PI and PI-COOH was carried out using a two-step polycondensation reaction, as depicted in Scheme 1. The detailed preparation process is as follows.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. First, DABA (1.37 g, 9 mmol) and ODA (0.20 g, 1 mmol) monomers were added to a 50 mL round-bottom flask containing 23 mL DMAc, and a transparent solution was then obtained by magnetic stirring at room temperature. After cooling to below 5 °C in an ice water bath, ODPA (3.10 g, 10 mmol) was gradually added. The solution was stirred for 5 h below 5 °C to acquire a viscous homogeneous transparent polyamide acid solution containing carboxylic acid (PAA-COOH) with a solid content of 20 wt%. The PAA-COOH solution was uniformly cast onto a clean glass sheet, and the solvent was removed at 80 °C for 6 h to form a PAA-COOH membrane. Finally, the PAA-COOH membrane was transferred to a muffle furnace for thermal imidization and treated step-by-step at 100 °C for 1 h, 200 °C for 2 h, and 250 °C for 0.5 h to form a light-yellow brown PI-COOH membrane. The synthesis of PI-COOH is shown in Scheme 1b.
1. First, DABA (1.37 g, 9 mmol) and ODA (0.20 g, 1 mmol) monomers were added to a 50 mL round-bottom flask containing 23 mL DMAc, and a transparent solution was then obtained by magnetic stirring at room temperature. After cooling to below 5 °C in an ice water bath, ODPA (3.10 g, 10 mmol) was gradually added. The solution was stirred for 5 h below 5 °C to acquire a viscous homogeneous transparent polyamide acid solution containing carboxylic acid (PAA-COOH) with a solid content of 20 wt%. The PAA-COOH solution was uniformly cast onto a clean glass sheet, and the solvent was removed at 80 °C for 6 h to form a PAA-COOH membrane. Finally, the PAA-COOH membrane was transferred to a muffle furnace for thermal imidization and treated step-by-step at 100 °C for 1 h, 200 °C for 2 h, and 250 °C for 0.5 h to form a light-yellow brown PI-COOH membrane. The synthesis of PI-COOH is shown in Scheme 1b.
2.3 Preparation of PI-COOH and OPBI acid-base composite membranes
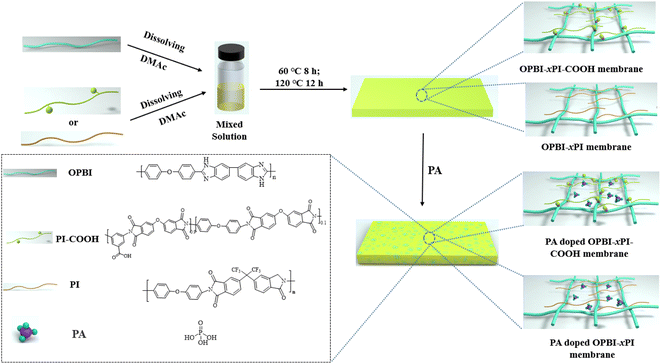
As described in Scheme 2, the acid-base composite membranes composed of PI-COOH and OPBI in different proportions were prepared by dint using the solution casting method. The as-prepared membranes were named OPBI-xPI-COOH, where x denotes the weight percentage of PI-COOH in the composite membranes. In contrast, pure linear OPBI and OPBI-40PI composite membranes in Scheme 2 (40 represents the weight percentage of PI in the composite membrane, which is 40%) were also prepared. The preparation process is described as follows.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 6
5, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 7
4, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 8
3, and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The detailed preparation process of the OPBI-50PI-COOH membrane is illustrated below as an example, and the other ratio composite membranes were prepared in a similar manner. OPBI (0.29 g) powder was dissolved in 7 mL DMAc under vigorous magnetic stirring at 80 °C. Subsequently, the PI-COOH membrane was mixed with OPBI solution for 1 h at 80 °C. The OPBI-50PI-COOH membrane was formed by casting the mixed solution, and after volatilizing the solvent for 8 h at 60 °C, then drying it in an oven for 24 h at 100 °C.
2. The detailed preparation process of the OPBI-50PI-COOH membrane is illustrated below as an example, and the other ratio composite membranes were prepared in a similar manner. OPBI (0.29 g) powder was dissolved in 7 mL DMAc under vigorous magnetic stirring at 80 °C. Subsequently, the PI-COOH membrane was mixed with OPBI solution for 1 h at 80 °C. The OPBI-50PI-COOH membrane was formed by casting the mixed solution, and after volatilizing the solvent for 8 h at 60 °C, then drying it in an oven for 24 h at 100 °C.
2.4 Characterization
 | (1) |
The dry membranes were soaked in 85 wt% PA solution for 12 h at 120 °C. After removing the membrane from the PA solution, surplus PA was wiped from the surface of the membrane with tissue paper, and the corresponding weight was measured. At least three samples were obtained from each membrane, and the average value was calculated. The adsorption capacity of PA and the volumetric swelling ratio for membranes was measured by eqn (2) and (3):
 | (2) |
 | (3) |
The PA retention of PA-doped membranes was measured by means of the water vapor method.45 Relative membranes were placed in water vapor at 80 °C and 30% relative humidity for 36 h employing a programmable constant temperature and humidity testing machine, PT-2090DZ0, and the weights of PA-doped membranes were measured at 16 h, 28 h, 32 h, and 36 h. The PA retention rate of the membrane was computed according to eqn (4):
 | (4) |
OS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (%)=W3/W2×100 (%)=W3/W2×100 | (5) |
Polarization curves were collected from a HT-PEMFC testing system by gradually controlling current densities from 0 (open circuit) to 1600 mA cm−2 or until the voltage dropped below 0.3 V. The short-term durability was also tested for PA-doped OPBI-50PI-COOH membrane fuel cells at an identical current density of 150 mA cm−2 operated at 140 °C without humidity or backpressure.
3 Results and discussion
3.1 Structural characterization of PI and PI-COOH
To obtain soluble polyimide polymers, the dianhydride monomers (6FDA and ODPA) and diamino monomer (ODA) containing flexible groups (–C(CF3)2– or O–) were used for PI and PI-COOH, respectively. A carboxylic acid group-containing diamino monomer (DABA) was used to copolymerize with ODA to synthesize PI-COOH. The molar ratio of DABA to ODA was controlled at 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to produce an optimal interaction between PI-COOH and OPBI in the composite membranes.
1 to produce an optimal interaction between PI-COOH and OPBI in the composite membranes.
With regard to the characterization of molecular weight correlation, the GPC results showed that the weight-average molecular weights of the synthesized PI and PI-COOH were 305![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1 and 490
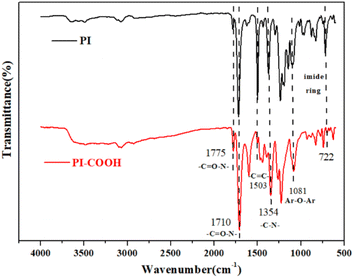
700 g mol−1 and 490![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1, respectively, suggesting that a high molecular weight was acquired. The structures of the synthesized PI and PI-COOH were confirmed by FT-IR spectroscopy. PI and PI-COOH generally represent specific vibrational bands, including C
500 g mol−1, respectively, suggesting that a high molecular weight was acquired. The structures of the synthesized PI and PI-COOH were confirmed by FT-IR spectroscopy. PI and PI-COOH generally represent specific vibrational bands, including C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N symmetrical stretching vibration, symmetric and asymmetric vibration of C
N symmetrical stretching vibration, symmetric and asymmetric vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and deformation vibration from the imide five rings. As revealed in Fig. 2, for PI and PI-COOH, some distinct characteristic absorption peaks for imide appear at approximately 1791 cm−1 (imide asymmetric C
O, and deformation vibration from the imide five rings. As revealed in Fig. 2, for PI and PI-COOH, some distinct characteristic absorption peaks for imide appear at approximately 1791 cm−1 (imide asymmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration), approximately 1714 cm−1 (imide symmetric C
O stretching vibration), approximately 1714 cm−1 (imide symmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration), approximately 1354 cm−1 (C-N stretching vibration), and approximately 722 cm−1 (deformation vibration from the imide five rings). The absorption band at approximately 1503 cm−1 denotes C
O stretching vibration), approximately 1354 cm−1 (C-N stretching vibration), and approximately 722 cm−1 (deformation vibration from the imide five rings). The absorption band at approximately 1503 cm−1 denotes C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in aromatic rings, and the noticeable vibration band at approximately 1081 cm−1 was assigned to the ArO–Ar linkage structure. Additionally, both polymers exhibited a broadband absorption between 3200 cm−1 and 3700 cm−1, which can be ascribed to the symmetrical stretching vibration of O–H from –COOH and H2O.
C bonds in aromatic rings, and the noticeable vibration band at approximately 1081 cm−1 was assigned to the ArO–Ar linkage structure. Additionally, both polymers exhibited a broadband absorption between 3200 cm−1 and 3700 cm−1, which can be ascribed to the symmetrical stretching vibration of O–H from –COOH and H2O.
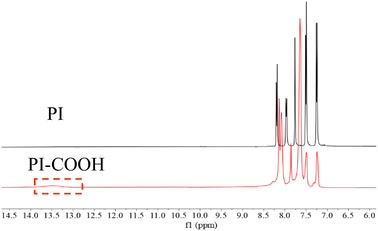
The 1H NMR (500 MHz, DMSO-d6) spectra of the prepared PI and PI-COOH are depicted in Fig. 3 to further confirm their structure. The chemical shift of the aromatic protons of both polyimides clearly appear at 7.0–8.5 ppm. Moreover, for PI-COOH, the hydrogen resonance signal of carboxylic acid groups is at 13.5 ppm. According to the data analysis of the FT-IR and NMR spectra, both polyimides were successfully synthesized.
3.2 Thermal stability
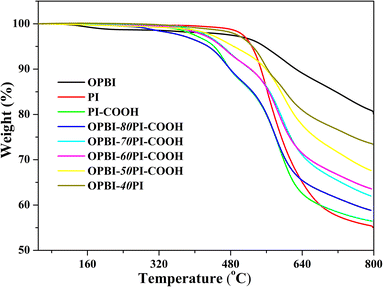
TGA was used to measure the thermal stability of OPBI-xPI–COOH–10% blend membranes under a N2 atmosphere at a heating rate of 10 °C min−1. As represented in Fig. 4, the PI-COOH and OPBI-xPI-COOH composite membranes decomposed in two stages. In terms of the PI-COOH membrane, the first weight loss at 400 °C or so is ascribed to the disintegration of carboxyl acid groups, while the second major weight loss at approximately 500 °C results from the decomposition of the main chains of PI-COOH. For the OPBI-xPI-COOH composite membranes, the addition of PBI can lead to decomposition at higher temperatures regardless of the presence of carboxyl acid groups or PI-COOH main chains. Furthermore, with increasing OPBI content, the residual free carboxyl acid groups decreased due to the acid-base interaction between the carboxyl acid group and the active sites (–NH– and –N = groups) on the imidazole ring. Therefore, the first step becomes decreasingly obvious or even disappears when the weight percentage ratio of OPBI increases from 20% to 50%.It is noteworthy that the TG curve of OPBI-xPI-COOH is significantly inconsistent with OPBI-0.4PI. With regard to the latter, its degradation trend and platform are nearly the same as that of the pure PI membrane. The reason for this is because there is no internal interaction between PI and OPBI, which results in poor stability of the OPBI-0.4PI composite membrane. These results imply that the thermal stability of the OPBI-xPI-COOH composite membranes is superior to that of pure PI-COOH. Moreover, the greater the amount of OPBI, the stronger the acid-base internal interaction, i.e., the greater the stability. In addition, the temperatures at 5% weight loss (T−5%) for each sample from the TG curves are all greater than 400 °C, which shows that they can meet the application requirements of HT-PEMs.
3.3 Proton conductivity, PA uptake, and retention
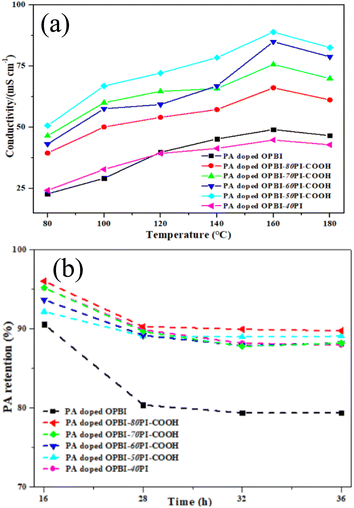
Fig. 5a compares the temperature dependence of the proton conductivity for different PA-doped membranes without humidification. The results show that the proton conductivity is positively correlated with temperatures between 80 °C and 160 °C, whereas the slight decrease above 160 °C is mainly dependent on the dimerization of PA.46 Significantly, as it is clear in Fig. 5a, the PA-doped OPBI-xPI-COOH composite membranes exhibited higher conductivities than the PA-doped OPBI and OPBI-40PI membranes on the basis of the same test criteria.Table 1 shows the proton conductivities and volume swelling ratios of the PA-doped membranes at 160 °C. Interestingly, the proton conductivity of the OPBI-50PI-COOH acid-base composite membrane with 109% PA uptake was as high as 89 mS cm−1, which is superior to that of the OPBI membrane with 250% PA uptake (49 mS cm−1). Moreover, the volumetric swelling ratios of the OPBI-50PI-COOH composite membrane were reduced to only 34% of that of OPBI, and high proton conductivity continued. This realizes the goal of higher proton conductivity of the membrane at lower PA adsorption capacity, which can alleviate the deterioration of cell performance caused by loss of excessive free PA.
| Sample | PA uptake/% | S volume (%) | Proton conductivity/mS cm−1 (160 °C) |
|---|---|---|---|
| PA-OPBI | 250.0 | 128 ± 12 | 49 |
| PA-OPBI-40PI | 145.0 | 50 ± 2 | 45 |
| PA-OPBI-80PI-COOH | 41.0 | 12 ± 2 | 66 |
| PA-OPBI-70PI-COOH | 85.7 | 25 ± 5 | 76 |
| PA-OPBI-60PI-COOH | 86.4 | 27 ± 7 | 85 |
| PA-OPBI-50PI-COOH | 109.0 | 43 ± 4 | 89 |
For HT-PEMFC systems with PA as the medium, once the acid overflows, it cannot be retrieved. Under long-term operation, PA loss may occur because of the formation of water at the cathode end. Therefore, the retention capacity of PA is critical to maintaining the performance of PEMs. PA leaching tests were carried out to identify their retention, and PA retention of all the PA-doped membranes is shown in Fig. 5b. According to the calculation results at different times, it can be concluded that there was more rapid PA loss in all the PA-doped membranes during the first 16 h, with stability at 28 h, and the difference was small compared with 36 h. It should be noted that the composite membranes presented much higher PA retention, compared to the neat OPBI membrane.
The above results are considered to be caused by the intermolecular interaction among the nitrogen groups of PBI, carboxyl acid groups, and free PA. The active nitrogen sites (–NH– and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) groups) of the imidazole ring in the OPBI skeleton play a vital role in such phenomenon, because nitrogen can serve as a proton donor and acceptor, promoting proton conduction by a Grotthuss-type mechanism via acid-base intermolecular interaction without humidification. Also, the proton conductivity increases with the increase in the OPBI content, which further demonstrates the formation of a more continuous hydrogen bond network that will facilitate proton transport and high PA retention.
groups) of the imidazole ring in the OPBI skeleton play a vital role in such phenomenon, because nitrogen can serve as a proton donor and acceptor, promoting proton conduction by a Grotthuss-type mechanism via acid-base intermolecular interaction without humidification. Also, the proton conductivity increases with the increase in the OPBI content, which further demonstrates the formation of a more continuous hydrogen bond network that will facilitate proton transport and high PA retention.
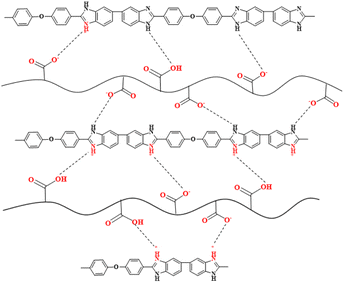
The possible proton transport mechanism for OPBI-xPI-COOH membranes under anhydrous conditions is revealed in Scheme 3, where the proton exchange between ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH+– on the OPBI backbone and -COO– carboxylate groups on the PI-COOH is helpful for the transport of protons through the Grotthuss mechanism. At the same time, the intermolecular proton jumping occurring between acidic –COOH groups of PI-COOH also facilitates the movement of protons within membranes.
NH+– on the OPBI backbone and -COO– carboxylate groups on the PI-COOH is helpful for the transport of protons through the Grotthuss mechanism. At the same time, the intermolecular proton jumping occurring between acidic –COOH groups of PI-COOH also facilitates the movement of protons within membranes.
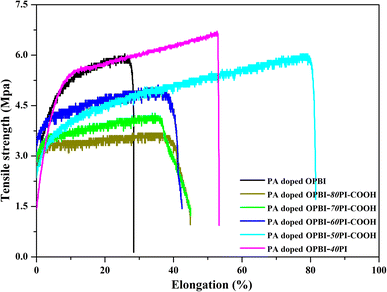
3.4 Mechanical and oxidative stability
The excellent mechanical performance and chemical stability of the PA-doped membranes are necessary for cell operation. The stress–strain curves of membranes are illustrated in Fig. 6. The tensile strengths of the PA-doped OPBI-xPI-COOH composite membranes increase with the increasing OPBI content. The mechanical strength of the PA-doped OPBI-50PI-COOH membrane is comparable to that of the PA-doped OPBI membrane, and it is assigned to the generation of hydrogen bond interactions. The existence of PI-COOH results in disarray in the OPBI chains, and the generation of hydrogen bonds between the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) (or –NH–) groups of OPBI and the –COOH group of PI-COOH enhances the blended cohesion.47 These parameters are adverse and balance out their influence, and thus no obvious change in tensile strength was viewed between PA-doped OPBI-50PI-COOH and the OPBI membranes. For the PA-doped OPBI-40PI composite membrane, its sturdiness was derived from the addition of high-strength PI. These results imply that the mechanical properties of OPBI-xPI-COOH composite membranes are strengthened by the increase in OPBI content due to the acid-base interaction, which assists in the utilization of the membrane under acidic operating conditions.
(or –NH–) groups of OPBI and the –COOH group of PI-COOH enhances the blended cohesion.47 These parameters are adverse and balance out their influence, and thus no obvious change in tensile strength was viewed between PA-doped OPBI-50PI-COOH and the OPBI membranes. For the PA-doped OPBI-40PI composite membrane, its sturdiness was derived from the addition of high-strength PI. These results imply that the mechanical properties of OPBI-xPI-COOH composite membranes are strengthened by the increase in OPBI content due to the acid-base interaction, which assists in the utilization of the membrane under acidic operating conditions.
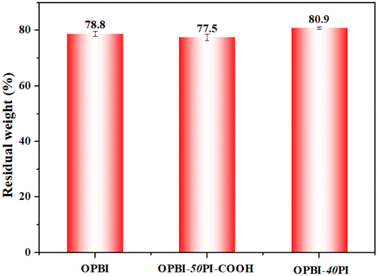
Another pivotal factor that affects the performance of HT-PEMs is the oxidative stability. As given in Fig. 7, the oxidative stability of the OPBI-50PI-COOH composite membrane is slightly poorer than that of the OPBI membrane. The aforementioned results are attributed to the high hydrophilicity of PI-COOH and its poor chemical stability as a result of the attack of hydroperoxyl and hydroxyl free radicals (˙OOH and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OH) because of the presence of its ether bonds and carboxyl groups. Similarly, OPBI-40PI composite membranes also exhibited a similar phenomenon. Table 2 presents the elapsed time for all PA-doped membranes to begin to break into pieces, and the PA-doped composites exhibit increased antioxidant stability. The data show that the oxidative stability of PA-doped membranes is the result of the combined effect of PA adsorption and mechanical properties, which is also consistent with our previous research.48
OH) because of the presence of its ether bonds and carboxyl groups. Similarly, OPBI-40PI composite membranes also exhibited a similar phenomenon. Table 2 presents the elapsed time for all PA-doped membranes to begin to break into pieces, and the PA-doped composites exhibit increased antioxidant stability. The data show that the oxidative stability of PA-doped membranes is the result of the combined effect of PA adsorption and mechanical properties, which is also consistent with our previous research.48
| Sample | Time/h |
|---|---|
| PA-OPBI | 2.0 |
| PA-OPBI-80PI-COOH | 2.5 |
| PA-OPBI-70PI-COOH | 4.0 |
| PA-OPBI-60PI-COOH | 6.0 |
| PA-OPBI-50PI-COOH | 7.0 |
| PA-OPBI-40PI | 2.5 |
3.5 HT-PEM fuel cell performance
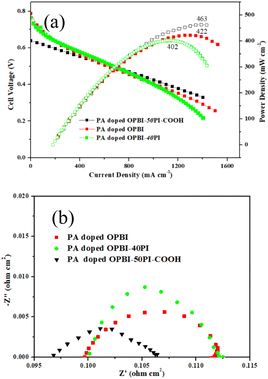
The fuel cell performances of PA-doped membranes were investigated at 180 °C without humidification, and the matching polarization curves are depicted in Fig. 8a. As shown in Fig. 8a, the OPBI-50PI-COOH composite membrane displayed a superior fuel cell performance as compared to the OPBI and OPBI-40PI membranes. The OPBI-50PI-COOH composite membranes with lower PA uptake (109%) exhibited a high peak power density of 463 mW cm−2 with a flow rate of 80- and 160 mL min−1 for non-humidified H2 and O2 without backpressure, respectively. However, under the same testing conditions, the pure OPBI and OPBI-40PI membranes with higher PA uptake (250% and 145%) delivered only 422 mW cm−2 and 402 mW cm−2 of the peak power density, respectively. Indeed, the formation of hydrogen bond interactions between OPBI and PI-COOH contributed to increased proton conductivity and high PA retention, which then enhanced the fuel cell performance. The comparison of the power density indicates that the obtained OPBI-50PI-COOH acid-base composite membrane is a promising candidate PEM for HT-PEMs.To further analyse the influence of different feature combinations on the electrochemical reaction performance of the MEA, the impedance of a single cell was tested at a voltage of 0.7 V (Fig. 7b). The Nyquist diagram shows that a decrease in the high-frequency resistance (HFR) at 0.7 V follows the trend of PA-doped OPBI-40PI membrane > PA-doped OPBI membrane > PA-doped OPBI-50PI-COOH membrane. This indicates that the lower HFR of the MEA with the PA-doped OPBI-50PI-COOH membrane can be assigned to increased proton transfer resulting from the use of PEM, with consequently a lower proton transport resistance. The lowest charge transfer resistance (RCT) was achieved by the MEA with a PA-doped OPBI-50PI-COOH membrane, which is consistent with the polarisation and power density curves in Fig. 8a.
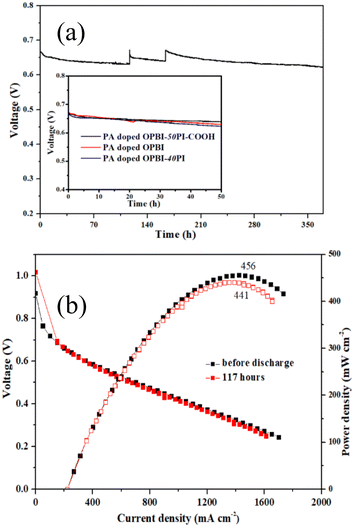
For the purpose of assessing the working stability of the PA-doped composite membrane fuel cell, the short-term durability of constant current discharge for the PA-doped OPBI-50PI-COOH membrane with optimal comprehensive performance was tested by a startup/shutdown procedure, as shown in Fig. 9a. The cell experienced a rapid voltage drop in the first 24 h short-term durability test, which may have been caused by the redistribution of PA in the membrane electrode49 and the agglomeration of Pt catalyst nanoparticles.50 The distribution of PA in the membrane electrode subsequently reached equilibrium, thus exhibiting a stable discharge behaviour, which is in accordance with the results for the PA retention rate.
Additionally, the working voltage of a single cell with PA-doped OPBI (1.0 mV h−1) and OPBI-40PI membranes (1.2 mV h−1) rapidly decreased, and the attenuation rate was approximately 2 times that of the PA-doped OPBI-50PI-COOH membrane (0.56 mV h−1) during the stability test of 50 h. This further indicates that the addition of PI-COOH can lengthen the working lifetime of a single cell with a PA-doped OPBI-based membrane. Moreover, the working voltage decay rate of the PA-doped OPBI-50PI-COOH membrane HT-PEMFC was 119 μV h−1 during the stability test of 369 h. Remarkably, the voltage can be restored to the same level as that during the initial state after each shutdown/startup procedure. That is, the performance of the PA-doped OPBI-50PI-COOH membrane HT-PEMFC was not affected by a shutdown during the different stages, indicating that the PA-doped OPBI-50PI-COOH membrane possesses admirable stability.
The peak power densities of the PA-doped OPBI-50PI-COOH membrane fuel cell at different operating times are shown in Fig. 9b. The peak power densities decrease from the initial 456 mW cm−2 to 441 mW cm−2 after 117 h, and the calculated attenuation rate was approximately 3.3%, which also suggests that its stability is excellent. Overall, the integral performance of the novel acid-base polymer composite membranes in this work is superior to that of the HT-PEMs listed in Table 3, with lower PA adsorption, higher proton conductivity, and superior single cell performance and stability. The above results demonstrate the promising application of the OPBI-50PI-COOH membrane for HT-PEMFCs.
| Membrane type | Membrane parameter | Fuel cell performance | Ref. | |||
|---|---|---|---|---|---|---|
| PA uptake (%) | Proton conductivity (mS cm−1) (T/°C, RH/%) | Voltage (V) vs. H2/O2 (Pt mg−1 cm−1−2, T/°C, RH/%) | Voltage decay rate (μV h−1) (approximate duration per h, current density per mA cm−2) | Maximum power density (%) (T/°C, RH/%) | ||
| OPBI-50PI-COOH | 109 | 89 (160, 0%) | 0.92 (0.4, 140, 0) | 119 (369, 150) | 456 (140, 0) | This work |
| PBI | 210 | — | 0.73 (0.4, 150, 0) | 676 (182, 200) | 290 (125, 0) | 51 |
| PBI | 95 | — | - (Cathode: 0.75& Anode:1.0, 160, 0) | 363 (70, 300) | — | 52 |
| Asymmetrically porous PBI | 307 | 82 (160, 0%) | 0.86 (0.2, 160, 0) | 283 (720, 200) | 835 (160, 0) | 53 |
| PES-PVP-NH | 245 | 152 (180, 5) | 0.9 (0.26, 160, 0) | 1400 (120, 200) | 480 (180, 0) | 54 |
| 6-c-sTiO2-PBI-OO (6 wt% sTiO2) | 392 | 98 (160, 5) | ∼1.0 (0.47, 160, 0) | 98 (300, 200) | 356 (160, 0) | 55 |
| SC-B-OPBI-10 | 223 | 44 (180, 0) | 0.94 (0.4, 160, 0) | 110 (200, 200) | 404 (160, 0) | 56 |
| Symmetric sponge-like porous OPBI | 545 | 71 (180, 0) | 0.86 (0.65, 160, 0) | Poor durability (∼72, 300) | 485 (160, 0) | 57 |
| PSf-TEA-110 | 238 | 78 (160, 0) | ∼0.60 (0.65, 160, 0) | Poor durability (<30, 300) | ∼400 (160, 0) | 58 |
| m-PBI/1%CeO2/g-C3N4 | 331 | 48 (140, 0) | 0.94 (0.65, 160, 0) | 188 (160, 300) | 504 (160, 0) | 59 |
4 Conclusions
In this work, PI-COOH with carboxyl functional groups was prepared and blended with OPBI for application as HT-PEMs. Some crucial performance indicators were characterized, including thermo-chemical stability, mechanical property, proton conductivity, PA uptake, and single cell performance. The OPBI-50PI-COOH acid-base composite membrane exhibited remarkable proton conductivity under lower PA uptake. The continuous hydrogen bond network between OPBI and PI-COOH promoted proton transport and resulted in high proton conductivity. In comparison with the neat OPBI, the OPBI-50PI-COOH acid-base composite membrane with low uptake (109%) displayed a power density of up to 463 mW cm−2 and proton conductivity of 89 mS cm−1 at 160 °C without humidification. Moreover, it exhibited excellent stability in short-term durability tests for HT-PEMFCs. The integral performance of the as-prepared acid-base composite membrane was far superior to that of HT-PEMs as recently reported, and was characterized by lower PA adsorption, higher proton conductivity, and more optimal single cell performance and stability, thus demonstrating that it is a promising candidate for HT-PEMFC application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program (2018YFA0702002), and the National Key Research and Development Program (Japan-China Joint Research Program) (2017YFE0197900).References
- A. A. Tahrim and I. N. H. M. Amin, J. Appl. Membr. Sci. Technol., 2018, 23, 37–62 Search PubMed.
- J. Fang, X. Lin, D. Cai, N. He and J. Zhao, J. Membr. Sci., 2016, 502, 29–36 CrossRef CAS.
- S. Rahman, M. S. Masdar, M. I. Rosli, E. H. Majlan, T. Husaini, S. K. Kamarudin and W. Daud, Renewable Sustainable Energy Rev., 2016, 66, 137–162 CrossRef CAS.
- P. Sun, Z. Li, F. Dong, S. Wang, X. Yin and Y. Wang, Int. J. Hydrogen Energy, 2017, 42, 486–495 CrossRef CAS.
- Z. Xia, L. Ying, J. Fang, Y. Y. Du, W. M. Zhang, X. Guo and J. Yin, J. Membr. Sci., 2017, 525, 229–239 CrossRef CAS.
- F. Altaf, R. Gill, R. Batool, M. Drexler and K. Jacob, Eur. Polym. J, 2019, 110, 155–167 CrossRef CAS.
- K. Hooshyari, H. Rezania, V. Vatanpour, P. Salarizadeh and M. Enhesari, J. Membr. Sci., 2020, 612, 118436–118451 CrossRef CAS.
- E. Qu, X. Hao, M. Xiao, D. Han, S. Huang, Z. Huang, S. Wang and Y. Meng, J. Power Sources, 2022, 533, 231386–231405 CrossRef CAS.
- D. Chen, S. Yu, X. Liu and X. Li, J. Power Sources, 2015, 282, 323–327 CrossRef CAS.
- K. Hooshyari, M. Javanbakht and M. Adibi, Electrochim. Acta, 2016, 205, 142–152 CrossRef CAS.
- K. Hooshyari, M. Moradi and P. Salarizadeh, Int. J. Energy Res., 2020, 44, 2617–2633 CrossRef CAS.
- M. Ja Van Bakht, Z. Rajabi, K. Houshyari, A. Badiei and M. Adibi, New J. Chem., 2020, 44, 5001–5018 RSC.
- M. Hazarika and T. Jana, ACS Appl. Mater. Interfaces, 2012, 4, 5256–5265 CrossRef CAS PubMed.
- S. Maity and T. Jana, Macromolecules, 2013, 46, 6814–6823 CrossRef CAS.
- J. Jiang, Z. Li, M. Xiao, S. Wang, K. Miyatake and Y. Meng, J. Membr. Sci., 2022, 660, 120878–120886 CrossRef CAS.
- U. Sen, O. Acar, A. Bozkurt and A. Ata, J. Appl. Polym. Sci., 2011, 120, 1193–1198 CrossRef CAS.
- E. Sevim ünügür, A. Bozkurt and S. S. Hosseini, Prog. Polym. Sci., 2012, 37, 1265–1291 CrossRef.
- Z. Taherkhani, M. Abdollahi and A. Sharif, Solid State Ionics, 2019, 337, 122–131 CrossRef CAS.
- Z. Taherkhani, M. Abdollahi and A. Sharif, J. Electrochem. Soc., 2020, 167, 104503–104513 CrossRef CAS.
- X. Hao, Z. Li, M. Xiao, D. Han, S. Huang, G. Xi, S. Wang and Y. Meng, J. Mater. Chem. A, 2022, 10, 10916–10925 RSC.
- S. Lee, K. Seo, R. V. Ghorpade, K. H. Nam and H. Han, Mater. Lett., 2019, 263, 127167–127171 CrossRef.
- J. Yang, X. Li, C. Shi, B. Liu and B. Liu, J. Membr. Sci., 2020, 620, 118855–118864 CrossRef.
- J. Zhang and S. P. Jiang, Funct. Org. Hybrid Nanostruct. Mater., 2018, 10, 383–418 Search PubMed.
- J. S. Yang, Q. F. Li, L. N. Cleemann, J. O. Jensen, C. Pan, N. J. Bjerrum and R. H. He, Adv. Energy Mater., 2013, 3, 622–630 CrossRef CAS.
- W. Li, B. Zla, L. Yi and W. Lei, J. Membr. Sci., 2019, 583, 110–117 CrossRef.
- P. Ngamsantivongsa, H. L. Lin and T. L. Yu, J. Polym. Res., 2016, 23, 22–33 CrossRef.
- G. A. Giffin, S. Galbiati, M. Walter, K. Aniol, C. Ellwein, J. Kerres and R. Zeis, J. Membr. Sci., 2017, 535, 122–131 CrossRef CAS.
- N. N. Krishnan, N. Duong, A. Konovalova, J. H. Jang and D. Henkensmeier, J. Membr. Sci., 2020, 614, 118494–118507 CrossRef CAS.
- B. Liu, Y. Zhang, Y. Jiang, P. Qian and H. Shi, J. Membr. Sci., 2019, 591, 117332–117739 CrossRef CAS.
- A. Zg, A. Mp, A. Jc, A. Zj and A. Smh, J. Energy Chem., 2021, 63, 393–429 CrossRef.
- J. H. Chun, G. K. Sang, Y. L. Ji, H. H. Dong and K. T. Park, Renewable Energy, 2013, 51, 22–28 CrossRef CAS.
- K. T. Park, J. H. Chun, G. K. Sang, B. H. Chun and S. H. Kim, Int. J. Hydrogen Energy, 2011, 36, 1813–1819 CrossRef CAS.
- Z. J. Xia, H. Xu, X. X. Guo and J. H. Fang, Adv. Mater. Res., 2011, 287–290, 2516–2521 CAS.
- I. Nicotera, V. Kosma, C. Simari, S. Angioni, P. Mustarelli and E. Quartarone, J. Phys. Chem. C, 2015, 119, 9745–9753 CrossRef CAS.
- C. H. Shen, L. C. Hsu, E. Bulycheva and N. Belomoina, J. Membr. Sci., 2012, 399–400, 11–15 CrossRef CAS.
- Q. Rong, S. Gu, G. H. He, X. M. Wu, Z. W. Hu and X. M. Yan, Polym. Mater.: Sci. Eng., 2009, 126–129 CAS.
- Y. Bai, M. Xiao, Z. Huang, D. Han, C. Wang, S. Wang and Y. Meng, J. Electrochem. Soc., 2021, 168, 114509–114516 CrossRef CAS.
- S. Wen, C. Gong, W. C. Tsen, Y. C. Shu and F. C. Tsai, Int. J. Hydrogen Energy, 2009, 34, 8982–8991 CrossRef CAS.
- Y. S. Ye, R. J. Huang, R. C. Cheng and R. C. Chang, Chem. Commun., 2010, 46, 7554–7556 RSC.
- S. S. Lim, R. Wan, J. M. Jahim, M. Ghasemi, P. S. Chong and M. Ismail, Int. J. Hydrogen Energy, 2012, 37, 11409–11424 CrossRef CAS.
- V. Narayanan, T. D. Dang, Z. W. Bai, V. K. McNier, J. N. DeCerbo, B. H. Tsao and J. T. Stricker, Mater. Sci. Eng. B, 2010, 168, 16–21 CrossRef.
- E. S. Weiser, T. F. Johnson, T. Clair, Y. Echigo and B. W. Grimsley, High Perform. Polym., 2000, 12, 1–12 CrossRef CAS.
- C. Zhao, W. Zhe, D. Bi, H. Lin, S. Ke, T. Fu, S. Zhong and N. Hui, Polymer, 2007, 48, 3090–3097 CrossRef CAS.
- D. Düsselberg, D. Verreault, P. Koelsch and C. Staudt, J. Mater. Sci., 2011, 46, 4872–4879 CrossRef.
- K. S. Lee, J. S. Spendelow, Y. K. Choe, C. Fujimoto and Y. S. Kim, Nat. Energy, 2016, 1, 16120–16126 CrossRef CAS.
- W. Ma, C. Zhao, H. Lin, Z. Gang, N. Jing, W. Jing, W. Shuang and N. Hui, J. Power Sources, 2011, 196, 9331–9338 CrossRef CAS.
- K. Hooshyari, H. Rezania, V. Vatanpour, R. Mohadese and H. R. Rajabi, Int. J. Energy Res., 2021, 1–19 Search PubMed.
- E. Qu, J. Jiang, M. Xiao, D. Han, S. Huang, Z. Huang, S. Wang and Y. Meng, Nanomaterials, 2022, 12, 773–789 CrossRef CAS PubMed.
- J. Hu, H. Zhang, Y. Zhai, L. Gang, J. Hu and B. Yi, Electrochim. Acta, 2007, 52, 394–401 CrossRef.
- J. Zhang, D. Aili, J. Bradley, H. Kuang, C. Pan, R. D. Marco, Q. Li and S. P. Jiang, J. Electrochem. Soc., 2017, 164, F1615–F1625 CrossRef CAS.
- F. J. Pinar, P. Canizares, M. A. Rodrigo, D. Ubeda and J. Lobato, J. Power Sources, 2011, 196, 4306–4313 CrossRef CAS.
- D. Schonvogel, M. Rastedt, P. Wagner, M. Wark and A. Dyck, Fuel Cells, 2016, 16, 480–489 CrossRef CAS.
- L.-C. Jheng, W. J.-Y. Chang, S. L.-C. Hsu and P.-Y. Cheng, J. Power Sources, 2016, 323, 57–66 CrossRef CAS.
- J. Zhang, S. Lu, H. Zhu, K. Chen, Y. Xiang, J. Liu and M. Forsyth, RSC Adv., 2016, 6, 86575–86585 RSC.
- N. N. Krishnan, S. Lee, R. V. Ghorpade, A. Konovalova, J. H. Jang, H. J. Kim, J. Han, D. Henkensmeier and H. Han, J. Membr. Sci., 2018, 560, 11–20 CrossRef CAS.
- M. Hu, J. Ni, B. Zhang, S. Neelakandan and L. Wang, J. Power Sources, 2018, 389, 222–229 CrossRef CAS.
- K. Geng, H. Tang, Q. Ju, H. Qian and N. Li, J. Membr. Sci., 2021, 620, 118981–118988 CrossRef CAS.
- H. Tang, K. Geng, J. Hao, X. Zhang, Z. Shao and N. Li, J. Power Sources, 2020, 475, 228521–228530 CrossRef CAS.
- B. Lv, K. Geng, H. Yin, C. Yang, J. Hao, Z. Luan, Z. Huang, X. Qin, W. Song and N. Li, J. Membr. Sci., 2021, 639, 119760–119769 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |