Identification of catalytic activity descriptors for selective 5-hydroxymethyl furfural electrooxidation to 2,5-furandicarboxylic acid†
William Hadinata
Lie
 a,
Yuwei
Yang
a,
Jodie A.
Yuwono
a,
Yuwei
Yang
a,
Jodie A.
Yuwono
 ab,
Constantine
Tsounis
ab,
Constantine
Tsounis
 a,
Muhammad
Zubair
a,
Muhammad
Zubair
 a,
Joshua
Wright
a,
Joshua
Wright
 c,
Lars
Thomsen
d,
Priyank
Kumar
a and
Nicholas
Bedford
c,
Lars
Thomsen
d,
Priyank
Kumar
a and
Nicholas
Bedford
 *a
*a
aSchool of Chemical Engineering, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: n.bedford@unsw.edu.au
bCollege of Engineering, Computing and Cybernetics, Australian National University, Canberra ACT 2601, Australia
cDepartment of Physics, Illinois Institute of Technology, Chicago, Illinois 60616, USA
dAustralian Synchrotron, Australian Nuclear Science and Technology Organisation, 800 Blackburn Road, Clayton, VIC 3168, Australia
First published on 20th January 2023
Abstract
Several transition metal oxides and hydroxides have been reported as active electrocatalysts towards the oxidation of 5-hydroxymethyl furfural (HMF), an important biomass-derived compound for downstream sustainable chemical and transport fuel production. However, few studies have investigated the activity descriptors influencing the reactivity and selectivity of these materials towards HMF electrooxidation (HMFOR). Herein, Co, Ni and Cu-based Prussian blue analogue (PBA) electrocatalysts were systematically investigated to identify the intrinsic electronic contributions of these redox species towards HMF electrooxidation activity. Cu-phase PBAs exhibited the highest faradaic efficiency of 97.4%, followed by Co-phase (90.5%) and Ni-phase (82.6%) PBAs towards generating 2,5-furandicarboxylic acid (FDCA) at 1.42 V vs. RHE in 1.0 M KOH. This activity trend is found to be influenced by the amount of nucleophilic OH* species expressed on the electrocatalyst's surface. A higher expression of these surface species results in a lower activation energy barrier for the rate limiting step, leading to increased selectivity towards FDCA. Analysis of the bulk electronic properties shows that a strong M–O bond covalency and high d-orbital occupancy contribute to this high expression of electrophilic sites. These findings establish a basis for rationalising the origins of FDCA selectivity in HMFOR catalysts, which importantly eschews using catalysts with high oxygen evolution reaction (OER) activity as a prerequisite for high HMFOR performance.
Introduction
Recently, the electrooxidation of biomass compounds has been studied as a sustainable alternative to conventional petroleum refining for producing platform chemicals.1,2 One notable example is the electrooxidation of 5-hydroxymethyl furfural (HMF), a sugar-derived furan compound to 2,5-furandicarboxylic acid (FDCA).3 FDCA is a key platform chemical used in the production of monomers, agrochemicals, pharmaceuticals and specialty chemicals.4 Owing to FDCA's wide applications, researchers have devoted significant efforts to achieve selective oxidation of HMF to FDCA. The electrooxidation of 5-HMF to FDCA is a multi-step oxidation pathway involving the oxidation of its aldehyde and alcohol functional groups.5 In the literature, this process is known to be less kinetically demanding and has a lower potential requirement (0.113 V vs. NHE) when compared to another similar reaction, the oxygen evolution reaction (OER) at 1.23 V vs. NHE.6 When coupled with the hydrogen evolution reaction (HER) in water electrolysis, it can prospectively enable a more cost-efficient pathway for producing green H2 and platform chemicals.7–9Current studies have reported several transition metal-based materials as highly active catalysts towards 5-HMF electrooxidation.10,11 These materials are preferred over their noble metal counterparts due to their substantially reduced costs, relative abundance, and superior performance. Most currently reported materials are also OER active, where pre-existing materials and associated synthesis strategies are implemented for HMF oxidation electrocatalysts. While these studies demonstrate the feasibility of HMF electrooxidation, thorough investigations into activity descriptor(s) for 5-HMF electrooxidation remain largely scarce, potentially negating possible catalyst candidates for selective 5-HMF electrooxidation.
Although discussions on electronic structure contribution of catalysts towards HMF electrooxidation activity are limited, some insights can be drawn from OER studies involving transition metal oxides. Using density functional theory (DFT) calculations, Moltved and Kepp proposed the inverse relationship between metal species electronegativity and its oxophilicity.12,13 Metals with low electronegativity were found to have higher oxophilicity and therefore stronger binding energies towards surface oxygen species.12 These adsorbed oxygen species form metal oxo/oxyl complexes which act as electrophilic sites during the rate determining step of the OER to form O–O bonds.14,15 Several studies have shown that optimal OER activity is achieved when the binding strength of surface oxygen is neither too strong nor too weak, exhibiting a volcano relationship.16–18 In transition metal oxides, the oxygen adsorption energy trend in the volcano plot is found to correlate with the eg orbital occupancy.19 However, this scaling relationship between the oxygen species binding energy and OER activity has been disputed in recent years, especially in late-transition metal oxides.
Grimaud et al. using in situ18O isotope labelling found that O2 gas can also originate from the lattice oxygen species during the OER.20 The study found that lattice oxygen participation during the OER is driven by the covalency of the metal–oxygen bond, quantifiable by the charge transfer energy gap between the transition metal 3d and O 2p-states. DFT calculations by Rong et al. further showed that the lattice oxygen in perovskites can reversibly form surface oxygen vacancies (VO) by shifting out of plane and interacting with the adsorbed OH*.21 A separate DFT study by Lee et al. reported that the bulk O p-band centre can be used as a reliable descriptor for the VO formation in metal oxides and its OER activity.22 Fundamentally, the lattice oxygen participation mechanism (LOM) indicates that OER activity is more influenced by the bulk electronic structure of the material than the adsorption energies of the oxygen species.
On identifying a descriptor for biomass electrooxidation, Chen et al. similarly discovered the role of lattice oxygen species (Mδ+–O) and adsorbed oxygen species (OH*) in Ni-based catalysts as electrophilic active sites during alcohol electrooxidation.23 These electrophilic sites were found to facilitate the removal of H+ (i.e. hydride transfer) and e− from the substrate molecule by activating the C–H bond or cleaving the O–H bond. Given the identical active sites in both the OER and alcohol electrooxidation, we hypothesise that the ideal descriptor(s) for HMF electrooxidation is linked to electron orbital filling and metal–oxygen bond covalency of the active phase.24,25 Understanding the relationship between these descriptors and FDCA selectivity can enable a more rational approach for designing transition metal-based HMFOR electrocatalysts.
To investigate the above, we chose to experiment with Prussian blue analogues (PBAs), a type of synthetic coordination compound with reported OER activity.26–31 PBAs are typically composed of dual metal sites coordinated with cyanide ligand bridges, representable with the general formula, Ma1[M2(CN)b]c·dH2O (where, M = transition metal & a, b, c, d = stoichiometric ratios). These compounds are highly suited for HMF electrooxidation due to their tuneable metal compositions, ordered framework structure and facile synthesis.32 In alkaline solutions, PBAs often reconstruct into metal (oxy)hydroxides, which rival the catalytic performance of state-of-the-art metal oxide OER electrocatalysts.33,34 This phenomenon highlights PBAs as ideal templates for engineering features or defects into electrocatalysts. In our previous study involving CoFe PBAs, we established a link between the unsaturated Co sites detected in the pulse electrodeposited films with its relatively high HMF electrooxidation activity.35 We demonstrated that unsaturated Co sites promote the expression of electrophilic nucleophiles integral to efficient charge transport. Substituting the Co-site with a more electronegative species could induce a more robust expression of these electrophilic sites.
Herein, Co, Ni and Cu-hexacyanoferrate PBA films were synthesised via pulse electrodeposition to investigate the contribution of the primary redox species (i.e. M = Co, Ni, Cu) towards HMF electrooxidation activity. The Co, Ni and Cu species were chosen due to their established electronegativity trend. Specifically, the number of d valence electrons in each metal species can influence the metal–oxygen bond covalency.36
In oxides, Cu typically exhibits a +2 valence state and does not readily form +3 or +4 valence states like Co and Ni-oxides at high anodic potentials.37,38 This phenomenon results in metal active sites with different coordination geometries which can affect HMF adsorption affinity during electrooxidation.39
Although recent HMF electrooxidation studies have highlighted the role of HMF adsorption strength as an activity descriptor,40,41 the contribution of the catalyst's electronic structure towards this interaction has not been thoroughly explored. The contribution of the oxidation states and local atomic structure in the post-reaction samples was characterised using a suite of spectroscopic techniques, electron microscopy and oxidation performance tests. Combining our experimental data with density functional theory (DFT) calculations, we established that a metal species with a high d-electron count, low charge transfer energy gap and short M–O bond can promote a high expression of electrophilic lattice oxygen species. Such electronic structure contributions accelerate the rate determining step of HMF oxidation, improving its kinetics and selectivity. With these optimal parameters, the PBA-derived Cu-phase exhibits the highest conversion of HMF to FDCA in 1.0 M KOH at 1.42 V vs. RHE. These findings highlight the significance of the metal–oxygen coordination environment in determining the charge transfer kinetics of the HMF electrooxidation and its selectivity towards full conversion to FDCA. The exceptional performance of the Cu-phase also sheds light on the possible application of Cu-based materials for other biomass oxidation reactions.
Results and discussion
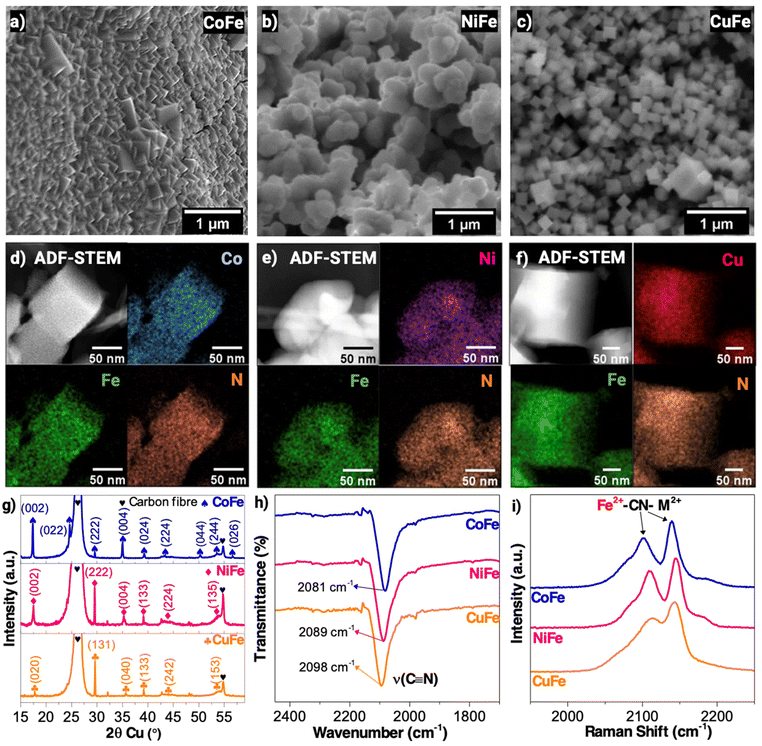
M2[Fe(CN)6] (M: Co, Ni, Cu) thin films were pulse-electrodeposited on Ni-foam substrates using a one-step protocol as described previously.35 For the sake of clarity, the PBA-modified Ni-foam anodes are abbreviated in this study as CoFe, NiFe and CuFe. Electrodeposition times were modified for each PBA system to achieve an equivalent mass-loading (23.4 ± 0.4 mg mg−1 Ni-foam) before electrochemical testing. Fig. 1a–c (top row) show the scanning electron microscope (SEM) images taken for each pristine PBA film. The CoFe and CuFe film images show nanocube structures typical of PBAs, whereas the NiFe film exhibits a truncated nanocube morphology.42 Scanning transmission electron microscope (STEM)-electron dispersive X-ray (EDX) element maps (Fig. 1d–f) show homogeneous distributions of M: (Co, Ni and Cu), Fe, and N in the electrocatalysts. X-ray diffraction (XRD) was used to characterize the crystal structures of these films. The diffraction peaks of the pristine films (Fig. 1g) agree in general with reference patterns of CoFe (#ICSD: 81200), NiFe (#ICSD: 8199) and CuFe (#ICSD: 85961) PBAs.43 The sharp peaks seen in the XRD patterns also show that the PBA phases are highly crystalline.X-ray photoelectron spectroscopy (XPS) was used to characterise the metal species present and their oxidation states. The deconvoluted XPS spectra (Fig. S1 and S2, ESI†) show that the Co,44 Ni,45 Cu46,47 and Fe-sites48 in the synthesised PBA films exhibit the +2 oxidation state. Fourier-transform infrared (FTIR) spectroscopy was used to confirm the presence of cyanide bonds in the film structures. The FTIR scans (Fig. 1h) showed that the CoFe, NiFe and CuFe PBA films contain cyanide stretching peaks at 2081 cm−1, 2088 cm−1 and 2098 cm−1, respectively, which is consistent with the XPS scans and prior characterisation reports of associated PBA variants with MII–CN–FeII moieties.49–51 The Raman peaks (Fig. 1i) observed at ∼2100 cm−1 and ∼2120 cm−1 for the three PBAs are assignable to the Fe2+–CN–M2+.52 Like the FTIR results, the Raman measurements showed that switching from Co → Ni → Cu resulted in a progressive increase in the cyanide bond stretching frequency of the PBA structure. A DFT study by Alsaç et al. proposed that a higher cyanide stretching frequency in PBAs corresponds to a lower electron density of the primary metal site (i.e. M1 = Co, Ni, Cu) from the main precursor.53 In their study, a lower electron density was shown to benefit the nucleophilic attack of water on the oxo/oxyl radical intermediate during the rate determining step of the OER. The effect of this change in the PBA electronic structure towards HMF oxidation activity is discussed below.
Linear sweep voltammetry (LSV) scans were done following anode pre-treatment in 1.0 M KOH to first study their activity towards the OER in the absence of HMF. Fig. S4, ESI† shows the potential needed to reach J = 2.0 mA cm−2 in increasing order: CuFe < NiFe < CoFe. Following the addition of 10 mM HMF (Fig. 2a), the potential order remains similar. However, the CoFe anode requires significantly more potential to achieve higher current densities (J > 2.0 mA cm−2) than the NiFe and CuFe anodes. To further probe HMF electrooxidation activity, we conducted a Tafel analysis in the onset potential region of the LSV plots. Fig. 2b shows that the Tafel slope trend is CoFe > CuFe > NiFe. A lower value such as the one exhibited by the NiFe electrode suggests a more efficient charge transfer kinetics during an electrooxidation process.54
Electrochemical impedance spectroscopy (EIS) was used to study the electron transfer behaviours of the electrocatalyst films. Fig. 2c shows the Nyquist plots measured for each PBA anode at 1.42 V vs. RHE with 10 mM HMF. EIS fitting (Table S1, ESI†) of the measured plot data was done using an equivalent circuit model shown in Fig. S7, ESI†.23 Based on the optimum fitting parameters, the CuFe anode has the lowest charge transfer resistance (Rct) at 0.318 Ω, followed by the CoFe anode (0.693 Ω) and NiFe anode (1.18 Ω). The Rct trend suggests that the CuFe anode is the most conductive and has the strongest interfacial adhesion with the Ni-foam substrate. However the fitted polarisation resistance (Rp) values show the NiFe anode with the highest value (1.18 Ω) followed by the CuFe anode (1.00 Ω) and CoFe anode (0.790 Ω). A higher RP value implies a higher mass transfer occurring at the catalyst–electrolyte interface from an oxidation reaction.55 The EIS trends observed are consistent with the initial Tafel analysis. Altogether, it can be inferred that a conductive structure is generally beneficial towards the HMF electrooxidation kinetics.
To study the impact of the charge transfer rate towards reaction selectivity, constant potential oxidation experiments were conducted. Fig. S8, ESI† shows the charge (Q) values recorded for each PBA anode during a 120-minute reaction with 10 mM HMF at 1.42 V vs. RHE. The charge plots generally exhibit a logarithmic profile consisting of a ‘growth phase’ and a ‘lag’ phase common with the electrooxidation of HMF.56 Comparison of the ‘growth’ phase plot regions in the first 90 minutes shows that the CuFe anode has the highest rate of charge accumulation during reaction. Interestingly, the CoFe anode shared a similar growth profile during the first hour whereafter it decreased below the CuFe anode. In contrast, the NiFe anode has the slowest charge accumulation among the three tested anodes. This result supports the earlier EIS results that a higher conductivity improves the kinetics of HMF electrooxidation.
High-performance liquid chromatography (HPLC) was used to analyse the product formation of aliquots collected periodically during the reaction. The chromatograms (Fig. S9†) show FDCA as the dominant product for each PBA anode following 90 minutes of reaction. Trace amounts of 5-formyl-2-furandicarboxylic acid (FFCA) were also detected in the initial 30 minutes of the reaction for the NiFe and CuFe anodes based on the elution order and absorption wavelength (Fig. S10†). The absence of 5-hydroxymethyl-2-furandicarboxylic acid (HMFCA), the immediate oxidation by-product of HMF, in all the chromatograms also suggests the rapid conversion of HMF to FFCA on the PBA anodes. Fig. 2d and e show the concentration of FDCA detected by HPLC and the calculated faradaic efficiencies (FE%). These results show the conversion performance from the lowest to highest: NiFe < CoFe < CuFe, which is on par with reported HMFOR catalysts (Table S2, ESI†). Additional stability tests (Fig. 2f) with 5 mM HMF at fixed 1 hour durations also exhibit a similar performance trend and high FE% towards FDCA with minimal degradation after four batch cycles. These conversion trends diverge from the Tafel results, indicating that FDCA selectivity is not driven by the rate of charge transfer at the catalyst interface. Recent in situ EIS studies proposed that the amount of adsorbed OH− is linked to the amount of electrophilic oxygen generated, which drives the removal of electrons and protons from adsorbed HMF molecules.55,57 The trends reflected in our experimental data suggest that the affinity of the electrocatalyst surface towards both the adsorbate species is influenced by the atomic and electronic structure of the different redox species.
We conducted post-reaction characterisation of the PBA films using transmission electron microscopy (TEM), Raman, and X-ray absorption spectroscopy (XAS) to elucidate the link between the redox species and the HMF electrooxidation activity. TEM images (Fig. 3a–c) show a clear evolution of nanosheet-like structures from the previously observed nanocubes. The inset selected area electron diffraction (SAED) images obtained near the edges of the nanosheet structures indicate the polycrystalline nature of the crystal lattice. The STEM-EDS maps further show that the nanosheet structures mostly contain their primary metal elements (M: Co, Ni and Cu), O, and only trace amounts of Fe. The TEM results collectively show that the observed structures are no longer PBAs and have transformed into defective layered (oxy)hydroxide nanosheets.35,58,59
Raman analysis was used to characterise the possible phases present in the nanosheet structures. The post-reaction CoFe film (Fig. 3d) exhibits Raman peaks at 192, 473, 516, 610 and 679 cm−1 that can be assigned to a Co3O4 phase.60,61 The post-reaction NiFe film (Fig. 3e) showed characteristic peaks at 478 and 558 cm−1 belonging to NiOOH.62,63 The small peaks at 184 and 637 cm−1 are consistent with prior reports Ni(OH)2 in the literature.64 The post-reaction CuFe film (Fig. 3f) exhibits peaks at 289, 338, and 623 cm−1 belonging to CuO.65 The spectra also contain a small peak at 141 cm−1 which can be co-assigned with the 623 cm−1 peak to a minor Cu2O phase.66 The Raman results indicate that the surface species on each electrocatalyst film are in varying degrees of oxidation.
The electronic structure and local atomic coordination of the nanosheets were further analysed using X-ray absorption near edge spectroscopy (XANES) and extended X-ray absorption fine structure (EXAFS) measurements. Fig. 4a shows the Co K-edge XANES data collected for the pristine and post-reaction CoFe films compared against a prepared Co3O4 reference. The second derivative (Fig. S11, ESI†) of the Co K-edge XANES spectra shows the pristine film with an edge position at 7717.1 eV. After the reaction, the edge position shifts to 7721.5 eV. The two edge positions indicate oxidation from Co2+ → Co3+ species respectively based on matching peaks in the Co3O4 derivative plot (Fig. S11c, ESI†).67 This observation is supported by the Co 2p XPS scan of the post-reaction film (Fig. S3a, ESI†), which showed a Co 2p3/2 peak (779.5 eV) and Co 2p1/2 peak (794.7 eV).68 The XANES spectra of the post-reaction CoFe film exhibit a weak pre-edge feature at 7709 eV. This peak's intensity is weaker than that of the Co3O4 pre-edge peak which implies a slight deviation from centrosymmetric coordination geometry. Together, the XANES features in the post-reaction sample resemble reported profiles of CoOOH, consistent with reports of Co-based electrocatalysts in alkaline solutions.69,70
Examination of the Co K-edge EXAFS region (Fig. 4d) showed two prominent peak positions detected in the post-reaction CoFe spectrum. These peaks arise from Co–O (1.922 ± 0.007 Å), and Co–Co (2.860 ± 0.007 Å), which is consistent with the reported EXAFS analysis of CoOOH.71 The EXAFS fittings (Table S3, ESI†) revealed that the Co–O peak has a coordination number of 5.207 ± 0.4. This value suggests that there are 5 oxygen atoms bound to each Co3+ site on average, which coupled with a weak pre-edge feature indicates it is more likely that the CoOOH adopts a distorted octahedral coordination.
Fig. 4b shows the Ni K-edge XANES profiles for the pristine and post-reaction NiFe PBA films compared against a prepared NiFe LDH. The second derivative of the Ni K-edge XANES spectra (Fig. S12, ESI†) shows that the post-reaction film contains a peak at 8346 eV (edge) similar to the one in the NiFe LDH, assignable to the Ni2+ species.72 The Ni 2p3/2 and 2p1/2 doublet (855.6 eV and 873.0 eV) in the Ni 2p XPS spectra (Fig. S3b, ESI†) confirms this finding.73 Analysis of the XANES pre-edge region (8331–8335 eV) shows that the post-reaction NiFe film does not exhibit any noticeable peaks. This observation indicates that the Ni2+ species adopts an octahedral coordination geometry, which corresponds with the reported structures of β-Ni(OH)2.74
The Ni K-edge EXAFS plots (Fig. 3e) show that the post-reaction film contains a Ni–O peak at 2.050 ± 0.02 Å and a weak Ni–Ni peak at 3.099 ± 0.02 Å. These values are consistent with reported fitted bond lengths of Ni(OH)2.75 EXAFS fittings (Table S3, ESI†) further revealed that the post-reaction structure has a very low Ni–Ni coordination number (1.084 ± 0.7) relative to its Ni–O coordination number (6.366 ± 0.5). This observation suggests that the Ni2+ sites in the Ni(OH)2 phase have fewer 2nd shell neighbors which could lead to a distorted local octahedral coordination geometry and/or a disordered Ni–O–Ni network.
Fig. 4c shows the Cu K-edge XANES measurements for the pristine and post-reaction CuFe films compared against a CuO reference and simulated Cu(OH)2 spectrum. The second derivative plot of the XANES spectra (Fig. S13, ESI†) shows that the post-reaction CuFe film has an edge position at 8992.48 eV, linked to the Cu2+ species.76,77 This position is also present in the pristine film and the two reference spectra. The XANES spectra of the post-reaction film also exhibit a very weak pre-edge (Fig. S14, ESI†) peak at 8978.5 eV representing the forbidden dipole 1s → 3d transition, typical of tetrahedral Cu2+ complexes.78,79
Between the two reference XANES spectra, the post-reaction CuFe film bears more similarity to the CuO reference based on the pre-edge feature and shoulder on the rising part of the edge. However, the similarity of the post-reaction film's white line peak position with the Cu(OH)2 spectra suggests that the structure is likely a mixture of CuO and Cu(OH)2 phases.
Analysis of the Cu K-edge EXAFS region (Fig. 4f) shows that the post-reaction CuFe spectrum contains a Cu–O peak at 1.974 ± 0.008 Å and a Cu–Cu peak at 2.918 ± 0.03 Å. Due to the similarity of first shell coordination of CuO and Cu(OH)2, it is difficult to distinguish these phases in the post-reaction CuFe film using EXAFS analysis alone.80,81 Pair distribution functions (PDFs) of the Cu–O and Cu–Cu pathways in the reference CuO and Cu(OH)2 reference samples were compared against the phase-corrected EXAFS spectra of the post-reaction film. Fig. S16, ESI† shows the post-reaction film matching closely with the Cu(OH)2 sample based on the proximity of the Cu–Cu bond length. The fitting results (Table S3, ESI†) for the Cu–O coordination number (3.950 ± 0.4) show 4-fold coordination, confirming the tetrahedral nature of the structure. However, the low Cu–Cu CN (0.870 ± 0.7) indicates a high degree of disorder in the crystal lattice.
We also performed XAS measurements at the O K-edge to probe the local oxygen bonding environment in the PBA films. Under pristine conditions (Fig. S17, ESI†), all the PBA films exhibit a common pre-edge peak at ∼531.8 eV which corresponds to the electron transition from the O 1s to the empty M 3d–O 2p hybrid orbital.82 The plots also exhibit broad high energy peaks at ∼535 eV belonging to the electron transition from O 2p to the metal 4s and 4p orbitals.83 Unlike the metal K-edge, the O K-edge is more sensitive to surface oxidation products.84 Given the strong evidence in Fig. 1 that the pristine films are PBAs, it is likely the pre-edge features in Fig. S17, ESI† belong to surface oxygen contamination rather than local M–O bonds within the PBA lattice.
Fig. 5 shows the O K-edge XANES plots of the PBA films taken following reaction in 1.0 M KOH. In contrast to the NiFe and CuFe films, the CoFe film exhibits distinct pre-edge features linked to the formation of a CoOOH phase.85 The double peaks at 530 eV and 531.5 eV can be linked to the transitions of the hybridised Co 3d–O 2p states with the t2g and eg orbital symmetry.86 An additional peak is seen at ∼533 eV which appears in both the NiFe and CuFe film plots. In the post-reaction NiFe film plot, a small peak at ∼528 eV prior to the intense peak at ∼533 eV can be seen. This peak can be attributed to the electron transition from O 1s to the nickel vacancies induced O 2p states.87 This observation is consistent with the low Ni–Ni coordination number (1.08 ± 0.74) seen in the Ni K-edge EXAFS fitting results (Table S3, ESI†). However, the post-reaction CuFe plot shows a small shoulder peak at ∼532.2 eV linked to the O 1s → Cu 3d transition.88 The common feature seen in the post-reaction films at ∼533 eV originates from an electron hole feature which transitions from the O 2p → metal 3d orbital specific to each species.89,90 The strength of this feature corresponds to the density of unoccupied O 2p states and can be used to probe the covalency of the M–O bond.83,91 From Fig. 5, it can be surmised that the Cu–O bond in the post-reaction CuFe film has the strongest covalent character among the metal species post-reaction.
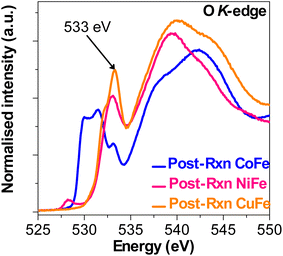 | ||
| Fig. 5 Normalised O–K-edge XANES plots of post-reaction CoFe, NiFe, and CuFe PBA films after reaction in 1.0 M KOH solution. | ||
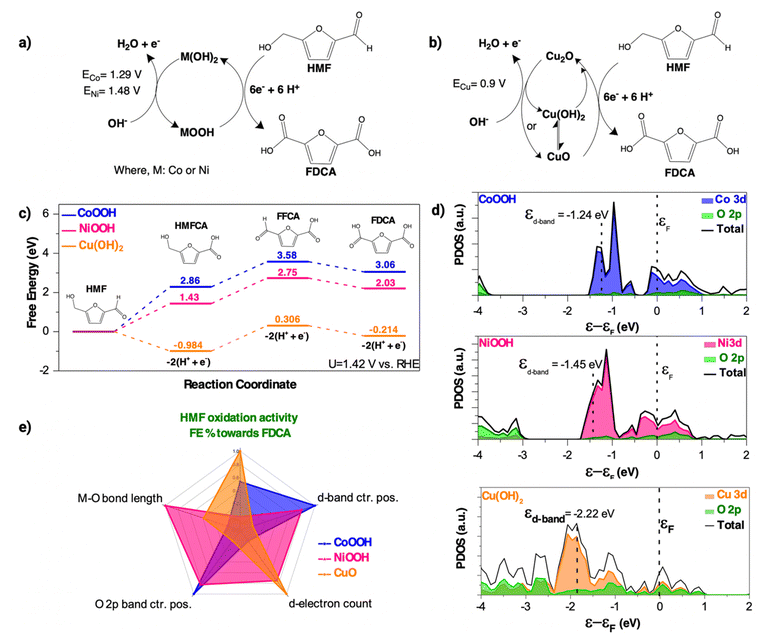
To account for possible phase transitions in various applied potentials, we conducted further cyclic voltammetry (CV) measurements of the stabilised post-reaction film in 1.0 M KOH. The CV plot (Fig. S15a, ESI†) of the post-reaction CoFe film shows an anodic peak at 1.29 V vs. RHE, belonging to the Co2+ → Co3+ oxidation step.92 The plot also shows a cathodic peak at ∼1.37 V vs. RHE, indicating the reversibility of the above process. Based on the collected data, we propose that CoOOH is the active phase in the post-reaction CoFe film during HMF oxidation at 1.42 V vs. RHE.
Several in situ OER studies of Ni-based catalysts in alkaline solutions have reported NiOOH as the active phase during reaction. The presence of the Ni(OH)2 phase in our experiment indicates that this NiOOH phase is not stable without applied bias.93,94 CV scan of the stable post-reaction NiFe film (Fig. S15b, ESI†) confirms the presence of an anodic peak at 1.48 V vs. RHE linked to the Ni2+ → Ni3+ oxidation step.95 The cathodic peak at ∼1.18 V vs. RHEE shows the reverse of the above process. Therefore, we propose that NiOOH is the active phase during HMF oxidation for the post-reaction NiFe film. The data collected so far show that the CoFe and NiFe PBA-derived hydroxides follow a similar redox transition between M(OH)2 and MOOH phases during reaction (Fig. 6a). It also shows that the Co-system requires a lower anodic potential to transition from a +2 → +3 oxidation state than the Ni-system. The mechanistic implications of this lower energetic requirement will be discussed later in this study.
The CV scan of the post-reaction CuFe film (Fig. S15c, ESI†) shows a prominent anodic peak at 0.89 V vs. RHE belonging to the oxidation of the Cu+ species to Cu2+. The reversibility of this process is indicated by a cathodic peak at ∼0.04 V vs. RHE. The presence of both the Cu+ and Cu2+ species is confirmed by further XPS analysis of the Cu 2p region (Fig. S3c, ESI†). The Cu 2p3/2 peak at 932.5 eV and the Cu 2p1/2 peak at 953.2 eV can be linked to a Cu2O phase.96 However, the Cu 2p3/2 and 2p1/2 doublet at 934.4 eV and Cu 2p1/2 at 954.5 eV can be linked to Cu(OH)2 observed in the XAS measurements.97 In alkaline solutions, Cu(OH)2 can further transform to a more thermodynamically stable CuO phase.98 The presence of CuO in the post-reaction CuFe film can be assigned to the Cu 2p3/2 peak at 936.3 eV and the Cu 2p1/2 peak at 956.1 eV.99 Both CuO and Cu2O phases were not observed in the XANES profile due to the bulk nature of the measurement. Based on the characterisation data, we propose that CuO likely supersedes Cu(OH)2 as the dominant active phase (Fig. 6b) during HMF oxidation after prolonged reaction time.
As shown above, the Co, Ni, and Cu-precursors in the PBA films result in different phase evolutions during HMF electrooxidation. These structural changes are the origin of the activity and selectivity trends observed during the experiment. In this section, we investigate the relationship between the electrocatalysts' structural and electronic properties with HMFOR selectivity. The electrooxidation of HMF to FDCA proceeds through a 6-electron transfer pathway. Initially, HMF molecules bind on the electrocatalyst surface and form a geminal diol intermediate via hydration. This intermediate is then oxidised either through its aldehyde group or alcohol group via interaction with the surface-adsorbed OH* species. Prior studies of HMFOR demonstrated that the aldehyde route is energetically preferential in highly alkaline solutions (pH > 13.0) forming 5-hydroxymethyl-2-furancarboxylic acid (HMFCA).41,100 The aldehyde group of HMFCA would then undergo a similar interaction with the surface OH*, causing the deprotonation of the alcohol group to form a carboxyl group. This step forms 5-formyl-2-furancarboxylic acid (FFCA) as an intermediate. In the final step, the FFCA's aldehyde group forms a geminal diol intermediate and undergoes the same deprotonation step to form FDCA.
To elucidate the selectivity towards a 6-electron process, we conducted a thermodynamic analysis of the full reaction pathway using density functional theory (DFT) calculations. Owing to the absence of the Fe-species in the post-reaction TEM, XPS, and XAS characterization data, established crystal structures of conventional oxides and oxyhydroxides of the proposed active phases (Fig. 6a) were used to simplify the calculations (Fig. S18, ESI†). Fig. 6c shows the DFT-calculated free energy diagram for HMF electrooxidation at an applied potential of 1.42 V vs. RHE. The energy profiles show that the electrooxidation of HMF to FDCA is generally an energetically uphill process for the PBA-derived CoOOH and NiOOH. In comparison, the CuO phase mostly exhibits negative values which show the process being thermodynamically favourable. The plots also show different rate limiting steps for the metal systems. For the CoOOH and NiOOH phases, the largest activation energy barrier is the conversion of HMF → HMFCA. However for the CuO phase, it is the HMFCA → FFCA reaction step. These observations suggest that the high selectivity of the PBA-derived CuO stems from its ability to oxidise the aldehyde group at a low activation energy.
Projected density of states (PDOS) analysis of the proposed active phases (Fig. 6d) was conducted to study the distribution of the transition metal 3d states and O 2p-states in relation to the energy trends. The results show that switching from Co to Cu moves the d-band centre position further away from the Fermi level. PDOS analysis (Fig. S20, ESI†) on Fe-doped crystal structures (Fig. S19, ESI†) yielded similar d-band values and order, ruling out the possible contribution of trace Fe in the catalyst's structures. The d-band centre position relative to the Fermi level can be related to the HMF adsorption strength on the electrocatalyst surface in transition metal-based materials.101,102 A d-band centre position further away from the Fermi level indicates more electrons populating the antibonding orbital, weakening the adsorption strength of HMF molecules,103 consequently leading to a lower electrooxidation activity. The PBA-derived CoOOH and NiOOH follow this d-band mechanism (Fig. S21a, ESI†) based on their activity order. However, the PBA-derived CuO's enhanced performance contradicts this relationship.
Xin and Linic proposed that d9 and d10 transition metals are exceptions to the adsorption energy trends in the d-band model when they interact with an electronegative species like OH−.104 In the literature, the adsorption of OH* on the low valence metal redox species is a rate determining step for the deprotonation of C–H/O–H bonds in potential dependent HMF oxidation.10 The oxygen atom in the OH* species is electron rich from transfer due to its higher electronegativity than hydrogen. This environment narrows the spread between the bonding and antibonding states of the M–OH coupling, placing the antibonding states below the Fermi level. In this configuration, the interaction between the metal d-states and the renormalised adsorbate states occurs via Pauli repulsion instead of covalent attraction (Fig. S21c, ESI†).105,106 As a result, a downshift in the d-band centre in the CuO results in a corresponding decrease of electrons filling the antibonding orbital, subsequently increasing the binding strength between the surface OH* and Cu2+ sites. Based on the activity trends, we inferred that the PBA-derived CuO has a higher adsorption affinity towards the OH* than the PBA-derived CoOOH and NiOOH. The charge transfer energy gap (Δ) between the transition metal 3d states and O 2p-states was further examined to study the influence of the bulk electronic structure towards HMF oxidation activity. Table S4, ESI† shows the DFT-calculated energy gap for the proposed active phases. The results (Fig. S22a, ESI†) show that a lower Δ increases the HMF oxidation activity, except for the NiOOH phase. In transition metal oxides, a lower Δ value is associated with higher covalency of the metal–oxygen bond, which lowers the Fermi level relative to the O 2p-band.107 The O 2p-band centre position itself is a descriptor of the amount of electrons that can be shuttled by the M–O bond.108 As the O 2p-band centre moves closer to the Fermi level, there is a tendency for electron holes to form which increases the surface affinity towards the hydroxide species.22 Examination of the calculated O 2p-band centres (Fig. S22b, ESI†) shows that this relationship is consistent with the activity order of the Co and Ni species. Interestingly, the Cu-species O 2p-band centre diverges from this trend. Hong et al. reported that p-type semiconductors (i.e. CuO) do not exhibit a direct scaling relationship between the O 2p band centre and covalency.109 However, a study by Suntivich et al. demonstrated that a high d-electron count in late transition metals correlates with an increased hybridisation between transition metal 3d and O 2p-states.110 Considering the above, we propose that the CuO phase has the highest M–O bond covalency which translates to more electrophilic lattice oxygen sites for HMF oxidation. Experimental evidence of this higher active site expression can be inferred from O K-edge measurements of the post-reaction PBA films (Fig. 5).
The activity of the electrophilic lattice oxygen sites was further analysed using the M–O bond lengths from the EXAFS fitting results. Fig. S23a, ESI† show that the Co and Cu-species have shorter M–O bond lengths than the Ni-species which correlates to a higher FE%. Generally, a shorter M–O bond length is associated with a better chemisorption behaviour due to a shorter electron transfer path.111 This result is largely consistent with the proposed covalency trend above. Fig. 6f shows the proposed radar plot illustrating the impact of each structural descriptor trend discussed so far in this study. From the plot, it can be concluded that a combination of a near occupied d-orbital, low charge transfer energy gap, and a short M–O bond in the Cu species contributed to a higher adsorption of surface hydroxide species and, consequently, leading to an increased coupling interaction with the aldehyde functional group of HMF and its oxidative products, thus promoting a low activation energy for the full transfer of 6 electrons for generating FDCA from HMF. The significant performance gap of the Cu-species over the Co and Ni-species alone highlights the strong influence of the d-electron configuration towards the electrocatalyst bulk electronic properties and its ability to shuttle electrons during HMF oxidation.
Conclusions
In summary, we synthesized Co, Ni, and Cu-based PBA electrocatalysts using pulse electrodeposition to study the activity and selectivity trends for HMF electrooxidation. The results show the Cu-phase achieving a 97.4% FE towards FDCA generation, outperforming both the Co-phase (90.5%) and the Ni-phase (82.6%) at 1.42 V vs. RHE. Combining experimental data and DFT calculations, we identified the d-electron count, charge transfer energy gap, and M–O bond length as probable descriptors for HMF electrooxidation activity in these materials. Free energy calculations show that an optimal combination of these descriptors in the Cu-species contributes to a high expression of electrophilic active sites which significantly lowers the energy barrier for a 6-electron transfer pathway to FDCA. Our findings present a simple framework for forecasting the HMF oxidation activity of transition metal-based materials using their bulk electronic properties and aid in the rational design of HMF oxidation electrocatalysts, including those with high d-electron count for future work (Zn, Ag, etc.). Importantly, this approach can include catalysts that are not traditionally used for the OER, providing a pathway toward tuning biomass electrooxidation for a range of prospective applications and chemical reactions.With the growing significance of green hydrogen in renewable economies, future studies should investigate PBA materials as bifunctional electrocatalysts towards the HER and HMFOR in flow electrolyzer cells. The excellent performance stability of these materials in this study potentially enables easier scale-up investigations in larger current densities paving the way towards future commercial applications.
Author contributions
William Hadinata: experiment design and execution, data analysis, manuscript preparation. Yuwei-Yang: XAS data processing and analysis, manuscript preparation. Jodie Yuwono: DFT PDOS and Gibbs free energy calculations and data analysis, manuscript preparation. Priyank Kumar: DFT computing resource allocation, manuscript preparation. Constantine Tsounis: TEM, STEM-EDX imaging of samples, data analysis, and manuscript preparation. Muhammad Zubair: experimental NEXAFS data analysis, theoretical XANES & EXAFS simulation of the Cu(OH)2 reference spectrum, and manuscript preparation. Joshua Wright: XAS measurements and data processing. Lars Thomsen: NEXAFS measurements and data processing. Nicholas Bedford: project planning, interpretation of results, manuscript editing.Conflicts of interest
The authors declare no conflicts of interests.Acknowledgements
This work was financially supported in part by the Australia Renewable Energy Agency's (ARENA) Hydrogen Program (2018/RND015) and the Faculty of Engineering at UNSW. WHL acknowledges scholarship support from the Australian Commonwealth Government for the Research Training Program (RTP), while MZ acknowledges scholarship support from the UNSW Scientia Scholarship Program. JAY and PK acknowledge computational resources provided by the Australian Government through National Computational Infrastructure (NCI). The authors acknowledge the scientific and technical assistance from Mark Wainwright Analytical Centre (MWAC) at UNSW for the use of instruments at the Electron Microscope Unit (EMU) and other spectroscopy facilities. XAS experiments were performed at the 10-ID-B beamline of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Facilities at 10-ID-B are further supported by the Materials Research Collaborative Access Team and its member institutions. NEXAFS measurements were performed at the SXR beamline at the Australian Synchrotron, an ANSTO facility.Notes and references
- Z. Fang and X. Qi, Production of Platform Chemicals from Sustainable Resources, Springer, 2017 Search PubMed.
- F. W. S. Lucas, R. G. Grim, S. A. Tacey, C. A. Downes, J. Hasse, A. M. Roman, C. A. Farberow, J. A. Schaidle and A. Holewinski, ACS Energy Lett., 2021, 6, 1205–1270 CrossRef CAS.
- R.-J. van Putten, J. C. Van Der Waal, E. De Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- E. de Jong, M. A. Dam, L. Sipos and G. J. M. Gruter, in Biobased Monomers, Polymers, and Materials, American Chemical Society, 2012, vol. 1105, ch. 1, pp. 1–13 Search PubMed.
- T. Su, D. Zhao, Y. Wang, H. Lü, R. S. Varma and C. Len, ChemSusChem, 2021, 14, 266–280 CrossRef CAS PubMed.
- Y. Xie, Z. Zhou, N. Yang and G. Zhao, Adv. Funct. Mater., 2021, 31, 2102886 CrossRef CAS.
- B. You, X. Liu, N. Jiang and Y. Sun, J. Am. Chem. Soc., 2016, 138, 13639–13646 CrossRef CAS PubMed.
- B. You, X. Liu, X. Liu and Y. Sun, ACS Catal., 2017, 7, 4564–4570 CrossRef CAS.
- W. Li, N. Jiang, B. Hu, X. Liu, F. Song, G. Han, T. J. Jordan, T. B. Hanson, T. L. Liu and Y. Sun, Chem, 2018, 4, 637–649 CAS.
- Y. Yang and T. Mu, Green Chem., 2021, 23, 4228–4254 RSC.
- O. Simoska, Z. Rhodes, S. Weliwatte, J. R. Cabrera-Pardo, E. M. Gaffney, K. Lim and S. D. Minteer, ChemSusChem, 2021, 14(7), 1674–1686 CrossRef CAS PubMed.
- K. P. Kepp, Inorg. Chem., 2016, 55, 9461–9470 CrossRef CAS PubMed.
- K. A. Moltved and K. P. Kepp, J. Phys. Chem. C, 2019, 123, 18432–18444 CrossRef CAS.
- M. Zhang, M. De Respinis and H. Frei, Nat. Chem., 2014, 6, 362–367 CrossRef CAS PubMed.
- X.-P. Zhang, A. Chandra, Y.-M. Lee, R. Cao, K. Ray and W. Nam, Chem. Soc. Rev., 2021, 50, 4804–4811 RSC.
- R. Subbaraman, D. Tripkovic, K.-C. Chang, D. Strmcnik, A. P. Paulikas, P. Hirunsit, M. Chan, J. Greeley, V. Stamenkovic and N. M. Markovic, Nat. Mater., 2012, 11, 550–557 CrossRef CAS PubMed.
- T. Reier, M. Oezaslan and P. Strasser, ACS Catal., 2012, 2, 1765–1772 CrossRef CAS.
- X. Mu, Y. Zhu, X. Gu, S. Dai, Q. Mao, L. Bao, W. Li, S. Liu, J. Bao and S. Mu, J. Energy Chem., 2021, 62, 546–551 CrossRef CAS.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383–1385 CrossRef CAS PubMed.
- A. Grimaud, K. J. May, C. E. Carlton, Y.-L. Lee, M. Risch, W. T. Hong, J. Zhou and Y. Shao-Horn, Nat. Commun., 2013, 4, 2439 CrossRef PubMed.
- X. Rong, J. Parolin and A. M. Kolpak, ACS Catal., 2016, 6, 1153–1158 CrossRef CAS.
- Y.-L. Lee, J. Kleis, J. Rossmeisl, Y. Shao-Horn and D. Morgan, Energy Environ. Sci., 2011, 4, 3966 RSC.
- W. Chen, C. Xie, Y. Wang, Y. Zou, C.-L. Dong, Y.-C. Huang, Z. Xiao, Z. Wei, S. Du and C. Chen, Chem, 2020, 6, 2974–2993 CAS.
- W. T. Hong, R. E. Welsch and Y. Shao-Horn, J. Phys. Chem. C, 2016, 120, 78–86 CrossRef CAS.
- T. G. Yun, Y. Heo, H. Bin Bae and S.-Y. Chung, Nat. Commun., 2021, 12, 824 CrossRef CAS PubMed.
- Y. Wang, J. Ma, J. Wang, S. Chen, H. Wang and J. Zhang, Adv. Mater., 2019, 9, 1802939 Search PubMed.
- Z.-Y. Yu, Y. Duan, J.-D. Liu, Y. Chen, X.-K. Liu, W. Liu, T. Ma, Y. Li, X.-S. Zheng and T. Yao, Nat. Commun., 2019, 10, 1–9 CrossRef PubMed.
- Z. Chen, B. Fei, M. Hou, X. Yan, M. Chen, H. Qing and R. Wu, Nano Energy, 2020, 68, 104371 CrossRef CAS.
- L.-M. Cao, Y.-W. Hu, S.-F. Tang, A. Iljin, J.-W. Wang, Z.-M. Zhang and T.-B. Lu, Adv. Sci., 2018, 5, 1800949 CrossRef PubMed.
- X. Zhou, L. Xu, Y. Gao, L. Li, J. Tang and J. Yang, Sustainable Energy Fuels, 2020, 4, 2411–2421 RSC.
- X. Xing, Y. Song, W. Jiang and X. Zhang, Sustainable Energy Fuels, 2020, 4, 3985–3991 RSC.
- W. J. Li, C. Han, G. Cheng, S. L. Chou, H. K. Liu and S. X. Dou, Small, 2019, 15, 1900470 CrossRef PubMed.
- B. He, P. Kuang, X. Li, H. Chen, J. Yu and K. Fan, Chem.–Eur. J., 2020, 26, 4052–4062 CrossRef CAS PubMed.
- B. K. Kang, M. H. Woo, J. Lee, Y. H. Song, Z. Wang, Y. Guo, Y. Yamauchi, J. H. Kim, B. Lim and D. H. Yoon, J. Mater. Chem. A, 2017, 5, 4320–4324 RSC.
- W. H. Lie, C. Deng, Y. Yang, C. Tsounis, K.-H. Wu, M. V. Chandra-Hioe, N. M. Bedford and D. Wang, Green Chem., 2021, 23(12), 4333–4337 RSC.
- X. Guo, L. Li, Z. Liu, D. Yu, J. He, R. Liu, B. Xu, Y. Tian and H.-T. Wang, J. Appl. Phys., 2008, 104, 023503 CrossRef.
- Y. Deng, A. D. Handoko, Y. Du, S. Xi and B. S. Yeo, ACS Catal., 2016, 6, 2473–2481 CrossRef CAS.
- Q. Chen, Y. Hao, C. Cao and J. Zhang, Nanoscale, 2022, 15(2), 450–460 Search PubMed.
- Y. Lu, C.-L. Dong, Y.-C. Huang, Y. Zou, Z. Liu, Y. Liu, Y. Li, N. He, J. Shi and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 19215–19221 CrossRef CAS PubMed.
- Y. Yang, D. Xu, B. Zhang, Z. Xue and T. Mu, Chem. Eng. J., 2021, 133842 Search PubMed.
- X. Lu, K. H. Wu, B. Zhang, J. Chen, F. Li, B. J. Su, P. Yan, J. M. Chen and W. Qi, Angew. Chem., 2021, 133, 14649–14656 CrossRef.
- M. Cao, X. Wu, X. He and C. Hu, Chem. Commun., 2005, 2241, 10.1039/b500153f.
- I. Levin, NIST Standard Reference Database Number 3, 2018, DOI:10.18434/M32147, accessed 07-11-2021.
- X. Yang, H. Mi, X. Ren, P. Zhang and Y. Li, Nanoscale Res. Lett., 2020, 15(82), 1–12 Search PubMed.
- A. Nadeema, V. M. Dhavale and S. Kurungot, Nanoscale, 2017, 9, 12590–12600 RSC.
- Y. Duan, F. Gao and A. V. Teplyakov, J. Phys. Chem. C, 2015, 119, 27018–27027 CrossRef CAS PubMed.
- R. J. Deokate, H. S. Chavan, S. C. Bulakhe, S. B. Tanwade, S. H. Mujawar, S. S. Mali, C. K. Hong, H. Im and A. I. Inamdar, Int. J. Energy Res., 2022, 46, 5269–5279 CrossRef CAS.
- D. Baster, E. Oveisi, P. Mettraux, S. Agrawal and H. H. Girault, Chem. Commun., 2019, 55, 14633–14636 RSC.
- D. O. Ojwang, J. Grins, D. Wardecki, M. Valvo, V. Renman, L. Häggström, T. Ericsson, T. Gustafsson, A. Mahmoud, R. P. Hermann and G. Svensson, Inorg. Chem., 2016, 55, 5924–5934 CrossRef CAS PubMed.
- T. Lara-Estrada, F. Carvajal-Ramos, M. G. Pérez-García, E. D. Moreno-Medrano, J. B. Pelayo-Vázquez, M. Bárcena-Soto and A. Gutiérrez-Becerra, Colloids Surf., A, 2018, 544, 1–7 CrossRef CAS.
- Y. Xu, M. Chang, C. Fang, Y. Liu, Y. Qiu, M. Ou, J. Peng, P. Wei, Z. Deng, S. Sun, X. Sun, Q. Li, J. Han and Y. Huang, ACS Appl. Mater. Interfaces, 2019, 11, 29985–29992 CrossRef CAS PubMed.
- Y. Zeng, G.-F. Chen, Z. Jiang, L.-X. Ding, S. Wang and H. Wang, J. Mater. Chem. A, 2018, 6, 15942–15946 RSC.
- E. P. Alsaç, E. Ülker, S. V. K. Nune, Y. Dede and F. Karadas, Chem.–Eur. J., 2018, 24, 4856–4863 CrossRef PubMed.
- T. Shinagawa, A. T. Garcia-Esparza and K. Takanabe, Sci. Rep., 2015, 5, 13801 CrossRef PubMed.
- B. Zhou, C.-L. Dong, Y.-C. Huang, N. Zhang, Y. Wu, Y. Lu, X. Yue, Z. Xiao, Y. Zou and S. Wang, J. Energy Chem., 2021, 61, 179–185 CrossRef CAS.
- R. Latsuzbaia, R. Bisselink, A. Anastasopol, H. van der Meer, R. van Heck, M. S. Yagüe, M. Zijlstra, M. Roelands, M. Crockatt and E. Goetheer, J. Appl. Electrochem., 2018, 48, 611–626 CrossRef CAS.
- Y. Lu, T. Liu, C. L. Dong, C. Yang, L. Zhou, Y. C. Huang, Y. Li, B. Zhou, Y. Zou and S. Wang, Adv. Mater., 2021, 2107185, DOI:10.1002/adma.202107185.
- H. Fang, T. Huang, D. Liang, M. Qiu, Y. Sun, S. Yao, J. Yu, M. M. Dinesh, Z. Guo, Y. Xia and S. Mao, J. Mater. Chem. A, 2019, 7, 7328–7332 RSC.
- A. Indra, U. Paik and T. Song, Angew. Chem., Int. Ed., 2018, 57, 1241–1245 CrossRef CAS.
- J. Yang, H. Liu, W. N. Martens and R. L. Frost, J. Phys. Chem. C, 2010, 114, 111–119 CrossRef CAS.
- J. Jiang and L. Li, Mater. Lett., 2007, 61, 4894–4896 CrossRef CAS.
- R. Kostecki and F. McLarnon, J. Electrochem. Soc., 1997, 144, 485–493 CrossRef CAS.
- R. N. Dürr, P. Maltoni, H. Tian, B. Jousselme, L. Hammarström and T. Edvinsson, ACS Nano, 2021, 15, 13504–13515 CrossRef PubMed.
- B.-H. Chen, Z.-S. Chao, H. He, C. Huang, Y.-J. Liu, W.-J. Yi, X.-L. Wei and J.-F. An, Dalton Trans., 2016, 45, 2720–2739 RSC.
- J. Xu, W. Ji, Z. Shen, W. Li, S. Tang, X. Ye, D. Jia and X. Xin, J. Raman Spectrosc., 1999, 30, 413–415 CrossRef CAS.
- A. Singhal, M. R. Pai, R. Rao, K. T. Pillai, I. Lieberwirth and A. K. Tyagi, Eur. J. Inorg. Chem., 2013, 2013, 2640–2651 CrossRef CAS.
- B. Yildirim and H. Riesen, J. Phys.: Conf. Ser., 2013, 430, 2011 CrossRef.
- T. Sattar, S.-J. Sim, B.-S. Jin and H.-S. Kim, Sci. Rep., 2021, 11, 1–9 CrossRef PubMed.
- H.-Y. Chen, Y.-C. Chang, J.-F. Lee, C.-W. Pao, H.-C. Huang and C.-H. Wang, ACS Sustainable Chem. Eng., 2021, 9, 12300–12310 CrossRef CAS.
- Y. Huang, X. Zhao, F. Tang, X. Zheng, W. Cheng, W. Che, F. Hu, Y. Jiang, Q. Liu and S. Wei, J. Mater. Chem. A, 2018, 6, 3202–3210 RSC.
- A. Bergmann, T. E. Jones, E. Martinez Moreno, D. Teschner, P. Chernev, M. Gliech, T. Reier, H. Dau and P. Strasser, Nat. Catal., 2018, 1, 711–719 CrossRef CAS.
- D. Drevon, M. Görlin, P. Chernev, L. Xi, H. Dau and K. M. Lange, Sci. Rep., 2019, 9, 1532 CrossRef PubMed.
- K. Fan, H. Chen, Y. Ji, H. Huang, P. M. Claesson, Q. Daniel, B. Philippe, H. Rensmo, F. Li, Y. Luo and L. Sun, Nat. Commun., 2016, 7, 11981 CrossRef CAS PubMed.
- D. S. Hall, D. J. Lockwood, C. Bock and B. R. MacDougall, Proc. R. Soc. A, 2015, 471, 20140792 CrossRef PubMed.
- X. Su, Y. Wang, J. Zhou, S. Gu, J. Li and S. Zhang, J. Am. Chem. Soc., 2018, 140, 11286–11292 CrossRef CAS PubMed.
- L. S. Kau, D. J. Spira-Solomon, J. E. Penner-Hahn, K. O. Hodgson and E. I. Solomon, J. Am. Chem. Soc., 1987, 109, 6433–6442 CrossRef CAS.
- S. H. Liu, H. P. Wang, C. H. Huang and T. L. Hsiung, J. Synchrotron Radiat., 2010, 17, 202–206 CrossRef CAS PubMed.
- D. T. Bowron, M. Amboage, R. Boada, A. Freeman, S. Hayama and S. Díaz-Moreno, RSC Adv., 2013, 3, 17803 RSC.
- A. Gaur, W. Klysubun, N. Nitin Nair, B. D. Shrivastava, J. Prasad and K. Srivastava, J. Mol. Struct., 2016, 1118, 212–218 CrossRef CAS.
- E. M. C. Alayon, M. Nachtegaal, A. Bodi, M. Ranocchiari and J. A. van Bokhoven, Phys. Chem. Chem. Phys., 2015, 17, 7681–7693 RSC.
- J. Timoshenko, H. S. Jeon, I. Sinev, F. T. Haase, A. Herzog and B. R. Cuenya, Chem. Sci., 2020, 11, 3727–3736 RSC.
- Y. Satou, S. Komine and S. Shimizu, AIP Adv., 2017, 7, 095117 CrossRef.
- F. Frati, M. O. J. Y. Hunault and F. M. F. de Groot, Chem. Rev., 2020, 120, 4056–4110 CrossRef CAS PubMed.
- J. A. Bradley, P. Yang, E. R. Batista, K. S. Boland, C. J. Burns, D. L. Clark, S. D. Conradson, S. A. Kozimor, R. L. Martin, G. T. Seidler, B. L. Scott, D. K. Shuh, T. Tyliszczak, M. P. Wilkerson and L. E. Wolfsberg, J. Appl. Chem. Sci., 2010, 132, 13914–13921 CAS.
- J. Zhou, Y. Wang, X. Su, S. Gu, R. Liu, Y. Huang, S. Yan, J. Li and S. Zhang, Energy Environ. Sci., 2019, 12, 739–746 RSC.
- Y. Chin, Z. Hu, H.-J. Lin, S. Agrestini, J. Weinen, C. Martin, S. Hébert, A. Maignan, A. Tanaka and J. Cezar, Phys. Rev. B, 2019, 100, 205139 CrossRef CAS.
- H. Y. Peng, Y. F. Li, W. N. Lin, Y. Z. Wang, X. Y. Gao and T. Wu, Sci. Rep., 2012, 2, 442 CrossRef PubMed.
- E. C. Todd, D. M. Sherman and J. A. Purton, Geochim. Cosmochim. Acta, 2003, 67, 2137–2146 CrossRef CAS.
- D. K. Bora, A. Braun, S. Erat, A. K. Ariffin, R. Löhnert, K. Sivula, J. Töpfer, M. Grätzel, R. Manzke, T. Graule and E. C. Constable, J. Phys. Chem., 2011, 115, 5619–5625 CAS.
- P. Hillyard, S. Kuchibhatla, T. Glover, M. Hertlein, N. Huse, P. Nachimuthu, L. Saraf, S. Thevuthasan and K. Gaffney, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 125210 CrossRef.
- F. M. F. de Groot, M. Grioni, J. C. Fuggle, J. Ghijsen, G. A. Sawatzky and H. Petersen, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 40, 5715–5723 CrossRef CAS PubMed.
- R. Samal, B. Dash, C. Sarangi, K. Sanjay, T. Subbaiah, G. Senanayake and M. Minakshi, Nanomaterials, 2017, 7, 356 CrossRef PubMed.
- A. Van Der Ven, D. Morgan, Y. S. Meng and G. Ceder, J. Electrochem. Soc., 2006, 153, A210 CrossRef CAS.
- Š. Trafela, J. Zavašnik, S. Šturm and K. Ž. Rožman, Electrochim. Acta, 2019, 309, 346–353 CrossRef.
- O. Diaz-Morales, D. Ferrus-Suspedra and M. T. M. Koper, Chem. Sci., 2016, 7, 2639–2645 RSC.
- S. Felix, P. Kollu, B. P. Raghupathy, S. K. Jeong and A. N. Grace, J. Chem. Sci., 2014, 126, 25–32 CrossRef CAS.
- A. A. Dubale, W.-N. Su, A. G. Tamirat, C.-J. Pan, B. A. Aragaw, H.-M. Chen, C.-H. Chen and B.-J. Hwang, J. Mater. Chem. A, 2014, 2, 18383–18397 RSC.
- Y. Cudennec and A. Lecerf, Solid State Sci., 2003, 5, 1471–1474 CrossRef CAS.
- G. Su, T. Feng, Z. Huang, Y. Zheng, W. Zhang, G. Liu, W. Wang, H. Wei and L. Dang, Chem. Eng. J., 2022, 436, 135119 CrossRef CAS.
- N. Heidary and N. Kornienko, Chem. Sci., 2020, 11, 1798–1806 RSC.
- S. Li, X. Sun, Z. Yao, X. Zhong, Y. Cao, Y. Liang, Z. Wei, S. Deng, G. Zhuang, X. Li and J. Wang, Adv. Funct. Mater., 2019, 29, 1904780 CrossRef CAS.
- Y. Zhang, Z. Xue, X. Zhao, B. Zhang and T. Mu, Green Chem., 2022, 24, 1721–1731 RSC.
- L. Yu, Q. Yan and A. Ruzsinszky, Phys. Rev. Mater., 2019, 3(9), 092801 CrossRef CAS.
- H. Xin and S. Linic, J. Chem. Phys., 2010, 132, 221101 CrossRef PubMed.
- B. Hammer and J. K. Norskov, Nature, 1995, 376, 238–240 CrossRef CAS.
- B. Hammer and J. K. Nørskov, Adv. Catal., 2000, 45, 71–129 CAS.
- J. Zaanen, G. A. Sawatzky and J. W. Allen, Phys. Rev. Lett., 1985, 55, 418–421 CrossRef CAS PubMed.
- H. Lee, O. Gwon, K. Choi, L. Zhang, J. Zhou, J. Park, J.-W. Yoo, J.-Q. Wang, J. H. Lee and G. Kim, ACS Catal., 2020, 10, 4664–4670 CrossRef CAS.
- W. T. Hong, K. A. Stoerzinger, Y.-L. Lee, L. Giordano, A. Grimaud, A. M. Johnson, J. Hwang, E. J. Crumlin, W. Yang and Y. Shao-Horn, Energy Environ. Sci., 2017, 10, 2190–2200 RSC.
- J. Suntivich, W. T. Hong, Y.-L. Lee, J. M. Rondinelli, W. Yang, J. B. Goodenough, B. Dabrowski, J. W. Freeland and Y. Shao-Horn, J. Phys. Chem. C, 2014, 118, 1856–1863 CrossRef CAS.
- A. Kulkarni, S. Siahrostami, A. Patel and J. K. Nørskov, Chem. Rev., 2018, 118, 2302–2312 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta08306j |
| This journal is © The Royal Society of Chemistry 2023 |