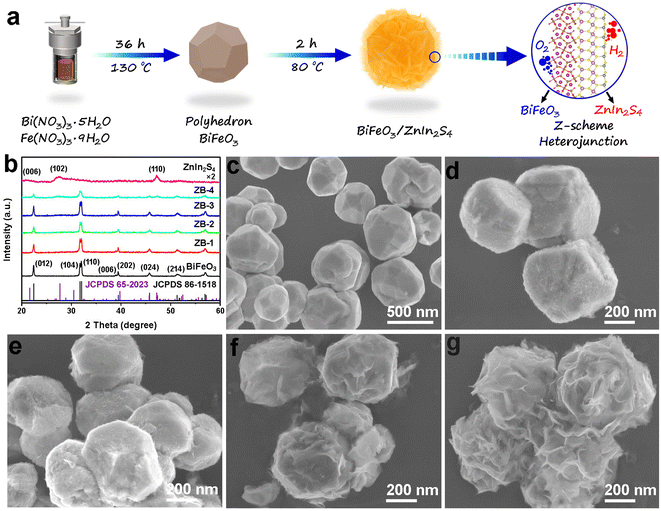
Synergizing the internal electric field and ferroelectric polarization of the BiFeO3/ZnIn2S4 Z-scheme heterojunction for photocatalytic overall water splitting†
Jian
Zhang
 *ab,
Ying
Zhang
a,
Lutao
Li
b,
Wei
Yan
a,
Haiyun
Wang
a,
Weiwei
Mao
*ab,
Ying
Zhang
a,
Lutao
Li
b,
Wei
Yan
a,
Haiyun
Wang
a,
Weiwei
Mao
 *a,
Yan
Cui
*c,
Yonghua
Li
b and
Xinbao
Zhu
d
*a,
Yan
Cui
*c,
Yonghua
Li
b and
Xinbao
Zhu
d
aNew Energy Technology Engineering Lab of Jiangsu Province, College of Science, Nanjing University of Posts & Telecommunications (NUPT), Nanjing 210023, P. R. China. E-mail: iamjzhang@njupt.edu.cn; maoww@njupt.edu.cn
bState Key Laboratory of Organic Electronics and Information Displays and Institute of Advanced Materials (IAM), Nanjing University of Posts and Telecommunications (NUPT), Nanjing 210023, Jiangsu, P. R. China
cKey Laboratory of Broadband Wireless Communication and Sensor Network Technology, Ministry of Education, Nanjing University of Posts and Telecommunications, Nanjing 210003, P. R. China. E-mail: Cuiyan@njupt.edu.cn
dCollege of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, P. R. China
First published on 29th November 2022
Abstract
Coupling the internal electric field and ferroelectric polarization via the modulation of surface electronic structures to achieve high carrier separation and migration efficiency is attractive but challenging in solar-to-chemical energy conversion. Herein, we report that coating ultrathin ZnIn2S4 nanoflakes on BiFeO3 polyhedron microparticles constructs a ferroelectric polarization and internal electric field enhanced Z-scheme heterojunction photocatalyst (BiFeO3/ZnIn2S4). Benefiting from the efficient photogenerated carrier separation and migration processes triggered by powerful spontaneous polarization and internal electric field, respectively, the nanocomposites exhibit strong half-reaction activity over hydrogen and oxygen evolution. Importantly, the optimal nanohybrid photocatalyst (ZB-3) delivered superior photocatalytic overall water splitting activity (H2: 87.3 μmol g−1 h−1, O2: 42.3 μmol g−1 h−1) with an apparent quantum efficiency of 1.12% at 420 nm in pure water under visible light irradiation, the highest activity reported thus far for ferroelectric-based heterojunction photocatalysts. The novel strategy of synergizing internal electric field and ferroelectric polarization via hybridization has been proposed to boost the separation and transfer efficiency of photogenerated charges in ferroelectric materials.
1 Introduction
Among the potential green fuels from artificial photosynthesis, hydrogen is one of the most attractive candidates owing to its high combustion value and zero-carbon emission.1–3 Ever since TiO2 was employed to split water under solar energy in 1972,4 hydrogen obtained from photocatalytic water splitting over particulate photocatalysts has been considered to hold great promise owing to its obvious advantages such as its sustainability, environment-friendly nature and scalability. Typically, the photocatalytic overall water splitting process is a thermodynamically uphill reaction, and the splitting efficiency is determined by (i) the light absorption capacity, (ii) charge separation and migration efficiency of the photocatalyst and (iii) activity of catalytic sites. Considering the practical requirements of commercial competitiveness with traditional industrial hydrogen generation methods, an energy conversion efficiency of over 10% with long-term stability is vital, which makes it urgent to design and develop versatile photocatalysts.5 In the past half century, various modified methods have been developed to improve charge separation and transfer efficiency on semiconductor-based photocatalysts, such as doping heteroatoms, loading cocatalysts, Z-scheme construction, phase junction, and heterojunction assembly.6–12Among the numerous reported semiconductors, perovskite-type ABO3 ferroelectric semiconductors (e.g., BiFeO3) have drawn increasing attention in photocatalysis compared with conventional metallic compound photocatalysts in recent years owing not only to their strong absorption of photons but also to their adjustable spontaneous polarization to influence charge behavior.13 Spontaneous polarization, as a peculiar intrinsic property of ferroelectrics, has been demonstrated as a more efficient driving force of charge separation than the universal junction method in related applications of photogenerated carriers, such as photovoltaics and photocatalysis.14,15 In this regard, the open-circuit voltage and short-circuit current density of the BiLaFeO3 photovoltaic cell increased by 8.4% and 54.7%, respectively, via the enhancement of ferroelectric polarization.16 Similarly, Li et al. reported that the photocatalytic hydrogen evolution activity of PbTiO3 was considerably improved when the particle size increased along the polarization direction, which directly contributed to the driving force of charge separation.17 Despite exhibiting fascinating modulation between spontaneous polarization and carrier features, particulate BiFeO3 normally exhibits low photocurrent and poor activity, and the underlying mechanism is still under debate. Besides, the bottleneck of photocatalytic applications can also be ascribed to some intrinsic obstacles in the preparation process of BiFeO3, which usually contains crystal phase, defect concentration, and domain wall distribution.18–20 These all brought a tough challenge to us in illustrating clearly the photocatalytic mechanism of particulate ferroelectric photocatalysts.
In parallel, surface features of catalysts including functional group,21 atomic defects,22,23 and junctions24–27 have a great impact on charge migration and utilization. Considering the fact that the band of BiFeO3 straddles the water redox energy only, loading noble-metal-based water redox cocatalysts just enhanced oxygen evolution activity, difficult to finish the overall water splitting. Theoretically, merging another band-matched semiconductor to fabricate a Z-scheme heterojunction can fill the intrinsic gap of BiFeO3 towards overall water-splitting application. Unfortunately, BiFeO3 Z-scheme heterojunction photocatalysts for overall water splitting are still difficult to achieve due to their intrinsic sluggish dynamics. Although BiFeO3 particulate as photocatalyst and photoelectrocatalyst for half-reaction water splitting has been already reported, with assistance from polarization modulation or heterojunction hybrid assemblage,28,29 synergistic effect between ferroelectric polarization and internal electric field for efficient photocatalytic overall water splitting without any sacrificial agent and cocatalysts has never been realized to date and still remains a significant basic science challenge. ZnIn2S4 has been verified as an effective visible light photocatalyst to achieve hydrogen evolution from water. On the other hand, the ultrathin ZnIn2S4 nanosheets for core/shell heterogeneous construction has some significant advantage, including the large amounts of the reactive active site, the compact interface, and the stable steric configuration.
In this work, we fabricated visible light-driven overall water-splitting Z-scheme heterojunction photocatalysts of BiFeO3/ZnIn2S4 by a moderate two-step solvothermal process (Fig. 1a). Such a core/shell configuration delivered accelerated surface reaction kinetics stemming from the enhanced ferroelectric polarization and internal electric field. As a result, the good activities of half-reaction photocatalytic water splitting over these BiFeO3/ZnIn2S4 nanohybrids with different ratios were monitored by hydrogen evolution and oxygen evolution under different charge sacrificial agents. Then, under visible light irradiation (λ > 420 nm), the optimal photocatalytic performance from the core/shell heterojunction labeled ZB-3 exhibited remarkable overall water splitting performance with hydrogen and oxygen generation rates of up to 87.3 and 42.3 μmol g−1 h−1, respectively, and with an apparent quantum efficiency of 1.12% at 420 nm in pure water. The quantitative study on ferroelectric spontaneous polarization, transient absorption kinetics results, and surface photovoltage demonstrated the rational design to boost overall water-splitting performance. This work offers a novel strategy for the rational design of ferroelectric semiconductors targeting the direct conversion of solar into hydrogen energy.
2 Results and discussion
2.1. Characterizations of the as-prepared photocatalysts
The X-ray diffraction (XRD) patterns of the as-prepared BiFeO3, ZnIn2S4, and different ratios of BiFeO3/ZnIn2S4 heterojunctions (ZB-1, ZB-2, ZB-3 and ZB-4, see Experimental section for details) are shown in Fig. 1b. Firstly, the eight obvious diffraction peaks of the pure BiFeO3 curve are in good accordance with the standard card of rhombohedral R3c type BiFeO3 (JCPDS 86-1518)30 without any impurity phase, indicating that the pure phase of BiFeO3 can also grow successfully under lower temperatures (130 °C) compared to that in other reports that involve the annealing process.22,31 For single ZnIn2S4, the peaks recorded at 2 theta values of 21.2°, 27.6°, 30.3°, and 47.3° are ascribed to the (006), (102), (104), and (110) crystallite planes of the hexagonal ZnIn2S4 (JCPDS No. 65-2023), respectively.32 In the case of hybrids (ZB-1, ZB-2, ZB-3, and ZB-4), it is not difficult to find that the diffraction peak position of the composites were kept well and the strength of the diffraction signals from ZnIn2S4 became gradually stronger as the proportion increased. The surface morphology and size of samples were investigated by field emission scanning electron microscopy (FESEM) (Fig. 1c–g and S1†). The bare BiFeO3 displayed the distinct polyhedral particles configuration with a highly crystalline smooth surface and the particle size ranging from 200 to 500 nm, as shown in Fig. 1c. The FESEM (Fig. S1†) and transmission electron microscopy (TEM) (Fig. S2†) images of the single ZnIn2S4 presented uniform nanosheet structures and the diameters of the flakes were over 300 nm, different from those in the other reports involving ZnIn2S4 nanospheres or nanoflowers.33 The flexible lamellar characteristics of ZnIn2S4 are beneficial to grow and coat on the other material, forming close contact composites after in situ nucleation on the interface.34 The FESEM images of the as-obtained BiFeO3/ZnIn2S4 heterostructures with different proportions (ZB-1, ZB-2, ZB-3 and ZB-4) are exhibited in Fig. 1d–g. As expected, at a relatively low loading ratio (ZB-1), a small amount of ZnIn2S4 nanoflakes were observed on the surface of BiFeO3 (Fig. 1d). With a further increase in the amount of ZnIn2S4, as shown in Fig. 1e–g, a gradual increase of the ZnIn2S4 nanosheets growing densely was observed until they were completely wrapping the BiFeO3 surface. It is clear for the ZB-4 sample from Fig. 1g that the excess ZnIn2S4 nanosheets crimp and crosslink each other finally destroying the monodispersity of the BiFeO3 particles.Subsequently, TEM and high-resolution TEM (HRTEM) images were exhibited to further reveal the morphology and microstructure details of the as-prepared nanocomposites. Specially, we focussed on the ZB-3 nanohybrid sample because it showed the best catalytic activity and durability in photocatalytic overall water splitting, not only beyond that of other synthesized photocatalysts reported in this work, but also surpassing the performance of the reported photocatalysts thus far for the ferroelectric-based heterojunction photocatalysts. Fig. 2a shows the typical TEM image of the ZB-3 sample. According to Fig. 2a, the configurational relationship between BiFeO3 and ZnIn2S4 is clearly uncovered in that the BiFeO3 polyhedron is completely coated by ZnIn2S4 nanosheets (the contact tightly between ZnIn2S4 and BiFeO3) through a strong contrast between the darker core and brighter shell. The HRTEM images (Fig. 2b and c) from the inset of Fig. 2a (I and II) reveal that the regular lattice fringes with an interplanar spacing of 0.27, 0.28, and 0.39 nm are corresponding to the (110), (104) and (012) crystal faces of the rhombohedral BiFeO3, respectively, and 0.32 nm are in good agreement with the (102) planes of the hexagonal ZnIn2S4.35,36 From the same area, the selective area electron diffraction (SAED) was implemented and the results were presented in the insets of Fig. 2b and c. The monocrystalline structure of the BiFeO3 core and polycrystalline structure of the ZnIn2S4 shell were thoroughly verified from the SAED patterns, which are in accordance with the XRD results and the previous reports.37,38 It is well known that a monocrystal is favorable to the adjustment of spontaneous polarization in ferroelectric particles due to the unified intrinsic polarization orientation from a small amount of crystal boundary. The elemental composition and spatial distribution of the ZB-3 sample were confirmed from the corresponding energy dispersive spectroscopy (EDS) elemental mapping analysis, as shown in Fig. 2d–k. Scanning transmission electron microscopy with high-angle annular dark field (STEM-HADDF) (Fig. 2d) and EDS elemental mapping (Fig. 2e–k) displayed a homogeneous distribution of the Bi, Fe, and O throughout the core of the nanohybrid (Fig. 2e–g), while, Zn, In, and S elements were homogeneously distributed throughout the whole nanocomposite (Fig. 2h–j), which is consistent with the above SEM and TEM results, demonstrating the assembling of intimate contact interface between BiFeO3 and ZnIn2S4. Meanwhile, the core/shell structure was further proved through the visualized EDS line-scan profile of Bi and In elements (Fig. 2l) across the nanoparticles along the green dotted line in Fig. 3d. Quantitatively, the atomic ratio of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In was normalized as about 9
In was normalized as about 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in accordance with EDS results (Fig. S3†). In addition, as a precise element, the quantitative characterization technique inductively coupled plasma atomic emission spectrometry (ICP-AES) results (Table S1†) also confirmed the similar element content of the ZB-3 sample.
1, in accordance with EDS results (Fig. S3†). In addition, as a precise element, the quantitative characterization technique inductively coupled plasma atomic emission spectrometry (ICP-AES) results (Table S1†) also confirmed the similar element content of the ZB-3 sample.
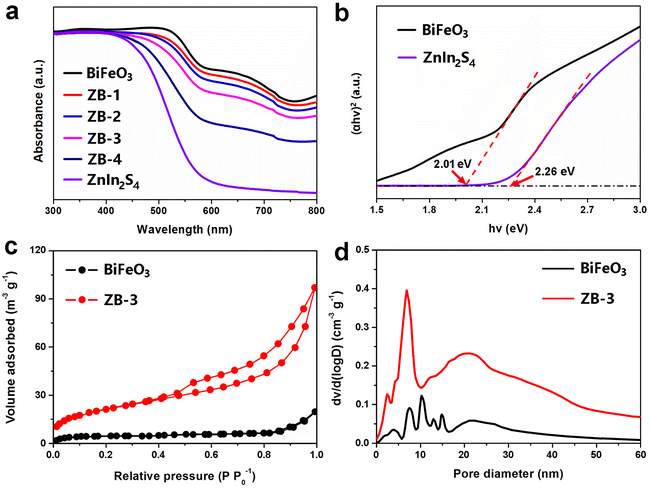
To investigate the light absorption capacity of the different photocatalysts, the UV-vis diffuse reflectance spectra (DRS) of original BiFeO3, ZnIn2S4, and various hybrids were acquired in Fig. 3a. For pure ZnIn2S4, the absorption edge of the approximately 570 nm peak displayed the representative absorption characteristics of layered ZnIn2S4. After hybridization, the absorption intensities of the BiFeO3/ZnIn2S4 composites gradually increased with increasing ZnIn2S4 content in the visible light region. The optimal visible light absorption ability obtained from bare BiFeO3 sample indicated that it can serve as a benchmark visible light absorbing ferroelectric photocatalyst. The corresponding linearity of the Tauc plots ((αhν)2–hν) confirmed the optical bandgap of BiFeO3 and ZnIn2S4 (Fig. 3b). From the intercept of the tangent to hν, the bandgap values of BiFeO3 and ZnIn2S4 were estimated to be 2.01 and 2.26 eV, respectively in a neutral environment. The Brunauer–Emmett–Teller (BET) measurements were conducted on BiFeO3 and ZB-3 samples through nitrogen adsorption–desorption isotherms and Barrett–Joyner–Halenda (BJH) pore size distribution analysis, as shown in Fig. 3c and d, respectively. The pure BiFeO3 exhibited a type I isotherm with no hysteresis, indicating the microporous structure, which is attributed to particle accumulation. For the ZB-3 sample, the curve shape belonged to type IV isotherm with a large hysteresis loop characteristic of the mesoporous structure, which will facilitate exposure of adequate catalytic active sites and the mass transfer for heterogeneous catalysis. Specifically, the BET surface area of ZB-3 was calculated to be 46.13 m2 g−1, almost 7 times higher than that of pure BiFeO3 (5.02 m2 g−1), which could be ascribed to the anchoring of layered ZnIn2S4 on the surfaces of BiFeO3. From the corresponding pore size distribution curves of the two samples (Fig. 3d), the pore size mainly ranged from 3 to 10 nm and the peaks of the ZB-3 curve were wider and stronger than those of pure BiFeO3, indicating the coexistence of mesoporous (primary) and micropores (secondary). The increased specific surface area and abundant pore distribution patterns of the nanohybrids can offer more active sites and are conducive to the diffusion of charges, resulting in the increase of photocatalytic activity.
X-ray photoelectron spectroscopy (XPS) characterization was employed to analyze the surface atomic species and bonding modes in BiFeO3, ZnIn2S4, and ZB-3 hybrids. From the survey XPS spectra of the three samples (Fig. 4a), the same signals centered at the binding energy of 284.8 eV from the conductive substrates during the testing process, which was used for the calibration. Excluding the contribution of C 1s, three XPS curves shown in Fig. 4a prove the existence of Bi, Fe, and O in BiFeO3, Zn, In and S, in ZnIn2S4, and Bi, Fe, Zn, In, O and S in the ZB-3 Z-scheme heterojunction, based on our expectation. Fig. 4b shows high-resolution Bi 4f XPS spectra of bare BiFeO3 and ZB-3. For bare BiFeO3, two strong peaks at 158.6 and 163.8 eV are attributed to Bi 4f7/2 and Bi 4f5/2, respectively, induced by Bi3+ species.39 After loading ZnIn2S4, the binding energies of Bi 4f were shifted to a higher energy level (∼0.2 eV), strongly suggesting the heterojunction effect between BiFeO3 and ZnIn2S4.40 The rest of the peaks extracted from 158 to 166 eV can be assigned to S 2p XPS spectrum, which will be contrastively analyzed with those of pure ZnIn2S4, as shown below. In Fig. 4c, the Fe 2p spectrum from BiFeO3 can be divided into two typical splitting peaks centered at 708.2 and 724.1 eV, which are ascribed to Fe 2p3/2 and Fe 2p1/2, respectively, and a broad satellite peak is observed at 715.8 eV.12 Correspondingly, the signals of Fe 2p XPS from ZB-3 nanohybrids also shifted towards the higher binding energies (∼0.2 eV) in comparison with pure BiFeO3, implying that the surface lattice framework of BiFeO3 may have subtly changed after layered ZnIn2S4 coating and strong interfacial interaction between BiFeO3 and ZnIn2S4 could be forming.41 In the O 1s XPS spectrum of pure BiFeO3, as shown in Fig. 4d, the peaks focused on 529.2 and 531.5 eV are indexed to lattice oxygen and surface hydroxyl (–OH) groups in BiFeO3, respectively.41 Similarly, 0.5 eV of positive shift is marked by dash lines for O 1s XPS curve of ZB-3. Fig. 4e–g displays the different binding energies values of Zn 2p, In 3d, and S 2p for original ZnIn2S4, consistent with previous reports.42 In contrast, compared with pure ZnIn2S4, the binding energy values of Zn 2p, In 3d, and S 2p from the ZB-3 sample exhibit slight shifts towards lower energy levels (Fig. 4e–g), contrary to the results of BiFeO3, as discussed above. Actually, the changes in the element binding energy can directly reflect the changes in the electron density.43,44 Therefore, the preliminary conclusion that the electrons and holes effectively migrated and accumulated on the surface of ZnIn2S4 and BiFeO3, respectively can be obtained according to the above XPS analysis. All XPS results suggest the existence of the strong interfacial coupling effect between BiFeO3 and ZnIn2S4, which can enhance the separation and migration of photogenerated carriers, and further improving the photocatalytic activity of ZB-3 nanohybrids.45
 | ||
| Fig. 4 (a) XPS survey spectra of BiFeO3, ZnInS4 and ZB-3. High-resolution Bi 4f (b), Fe 2p (c), O 1s (d), Zn 2p (e), In 3d (f) and S 2p (g) XPS spectra for alone and hybrid samples. | ||
2.2. Photocatalytic performance measurements
Photocatalytic hydrogen evolution was first implemented to monitor the photocatalytic performance of the BiFeO3, ZnIn2S4, and different ratios of BiFeO3/ZnIn2S4 hybrids using Na2SO3 and Na2S as hole scavengers under visible light irradiation (λ > 420 nm) (Fig. S4†). Fig. 5a summarizes the comparison results on the hydrogen generation rate of the BiFeO3, ZnIn2S4, and various composites catalysts (ZB-1, ZB-2, ZB-3, and ZB-4, see Experimental section for details) within 5 h. As described in Fig. 5a, the pure BiFeO3 nanoparticles show no hydrogen evolution activities because of the more positive intrinsic conduction band potential than that of H+/H2 (0 V vs. NHE),46 while the pure ZnIn2S4 are active for photocatalytic water splitting with the hydrogen generation rate of 1.12 mmol g−1 h−1 for its suitable intrinsic conduction band potential. In sharp contrast, all the nanohybrids photocatalysts (ZB-1, ZB-2, ZB-3, and ZB-4) displayed substantial improvement in hydrogen generation rates after growing the ZnIn2S4 shell on the BiFeO3 core. Strikingly, the ZB-3 composite manifested a satisfactory hydrogen evolution rate of 11.05 mmol g−1 h−1 without any cocatalysts, which is almost 10 times higher than that of the pure ZnIn2S4 photocatalyst (Fig. S5†). This enhancement suggests that the strong interfacial coupling effect between BiFeO3 nanoparticles and ZnIn2S4 nanoflakes is extremely beneficial for the photogenerated charge transfer. Besides, a decreased hydrogen evolution rate (5.26 mmol g−1 h−1) was recorded for ZB-4, indicating that the superfluous ZnIn2S4 shell may reduce the interface transfer efficiency of the carrier, which is caused by the inadequate light absorption of BiFeO3. As shown in Fig. 5b, visible light-driven photocatalytic oxygen evolution was further carried out on the as-prepared six catalysts containing NaIO3 as an electron scavenger. Compared with the complete inert on oxygen evolution from pure ZnIn2S4 sample, bare BiFeO3 presents intrinsic photocatalytic activity with an oxygen evolution rate of 60.2 μmol g−1 h−1 under identical reaction conditions. Notably, after combination with the ZnIn2S4 shell, all the hybrids displayed obvious enhancement in oxygen generation rates. With increasing ZnIn2S4 proportion, similar to the results of hydrogen generation, the photocatalytic oxygen generation rate increases gradually, ultimately reaching the maximum value of 365.9 μmol g−1 h−1, which is over 6-fold higher than that of the separate BiFeO3 catalyst. However, overloading ZnIn2S4 decreased the oxygen generation rate (275.1 μmol g−1 h−1) distinctly (ZB-4 curve), which might be triggered by the following factors: (i) excessive ZnIn2S4 coating inhibited visible light absorption of BiFeO3 and (ii) overload of the ZnIn2S4 shell crimped and crosslinked with each other destroying the monodispersity of the BiFeO3 particles and, finally, partially covering the catalytic active sites, which has been discussed in FESEM results (Fig. 1g).
Fig. 5c shows the visible light (λ > 420 nm) photocatalytic activity of overall pure water splitting on ZB-3 Z-scheme heterostructures in the absence of any sacrificial reagents, delivered the optimal photocatalytic activity towards half-reactions of water splitting. The averaged hydrogen and oxygen generation rates were calculated as 89.2 and 43.7 μmol g−1 h−1, respectively, giving hydrogen![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxygen ratio very close to the expected 2
oxygen ratio very close to the expected 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for overall water splitting, as expected, surpassing that of other composites (Fig. S6†). All heterojunctions exhibit better photocatalytic activity than that of the physical mixture sample (Fig. S7†). Moreover, action spectra of ZB-3 were then collected and the results are illustrated in Fig. 5d. From Fig. 5d, we observe that the trend of apparent quantum efficiency (AQE) values is well coincident with the UV-vis DRS spectra of ZnIn2S4, not with that of ZB-3, demonstrating that water reduction over the ZB-3 Z-scheme heterojunction is essentially photon-driven. Specifically, a considerable AQE of 1.13% at 420 nm and 0.009% at 560 nm was achieved, suggesting attractive photocatalytic activities in the wavelength range of 560 nm. These activity results are competitive with the reported semiconductor-based heterojunction photocatalysts toward overall water splitting (Table S2†). The photocatalytic cycle test is a benchmark to evaluate photostability for practical applications. As shown in Fig. 5e, the ZB-3 nanohybrid photocatalyst exhibited favorable stability for long-term photocatalytic overall pure water splitting reaction. Even after storing in the dark for 24 h after 12 cycles, the hybrids catalyst retained ∼95.6% of its original photocatalytic water splitting activity after 138 h discontinuous tests, suggesting the relatively high durability of the ZB-3 sample. Furthermore, no obvious change was found in morphology, crystal structure, and coordination states of the optimal ZB-3 photocatalyst from SEM (Fig. S8†), TEM (Fig. S9†), XRD (Fig. S10†), and XPS (Fig. S11†), respectively, after the cycle tests, further implying a superior long-term photocatalytic stability. The ZB-3 sample also has conspicuous stability as a half-reaction photocatalyst (Fig. S12†).
1 stoichiometry for overall water splitting, as expected, surpassing that of other composites (Fig. S6†). All heterojunctions exhibit better photocatalytic activity than that of the physical mixture sample (Fig. S7†). Moreover, action spectra of ZB-3 were then collected and the results are illustrated in Fig. 5d. From Fig. 5d, we observe that the trend of apparent quantum efficiency (AQE) values is well coincident with the UV-vis DRS spectra of ZnIn2S4, not with that of ZB-3, demonstrating that water reduction over the ZB-3 Z-scheme heterojunction is essentially photon-driven. Specifically, a considerable AQE of 1.13% at 420 nm and 0.009% at 560 nm was achieved, suggesting attractive photocatalytic activities in the wavelength range of 560 nm. These activity results are competitive with the reported semiconductor-based heterojunction photocatalysts toward overall water splitting (Table S2†). The photocatalytic cycle test is a benchmark to evaluate photostability for practical applications. As shown in Fig. 5e, the ZB-3 nanohybrid photocatalyst exhibited favorable stability for long-term photocatalytic overall pure water splitting reaction. Even after storing in the dark for 24 h after 12 cycles, the hybrids catalyst retained ∼95.6% of its original photocatalytic water splitting activity after 138 h discontinuous tests, suggesting the relatively high durability of the ZB-3 sample. Furthermore, no obvious change was found in morphology, crystal structure, and coordination states of the optimal ZB-3 photocatalyst from SEM (Fig. S8†), TEM (Fig. S9†), XRD (Fig. S10†), and XPS (Fig. S11†), respectively, after the cycle tests, further implying a superior long-term photocatalytic stability. The ZB-3 sample also has conspicuous stability as a half-reaction photocatalyst (Fig. S12†).
2.3. Determination of interfacial charge transfer
To obtain insight into the intrinsic enhancement causation for photocatalysis water splitting performance of the heterojunctions system, the photoluminescence (PL) was executed (350 nm excitation). As illustrated in Fig. 6a, the strong emission peaks from pure BiFeO3 and ZnIn2S4 located at ∼550 and 515 nm, respectively,47 meaning that the photo-excited electrons and holes in BiFeO3 and ZnIn2S4 will recombine rapidly. The PL intensity of ZB-3 composites quenches explicitly compared with the pure samples, uncovering the rapid transfer of photo-excited carriers between BiFeO3 and ZnIn2S4. Meanwhile, the charge carrier behaviors of the ZB-3 photocatalyst were investigated in comparison with BiFeO3 and ZnIn2S4 through time-resolved PL spectroscopy, as shown in Fig. 6b. Clearly, the ZB-3 Z-scheme photocatalyst delivered a longer average lifetime (5.58 ns) than those of BiFeO3 (4.49 ns) and ZnIn2S4 samples (4.02 ns), demonstrating that the assembled heterostructures can effectively restrain the recombination of charge carriers. Furthermore, the transient photocurrent responses and electrochemical impedance spectra (EIS) of the as-obtained samples were administrated to explore the enhanced separation efficiency of photoinduced charge carriers under visible light irradiation using a 300 W xenon lamp equipped with a λ > 420 nm cutoff filter (Fig. 6c). From Fig. 6c, transient photocurrent responses experiments were conducted with six 50 s light on/off cycles, the stable photocurrent density values of various photocatalysts can be achieved instantly once the light is turned on, indicating the high sensitivity to visible light. The consistent spikes of the photocurrent density curve appear when the light is turned on, which is caused by the transient accumulation of photoinduced charges, implying that abundant photogenerated carriers from these BiFeO3/ZnIn2S4 nanohybrids are produced in photocatalysts rather with low recombination.48 Subsequently, electrochemical impedance spectroscopy (EIS) results (Fig. 6d) displayed that the ZB-3 heterojunctions possess a smaller semicircle radius compared to pure catalysts and other composites catalysts, once again confirming the faster electron transfer in the ZB-3 composites, which was confirmed in the above photocatalytic characterization section (Fig. 5). Besides, the dynamics of photogenerated charge carriers in pure ZnIn2S4 and ZB-3 composites were quantitatively performed through transient absorption (TA) spectra (Fig. 6e and f). To match the time scales of water oxidation, which is generally considered as the rate-determining step of water splitting reaction, microsecond–second time scale TA kinetics was adopted under a relatively low-intensity incident pump intensity of 300 μJ cm−2 (410 nm).49 From Fig. 6e and f, two positive photoinduced absorptions signals from two samples were fitted by the power law with ΔOD − t−α, respectively, which were caused by the trapped holes, similar to the previous reports including metal oxides and metal chalcogenides.50–52 The larger initial amplitude from the ZB-3 sample signifies that the hybrid catalyst possesses a higher hole-trapping density than that of the pure ZnIn2S4. More importantly, the decay kinetics from the ZB-3 sample is considerably slower than that of pure ZnIn2S4 through the comparison of t50% lifetimes (635 and 76 μs for ZB-3 and ZnIn2S4, respectively), indicating that the photogenerated holes in ZB-3 are more likely to participate in the water oxidation reaction.To validate the Z-scheme charge transfer migration pathway across the staggered heterojunction, surface photovoltage (SPV) tests of BiFeO3, ZnIn2S4, and ZB-3 films (a) and single nanoparticles were implemented, as shown in Fig. 7a and b, respectively. In the SPV spectra of the films (Fig. 7a), compared with the weak photovoltage signals from BiFeO3 and ZnIn2S4 films, a significantly increased photovoltage amplitude at 320–550 nm emerged in the ZB-3 film upon light illumination. This enhancement confirmed that the photoinduced electrons from ZnIn2S4 and holes of BiFeO3 were transferred to the illumination and backlight side, respectively, while the remaining electrons from BiFeO3 and holes from ZnIn2S4 recombined on the heterojunction interface (inset of Fig. 7a), that is the Z-scheme mechanism.53 Line-scan SPV of single nanoparticles BiFeO3 and ZB-3 were then measured under 400 nm excitation. When the probe only scans the particle diameter, the ZB-3 sample exhibits arch-shaped characteristic SPV spectra and the maximum photovoltage value was marked as large as ∼15 mV, much more significant than that of the BiFeO3 particle under the same measurement conditions. Such variation of SPV signals demonstrates the existence of a considerable internal electric field in the ZB-3 catalyst that is competitive with other research studies, which were triggered by the phase junction, cocatalyst loading, and crystal architecture.54–56 The internal electric field values of ZnIn2S4, BiFeO3 and ZB-3 were approximately estimated using the following equation.57
| Fs = (−2Vsρ/εε0)1/2 |
In parallel, as an effective booster for the efficiency of photoinduced charge carries separation and migration, spontaneous polarization may promote the photocatalytic performance of ferroelectric semiconductors even more efficiently than the conventional junction strategies.17 Based on the definite results of the photoexcited charge separation and transfer behaviors analyzed above (Fig. 6 and 7a–c), ferroelectric polarization of the nature ferroelectrics BiFeO3 and ZB-3 was also monitored by recording the polarization–electric field (P–E) hysteresis loop characteristics and leakage current behaviors. As illustrated in Fig. 7d, the open-mouth-shaped P–E hysteresis loop can be achieved on a pure BiFeO3 sample at 1 kHz, which is attributed to the resistive leakage of ferroelectric constructions. From Fig. 7f, correspondingly, pure BiFeO3 film outputs a relatively high leakage current value as 10−3 A, which determines that just a small electric field (4.0 V) can be employed to measure the ferroelectric characters of the single BiFeO3. In contrast, the ZB-3 sample displays a much lower leakage current of ∼10−7 A at the same applied ac voltage (10 V) (Fig. 7f), guaranteeing that a higher voltage above 180 V can be adopted to investigate the polarization loop of the heterojunction film (Fig. 7e). The ferroelectric polarization of ZB-3 increases obviously compared with pure BiFeO3, even though it is lower than that of bulk BiFeO3 films, which were prepared by an epitaxial growth strategy.58,59 This reason for this difference is misunderstood: the P–E hysteresis loops of BiFeO3 and ZB-3 were obtained under the unsaturation voltage (3 and 200 kV m−1), much lower than that of bulk BiFeO3 (7 MV m−1).60 Overall, distinguishable ferroelectric polarization and much lower leakage current from the ZB-3 sample implied that the merging ZnIn2S4 plays an important role in adjusting the spontaneous polarization of ferroelectric BiFeO3 to realize efficient photogenerated charge separation and transfer.
2.4. Photocatalytic mechanism analysis
The electron spin resonance (EPR) spin-trapping technique was carried out to further support the Z-scheme charge transfer mechanism in BiFeO3/ZnIn2S4 heterojunctions photocatalysts. DMPO was used as a radical capture reagent to capture superoxide radical (˙O2−) and hydroxyl radical (˙OH) in methanol and water, respectively. As displayed in Fig. 8a and b, almost no DMPO–˙O2− or DMPO–˙OH signals can be found over ZB-3 photocatalysts under dark conditions. After 5 min illumination, the characteristic peaks of DMPO–˙O2− could still not be detected from single BiFeO3 because the band gap of BiFeO3 straddles the water redox energy only,46 resulting in the electrons in the CB of BiFeO3 not able to reduce oxygen to ˙O2−. However, the ˙O2− signal of pure ZnIn2S4 appeared for the appropriate CB potential. Significantly, the ZB-3 heterojunction exhibits stronger DMPO–˙O2− peaks, speculating that the accumulated photoinduced electrons were present on the CB of ZnIn2S4 rather than BiFeO3. In contrast, similar features are recorded for DMPO–˙OH, as shown in Fig. 8b. Specifically, the weak oxidation of photoinduced hole from BiFeO3 produces inconspicuous DMPO–˙OH signals. The signals of DMPO–˙OH from ZnIn2S4 can be attributed to the reduction of O2 on CB (O2 + 4e− + 2H+ → ˙OH + OH−).41 For ZB-3 heterojunctions photocatalysts, the strongest signals of DMPO–˙OH are also abstracted, demonstrating that the photoinduced holes are mainly locked in VB of BiFeO3, thus verifying the direct Z-scheme charge migration mechanism we proposed.Furtherly, after the Kubelka–Munk formula calculation, as shown in Fig. 4b, 2.01 and 2.26 eV of energy gap (Eg) values were confirmed for pure BiFeO3 and ZnIn2S4, respectively. Based on Mott–Schottky test results, as shown in Fig. 8c and d, the flat band potentials (Ef) of BiFeO3 and ZnIn2S4 were determined to be 0.15 and −1.25 V (vs. NHE), respectively. According to the n-type semiconductor traits,61 the conduction band potential (ECB) of BiFeO3 and ZnIn2S4 were calculated to be 0.35 and −1.05 V, respectively. Combined with the Eg data (EVB = ECB + Eg), the valence band potential (EVB) of the BiFeO3 and ZnIn2S4 can be estimated to be 1.60 and 1.21 V, respectively. The work function (ϕ) is an important parameter for reflecting the escaping ability of free electrons from the Fermi level (Ef) to the vacuum level, which was obtained through ultraviolet photoelectron spectroscopy (UPS) equipment with He I as the excitation source. As illustrated in Fig. S13 and S14,† the ϕ of BiFeO3 and ZnIn2S4 can be calculated as 4.74 and 3.37 eV, respectively based on the cutoff binding energy (Ecutoff) (16.48 eV for BiFeO3 and 17.85 eV for ZnIn2S4) and the formula of ϕ = hν (21.22) − Ecutoff.62 Based on the above calculation results, the detailed band structure of BiFeO3 and ZnIn2S4 before and after contact are exhibited in Fig. 8e and f. The Ef of BiFeO3 is below that of ZnIn2S4 before contact (Fig. 8e); when an intimate interface is established between BiFeO3 and ZnIn2S4, the photoinduced electrons in ZnIn2S4 would diffuse unobstructed to BiFeO3 until a new equilibrium state Ef is formed. The electron drifting from ZnIn2S4 to BiFeO3 accelerates the charge redistribution on the BiFeO3/ZnIn2S4 heterojunction interface, in which the electrons and holes distribute on the BiFeO3 and ZnIn2S4 interfaces, respectively. Therefore, a reasonable Z-scheme system was put forward, as shown in Fig. 8f. The photogenerated electrons in the CB of BiFeO3 would fast transfer and recombine with the photoinduced hole in the VB of ZnIn2S4 through the contact interface, which was derived by a powerful internal electric field. Meanwhile, the oxygen generation reaction can be accelerated due to the sufficient holes from the VB of BiFeO3, which is guaranteed by the enhanced ferroelectric polarization of BiFeO3. Overall, synergizing internal electric field and ferroelectric polarization on the BiFeO3/ZnIn2S4 Z-scheme heterojunction realized high-efficiency photocatalytic overall water splitting applications.
3 Conclusions
In summary, we successfully demonstrated an internal electric field and ferroelectric polarization synergetic modulated BiFeO3/ZnIn2S4 Z-scheme heterojunction photocatalyst through a moderate two-step solvothermal process for high-efficiency photocatalytic overall pure water splitting under visible light. These two synergistic effects from the reasonable design favorably boosted the reduction and oxidation ability of photoinduced electrons and holes, simultaneously. Benefitting from the efficient photogenerated carrier separation and migration processes, triggered by enhanced spontaneous polarization and powerful internal electric field, respectively, BiFeO3/ZnIn2S4 heterojunction photocatalysts displayed not only the firm half-reaction performance over hydrogen and oxygen evolution but also considerable activity on overall water splitting. Specifically, the optimized photocatalyst delivered a superior photocatalytic overall water-splitting activity (H2: 87.3 μmol g−1 h−1, O2: 42.3 μmol g−1 h−1) with an apparent quantum efficiency of 1.12% at 420 nm in pure water under visible light irradiation, the highest activity reported so far for ferroelectric-based heterojunction photocatalysts. Additionally, BiFeO3/ZnIn2S4 composite demonstrated high stability and recyclability, holding great promise for practical applications in photocatalytic water splitting. These results provide new insights into deep understanding and rational design of ferroelectric-based photocatalysts with high performance for solar-to-hydrogen energy conversion.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this study.Acknowledgements
We acknowledge the National Natural Science Foundation of China (52072182 and 51872145), the Natural Science Foundation of Jiangsu Province (BK20211278), the China Post-doctoral Science Foundation (No. 2019M650120 and 2020M671554), and the National Synergetic Innovation Center for Advanced Materials (SICAM).Notes and references
- Z. Wang, C. Li and K. Domen, Chem. Soc. Rev., 2019, 48, 2109–2125 RSC.
- J. Li, J. Luo, H. Chen, B. Qin, C. Yuan, N. Wu, G. Liu and X. Liu, Chem. Commun., 2022, 25, 9918–9921 RSC.
- J. Li, Q. Zhang, J. Zhang, N. Wu, G. Liu, H. Chen, C. Yuan and X. Liu, J. Colloid Interface Sci., 2022, 627, 862–871 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS.
- Q. Wang, M. Nakabayashi, T. Hisatomi, S. Sun, S. Akiyama, Z. Wang, Z. Pan, X. Xiao, T. Watanabe, T. Yamada, N. Shibata, T. Takata and K. Domen, Nat. Mater., 2019, 18, 827–832 CrossRef CAS PubMed.
- T. Hisatomi and K. Domen, Nat. Catal., 2019, 2, 387–399 CrossRef CAS.
- C. Yang, H. Huang, H. He, L. Yang, Q. Jiang and W. Li, Coord. Chem. Rev., 2021, 435, 213806 CAS.
- J. Li, X. Liu and J. Zhang, ChemSusChem, 2020, 13, 2996–3004 CAS.
- W. Chen, G. Huang, H. Song and J. Zhang, J. Mater. Chem. A, 2020, 8, 20963–20969 CAS.
- C. Yang, Q. Jiang, H. Liu, L. Yang, H. He, H. Huang and W. Li, J. Mater. Chem. A, 2021, 9, 15432–15440 CAS.
- G. Liu, F. Xiao, T. Zhang, Y. Gu, J. Li, D. Guo, M. Xu, N. Wu, A. Cao and X. Liu, Appl. Surf. Sci., 2022, 597, 153688 CAS.
- J. Zhang, F. Feng, Y. Pu, X. Li, C. Lau and W. Huang, ChemSusChem, 2019, 12, 2651–2659 CAS.
- S. Monny, Z. Wang, M. Konarova and L. Wang, J. Energy Chem., 2021, 6, 517–530 Search PubMed.
- T. Rangel, B. Fregoso, B. Mendoza, T. Morimoto, J. Moore and J. Neaton, Phys. Rev. Lett., 2017, 119, 067402 Search PubMed.
- X. Huang, K. Wang, Y. Wang, B. Wang, L. Zhang, F. Gao, Y. Zhao, W. Feng, S. Zhang and P. Liu, Appl. Catal., B, 2018, 227, 322–329 CAS.
- Y. Zhang, L. Yang, Y. Zhang, Z. Ding, M. Wu, Y. Zhou, C. Diao, H. Zheng, X. Wang and Z. Wang, ACS Nano, 2020, 14, 10723–10732 CAS.
- Y. Liu, S. Ye, H. Xie, J. Zhu, Q. Shi, N. Ta, R. Chen, Y. Gao, H. An, W. Nie, H. Jing, F. Fan and C. Li, Adv. Mater., 2020, 32, 1906513 CAS.
- K. Saravanakumar and C. Park, Chem. Eng. J., 2021, 423, 130076 CAS.
- Y. Sun, X. Li, A. Vijayakumar, H. Liu, C. Wang, S. Zhang, Z. Fu, Y. Lu and Z. Cheng, ACS Appl. Mater. Interfaces, 2021, 13, 11050–11057 CAS.
- A. Kumar, G. Sharma, M. Naushad, T. Ahamad and R. Veses, Chem. Eng. J., 2019, 270, 148–165 Search PubMed.
- Y. Cui, X. Guo, J. Zhang, X. Li, X. Zhu and W. Huang, Nano Res., 2022, 15, 677–684 CAS.
- J. Zhang, N. Zhou, M. Du, Y. Li, Y. Cui, X. Li, X. Zhu and W. Huang, J. Mater. Chem. A, 2022, 10, 296–303 CAS.
- H. Yang, Z. Zhao, Y. Yang, Z. Zhang, W. Chen, R. Yang, Y. Jin and J. Zhang, Sep. Purif. Technol., 2022, 300, 121846 CAS.
- J. Li, H. Huang, X. Cao, H. Wu, K. Pan, Q. Zhang, N. Wu and X. Liu, Chem. Eng. J., 2021, 416, 127677 CAS.
- C. Pei, T. Li, M. Zhang, J. Wang, L. Chang, X. Xiong, W. Chen, G. Huang and D. Han, Sep. Purif. Technol., 2022, 290, 120875 CAS.
- W. Meng, H. He, L. Yang, Q. Jiang, B. Yuliarto, Y. Yamauchi, X. Xu and H. Huang, Chem. Eng. J., 2022, 450, 137932 CAS.
- C. Xu, Y. Kong, W. Zhang, M. Yang, K. Wang, L. Chang, W. Chen, G. Huang and J. Zhang, Sep. Purif. Technol., 2022, 303, 122266 CAS.
- K. Tan, Y. Chen, C. Van, H. Wang, J. Chen, F. Lim, K. Chew, Q. Zhao, C. Wu, S. Chai, Y. Chu and W. Chang, ACS Appl. Mater. Interfaces, 2019, 11, 1655–1664 CAS.
- Y. Yin, J. Wang, B. Chen, P. Zhang, G. Li, W. Sun, F. Hu and C. Li, Nanoscale, 2022, 14, 2686–2695 CAS.
- J. Tang, R. Wang, M. Liu, Z. Zhang, Y. Song, S. Xue, Z. Zhao and D. Dionysios, Chem. Eng. J., 2018, 351, 1056–1066 CAS.
- J. Xu, T. Qin, W. Chen, J. Lv, X. Zeng, J. Sun, Y. Li and J. Zhou, Nano Energy, 2021, 89, 106317 CAS.
- H. Yang, P. Cao, P. Sun, J. Yin, S. Zhang and J. Xu, Appl. Catal., B, 2018, 265, 117862 Search PubMed.
- J. Hu, C. Chen, Y. Zheng, G. Zhang, C. Guo and C. Li, Small, 2020, 16, 2002988 CAS.
- Q. Han, L. Li, W. Gao, Y. Shen, L. Wang, Y. Zhang, X. Wang, Q. Shen, Y. Xiong, Y. Zhou and Z. Zou, ACS Appl. Mater. Interfaces, 2021, 13, 15092–15100 CAS.
- Q. Zhang, J. Zhang, X. Wang, L. Li, T. Li and W. Dai, ACS Catal., 2021, 11, 6276–6289 CAS.
- S. Patel, J. Lee, M. Kim, B. Bhoi and S. Kim, J. Mater. Chem. C, 2018, 6, 526–534 CAS.
- C. Du, B. Yan, Z. Lin and G. Yang, J. Mater. Chem. A, 2020, 8, 207–217 CAS.
- J. Yang, D. Wang, H. Han and C. Li, Acc. Chem. Res., 2013, 46, 1900–1909 CAS.
- J. Li, H. Yuan, J. Li, W. Zhang, Y. Liu, N. Liu, H. Cao and Z. Jiao, Appl. Catal., B, 2021, 285, 119833 CAS.
- C. Li, H. Che, Y. Yan, C. Liu and H. Dong, Chem. Eng. J., 2020, 398, 125523 CAS.
- D. Kong, H. Fan, D. Yin, D. Zhang, X. Pu, S. Yao and C. Su, ACS Sustainable Chem. Eng., 2021, 9, 2673–2683 CAS.
- S. B. Sun, J. Bu, Y. Du, X. Chen, Z. Li and W. Zhou, ACS Appl. Mater. Interfaces, 2021, 13, 37545–37552 Search PubMed.
- L. Wang, B. Cheng, L. Zhang and J. Yu, Small, 2021, 17, 2103447 CAS.
- J. Low, B. Dai, T. Tong, C. Jiang and J. Yu, Adv. Mater., 2019, 31, 1802981 Search PubMed.
- L. Wang, B. Zhu, B. Cheng, J. Zhang, L. Zhang and J. Yu, Chin. J. Catal., 2021, 42, 1648–1658 CAS.
- J. Shah, B. Huang, A. Idris, Y. Liu, A. Malik, W. Hu, Z. Zhang, H. Han and C. Li, Small, 2020, 16, 2003361 CAS.
- G. Zhang, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Angew. Chem., Int. Ed., 2020, 59, 8255–8261 CAS.
- S. Wang, Y. Wang, S. Zhang, S. Zang and X. Lou, Adv. Mater., 2019, 31, 1903404 CAS.
- S. Pendlebury, M. Barroso, A. Cowan, K. Sivula, J. Tang, M. Grätzel, D. Klug and J. Durrant, Chem. Commun., 2011, 47, 716–718 CAS.
- Y. Ma, S. Pendlebury, A. Reynal, F. Le Formal and J. Durrant, Chem. Sci., 2014, 5, 2964–2973 CAS.
- W. Yang, Y. Liu, J. McBride and T. Lian, Nano Lett., 2021, 21, 453–461 CAS.
- R. Pan, M. Hu, J. Liu, D. Li, X. Wan, H. Wang, Y. Li, X. Zhang, X. Wang, J. Jiang and J. Zhang, Nano Lett., 2021, 21, 6228–6236 CAS.
- X. Chen, J. Wang, Y. Chai, Z. Zhang and Y. Zhu, Adv. Mater., 2021, 33, 2007479 CAS.
- Y. Gao, J. Zhu, H. An, P. Yan, B. Huang, R. Chen, F. Fan and C. Li, J. Phys. Chem. Lett., 2017, 8, 1419–1423 CAS.
- J. Zhang, R. Cui, C. Gao, L. Bian, Y. Pu, X. Zhu, X. Li and W. Huang, Small, 2019, 15, 1904688 CAS.
- J. Zhang, J. Li, H. Huang, W. Chen, Y. Cui, Y. Li, W. Mao, X. Zhu and X. Li, Small, 2022, 18, 2204557 CAS.
- G. Morello, F. Della Sala, L. Carbone, L. Manna, G. Maruccio, R. Cingolani and M. De Giorgi, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 195313 Search PubMed.
- D. Sando, M. Han, V. Govinden, O. Paull, F. Appert, C. Carretero, J. Fischer, A. Barthelemy, M. Bibes, V. Garcia, S. Fusil, B. Dkhil, J. Juraszek, Y. Zhu, X. Ma and V. Nagarajan, Adv. Funct. Mater., 2020, 30, 2000343 CAS.
- B. Yang, L. Jin, R. Wei, X. Tang, L. Hu, P. Tong, J. Yang, W. Song, J. Dai, X. Zhu, Y. Sun, Y. Sun, S. Zhang, X. Wang and Z. Cheng, Small, 2021, 17, 1903663 CAS.
- S. Zhang, M. Lu, D. Wu, Y. Chen and N. Ming, Appl. Phys. Lett., 2005, 87, 262907 Search PubMed.
- A. Meng, W. Tian, H. Yang, X. Wang, X. Wang and Z. Li, J. Hazard. Mater., 2021, 413, 125400 CAS.
- J. Lee, J. Baek, T. Gill, X. Shi, S. Lee, I. Cho, H. Jung and X. Zheng, J. Mater. Chem. A, 2019, 7, 9019–9024 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta07976c |
| This journal is © The Royal Society of Chemistry 2023 |