Super-flexible phase change materials with a dual-supporting effect for solar thermoelectric conversion in the ocean environment†
Yi-Cun
Zhou
,
Jie
Yang
*,
Lu
Bai
,
Rui-Ying
Bao
 ,
Ming-Bo
Yang
,
Ming-Bo
Yang
 and
Wei
Yang
and
Wei
Yang
 *
*
College of Polymer Science and Engineering, Sichuan University, State Key Laboratory of Polymer Materials Engineering, Chengdu 610065, Sichuan, People's Republic of China. E-mail: psejieyang@scu.edu.cn; weiyang@scu.edu.cn; Tel: +86-2885460130
First published on 25th November 2022
Abstract
Solar thermoelectric generators (STEGs) based on phase change materials (PCMs) are an emerging advanced technology to collect and utilize solar energy. However, it is still challenging for photo-driven PCMs to make use of the huge amount of solar energy in oceans due to their limited shape stability and stability underwater. Herein, dual-supporting super-flexible PCMs (SFPCMs) comprising a natural rubber (NR) continuous network and island-like dispersed paraffin wax (PW) microcapsules were developed. The interaction between the NR matrix and phase change microcapsules (PCMCs) was further improved by incorporating a small amount of MXene to enhance the photo-absorption ability, mechanical performance, and thermal conductivity of the composite PCMs. In addition to excellent stretchability and compressibility, this dual-supporting structure consisting of a continuous NR network and encapsulated polyurethane (PU) shell endowed SFPCMs with unprecedented leakage resistance and underwater stability, even in various harsh environments. More impressively, a proof-of-concept STEG based on SFPCMs with a photothermal conversion efficiency of up to 92% was proposed, exhibiting excellent cycling output stability in a simulated ocean environment with an output voltage of 410 mV. This work expands the application potential of photo-driven PCMs to efficiently harvest solar energy from the ocean environment.
1. Introduction
The collection, storage, and conversion of solar energy are significant strategies to relieve the current energy crisis.1 However, the utilization of solar energy is greatly limited because of its uneven distribution in time and space.2 Currently, the strategies for the utilization of solar energy predominately include photothermal,3 photoelectric,4 and photochemical5 conversion. Multiple photothermal conversion effects, including electron excitation relaxation,6 electron hole separation,7 and plasmon resonance,8 lead to a variety of photothermal materials and devices. Therefore, solar-thermal conversion has a wide range of applications in the fields of solar thermal-power generation,9 water purification,10 and solar buildings.11 Among the various photothermal conversion devices, solar thermoelectric generators (STEGs) based on phase change materials (PCMs) and thermoelectric modules capable of directly converting heat into electricity have attracted significant attention.12 This is because they are capable of converting solar energy into thermal energy and storing it in the form of latent heat through phase transition, further generating electricity for a prolonged time and mitigating the intermittency of solar energy in time and space.13–16At present, the construction of solar thermoelectric systems with regard to energy conversion on land has been widely studied.17–19 As is known, 71% of the Earth's surface is covered by oceans, on which the solar energy density is close to 1367 W m−2.20,21 However, most of the solar energy radiated on the ocean surface is directly dissipated to change the ocean temperature and salinity.22,23 Thus, making full use of this part of energy is of great significance to improve the utilization efficiency of solar energy and alleviate the energy crisis. However, the implementation of STEGs in the marine environment is hampered by several limiting factors originating from photothermal phase change energy storage units,24–26 including low photothermal conversion capability,27 insufficient shape stability during phase transition,28,29 and poor underwater stability.30 In addition, most of the composite PCMs previously reported in the literature lack flexibility,31–35 giving rise to low adaptability to large-scale assembly of STEGs36 and application in various scenarios. Therefore, the preparation of leakage-proof flexible PCMs with excellent photothermal conversion properties and underwater shape stability is promising for the collection and utilization of solar energy in the ocean environment.37 Generally, flexible organic PCMs can be mainly divided into two categories, i.e., intrinsic flexible PCMs and composite flexible PCMs.38–41 Typical intrinsic flexible PCMs with phase transition properties are synthesized by connecting polyethylene glycol molecules to polymer chains.42–44 However, the preparation of intrinsic flexible PCMs involves the use of toxic reagents and a decrease in phase change enthalpy. Composite flexible PCMs are usually obtained by confining PCMs in a flexible polymer supporting network,45 which are manifested as fiber membranes, foam composites, microcapsules, etc.46–52
In our previous work, an effective strategy was proposed to fabricate composite flexible PCMs with excellent flexibility and enhanced thermal conductivity for the thermal management of electronics and buildings through swelling phase change components into polymer elastomers on a micrometer scale.53,54 Similarly, we developed flexible phase change hydrogels based on paraffin wax (PW) microspheres and polyvinyl alcohol (PVA) hydrogels.55 It is noteworthy that although lots of composite PCMs with enhanced shape stability have been prepared by introducing capillary force, hydrogen bonding, and dipole interaction, only a few reported thus far can maintain shape stability under water without any leakage, which is mainly ascribed to the solubility of the commonly used PCMs in water and the limited confining effect of the supporting materials.49 Although there have been attempts to employ hydrogel-based supporting networks to accommodate PCMs underwater, PCMs inevitably leak under prolonged water conditions.56 Therefore, it remains a huge challenge to achieve complete leakage resistance when adapting to different application scenarios.
In this contribution, a dual-supporting structure confining polymer-encapsulated core–shell PCMs in a flexible natural rubber (NR) supporting network was designed to yield super-flexible PCMs (SFPCMs) with complete leakage resistance and underwater stability, followed by introducing MXene nanosheets to endow the resulting composites with salient photothermal conversion ability,57,58 improved mechanical performance, and high thermal conductivity. Furthermore, a self-floating STEG was assembled by integrating the SFPCMs with a commercial thermoelectric modulus. The preparation of high-performance flexible PCMs and the rational design of advanced thermoelectric technology provide an idea for the extensive and efficient collection and conversion of solar energy in the ocean environment.
2. Results and discussion
2.1. Fabrication of SFPCMs
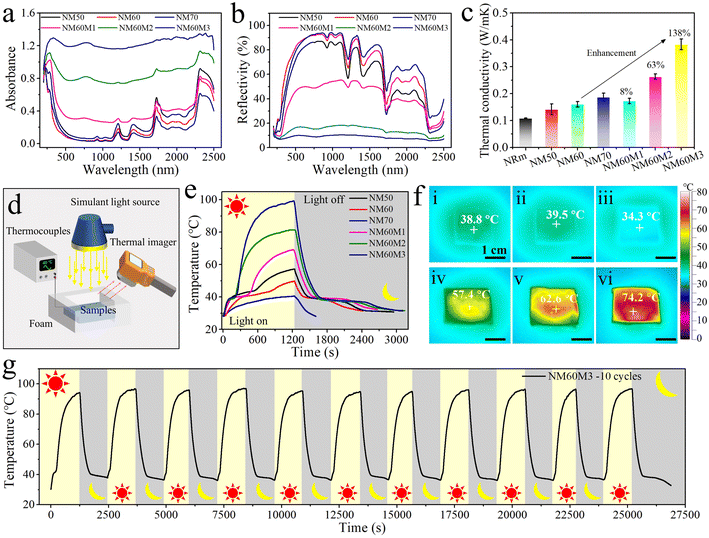
As shown in Fig. 1a, the SFPCMs were fabricated by mixing the as-prepared Ti3C2Tx MXene nanosheets in NR latex (NRl) solution, followed by introducing PW-based phase change microcapsules (PCMCs) in the mixture. After heat treatment and demolding, the samples were obtained with a cross-linked network originating from the unsaturated bonds of NR. The obtained samples were denoted as NMx or NMxMy, where x (50, 60, and 70) and y (1, 2, and 3) represent the PCMC loading and MXene loading, respectively. For example, NM60M3 refers to the SFPCMs with the PCMC loading of 60 wt% and the MXene loading of 0.6 wt%. Detailed information can be found in the ESI.† The PCMCs with an average particle size of 61.7 μm could maintain their shape stability before and after the phase change process, as shown in Fig. 1b–d and S1a.† The dual encapsulation of PW supplied by the polyurethane (PU) shell (Fig. S1b†) and the NR network ensured the shape stability of the phase change components in the flexible composites during the energy conversion process. The atomic force microscopy (AFM) image and corresponding height curve of the Ti3C2Tx MXene nanosheets are presented in Fig. 1e and f, respectively, showing that MXene nanosheets with a thickness of about 3 nm were prepared after the Al phase in the MAX raw material was successfully etched. Owing to its great water dispersibility,59,60 MXene is predominately distributed in the continuous NR phase.With an increase in the content of PCMCs, more interfaces and defects between the NR matrix and PCMCs were observed, as shown in Fig. 1g–i. Specifically, the PCMCs in NM70 were in a relatively loose state when the mass fraction of the PCMCs reached 70%. Interestingly, the PCMCs were more tightly bound to the NR matrix with an increase in MXene content in NM60 (Fig. 1j and S2†) owing to the fact that the MXene nanosheets with abundant oxygen-containing functional groups can act as bridges between the PU shell of the PCMCs and the proteins in NR through strong intermolecular forces and hydrogen bonds.61,62 The blue shift in the corresponding peak of the –N–H group in the SFPCMs with the introduction of MXene corroborates the enhancement of these interaction forces (Fig. 1k and l).63–65 In addition, the cross-sectional SEM images of the SFPCMs visually demonstrate the dual-supporting network formed by the PU shell and the NR continuous matrix. Furthermore, both G′ and G′′ of NM60 and NM60M3 are independent of the frequency before and after the phase transition, presenting a solid-like viscoelastic behavior, corroborating the existence of the supporting network structure in SFPCMs (Fig. S3†). The difference between the NRl and NR membrane (NRm) is mainly manifested in the water peak intensity at 3350 cm−1. The peaks corresponding to each component in NM60M3 remained intact and no new peaks were observed, indicating that there was no chemical reaction among MXene, PCMCs, and NR. The X-ray diffraction (XRD) patterns (Fig. 1m) clearly show the typical diffraction planes (110/220) of the PW crystals (2θ = 23.4°/24.9°), which indicate that the PW in the SFPCMs maintains a well-established crystalline structure for considerable energy storage density.
2.2. Mechanical properties of SFPCMs
The NR matrix as a continuous phase provides stretchability for SFPCMs, while the island-like dispersed PCMCs make SFPCMs compressible. The stretchability and compressibility endow the SFPCMs with excellent flexibility. As shown in Fig. 2a, the tensile strength decreased and the elongation at break increased for NM50 relative to NRm after introducing 50 wt% PCMCs. This is because the introduction of PCMCs induced some interfacial defects in the intact NRm, which led to a decrease in the tensile strength. The island-like dispersed PCMCs can act as a concentration point for macroscopic deformation during the stretching process, giving rise to a longer stretching deformation for SFPCMs.66 In addition, the interface induced by PCMCs significantly reduces the elastic modulus of SFPCMs (Fig. S4a†). However, with a gradual increase in the content of PCMCs, the interfacial defects in SFPCMs obviously increased and the continuity of NR decreased, resulting in deteriorated mechanical properties for NM70. Impressively, the tensile strength and elongation at break of SFPCMs were significantly improved with an increase in the MXene loading. Specifically, the tensile strength and elongation at break of NM60M3 reached 0.54 MPa and 708% (Fig. 2b and S4b†), respectively. MXene acts as a bridge to enhance the interfacial interaction between PCMCs and NR matrix, further improving the mechanical properties of NM60M3 and maintaining its low elastic modulus of 2.9 MPa.As shown in Fig. 2c, NM60M3 with a dark appearance at room temperature could be easily bent into a ring or twisted by 720° under the action of external force, which further confirms its flexibility. Moreover, NM60M3 exhibits a permanent deformation of 14.3% after multiple cycles of stretching and good springback properties in a wide range of elongation (Fig. 2d), maintaining good stretchability after cyclic tensile tests, namely, elongation at break of 445% and tensile strength of 0.61 MPa (Fig. 2e). Meanwhile, the SFPCMs exhibited over 300% stretchability and sufficient tensile strength under the phase transition state at high temperature, as shown in Fig. 2f and S4c.† The introduction of core–shell PCMCs had a limited effect on the hardness of the SFPCMs, but the further addition of MXene increased the hardness owing to the enhancement of the interfacial interactions between the PCMCs and NR matrix, as shown in Fig. 2g.
Furthermore, the island-like dispersed PCMCs in the continuous NR matrix endowed the SFPCMs with considerable compressibility, as shown in Fig. 2h and i. Under the action of compressive force, the NR continuous phase and the PU shell of PCMCs first underwent elastic deformation. Subsequently, relative slip occurs between them. When the molecular chains of NR and PU were compressed to the critical density, the PCMs in PCMCs were compressed and broken, followed by a sharp increase in strength with the compressed overall bulk density of SFPCMs. Among them, NM50 and NM60M3 exhibited relatively high compressive strengths, reaching 31.2 and 29.4 MPa, respectively. The excellent stretchability and compressibility enable SFPCMs to be processed, shaped and adapted to more complex application scenarios.
2.3. Thermophysical properties of SFPCMs
As shown in Fig. 3a, the solar energy converted to latent heat is mainly stored in PCMCs and NR has no phase transition process in the temperature range of 0 to 240 °C. The enthalpy of SFPCMs obviously increased with an increase in the content of PCMCs (Fig. 3b and Table S1†). Considering the mechanical properties and enthalpy values, NM60 with an enthalpy of 125.6 J g−1 was selected as the object for further introducing MXene. Given that the loading of MXene is ultralow, the crystallization and melting enthalpies of NM60 were basically the same as that of NM60M1, NM60M2 and NM60M3 (Fig. 3c and Table S1†). In addition, a crystallization/melting cyclic test was performed on NM60 and NM60M3 to simulate the thermal cycling state of the SFPCMs during the phase transition processes (Fig. 3d and e), respectively. Consequently, the phase change enthalpies of NM60 and NM60M3 remained stable after 200 cycles (Table S2†), which indicates that the dual-supporting strategy with core–shell structure and flexible polymer network can ensure the thermal cycling stability of SFPCMs. Moreover, the results of the FT-IR spectra before and after thermal cycling corroborate that the chemical structures of NM60 and NM60M3 also maintain the initial state after multiple thermal cycles (Fig. 3f). | ||
| Fig. 3 Thermophysical properties of SFPCMs. (a) Differential scanning calorimetry (DSC) first heating curves of PCMCs and NRm. DSC cooling and secondary heating curves of SFPCMs (b) without and (c) with MXene. DSC heating and cooling curves of (d) NM60 and (e) NM60M3 after 200 cycles. (f) FT-IR spectra of NM60 and NM60M3 after 200 heating–cooling cycles. Thermogravimetric analysis (TGA) and derivative thermogravimetry (DTG) curves of SFPCMs (g) without and (h) with MXene under N2 atmosphere at 10 °C min−1. (i) Comparison of elongation at break with respect to melting enthalpy of our works with composite flexible PCMs reported in the literature, including 1# cellulose/poly(hexadecyl acrylate)/graphene,67 2# polyolefin elastomer (POE)/PW/graphene nanoplatelets,53 3# PVA/water-borne polyurethane/PW,48 4# POE/styrene-b-(ethyleneco-butylene)-b-styrene triblock copolymer/PW/BN,54 5# ethylene-a-octene block copolymer/PW/carbon nanotubes,68 6# thermoplastic polyurethane/carbon cloth/PW,69 7# thermoplastic elastomer/expanded graphite/PW,70 8# polyurethane/n-docosane,71 9# polyurethane/PW,72 10# PW/styrene–butadiene–styrene block copolymer/CNT,73 11# polycaprolactone/PEG,74 and 12# CNT/h-BN/n-docosane/styrene–ethylene–propylene–styrene tri-block copolymer.75 | ||
The high-temperature thermal stability of SFPCMs is equally important. As shown in Fig. 3g and h, there is only one distinct degradation peak for NRm with a degradation peak temperature of 370 °C, indicating that NR can work as a flexible substrate with sufficient thermal stability. The decomposition temperatures of the PCMCs are around 226 °C and 278 °C, corresponding to the decomposition processes of the PU shell and PW core, respectively. The degradation process of the SFPCMs presents a combination of the degradation behaviors of NR and PCMCs with degradation peak temperatures over 200 °C. Compared to NM60, no obvious change in the decomposition peak temperature of SFPCMs is observed after introducing MXene, indicating that the presence of thimbleful MXene has no effect on their decomposition behaviors. These results suggest that the high-temperature thermal stability of SFPCMs is sufficient to enable them to work in various energy conversion environments. Moreover, compared with the materials reported in the literature,48,53,54,67–71 the SFPCMs with considerable energy storage density show the maximum elongation at break, corroborating their superior flexibility for thermal energy storage technology (Fig. 3i).
2.4. Salient stability of SFPCMs
Three-dimensional supporting materials have been employed to fabricate composite PCMs with improved shape stability,15,76–78 but a small part of the melt on the surface inevitably leaks during phase transition. Herein, two common composite PCMs, melamine foam-supporting PCMs (MFPCMs) and boron nitride (BN)/cellulose scaffold-supporting PCMs (SPCMs), were selected for comparison with the SFPCMs. As shown in Fig. S5,† it was observed that a small amount of phase change melt leaked from the MFPCMs and SPCMs on an 80 °C hot stage, while more melt leakage was absorbed by a blotting paper under the pressure of a 500 g load. Therefore, it is still a huge challenge to develop completely leakage-proof and water-resistant composite PCMs. In this work, the SFPCMs exhibited unprecedented shape stability without any leakage after reaching the phase transition temperature and the blotting paper remained dry (Fig. 4a), even under the pressure of a 500 g weight (Fig. 4b). To further quantify the leakage behaviors of the samples, they were placed in an 80 °C oven for melting/crystallization cycles with the calculation of mass loss (Fig. S6a†). After 10 cycles, the residual weights of the MFPCMs and SPCMs were 41% and 60% of their initial mass, respectively, whereas there was almost no mass loss for NM60 and NM60M3 owing to the dual-supporting effect, intuitively confirming the excellent leakage resistance of SFPCMs.In addition, the phase transition stability of the SFPCMs in various water environments was examined, aiming at expanding the use of STEGs for solar energy harvesting in the ocean environment. NM60 and NM60M3 were subjected to accelerated stability experiments under simulated water environments at high temperature (50 °C), salinity (saturation), acidity (2 M HCl), and basicity (2 M NaOH), as illustrated in Fig. 4c. Consequently, the tensile strengths of NM60 and NM60M3 were significantly improved after accelerated experiments (Fig. 4d and S7†), which is ascribed to the high temperature, salt, acid and base environments, which caused further cross-linking reactions of the unsaturated bonds in SFPCMs.79,80 One prominent feature is the reduction effect of saturated NaCl on the elongation at break of the SFPCMs because of the decrease in the mobility of the NR chains originating from the penetration of the high-salt ion solution in the NR matrix. Although the SFPCMs are in a phase transition state during the 120 h accelerated stability experiments, the elongation at break of the SFPCMs was maintained over 350% after these treatments. The crystallization/melting enthalpies of NM60 and NM60M3 before and after the accelerated stability experiments were consistent due to the effective supporting effect of the PU shell and the NR continuous network structure (Fig. 4e and Table. S3†). The results of FT-IR spectroscopy reveal that the main chemical structures of the NR and PCMCs in SFPCMs did not significantly decompose after the accelerated stability tests, as shown in Fig. 4f. These results demonstrate the excellent long-term phase transition stability and leakage resistance of SFPCMs in harsh underwater environments.
To more intuitively clarify the underwater phase transition stability of SFPCMs, the samples were immersed in 80 °C deionized water, followed by recording their leakage using thermal imaging technology. According to the digital and infrared images shown in Fig. 4g, the MFPCMs and SPCMs exhibited visible leakage in 80 °C water with the PW droplets floating on the water surface, and there were holes on the surface of the samples due to the leakage of PW, thus showing a relatively low surface temperature. In contrast, NM60 and NM60M3 showed complete leakage resistance, even pressurized by a 500 g load under water (Fig. 4h and S6b, c†). Moreover, SFPCMs exhibited great stretchability under water at 80 °C (Fig. 4i). The underwater phase transition stability of SFPCMs is ultimately attributed to the dual encapsulation from the PU shell and NR network, making them resistant to salt, acid, base, and external force.
2.5. Solar energy conversion of SFPCMs
The absorbance and reflectivity spectra of SFPCMs are shown in Fig. 5a and b, respectively. Because of the existence of polar components in NR, which are related to the absorption of ultraviolet-visible (UV-vis) light, a slight increase in light adsorption was observed for SFPCMs. After introducing MXene in NM60, its photo-absorption performance was significantly improved owing to the strong light absorption properties of MXene with a surface localized plasmon resonance effect.81 The photon capturing ability of SFPCMs increased with an increase in MXene content. Among them, NM60M3 exhibited the highest light absorption efficiency of about 94% in the UV-vis near-infrared (UV-vis-NIR) spectral range, enabling it to realize efficient solar energy conversion. Moreover, the thermal conductivity, an important parameter for PCMs, was evaluated (Fig. 5c and S8†). Consequently, it was found that the thermal conductivity of NRm was as low as 0.11 W m−1 K−1 and was improved after the incorporation of PCMCs, which is attributed to the high thermal conductivity of the PW in the PCMCs. The thermal conductivity of SFPCMs was further improved after adding MXene with superior thermal conductivity. In addition, the interfacial interaction between PCMCs and NR was improved due to the presence of MXene nanosheets, weakening the phonon scattering effect at the interfaces. The thermal conductivity of NM60M3 (0.38 W m−1 K−1) increased by 138% compared to that of NM60 (0.16 W m−1 K−1), which is beneficial to accelerating the storage and release of energy in SFPCMs.To further verify the ability of the SFPCMs to collect and store solar energy, the apparatus shown in Fig. 5d was designed to simulate solar energy conversion with PCMs. The samples were irradiated under a simulated sunlight with an intensity of 150 mW cm−2 for 20 min, and the temperature evolution curves of the samples were recorded in real time, as shown in Fig. 5e. NM70 with strong light reflection ability was unable to effectively absorb photons and convert them into latent heat, making its maximum temperature as low as 40.1 °C. With an increase in the NR content, the improved UV-vis light absorption of NR caused the maximum temperatures of NM50 and NM60 to reach 57.3 and 50.4 °C, respectively. After the introduction of MXene in NM60, the capture capacity of photons and the thermal charging rate were significantly enhanced. The maximum temperatures of NM60M1, NM60M2 and NM60M3 reached 68.6 °C, 81.4 °C and 97.8 °C, respectively. This is ascribed to the enhanced photothermal conversion capability and thermal conductivity, and thus SFPCMs with MXene delivered a desirable photothermal conversion efficiency. Among them, the photothermal conversion efficiency of NM60M3 in the latent heat storage process was as high as 92% (Fig. S9†). After removing the light source, SFPCMs started to release latent heat at around 32 °C and kept a constant temperature for a long time. The significant difference in photothermal conversion ability among the samples is shown in the thermal infrared images after irradiation for 6 min (Fig. 5f). The temperature of NM60M3 was close to 70 °C, whereas that of NM70 was basically the same as the ambient temperature. Thus, based on the above-mentioned results, NM60M3 was selected for 10 photothermal cycling tests, as shown in Fig. 5g. The cyclic curves with high consistency manifest the excellent photothermal stability of the photo-driven SFPCMs.
2.6. Solar-thermoelectric energy conversion of SFPCMs
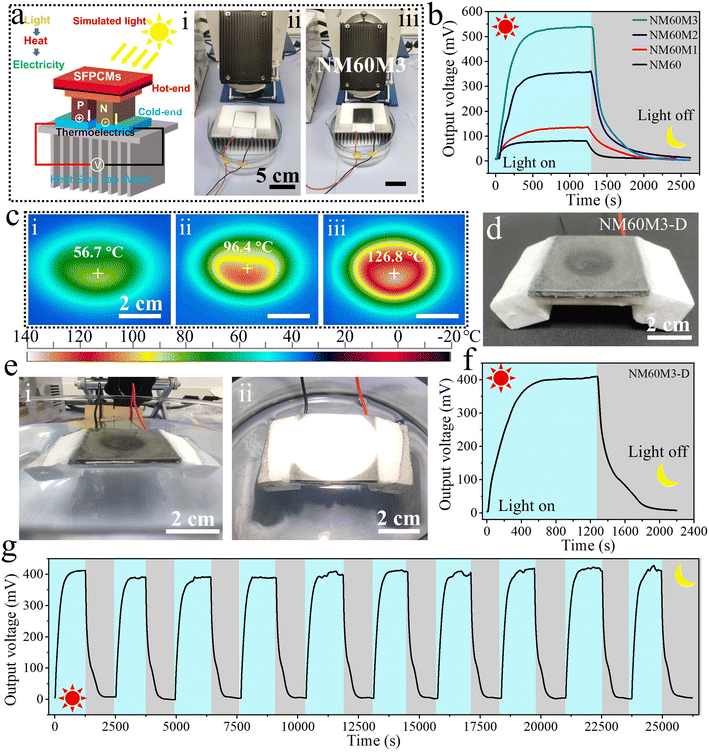
SFPCMs capable of efficiently converting solar energy into heat are a value-added component in STEGs. Herein, an STEG system with SFPCMs was designed to perform solar-thermoelectric energy conversion, as shown in Fig. 6a. SFPCMs and a stainless–steel radiator immersed in water were employed as the heat and cold sources, respectively, and the light source simulating solar energy was provided by a Xenon lamp. The curves of the output voltage of the STEGs as a function of the illumination time were recorded in real time (Fig. 6b). Owing to the poor photothermal conversion ability, the maximum output voltage produced by NM60 was only 79 mV, and the output voltage decayed rapidly after the removal of the light source. The maximum output voltages of SFPCMs in thermal equilibrium increased with an increase in the MXene loading, attaining 135, 357 and 539 mV for NM60M1, NM60M2 and NM60M3, respectively.Under the same cold source (about 17 °C), the energy released by the SFPCMs at the heat end of the thermoelectric module determined the final output voltage of the STEGs. Therefore, NM60M3 with an excellent light-to-heat conversion capability had a superior output voltage. Fig. 6c visually shows the thermal equilibrium temperature of the SFPCMs, which is consistent with results of the voltage output. The equilibrium temperature of NM60M3 at the center of the simulated light source spot is up to 130 °C. After turning off the light, the output voltage rapidly dropped to 0 mV, but it lasted longer for the SFPCMs with MXene owing to their higher equilibrium temperature and the release of stored energy. In addition, the specific structure of the thermoelectric unit is shown in Fig S10.† The P-type and N-type legs were packed using polymer adhesives and alumina ceramic sheets, which can hinder the corrosion from seawater to a certain extent.
A proof-of-concept self-floating STEG (NM60M3-D) was assembled based on NM60M3 and commercial thermoelectric modules (Fig. 6d and S11†), in which NM60M3 was used to absorb solar energy and convert it into thermal energy for storage and release. NM60M3-D could float on the water surface to harvest energy from the simulated light source (Fig. 6e) directly utilizing the water-cooling effect as the cold source. A stable output voltage of 410 mV for NM60M3-D was achieved after reaching thermal equilibrium, as shown in Fig. 6f. After the light source was removed, NM60M3-D could maintain the output voltage for 10 min by means of the release of stored heat. Finally, the cyclic stability of a self-floating STEG (NM60M3-D) was examined (Fig. 6g), and NM60M3-D maintained a stable output voltage during multiple cycles. These attempts demonstrate that NM60M3-D with sufficient stability exhibits great application potential in energy conversion in the ocean environment.
3. Conclusion
In summary, we developed dual-supporting SFPCMs comprised of island-like dispersed PCMCs and continuous supporting NR network. The resulting SFPCMs with excellent stretchability and compressibility exhibited salient leakage resistance and underwater stability, even in salt, acid, and alkali environments. The introduction of MXene not only enhanced the mechanical properties and thermal conductivity of SFPCMs, but also endowed them with improved photothermal conversion capabilities, achieving a high photothermal conversion efficiency of 92%. Based on the enhanced comprehensive properties of SFPCMs, a proof-of-concept self-floating STEG was devised to realize photoelectric energy conversion on the surface of water. Thus, this work presents new insights into the development of multifunctional flexible PCMs and emerging STEGs capable of making full use of solar energy in the ocean environment.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (NNSFC Grants 52003170 and 52125301) and the Fundamental Research Funds for the Central Universities.References
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed
.
- S. Lewis Nathan and G. Nocera Daniel, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed
.
- J. U. Kim, S. Lee, S. J. Kang and T.-i. Kim, Nanoscale, 2018, 10, 21555–21574 RSC
.
- X. Liu, Y. Feng, H. Cui, F. Liu, X. Hao, G. Conibeer, D. B. Mitzi and M. Green, Prog. Photovolt: Res. Appl., 2016, 24, 879–898 CrossRef
.
- Z. Zou, J. Ye, K. Sayama and H. Arakawa, Nature, 2001, 414, 625–627 CrossRef CAS PubMed
.
- H. Ren, M. Tang, B. Guan, K. Wang, J. Yang, F. Wang, M. Wang, J. Shan, Z. Chen, D. Wei, H. Peng and Z. Liu, Adv. Mater., 2017, 29, 1702590 CrossRef PubMed
.
- W. Guo, C. Guo, N. Zheng, T. Sun and S. Liu, Adv. Mater., 2017, 29, 1604157 CrossRef PubMed
.
- X. Zhao, L.-M. Peng, C.-Y. Tang, J.-H. Pu, X.-J. Zha, K. Ke, R.-Y. Bao, M.-B. Yang and W. Yang, Mater. Horiz., 2020, 7, 855–865 RSC
.
- H. Liu, J. Tang, L. Dong, H. Wang, T. Xu, W. Gao, F. Zhai, Y. Feng and W. Feng, Adv. Funct. Mater., 2020, 31, 2008496 CrossRef
.
- G. Ni, G. Li, S. V. Boriskina, H. Li, W. Yang, T. Zhang and G. Chen, Nat. Energy, 2016, 1, 16126 CrossRef CAS
.
- B. M. Diaconu, Renewable Energy, 2012, 37, 266–276 CrossRef
.
- A. R. Muchtar, B. Srinivasan, S. L. Tonquesse, S. Singh, N. Soelami, B. Yuliarto, D. Berthebaud and T. Mori, Adv. Energy Mater., 2021, 11, 2170110 CrossRef
.
- D. Liu, C. Lei, K. Wu and Q. Fu, ACS Nano, 2020, 14, 15738–15747 CrossRef PubMed
.
- J. Yang, P. Yu, L. S. Tang, R. Y. Bao, Z. Y. Liu, M. B. Yang and W. Yang, Nanoscale, 2017, 9, 17704–17709 RSC
.
- J. Yang, L.-S. Tang, L. Bai, R.-Y. Bao, Z.-Y. Liu, B.-H. Xie, M.-B. Yang and W. Yang, Mater. Horiz., 2019, 6, 250–273 RSC
.
- W. Aftab, J. Shi, M. Qin, Z. Liang, F. Xiong, A. Usman, S. Han and R. Zou, Energy Storage Mater., 2022, 52, 284–290 CrossRef
.
- J. Yang, G.-Q. Qi, Y. Liu, R.-Y. Bao, Z.-Y. Liu, W. Yang, B.-H. Xie and M.-B. Yang, Carbon, 2016, 100, 693–702 CrossRef CAS
.
- L.-S. Tang, Y.-C. Zhou, L. Zhou, J. Yang, L. Bai, R.-Y. Bao, Z.-Y. Liu, M.-B. Yang and W. Yang, Chem. Eng. J., 2022, 430, 132773 CrossRef CAS
.
- Y. He, Y. Wei, Y. Qian, C. Wang, Y. Liu, Z. Ye and G. Chen, J. Mater. Chem. A, 2022, 10, 18856–18865 RSC
.
-
H. D. Holland, The Chemical Evolution of the Atmosphere and Oceans, Princeton University Press, 2020 Search PubMed
.
-
Z. Qiu and P. Li, in Advanced Energy Efficiency Technologies for Solar Heating, Cooling and Power Generation, ed. X. Zhao and X. Ma, Springer International Publishing, Cham, 2019, pp. 1–30 Search PubMed
.
- S. Olson, M. F. Jansen, D. S. Abbot, I. Halevy and C. Goldblatt, Geophys. Res. Lett., 2022, 49, e2021GL095748 CrossRef CAS PubMed
.
- R. J. Leamon, S. W. McIntosh and D. R. Marsh, Earth Space Sci., 2021, 8, e2020EA001223 Search PubMed
.
- X. Chen, H. Gao, M. Yang, W. Dong, X. Huang, A. Li, C. Dong and G. Wang, Nano Energy, 2018, 49, 86–94 CrossRef CAS
.
- Z. Tang, H. Gao, X. Chen, Y. Zhang, A. Li and G. Wang, Nano Energy, 2021, 80, 105454 CrossRef CAS
.
- G. Qi, J. Yang, R. Bao, D. Xia, M. Cao, W. Yang, M. Yang and D. Wei, Nano Res., 2016, 10, 802–813 CrossRef
.
- Y. Li, Y. A. Samad, K. Polychronopoulou, S. M. Alhassan and K. Liao, J. Mater. Chem. A, 2014, 2, 7759–7765 RSC
.
- D. G. Atinafu, W. Dong, C. Wang and G. Wang, J. Mater. Chem. A, 2018, 6, 8969–8977 RSC
.
- A. R. Muchtar, C. L. Hassam, B. Srinivasan, D. Berthebaud, T. Mori, N. Soelami and B. Yuliarto, J. Energy Storage, 2022, 52, 104974 CrossRef
.
- S. Gong, X. Li, M. Sheng, S. Liu, Y. Zheng, H. Wu, X. Lu and J. Qu, ACS Appl. Mater. Interfaces, 2021, 13, 47174–47184 CrossRef CAS PubMed
.
- J. Yang, Y.-C. Zhou, L.-Y. Yang, C.-P. Feng, L. Bai, M.-B. Yang and W. Yang, Adv. Funct. Mater., 2022, 32, 2200792 CrossRef CAS
.
- F. Xue, X.-d. Qi, T. Huang, C.-y. Tang, N. Zhang and Y. Wang, Chem. Eng. J., 2021, 419, 129620 CrossRef CAS
.
- D. G. Atinafu, B. Y. Yun, Y. U. Kim, S. Yang, S. Wi and S. Kim, Chem. Eng. J., 2021, 420, 130374 CrossRef CAS
.
- P. Min, J. Liu, X. Li, F. An, P. Liu, Y. Shen, N. Koratkar and Z.-Z. Yu, Adv. Funct. Mater., 2018, 28, 1805365 CrossRef
.
- M. Li, L. Li, Y. Qin, X. Wei, X. Kong, Z. Zhang, S. Xiong, H. Do, J. C. Greer, Z. Pan, T. Cai, W. Dai, C.-T. Lin, N. Jiang and J. Yu, J. Mater. Chem. A, 2022, 10, 593–601 RSC
.
- Y. Zhang, R. Lu, S. Zhang and B. Tang, Chem. Eng. J., 2021, 423, 130260 CrossRef CAS
.
- X. Hu, C. Zhu, H. Wu, X. Li, X. Lu and J. Qu, Composites, Part A, 2022, 154, 106770 CrossRef CAS
.
- J. Shi, M. Qin, W. Aftab and R. Zou, Energy Storage Mater., 2021, 41, 321–342 CrossRef
.
- X. Chen, H. Gao, G. Hai, D. Jia, L. Xing, S. Chen, P. Cheng, M. Han, W. Dong and G. Wang, Energy Storage Mater., 2020, 26, 129–137 CrossRef
.
- A. Usman, F. Xiong, W. Aftab, M. Qin and R. Zou, Adv. Mater., 2022, 34, 2202457 CrossRef CAS PubMed
.
- J. Shi, W. Aftab, Z. Liang, K. Yuan, M. Maqbool, H. Jiang, F. Xiong, M. Qin, S. Gao and R. Zou, J. Mater. Chem. A, 2020, 8, 20133–20140 RSC
.
- X. Lu, C. Fang, X. Sheng, L. Zhang and J. Qu, Ind. Eng. Chem. Res., 2019, 58, 3024–3032 CrossRef CAS
.
- J. Cui, W. Li, Y. Wang, H. Yu, X. Feng, Z. Lou, W. Shan and Y. Xiong, Adv. Funct. Mater., 2021, 32, 2108000 CrossRef
.
- Y. Kou, K. Sun, J. Luo, F. Zhou, H. Huang, Z.-S. Wu and Q. Shi, Energy Storage Mater., 2021, 34, 508–514 CrossRef
.
- H. Wu, X. Hu, X. Li, M. Sheng, X. Sheng, X. Lu and J. Qu, Composites, Part A, 2022, 152, 106713 CrossRef CAS
.
- Y. Bao, J. Lyu, Z. Liu, Y. Ding and X. Zhang, ACS Nano, 2021, 15, 15180–15190 CrossRef CAS PubMed
.
- X. Yu, Y. Li, X. Yin, X. Wang, Y. Han, Y. Si, J. Yu and B. Ding, ACS Appl. Mater. Interfaces, 2019, 11, 39324–39333 CrossRef CAS PubMed
.
- Y. He, W. Li, N. Han, J. Wang and X. Zhang, Appl. Energy, 2019, 247, 615–629 CrossRef CAS
.
- D. G. Atinafu, B. Y. Yun, S. Yang, H. Yuk, S. Wi and S. Kim, Energy Storage Mater., 2021, 42, 164–184 CrossRef
.
- W. Aftab, A. Mahmood, W. Guo, M. Yousaf, H. Tabassum, X. Huang, Z. Liang, A. Cao and R. Zou, Energy Storage Mater., 2019, 20, 401–409 CrossRef
.
- K. Sun, Y. Kou, H. Dong, S. Ye, D. Zhao, J. Liu and Q. Shi, J. Mater. Chem. A, 2021, 9, 1213–1220 RSC
.
- C. Chang, X. Nie, X. Li, P. Tao, B. Fu, Z. Wang, J. Xu, Q. Ye, J. Zhang, C. Song, W. Shang and T. Deng, J. Mater. Chem. A, 2020, 8, 20970–20978 RSC
.
- F. Wei, C.-P. Feng, J. Yang, L.-Y. Yang, L. Bai, R.-Y. Bao, Z.-Y. Liu, M.-B. Yang and W. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 59364–59372 CrossRef CAS PubMed
.
- L.-Y. Yang, C.-P. Feng, L. Bai, R.-Y. Bao, Z.-Y. Liu, M.-B. Yang and W. Yang, Chem. Eng. J., 2021, 425, 131466 CrossRef CAS
.
- Y.-C. Zhou, J. Yang, L. Bai, R.-Y. Bao, M.-B. Yang and W. Yang, Chem. Eng. J., 2022, 446, 137463 CrossRef CAS
.
- L. Zhou, X.-f. Tao, L.-s. Tang, M.-B. Yang and W. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 53365–53375 CrossRef CAS PubMed
.
- X. Fan, L. Liu, X. Jin, W. Wang, S. Zhang and B. Tang, J. Mater. Chem. A, 2019, 7, 14319–14327 RSC
.
- X. Zhao, X.-J. Zha, L.-S. Tang, J.-H. Pu, K. Ke, R.-Y. Bao, Z.-y. Liu, M.-B. Yang and W. Yang, Nano Res., 2019, 13, 255–264 CrossRef
.
- W. Chen, L.-X. Liu, H.-B. Zhang and Z.-Z. Yu, ACS Nano, 2021, 15, 7668–7681 CrossRef CAS PubMed
.
- Y. Zhang, M.-K. Xu, Z. Wang, T. Zhao, L.-X. Liu, H.-B. Zhang and Z.-Z. Yu, Nano Res., 2022, 15, 4916–4924 CrossRef CAS
.
- S. Abdolhosseinzadeh, X. Jiang, H. Zhang, J. Qiu and C. Zhang, Mater. Today, 2021, 48, 214–240 CrossRef CAS
.
- Q. Guo, X. Zhang, F. Zhao, Q. Song, G. Su, Y. Tan, Q. Tao, T. Zhou, Y. Yu, Z. Zhou and C. Lu, ACS Nano, 2020, 14, 2788–2797 CrossRef CAS PubMed
.
- P. Hobza and Z. Havlas, Chem. Rev., 2000, 100, 4253–4264 CrossRef CAS PubMed
.
- I. V. Alabugin, M. Manoharan, S. Peabody and F. Weinhold, J. Am. Chem. Soc., 2003, 125, 5973–5987 CrossRef CAS PubMed
.
- X. Li, L. Liu and H. B. Schlegel, J. Am. Chem. Soc., 2002, 124, 9639–9647 CrossRef CAS PubMed
.
- Z. Yan, F. Zhang, J. Wang, F. Liu, X. Guo, K. Nan, Q. Lin, M. Gao, D. Xiao, Y. Shi, Y. Qiu, H. Luan, J. H. Kim, Y. Wang, H. Luo, M. Han, Y. Huang, Y. Zhang and J. A. Rogers, Adv. Funct. Mater., 2016, 26, 2629–2639 CrossRef CAS PubMed
.
- Y. Qian, N. Han, Z. Zhang, R. Cao, L. Tan, W. Li and X. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 45832–45843 CrossRef CAS PubMed
.
- X.-d. Qi, Y.-w. Shao, H.-y. Wu, J.-h. Yang and Y. Wang, Compos. Sci. Technol., 2019, 181, 107714 CrossRef CAS
.
- M. M. Umair, Y. Zhang, S. Zhang, X. Jin and B. Tang, J. Mater. Chem. A, 2019, 7, 26385–26392 RSC
.
- Z. Cai, J. Liu, Y. Zhou, L. Dai, H. Wang, C. Liao, X. Zou, Y. Chen and Y. Xu, Sol. Energy Mater. Sol. Cells, 2021, 219, 110728 CrossRef CAS
.
- X. Li, C. Wang, Y. Wang, B. Wang, X. Feng, Z. Mao and X. Sui, Sol. Energy Mater. Sol. Cells, 2021, 227, 111111 CrossRef CAS
.
- J. Wu, M. Wang, L. Dong, J. Shi, M. Ohyama, Y. Kohsaka, C. Zhu and H. Morikawa, ACS Nano, 2022, 16, 12801–12812 CrossRef CAS PubMed
.
- D. Hu, L. Han, W. Zhou, P. Li, Y. Huang, Z. Yang and X. Jia, Chem. Eng. J., 2022, 437, 135056 CrossRef CAS
.
- X. Chai, T. Zhao, J. Yue, J. Yang, S. Zhang, J. Du, A. Zhang and Z. Feng, React. Funct. Polym., 2022, 181, 105451 CrossRef CAS
.
- Y. Ma, H. Wang, L. Zhang, X. Sheng and Y. Chen, Composites, Part A, 2022, 163, 107203 CrossRef CAS
.
- H. Gao, J. Wang, X. Chen, G. Wang, X. Huang, A. Li and W. Dong, Nano Energy, 2018, 53, 769–797 CrossRef CAS
.
- P. Liu, F. An, X. Lu, X. Li, P. Min, C. Shu, W. Li and Z.-Z. Yu, Compos. Sci. Technol., 2021, 201, 108492 CrossRef CAS
.
- J. Yang, X. Li, S. Han, R. Yang, P. Min and Z.-Z. Yu, J. Mater. Chem. A, 2018, 6, 5880–5886 RSC
.
- H. Zhang, L. Zhang, X. Chen, Y. Wang, F. Zhao, M. Luo and S. Liao, Polymers, 2020, 12, 2880 CrossRef CAS PubMed
.
- J. Yunyongwattanakorn, J. T. Sakdapipanich, S. Kawahara, M. Hikosaka and Y. Tanaka, J. Appl. Polym. Sci., 2007, 106, 455–461 CrossRef CAS
.
- L. Tang, X. Zhao, C. Feng, L. Bai, J. Yang, R. Bao, Z. Liu, M. Yang and W. Yang, Sol. Energy Mater. Sol. Cells, 2019, 203, 110174 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta07885f |
| This journal is © The Royal Society of Chemistry 2023 |