Lignin-derived electrode materials for supercapacitor applications: progress and perspectives
Yao
Tong
a,
Junyu
Yang
b,
Jiajun
Li
a,
Ziyang
Cong
a,
Li
Wei
a,
Miaomiao
Liu
a,
Shangru
Zhai
a,
Kai
Wang
 *b and
Qingda
An
*a
*b and
Qingda
An
*a
aFaculty of Light Industry and Chemical Engineering, Dalian Polytechnic University, Dalian 116034, China. E-mail: anqingda@dlpu.edu.cn
bDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China. E-mail: wangkai@dicp.ac.cn
First published on 7th December 2022
Abstract
Lignin is one of the most abundant natural polymers and is affordable, has high carbon content and abundant active functional groups. At present, lignin-derived carbon is considered an ideal, promising electrode material for supercapacitor applications and this route is showing a vigorous development trend. In this review, we summarize the progress of lignin-derived materials for supercapacitor applications. Firstly, the concept, classification strategy and basic chemistry of lignin are introduced in brief. Then, the up-to-date developments of the synthesis strategies of carbon electrodes from lignin for supercapacitors are reviewed in detail. Finally, the work is summarized and the major challenges of the lignin-based supercapacitors are discussed. This review is presented to guide the synthesis of lignin-based electrodes for supercapacitors and facilitate their widespread application.
1. Introduction
The dwindling supply of fossil fuels and the growing demand for energy resources have stimulated the development of renewable energy and new types of energy storage devices.1–6 Compared with other energy storage devices, supercapacitors are fascinating owing to their integrated advantages, such as high power density (>10 kW kg−1), exceptionally fast charge/discharge rate (in seconds) in conjunction with an extremely long cycle life (>105).7–11 Nevertheless, their subpar energy density restrains their large-scale applications; thus, one of the paramount challenges for supercapacitors is to enhance the energy density as much as possible without sacrificing the original high power density and ultra-long cycle stability.12–14 Besides, most of the electrode materials for supercapacitor applications are extracted from unrenewable and unsustainable coal or fossil oil materials.15–18 In this regard, the development of various renewable and sustainable resources as alternative precursors for the preparation of supercapacitors is indispensable.Lignin is a heterogeneous and amorphous polymer. At present, approximately 70 million tons of lignin are produced during the extraction of cellulose for the paper industry each year. The affordability and bio-renewable properties make lignin a desirable candidate material for numerous applications.19–24 Despite the huge possibilities, lignin has not yet been converted into high-value-added products on a large scale thus far.25–28 Only a tiny percentage, approximately less than 2% of the gross product is applied as stabilizing agents,29,30 concrete additives or surfactants and dispersants.31–35 The rest is thrown away directly as waste or burned as low-quality fuel.36–38 On the other hand, due to the integrated advantages of lignin, such as high carbon content, high thermal stability, biodegradability and feasible stiffness, deriving more value-added products, such as electrode materials, from lignin for supercapacitors is a potential alternative.39–42 At present, massive efforts have already been devoted to converting lignin into carbon materials for the design of safe and reliable supercapacitors.
This review covers the recent progress in the transformation of lignin into valuable products applied in the field of supercapacitors. We shall first briefly introduce the categorization and isolation processes of lignin materials. Subsequently, the synthesis of electrode materials from lignin and their applications for supercapacitors are elaborated. Finally, the challenges and possible future directions are presented to spark new thoughts for bringing supercapacitors into practical and daily-life applications.
2. Background and isolation process of lignin
Uncovering the specific structure, categorization, source and preparation methods of various kinds of lignin is of paramount significance in investigating their potential applications. As such, we will discuss the origin and fundamental structures of various kinds of lignin, as well as the isolation process.2.1 Origin and fundamental structures of lignin
Lignin is the most abundant aromatic polymer compound in nature, which is extensively present in softwoods, hardwoods, grasses and other plants. Softwoods are comprised of the most lignin (25–35%), hardwoods are comprised of moderate lignin (20–25%), and Gramineae plants are comprised of less lignin (15–25%).43–46 In general, lignin is chemically interwoven with hemicellulose and then wraps the outside of cellulose fibers.47 As indicated in Fig. 1a, the bonding and strengthening between lignin, cellulose and hemicellulose play a decisive role in the strength and toughness of plant cell walls.48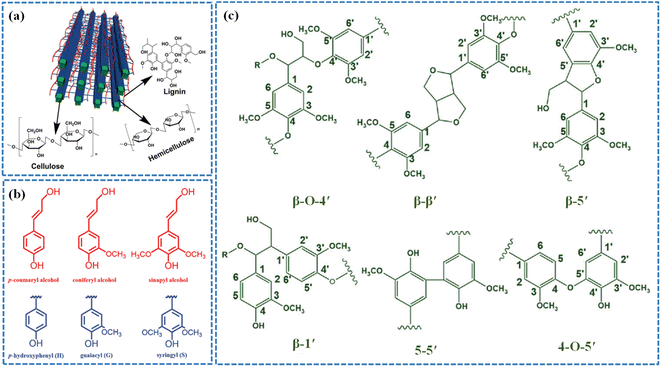 | ||
| Fig. 1 (a) The natural structure of cellulose, hemicellulose and lignin in the lignocellulosic biomass. Adapted with permission from ref. 53. Copyright 2015 Royal Society of Chemistry. (b) Lignin monomer units and their precursors. (c) The linkages of lignin. Adapted with permission from ref. 54. Copyright 2017 Royal Society of Chemistry. | ||
In terms of molecular structures, lignin is an amorphous polymer with a three-dimensional network structure consisting of phenyl-propane structural units connected by ether bonds and carbon–carbon bonds.49–52 Its phenyl-propane structural unit is usually comprised of three basic phenylpropanolic monomers, i.e., sinapyl, p-coumaryl and coniferyl alcohols, corresponding to the three types of lignin structural units, namely, guaiacyl (G), p-hydrophenyl (H), and syringyl units (S), respectively (Fig. 1b).55–58 The β-O-4′ ether linkages are the main type in lignin that account for more than 50% of the linkage structures and are certainly a critical criterion in the degradation studies.57 The 4-O-5′, β-β′, β-5′, β-1′ and 5-5′ linkages also exist in lignin but they account for smaller percentages (Fig. 1c). The proportion of structural units and connection structures in various lignins are dissimilar depending on the plant species, growth parts, growth environment, and interference from external factors. As an example, G units constitute softwood, GS units are abundant in hardwood, while the grasses are comprised of the three monolignols, HGS-units.23,59
2.2 Isolation process of lignin
Lignin can be extracted from the chemical processes of pulping. The various extraction processes will affect the chemical functional groups and properties of lignin. The most crucial chemical functional groups in lignin include different numbers and proportions of hydroxyl, methoxyl, carbonyl and carboxyl groups, depending on the source of lignin and the extraction process. There are generally two strategies for separating lignin from biomass as follows: (i) polysaccharides are selectively hydrolyzed and lignin remains in the solid residues along with the decomposition products of some condensed carbohydrates;60–62 (ii) lignin is degraded into soluble fragments and the solid residue is removed from the liquor, from which the lignin is extracted by using an appropriate solvent. At present, all the industrial pulping processes belong to the latter, resulting in the generation of technical lignin, kraft lignin, lignosulfonate and organosolv lignin.Industrial lignin can be divided into four different types depending on whether it is subjected to the sulphuring process.36 Kraft lignin is the dominant industrially processed lignin worldwide.63,64 During the kraft process, the structure of pristine lignin is dramatically broken. As a consequence, kraft lignin contains more phenolic –OH groups and more condensed C–C structures.65 Lignosulfonate is mainly derived from the waste liquid produced during the pulping process, which usually consists of active groups including phenolic hydroxyl, aliphatic hydroxyl and carbonyl. The sulfur content of lignosulfonate is rather high (i.e. 4–8%), most of which is in the form of sulfonate.66,67 Organosolv lignin is derived from the by-products of the organosolv pulping process, which exhibit smaller molecular weights and possess a higher concentration of phenolic hydroxyl groups.36,68 Aside from these, soda lignin is mainly derived from the soda or soda–anthraquinone pulping process.54 In comparison with kraft pulping, soda lignin has no sulfur and is more like native lignin, thus soda lignin possesses more active sites for chemical modification.36,69 Enzymatic hydrolysis lignin is a new type of industrial lignin that is extracted from crop waste obtained during the production of bioethanol. It preserves the original lignin structure to a large extent and has higher hydrophilicity as compared to other lignin.70,71
3. Lignin-based electrodes for supercapacitors
Owing to their efficient, safe and reliable advantages, supercapacitors have successfully drawn extensive research interest during the past couple of decades.72,73 Generally, supercapacitors mainly comprise electrodes, electrolytes and separators.6,74 Among them, electrodes play a decisive role in determining the electrochemical performance of supercapacitors.75 Supercapacitors can be normally categorized into electrical double-layer capacitors (EDLCs) and pseudo-capacitors according to their energy-storage mechanisms.12 As shown in Fig. 2a, EDLCs rely on ion adsorption–desorption at the surface/interfaces or inner pores of electrode materials to store charges.76,77 In recent years, a variety of carbon-based materials, such as porous carbons (PCs),78 activated carbons (ACs),79 carbon nanotubes (CNTs),80 carbon aerogels (CAs),81 carbon fibers (CFs)82 and graphene83 are preferred for EDLCs electrodes ascribed to their convincing merits of tunable porosity, high specific surface area (SSA), non-toxicity, superior electronic conductivity, and excellent chemical and thermal stability. Carbon-based materials can form a short electron or ion transmission distance at high energy transfer conditions, endowing EDLCs with high power density and excellent cycle stability.84 As displayed in Fig. 2b, in comparison with EDLCs, pseudo-capacitors store energy through the reversible redox or faradaic reactions between the electrodes and electrolytes.76 As such, pseudo-capacitors possess a higher specific capacitance and superior energy density but they suffer from lower power density and inferior cycle stability. Transition metal oxides/hydroxides and conducting polymers are typical candidates for electrode materials in pseudo-capacitors.85 Recently, increasing attention has been devoted to the synthesis of lignin-derived carbon materials for EDLCs, benefiting from the perfect combination of the high carbon content and low-cost feature. Moreover, the quinone functional group existing in the lignin and its derivatives display strong redox activity, which is conducive to the charge transfer reactions between the electrode surface and soluble species.86,87 Consequently, lignin and its derivatives can be utilized to prepare pseudo-capacitors as well. Table 1 summarizes in detail the recent research contributions in lignin-derived electrode materials utilized for supercapacitors.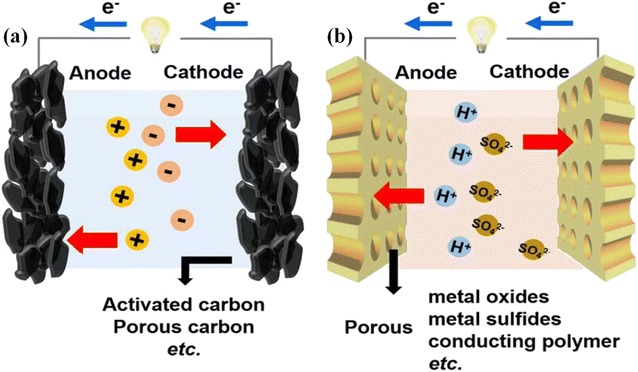 | ||
| Fig. 2 Schematic diagram of (a) EDLCs and (b) pseudo-capacitors. Adapted with permission from ref. 76. Copyright 2016 Royal Society of Chemistry. | ||
| Electrode materials | Structural properties | Synthesis | Electrolytes | Specific capacitance | Energy density | Cycle stability | Ref. |
|---|---|---|---|---|---|---|---|
| Lignin/PAN carbon nanofiber | S BET: 1176.0 m2 g−1 | Electrospinning and self-activation process | Lignin hydrogel electrolyte | 129.23 F g−1 at 0.5 A g−1 | 4.49 W h kg−1 at 2.63 kW kg−1 | 95% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
18 |
| V t: 0.394 cm3 g−1 | 108.90 F g−1 at 5 A g−1 | ||||||
| LFW@PANI | — | In situ self-polymerization | 1 M H2SO4 | 800 F g−1 at 10 A g−1 | 46 W h kg−1 at 68 kW kg−1 | 96% after 5000 cycles at 50 mV s−1 | 39 |
| LCA/Ni cubic carbon | S BET: 892 m2 g−1 | ZnCl2 activation and template | 6 M KOH | 26.6 F cm−2 at 1 mA cm−2 | — | 98.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 200 mA cm−2 000 cycles at 200 mA cm−2 |
42 |
| V t: 0.53 cm3 g−1 | 16.4 F cm−2 at 200 mA cm−2 | ||||||
| HPC | S BET: 1504 m2 g−1 | Hydrothermal carbonization and KOH activation | 6 M KOH | 324 F g−1 at 0.5 A g−1 | 17.9 W h kg−1 at 458 W kg−1 | 98.07% after 5000 cycles at 5 A g−1 | 70 |
| V t: 0.757 cm3 g−1 | 249 F g−1 at 50 A g−1 | ||||||
| HPC/WO3 | S BET: 1430 m2 g−1 | Carbonization and solvothermal process | 1 M H2SO4 | 432 F g−1 at 0.5 A g−1 | 34.2 W h kg−1 at 243 W kg−1 | 86.6% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
71 |
| V t: 0.41 cm3 g−1 | 214 F g−1 at 20 A g−1 | ||||||
SC-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
S BET: 1886 m2 g−1 | KOH activation | EMIBF4 | 231 F g−1 at 1 A g−1 | — | 50% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 000 cycles at 1 A g−1 |
91 |
| V t: 0.86 cm3 g−1 | 203 F g−1 at 10 A g−1 | ||||||
| LAC4 | S BET: 3775 m2 g−1 | Carbonization and KOH activation | 6 M KOH | 286.7 F g−1 at 0.2 A g−1 | 8.87 W h kg−1 at 51.92 W kg−1 | — | 97 |
| V t: 2.703 cm3 g−1 | 207.1 F g−1 at 8 A g−1 | ||||||
| LHC-3K | S BET: 1660 m2 g−1 | Hydrothermal carbonization and KOH activation | 6 M KOH | 420 F g−1 at 0.1 A g−1 | 10 W h kg−1 at 50 W kg−1 | 99% after 5000 cycles at 5 A g−1 | 103 |
| V t: 0.78 cm3 g−1 | 284 F g−1 at 100 A g−1 | ||||||
| PCS | S BET: 1590 m2 g−1 | Spray drying; KOH activation | 3 M KOH | 345 F g−1 at 0.5 A g−1 | 9.7 W h kg−1 at 250 W kg−1 | 94.5% after 5000 cycles at 4 A g−1 | 198 |
| V t: 0.89 cm3 g−1 | 245 F g−1 at 10 A g−1 | ||||||
| S–PC–L-900 | S BET: 1054 m2 g−1 | Silica template | 6 M KOH | 328 F g−1 at 0.2 A g−1 | 6.9 W h kg−1 at 50 W kg−1 | 94% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 A g−1 000 cycles at 2 A g−1 |
161 |
| V t: 1.73 cm3 g−1 | 192 F g−1 at 20 A g−1 | ||||||
| CO2-activated mesoporous carbon from lignin | S BET: 1148 m2 g−1 | Surfactant Pluronic F127/CO2-activated | 6 M KOH | 102.3 F g−1 at 1 mVs−1 | — | — | 107 |
| V t: 1.00 cm3 g−1 | |||||||
| SLC | S BET: 803 m2 g−1 | Dual template of P123 and KIT-6 | 6 M KOH | 3.0 F cm−2 at 0.1 A g−1 | 0.16 mW h cm−2 at 1.75 mW cm−2 | 96% after 1500 cycles at 2 A g−1 | 109 |
| V t: 0.86 cm3 g−1 | 1.4 F cm−2 at 10 A g−1 | ||||||
| CNSs | Thickness: 50–100 nm | Freeze-casting lignin aqueous dispersion | 1 M H2SO4 | 281 F g−1 at 0.5 A g−1 | 25.1 W h kg−1 at 583 W kg−1 | 91% after 5000 cycles at 1 A g−1 | 199 |
| S BET: 854.7 m2 g−1 | 153 F g−1 at 20 A g−1 | ||||||
LCNFs–PRL (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
S BET: 1063 m2 g−1 | Electrospinning PAN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lignin (5 lignin (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
6 M KOH | 339.2 F g−1 at 0.1 A g−1 | 56.9 W h kg−1 at 339 W kg−1 in 1 M Na2SO4 | 90.52% after 5000 cycles at 1 A g−1 in 1 M Na2SO4 | 112 |
| V t: 0.53 cm3 g−1 | 289.6 F g−1 at 5 A g−1 | ||||||
| Carbon nanofiber mats | Thickness: 50–174 μm | Electrospinning process | 6 M KOH | 130 F cm−3 at 0.1 A g−1 | 3.4 W h L−1 at 10 kW L−1 | Over 90% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
116 |
| 102.7 F g−1 at 100 A g−1 | |||||||
| ML-7 K CFs | S BET: 2042.86 m2 g−1 | Modification and fractionation | 6 M KOH | 442.2 F g−1 at 1 A g−1 | 37.1 W h kg−1 at 400 W kg−1 | 97.1% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles in 1 M Na2SO4 000 cycles in 1 M Na2SO4 |
118 |
| 384 F g−1 at 10 A g−1 | |||||||
| AILCFN/Ni–Co–S | S BET: 715.38 m2 g−1 | Electrospinning and thermal treatment | 6 M KOH | 1140.0 C g−1 at 10 A g−1 | 30.8 W h kg−1 at 800 W kg−1 | 84.7% after 3000 cycles at 10 mA cm−2 | 123 |
| V t: 0.3240 mL g−1 | |||||||
| K-ACFs | — | Electrospinning, carbonization and KOH activation | 6 M KOH | 344 F g−1 at 10 mv s−1 | 8.1 W h kg−1 at 50 mV s−1 | 96.5% after 5000 cycles | 125 |
| 196 F g−1 at 50 mV s−1 | |||||||
| Lignin–cellulose-based CFs-5 | S BET: 837.4 m2 g−1 | Preoxidation and carbonization treatment | 6 M KOH | 346.6 F g−1 at 0.1 A g−1 | 31.5 W h kg−1 at 400 W kg−1 in 1 M Na2SO4 | — | 126 |
| V t: 0.488722 cm3 g−1 | 285.3 F g−1 at 5 A g−1 | ||||||
| Lignin–NiWO4 | — | Depositing | PVA/H3PO4 gel | 129.7 mF cm−2 at 0.013 A g−1 | 2 W h cm−2 at 100 W cm−2 | 84% after 2000 cycles | 139 |
| 6.39 mF cm−2 at 0.128 A g−1 | |||||||
ECNF/MnO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mat 1) mat |
S BET: 583 m2 g−1 fiber diameters of ∼200 nm | Electrospinning | 1 M LiPF6 | 83.3 F g−1 at 250 mA g−1 | 84.3 W h kg−1 at 5.72 kW kg−1 | 99% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2000 mA g−1 000 cycles at 2000 mA g−1 |
142 |
| MCNFs@SnO2 | S BET: 659 m2 g−1 | Co-electrospinning | 6 M KOH | 406 F g−1 at 0.5 A g−1 | 11.5 W h kg−1 at 451 W kg−1 | 95% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
144 |
| 128 F g−1 at 50 A g−1 | |||||||
| LDC/ZnO | S BET: 372.6 m2 g−1 | Electrostatic self-assembling carbonization process | PVA/KOH gel | 193 F g−1 at 0.5 A g−1 | 6.7 W h kg−1 at 197.7 W kg−1 | 94.2% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 A g−1 000 cycles at 2 A g−1 |
145 |
| 151 F g−1 at 20 A g−1 | |||||||
| PC–Ni/MnO2-1 | S BET: 80.14 m2 g−1 | Carbonization process | 6 M KOH | 267.34 F g−1 at 0.1 A g−1 | 28 W h kg−1 at 360 W kg−1 | 83.6% after 5000 cycles at 1 A g−1 | 146 |
182.16 F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g−1 at 2 A g−1 g−1 at 2 A g−1 |
|||||||
| KL/TAC | — | Ultrasonic-assisted deposition method | 1 M H2SO4 | 293 F g−1 at 1 A g−1 | — | 98.1% after 1000 cycles at 1 A g−1 | 200 |
| HLRGO11 | S BET: 1804 m2 g−1 | Hydrothermal process | 6 M KOH | 190 F g−1 at 0.5 A g−1 | — | 86.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
133 |
| 133.9 F g−1 at 10 A g−1 | |||||||
| Lig–GHs | S BET: 109 m2 g−1 | Hydrothermal process | 0.1 M HClO4 | 549.5 F g−1 at 1 A g−1 | — | 83.7% after 1000 cycles at 20 A g−1 | 132 |
| 335 F g−1 at 20 A g−1 | |||||||
| C–LRGOs | S BET: 444.29 m2 g−1 | Hydrothermal reaction and carbonization | 1 M H2SO4 | 330 F g−1 at 1 A g−1 | 11.3 W h kg−1 at 254 W kg−1 | 100% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 50 mV s−1 000 cycles at 50 mV s−1 |
134 |
| V t: 0.773 cm3 g−1 | 265 F g−1at 10 A g−1 | ||||||
| HPCSLS-700-1 | S BET: 903 m2 g−1 | Direct carbonization | 7 M KOH | 247 F g−1 at 0.05 A g−1 | 8.6 W h kg−1 at 14.3 W kg−1 | 92% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 A g−1 000 cycles at 2 A g−1 |
162 |
| V t: 0.53 cm3 g−1 | 104 F g−1 at 20 A g−1 | ||||||
| NHPC1:1-3-800 | S BET: 1867.4 m2 g−1 | Hydrothermal crosslinking reaction and KOH activation | 6 M KOH | 440 F g−1 at 0.5 A g−1 | 18.5 W h kg−1 at 300 W kg−1 in 6 M KOH | 95.1% after 3000 cycles at 20 A g−1 in 6 M KOH | 163 |
| V t: 0.997 cm3 g−1 | 1 M H2SO4 | 331 F g−1 at 1 A g−1 | |||||
| N, S–HPC-1 | S BET: 1454.7 m2 g−1 | Carbonization and KOH activation | 6 M KOH | 269 F g−1 at 0.5 A g−1 | 37.4 W h kg−1 at 62 W kg−1 | 98.4% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
164 |
| V t: 0.894 cm3 g−1 | 168 F g−1 at 50 A g−1 | ||||||
| L–U | S BET: 3130 m2 g−1 | Carbonization and KOH activation | KOH–PVA | 306 F g−1 at 0.1 A g−1 | 17 W h kg−1 | 96.8% after 5000 cycles at 0.1 to 10 A g−1 | 172 |
| V t: 1.67 cm3 g−1 | 251 F g−1 at 10 A g−1 | ||||||
| PL-700 | S BET: 2265 m2 g−1 | KOH activation | 6 M KOH | 333 F g−1 at 20 mV s−1 | — | 100% after 1000 cycles at 1 A g−1 | 173 |
| GNs–N–S co-doped ACNFs-5 | S BET: 2439 m2 g−1 | Electrospinning, carbonization and activation process | 6 M KOH | 267.32 F g−1 at 5 mV s−1 | 9.28 W h kg−1 at 493 W kg−1 | 96.7% after 5000 cycles | 175 |
| V t: 1.2882 cm3 g−1 | 148.47 F g−1 at 50 mV s−1 | ||||||
| ONS–HPCs–L-700 | S BET: 1269 m2 g−1 | Direct pyrolysis | 6 M KOH | 300.5 F g−1 at 0.5 A g−1 | 66.8 W h kg−1 at 1750 W kg−1 in EMIM BF4 | 91.6% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 in 6 M KOH 000 cycles at 5 A g−1 in 6 M KOH |
180 |
| V t: 0.598 cm3 g−1 | 243 F g−1 at 60 A g−1 | ||||||
| CKL-1098 | S BET: 1092 m2 g−1 | Direct carbonization | 1 M H2SO4 | 114 F g−1 at 5 mV s−1 | 12.8 W h kg−1 at 1006 W kg−1 | 80% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 50 mV s−1 000 cycles at 50 mV s−1 |
201 |
| 76 F g−1 at 100 mV s−1 | |||||||
| Ca-800 | S BET: 1362 m2 g−1 | Carbonization | 6 M KOH | 182 F g−1 at 1 mA cm−2 | 25 W h kg−1 at 150 W kg−1 | 95% after 1000 cycles at 10 mA cm−2 | 202 |
| V t: 0.83 cm3 g−1 | 122 F g−1 at 20 mA cm−2 | ||||||
| CHMS-5-700–N | S BET: 991 m2 g−1 | Carbonization and post-nitric acid modification | 7 M KOH | 284 F g−1 at 0.1 A g−1 | 2.9 W h L−1 at 11.3 W L−1 | 93.4% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 A g−1 000 cycles at 2 A g−1 |
203 |
| V t: 0.75 cm3 g−1 | 124 F g−1 at 20 A g−1 | ||||||
| HPCS-700 | S BET: 1255 m2 g−1 | Thermostabilization and carbonization | 7 M KOH | 276 F g−1 at 0.1 A g−1 | 34.3 W h kg−1 at 9.4 kW kg−1 in 1 M SBPBF4/PC | 99.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 A g−1 000 cycles at 2 A g−1 |
204 |
| V t: 0.87 cm3 g−1 | 155 F g−1 at 20 A g−1 | ||||||
| HAPC-11-900 | S BET: 856 m2 g−1 | Template and chemical activation | 6 M KOH | 286 F g−1 at 0.25 A g−1 | 13 W h kg−1 at 27 kW kg−1 | 89.4% after 2000 cycles at 4 A g−1 | 205 |
| V t: 0.17 cm3 g−1 | 141 F g−1 at 10 A g−1 | ||||||
| PNG | S BET: 515 m2 g−1 | Co-pyrolysis | 6 M KOH | 170 F g−1 at 0.2 A g−1 | — | 92.5% after 1000 cycles at 100 mV s−1 | 206 |
| 138.5 F g−1 at 5 A g−1 | |||||||
| HPGC-160-8 | S BET: 856.84 m2 g−1 | Cross-linking and carbonization | 6 M KOH | 331 F g−1 at 5 mV s−1 | — | 78% after 1000 cycles at 20 mV−1 | 207 |
| V t: 0.425 cm3 g−1 | 202 F g−1 at 100 mV s−1 | ||||||
| LHPC | SBET: 907 m2 g−1 | Template-free and KOH activation | 1 M H2SO4 | 165 F g−1 at 0.05 A g−1 | 3.75 W h kg−1 at 1070 W kg−1 | 97.45% after 5000 cycles at 1 A g−1 | 208 |
| V t: 0.515 cm3 g−1 | 123.5 F g−1 at 10 A g−1 | ||||||
| NiO/HMPC NSs | S BET: 851.8 m2 g−1 | Self-assembly and carbonization | 6 M KOH | 508 F g−1 at 20 mV s−1 | — | 92% after 2000 cycles at 20 mV s−1 | 209 |
| V t: 0.16 cm3 g−1 | |||||||
| 1-HPC600-600-5 | S BET: 2753.9 m2 g−1 | Carbonization and KOH activation | 6 M KOH | 428 F g−1 at 0.04 A g−1 | 12 W h kg−1 at 9908 W kg−1 | 96% after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
210 |
| V t: 1.43 cm3 g−1 | 288 F g−1 at 10 A g−1 | ||||||
| CA-L20 | S BET: 779 m2 g−1 | Carbonization and KOH activation | 6 M KOH | 142.8 F g−1 at 0.5 A g−1 | — | 96% after 2000 cycles at 10 A g−1 | 211 |
| V t: 0.48 cm3 g−1 | 112.5 F g−1 at 10 A g−1 | ||||||
| PANI/LGS | — | — | 1 M H2SO4 | 502.1 F g−1 at 0.1 A g−1 | — | 74.3% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 000 cycles at 1 A g−1 |
212 |
| 377.2 F g−1 at 10 A g−1 | |||||||
| PANI/LG | — | In situ oxidation polymerization | 1 M HClO4 | 485.3 F g−1 at 0.5 A g−1 | — | 67.4% after 5000 cycles at 1 A g−1 | 213 |
| 284.4 F g−1 at 30 A g−1 | |||||||
| ECNFs (70/30) | S BET: 583 m2 g−1 | Electrospinning, stabilization and carbonization | 6 M KOH | 64 F g−1 at 0.4 A g−1 | 5.67 W h kg−1 at 94.19 W kg−1 | 90% after 6000 cycles at 2 A g−1 | 214 |
| V t: 0.289 cm3 g−1 | 50 F g−1 at 2 A g−1 | ||||||
| NiCo2O4@CNF55 | Fiber diameters of 1830 ± 155 nm | Electrospinning, stabilization and carbonization | 2 M KOH | 1757 F g−1 at 2 mA cm−2 | 47.75 W h kg−1 at 799.53 W kg−1 | 138% after 5000 cycles at 7 mA cm−2 | 215 |
| 1304 F g−1 at 50 mA cm−2 | |||||||
| LCNFs-2 | S BET: 1140 m2 g−1 | Electrospinning PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg(NO3)2·6H2O Mg(NO3)2·6H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lignin (2 lignin (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6 M KOH | 248 F g−1 at 0.2 A g−1 | — | 97% after 1000 cycles at 20 A g−1 | 216 |
| V t: 0.627 cm3 g−1 | 146 F g−1 at 20A g−1 | ||||||
| MnO2–CNFM4 | — | Electrospinning, carbonization | 1 M Na2SO4 | 171.6 F g−1 at 5 mV?s−1 | 6.0 W h kg−1 at 160 W kg−1 | 98.95% after 1000 cycles at 0.5 A g−1 | 217 |
| 88.4 F g−1 at 50 mV s−1 | |||||||
| MnO2–LCF-800 mat | S BET: 273.70 m2 g−1 | Electrospinning, stabilization and carbonization | 1 M Na2SO4 | 131.28 F g−1 at 0.3 A g−1 | 14.77 W h kg−1 at 135.01 W kg−1 | — | 218 |
| V t: 0.09 cm3 g−1 | |||||||
| LUDC_0.5 | S BET: 764 m2 g−1 | DES-templated | 6 M KOH | 177.5 F g−1 at 0.5 A g−1 | — | 96% after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
219 |
| V t: 0.47 cm3 g−1 | 136.8 F g−1 at 10 A g−1 | ||||||
| BALC-9 | S BET: 1831 m2 g−1 | Carbonization and bacterial activation | 6 M KOH | 428 F g−1 at 1 A g−1 | 66.18 W h kg−1 at 312 W kg−1 in EMIM TFSI | 96.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 in 6 M KOH 000 cycles at 5 A g−1 in 6 M KOH |
104 |
| V t: 1.53 cm3 g−1 | EMIM TFSI | 289 F g−1at 1 A g−1 | |||||
| ARS/PGLS-1 | S BET: 1727.7 m2 g−1 | Carbonization and graphitization | 6 M KOH | 469.5 F g−1 at 0.5 A g−1 | 9.45 W h kg−1 at 100.06 W kg−1 in PVA/KOH/ARS | 99.7% after 2000 cycles at 2 A g−1 in PVA/KOH/ARS | 220 |
| V t: 1.33 cm3 g−1 | 200.2 F g−1 at 10 A g−1 | ||||||
| LSG-P36–Au | S BET: 338.3 m2 g−1 | One-step CO2 laser irradiation | H2SO4/PVA gel | 11.9 mF cm−2 at 0.02 mA cm−2 | — | 98.47% after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 mA cm−2 000 cycles at 2 mA cm−2 |
221 |
| V t: 0.232 cm3 g−1 | |||||||
| sLIG-O/S14 | S BET: 181.37 m2 g−1 | A duplicated laser scribing process | PVA/H2SO4 gel | 53.2 mF cm−2 at 0.08 mA cm−2 | 0.45 mW h cm−3 at 1.6 mW cm−2 | 81.3% after 8000 cycles at 50 mV s−1 | 222 |
| V t: 0.351 cm3 g−1 | |||||||
| N–BLPC | S BET: 2646 m2 g−1 | KOH activation | 6 M KOH | 337 F g−1 at 0.5 A g−1 | 9.34 W h kg−1 at 250 W kg−1 | 98% after 3000 cycles at 10 A g−1 | 223 |
| V t: 1.285 cm3 g−1 | 254 F g−1 at 20 A g−1 | ||||||
| LPC-3 | S BET: 2866 m2 g−1 | Microwave heating | 6 M KOH | 216 F g−1 at 0.5 A g−1 | 55.5 W h kg−1 at 1.1 kW kg−1 | 99.9% after 2000 cycles at 5 A g−1 in gel-like PVA/LiCl electrolyte | 224 |
| V t: 2.02 cm3 g−1 | 188 F g−1 at 10 A g−1 | ||||||
| NSC-700 | S BET: 1199 m2 g−1 | Fe3O4 template and KOH activation | 6 M KOH | 241 F g−1 at 1 A g−1 | 27.2 W h kg−1 at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 000 W kg−1 |
95% after 3000 cycles at 10 A g−1 | 225 |
| 196 F g−1 at 20 A g−1 | |||||||
| E-CNFs | S BET: 2313 m2 g−1 | Esterification, electrospinning, and carbonization processes | 6 M KOH | 320 F g−1 at 1 A g−1 | 17.92 W h kg−1 at 800 W kg−1 in 1 M Na2SO4 | 94.5% after 5000 cycles at 1 A g−1 in 1 M Na2SO4 | 226 |
| 200.4 F g−1 at 20 A g−1 | |||||||
| LUPCF-2 | S BET: 1363 m2 g−1 | Electrospinning, pre-oxidation, carbonization, and pickling processes | 6 M KOH | 289 F g−1 at 0.1 A g−1 | — | 92% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
227 |
| V t: 0.689 cm3 g−1 | 162 F g−1 at 20 A g−1 | ||||||
| PLC-650-2 | S BET: 1069 m2 g−1 | Zinc oxalate-assisted gas-exfoliation and in situ templating | 6 M KOH | 365 F g−1 at 0.5 A g−1 | 9.75 W h kg−1 at 6157.9 W kg−1 in PVA/KOH gel electrolytes | 93.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 000 cycles at 5 A g−1 |
105 |
| V t: 1.375 cm3 g−1 | 260 F g−1 at 20 A g−1 | ||||||
| CNFs-6 | S BET: 1061.7 m2 g−1 | ECH modification and heat treatment | 6 M KOH | 320.3 F g−1 at 1 A g−1 | 30.2 W h kg−1 at 400 W kg−1 in 1 M Na2SO4 | — | 228 |
| V t: 0.57 cm3 g−1 | |||||||
| L–CNFs@Fe3O4 nanofibers | — | Electrospinning, stabilized and carbonized | 1 M Na2SO4 | 216 F g−1 at 0.1 A g−1 | 43 W h kg−1 at 242 W kg−1 | 96.7% after 1000 cycles at 1 A g−1 | 229 |
| C800-SS | S BET: 28.3 m2 g−1 | Electrospinning and carbonization | PVA-1 M H2SO4 | 451.1 F g−1 at 1 A g−1 | 62.6 W h kg−1 at 1250 W kg−1 | 99.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
230 |
| V t: 0.068 cm3 g−1 | |||||||
| HPCS-X-3 | S BET: 3406 m2 g−1 | Simple spray drying and carbonization-activation | 6 M KOH | 236.2 F g−1 at 0.2 A g−1 | — | 91.7% after 5000 cycles at 20 A g−1 | 231 |
| V t: 2.46 cm3 g−1 | 130.6 F g−1 at 20 A g−1 | ||||||
| LPC-700 | S BET: 529 m2 g−1 | Carbonization-induced self-template method | 6 M KOH | 170 F g−1 at 0.5 A g−1 | — | 81% after 5000 cycles at 1 A g−1 | 232 |
89 F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g−1at 10 A g−1 g−1at 10 A g−1 |
|||||||
| NCA-12 | S BET: 482 m2 g−1 | Co-electrospinning, freeze-casting and freeze-drying, carbonization | 6 M KOH | 282 F g−1 at 0.2 A g−1 | 32 W h kg−1 at 125 W kg−1 | 96% after 5000 cycles at 5 A g−1 | 233 |
| 60 F g−1 at 10 A g−1 | |||||||
| LPCs | S BET: 3178.36 m2 g−1 | Hydrothermal and KOH-assisted synthesis | 6 M KOH | 201.69 F g−1 at 0.5 A g−1 | — | 90.21% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
234 |
| 171.88 g−1 at 10 A g−1 | |||||||
| LCNS | S BET: 736 m2 g−1 | Self-assembly, stabilization treatment, and carbonization | 6 M KOH | 147 F g−1 0.5 A g−1 | 3.5 W h kg−1 at 62.6 W kg−1 | 85.37% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 000 cycles at 10 A g−1 |
235 |
| 86 F g−1 at 20 A g−1 | |||||||
| LSC–ZnC2O4/PANI | — | Carbonization and in situ polymerization | 1 M H2SO4 | 643 F g−1 at 1.0 A g−1 | 36.3 W h kg−1 at 850.2 W kg−1 in 1 M Na2SO4 | 88.0% after 5000 cycles at 5.0 A g−1 | 236 |
| 390 F g−1 at 30 A g−1 |
3.1 Lignin-based electrodes for EDLCs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 displayed the champion SSA of 3775 m2 g−1, and delivered a considerable specific capacitance of 286.7 F g−1 at 0.2 A g−1 in 6 M KOH.
1 displayed the champion SSA of 3775 m2 g−1, and delivered a considerable specific capacitance of 286.7 F g−1 at 0.2 A g−1 in 6 M KOH.
Of special note, the activator plays a paramount role in the ultimate SSA and pore structure of the lignin-based carbon materials. Wu et al. demonstrated that the electrochemical performance of lignin-based ACs depends on the various activators (ZnCl2, KOH, K2CO3).98 Interestingly, the ZnCl2-activated ACs exhibited the smallest SSA because ZnCl2 only acts as a catalyst for dehydroxylation and dehydration in the activation process, whereas both KOH and K2CO3 serve to dehydrate and oxidize lignin. Furthermore, K2CO3 decomposes into CO2 and introduces physical activation. As such, the K2CO3-activated ACs exhibited the maximum SSA of 1585 m2 g−1 and an excellent specific capacitance of 263.5 F g−1 at 40 mA g−1 in 6 M KOH.
A high SSA is of significance for raising the performance of lignin-based ACs, but a suitable pore structure is a prerequisite to the high utilization of the SSA.99 For example, most high SSA materials are usually microporous with a narrow pore size distribution of 0.5–1.5 nm, which severely restrains the diffusion of electrolyte ions, and undermines the rate capability.100–102 To tackle these issues, hierarchical porous carbons (HPCs) combining micro-, meso- and macro-pores have been utilized as EDLCs electrode materials as they are capable of offering effective diffusion paths. In HPCs, the macropores provide a high buffer capacity for electrolyte ions, the mesoporous channels are conducive to the rapid transport of ions, and the micropores can further enhance the ion-accessible surface area for the construction of an electric double layer, resulting in excellent electrochemical performance.101 Lignins enjoy a loose structure that is conducive to the formation of porous structures during pyrolysis. In this regard, tremendous efforts have been devoted to constructing HPCs via appropriate pyrolysis and activation strategies by adopting lignin as a carbon source. Guo and co-workers configured a 3D HPC from the enzymatic hydrolysis of lignin via hydrothermal carbonization followed by a KOH activation process (Fig. 3a).103 The unique 3D porous network (Fig. 3b) exhibited a high SSA of 1660 m2 g−1 and realized a superior specific capacitance of 420 F g−1 at 0.1 A g−1, together with considerable rate performance in 6 M KOH (Fig. 3c). Moreover, it can retain 99% of the pristine capacitance upon undergoing 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1, indicative of excellent structural stability. Strikingly, the assembled symmetric supercapacitor exhibited an outstanding energy density of 46.8 W h kg−1 in ionic liquid systems (Fig. 3d). Based on this work, our group systematically studied the effects of hydrothermal carbonization conditions on the structure and properties of HPCs according to the Taguchi method.70 The optimized sample enjoyed a favorable SSA of 1504 m2 g−1 and the corresponding supercapacitors delivered a desirable specific capacitance of 324 F g−1 at 0.5 A g−1 in 6 M KOH coupled with decent rate performance and excellent cycle stability. Zhang et al. developed a green bacterial activation strategy to prepare HPCs.104 It is worth noting that bacterial activation can break the β-O-4, β-β′, and β-5 linkages in lignin, reduce the molecular weight, and promote the carbonization and graphitization of lignin. As such, an enhanced specific capacitance of 428 F g−1 at 1 A g−1 in 6 M KOH was achieved. In addition, the symmetric supercapacitor can deliver a superior energy density of 66.18 W h kg−1 at a power density of 312 W kg−1 in the ionic liquid system. It also achieved a decent cycle performance, with a capacitance retention of up to 96.7% after 10
000 cycles at 5 A g−1, indicative of excellent structural stability. Strikingly, the assembled symmetric supercapacitor exhibited an outstanding energy density of 46.8 W h kg−1 in ionic liquid systems (Fig. 3d). Based on this work, our group systematically studied the effects of hydrothermal carbonization conditions on the structure and properties of HPCs according to the Taguchi method.70 The optimized sample enjoyed a favorable SSA of 1504 m2 g−1 and the corresponding supercapacitors delivered a desirable specific capacitance of 324 F g−1 at 0.5 A g−1 in 6 M KOH coupled with decent rate performance and excellent cycle stability. Zhang et al. developed a green bacterial activation strategy to prepare HPCs.104 It is worth noting that bacterial activation can break the β-O-4, β-β′, and β-5 linkages in lignin, reduce the molecular weight, and promote the carbonization and graphitization of lignin. As such, an enhanced specific capacitance of 428 F g−1 at 1 A g−1 in 6 M KOH was achieved. In addition, the symmetric supercapacitor can deliver a superior energy density of 66.18 W h kg−1 at a power density of 312 W kg−1 in the ionic liquid system. It also achieved a decent cycle performance, with a capacitance retention of up to 96.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1.
000 cycles at 5 A g−1.
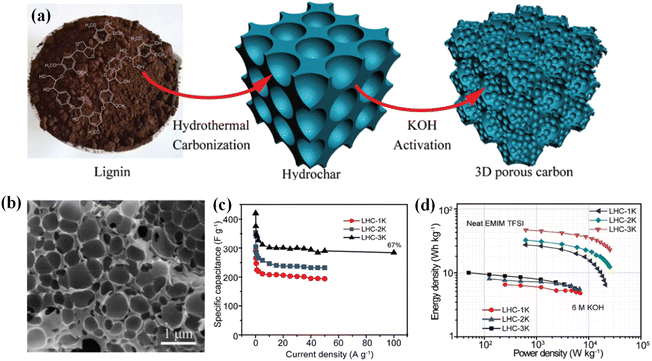 | ||
| Fig. 3 (a) Schematic of the synthesis of LHC samples. (b) SEM image of the LHC-3K. (c) Rate performance of the LHC-based electrodes. (d) Ragone profiles of LHC-based supercapacitors (LHC denoted as enzymatic hydrolysis lignin-derived 3D HPCs). Reproduced with permission from ref. 103. Copyright 2017 Royal Society of Chemistry. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and a volumetric capacitance of 75 F cm−3 at 20 A g−1.
000 cycles and a volumetric capacitance of 75 F cm−3 at 20 A g−1.
However, the synthesis and removal of the hard template are complicated, and the properties of PCs largely depend on the properties of templates. Recently, the soft template method has attracted much more attention because it is unnecessary to remove the soft templates after carbonization. The soft template and abundant oxygen-containing functional groups in lignin can self-assemble into precursors through hydrogen bond interactions, and the mesoporous materials can be synthesized by subsequently carbonizing the precursors. Saha et al.107 prepared lignin-based ordered mesoporous carbons (OMCs) by adopting Pluronic F127 as the template. The OMCs showed an SSA of 1148 m2 g−1, with a mesoporosity of 66%. The OMCs electrode activated by CO2 delivered a capacitance of 102.3 F g−1 and excellent rate performance in 6 M KOH. Moreover, Herou et al.108 adopted the dual precursor system comprising equal-weight phloroglucinol and lignin to prepare OMCs through a soft template strategy. The synthesized OMCs displayed superior electrochemical performance as compared to the material prepared by only utilizing phloroglucinol because the smaller mesopores in the former boosted the electrolyte diffusion rate.
Compared with the hard template method, the soft template method shows an underdeveloped capability of selectively regulating the pore structure of PCs and thus, the prepared PCs possess fewer micropores and smaller SSA. With the above information in mind, an efficient strategy is to couple the hard template with the soft template method to achieve carbon electrodes possessing appropriate pore structures. Li et al. configured large-scale hierarchical porous carbon monoliths (HPCMs), by effectively carbonizing the mixture of P123, lignin and mesoporous silica (hereafter denoted as SLC), as shown in Fig. 4a.109 The SLC displays a favorable microstructure and excellent conductivity. As expected, the assembled symmetric supercapacitor exhibited a superior areal capacity of 3.0 F cm−2 and a volume capacity of 97.1 F cm−3 at a current density of 1.4 mA cm−2 in 6 M KOH without utilizing binders and conductive additives (Fig. 4b). Moreover, a considerable areal energy density of 0.16 mW h cm−2 at 1.75 mW cm−2 was achieved (Fig. 4c). In another study, Song et al. constructed the mesostructured carbons by employing MgO and Pluronic F127 as templates. They revealed that both the space-occupying effect of the MgO template and the mass ratio of lignin/MgO are critical for regulating the porosity of carbons. Eventually, the obtained material achieved a high SSA of 712 m2 g−1 and the fabricated electrodes yielded a larger specific capacitance of 186.3 F g−1 at a current density of 0.1 A g−1 in 1 M H2SO4.111
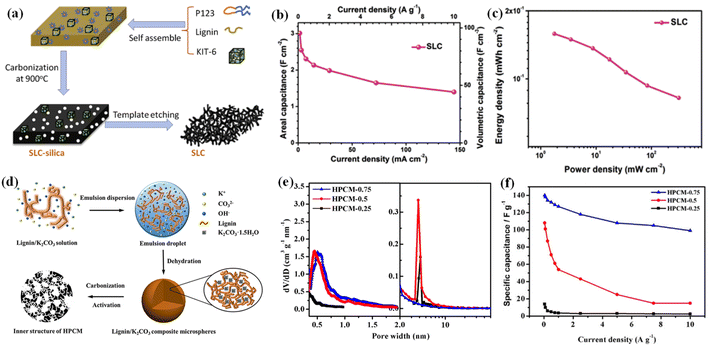 | ||
| Fig. 4 (a) Schematic illustration of the SLC process. (b) Areal/volumetric capacitances of SLC. (c) Areal Ragone plots (energy density vs. power density) for the full cell. Reprinted with permission from ref. 109. Copyright 2016 Elsevier. (d) Schematic of the formation of lignin/K2CO3 composite microspheres. (e) Micropore and mesopore size distribution of HPCMs. (f) The rate performance of various HPCMs at different scan rates. Reprinted with permission from ref. 110. Copyright 2018 Wiley Online Library. | ||
Recent studies have demonstrated that the templates not only construct pores but also play other roles in the preparation of lignin-based carbon materials.110 As illustrated in Fig. 4d, Wang and coworkers reported an inverse phase dehydration method to effectively synthesize the HPCMs.110 In this work, K2CO3 firstly acts as a pH regulator to promote the dissolution of lignin, and subsequently serves as the mesopore template and activator to promote the formation of abundant micropores of approximately 4 nm (Fig. 4e). The supercapacitor fabricated based on the optimal sample (HPCM-0.75) delivered a remarkable capacitance of 140 F g−1 at 0.05 A g−1 and excellent rate performance where the electrolyte used was 1 M tetraethylammonium tetrafluoroborate salt solution in propylene carbonate (Fig. 4f).
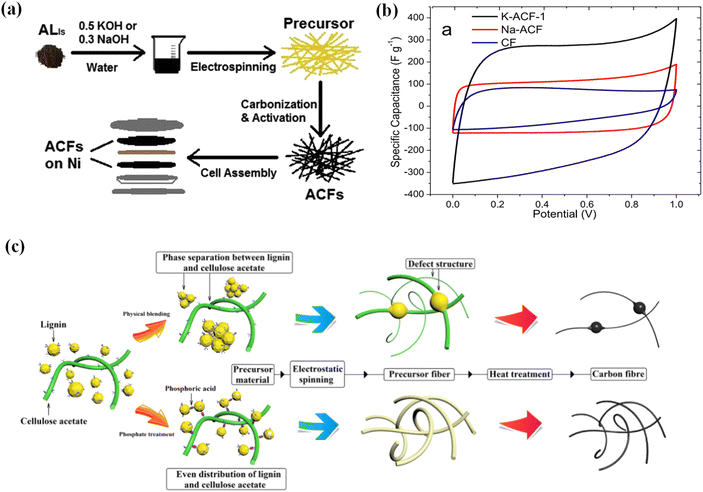 | ||
| Fig. 5 (a) Schematic diagrams of the preparation of lignin-based porous submicron ACFs. (b) CV profiles of various ACFs. Adapted with permission from ref. 125. Copyright 2014 Elsevier. (c) Proposed mechanism of phosphoric acid-functionalized ACFs. Adapted with permission from ref. 126 Copyright 2020 American Chemical Society. | ||
The low molecular weight, high heterogeneity and large dispersion index of lignin hinder the preparation of high-quality CFs from pure lignin.118,127 Zhou and coworkers utilized the reaction between the hydroxyl groups in lignin and the isocyanate groups in isophorone diisocyanate to enhance the molecular weight and reduce the heterogeneity of lignin, thus decreasing the carbon weight loss of precursor fibers and maintaining the morphology of lignin-based CFs.118 Correspondingly, the prepared CFs exhibited an outstanding SSA of 2042.86 m2 g−1. The assembled optimized supercapacitors displayed a high specific capacitance of 428.9 F g−1 at 1 A g−1 in 6 M KOH. Also, an excellent energy density of 37.1 W h kg−1 at a power density of 400 W kg−1 was realized in 1 M Na2SO4 electrolyte. In another study, Cao and co-workers modified lignin with cellulose-acetate to obtain precursor fibers by employing a facile phosphating process (Fig. 5c).126 Thereafter, lignin-based CFs were subjected to pre-oxidizing and carbonizing processes, thereby obtaining electrodes with high SSA and excellent electrochemical performance. Accordingly, the fabricated supercapacitor exhibited a capacitance of up to 346.6 F g−1 at 0.1 A g−1 in 6 M KOH. A superior energy density of 31.5 W h kg−1 at a power density of 400 W kg−1 was achieved in 1 M Na2SO4 electrolyte as well. Notably, the energy density of the supercapacitor was astonishingly maintained at 24.3 W kg−1 when the power density increased to 4000 W kg−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) in 1 M H2SO4. Li et al. manufactured a flexible metal-free supercapacitor by employing lignin-functionalized graphene hydrogel (Fig. 6c–f).135 The integrated flexible solid-state supercapacitor device utilizing H2SO4–PVA gel as the electrolyte achieved a high specific capacitance (408 F g−1 at 1 A g−1), outstanding cycle stability (84.4% after 10
000 cycles) in 1 M H2SO4. Li et al. manufactured a flexible metal-free supercapacitor by employing lignin-functionalized graphene hydrogel (Fig. 6c–f).135 The integrated flexible solid-state supercapacitor device utilizing H2SO4–PVA gel as the electrolyte achieved a high specific capacitance (408 F g−1 at 1 A g−1), outstanding cycle stability (84.4% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1), and a considerable energy density of 13.8 W h kg−1 at a power density of 500 W kg−1, thanks to the reversible redox charge transfer of quinone groups in lignin.
000 cycles at 10 A g−1), and a considerable energy density of 13.8 W h kg−1 at a power density of 500 W kg−1, thanks to the reversible redox charge transfer of quinone groups in lignin.
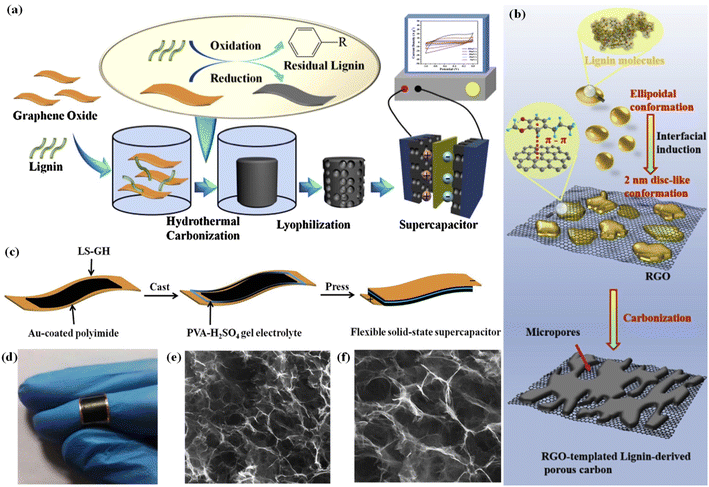 | ||
| Fig. 6 (a) Schematic of the synthesis of the lignin–RGO composite. Adapted with permission from ref. 133. Copyright 2017 Elsevier. (b) A schematic diagram of the molecular conformation conversion of lignin and the preparation of micropores in RGO-templated pseudo-capacitors. Adapted with permission from ref. 134. Copyright 2020 Elsevier. (c) Illustration of the preparation of LS–GH flexible solid-state supercapacitors. (d) Digital photograph of the flexible device; SEM images of the interior microstructure at (e) low and (f) high magnification. Adapted with permission from ref. 135. Copyright 2017 Royal Society of Chemistry. | ||
3.2 Lignin-based electrodes for pseudo-capacitors
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 W kg−1. Additionally, the solid-state planar micro-supercapacitor device based on the HPC/WO3 composite also exhibited a high areal specific capacitance of 20 mF cm−2 in the H2SO4–polyvinyl alcohol (PVA) gel electrolyte. Besides, our group further synthesized Ni4−xCoxWO4/HPC composite materials with various Co/Ni ratios via a facile co-precipitation method.143 The optimal Ni3Co1WO4/HPC electrode exhibited a specific capacitance of up to 1084 F g−1 at 0.5 A g−1 in 6 M KOH, along with an outstanding rate capability, due to the large SSA and strong synergistic effect between Co and Ni ions. The Faraday reactions are listed as follows:
300 W kg−1. Additionally, the solid-state planar micro-supercapacitor device based on the HPC/WO3 composite also exhibited a high areal specific capacitance of 20 mF cm−2 in the H2SO4–polyvinyl alcohol (PVA) gel electrolyte. Besides, our group further synthesized Ni4−xCoxWO4/HPC composite materials with various Co/Ni ratios via a facile co-precipitation method.143 The optimal Ni3Co1WO4/HPC electrode exhibited a specific capacitance of up to 1084 F g−1 at 0.5 A g−1 in 6 M KOH, along with an outstanding rate capability, due to the large SSA and strong synergistic effect between Co and Ni ions. The Faraday reactions are listed as follows:| Ni2+ + 3OH− ↔ NiOOH + H2O + e− | (1) |
| Co2+ + 3OH− ↔ CoOOH + H2O + e− | (2) |
| CoOOH + OH− ↔ CoO2 + H2O + e− | (3) |
The assembled asymmetric supercapacitor can provide a wide voltage window of 1.6 V and give a superior energy density of 105.6 W h kg−1 at 400.5 W kg−1 in 6 M KOH, in conjunction with the considerable stability of 80.74% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Moreover, Cao et al.144 prepared the multi-channel carbon nanofiber (MCNFs)@SnO2 nanocomposites via the co-electrospinning method, whereby poly(vinyl pyrrolidone)–SnCl2·2H2O and lignin–poly(methyl methacrylate) were used as the shell and core, respectively (Fig. 7a). Notably, the hollow structures function as fast electron transfer pathways to achieve intimate contact with electrolyte ions. Besides, SnCl2·2H2O not only serves as a pore-forming agent to enhance the SSA but also acts as a precursor for SnO2 to provide pseudocapacitance for nanocomposites. Accordingly, the optimal nanocomposite electrode displays an excellent specific capacitance of 406 F g−1 at 0.5 A g−1 in 6 M KOH. Moreover, the specific capacitance can retain 95% after 10
000 cycles. Moreover, Cao et al.144 prepared the multi-channel carbon nanofiber (MCNFs)@SnO2 nanocomposites via the co-electrospinning method, whereby poly(vinyl pyrrolidone)–SnCl2·2H2O and lignin–poly(methyl methacrylate) were used as the shell and core, respectively (Fig. 7a). Notably, the hollow structures function as fast electron transfer pathways to achieve intimate contact with electrolyte ions. Besides, SnCl2·2H2O not only serves as a pore-forming agent to enhance the SSA but also acts as a precursor for SnO2 to provide pseudocapacitance for nanocomposites. Accordingly, the optimal nanocomposite electrode displays an excellent specific capacitance of 406 F g−1 at 0.5 A g−1 in 6 M KOH. Moreover, the specific capacitance can retain 95% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, indicative of exceptionable cycle stability.
000 cycles, indicative of exceptionable cycle stability.
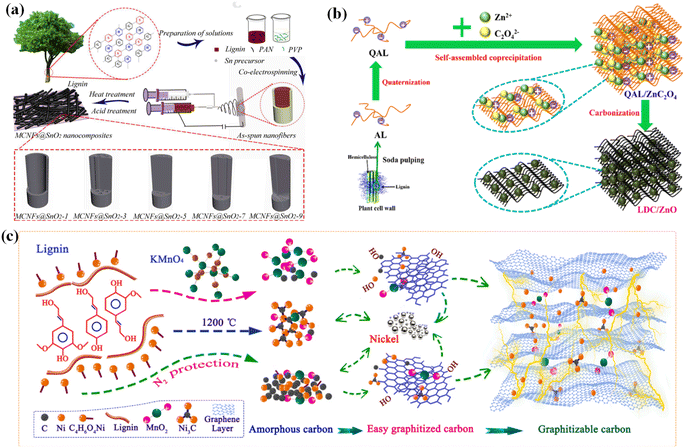 | ||
| Fig. 7 (a) Schematic procedure for the preparation of MCNFs@SnO2 nanocomposites. Adapted with permission from ref. 144. Copyright 2020 Elsevier. (b) Illustration of the fabrication mechanism of the LDC/ZnO materials. Reprinted with permission from ref. 145. Copyright 2019 American Chemical Society. (c) Proposed catalytic graphitization mechanism of Ni. Reprinted with permission from ref. 146. Copyright 2021 Elsevier. | ||
In the past two decades, due to the considerable specific energy density, good electrochemical activity, nontoxicity, and environmental friendliness, ZnO has become one of the promising metal oxides for supercapacitors.147–149 Fu et al. successfully constructed a lignin-derived carbon/ZnO (LDC/ZnO) composite with a 3D porous structure via electrostatic self-assembly. The composite was in situ carbonized by using ZnC2O4 particles as the catalyst and activator, leading to a large SSA and excellent conductivity (Fig. 7b).145 The fabricated symmetric supercapacitor device based on LDC/ZnO electrodes achieved a capacitance of up to 193 F g−1 at 0.5 A g−1 using the PVA/KOH gel as the electrolyte, with considerable cycle stability. Furthermore, Ji and co-workers incorporated Ni and MnO2 in lignin-based porous carbon materials (PC–Ni/MnO2),146 whereby C4H6O4Ni·4H2O and KMnO4 were utilized as a catalyst and a dopant, respectively. As illustrated in Fig. 7c, lignin is converted to highly graphitized carbon nanosheets and multilayer graphene due to the catalytic effect of Ni during the carbonization process, while KMnO4 decomposes into MnO2 loading on the carbon material. As a result, the PC–Ni/MnO2 can realize a superior gravimetric capacitance of 267.34 F g−1 at 0.1 A g−1 in 6 M KOH. Furthermore, the assembled symmetrical supercapacitor displays an energy density of 28 W h kg−1 and a power density of 360 W kg−1, with a favorable cycle stability after 5000 cycles in 6 M KOH.
| PPy0(Lig–QH2) → PPy+(ClO4−)(Lig–QH2) + e− | (4) |
| PPy+(ClO4−)(Lig–QH2) → PPy+(ClO4−)(Lig–Q) + 2e− + 2H+ | (5) |
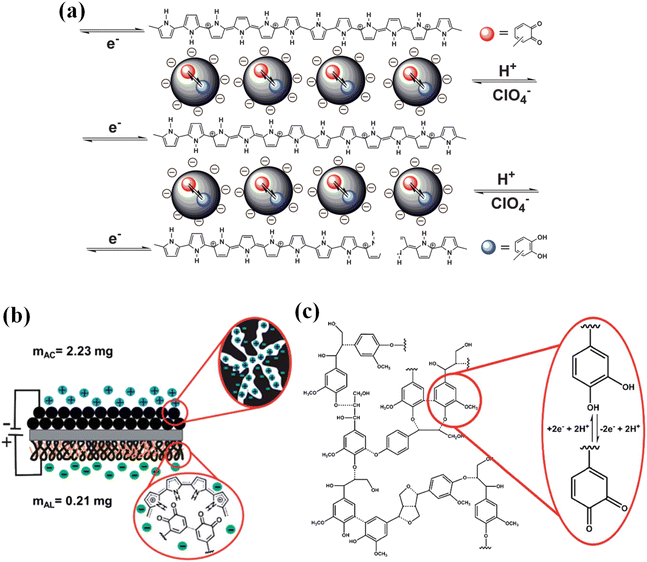 | ||
| Fig. 8 (a) The electrochemical redox reaction of quinone functions in an LS biopolymer within a PPy matrix. Reprinted with permission from ref. 86. Copyright 2012 Science. (b) Schematic diagram of the PPy/AL–AC cell. (c) Illustrative schematic of the lignin structure along with the redox processes occurring between QH2/Q. Adapted with permission from ref. 159. Copyright 2015 Royal Society of Chemistry. | ||
Rojo et al. fabricated the full-cell supercapacitor device composed of lignin/PEDOT as the positive electrode and partially reduced graphite oxide (prGrO) as the negative electrode,156 which delivered a higher capacitance of 34.6 F g−1 at 0.1 A g−1 in 0.1 M HClO4 electrolyte in comparison with the symmetric cells of lignin/PEDOT and prGrO. The enhanced electrochemical properties of the asymmetric device can be ascribed to the synergistic effect between the homogeneous distribution of the pseudocapacitive centers and enhanced electrical conductivity triggered by the intimate combination of both materials in the same electrode. Ajjan et al. synthesized bio-composites via both oxidative chemical and electrochemical polymerization of poly(3,4-ethylenedioxythiophene) (PEDOT) by adopting lignin sulfonate as the dopant and surfactant.157 Compared to reference PEDOT electrodes, the synthesized PEDOT/lignin composite electrodes exhibited an increased specific capacitance from 80.4 F g−1 to 170.4 F g−1 in a 0.1 M HClO4/water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (9
acetonitrile (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent, which is attributed to the additional pseudocapacitance of quinone moieties in lignin. In order to further improve the capacitance of the composite electrode, they fabricated a trihybrid electrode (PEDOT/lignin/poly(aminoanthraquinone) (PAAQ)) by introducing a third component of PAAQ to provide more pseudocapacitance.158 The trihybrid electrode manifested an excellent specific capacitance of 418 F g−1 at a current density of 1 A g−1 in 0.1 M HClO4. The asymmetric supercapacitor device was assembled, wherein the PEDOT/lignin/PAAQ and PEDOT/PAAQ were employed as the positive and negative electrodes, respectively. Benefitting from the synergistic effect of the two electrodes, it demonstrated excellent electrochemical performance, allowing for a specific capacitance of 74 F g−1 in 0.1 M HClO4. Additionally, a capacitance retention rate of 80% was identified after 10
1) mixed solvent, which is attributed to the additional pseudocapacitance of quinone moieties in lignin. In order to further improve the capacitance of the composite electrode, they fabricated a trihybrid electrode (PEDOT/lignin/poly(aminoanthraquinone) (PAAQ)) by introducing a third component of PAAQ to provide more pseudocapacitance.158 The trihybrid electrode manifested an excellent specific capacitance of 418 F g−1 at a current density of 1 A g−1 in 0.1 M HClO4. The asymmetric supercapacitor device was assembled, wherein the PEDOT/lignin/PAAQ and PEDOT/PAAQ were employed as the positive and negative electrodes, respectively. Benefitting from the synergistic effect of the two electrodes, it demonstrated excellent electrochemical performance, allowing for a specific capacitance of 74 F g−1 in 0.1 M HClO4. Additionally, a capacitance retention rate of 80% was identified after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles under a voltage window of 0.7 V. Alkali lignin (AL) accounts for 98% of lignin from paper-pulping; however, it encounters its own limitations due to its underdeveloped solubility in inorganic acids. To address this issue, Leguizamon et al. employed organic acid as the deposition solvent to fabricate PPy/AL electrodes (Fig. 8b).159 The PPy/AL electrode delivers an improved capacitance of 444 F g−1 in 0.5 M H2SO4, which is 30% higher than the electrodes containing identical compositions of sodium lignosulfate and 56% higher than pure PPy electrodes. Fig. 8c shows the redox processes occurring between hydroquinone/quinone (QH2/Q).
000 cycles under a voltage window of 0.7 V. Alkali lignin (AL) accounts for 98% of lignin from paper-pulping; however, it encounters its own limitations due to its underdeveloped solubility in inorganic acids. To address this issue, Leguizamon et al. employed organic acid as the deposition solvent to fabricate PPy/AL electrodes (Fig. 8b).159 The PPy/AL electrode delivers an improved capacitance of 444 F g−1 in 0.5 M H2SO4, which is 30% higher than the electrodes containing identical compositions of sodium lignosulfate and 56% higher than pure PPy electrodes. Fig. 8c shows the redox processes occurring between hydroquinone/quinone (QH2/Q).
Despite these achievements, the cycle stability of the composite electrodes based on lignin with various conductive polymers is still restrained due to the instability of the conductive polymer during the electrochemical charging/discharging process. A ternary composite electrode comprised of phosphomolybdic acid (HMA, H3PMo12O40·nH2O), lignin and PPy was fabricated by Admassie's group via one-step simultaneous electrochemical deposition.160 The incorporation of HMA enabled the specific capacitance of the PPy–lignin composite to increase from 477 to 682 F g−1 at 1 A g−1 in 0.1 M HClO4.
Co-doping with two or more kinds of heteroatoms is an efficient way to further boost the device performance through synergistic effects.175–179 Tian et al. synthesized the nitrogen and sulfur co-doped 3D multilevel hierarchical porous carbons (N, S–HPCs) by utilizing sodium lignosulfonate as the C and S sources, while the polyaniline-coated polystyrene spheres were spotlighted as the N precursor and template for macropores (Fig. 9a).164 Startlingly, this high N, S-doping content (up to 2.1 and 4.3 wt%) and lower internal series resistance boosted the specific capacitance of the N, S–HPC electrode material to 269 F g−1 (at 0.5 A g−1) from 16.9 F g−1 of solely carbonized lignin in 6 M KOH (Fig. 9b and c). As depicted in Fig. 9d, this N–S–HPC electrode also demonstrated outstanding rate performance (62% capacitance retention at 50 A g−1) and cycle stability (98.4% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) in 6 M KOH. Moreover, Liu et al. fabricated the O–N–S co-doped HPCs with a large SSA (338–1307 m2 g−1) by directly pyrolyzing kraft lignin.180 As expected, the fabricated symmetric supercapacitor in aqueous electrolyte delivered a high energy density of 66.8 W h kg−1 at a power density of 1.75 kW kg−1 while utilizing EMIMBF4 as the electrolyte. Our group successfully synthesized O, N and S co-doped HPC via a carbonization activation route using enzymatically hydrolyzed lignin as the carbon source.181 The obtained HPC has large SSA, abundant multiscale pores and high O, N, and S doping density. The faradaic redox reactions of N–O–S-containing functional groups in KOH electrolyte are shown in the following equations:182–186
000 cycles) in 6 M KOH. Moreover, Liu et al. fabricated the O–N–S co-doped HPCs with a large SSA (338–1307 m2 g−1) by directly pyrolyzing kraft lignin.180 As expected, the fabricated symmetric supercapacitor in aqueous electrolyte delivered a high energy density of 66.8 W h kg−1 at a power density of 1.75 kW kg−1 while utilizing EMIMBF4 as the electrolyte. Our group successfully synthesized O, N and S co-doped HPC via a carbonization activation route using enzymatically hydrolyzed lignin as the carbon source.181 The obtained HPC has large SSA, abundant multiscale pores and high O, N, and S doping density. The faradaic redox reactions of N–O–S-containing functional groups in KOH electrolyte are shown in the following equations:182–186
| –C–N–C− + H2O + e− ↔ –C–NH–C– + OH− | (6) |
| –C–S–C− + H2O + 3e− ↔ –C–SO2–C– + 3OH− | (7) |
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + H2O + 2e− ↔ –CH–O− + OH− O + H2O + 2e− ↔ –CH–O− + OH− | (8) |
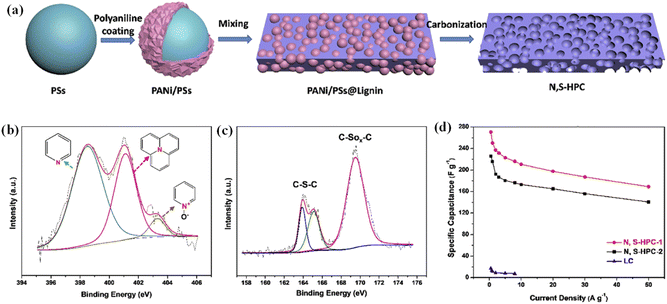 | ||
| Fig. 9 (a) Schematic preparation procedure for N, S–HPC. High-resolution N1s (b), S2p (c) XPS of N, S–HPC-1. (d) The specific capacitances of the samples at various current densities. Adapted with permission from ref. 164. Copyright 2019 Elsevier. | ||
Consequently, the HPC electrode delivered a superior specific capacitance of 318 F g−1 at 0.5 A g−1 and excellent rate performance (62% retention at 50 A g−1) in 6 M KOH. The assembled asymmetric supercapacitor yielded a high energy density of 16.7 W h kg−1 at a power density of 249 W kg−1 and outstanding cycle stability (99.58% retention over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) in 6 M KOH.
000 cycles) in 6 M KOH.
3.3 Applications of lignin-based supercapacitors
Owing to their excellent flexibility, fast charging and discharging capabilities, and durable service lifetime, flexible supercapacitors (FSCs) have successfully attracted broad research interest with an increasing demand for flexible and wearable electronics.9,187,188 Commonly used electrode materials for FSCs can be categorized into carbon materials (i.e., ACs, CNTs, and graphene), transition metal oxides/hydroxides, and conductive polymers.189–193 Among them, graphene and CNTs are suitable for preparing FSCs due to their high SSA, excellent conductivity and mechanical properties. Nevertheless, when they are utilized to prepare large-area electrodes, the SSA and capacitance performance will be compromised significantly due to undesirable agglomeration.Thanks to the unique structural characteristics, lignin can interact with graphene and CNTs through π–π effects to offset this irreversible agglomeration, having exhibited a huge potential for FSCs.194,195 Peng et al. fabricated FSCs by utilizing a lignosulfonate/single-walled CNT composite as the electrode and cellulose/Li2SO4 hydrogel as the electrolyte, which delivered an outstanding specific capacitance of 292 F g−1 at 0.5 A g−1 coupled with excellent rate capability, and a high energy density of 17.1 W h kg−1 at the power density of 324 W kg−1.196 The FSCs also exhibited excellent mechanical stability, retaining 98% of the initial capacitance after 1000 bending cycles. This excellent performance can be attributed to the synergistic effect of lignosulfonate-based carbon and CNT, as well as the superior 3D porous network structure. Moreover, Jha et al. assembled FSCs composed of Al/AC/lig–MnO2 as the anode, Al/AC as the cathode, and (PVA)/H3PO4 gel as the electrolyte.197 Due to their synergistic effect, the FSCs yielded a high specific capacitance of 5.52 mF cm−2, excellent mechanical stability (97.5% retention after 2000 cycles), and a favorable energy density of 14.11 W h kg−1 at the power density of 1 kW kg−1.
In the quest for renewable and highly efficient energy storage devices, Park et al. prepared all-lignin-based FSCs based on lignin hydrogel electrolytes and electrospun lignin/PAN nanofiber electrodes.18 The lignin hydrogel electrolytes display high ionic conductivity and excellent mechanical integrity, and the lignin-based carbon/PAN composite electrode possessing interconnected porous channels exhibits exceptional charge storage capability and kinetics. The FSCs attained a high capacitance of 129.23 F g−1 and beneficial capacitance retention of 95% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, as well as excellent flexibility and durability under diverse bending angles. Moreover, a maximum energy density of 4.49 W h kg−1 at the power density of 2.63 kW kg−1 was achieved. Accordingly, utilizing renewable lignin-based materials to assemble environmentally friendly and biocompatible FSCs provides a novel option for the development of sustainable energy storage systems.
000 cycles, as well as excellent flexibility and durability under diverse bending angles. Moreover, a maximum energy density of 4.49 W h kg−1 at the power density of 2.63 kW kg−1 was achieved. Accordingly, utilizing renewable lignin-based materials to assemble environmentally friendly and biocompatible FSCs provides a novel option for the development of sustainable energy storage systems.
4. Conclusion and prospectives
This review has comprehensively summarized the recent developments in the design and fabrication of lignin-based electrode materials for supercapacitors. It has been validated that lignin can be utilized as precursors to fabricate efficient PCs (e.g. ACs, templated carbon and CFs), which are prospective electrode materials for supercapacitors. Nevertheless, despite the progress to date, the study of lignin-based electrode materials is still in its infancy, and there are still some prospects that are worthy of attention in the future.(1) The physicochemical properties of lignin (e.g., molecular weight, solubility, and purity) depending on the natural sources and extraction methods will play a decisive role in the properties of the derivative carbon materials. Thus, further endeavors should be devoted to developing feasible methods to attain stable and homogeneous lignin, in favor of optimizing the properties of carbon materials for specific applications.
(2) The carbon materials converted from lignin generally possess controllable microstructures and diverse morphologies. However, the current converting strategies are complex and costly, which severely limits their large-scale applications. Of special note, the commonly utilized alkali activators are highly corrosive to the equipment and effortlessly lead to environmental pollution. Therefore, future research should focus on developing a green route for the large-scale production of lignin carbon materials.
(3) The structure–function relationship between the microstructure of lignin-based carbons and their charge storage performance/mechanisms remains ambiguous. Operando characterization techniques, such as ATR-FTIR, EQCM-D and in situ NMR, etc., are highly desirable to investigate the alteration of surface states of lignin-based PCs during the electrochemical process, which is beneficial to expound the energy storage mechanism and lay a foundation for designing and optimizing lignin-based energy storage materials.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research is financially supported by the National Natural Science Foundation of China (21908013, U20A20252, 22279140), the innovation fund project of Dalian Institute of Chemical Physics (DICP I202025), the Cooperation Foundation of Dalian National Laboratory for Clean Energy of the Chinese Academy of Sciences (DNL202015), Natural Science Foundation of Liaoning Province of China (2021-MS-016), Research Project of Education Department of Liaoning Province (LJKQZ2021115), Dalian Young Star of Science and Technology Project (2021RQ020, 2021RQ121).References
- N. Chang, T. Li, R. Li, S. Wang, Y. Yin, H. Zhang and X. Li, Energy Environ. Sci., 2020, 13, 3527–3535 RSC.
- T. Xu, H. Du, H. Liu, W. Liu, X. Zhang, C. Si, P. Liu and K. Zhang, Adv. Mater., 2021, 33, 2101368 CrossRef.
- S. Chen, L. Qiu and H.-M. Cheng, Chem. Rev., 2020, 120, 2811–2878 CrossRef CAS.
- J. Xiao, J. Han, C. Zhang, G. Ling, F. Kang and Q.-H. Yang, Adv. Energy Mater., 2022, 12, 2100775 CrossRef CAS.
- H. Li, Z. Tang, Z. Liu and C. Zhi, Joule, 2019, 3, 613–619 CrossRef.
- W. Guo, C. Yu, S. Li and J. Qiu, Energy Environ. Sci., 2021, 14, 576–601 RSC.
- M. Salanne, B. Rotenberg, K. Naoi, K. Kaneko, P. L. Taberna, C. P. Grey, B. Dunn and P. Simon, Nat. Energy, 2016, 1, 16070 CrossRef CAS.
- W. Zuo, R. Li, C. Zhou, Y. Li, J. Xia and J. Liu, Adv. Sci., 2017, 4, 1600539 CrossRef.
- Y. Wang, X. Wu, Y. Han and T. Li, J. Energy Storage, 2021, 42, 103053 CrossRef.
- J. Chmiola, C. Largeot, P.-L. Taberna, P. Simon and Y. Gogotsi, Science, 2010, 328, 480–483 CrossRef CAS PubMed.
- X. Jin, L. Song, H. Yang, C. Dai, Y. Xiao, X. Zhang, Y. Han, C. Bai, B. Lu, Q. Liu, Y. Zhao, J. Zhang, Z. Zhang and L. Qu, Energy Environ. Sci., 2021, 14, 3075–3085 RSC.
- S. Kumar, G. Saeed, L. Zhu, K. N. Hui, N. H. Kim and J. H. Lee, Chem. Eng. J., 2021, 403, 126352 CrossRef CAS.
- W. Raza, F. Ali, N. Raza, Y. Luo, K.-H. Kim, J. Yang, S. Kumar, A. Mehmood and E. E. Kwon, Nano Energy, 2018, 52, 441–473 CrossRef CAS.
- L. Fan, K. Lin, J. Wang, R. Ma and B. Lu, Adv. Mater., 2018, 30, 1800804 CrossRef.
- P. Divya and R. Rajalakshmi, J. Energy Storage, 2020, 27, 101149 CrossRef.
- H. H. Rana, J. H. Park, G. S. Gund and H. S. Park, Energy Storage Mater., 2020, 25, 70–75 CrossRef.
- D. Zhao, C. Chen, Q. Zhang, W. Chen, S. Liu, Q. Wang, Y. Liu, J. Li and H. Yu, Adv. Energy Mater., 2017, 7, 1700739 CrossRef.
- J. H. Park, H. H. Rana, J. Y. Lee and H. S. Park, J. Mater. Chem. A, 2019, 7, 16962–16968 RSC.
- C. G. Yoo, X. Meng, Y. Pu and A. J. Ragauskas, Bioresour. Technol., 2020, 301, 122784 CrossRef CAS.
- J. Ralph, C. Lapierre and W. Boerjan, Curr. Opin. Biotechnol., 2019, 56, 240–249 CrossRef PubMed.
- C. Chio, M. Sain and W. Qin, Renewable Sustainable Energy Rev., 2019, 107, 232–249 CrossRef.
- D. S. Bajwa, G. Pourhashem, A. H. Ullah and S. G. Bajwa, Ind. Crops Prod., 2019, 139, 111526 CrossRef.
- K. Liu, H. Du, T. Zheng, W. Liu, M. Zhang, H. Liu, X. Zhang and C. Si, Green Chem., 2021, 23, 9723–9746 RSC.
- H. Wang, F. Fu, M. Huang, Y. Feng, D. Han, Y. Xi, W. Xiong, D. Yang and L. Niu, Nano Mater. Sci., 2022 DOI:10.1016/j.nanoms.2022.01.002.
- L. T. Nguyen, D.-P. Phan, A. Sarwar, M. H. Tran, O. K. Lee and E. Y. Lee, Ind. Crops Prod., 2021, 161, 113219 CrossRef.
- R. Radhakrishnan, P. Patra, M. Das and A. Ghosh, Renewable Sustainable Energy Rev., 2021, 149, 111368 CrossRef.
- J. Ni, Y.-T. Wu, F. Tao, Y. Peng and P. Xu, J. Am. Chem. Soc., 2018, 140, 16001–16005 CrossRef CAS.
- M. Chen, F. Malaret, A. E. J. Firth, P. Verdía, A. R. Abouelela, Y. Chen and J. P. Hallett, Green Chem., 2020, 22, 5161–5178 RSC.
- S. Hu and Y.-L. Hsieh, Int. J. Biol. Macromol., 2016, 82, 856–862 CrossRef CAS.
- B. M. Cerrutti, C. S. de Souza, A. Castellan, R. Ruggiero and E. Frollini, Ind. Crops Prod., 2012, 36, 108–115 CrossRef CAS.
- C. Cai, Y. Bao, X. Zhan, X. Lin, H. Lou, Y. Pang, Y. Qian and X. Qiu, Green Chem., 2019, 21, 1141–1151 RSC.
- J.-H. Choi, S.-K. Jang, J.-H. Kim, S.-Y. Park, J.-C. Kim, H. Jeong, H.-Y. Kim and I.-G. Choi, Renewable Energy, 2019, 130, 952–960 CrossRef CAS.
- M. Parit and Z. Jiang, Int. J. Biol. Macromol., 2020, 165, 3180–3197 CrossRef CAS.
- B. M. Upton and A. M. Kasko, Chem. Rev., 2016, 116, 2275–2306 CrossRef CAS.
- W. Pei, W. Shang, C. Liang, X. Jiang, C. Huang and Q. Yong, Ind. Crops Prod., 2020, 154, 112638 CrossRef CAS.
- D. Kai, M. J. Tan, P. L. Chee, Y. K. Chua, Y. L. Yap and X. J. Loh, Green Chem., 2016, 18, 1175–1200 RSC.
- M. Luo, H. Lin, B. Li, Y. Dong, Y. He and L. Wang, Bioresour. Technol., 2018, 259, 312–318 CrossRef CAS.
- I. P. Pérez, A. M. Rodríguez Pasandín, J. C. Pais and P. A. Alves Pereira, J. Cleaner Prod., 2019, 220, 87–98 CrossRef.
- C. Xiong, M. Li, S. Nie, W. Dang, W. Zhao, L. Dai and Y. Ni, J. Power Sources, 2020, 471, 228448 CrossRef CAS.
- W. Zhang, J. Yin, C. Wang, L. Zhao, W. Jian, K. Lu, H. Lin, X. Qiu and H. N. Alshareef, Small Methods, 2021, 5, 2100896 CrossRef CAS PubMed.
- J. Zhu, C. Yan, X. Zhang, C. Yang, M. Jiang and X. Zhang, Prog. Energy Combust. Sci., 2020, 76, 100788 CrossRef.
- S. Guo, H. Li, X. Zhang, H. Nawaz, S. Chen, X. Zhang and F. Xu, Carbon, 2021, 174, 500–508 CrossRef CAS.
- W. Schutyser, T. Renders, S. Van den Bosch, S. F. Koelewijn, G. T. Beckham and B. F. Sels, Chem. Soc. Rev., 2018, 47, 852–908 RSC.
- R. Liu, L. Dai, C. Xu, K. Wang, C. Zheng and C. Si, ChemSusChem, 2020, 13, 4266–4283 CrossRef CAS PubMed.
- W. Lin, S. Xing, Y. Jin, X. Lu, C. Huang and Q. Yong, Bioresour. Technol., 2020, 306, 123163 CrossRef.
- L. Zheng, P. Yu, Y. Zhang, P. Wang, W. Yan, B. Guo, C. Huang and Q. Jiang, Int. J. Biol. Macromol., 2021, 176, 13–25 CrossRef PubMed.
- L. Dai, M. Ma, J. Xu, C. Si, X. Wang, Z. Liu and Y. Ni, Chem. Mater., 2020, 32, 4324–4330 CrossRef.
- A. Naseem, S. Tabasum, K. M. Zia, M. Zuber, M. Ali and A. Noreen, Int. J. Biol. Macromol., 2016, 93, 296–313 CrossRef PubMed.
- S. Gharehkhani, Y. Zhang and P. Fatehi, Prog. Energy Combust. Sci., 2019, 72, 59–89 CrossRef.
- H. Wang, Y. Pu, A. Ragauskas and B. Yang, Bioresour. Technol., 2019, 271, 449–461 CrossRef PubMed.
- H. Liu, T. Xu, K. Liu, M. Zhang, W. Liu, H. Li, H. Du and C. Si, Ind. Crops Prod., 2021, 165, 113425 CrossRef CAS.
- A. K. Mondal, D. Xu, S. Wu, Q. Zou, W. Lin, F. Huang and Y. Ni, Int. J. Biol. Macromol., 2022, 207, 48–61 CrossRef CAS PubMed.
- W.-J. Liu, H. Jiang and H.-Q. Yu, Green Chem., 2015, 17, 4888–4907 RSC.
- W. Fang, S. Yang, X.-L. Wang, T.-Q. Yuan and R.-C. Sun, Green Chem., 2017, 19, 1794–1827 RSC.
- M. Norgren and H. Edlund, Curr. Opin. Colloid Interface Sci., 2014, 19, 409–416 CrossRef CAS.
- D. Gan, W. Xing, L. Jiang, J. Fang, C. Zhao, F. Ren, L. Fang, K. Wang and X. Lu, Nat. Commun., 2019, 10, 1487 CrossRef PubMed.
- P. Figueiredo, K. Lintinen, J. T. Hirvonen, M. A. Kostiainen and H. A. Santos, Prog. Mater. Sci., 2018, 93, 233–269 CrossRef CAS.
- V. K. Ponnusamy, D. D. Nguyen, J. Dharmaraja, S. Shobana, J. R. Banu, R. G. Saratale, S. W. Chang and G. Kumar, Bioresour. Technol., 2019, 271, 462–472 CrossRef CAS PubMed.
- J. Xu, C. Li, L. Dai, C. Xu, Y. Zhong, F. Yu and C. Si, ChemSusChem, 2020, 13, 4284–4295 CrossRef CAS.
- N. Kamimura, S. Sakamoto, N. Mitsuda, E. Masai and S. Kajita, Curr. Opin. Biotechnol., 2019, 56, 179–186 CrossRef CAS PubMed.
- J. L. Espinoza-Acosta, P. I. Torres-Chávez, J. L. Olmedo-Martínez, A. Vega-Rios, S. Flores-Gallardo and E. A. Zaragoza-Contreras, J. Energy Chem., 2018, 27, 1422–1438 CrossRef.
- J. Mei, X. Shen, L. Gang, H. Xu, F. Wu and L. Sheng, Bioresour. Technol., 2020, 310, 123445 CrossRef CAS.
- C. Crestini, H. Lange, M. Sette and D. S. Argyropoulos, Green Chem., 2017, 19, 4104–4121 RSC.
- G. Gellerstedt, Ind. Crops Prod., 2015, 77, 845–854 CrossRef CAS.
- S. Sen, S. Patil and D. S. Argyropoulos, Green Chem., 2015, 17, 4862–4887 RSC.
- R. Deshpande, L. Sundvall, H. Grundberg, G. Henriksson and M. Lawoko, Ind. Crops Prod., 2022, 177, 114391 CrossRef CAS.
- N.-E. E. Mansouri and J. Salvadó, Ind. Crops Prod., 2006, 24, 8–16 CrossRef.
- M. N. Collins, M. Nechifor, F. Tanasă, M. Zănoagă, A. McLoughlin, M. A. Stróżyk, M. Culebras and C.-A. Teacă, Int. J. Biol. Macromol., 2019, 131, 828–849 CrossRef PubMed.
- A. G. Vishtal and A. Kraslawski, Bioresources, 2011, 6, 22 Search PubMed.
- H. Li, F. Shi, Q. An, S. Zhai, K. Wang and Y. Tong, Int. J. Biol. Macromol., 2021, 166, 923–933 CrossRef PubMed.
- F. Shi, J. Li, J. Xiao, X. Zhao, H. Li, Q. An, S. Zhai, K. Wang, L. Wei and Y. Tong, Int. J. Biol. Macromol., 2021, 190, 11–18 CrossRef.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- S. S. Mofarah, E. Adabifiroozjaei, Y. Yao, P. Koshy, S. Lim, R. Webster, X. Liu, R. Khayyam Nekouei, C. Cazorla, Z. Liu, Y. Wang, N. Lambropoulos and C. C. Sorrell, Nat. Commun., 2019, 10, 2594 CrossRef.
- A. Muzaffar, M. B. Ahamed, K. Deshmukh and J. Thirumalai, Renewable Sustainable Energy Rev., 2019, 101, 123–145 CrossRef CAS.
- Y. Wang, Y. Song and Y. Xia, Chem. Soc. Rev., 2016, 45, 5925–5950 RSC.
- E. Lim, C. Jo and J. Lee, Nanoscale, 2016, 8, 7827–7833 RSC.
- D. Sheberla, J. C. Bachman, J. S. Elias, C.-J. Sun, Y. Shao-Horn and M. Dincă, Nat. Mater., 2017, 16, 220–224 CrossRef CAS PubMed.
- G. Zhao, C. Chen, D. Yu, L. Sun, C. Yang, H. Zhang, Y. Sun, F. Besenbacher and M. Yu, Nano Energy, 2018, 47, 547–555 CrossRef CAS.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250–1280 RSC.
- R. Rao, C. L. Pint, A. E. Islam, R. S. Weatherup, S. Hofmann, E. R. Meshot, F. Wu, C. Zhou, N. Dee, P. B. Amama, J. Carpena-Nuñez, W. Shi, D. L. Plata, E. S. Penev, B. I. Yakobson, P. B. Balbuena, C. Bichara, D. N. Futaba, S. Noda, H. Shin, K. S. Kim, B. Simard, F. Mirri, M. Pasquali, F. Fornasiero, E. I. Kauppinen, M. Arnold, B. A. Cola, P. Nikolaev, S. Arepalli, H.-M. Cheng, D. N. Zakharov, E. A. Stach, J. Zhang, F. Wei, M. Terrones, D. B. Geohegan, B. Maruyama, S. Maruyama, Y. Li, W. W. Adams and A. J. Hart, ACS Nano, 2018, 12, 11756–11784 CrossRef CAS PubMed.
- J.-H. Lee and S.-J. Park, Carbon, 2020, 163, 1–18 CrossRef CAS.
- H. Li, J. Liang, H. Li, X. Zheng, Y. Tao, Z.-H. Huang and Q.-H. Yang, J. Energy Chem., 2019, 31, 95–100 CrossRef.
- M. F. El-Kady, Y. Shao and R. B. Kaner, Nat. Rev. Mater., 2016, 1, 16033 CrossRef CAS.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed.
- M. Boota and Y. Gogotsi, Adv. Energy Mater., 2019, 9, 1802917 CrossRef.
- G. Milczarek and O. Inganäs, Science, 2012, 335, 1468–1471 CrossRef CAS.
- A. Ehsani, M. K. Moftakhar and F. karimi, J. Energy Storage, 2021, 35, 102291 CrossRef.
- Z. Liao, Y.-H. Zhu, G.-T. Sun, L. Qiu and M.-Q. Zhu, Ind. Crops Prod., 2022, 175, 114266 CrossRef CAS.
- M. L. Aparna, G. R. Rao and T. Thomas, J. Energy Storage, 2022, 48, 104048 CrossRef.
- B. Li, F. Dai, Q. Xiao, L. Yang, J. Shen, C. Zhang and M. Cai, Energy Environ. Sci., 2016, 9, 102–106 RSC.
- M. Klose, R. Reinhold, F. Logsch, F. Wolke, J. Linnemann, U. Stoeck, S. Oswald, M. Uhlemann, J. Balach, J. Markowski, P. Ay and L. Giebeler, ACS Sustainable Chem. Eng., 2017, 5, 4094–4102 CrossRef.
- Y. Wu, J.-P. Cao, X.-Y. Zhao, Q.-Q. Zhuang, Z. Zhou, Y. Huang and X.-Y. Wei, Appl. Surf. Sci., 2020, 508, 144536 CrossRef.
- Y. Xi, D. Yang, X. Qiu, H. Wang, J. Huang and Q. Li, Ind. Crops Prod., 2018, 124, 747–754 CrossRef.
- S. Li, K. Han, J. Li, M. Li and C. Lu, Microporous Mesoporous Mater., 2017, 243, 291–300 CrossRef.
- B. Szczęśniak, J. Phuriragpitikhon, J. Choma and M. Jaroniec, J. Mater. Chem. A, 2020, 8, 18464–18491 RSC.
- W. Jian, W. Zhang, B. Wu, X. Wei, W. Liang, X. Zhang, F. Wen, L. Zhao, J. Yin, K. Lu and X. Qiu, ACS Appl. Mater. Interfaces, 2022, 14, 5425–5438 CrossRef.
- W. Zhang, M. Zhao, R. Liu, X. Wang and H. Lin, Colloids Surf., A, 2015, 484, 518–527 CrossRef CAS.
- Y. Wu, J.-P. Cao, Z.-Q. Hao, X.-Y. Zhao, Q.-Q. Zhuang, J.-S. Zhu, X.-Y. Wang and X.-Y. Wei, Int. J. Electrochem. Sci., 2017, 12, 7227–7239 CrossRef CAS.
- C. Chen, D. Yu, G. Zhao, B. Du, W. Tang, L. Sun, Y. Sun, F. Besenbacher and M. Yu, Nano Energy, 2016, 27, 377–389 CrossRef CAS.
- B. Chang, Y. Guo, Y. Li, H. Yin, S. Zhang, B. Yang and X. Dong, J. Mater. Chem. A, 2015, 3, 9565–9577 RSC.
- L. Qie, W. Chen, H. Xu, X. Xiong, Y. Jiang, F. Zou, X. Hu, Y. Xin, Z. Zhang and Y. Huang, Energy Environ. Sci., 2013, 6, 2497–2504 RSC.
- A. B. Fuertes and M. Sevilla, ACS Appl. Mater. Interfaces, 2015, 7, 4344–4353 CrossRef CAS.
- N. Guo, M. Li, X. Sun, F. Wang and R. Yang, Green Chem., 2017, 19, 2595–2602 RSC.
- K. Zhang, M. Liu, T. Zhang, X. Min, Z. Wang, L. Chai and Y. Shi, J. Mater. Chem. A, 2019, 7, 26838–26848 RSC.
- F. Fu, D. Yang, W. Zhang, H. Wang and X. Qiu, Chem. Eng. J., 2020, 392, 123721 CrossRef CAS.
- R. Ruiz-Rosas, M. J. Valero-Romero, D. Salinas-Torres, J. Rodríguez-Mirasol, T. Cordero, E. Morallón and D. Cazorla-Amorós, ChemSusChem, 2014, 7, 1458–1467 CrossRef CAS PubMed.
- D. Saha, Y. Li, Z. Bi, J. Chen, J. K. Keum, D. K. Hensley, H. A. Grappe, H. M. Meyer, S. Dai, M. P. Paranthaman and A. K. Naskar, Langmuir, 2014, 30, 900–910 CrossRef CAS.
- S. Herou, M. C. Ribadeneyra, R. Madhu, V. Araullo-Peters, A. Jensen, P. Schlee and M. Titirici, Green Chem., 2019, 21, 550–559 RSC.
- H. Li, D. Yuan, C. Tang, S. Wang, J. Sun, Z. Li, T. Tang, F. Wang, H. Gong and C. He, Carbon, 2016, 100, 151–157 CrossRef CAS.
- Y. Zhang, B. Yu, J. Zhang, X. Ding, J. Zeng, M. Chen and C. Wang, ChemElectroChem, 2018, 5, 2142–2149 CrossRef CAS.
- Y. Song, J. Liu, K. Sun and W. Xu, RSC Adv., 2017, 7, 48324–48332 RSC.
- B. Du, H. Zhu, L. Chai, J. Cheng, X. Wang, X. Chen, J. Zhou and R.-C. Sun, Ind. Crops Prod., 2021, 170, 113745 CrossRef.
- M. B. Poudel and H. J. Kim, Chem. Eng. J., 2022, 429, 132345 CrossRef.
- H. Wang, H. Niu, H. Wang, W. Wang, X. Jin, H. Wang, H. Zhou and T. Lin, J. Power Sources, 2021, 482, 228986 CrossRef.
- P. Schlee, O. Hosseinaei, C. A. O' Keefe, M. J. Mostazo-López, D. Cazorla-Amorós, S. Herou, P. Tomani, C. P. Grey and M.-M. Titirici, J. Mater. Chem. A, 2020, 8, 23543–23554 RSC.
- S. Hérou, J. J. Bailey, M. Kok, P. Schlee, R. Jervis, D. J. L. Brett, P. R. Shearing, M. C. Ribadeneyra and M. Titirici, Adv. Sci., 2021, 8, 2100016 CrossRef PubMed.
- W. Qu, P. Hu, J. Liu, H. Jin and K. Wang, J. Cleaner Prod., 2022, 343, 131030 CrossRef CAS.
- M. Zhu, H. Liu, Q. Cao, H. Zheng, D. Xu, H. Guo, S. Wang, Y. Li and J. Zhou, ACS Sustainable Chem. Eng., 2020, 8, 12831–12841 CrossRef CAS.
- T. Mukhiya, B. Dahal, G. P. Ojha, D. Kang, T. Kim, S.-H. Chae, A. Muthurasu and H. Y. Kim, Chem. Eng. J., 2019, 361, 1225–1234 CrossRef CAS.
- E. Svinterikos, I. Zuburtikudis and M. Al-Marzouqi, ACS Sustainable Chem. Eng., 2020, 8, 13868–13893 CrossRef CAS.
- U. Kurtan, H. Aydın, B. Büyük, U. Şahintürk, M. A. Almessiere and A. Baykal, J. Energy Storage, 2020, 32, 101671 CrossRef.
- P. Schlee, S. Herou, R. Jervis, P. R. Shearing, D. J. L. Brett, D. Baker, O. Hosseinaei, P. Tomani, M. M. Murshed, Y. Li, M. J. Mostazo-Lopez, D. Cazorla-Amoros, A. B. Jorge Sobrido and M. M. Titirici, Chem. Sci., 2019, 10, 2980–2988 RSC.
- M. Zhou, A. Bahi, Y. Zhao, L. Lin, F. Ko, P. Servati, S. Soltanian, P. Wang, Y. Yu, Q. Wang and Z. Cai, Chem. Eng. J., 2021, 409, 128214 CrossRef CAS.
- R. A. Perera Jayawickramage, K. J. Balkus and J. P. Ferraris, Nanotechnology, 2019, 30, 355402 CrossRef PubMed.
- S. Hu, S. Zhang, N. Pan and Y.-L. Hsieh, J. Power Sources, 2014, 270, 106–112 CrossRef CAS.
- Q. Cao, M. Zhu, J. Chen, Y. Song, Y. Li and J. Zhou, ACS Appl. Mater. Interfaces, 2020, 12, 1210–1221 CrossRef CAS.
- Z. Dai, Q. Cao, H. Liu, X. Shi, X. Wang, H. Li, Y. Han, Y. Li and J. Zhou, ACS Sustainable Chem. Eng., 2019, 7, 16084–16093 CrossRef CAS.
- Z. Sun, S. Fang and Y. H. Hu, Chem. Rev., 2020, 120, 10336–10453 CrossRef CAS.
- Z. Li, S. Gadipelli, H. Li, C. A. Howard, D. J. L. Brett, P. R. Shearing, Z. Guo, I. P. Parkin and F. Li, Nat. Energy, 2020, 5, 160–168 CrossRef CAS.
- F. Torres-Canas, A. Bentaleb, M. Föllmer, J. Roman, W. Neri, I. Ly, A. Derré and P. Poulin, Carbon, 2020, 163, 120–127 CrossRef CAS.
- X. Sun, X. Liu and F. Li, Appl. Surf. Sci., 2021, 551, 149438 CrossRef CAS.
- C. Xiong, W. Zhong, Y. Zou, J. Luo and W. Yang, Electrochim. Acta, 2016, 211, 941–949 CrossRef CAS.
- W. Ye, X. Li, J. Luo, X. Wang and R. Sun, Ind. Crops Prod., 2017, 109, 410–419 CrossRef CAS.
- C. Jiang, Z. Wang, J. Li, Z. Sun, Y. Zhang, L. Li, K.-S. Moon and C. Wong, Electrochim. Acta, 2020, 353, 136482 CrossRef CAS.
- F. Li, X. Wang and R. Sun, J. Mater. Chem. A, 2017, 5, 20643–20650 RSC.
- M. Zhi, C. Xiang, J. Li, M. Li and N. Wu, Nanoscale, 2013, 5, 72–88 RSC.
- J. Li, D. Xiao, Y. Ren, H. Liu, Z. Chen and J. Xiao, Electrochim. Acta, 2019, 300, 193–201 CrossRef.
- W.-J. Youe, S. J. Kim, S.-M. Lee, S.-J. Chun, J. Kang and Y. S. Kim, Int. J. Biol. Macromol., 2018, 112, 943–950 CrossRef PubMed.
- S. Jha, S. Mehta, Y. Chen, P. Renner, S. S. Sankar, D. Parkinson, S. Kundu and H. Liang, J. Mater. Chem. C, 2020, 8, 3418–3430 RSC.
- X. Yang, L. Mao, W. Peng, J. Jin, S. Yang and G. Li, ChemistrySelect, 2020, 5, 2602–2609 CrossRef.
- A. K. Mondal, D. Xu, S. Wu, Q. Zou, F. Huang and Y. Ni, Biomacromolecules, 2022, 23, 766–778 CrossRef CAS PubMed.
- X. Ma, P. Kolla, Y. Zhao, A. L. Smirnova and H. Fong, J. Power Sources, 2016, 325, 541–548 CrossRef CAS.
- F. Shi, S. Zhao, J. Yang, Y. Tong, J. Li, S. Zhai, X. Zhao, S. Wu, H. Li, Q. An and K. Wang, J. Mater. Chem. A, 2022, 10, 12679–12691 RSC.
- M. Cao, W. Cheng, X. Ni, Y. Hu and G. Han, Electrochim. Acta, 2020, 345, 136172 CrossRef CAS.
- F. Fu, D. Yang, H. Wang, Y. Qian, F. Yuan, J. Zhong and X. Qiu, ACS Sustainable Chem. Eng., 2019, 7, 16419–16427 CrossRef CAS.
- X. Ji, D. Sun, W. Zou, Z. Wang and D. Sun, J. Alloys Compd., 2021, 876, 160112 CrossRef CAS.
- Ü. Özgür, Y. I. Alivov, C. Liu, A. Teke, M. A. Reshchikov, S. Doğan, V. Avrutin, S.-J. Cho and H. Morkoç, J. Appl. Phys., 2005, 98, 041301 CrossRef.
- A. Sirelkhatim, S. Mahmud, A. Seeni, N. H. M. Kaus, L. C. Ann, S. K. M. Bakhori, H. Hasan and D. Mohamad, Nano-Micro Lett., 2015, 7, 219–242 CrossRef CAS.
- C. H. Kim and B.-H. Kim, J. Power Sources, 2015, 274, 512–520 CrossRef CAS.
- N. Casado, G. Hernández, H. Sardon and D. Mecerreyes, Prog. Polym. Sci., 2016, 52, 107–135 CrossRef CAS.
- X. Zhao, C. Huang, D. Xiao, P. Wang, X. Luo, W. Liu, S. Liu, J. Li, S. Li and Z. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 7600–7607 CrossRef CAS PubMed.
- W. Yang, Y. Qu, B. Zhou, C. Li, L. Jiao and H. Dai, Ind. Crops Prod., 2021, 171, 113848 CrossRef CAS.
- D. H. Nagaraju, T. Rebis, R. Gabrielsson, A. Elfwing, G. Milczarek and O. Inganäs, Adv. Energy Mater., 2014, 4, 1300443 CrossRef.
- B. Zhou, J. Li, W. Liu, H. Jiang, S. Li, L. Tan, L. Dong, L. She and Z. Wei, ChemSusChem, 2020, 13, 2628–2633 CrossRef CAS PubMed.
- S. Chaleawlert-umpon, T. Berthold, X. Wang, M. Antonietti and C. Liedel, Adv. Mater. Interfaces, 2017, 4, 1700698 CrossRef.
- A. M. Navarro-Suárez, N. Casado, J. Carretero-González, D. Mecerreyes and T. Rojo, J. Mater. Chem. A, 2017, 5, 7137–7143 RSC.
- F. N. Ajjan, N. Casado, T. Rębiś, A. Elfwing, N. Solin, D. Mecerreyes and O. Inganäs, J. Mater. Chem. A, 2016, 4, 1838–1847 RSC.
- F. N. Ajjan, M. Vagin, T. Rębiś, L. E. Aguirre, L. Ouyang and O. Inganäs, Adv. Sustainable Syst., 2017, 1, 1700054 CrossRef.
- S. Leguizamon, K. P. Díaz-Orellana, J. Velez, M. C. Thies and M. E. Roberts, J. Mater. Chem. A, 2015, 3, 11330–11339 RSC.
- S. Admassie, A. Elfwing, E. W. H. Jager, Q. Bao and O. Inganäs, J. Mater. Chem. A, 2014, 2, 1974–1979 RSC.
- J. Tian, Z. Liu, Z. Li, W. Wang and H. Zhang, RSC Adv., 2017, 7, 12089–12097 RSC.
- J. Pang, W. Zhang, J. Zhang, G. Cao, M. Han and Y. Yang, Green Chem., 2017, 19, 3916–3926 RSC.
- W. Zhang, C. Yu, L. Chang, W. Zhong and W. Yang, Electrochim. Acta, 2018, 282, 642–652 CrossRef.
- J. Tian, C. Liu, C. Lin and M. Ma, J. Alloys Compd., 2019, 789, 435–442 CrossRef.
- H. Zhu, X. Gan, A. McCreary, R. Lv, Z. Lin and M. Terrones, Nano Today, 2020, 30, 100829 CrossRef.
- X. Meng, C. Yu, X. Song, J. Iocozzia, J. Hong, M. Rager, H. Jin, S. Wang, L. Huang, J. Qiu and Z. Lin, Angew. Chem., Int. Ed., 2018, 57, 4682–4686 CrossRef CAS PubMed.
- S. Ghosh, S. Barg, S. M. Jeong and K. Ostrikov, Adv. Energy Mater., 2020, 10, 2001239 CrossRef CAS.
- X. Feng, Y. Bai, M. Liu, Y. Li, H. Yang, X. Wang and C. Wu, Energy Environ. Sci., 2021, 14, 2036–2089 RSC.
- B. Hu, K. Wang, L. Wu, S.-H. Yu, M. Antonietti and M.-M. Titirici, Adv. Mater., 2010, 22, 813–828 CrossRef CAS.
- X. Zhao, Q. Zhang, C.-M. Chen, B. Zhang, S. Reiche, A. Wang, T. Zhang, R. Schlögl and D. Sheng Su, Nano Energy, 2012, 1, 624–630 CrossRef CAS.
- M. Liu, S. Wang and L. Jiang, Nat. Rev. Mater., 2017, 2, 17036 CrossRef CAS.
- K. Wang, M. Xu, Y. Gu, Z. Gu and Q. H. Fan, J. Power Sources, 2016, 332, 180–186 CrossRef CAS.
- K. Wang, Y. Cao, X. Wang, M. A. Castro, B. Luo, Z. Gu, J. Liu, J. D. Hoefelmeyer and Q. Fan, J. Power Sources, 2016, 307, 462–467 CrossRef CAS.
- J. Yang, Y. Wang, J. Luo and L. Chen, ACS Omega, 2018, 3, 4647–4656 CrossRef CAS.
- Z. Dai, P.-G. Ren, Y.-L. Jin, H. Zhang, F. Ren and Q. Zhang, J. Power Sources, 2019, 437, 226937 CrossRef CAS.
- Q. Ma, H. Xi, F. Cui, J. Zhang, P. Chen and T. Cui, J. Energy Storage, 2022, 45, 103509 CrossRef.
- X. Cai, Y. Xiao, W. Sun and F. Yang, Electrochim. Acta, 2022, 406, 139861 CrossRef CAS.
- J. Zhang, H. Zhao, J. Li, H. Jin, X. Yu, Y. Lei and S. Wang, Adv. Energy Mater., 2019, 9, 1803221 CrossRef.
- G. Zhao, Y. Li, G. Zhu, J. Shi, T. Lu and L. Pan, ACS Sustainable Chem. Eng., 2019, 7, 12052–12060 CAS.
- F. Liu, Z. Wang, H. Zhang, L. Jin, X. Chu, B. Gu, H. Huang and W. Yang, Carbon, 2019, 149, 105–116 CrossRef CAS.
- F. Shi, Y. Tong, H. Li, J. Li, Z. Cong, S. Zhai, Q. An and K. Wang, J. Energy Storage, 2022, 52, 104992 CrossRef.
- J. Zhou, H. Shen, Z. Li, S. Zhang, Y. Zhao, X. Bi, Y. Wang, H. Cui and S. Zhuo, Electrochim. Acta, 2016, 209, 557–564 CrossRef CAS.
- B. Liu, Y. Liu, H. Chen, M. Yang and H. Li, J. Power Sources, 2017, 341, 309–317 CrossRef.
- W. Zhang, Z. Chen, X. Guo, K. Jin, Y. Wang, L. Li, Y. Zhang, Z. Wang, L. Sun and T. Zhang, Electrochim. Acta, 2018, 278, 51–60 CrossRef.
- Z. Zhao and Y. Xie, J. Power Sources, 2018, 400, 264–276 CrossRef.
- Y. Ma, X. Zhang, Z. Liang, C. Wang, Y. Sui, B. Zheng, Y. Ye, W. Ma, Q. Zhao and C. Qin, Electrochim. Acta, 2020, 337, 135800 CrossRef.
- X. Zhang, C. Jiang, J. Liang and W. Wu, J. Mater. Chem. A, 2021, 9, 8099–8128 RSC.
- J. Liang, C. Jiang and W. Wu, Appl. Phys. Rev., 2021, 8, 021319 Search PubMed.
- K. Qi, R. Hou, S. Zaman, B. Y. Xia and H. Duan, J. Mater. Chem. A, 2018, 6, 3913–3918 RSC.
- S. Sun, T. Zhai, C. Liang, S. V. Savilov and H. Xia, Nano Energy, 2018, 45, 390–397 CrossRef CAS.
- J. E. ten Elshof and Y. Wang, Small Methods, 2019, 3, 1800318 CrossRef.
- H. Park, J. W. Kim, S. Y. Hong, G. Lee, D. S. Kim, J. h. Oh, S. W. Jin, Y. R. Jeong, S. Y. Oh, J. Y. Yun and J. S. Ha, Adv. Funct. Mater., 2018, 28, 1707013 CrossRef.
- T. He, W. Zhang, P. Manasa and F. Ran, J. Alloys Compd., 2020, 812, 152138 CrossRef CAS.
- H.-M. Wang, T.-Q. Yuan, G.-Y. Song and R.-C. Sun, Green Chem., 2021, 23, 3790–3817 RSC.
- P. Gu, W. Liu, Q. Hou and Y. Ni, J. Mater. Chem. A, 2021, 9, 14233–14264 RSC.
- Z. Peng, Y. Zou, S. Xu, W. Zhong and W. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 22190–22200 CrossRef CAS.
- S. Jha, S. Mehta, Y. Chen, L. Ma, P. Renner, D. Y. Parkinson and H. Liang, ACS Sustainable Chem. Eng., 2020, 8, 498–511 CrossRef CAS.
- Y. Chen, G. Zhang, J. Zhang, H. Guo, X. Feng and Y. Chen, J. Mater. Sci. Technol., 2018, 34, 2189–2196 CrossRef CAS.
- W. Liu, Y. Yao, O. Fu, S. Jiang, Y. Fang, Y. Wei and X. Lu, RSC Adv., 2017, 7, 48537–48543 RSC.
- B. Zhou, W. Liu, Y. Gong, L. Dong and Y. Deng, Electrochim. Acta, 2019, 320, 134640 CrossRef CAS.
- J.-W. Jeon, L. Zhang, J. L. Lutkenhaus, D. D. Laskar, J. P. Lemmon, D. Choi, M. I. Nandasiri, A. Hashmi, J. Xu, R. K. Motkuri, C. A. Fernandez, J. Liu, M. P. Tucker, P. B. McGrail, B. Yang and S. K. Nune, ChemSusChem, 2015, 8, 428–432 CrossRef CAS PubMed.
- X. Y. Chen and Q. Q. Zhou, Electrochim. Acta, 2012, 71, 92–99 CrossRef CAS.
- J. Pang, W.-F. Zhang, J.-L. Zhang, H.-M. Zhang, G.-P. Cao, M.-F. Han and Y.-S. Yang, ChemElectroChem, 2018, 5, 1306–1320 CrossRef CAS.
- J. Pang, W. Zhang, H. Zhang, J. Zhang, H. Zhang, G. Cao, M. Han and Y. Yang, Carbon, 2018, 132, 280–293 CrossRef.
- Z. Zhao, S. Hao, P. Hao, Y. Sang, A. Manivannan, N. Wu and H. Liu, J. Mater. Chem. A, 2015, 3, 15049–15056 RSC.
- H.-B. Zhao, W.-D. Wang, Q.-F. Lü, T.-T. Lin, Q. Lin and H. Yang, Bioresour. Technol., 2015, 176, 106–111 CrossRef PubMed.
- F. Chen, Z. Zhou, L. Chang, T. Kuang, Z. Zhao, P. Fan, J. Yang and M. Zhong, Microporous Mesoporous Mater., 2017, 247, 184–189 CrossRef.
- W. Zhang, H. Lin, Z. Lin, J. Yin, H. Lu, D. Liu and M. Zhao, ChemSusChem, 2015, 8, 2114–2122 CrossRef PubMed.
- Z. Zhou, F. Chen, T. Kuang, L. Chang, J. Yang, P. Fan, Z. Zhao and M. Zhong, Electrochim. Acta, 2018, 274, 288–297 CrossRef.
- Z.-Q. Hao, J.-P. Cao, Y.-L. Dang, Y. Wu, X.-Y. Zhao and X.-Y. Wei, ACS Sustainable Chem. Eng., 2019, 7, 4037–4046 CrossRef CAS.
- J. Xu, X. Zhou, M. Chen, S. Shi and Y. Cao, Microporous Mesoporous Mater., 2018, 265, 258–265 CrossRef CAS.
- H. Xu, H. Jiang, X. Li and G. Wang, RSC Adv., 2015, 5, 76116–76121 RSC.
- L. Wang, X. Li, H. Xu and G. Wang, Synth. Met., 2019, 249, 40–46 CrossRef CAS.
- C. Lai, Z. Zhou, L. Zhang, X. Wang, Q. Zhou, Y. Zhao, Y. Wang, X.-F. Wu, Z. Zhu and H. Fong, J. Power Sources, 2014, 247, 134–141 CrossRef CAS.
- D. Lei, X.-D. Li, M.-K. Seo, M.-S. Khil, H.-Y. Kim and B.-S. Kim, Polymer, 2017, 132, 31–40 CrossRef CAS.
- C. Ma, Z. Li, J. Li, Q. Fan, L. Wu, J. Shi and Y. Song, Appl. Surf. Sci., 2018, 456, 568–576 CrossRef CAS.
- W. J. Youe, S. J. Kim, S. M. Lee, S. J. Chun, J. Kang and Y. S. Kim, Int. J. Biol. Macromol., 2018, 112, 943–950 CrossRef CAS PubMed.
- C. Guo, H. Ma, Q. Zhang, M. Li, H. Jiang, C. Chen, S. Wang and D. Min, Nanomaterials, 2020, 10, 594 CrossRef CAS.
- L. Chen, J. Deng, Y. Song, S. Hong and H. Lian, Mater. Res. Bull., 2020, 123, 110708 CrossRef CAS.
- T. Wang, S. Hu, D. Wu, W. Zhao, W. Yu, M. Wang, J. Xu and J. Zhang, J. Mater. Chem. A, 2021, 9, 11839–11852 RSC.
- W. Zhang, Y. Lei, F. Ming, Q. Jiang, P. M. F. J. Costa and H. N. Alshareef, Adv. Energy Mater., 2018, 8, 1801840 CrossRef.
- M. Yuan, F. Luo, Y. Rao, Y. Wang, J. Yu, H. Li and X. Chen, J. Power Sources, 2021, 513, 230558 CrossRef CAS.
- L. Zhu, F. Shen, R. L. Smith, L. Yan, L. Li and X. Qi, Chem. Eng. J., 2017, 316, 770–777 CrossRef CAS.
- W. Chen, X. Wang, M. Feizbakhshan, C. Liu, S. Hong, P. Yang and X. Zhou, J. Colloid Interface Sci., 2019, 540, 524–534 CrossRef CAS.
- W.-M. Yin, L.-F. Tian, B. Pang, Y.-R. Guo, S.-J. Li and Q.-J. Pan, Int. J. Biol. Macromol., 2020, 156, 988–996 CrossRef CAS PubMed.
- Z. Dai, P.-G. Ren, W. He, X. Hou, F. Ren, Q. Zhang and Y.-L. Jin, Renewable Energy, 2020, 162, 613–623 CrossRef CAS.
- C. Ma, L. Wu, M. Dirican, H. Cheng, J. Li, Y. Song, J. Shi and X. Zhang, J. Colloid Interface Sci., 2021, 586, 412–422 CrossRef.
- Q. Cao, Y. Zhang, J. Chen, M. Zhu, C. Yang, H. Guo, Y. Song, Y. Li and J. Zhou, Ind. Crops Prod., 2020, 148, 112181 CrossRef.
- P. Butnoi, A. Pangon, R. Berger, H.-J. Butt and V. Intasanta, J. Mater. Res. Technol., 2021, 12, 2153–2167 CrossRef.
- M. Singh, A. Gupta, S. Sundriyal, K. Jain and S. R. Dhakate, Mater. Chem. Phys., 2021, 264, 124454 CrossRef.
- S. Liu, S. Wu, H. Cheng, W. Wei and F. Zhang, Ind. Crops Prod., 2022, 179, 114657 CrossRef.
- X. Zhang, W. Jian, L. Zhao, F. Wen, J. Chen, J. Yin, Y. Qin, K. Lu, W. Zhang and X. Qiu, Colloids Surf., A, 2022, 636, 128191 CrossRef.
- M. Cao, Y. Hu, W. Cheng, S. Huan, T. Bai, Z. Niu, Y. Zhao, G. Yue, Y. Zhao and G. Han, Chem. Eng. J., 2022, 436, 135233 CrossRef CAS.
- L. Wang, X. Feng, X. Li, H. Wang, J. Wu, H. Ma and J. Zhou, J. Mater. Res. Technol., 2022, 16, 570–580 CrossRef CAS.
- H. Wang, F. Xiong, J. Yang, B. Ma, Y. Qing, F. Chu and Y. Wu, Ind. Crops Prod., 2022, 179, 114689 CrossRef CAS.
- F. Fu, H. Wang, D. Yang, X. Qiu, Z. Li and Y. Qin, J. Colloid Interface Sci., 2022, 617, 694–703 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |
