Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Towards sustainable synthesis: a life cycle assessment of polymer of intrinsic microporosity (PIM-1) by green mechanosynthesis†
Ching Yoong
Loh
 a,
Rui
Huang
b,
Roy
Bell
c and
Ming
Xie
a,
Rui
Huang
b,
Roy
Bell
c and
Ming
Xie
 *a
*a
aDepartment of Chemical Engineering, University of Bath, Bath, BA2 7AY, UK. E-mail: m.xie2@bath.ac.uk
bGuangdong Water Co., Ltd, Shenzhen, China 518001
cPA Consulting, 50 Farringdon Rd, London EC1M 3HE, UK
First published on 26th October 2023
Abstract
Mechanochemistry represents an emerging technology that facilitates chemical reactions through the application of mechanical energy. This straightforward technique enhances reaction efficiency, expediting the process in an environmentally friendly, solvent-free manner. Polymers of intrinsic microporosity (PIMs) belongs to a class of polymers characterized by intrinsic microporosity, remarkable processability, and high adsorption capacity, rendering them well-suited for gas-related applications. However, conventional wet chemical synthesis methods of PIM-1 often necessitate substantial solvent usage, leading to significant and enduring environmental impacts. In this study, we present an alternative approach, harnessing green mechanochemical reactions to produce PIM-1. Furthermore, we conducted a comprehensive Life Cycle Assessment (LCA) to compare and simulate the environmental impacts of both wet chemical and mechanosynthesis methods. Our findings indicate the successful qualitative synthesis of PIM-1 through mechanochemistry, resulting in a notable reduction of environmental impacts, approximately 1.5 times less compared to the conventional wet chemical synthesis route. This advancement holds great promise for advancing sustainable and eco-friendly polymer synthesis methods.
Sustainability spotlightPolymer of intrinsic microporosity (PIM) has major implications with gas applications and high potential in the fabrication of liquid separation membranes as a material. However, in principle, the synthesis of the highly adsorptive material rely on wet chemical methods, which poses a hazard to the environment due to the involvement of toxic chemicals with exceeding amount. As a notable alternative, mechanosynthesis has emerged as a green technology that minimizes or eliminates the need for solvents, thereby substantially reducing the adverse environmental impacts associated with chemical reactions. In our research, we have successfully synthesis PIM-1 via mechanochemistry reaction, involving minimal solvents in the process. Furthermore, we conducted a comprehensive environmental assessment employing the Life Cycle Assessment (LCA) method, which showed that the former generates more negative impact to the environment. This study aligns seamlessly with the United Nations Sustainable Development Goals (SDGs), particularly emphasizing for responsible consumption and production (SDG 12), life below water (SDG 14) and life on land (SDG 15). |
1 Introduction
Polymers of intrinsic microporosity (PIMs) is a distinctive class of porous materials that exhibit great potential as adsorbents in physical adsorption processes.1 Unlike conventional polymers, which typically possess tightly packed structures with limited void space due to strong intermolecular forces, PIMs demonstrate a remarkable deviation by offering significant internal voids. These voids form a continuous network of interconnected intermolecular spaces, giving rise to the term ‘intrinsic microporosity’ and endowing the material with unique microporous characteristics.2,3 This intrinsic microporosity grants PIMs significant microporous characteristics, allowing them to act as molecular sieves. Among the various PIMs, PIM-1 stands out due to its excellent solubility in solvents like chloroform and tetrahydrofuran (THF), enabling its transformation into different forms, from granules to films.4 Additionally, PIM-1 demonstrates high surface areas and remarkable adsorption capacities.5 Consequently, PIM-1 has found wide-ranging applications in fields such as hydrogen storage,6 gas separation,7 and energy storage,8 owing to its unique properties and versatility.PIM-1 can be synthesized through two primary methods: the low-temperature method,1 and the high-temperature method.9 More recently, Zhang et al.10 proposed an alternative synthesis approach utilizing a mechanochemical reaction that remarkably reduces the synthesis time to just 15 minutes. This method involves initially combining the reactants, namely 5,5′,6,6′-tetrahydroxy-3,3,3′,3′-tetramethyl-1,1′-spirobisindane (TTSBI) and tetrafluoroterephthalonitrile (TFTPN), along with the addition of K2CO3 as a catalyst. The reaction mixture, along with suitable balling media, is then subjected to grinding in a ball milling machine. Notably, the conventional low-temperature and high-temperature methods typically require 24–72 h for PIM-1 synthesis, whereas the mechanochemical approach achieves a significantly shorter reaction time, yielding PIM-1 with a remarkable 98% efficiency.10
On the other hand, mechanochemistry, as an emerging technology, has gained prominence in the field of green chemistry over the past decade.11 Defined by IUPAC as ‘chemical reactions induced by mechanical energy,12 mechanochemistry often takes place without the need for solvents, making it a sustainable and environmentally friendly approach. Moreover, it has been utilized extensively, especially in the environmental sector, for instance, refining fly ash,13–15 reducing carbon dioxide,16–18 and dye adsorption.19–21 Therefore, employing mechanochemistry in the synthesis of PIM-1 not only offers significant time savings but also aligns with sustainable and green principles.
Mechanosynthesis has emerged as a promising green technology. However, the carbon footprint, particularly the environmental implications of this method has not been quantitatively analyzed, necessitating a thorough evaluation of its environmental impacts for informed decision-making regarding PIM-1 synthesis or, more broadly, other reactions and processes. In general, there are several metrics in green chemistry that could be utilized to quantitatively evaluate the environmental impacts, including atom economy, environmental factor, process mass intensity.22 The evaluation of these metrics could potentially promote the development of green technologies in laboratories and industries. Moreover, there are also generic assessments such as Life Cycle Assessment (LCA)23,24 or DOZN 2.0 (ref. 25) that are used extensively for the evaluations of environmental impacts of chemical processes. LCA is a widely employed quantitative tool, both in commercial and academic settings, to assess the environmental impacts of a given subject. It encompasses the evaluation of impacts occurring throughout the entire product life cycle, spanning from raw material extraction to product disposal.26 LCA serves to prevent the potential over-interpretation of environmental burdens during the development stages of a synthesis method.27 By providing a comprehensive and quantitative approach, LCA facilitates comparative studies and offers valuable insights into the environmental implications of various methods, fostering a more comprehensive understanding of their environmental impacts. In the context of PIM-1, Goh et al. had recently evaluated the environmental impacts of PIM-1 production as membrane materials using LCA.28 The group assessed the conventional low-temperature and high-temperature method of PIM-1 synthesis, emphasizing on solvent recovery and choosing alternative monomer.
In this study, we explored mechanosynthesis by synthesizing PIM-1 via mechanochemical reaction and further evaluate the environmental impacts of mechanosynthesis via LCA. This will provide a comprehensive understanding of the environmental performance of mechanochemistry and its potential as a sustainable synthesis approach for PIM-1 polymer and beyond. The findings of this study will contribute to the broader discussions on green synthesis methods and support the development of environmentally friendly strategies in materials science and chemistry.
2 Materials and methodology
2.1 Chemicals and materials
TTSBI (97%), TFTPN (98%), and potassium carbonate (anhydrous, 98%), were purchased from Alfa Aesar. Ethanol (analytical grade) was purchased from Fischer Scientific, and methanol (analytical grade) was purchased from VWR. De-ionized water was used for any aqueous usage and solution preparation.2.2 Green mechanosynthesis of PIM-1
PIM-1 was synthesized using TTSBI, TFTPN, and K2CO3 as reactants via mechanochemistry.10 The molar ratio of these reactants was set to 0.5 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 mmol
0.5 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 mmol, which corresponded to a near-complete synthesis reaction. All reactants were added into a stainless-steel reactor, with stainless-steel ball bearings. The stainless-steel reactor was placed into a Retsch PM 200 planetary ball mill (750 W, 500 rpm) for 20, 40 and 60 min to study the reaction kinetics of the synthesis of PIM-1. The reactant-to-ball ratio was controlled (1
1.5 mmol, which corresponded to a near-complete synthesis reaction. All reactants were added into a stainless-steel reactor, with stainless-steel ball bearings. The stainless-steel reactor was placed into a Retsch PM 200 planetary ball mill (750 W, 500 rpm) for 20, 40 and 60 min to study the reaction kinetics of the synthesis of PIM-1. The reactant-to-ball ratio was controlled (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 and 1
64 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128). The mixture resulting from the grinding procedure was collected and washed repeatedly using ethanol, methanol, and de-ionized water after centrifuge purification. After washing, the compound was dried by placing it on a watch glass in the oven overnight at a temperature of 55 °C. The resulting solids were sampled for characterization and were denoted as PIM-1.
128). The mixture resulting from the grinding procedure was collected and washed repeatedly using ethanol, methanol, and de-ionized water after centrifuge purification. After washing, the compound was dried by placing it on a watch glass in the oven overnight at a temperature of 55 °C. The resulting solids were sampled for characterization and were denoted as PIM-1.
2.3 Characterization of PIM-1
Proton nuclear magnetic resonance (1H NMR) analyses with samples dissolved in deuterated chloroform were performed (400 MHz Bruker NMR Spectrometer, Massachusetts, USA). Fourier-transform infrared (FT-IR) spectroscopy (PerkinElmer FT-IR Spectrometer Frontier, Massachusetts, USA) was performed to record the infrared spectra of the surface to determine the succession of the material deposits. The spectral resolution was 4 cm−1, and 16 scans were recorded for spectrum ranging from 4000–400 cm−1 for each sample after background analysis. Gel-Permeation Chromatography (GPC) was performed to determine the average molecular weight of the synthesized polymer. The PIM-1 samples were dissolved in THF, with a calibration standard of polystyrene. Scanning electron microscopy (SEM) was performed to study the overall morphology of PIM-1. The samples were degassed in a pressurized chamber overnight to remove unwanted particles for better imaging. The samples were coated with a layer of gold before carrying out SEM (Hitachi SU3900, Kyoto, Japan) to minimize a charge build-up in the sample. Brunauer–Emmett–Teller (BET) surface area analysis (Autosorb-iQ-C, Graz, Austria) was carried out to measure the specific surface area of the mechanosynthesis PIM-1. The samples were weighed and later degassed at 120 °C for 16 h before starting the analysis of adsorption and desorption isotherm with N2.2.4 LCA study of mechanosynthesis and wet chemical method
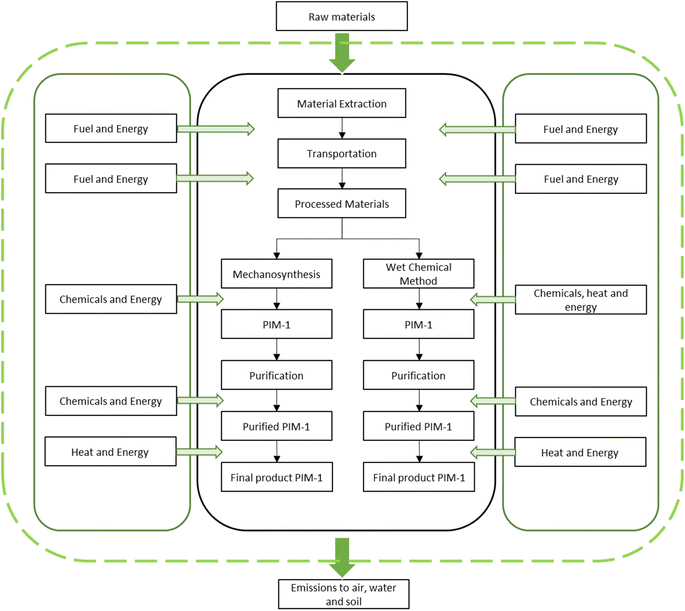
Life cycle assessment (LCA) was conducted between mechanosynthesis and wet chemical method for PIM-1 synthesis. OpenLCA (version 1.10.3) was utilized as a calculation and modelling software tool to further evaluate the report. The database, Eco-Invent 3.7 was used to provide necessary information on the inventory inputs and outputs of the assessment. In the final part of the modelling process, ReCiPe 2016 ‘H’ midpoint analysis was employed for the impact assessment section.In the assessment, the “cradle-to-gate” analysis technique was utilized to examine the chemical process of mechanochemical reaction and wet chemical reaction in the synthesis of PIM-1. Table 1 illustrated the experimental procedures of both mechanosynthesis and wet chemical method to produce PIM-1. The functional unit of this LCA report was assumed to be 1.0 g. To further elaborate, a competent contrast between the two methods was made by considering the production yield. Fig. 1 showed the system boundary of both mechanosynthesis and wet chemical method in the LCA model simulation.
| Mechanosynthesis | Wet chemical method | |
|---|---|---|
| Material inputs | TTSBI (C21H24O4), TFTPN (C6F4-1,4-(CN)2), potassium carbonate (K2CO3), ethanol (C2H5OH), methanol (CH3OH), de-ionized water (H2O) | TTSBI (C21H24O4), TFTPN (C6F4-1,4-(CN)2), potassium carbonate (K2CO3), dimethyl sulfoxide ((CH3)2SO), toluene (C6H5CH3), ethanol (C2H5OH), de-ionized water (H2O) |
| Energy inputs | Ball milling for 60 min, washing with ethanol, methanol and de-ionized water using a centrifuge, drying with oven for 24 h | Stirring for 3 min pre-reaction, heating and stirring for 8 h, washing with ethanol and de-ionized water using a centrifuge, drying using an oven for 24 h |
| Functional units | 1 g of PIM-1 | 1 g of PIM-1 |
| Yield | 98% | 93% |
| Reference | Zhang et al.,10 experimental data from this study | Ponomarev et al.29 |
3 Results and discussion
3.1 Key physicochemical properties of mechanosynthesized PIM-1
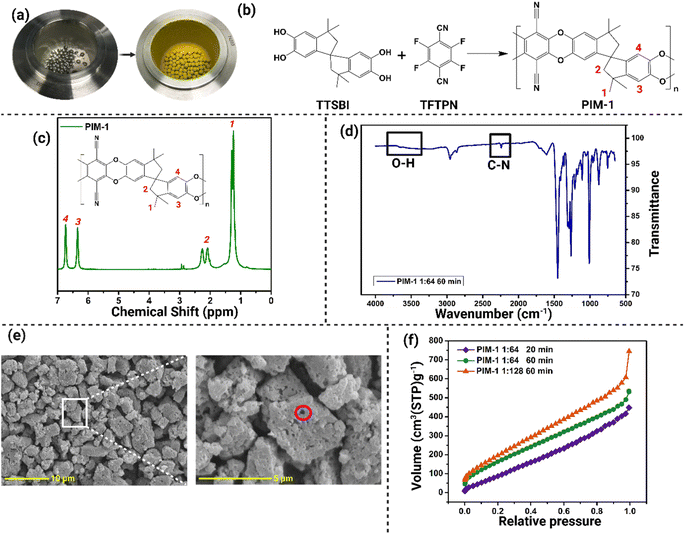
The successful mechanosynthesis of PIM-1 was visually confirmed through significant color transformation of the reactants, as depicted in Fig. 1a and b. Initially, the reactants exhibited a white powder appearance, which underwent a noticeable change to a yellow powder state, indicating the formation of PIM-1.The NMR analysis of the PIM-1 samples exhibited sharp peaks, demonstrating the solubility of PIM-1 in deuterated chloroform (CDCl3) as the solvent (Fig. 1c). The functional groups of the PIM-1 structure were assigned with the corresponding peaks in the NMR spectra (Fig. 2b and c). Notably, distinct peaks at 6.4 and 6.8 ppm were observed, which was attributed to the aromatic group presented in PIM-1.1,10
The NMR results revealed that the formation of PIM-1 was already evident after 20 minutes of the synthesis process, as evidenced by the identical NMR spectra obtained at different time intervals (40 min and 60 min) (Fig. S1†). Moreover, a comparison of the NMR spectra between samples with reactant-ball mass ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 and 1
64 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128 indicated no significant differences, suggesting that both samples represent the same compound (Fig. S2†).
128 indicated no significant differences, suggesting that both samples represent the same compound (Fig. S2†).
The FT-IR spectra displayed in Fig. 1d showed the functional group analysis of PIM-1 synthesized using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 reactant-ball ratio at 60 min. Notably, a peak observed at 2200 cm−1 confirmed the presence of the nitrile group (C–N) characteristic of PIM-1. Additionally, the absence of a peak in the range of 3000–3700 cm−1, where the alcohol O–H group from the TTSBI monomer would typically peak,1 further supported the identification of the product as PIM-1. The identical FT-IR spectra obtained for the three samples at different time intervals (Fig. S3†) confirmed that all samples were the same product. Furthermore, the intensity of the peaks increased proportionally with the reaction time, suggesting that the reaction progressed as the duration extended.
64 reactant-ball ratio at 60 min. Notably, a peak observed at 2200 cm−1 confirmed the presence of the nitrile group (C–N) characteristic of PIM-1. Additionally, the absence of a peak in the range of 3000–3700 cm−1, where the alcohol O–H group from the TTSBI monomer would typically peak,1 further supported the identification of the product as PIM-1. The identical FT-IR spectra obtained for the three samples at different time intervals (Fig. S3†) confirmed that all samples were the same product. Furthermore, the intensity of the peaks increased proportionally with the reaction time, suggesting that the reaction progressed as the duration extended.
The PIM-1 morphology and polymer structure were imaged in Fig. 1e, illustrating the microporous characteristics of the mechanosynthesized polymer. The hierarchical pores from PIM-1 shown in the SEM images (indicated by the red circle) are the intrinsic pores that confirmed the success of the synthesized polymer.31,32 Kinetics of the synthesis reaction is shown in Fig. S4.† At 20 min, the morphology of PIM-1 could still be seen as packed and there were no spaces between each PIM-1 particle (Fig. S4a†). Hence, the polymerization of PIM-1 was not complete because of the lack of free volume of the polymer that should be resulting in micropores. However, the voids became more obvious at the 40 min (Fig. S4b†) and finally, the pore structures were visible after 60 min (Fig. 1e). Regarding the case of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128 reactant-ball ratio, as shown in Fig. S4d,† the voids in each ‘granule’ of PIM-1 were smaller and appeared more frequently. This phenomenon proves that the greater the ball mass, the greater its efficiency and efficacy on the synthesis, and in this case, the overall surface area of the sample increases. The structure of these PIM-1 samples has slightly bigger void spaces and tighter packing formation, resulting in fewer surface areas. This could be attributed to the nature of the synthesis method, where there were enhanced polymer cohesion and packing during the progress of the reaction involving grinding of the solid-state.10,33
128 reactant-ball ratio, as shown in Fig. S4d,† the voids in each ‘granule’ of PIM-1 were smaller and appeared more frequently. This phenomenon proves that the greater the ball mass, the greater its efficiency and efficacy on the synthesis, and in this case, the overall surface area of the sample increases. The structure of these PIM-1 samples has slightly bigger void spaces and tighter packing formation, resulting in fewer surface areas. This could be attributed to the nature of the synthesis method, where there were enhanced polymer cohesion and packing during the progress of the reaction involving grinding of the solid-state.10,33
Table 2 presents the molecular weight of various PIM-1 samples. It is observed that a shorter polymerization time of 20 minutes results in a lower molecular weight of PIM-1, a consequence of the reduced time available for the polymerization of monomers. In contrast, after a duration of 60 minutes, the polymerization of PIM-1 appears more comprehensive. Specifically, at reactant-to-ball ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 and 1
64 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128, the average molecular weight (Mw) reached 41
128, the average molecular weight (Mw) reached 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 60
000 g mol−1 and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 991 g mol−1, respectively. While applications, particularly in membrane fabrication, often favor PIM-1 with a high molecular weight (>100 kg mol−1),34,35 it's noteworthy that Bhavsar et al. demonstrated the feasibility of CO2 gas separation membranes using PIM with an Mw of approximately 50 kg mol−1 by grafting polyethylene oxides onto PIM-1.35 Similarly, research by Kim and colleagues showcased the use of PIM-1, with an Mw of 50
991 g mol−1, respectively. While applications, particularly in membrane fabrication, often favor PIM-1 with a high molecular weight (>100 kg mol−1),34,35 it's noteworthy that Bhavsar et al. demonstrated the feasibility of CO2 gas separation membranes using PIM with an Mw of approximately 50 kg mol−1 by grafting polyethylene oxides onto PIM-1.35 Similarly, research by Kim and colleagues showcased the use of PIM-1, with an Mw of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, in the fabrication of carbonaceous membranes suited for saline water treatment.36
100 g mol−1, in the fabrication of carbonaceous membranes suited for saline water treatment.36
| Reaction conditions | Number average molecular weight, Mn (g mol−1) | Average molecular weight, Mw (g mol−1) |
|---|---|---|
PIM-1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64, 20 min 64, 20 min |
2274 | 4806 |
PIM-1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64, 60 min 64, 60 min |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939 939 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 028 028 |
PIM-1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128, 60 min 128, 60 min |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 644 644 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 991 991 |
3.2 Adsorption/desorption isotherms
Based on the IUPAC classification of adsorption isotherms, the adsorption/desorption isotherms of the mechanosynthesized PIM-1 (Fig. 1f and S5†) can be characterized as a composite of type I and type IV isotherms. This suggests a porosity that bridges between the microporous and mesoporous scales. In comparison to conventionally synthesized PIM-1, the ambiguous nature of the adsorption curve—oscillating between type I and type IV characteristics—might be attributed to suboptimal polymerization during the mechanosynthesis process. This inefficiency could result in increased free spaces among the monomers, thereby amplifying the porosity scale. The observations aligns with results from the SEM analysis, which further indicate that the PIM-1 samples manifest porosity at both macro and micro scales. Furthermore, the hysteresis of PIM-1 after 60 min exhibited a reduced magnitude compared to the PIM-1 at 20 min, as demonstrated in Fig. S5a and b.† As the level of hysteresis is subjected to the mesoporosity, this can be attributed to the reduction in mesoporosity of PIM-1 over the course of the reaction, which correlates with the kinetic profile of PIM-1 polymerization. Notably, under the condition of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128 reactants-to-balls ratio (Fig. S5c†), the hysteresis approached closure. This suggests that increased mechanical force contributes to the enthalpy necessary for polymerization, thereby leading to a heightened microporosity. On the other hand, there is an unlinked hysteresis phenomenon occurring at lower relative pressure. This is caused by the elastic changes of the polymer segment positions and acting solvation pressures, which commonly occur in most microporous polymers.37
128 reactants-to-balls ratio (Fig. S5c†), the hysteresis approached closure. This suggests that increased mechanical force contributes to the enthalpy necessary for polymerization, thereby leading to a heightened microporosity. On the other hand, there is an unlinked hysteresis phenomenon occurring at lower relative pressure. This is caused by the elastic changes of the polymer segment positions and acting solvation pressures, which commonly occur in most microporous polymers.37
Table 3 presents the BET surface area results of PIM-1 synthesized under different conditions. The analysis confirms that the resulting PIM-1 samples are microporous, falling within the typical range of 300–2000 m2 g−1 for microporous materials.38 In general, PIM materials exhibit surface areas ranging from 500–1000 m2 g−1.1 The results support the hypothesis that surface area increases with reaction time in mechanosynthesis, where it can be attributed to the continuous molecular collisions between reactants and balls throughout the reaction, resulting in a higher adsorption surface area. Similarly, the BET surface area of the samples also correlates with the reactants to ball mass ratio, indicating that higher ball masses lead to increased surface area. The greater number of collisions at higher ball masses promotes smaller molecule formation and, consequently, a higher BET surface area.
| Reaction conditions | BET surface area (m2 g−1) |
|---|---|
PIM-1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64, 20 min 64, 20 min |
545.1 |
PIM-1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64, 60 min 64, 60 min |
710.1 |
PIM-1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128, 60 min 128, 60 min |
855.5 |
4 Life cycle assessment of mechanosynthesis for PIM-1
4.1 Results of LCIA methods
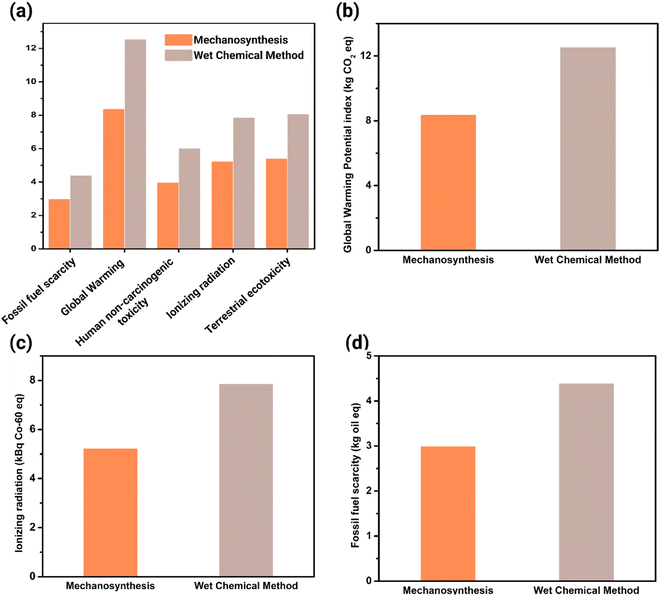
The 18 midpoint indicators in ReCiPe have diverse units representing different categories, making comparisons between indicators complicated. However, normalization is employed to enhance the comparability of data and assessment results. Fig. S5† illustrated the overall analysis, whereas Fig. 3a highlights the most contrasting impact categories in the analysis. The wet chemical method has significantly larger environmental impacts compared to mechanosynthesis when synthesizing 1 g of PIM-1. Notably, global warming, ionizing radiation, and fossil fuel scarcity emerge as major impact categories, exhibiting significant differences in PIM-1 synthesis.Global Warming Potential (GWP) is an indicator used to evaluate the impact of global warming. It represents the radiative energy emitted over time by 1 kg of greenhouse gas relative to the radiative energy emitted by 1 kg of CO2, with the unit of kilograms of CO2 equivalent (kg CO2 eq.).39 Ionizing radiation is characterized as a midpoint indicator relative to the radioactivity emitted by cobalt-60, a reference substance, to air, expressed in kilobecquerels of cobalt-60 equivalent (kBq Co-60 eq.).39 Fossil fuel scarcity is assessed using fossil fuel potential, which represents the energy content of the fossil resource in the evaluated product system relative to the energy content in crude oil. The unit for this indicator is kilograms of oil equivalent (kg oil eq.).39
Fig. 3b demonstrates that the wet chemical synthesis method contributes 50% more to the global warming index compared to mechanosynthesis. In a study by Goh et al., the global warming potential (GWP) was estimated to be approximately 2.5 kg CO2 eq. for the synthesis of 1 g of PIM-1 via a wet chemical method.28 In contrast, our findings indicate a GWP of around 12 kg CO2 eq. A significant source of this discrepancy can be attributed to the data values associated with the raw materials. In our study, the data for raw materials, including TTSBI and TFTPN, was inferred based on elemental composition. Conversely, Goh et al. synthesized these raw materials from scratch, providing a more robust and reliable dataset. It is essential to note, however, that the primary objective of our study was to juxtapose the environmental ramifications of different synthesis methods, and not to provide a precise envrionmental impacts measure. Thus, potential inaccuracies arising from raw material data were considered secondary and were not the focal point of our analysis.
Similarly, Fig. 3c and d show that the wet chemical method has index values 1.5 times higher than mechanosynthesis for ionizing radiation and fossil fuel scarcity, respectively. This consistent trend across different impact categories suggests a common underlying cause, which will be further discussed in the next subsection.
On the other hand, the high impact value of ionizing radiation in the wet chemical method can be attributed to its high electricity consumption. In the UK, the 13 nuclear reactors that are currently operating contribute 20% of the country's electricity generation as of 2020.41 Ionizing radiation is primarily generated through activities related to nuclear fuel mining, processing, waste disposal, as well as coal burning and phosphate rock extraction.39 Even at low radiation concentrations, radiation exposure can have severe health consequences, particularly for pregnant women, including cancer, growth retardation, and impaired brain function.42
The wet chemical method significantly depletes non-renewable fossil resources faster compared to mechanosynthesis. Fossil fuels such as natural gas, petroleum, and coal are commonly used as sources for electrical power generation. These fuels are considered non-renewable resources, meaning they will become increasingly scarce over time unless alternative energy sources are adopted. The depletion of fossil fuel reserves can have severe consequences for humanity, including inflation and disparities in wealth distribution. Therefore, it is crucial to consider the scarcity of fossil fuels when comparing the two synthesis methods.
When comparing the wet chemical method and mechanosynthesis in LCA, it is important to consider factors beyond energy consumption, such as the involvement of solvents. According to the system boundary depicted in Fig. 1, the chemicals used in PIM-1 synthesis for both methods are sourced from mineral extraction to the final product manufacturing stages. The additional solvents utilized in the reaction stage of the wet chemical method, such as DMSO and toluene were likely to contribute to three or more environmental impacts. Moreover, the manufacturing and mineral extraction processes require additional electricity and labor, amplifying the overall impact. Furthermore, transportation stages following mineral extraction and manufacturing can contribute to greenhouse gas emissions, intensifying global warming. Taking these factors into consideration is crucial for a comprehensive assessment of the differences between the two synthesis methods.
In the context of PIM-1's mechanosynthesis, certain methodological adjustments could offer significant environmental benefits. From a toxicological perspective, the primary concerns—both non-carcinogenic and carcinogenic—are intimately linked to the organic solvents employed during purification. It's imperative, from both an academic and practical standpoint, to investigate strategies that reduce or optimize these solvent volumes. Concurrently, land use considerations, as delineated in standard LCA frameworks, must be scrutinized. The elevated land usage can be attributed to three pivotal factors: transportation logistics associated with raw materials, the protocols adopted for waste solvent disposal, and the infrastructure requisites of the reaction process. A holistic approach that encompasses rigorous logistical planning, transportation optimization, and methodological refinement in reactions can potentially address these environmental challenges.
5 Conclusion
Experimental results and life cycle analysis reported here compared and underscored the mechanism of mechanosynthesis and its environmental implication. By synthesizing PIM-1 under different operating parameters, including varying reaction times (20, 40, 60 min) and reactants to balls mass ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64, 1
64, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128), the synthesis of PIM-1 samples has been confirmed through analyses. The synthesis of PIM-1 using mechanosynthesis, coupled with its positive environmental impact demonstrated through LCA, highlights the potential of this technique. The results of this assessment demonstrated that mechanosynthesis is generally more environmentally friendly, particularly in terms of energy usage and solvent involvement. Key environmental factors, such as fossil fuel scarcity, ionizing radiation, and global warming, were considered. However, further advancements are necessary to overcome existing limitations and optimize its implementation. The ongoing efforts in mechanosynthesis research hold promise for the development of a sustainable and efficient approach to chemical synthesis.
128), the synthesis of PIM-1 samples has been confirmed through analyses. The synthesis of PIM-1 using mechanosynthesis, coupled with its positive environmental impact demonstrated through LCA, highlights the potential of this technique. The results of this assessment demonstrated that mechanosynthesis is generally more environmentally friendly, particularly in terms of energy usage and solvent involvement. Key environmental factors, such as fossil fuel scarcity, ionizing radiation, and global warming, were considered. However, further advancements are necessary to overcome existing limitations and optimize its implementation. The ongoing efforts in mechanosynthesis research hold promise for the development of a sustainable and efficient approach to chemical synthesis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. Y. L. would like to thank the EPSRC for PhD studentship. R. H. would like to acknowledge the financial support from China Scholarship Council. Authors also thanked financial support from Royal Society of Chemistry (research fund R21-4839757049) and Royal Society International Exchange (IEC\NSFC\211021).References
- P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 230–231, 10.1039/B311764B.
- N. B. McKeown and P. M. Budd, Macromolecules, 2010, 43, 5163–5176 CrossRef CAS.
- B. Satilmis and T. Uyar, Appl. Surf. Sci., 2018, 453, 220–229 CrossRef CAS.
- S. H. Pang, M. L. Jue, J. Leisen, C. W. Jones and R. P. Lively, ACS Macro Lett., 2015, 4, 1415–1419 CrossRef CAS PubMed.
- N. B. McKeown, P. M. Budd, K. J. Msayib, B. S. Ghanem, H. J. Kingston, C. E. Tattershall, S. Makhseed, K. J. Reynolds and D. Fritsch, Chem.–Eur. J., 2005, 11, 2610–2620 CrossRef CAS PubMed.
- S. Rochat, K. Polak-Kraśna, M. Tian, T. J. Mays, C. R. Bowen and A. D. Burrows, Int. J. Hydrogen Energy, 2019, 44, 332–337 CrossRef CAS.
- N. Du, G. P. Robertson, J. Song, I. Pinnau, S. Thomas and M. D. Guiver, Macromolecules, 2008, 41, 9656–9662 CrossRef CAS.
- R. Ye, D. Henkensmeier, S. J. Yoon, Z. Huang, D. K. Kim, Z. Chang, S. Kim and R. Chen, J. Electrochem. Energy Convers. Storage, 2017, 15, 10801 CrossRef.
- J. Song, N. Du, Y. Dai, G. P. Robertson, M. D. Guiver, S. Thomas and I. Pinnau, Macromolecules, 2008, 41, 7411–7417 CrossRef CAS.
- P. Zhang, X. Jiang, S. Wan and S. Dai, J. Mater. Chem. A, 2015, 3, 6739–6741 RSC.
- P. Baláž, M. Achimovičová, M. Baláž, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutková, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Chem. Soc. Rev., 2013, 42, 7571–7637 RSC.
- K. Horie, M. Barón, R. B. Fox, J. He, M. Hess, J. Kahovec, T. Kitayama, P. Kubisa, E. Maréchal, W. Mormann, R. F. T. Stepto, D. Tabak, J. Vohlídal, E. S. Wilks and W. J. Work, Pure Appl. Chem., 2004, 76, 889–906 CrossRef CAS.
- J. H. Yan, Z. Peng, S. Y. Lu, X. D. Li, M. J. Ni, K. F. Cen and H. F. Dai, J. Hazard. Mater., 2007, 147, 652–657 CrossRef CAS PubMed.
- Y. Nomura, K. Fujiwara, M. Takada, S. Nakai and M. Hosomi, J. Mater. Cycles Waste Manage., 2008, 10, 14–18 CrossRef CAS.
- Z. Chen, S. Lu, M. Tang, X. Lin, Q. Qiu, H. He and J. Yan, Sci. Total Environ., 2019, 694, 133813 CrossRef CAS PubMed.
- S. Mori, W. C. Xu, T. Ishidzuki, N. Ogasawara, J. Imai and K. Kobayashi, Appl. Catal., A, 1996, 137, 255–268 CrossRef CAS.
- K. L. Chagoya, D. J. Nash, T. Jiang, D. Le, S. Alayoglu, K. B. Idrees, X. Zhang, O. K. Farha, J. K. Harper, T. S. Rahman and R. G. Blair, ACS Sustainable Chem. Eng., 2021, 9, 2447–2455 CrossRef CAS.
- C. V. Picasso, D. A. Safin, I. Dovgaliuk, F. Devred, D. Debecker, H.-W. Li, J. Proost and Y. Filinchuk, Int. J. Hydrogen Energy, 2016, 41, 14377–14386 CrossRef CAS.
- M. Vakili, W. Qiu, G. Cagnetta, J. Huang and G. Yu, Front. Environ. Sci. Eng., 2021, 15, 128 CrossRef CAS.
- H. Li, M. Dai, S. Dai, X. Dong and F. Li, Hum. Ecol. Risk Assess., 2018, 24, 2133–2141 CrossRef CAS.
- D. Tian, X. Zhang, C. Lu, G. Yuan, W. Zhang and Z. Zhou, Cellulose, 2014, 21, 473–484 CrossRef CAS.
- N. Fantozzi, J.-N. Volle, A. Porcheddu, D. Virieux, F. García and E. Colacino, Chem. Soc. Rev., 2023, 52, 6680–6714 RSC.
- O. Galant, G. Cerfeda, A. S. McCalmont, S. L. James, A. Porcheddu, F. Delogu, D. E. Crawford, E. Colacino and S. Spatari, ACS Sustainable Chem. Eng., 2022, 10, 1430–1439 CrossRef CAS.
- O. Galant, A. Aborome, A. S. McCalmont, S. L. James, M. Patrascu and S. Spatari, ACS Sustainable Chem. Eng., 2023, 11, 12155–12165 CrossRef CAS.
- A. DeVierno Kreuder, T. House-Knight, J. Whitford, E. Ponnusamy, P. Miller, N. Jesse, R. Rodenborn, S. Sayag, M. Gebel, I. Aped, I. Sharfstein, E. Manaster, I. Ergaz, A. Harris and L. Nelowet Grice, ACS Sustainable Chem. Eng., 2017, 5, 2927–2935 CrossRef CAS.
- I. V. Muralikrishna and V. Manickam, in Environmental Management, ed. I. V. Muralikrishna and V. Manickam, Butterworth-Heinemann, 2017, pp. 57–75, DOI:10.1016/B978-0-12-811989-1.00005-1.
- A. Bjørn, M. Owsianiak, C. Molin and A. Laurent, in Life Cycle Assessment: Theory and Practice, ed. M. Z. Hauschild, R. K. Rosenbaum and S. I. Olsen, Springer International Publishing, Cham, 2018, pp. 9–16, DOI:10.1007/978-3-319-56475-3_2.
- W. H. D. Goh, H. S. Lau and W. F. Yong, Sci. Total Environ., 2023, 892, 164582 CrossRef CAS PubMed.
- I. I. Ponomarev, D. Y. Razorenov, I. V. Blagodatskikh, A. V. Muranov, L. E. Starannikova, A. Y. Alent'ev, R. Y. Nikiforov and Y. P. Yampol'skii, Polym. Sci., Ser. B, 2019, 61, 605–612 CrossRef CAS.
- J. C. Bare and T. P. Gloria, Environ. Sci. Technol., 2006, 40, 1104–1113 CrossRef CAS PubMed.
- K. Polak-Kraśna, R. Dawson, L. T. Holyfield, C. R. Bowen, A. D. Burrows and T. J. Mays, J. Mater. Sci., 2017, 52, 3862–3875 CrossRef PubMed.
- P. Zhang, X. Jiang, S. Wan and S. Dai, J. Mater. Chem. A, 2015, 3, 6739–6741 RSC.
- T. Friščić, Chem. Soc. Rev., 2012, 41, 3493–3510 RSC.
- S. V. Gutiérrez-Hernández, F. Pardo, A. B. Foster, P. Gorgojo, P. M. Budd, G. Zarca and A. Urtiaga, J. Membr. Sci., 2023, 675, 121532 CrossRef.
- R. S. Bhavsar, T. Mitra, D. J. Adams, A. I. Cooper and P. M. Budd, J. Membr. Sci., 2018, 564, 878–886 CrossRef CAS.
- H. J. Kim, D.-G. Kim, K. Lee, Y. Baek, Y. Yoo, Y. S. Kim, B. G. Kim and J.-C. Lee, Sci. Rep., 2016, 6, 36078 CrossRef CAS PubMed.
- J. Jeromenok and J. Weber, Langmuir, 2013, 29, 12982–12989 CrossRef CAS PubMed.
- N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675–683 RSC.
- M. A. J. Huijbregts, Z. J. N. Steinmann, P. M. F. Elshout, G. Stam, F. Verones, M. Vieira, M. Zijp, A. Hollander and R. v. Zelm, Int. J. Life Cycle Assess., 2017, 22, 138–147 CrossRef.
- S. R. Weart, The Discovery of Global Warming, Harvard University Press, 2008 Search PubMed.
- S. Jack, New nuclear plant at Sizewell set for green light, https://www.bbc.co.uk/news/business-54754016, accessed 6 August 2021.
- N. F. Abu Bakar, S. Amira Othman, N. F. Amirah Nor Azman and N. Saqinah Jasrin, IOP Conf. Ser. Earth Environ. Sci., 2019, 268, 012005 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00340j |
| This journal is © The Royal Society of Chemistry 2023 |