Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Understanding the important variables to optimize glycolysis of polyethylene terephthalate with lanthanide-containing ionic liquids†
Nancy G.
Bush
 a,
Caitlin H.
Dinh
a,
Casandrah L.
Catterton
a and
Megan E.
Fieser
a,
Caitlin H.
Dinh
a,
Casandrah L.
Catterton
a and
Megan E.
Fieser
 *ab
*ab
aDepartment of Chemistry, University of Southern California, Los Angeles, CA 90089, USA. E-mail: fieser@usc.edu
bWrigley Institute for Environmental Studies, University of Southern California, Los Angeles, CA 90089, USA
First published on 2nd May 2023
Abstract
Glycolysis is a widely studied method for chemical recycling of polyethylene terephthalate (PET). Metal-containing ionic liquids (MILs) are attractive catalysts for the glycolysis of PET, as they have been shown to have high stability and can be easily recycled. While a range of MILs have been studied with varying cations and metal containing anions, there are no conclusive trends for the different variables in MIL catalyst design and how they affect glycolysis of PET. We report the use of lanthanide MILs to identify which catalyst design variables and reaction conditions can be tuned to make the biggest difference in the deconstruction of PET. Mixtures of ionic liquids (ILs) with lanthanide metal salts were found to lead to homogeneous MIL catalysts that are active for the glycolysis of PET. These studies identified that finding a metal salt and IL combination that leads to a cooperative MIL catalyst was more important than the basicity of the IL or metal salt anion itself. Finally, the high performance of MILs with a high ratio of IL to metal salt lowers the loading of metal salts with supply risk, while maintaining the value of MIL over metal-free IL catalysts.
Sustainability spotlightIonic liquids have been identified as promising catalysts for the glycolysis of poly(ethylene terephthalate) (PET) to its monomer precursor, bis(2-hydroxyethyl)terephthalate (BHET). Metal containing ionic liquids (MILs) have shown increased stability, recyclability and activity for this process over metal-free ionic liquids. However, using these MILs requires metals with supply risk, as they typically use only one or two equivalents of ionic liquid to metal salt. We identify that lanthanide-based MILs show the highest activity for PET glycolysis with a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ionic liquid to metal salt, significantly dropping the metal salt load. Along with reactions conducted in neat ethylene glycol, the lower metal loading in MIL catalysts aligns with the 12 principles of green chemistry and UN SDG:12. 1 ratio of ionic liquid to metal salt, significantly dropping the metal salt load. Along with reactions conducted in neat ethylene glycol, the lower metal loading in MIL catalysts aligns with the 12 principles of green chemistry and UN SDG:12.
|
Introduction
Polyethylene terephthalate (PET) is a semicrystalline polyester used commonly across many industries including textiles and packaging, making up 8% by weight and 12% by volume of the world's solid waste.1 Its lack of biodegradability has caused it to accumulate in landfills and the environment.2 Solvolysis of PET is the most-studied chemical recycling method for PET, as monomers can be produced that can be used to remake the same polymer.3–7 For example, glycolysis of PET forms bis(2-hydroxyethyl)terephthalate (BHET), which is an intermediate in the commercial synthesis of PET.The use of a catalyst is well-known to greatly improve the rate of PET glycolysis and the yield of BHET product.8 Catalysts tested have included heterogeneous materials, metal salts, and organocatalysts.8,9 While many of these catalysts often have several benefits, catalysts rarely follow the needed qualities for commercial use, such as fast rates at mild temperatures, low loadings, high yields of BHET, stability to additives and impurities in post-consumer plastics, and recyclability of the catalyst. Recently, volatile organic bases have shown promise, as the catalysts are reasonably stable to impurities and the catalysts can be recycled through sublimation (e.g., the VolCat catalyst that has been commercialized by IBM).10 However, many of these organic bases can be highly toxic and corrosive, and complete separation of the catalyst can be challenging and energy intensive.11
Ionic liquids (ILs), or inorganic salts with low melting points, have emerged as promising catalysts for the glycolysis of PET.12–17 In general, these liquid catalysts often show a bifunctional catalytic behavior in that the cation is proposed to activate the carbonyl for nucleophilic attack, while the anion participates in hydrogen bonding to improve the nucleophilicity of the ethylene glycol, Fig. 1.15 This proposed mechanism is supported by the observation that ionic liquids with more basic anions are more active catalysts than neutral or acidic ILs.3,16 However, the high cost and intensive synthesis were noted as a flaw for long term use.18 Metal-containing ILs (MILs) showed a significant improvement, as the anionic metal fragments were proposed to improve the nucleophilicity of the ethylene glycol similar to basic ILs, with less stability challenges.19–26 Additionally, efforts using iron and cobalt-containing ILs to heterogenize the catalyst and/or use magnets allowed for efficient recovery of the catalyst for reuse.27–30 To date, studies have mostly used imidazolium cations with first row transition metal chloride or acetate salts. Studies have not thoroughly investigated the role of IL cation and the metal containing anion (including the metal ion and ligands bound to the metal ion) for the rate of PET depolymerization or BHET selectivity. Additionally, the often 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ionic liquid to metal salt to isolate these transition metal MILs leads to a high consumption of metal salts, which is not ideal with many of these metals being endangered elements.31
1 ratio of ionic liquid to metal salt to isolate these transition metal MILs leads to a high consumption of metal salts, which is not ideal with many of these metals being endangered elements.31
 | ||
| Fig. 1 Proposed role for the cation [Cat+] and anion ([A]−) in the glycolysis of PET. [Cat] could be imidazolium, phosphonium, etc. | ||
The lanthanide series represents a unique class of metals to probe the desired trends for PET glycolysis. The ionic radius and Lewis acidity subtly change across the series,32 allowing for an understanding of how Lewis acidity and metal ion size impacts PET depolymerization. Additionally, lanthanide metals can allow a wide range of coordination environments,31 in which the ratio of IL to metal ion can be varied. While there are a few reports of lanthanide metal-containing MILs for luminescence and nuclear waste separation,33–35 none have been used for catalytic depolymerization of polymers. We hypothesized that lanthanide-containing MILs could be effective catalysts with a higher ratio of ionic liquids, lowering the endangered metal content for the catalyst without losing the added benefits of MILs over metal-free ILs. Herein we report the use of lanthanide salt/IL combinations to identify trends in PET glycolysis with MILs.
Results and discussion
Initial screening
Initially, lanthanide trichloride salts (LnCl3·xH2O, where Ln = La, Sm, Gd, Y, Tm and x = 6 or 7) were combined with a selection of imidazolium and phosphonium ILs to determine if they would form homogeneous liquids at 180 °C, Fig. 2. These metal choices represent a wide range of lanthanide metals to establish trends in ionic radii and Lewis acidity. While yttrium is a transition metal, it has similar reactivity to the lanthanide series and serves as a diamagnetic representation of a mid-sized lanthanide. While lutetium would represent the smallest lanthanide, it was eliminated from these studies due to the high cost of the metal salts. Additionally, lanthanides that pose a serious supply risk, due to their valuable use for sustainable energy and/or low abundance were not considered for this study.36 The ILs that were tested were selected based on low cost, low melting point, and diversity of cation and anion structure.Since lanthanides can accommodate a wider range of coordination environments than transition metals, the metal salts were combined with the ILs in two different ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and mixed at 180 °C. This is distinct from reported transition metal examples where only one or two equivalent of IL is mixed with the metal precursor. Mixtures that formed a homogeneous viscous liquid, indicative of the formation of a MIL, were selected for glycolysis of PET. Conditions in which the metal salt/IL mixture did not combine to homogeneous liquids were not considered further. Five initial ILs were found to effectively form homogeneous liquids at both ratios with the full range of metal ions studied, Fig. 2. For these reaction conditions, a consistent 5 wt% catalyst loading was used to determine the relevance of the metal salt/IL ratio for PET conversion and BHET selectivity. The EG
6) and mixed at 180 °C. This is distinct from reported transition metal examples where only one or two equivalent of IL is mixed with the metal precursor. Mixtures that formed a homogeneous viscous liquid, indicative of the formation of a MIL, were selected for glycolysis of PET. Conditions in which the metal salt/IL mixture did not combine to homogeneous liquids were not considered further. Five initial ILs were found to effectively form homogeneous liquids at both ratios with the full range of metal ions studied, Fig. 2. For these reaction conditions, a consistent 5 wt% catalyst loading was used to determine the relevance of the metal salt/IL ratio for PET conversion and BHET selectivity. The EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PET weight ratio was kept at 4
PET weight ratio was kept at 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the reaction was stirred for four hours at 180 °C, similar to conditions used for other MILs.25 Commercial PET cold drink lids, containing 25% recycled PET, from Eco-Products were used for most of the studies to gauge the practicality of the MIL catalyst system. PET conversion and BHET yields were calculated from isolated weights of PET and BHET from each reaction, as described in the ESI.† PET that does not break down fully to BHET can be found in the form of oligomers that are soluble in the ethylene glycol, which are easily separated from PET and BHET (see ESI† for characterization). Initial screening data can be seen in Fig. 3 and 4, with tabulated data present in Tables S1 and S2.†
1, and the reaction was stirred for four hours at 180 °C, similar to conditions used for other MILs.25 Commercial PET cold drink lids, containing 25% recycled PET, from Eco-Products were used for most of the studies to gauge the practicality of the MIL catalyst system. PET conversion and BHET yields were calculated from isolated weights of PET and BHET from each reaction, as described in the ESI.† PET that does not break down fully to BHET can be found in the form of oligomers that are soluble in the ethylene glycol, which are easily separated from PET and BHET (see ESI† for characterization). Initial screening data can be seen in Fig. 3 and 4, with tabulated data present in Tables S1 and S2.†
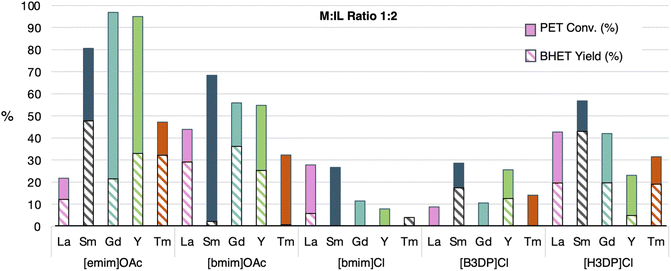
As shown in Fig. 3, reactions with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of LnCl3·xH2O (x = 6, 7) and IL showed that PET conversions of up to 97% could be achieved within the reaction time frame. It was noticeable that ionic liquids containing the acetate anions outperformed those with chloride anions. Phosphonium cations showed to be reasonably active, with longer alkyl substituents ([H3DP]Cl) performing better than those with shorter alkyl substituents ([B3DP]Cl). Alternatively, the imidazolium cation with the shorter substituent ([emim]OAc) performed better than the longer substituent ([bmim]OAc), which could relate to the higher basicity of [emim]OAc.37 The metals with the highest PET conversion varied depending on the ionic liquid used, with a general trend towards the bigger metals. This could be explained by the lowered Lewis acidity of the metal, causing a higher basicity of the anions bound to the metal. Alternatively, the pKa of these salts in aqueous solution, which increases as the metal ion gets bigger, could explain the observed results.38 However, the largest metal, lanthanum, often showed a drop in PET conversions, which may be a consequence of bridging or aggregate structures within the MIL caused by coordinative unsaturation.
2 ratio of LnCl3·xH2O (x = 6, 7) and IL showed that PET conversions of up to 97% could be achieved within the reaction time frame. It was noticeable that ionic liquids containing the acetate anions outperformed those with chloride anions. Phosphonium cations showed to be reasonably active, with longer alkyl substituents ([H3DP]Cl) performing better than those with shorter alkyl substituents ([B3DP]Cl). Alternatively, the imidazolium cation with the shorter substituent ([emim]OAc) performed better than the longer substituent ([bmim]OAc), which could relate to the higher basicity of [emim]OAc.37 The metals with the highest PET conversion varied depending on the ionic liquid used, with a general trend towards the bigger metals. This could be explained by the lowered Lewis acidity of the metal, causing a higher basicity of the anions bound to the metal. Alternatively, the pKa of these salts in aqueous solution, which increases as the metal ion gets bigger, could explain the observed results.38 However, the largest metal, lanthanum, often showed a drop in PET conversions, which may be a consequence of bridging or aggregate structures within the MIL caused by coordinative unsaturation.
BHET yields/selectivity most often followed the same trends as PET conversion, with imidazolium acetate ILs showing the highest BHET yields. There were some noticeable outliers, where Sm and Tm examples showed no production of BHET. The reasons for this erratic catalytic behavior are not entirely clear at the present but could be related to potentially facile variations in the active catalytic species that are primed by minor changes in the reaction conditions.
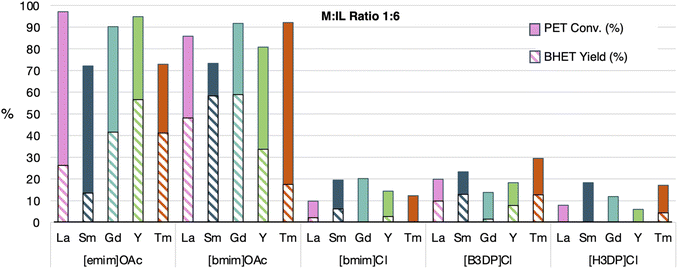
As shown in Fig. 4, reactions with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio of LnCl3·xH2O (x = 6, 7) and IL showed a significant improvement in the PET conversion for the conditions with ILs containing acetate anions, while the conditions with ILs with chloride anions showed a decrease in conversion. While the increase in conversion for acetate-containing ILs can be rationalized by the increased quantity of basic anions, it cannot explain the lower reactivity for chloride-containing ILs. We postulate that the lower conversion for the chloride containing ILs is due to a lowered metal salt content, which may be the main driver for glycolysis in this case. It was also noted that the higher IL ratio generally increased BHET yields for both acetate containing ILs, however, trends based on metal ion identity were less pronounced than in the case with the 1
6 ratio of LnCl3·xH2O (x = 6, 7) and IL showed a significant improvement in the PET conversion for the conditions with ILs containing acetate anions, while the conditions with ILs with chloride anions showed a decrease in conversion. While the increase in conversion for acetate-containing ILs can be rationalized by the increased quantity of basic anions, it cannot explain the lower reactivity for chloride-containing ILs. We postulate that the lower conversion for the chloride containing ILs is due to a lowered metal salt content, which may be the main driver for glycolysis in this case. It was also noted that the higher IL ratio generally increased BHET yields for both acetate containing ILs, however, trends based on metal ion identity were less pronounced than in the case with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. Interestingly, the largest (La) and smallest (Tm) metals performed much better with the 1
2 ratio. Interestingly, the largest (La) and smallest (Tm) metals performed much better with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio for PET conversion than they did with 1
6 ratio for PET conversion than they did with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. In this case, Sm and Tm continued to show erratic BHET yield, depending on the ionic liquid used. The indication that a higher ratio of IL to metal salt showing more effective rates of depolymerization suggests that the metal salt content can be decreased, improving the sustainability of these MIL catalysts.
2. In this case, Sm and Tm continued to show erratic BHET yield, depending on the ionic liquid used. The indication that a higher ratio of IL to metal salt showing more effective rates of depolymerization suggests that the metal salt content can be decreased, improving the sustainability of these MIL catalysts.
Based on these initial screenings, yttrium and gadolinium were identified to be the most promising metals to pursue further, as both showed high PET conversions with both ratios of metal salt to IL. However, yttrium is a much lighter metal than the rest of the lanthanides, which meant a higher mol% could be used for that metal. Since this could be one of the reasons for the high PET conversions, we chose to pursue further studies primarily with Gd. In some cases, lanthanum was used as an example that performed better with the high IL ratio. The imidazolium-based ILs were exclusively pursued further, as the acetate salts showed the highest impact on PET conversion and BHET yield. The next goal was to identify which reaction variables impacted the catalyst activity to optimize the MIL-catalyzed glycolysis of PET.
Cooperativity
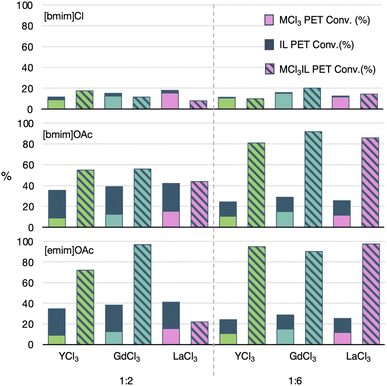
To show the value of the MIL over a metal-free IL, evidence for cooperativity between the metal salt and ionic liquid is important. First, control studies were conducted to confirm the cooperative catalysis from the MIL with [emim]OAc, [bmim]OAc and [bmim]Cl. These studies were performed with Gd, Y and La trichloride salts (LnCl3·xH2O, x = 6, 7) to identify if there were any distinctions between the lighter metal (Y), the most active catalyst (Gd) and the metal with more activity at a higher IL loading (La). Control reactions with just the IL and just the metal salt were conducted, and the conversions were added for comparison with that of the generated MIL. These controls were all carried out at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 conditions screened above (with MIL reactions maintaining 5 wt%). In the case of the metal salt control reactions, only one set of reactions were executed, with the weight of the metal salt matching weights used for [bmim]OAc IL. Since the metal salt weights for [emim]OAc and [bmim]Cl conditions were within 10% of the weights for [bmim]OAc, we did not expand the control reactions further. Comparisons are shown in Fig. 5 for the PET conversion (Fig. S3† for BHET yield), with detailed information shown in Tables S3–S8.†
6 conditions screened above (with MIL reactions maintaining 5 wt%). In the case of the metal salt control reactions, only one set of reactions were executed, with the weight of the metal salt matching weights used for [bmim]OAc IL. Since the metal salt weights for [emim]OAc and [bmim]Cl conditions were within 10% of the weights for [bmim]OAc, we did not expand the control reactions further. Comparisons are shown in Fig. 5 for the PET conversion (Fig. S3† for BHET yield), with detailed information shown in Tables S3–S8.†
As shown in Fig. 5, all 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reactions with [bmim]Cl were yielded similar PET conversions for control reactions and the MIL reactions. This shows limited cooperativity between the metal chloride salts and the chloride-containing IL. We hypothesize this is due to the less basic Cl anion being less likely to associate strongly with the metal ion, dropping the potential for a cooperative MIL.
2 reactions with [bmim]Cl were yielded similar PET conversions for control reactions and the MIL reactions. This shows limited cooperativity between the metal chloride salts and the chloride-containing IL. We hypothesize this is due to the less basic Cl anion being less likely to associate strongly with the metal ion, dropping the potential for a cooperative MIL.
However, reactions with [bmim]OAc showed significant cooperativity. For the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of metal salt to IL, Gd and Y both showed an approximate 50% improvement in PET conversion in comparison to combined results from the metal salt and IL run individually. In this 1
2 ratio of metal salt to IL, Gd and Y both showed an approximate 50% improvement in PET conversion in comparison to combined results from the metal salt and IL run individually. In this 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 case, the La-containing MIL showed no cooperativity in PET conversion. Extending to [emim]OAc showed an even larger cooperativity for Gd and Y with an approximate 100% improvement for the MIL over the controls. However, the La case showed a much lower conversion than the controls, consistent with the premise of aggregate formation that decreases acetate basicity. Alternatively, chelating coordination to an unsaturated La center could also lead to a decreased basicity of the acetate anions.
2 case, the La-containing MIL showed no cooperativity in PET conversion. Extending to [emim]OAc showed an even larger cooperativity for Gd and Y with an approximate 100% improvement for the MIL over the controls. However, the La case showed a much lower conversion than the controls, consistent with the premise of aggregate formation that decreases acetate basicity. Alternatively, chelating coordination to an unsaturated La center could also lead to a decreased basicity of the acetate anions.
When switching to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio of metal salt to IL, cooperativity still was not observed in reactions with [bmim]Cl. However, cooperativity was increased significantly for both [bmim]OAc and [emim]OAc for all three metals, with PET conversion increases reaching an approximate 300% increase in comparison to the controls. In this case, we hypothesize the demonstration of cooperativity for La is probably due to coordinative saturation that facilitates stronger metal interactions with the anions from the IL, leading to similar behavior to that of Gd and Y. No significant metal preference is identified for this condition, therefore the use of Gd was maintained. As mentioned previously, this cooperativity demonstrates the benefit of MIL catalysts over metal-free IL catalysts. However, these results also indicate the benefit of the metal can also be maintained with a much higher IL to metal salt ratio than previously described in the literature.
6 ratio of metal salt to IL, cooperativity still was not observed in reactions with [bmim]Cl. However, cooperativity was increased significantly for both [bmim]OAc and [emim]OAc for all three metals, with PET conversion increases reaching an approximate 300% increase in comparison to the controls. In this case, we hypothesize the demonstration of cooperativity for La is probably due to coordinative saturation that facilitates stronger metal interactions with the anions from the IL, leading to similar behavior to that of Gd and Y. No significant metal preference is identified for this condition, therefore the use of Gd was maintained. As mentioned previously, this cooperativity demonstrates the benefit of MIL catalysts over metal-free IL catalysts. However, these results also indicate the benefit of the metal can also be maintained with a much higher IL to metal salt ratio than previously described in the literature.
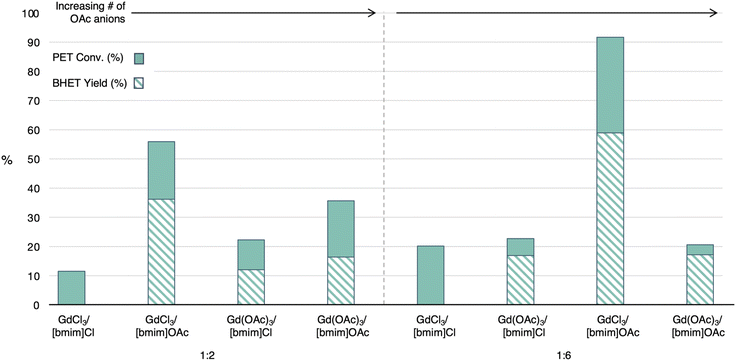
Effects of anion
Since the ILs with acetate anions were found to be the most promising, the studies were expanded to identify how the anion on the metal salt might impact both conversion of PET and selectivity for BHET. The four combinations of [bmim]OAc and [bmim]Cl with GdCl3·6H2O and Gd(OAc)3·2.5H2O allows for a gradual increase in acetate anions over chloride anions to identify the impact of anion on the depolymerization. As mentioned previously, an increase in acetate anions is proposed to increase PET conversion, as the acetate anion is more basic than the chloride anion, which has been proposed to better increase the nucleophilic attack from EG.3 Surprisingly, the expected trend was not observed. Instead, the combination of GdCl3·6H2O and [bmim]OAc showed to be the most active catalyst at both the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio, Fig. 6. Further, the 1
6 ratio, Fig. 6. Further, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 example of Gd(OAc)3·6H2O with [bmim]OAc showed a much higher PET conversion than the 1
2 example of Gd(OAc)3·6H2O with [bmim]OAc showed a much higher PET conversion than the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio, while BHET yield was similar for both conditions.
6 ratio, while BHET yield was similar for both conditions.
To identify the cause of the lowered reactivity of the all-acetate examples, cooperativity studies were also conducted for Ln(OAc)3·xH2O (Ln = Y, Gd and La) with [bmim]Cl, [bmim]OAc, and [emim]OAc combinations, analogous to those done about for the chloride metal salts (Fig. S4 and S5†). Interestingly, these studies showed no strong evidence for cooperativity between the metal acetate salts and the IL for both PET conversion and BHET selectivity. These results could be explained by the lowered Lewis acidity for the metal ions with acetate anions, leading to less interaction of the IL anions with the metal. These results suggest at a polydisperse catalyst speciation in solution that may be contingent on identity, quantity and source of basic anions, which can convolute observations of anticipated trends. In this regard, efforts to more deeply characterize the structures and speciation of the MILs studied herein are currently underway, using guidance from prior literature on lanthanide acetate species.39,40
Effects of metal salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) IL ratio
IL ratio
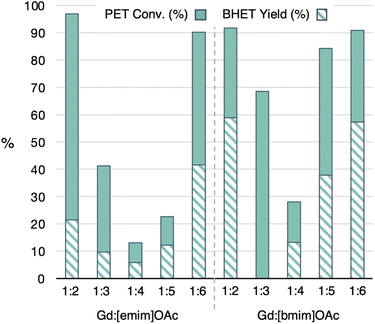
Using Y and Gd chloride salts as the most promising metal ions, the ratio of metal salt to ionic liquid was further varied between the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratios already studied for both [emim]OAc and [bmim]OAc. The wt% of the MIL was kept constant at 5 wt%. For both ILs, the PET conversion and BHET yield were highest at the 1
6 ratios already studied for both [emim]OAc and [bmim]OAc. The wt% of the MIL was kept constant at 5 wt%. For both ILs, the PET conversion and BHET yield were highest at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratios, Fig. 7. In the case of [emim]OAc, examples with 1
6 ratios, Fig. 7. In the case of [emim]OAc, examples with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratios showed a significantly lower catalyst performance. However, with [bmim]OAc, the 1
5 ratios showed a significantly lower catalyst performance. However, with [bmim]OAc, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratios were only just underperforming from the 1
5 ratios were only just underperforming from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratios. While the 1
6 ratios. While the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 metal salt to IL ratio were also the best conditions for Y, trends for the in-between ratios were rather different (Table S14†). These results further hint at possible variations in active catalyst species across different metal to IL ratios that dictate trends in PET conversion and BHET yield. Since analogous comparisons have not been made for transition metal-containing MILs, it is possible that the reactivity of catalysts in the literature can be tuned more.
6 metal salt to IL ratio were also the best conditions for Y, trends for the in-between ratios were rather different (Table S14†). These results further hint at possible variations in active catalyst species across different metal to IL ratios that dictate trends in PET conversion and BHET yield. Since analogous comparisons have not been made for transition metal-containing MILs, it is possible that the reactivity of catalysts in the literature can be tuned more.
Effects of temperature
Different MIL reports have used temperatures ranging from 140 to 190 °C. Using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
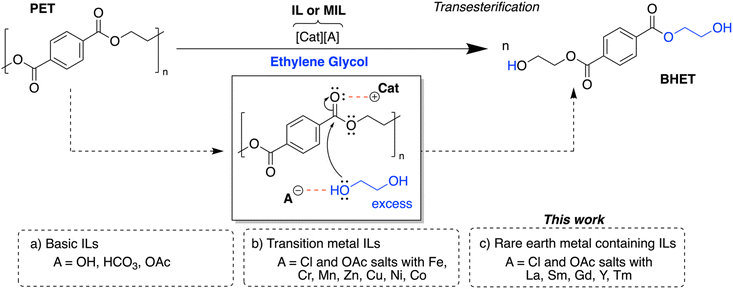
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 combination of GdCl3·6H2O and [emim]OAc, we probed the PET conversion and BHET selectivity at ranging temperatures for the same amount of time. As shown in Fig. 8, PET conversion remains low below 170 °C, with no observed BHET within 4 hours. PET conversion and BHET yield spike at 170 °C and increase again at 180 °C. While higher PET conversion was not seen from 180 °C to 190 °C, a large increase in BHET yield was observed. These results allude to potential pathways of establishing control over PET conversion vs. BHET yield.
6 combination of GdCl3·6H2O and [emim]OAc, we probed the PET conversion and BHET selectivity at ranging temperatures for the same amount of time. As shown in Fig. 8, PET conversion remains low below 170 °C, with no observed BHET within 4 hours. PET conversion and BHET yield spike at 170 °C and increase again at 180 °C. While higher PET conversion was not seen from 180 °C to 190 °C, a large increase in BHET yield was observed. These results allude to potential pathways of establishing control over PET conversion vs. BHET yield.
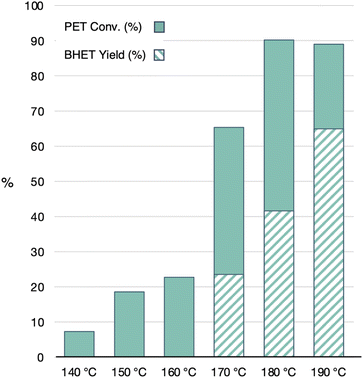 | ||
Fig. 8 PET conversion and BHET yield for GdCl3·6H2O/[emim]OAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio) across varying reaction temperatures. All reactions ran with 3 g of PET for 4 h with 5 wt% catalyst and a 1 6 ratio) across varying reaction temperatures. All reactions ran with 3 g of PET for 4 h with 5 wt% catalyst and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PET 4 PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG ratio. EG ratio. | ||
Effects of EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) PET ratio
PET ratio
Different MIL reports have also used different ratios of EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PET. Again, using 1
PET. Again, using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 combination of GdCl3·6H2O and [emim]OAc, we probed the PET conversion and BHET selectivity as the ratio of EG
6 combination of GdCl3·6H2O and [emim]OAc, we probed the PET conversion and BHET selectivity as the ratio of EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PET is changed, Fig. 9. To adjust to the higher EG volumes, these reactions were carried out in a 350 mL heavy-walled high-pressure flask. It was noted that all conditions showed slightly higher PET conversions than the 4
PET is changed, Fig. 9. To adjust to the higher EG volumes, these reactions were carried out in a 350 mL heavy-walled high-pressure flask. It was noted that all conditions showed slightly higher PET conversions than the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EG
1 EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PET ratio reactions done in a 20 mL vial. However, the PET conversions and BHET yields were rather similar for the ratios ranging from 4
PET ratio reactions done in a 20 mL vial. However, the PET conversions and BHET yields were rather similar for the ratios ranging from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 20
1 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with the slightly optimal condition being the 15
1, with the slightly optimal condition being the 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. This indicates that the EG
1 ratio. This indicates that the EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PET can adjust the results, making it difficult to compare to other reports at different conditions.
PET can adjust the results, making it difficult to compare to other reports at different conditions.
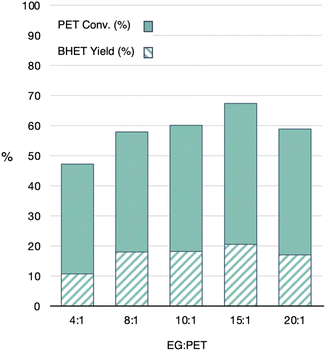 | ||
Fig. 9 PET conversion and BHET yield for GdCl3·6H2O/[emim]OAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio) with varying EG 6 ratio) with varying EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PET ratios. All reactions were done with 3 g of PET at 180 °C for 4 h with 5 wt% catalyst. PET ratios. All reactions were done with 3 g of PET at 180 °C for 4 h with 5 wt% catalyst. | ||
Comparisons to other ILs and MILs
It is a challenge to compare these lanthanide MIL catalysts to the other ionic liquid catalysts in the literature, as each study often uses different conditions. In fact, catalyst loadings have ranged from 1 to 50 wt%, EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PET ratios have ranged from 4
PET ratios have ranged from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, temperatures have ranged from 120 to 190 °C, and reactions were run from 50 min to 24 hours. Additionally, many reports only list the BHET selectivity, and not the yield. We therefore sought out to compare the 1
1, temperatures have ranged from 120 to 190 °C, and reactions were run from 50 min to 24 hours. Additionally, many reports only list the BHET selectivity, and not the yield. We therefore sought out to compare the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 molar ratio of GdCl3·6H2O/[emim]OAc to some of the most active and selective catalysts in the literature at their optimized conditions (Fig. 10).13,23,26,30,41 One important variable that could not be controlled for was the PET source, which can vary dramatically between reports. Additionally, since reaction vessel was found to impact conversion, lack of specific reaction details prevented complete reproduction of reported catalysis reactions. In these comparisons, the 1
6 molar ratio of GdCl3·6H2O/[emim]OAc to some of the most active and selective catalysts in the literature at their optimized conditions (Fig. 10).13,23,26,30,41 One important variable that could not be controlled for was the PET source, which can vary dramatically between reports. Additionally, since reaction vessel was found to impact conversion, lack of specific reaction details prevented complete reproduction of reported catalysis reactions. In these comparisons, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 molar ratio of GdCl3·6H2O/[emim]OAc catalyst combination was used with the PET lids used throughout the study, in which reaction conditions were mimicked as close as possible to the eight literature entries described in Fig. 10. The results of these reactions are shown in dark blue, while the values reported in the literature for the compared catalysts are shown in teal.
6 molar ratio of GdCl3·6H2O/[emim]OAc catalyst combination was used with the PET lids used throughout the study, in which reaction conditions were mimicked as close as possible to the eight literature entries described in Fig. 10. The results of these reactions are shown in dark blue, while the values reported in the literature for the compared catalysts are shown in teal.
 | ||
Fig. 10 PET conversion and BHET yield for GdCl3·6H2O/[emim]OAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio) using literature reaction conditions, compared to literature values. 6 ratio) using literature reaction conditions, compared to literature values. | ||
Interestingly, the Gd-containing MIL did not reach the performance of the reported literature examples when used at their optimized conditions. At its optimized condition however, this catalyst achieves comparable PET conversions and BHET yields to prior catalysts. While the goal of this work was not necessarily to find the best catalyst, it is clear that optimization of conditions for each catalyst dictates the obtained measures of catalytic activity and selectivity. That is, optimized conditions for one catalyst may only show slow catalysis for another. For example, the Gd-containing MIL did not perform as well at the higher catalyst loadings above 5 wt%. However, exhibiting higher performance at lower catalyst loadings would be considered valuable and potentially more sustainable. Therefore, relevant comparisons of catalyst activity should consider both optimized conditions for individual catalysts and conditions reported in the literature.
With several variables at play in this reaction, identifying those most critical for optimization will ultimately be necessary to inform catalyst design principles for PET glycolysis using MILs. In this regard, life cycle assessment and techno-economic assessment would be valuable to guide the establishment of appropriate priorities in route to MIL catalyst optimization. In doing so, attributes of each reaction variable can be weighed against overarching outcomes that define practicality and economic feasibility.
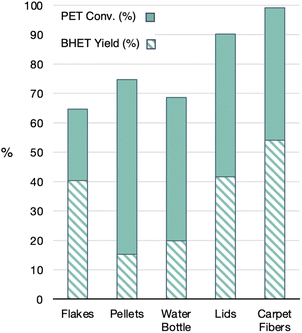
Effects of PET material
As noted in many studies, the starting PET material can have a large impact on the conversion of PET and the selectivity for BHET. With the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio of GdCl3·6H2O and [emim]OAc, the impact on various PET materials was studied, Fig. 11. The materials with the highest plastic thickness, including recycled PET flake, a chopped-up water bottle and melt-processed recycled PET pellets, showed the lowest conversions of PET (65–75%). Interestingly, the flake showed a higher BHET yield than that of the pellets or water bottle. It is unclear what led to this difference between the three materials. Used carpet fibers showed the highest efficiency for glycolysis, even in the presence of dyes and other additives, resulting in full conversion of PET and >50% yield of BHET. These results are consistent with prior literature reports that found surface area of the polymer to be critical for the reaction rates.26 These findings also show proof of concept that the catalysts can maintain their reactivity in the presence of additives. Further, these data validate the need for full disclosure about the types of plastic products used in reported glycolysis studies for relevant comparisons to other works in the literature.
6 ratio of GdCl3·6H2O and [emim]OAc, the impact on various PET materials was studied, Fig. 11. The materials with the highest plastic thickness, including recycled PET flake, a chopped-up water bottle and melt-processed recycled PET pellets, showed the lowest conversions of PET (65–75%). Interestingly, the flake showed a higher BHET yield than that of the pellets or water bottle. It is unclear what led to this difference between the three materials. Used carpet fibers showed the highest efficiency for glycolysis, even in the presence of dyes and other additives, resulting in full conversion of PET and >50% yield of BHET. These results are consistent with prior literature reports that found surface area of the polymer to be critical for the reaction rates.26 These findings also show proof of concept that the catalysts can maintain their reactivity in the presence of additives. Further, these data validate the need for full disclosure about the types of plastic products used in reported glycolysis studies for relevant comparisons to other works in the literature.
Sustainability of the metal salt choice
As shown in Fig. 4, the optimized 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 metal salt to IL ratio showed high conversions for many of the lanthanides. On one hand, Gd represents a highly paramagnetic metal ion that shows one of the lowest supply risks of the lanthanide metals, which could aid in recovery of the MIL after catalysis. While separation and recovery methods have been reported for supported Fe-containing MILs,27,30 the best methods to recover these low metal-loading Gd-based MILs are currently underway.
6 metal salt to IL ratio showed high conversions for many of the lanthanides. On one hand, Gd represents a highly paramagnetic metal ion that shows one of the lowest supply risks of the lanthanide metals, which could aid in recovery of the MIL after catalysis. While separation and recovery methods have been reported for supported Fe-containing MILs,27,30 the best methods to recover these low metal-loading Gd-based MILs are currently underway.
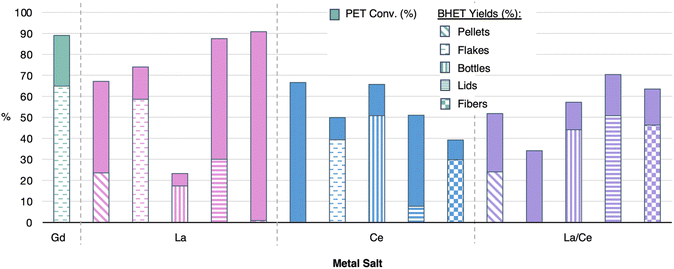
Results above also identify lanthanum as a promising metal ion, specifically when high ratios of IL are used. Since La and Ce represent the most abundant and inexpensive lanthanides, also with the lowest supply risks of the series, identification for their catalytic activity is warranted. Additionally, since La and Ce are difficult to separate, a MCl3·6H2O mixture with La and Ce, donated by a lanthanide metal processing company, that were not separated as oxide precursors was also pursued for PET glycolysis. As shown in Fig. 12, La, Ce and La/Ce mix (estimating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of La
1 ratio of La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce) MILs with a 1
Ce) MILs with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio of metal salt to [emim]OAc were tested for glycolysis of all five PET materials tested in Fig. 11. In this case, a reaction temperature of 190 °C was used, as it showed higher BHET selectivity for Gd, with all other conditions matching that of the Gd reaction shown in Fig. 8.
6 ratio of metal salt to [emim]OAc were tested for glycolysis of all five PET materials tested in Fig. 11. In this case, a reaction temperature of 190 °C was used, as it showed higher BHET selectivity for Gd, with all other conditions matching that of the Gd reaction shown in Fig. 8.
In general, the La MIL showed the highest conversion of PET for all materials tested, except the used plastic bottle which showed the highest PET conversion with Ce. However, BHET yields were very low for most of the materials with the La MIL, with the exception of the recycled flake. Ce MILs showed rather low PET conversions, in comparison to La, but had elevated BHET selectivity for some of the materials. Finally, the La/Ce mix showed lower conversions of PET than La, but showed the best BHET yields of the metals studied. This suggests that lanthanide metal salt mixtures, obtained without separation, could be promising alternatives to any one lanthanide metal in particular. While these results identify lanthanide metal mixtures in the MIL catalyst could lead to another improvement on catalyst sustainability, efforts to identify what mixtures are most effective and how conditions can be optimized for these MILs are currently underway.
Conclusions
Lanthanide metal-containing ionic liquids have been identified as active and tunable catalysts for the glycolysis of PET waste. Imidazolium acetate ionic ligands were found to show high cooperativity with LnCl3·xH2O (x = 6, 7) salts, showing significant improvements in the conversion of PET and the production of BHET in comparison to the sum of each individual component. A high ratio of ionic liquid to metal salt showed the highest degree of cooperativity, providing the benefits of MIL catalysts while minimizing the loading of endangered metals required. In general, catalytic activity for PET conversion and BHET yield were consistently most active for the mid-sized lanthanides, while the large and small, lanthanides only showed high catalytic activity with higher IL ratios. When changing the anion of the IL to chloride or the metal salts to acetate, the cooperativity and catalytic activity significantly drop. These results indicate that while choice of the components, such as the metal ion, metal salt anions, and the IL cation and anion, do make a difference on glycolysis of PET, identifying the combinations that lead to high cooperativity of the metal salt and ionic liquid isn't quite as straight forward.With an optimized MIL, further tuning reaction conditions revealed that reasonable conversions were achieved above 170 °C, with PET conversion and BHET yield generally increasing with temperature. PET conversion and BHET yield did not change much as EG amount was increased, suggesting any increase in rate from higher EG concentrations is countered by dilution of the MIL catalyst. Reactivity is reasonably maintained with different PET materials, including postconsumer recycled products that contain dyes and other additives. However, harsher conditions and longer reaction times are expected to be required for PET materials with low surface area and high density. Comparisons to optimized literature conditions emphasize that optimized conditions for one catalyst won't necessarily match that of another. Finally, La/Ce mixture-based MILs were found to have high BHET yields, encouraging the use of nonseparated or application-recovered lanthanide metal salts for this catalysis. These results show progress in improving the sustainability of MIL catalysts for the glycolysis of PET.
Efforts to optimize these catalysts and address the recycling of the MILs, as well as studies to understand the examples with high cooperativity, are currently underway. Additionally, identifying how MIL cooperativity changes with metal and IL choice will also be important for advances in MIL catalyst design for catalysis in general.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the USC Department of Chemistry and USC Women in Science and Engineering through start-up funds and the USC Wrigley Institute for Environmental Sciences through a 2021 Faculty Innovator Award. C. L. C. thanks the NSF REU program for funding her summer research in the group (CHE-1757942).References
- A. Atta, M. Abdel-Raouf, S. Elsaeed and A. Abdel-Azim, Prog. Org. Coat., 2006, 55, 50–59 CrossRef CAS.
- I. C. McNeill, M. Zulfiqar and T. Kousar, Polym. Degrad. Stab., 1990, 28, 131–151 CrossRef CAS.
- A. B. Raheem, Z. Z. Noor, A. Hassan, M. K. A. Hamid, S. A. Samsudin and A. H. Sabeen, J. Cleaner Prod., 2019, 225, 1052–1064 CrossRef CAS.
- M. Khoonkari, A. H. Haghighi, Y. Sefidbakht, K. Shekoohi and A. Ghaderian, Int. J. Polym. Sci., 2015, 2015, 124524 Search PubMed.
- D. Paszum and T. Spychaj, Ind. Eng. Chem. Res., 1997, 36, 1373–1383 CrossRef.
- L. Bartolome, M. Imran, B. G. Cho, W. A. Al-Masry and D. H. Kim, Recent developments in the chemical recycling of PET, material recycling – trends and perspectives, InTechOpen, London, 2012 Search PubMed.
- D. Carta, G. Cao and C. D'Angeli, Environ. Sci. Pollut. Res., 2003, 10, 390–394 CrossRef CAS PubMed.
- S. C. Kosloski-Oh, Z. A. Wood, Y. Manjarrez, J. P. de los Rios and M. E. Fieser, Mater. Horiz., 2021, 8, 1084–1129 RSC.
- C. Jehanno, M. M. Pérez-Madrigal, J. Demarteau, H. Sardon and A. Dove, Polym. Chem., 2019, 10, 172–186 RSC.
- A. Krishna, From seed to shelf: how IBM innovations will transform every stage of the food supply chain within the next five years, IBM Research Blog, 2019, https://research.ibm.com/blog/ibm-research-5-in-5-2019-food, accessed August 19, 2022 Search PubMed.
- S. Kaabel, J. P. Daniel Therien, C. E. Deschênes, D. Duncan, T. Friščić and K. Auclair, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2026452118 CrossRef CAS PubMed.
- H. Wang, Z. Li, Y. Liu, X. Zhang and S. Zhang, Green Chem., 2009, 11, 1568–1575 RSC.
- Q. F. Yue, C. X. Wang, L. N. Zhang, Y. Ni and Y. X. Jin, Polym. Degrad. Stab., 2011, 96, 399–403 CrossRef CAS.
- A. M. Al-Sabagh, F. Z. Yehia, A. M. F. Eissa, M. E. Moustafa, G. Eshaq, A. M. Rabie and A. E. El Metwally, Egypt. J. Chem., 2014, 57, 267–280 CrossRef.
- Z. Ju, W. Xiao, X. Lu, X. Liu, X. Yao, X. Zhang and S. Zhang, RSC Adv., 2018, 8, 8209–8219 RSC.
- H. Wang, Y. Liu, Z. Li, X. Zhang, S. Zhang and Y. Zhang, Eur. Polym. J., 2009, 45, 1535–1544 CrossRef CAS.
- A. Al-Sabagh, F. Yehia, A. Eissa, M. Moustafa, G. Eshaq, A. Rabie and A. E. El Metwally, Ind. Eng. Chem. Res., 2014, 53, 18443–18451 CrossRef CAS.
- M. A. Alnaqbi, M. A. Mohsin, R. M. Busheer and Y. Haik, J. Appl. Polym. Sci., 2014, 132, 41666 Search PubMed.
- F. Scé, I. Cano, C. Martin, G. Beobide, Ó. Castillo and I. de Pedro, New J. Chem., 2019, 43, 3476–3485 RSC.
- A. M. Al-Sabagh, F. Z. Yehia, A. M. F. Eissa, M. E. Moustafa, G. Eshaq, A. M. Rabie and A. E. El Metwally, Polym. Degrad. Stab., 2014, 110, 364–377 CrossRef CAS.
- C. Shuangjun, S. Weihe, C. Haidong, Z. Hao, Z. Zhenwei and F. Chaonan, J. Therm. Anal. Calorim., 2021, 143, 3489–3497 CrossRef.
- X. Zhou, X. Lu, Q. Wang, M. Zhu and Z. Li, Pure Appl. Chem., 2012, 84, 789–801 CAS.
- Q. Wang, X. Lu, X. Zhu, M. Zhou, H. He and X. Zhang, J. Appl. Polym. Sci., 2013, 129, 3574–3581 CrossRef CAS.
- S. Cot, M. K. Leu, A. Kalamiotis, G. Dimitrakis, V. Sans, I. de Pedro and I. Cano, ChemPlusChem, 2019, 84, 786–793 CrossRef CAS PubMed.
- H. Wang, R. Yan, Z. Li, X. Zhang and S. Zhang, Catal. Commun., 2010, 11, 763–767 CrossRef CAS.
- Q. Wang, Y. Geng, X. Lu and S. Zhang, ACS Sustainable Chem. Eng., 2015, 3, 340–348 CrossRef CAS.
- I. Cano, C. Martin, J. A. Fernandesa, R. W. Lodgea, J. Duponte, F. A. Casado-Carmonaf, R. Lucenaf, S. Cardenasf, V. Sansc and I. de Pedrod, Appl. Catal., B, 2020, 260, 118110 CrossRef CAS.
- T. Wang, C. Shen, G. Yu and X. Chen, Polym. Degrad. Stab., 2021, 194, 109751 CrossRef CAS.
- S. Najafi-Shoaa, M. Barikani, M. Ehsani and M. Ghaffari, Polym. Degrad. Stab., 2021, 192, 109691 CrossRef.
- A. M. Al-Sabagh, F. Z. Yehia, G. Eshaq and A. E. Metwally, Ind. Eng. Chem. Res., 2015, 54, 12474–12481 CrossRef CAS.
- D. Atwood, Sustainability and Endangered Elements, 2012, https://chem.as.uky.edu/datwood/sustainability-endangered-elements, accessed November 22, 2022 Search PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- D. Prodius and A.-V. Mudring, Coord. Chem. Rev., 2018, 363, 1–16 CrossRef CAS.
- N. M. Abbasi, V. R. Zeger, A. Biswas and J. L. Anderson, J. Mol. Liq., 2022, 361, 119530 CrossRef.
- B. Mallick, B. Balke, C. Felser and A.-V. Mudring, Angew. Chem., Int. Ed., 2008, 47, 7635–7638 CrossRef CAS PubMed.
- U.S. Department of Energy, P.I., Critical Materials Strategy, 2010 Search PubMed.
- C. Liu, Y. Li and Y. Hou, Int. J. Chem. Eng., 2018, 2018, 7501659 Search PubMed.
- D. D. Perrin, Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution, Pergamon Press, 1982 Search PubMed.
- A. Nayak, V. Smetana, A.-V. Mudring and R. D. Rogers, Cryst. Growth Des., 2021, 21, 2516–2525 CrossRef CAS.
- V. Smetana, S. Kelley, H. M. Titi, X. Hou, S. F. Tang, A.-V. Mudring and R. D. Rogers, Inorg. Chem., 2020, 59, 818–828 CrossRef CAS PubMed.
- Y. Liu, X. Yao, H. Yao, Q. Zhou, J. Xin, X. Lu and S. Zhang, Green Chem., 2020, 22, 3122–3131 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: General considerations, glycolysis reaction conditions, and detailed reaction data. See DOI: https://doi.org/10.1039/d3su00090g |
| This journal is © The Royal Society of Chemistry 2023 |