Open Access Article
Open Access ArticleCrosslinking strategies in modulating methylcellulose hydrogel properties
Lorenzo
Bonetti
 *a,
Luigi
De Nardo
*a,
Luigi
De Nardo
 ab and
Silvia
Farè
ab and
Silvia
Farè
 *ab
*ab
aDepartment of Chemistry, Materials and Chemical Engineering “G. Natta”, Politecnico di Milano, Piazza Leonardo da Vinci 22, 20133, Milan, Italy. E-mail: lorenzo.bonetti@polimi.it; silvia.fare@polimi.it
bNational Interuniversity Consortium of Materials Science and Technology (INSTM), 50121 Florence, Italy
First published on 11th October 2023
Abstract
Methylcellulose (MC) hydrogels are ideal materials for the design of thermo-responsive platforms capable of exploiting the environment temperature as a driving force to activate their smart transition. However, MC hydrogels usually show reduced stability in an aqueous environment and low mechanical properties, limiting their applications' breadth. A possible approach intended to overcome these limitations is chemical crosslinking, which represents a simple yet effective strategy to modify the MC hydrogels’ properties (e.g., physicochemical, mechanical, and biological). In this regard, understanding the selected crosslinking method's role in modulating the MC hydrogels’ properties is a key factor in their design. This review offers a perspective on the main MC chemical crosslinking approaches reported in the literature. Three main categories can be distinguished: (i) small molecule crosslinkers, (ii) crosslinking by high-energy radiation, and (iii) crosslinking via MC chemical modification. The advantages and limitations of each approach are elucidated, and special consideration is paid to the thermo-responsive properties after crosslinking towards the development of MC hydrogels with enhanced physical stability and mechanical performance, preserving the thermo-responsive behavior.
1. Introduction
1.1. Methylcellulose
Methylcellulose (MC) is one of the main non-ionic alkyl ethers of cellulose obtained by the partial substitution of the hydroxyl groups of cellulose with methoxy groups (Fig. 1).1,2 In this regard, the average number of hydroxyl groups substituted in each anhydroglucose unit (AGU) of cellulose is defined as the degree of substitution (DS), ranging from 0 for unsubstituted cellulose to 3 for fully substituted cellulose.3 Commercial MC is commonly obtained via a heterogenous process involving the dissolution of cellulose in an alkaline solution (NaOH) followed by the reaction with halogenated alkanes (e.g., CH3Cl) to achieve partial –OH substitution. Since the amorphous regions of cellulose chains are more receptive to methylation than the crystalline regions, MC obtained under heterogeneous conditions generally shows an uneven distribution of substituents along the macromolecular backbone (i.e., a variable DS).1,3,4 On a lab scale, homogeneously substituted MC has been obtained through different techniques, e.g., cellulose-selective solvents, multi-step methylation processes, and –OH capping groups to regio-selectively substitute methyl groups at C2, C3, or C6 positions.4 | ||
| Fig. 1 Chemical structure of MC. The three reactive hydroxyl groups of each AGU (at C2, C3, and C6 positions) are partially substituted with –CH3 groups (R = H or CH3). | ||
Independent of the substitution process, the disruption of intra- and inter-chain hydrogen bonds among the substituted cellulose chains is accountable for the water solubility of MC. In this regard, water solubility occurs for DS = 1.7–2.2 in commercial MC4,5 and for DS ∼ 0.9 in MC obtained under homogeneous conditions. MC's water solubility and viscosity allowed its use in several industrial fields, such as adhesives, solution thickeners, and binder agents, e.g., in food, cosmetic, and pharmaceutical industries.6
1.2. MC physical gelation
The partial methoxy substitution process is accountable for the thermo-responsive character of MC in water solution. MC solutions display a lower critical solution temperature (LCST) behavior, undergoing reversible sol–gel transition upon temperature increase.3,4 Specifically, the temperature at which this transition occurs is defined as the transition temperature (Tt or Tgel).4Different mechanisms for MC gelation have been proposed in the literature: early studies (i.e., before 2012) included physical crosslinking, micelle formation, and kinetically trapped phase separation.4 In particular, physical crosslinking, which attributes gelation to the formation of inter- and intra-molecular hydrophobic interactions (R1-CH3⋯H3C-R2) among the MC chains, has been the most credited mechanism.3,7–9 According to this theory, at low temperatures (T < Tt), hydrogen bonds between water and the –OH groups of MC dominate. Moreover, water molecules surround the –OCH3 groups of MC, forming water cage-like structures that prevent hydrophobic interactions among MC macromolecules. As a result, MC becomes water-soluble, i.e., a sol state.7,10,11 Upon heating (T > Tt), the previously formed water–polymer interactions and cage-like structures are disrupted, exposing the –CH3 groups of MC.
More recently, new experimental results (e.g., from cryo-TEM, SANS/SAX, and rheology) have shed new light on understanding the mechanism of thermo-induced MC physical gelation involving the formation of stiff fibrils that percolate into a fibrillar network.4,12,13 Upon heating, MC spontaneously self-assembles into fibrils with ∼60% by volume of water and a mean diameter of 15–20 nm, consisting of semicrystalline and amorphous regions (Fig. 2).14,15 Interestingly, the diameter of the fibrils was disclosed to be independent of the MC concentration, the gelation temperature, and the MC molecular weight (Mw). Instead, it has been found that the fibril length is a function of the MC Mw. Thus, lower molecular weight MC forms shorter fibrils, resulting in a less interconnected gel network having a lower gel strength.16
 | ||
| Fig. 2 Representation of the aggregation of MC into the fibrillar structure, composed of semicrystalline and amorphous regions. Reproduced with permission from ref. 13. Copyright 2023 American Chemical Society. | ||
However, the mechanism of MC self-assembly in the fibrillar network is still unclear. Despite the substantial efforts to understand this process, the exact mechanism of fibril nucleation and growth and the origin of the reproducible fibril diameter still need to be clarified. In this regard, computational modeling addressing the gelation process (i.e., fibrillar network structures, dimensions, and formation) also failed to account for the key experimental observations.4,12
Independent of the gelation mechanism, MC lends itself well to the design of responsive systems and devices due to its thermo-responsive nature and the easy tuning of its transition temperature. This last aspect is critical since pristine MC hydrogels (usually 2% w/v MC solution) display a Tt ∼ 55–60 °C, making them non-reactive to some thermal stimuli (e.g., body temperature). In this regard, it is possible to act on different parameters to fine-tune the Tt of MC, mainly the DS, the MC concentration, and the presence of ions or additives (e.g., polymers, sugars, and sugar alcohols) in solution.3,4 This is particularly interesting for biomedical applications, in which the body temperature can activate the sol–gel transition of MC hydrogels. In this regard, we have recently reported on the state-of-the-art of possible applications of thermo-responsive MC hydrogels in the biomedical area,3 identifying three prominent families of applications: (i) in situ gelling systems, (ii) 3D (bio)printing, and (iii) smart culture surfaces.
MC is a highly versatile polymer. Owing to the reversibility of the fibril formation process during MC physical gelation, MC hydrogels possess some distinctive properties, e.g., reversibility of the sol–gel transition, reduced cytotoxicity (due to the absence of chemical crosslinkers), and low costs.1,17
In this regard, the reversible nature of the physical gelation process can be smartly exploited, e.g., when MC is used as a “non-gelling” – or a sacrificial – component in different bioprinting approaches,18–21 and its selective dissolution is achieved simply by lowering the temperature.
However, it is clear that the formed bonds are weaker than those that could be obtained via chemical crosslinking, and the degree of crosslinking is usually lower than that of chemically crosslinked hydrogels.22 Consequently, non-crosslinked MC hydrogels typically suffer from limited stability in aqueous environment and reduced mechanical performance, restricting the breadth of their possible applications.23 In this regard, MC gels usually dissolve rapidly in an aqueous medium, reaching a gel fraction value of ∼50%24 at their swelling equilibrium (usually within 24 h) and an almost complete dissolution within 28 days.25
Thus, when MC is used alone or stable and tough gels are needed, chemical crosslinking can be considered an attractive strategy to overcome the above-mentioned limitations.
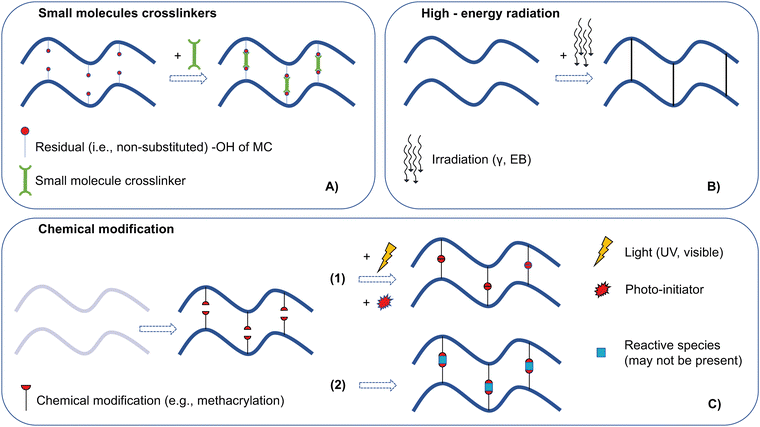
2. MC chemical crosslinking
Crosslinking technology is an area of significant importance in the design of hydrogel systems. Crosslinks are physical or chemical bonds among the functional groups (e.g., –OH, –NH2, and –COOH) of different polymer chains.26 Chemical crosslinking of MC is the most diffused and effective approach to overcome its physical and mechanical restrains.3,25,27 Chemical crosslinking can strongly influence the mechanical (e.g., tensile/compressive strength and stiffness), thermal (e.g., performance at high temperatures), biological (e.g., cell–material interactions), and physical (e.g., chemical and enzymatic degradation) properties of MC gels.26Hereafter, the main crosslinking approaches for MC (Fig. 3), each one presenting specific advantages and limitations (Table 1), will be described, classifying them as (i) small molecule crosslinkers, (ii) crosslinking by high-energy radiation, and (iii) crosslinking by MC chemical modification. For each crosslinking method, the envisaged applications will be described (Table 2). Particular attention will be paid to assessing the thermo-responsive properties of MC after crosslinking. It is well known that chemical crosslinking, regardless of the selected approach, can negatively affect the thermo-responsive behavior of the crosslinked hydrogel.3,25
| Crosslinker | Chemical structure | Concentration/dosage | Advantages | Disadvantages | Ref. | ||
|---|---|---|---|---|---|---|---|
| Small molecule crosslinkers | Glutaraldehyde (GA) |
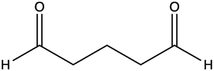
|
Films: 0.05–20 wt% (wGA/wpolymer) | Inexpensive, high crosslinking degree, effective for different polymers | High cytotoxicity, need of low pH, need of washing step, yellowing of polymer | 27, 31, 32 and 84–86 | |
| Microspheres: 2.5–10% (vGA/vparaffin), liquid paraffin bath*, *starting from a 25% (v/v) GA solution | 33–37 | ||||||
| Divinyl sulfone (DVS) |

|
1.2 wt% (wDVS/wpolymer) | Inexpensive, reactivity, effective for different polymers | Need of high pH, cytotoxicity at high concentration | 22 | ||
| Hexamethylene diisocyanate (HDI) |

|
0.2 NCO/OH ratio (mol/mol) | Reactivity, effective for different polymers | Need of high temperatures (70 °C), urea formation, need of a catalyst | 87 | ||
| N,N′-Methylene-bis-acrylamide (MBA) |
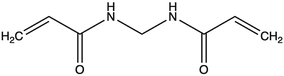
|
0.25–10 wt% (wMBA/wpolymer) | Mild conditions (T, pH), effective for different polymers | γ-rays as initiator, need of washing step | 88 | ||
| N,N′-Carbonyldiimidazole (CDI) |

|
50–150 wt% (wCDI/wpolymer) | Mild conditions (T, pH), effective for different polymers | Need of washing step | 89 | ||
| Sodium trimetaphosphate (STMP) |
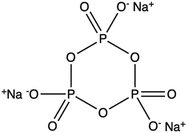
|
Immersion in STMP water solution: 0.1–0.5 wt% (wSTMP/wwater) | Non-toxic, mild conditions (T, pH), effective for different polymers | Long crosslinking time | 90 | ||
| Citric acid (CA) |

|
1–5 wt% (wCA/wpolymer) | Inexpensive, biocompatible, effective for different polymers, self-mineralization ability | Need of high temperatures (>165 °C), need of a catalyst | 25, 47 and 50 | ||
| Irradiation-based crosslinking | γ or EB irradiation | 5–200 kGy | Chemical-free, mild conditions (T, pH), effective for different polymers | Irradiation instrumentation, degradation by scission in the main chain | 27, 54, 55 and 91 | ||
| Photo-crosslinking | UV light | MC-MA + UV: λ = 365–368 nm, I = 2400 mW cm−2, t = 10–20 min + Irgacure 2959 | Fast, localized, mild conditions (T, pH) | Chemical modification required (time-consuming), photo-initiator required, possible DNA damage (UV light) | 66 | ||
| MC-allyl + UV: λ = 365 nm, t = 20 min + Irgacure 2959 | 67 | ||||||
| MC-NB + UV: λ = 365 nm, 5 mW cm−2, t = 2 min + LAP (and PEG4SH) | 69 | ||||||
| Visible light | MC-Try + visible light: λ = 440 nm, I = 2500 mW cm−2, t = 2 min + riboflavin (and SPS as co-initiator) | 68 | |||||
| Chemical modification | APS/AA |

|
MC-MA + APS: 5.7–11.4 wt% (wAPS/wpolymer) + AA: 4.4–8.8 wt% (wAA/wpolymer) | Physiological conditions, no need of a catalyst, in situ crosslinking | Chemical modification required (time-consuming) | 57 | |
| APS/TEMED |

|
MC-MA + APS: 5.7–11.4 wt% (wAPS/wpolymer) + TEMED: 2.9–5.8 wt% (wTEMED/wpolymer) | 58 | ||||
| PEGMI2 |

|
MC-SH + PEGMI2: 0.25–1.25 maleimide/thiol molar ratio (mol/mol) | 60 | ||||
| — | — | MC-NB + MC-DTP-Tz | 62 | ||||
| Crosslinking approaches | Crosslinkers | MC properties | Physical state | Applications | Ref. | |
|---|---|---|---|---|---|---|
| [MC] | Viscosity (cP) or Mw | |||||
| Small molecule crosslinkers | GA | 1% (w/v) | — | Film | Food packaging | 85 and 86 |
| 10–30% (w/v) | — | Microspheres | Drug delivery | 33–37 | ||
| 2% (w/w) | — | 34 | ||||
| 2% (w/w) | — | 35 | ||||
| 10–20% (w/w) | 350–550 | 36 | ||||
| 10–30% (w/w) | 350–550 | 37 | ||||
| 2% (w/v) | — | 92 | ||||
| DVS | 10% (w/v) | 3000–5500 | Gel | Thermo-responsive gels | 22 | |
| HDI | 0.1–4% (w/w) | 100, 400, 1500, 4000 | 87 | |||
| MBA | 4–40% (w/w) | 3000–5500 | Superabsorbent gels | 88 | ||
| CDI | 80% (w/w) | — | Scaffold | Bone tissue engineering | 89 | |
| STMP | 3% (w/w) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Film | Food packaging | 90 | |
| CA | 8% | 3000–5500 | Gel | Smart culture surfaces | 47 | |
| 8% | 3000–5500 | Smart drug delivery | 50 | |||
| Irradiation-based crosslinking | γ or EB rays | 5–40% (w/w) | 3000–5500 | Superabsorbent gels | 24 | |
| Photo-crosslinking | UV | 2–6% (w/w) | ∼15 (Mw = 15 kDa) | Soft tissue regeneration/augmentation | 66 | |
| 3% (w/v) | 1500 | Gel polymer electrolyte | 80 | |||
| 6–8% (w/w) | 15 | Bioink | 69 | |||
| Visible light | 8% (w/w) | 15 | Bioink | 68 | ||
| Chemical modification | APS/AA | 2–4% (w/v) | ∼15 (Mw = 14 kDa) | Soft tissue regeneration/augmentation | 57 | |
| APS/TEMED | 2–4% (w/v) | ∼15 (Mw = 14 kDa) | 58 | |||
| PEGMI2 | 5% (w/w) | (Mw = 300 kDa) | Drug delivery | 60 | ||
| — (MC-NB + MC-DTP-Tz) | 1% (w/v) | (Mw = 300 kDa) | Cell/drug delivery | 62 | ||
2.1. Small molecule crosslinkers
Small molecule crosslinkers (Table 1) are molecules involved in the crosslinking process and generally incorporated into the polymer network (i.e., non-zero length type crosslinkers).26 Hereunder, some of the most widely investigated small molecule crosslinkers for MC crosslinking will be described. | ||
| Fig. 4 Aldehyde-based crosslinking of water-soluble polymers with hydroxyl groups. R = polymer chains and X = spacer (e.g. (CH2)3 for glutaraldehyde). | ||
GA has been demonstrated to be an effective way to tune the physicochemical and mechanical properties of MC27,31 and has been mainly employed in preparing crosslinked MC films and microspheres.
MC films have been produced by adding GA directly into the polymer solution before casting it on a plate to obtain the crosslinked film. Acidic conditions are needed to achieve crosslinking to protonate the oxygen atoms of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of GA, promoting the formation of acetal bridges (Fig. 4). Thus, HCl (0.1–1 M) is usually added as a catalyst in the polymer solution with GA.31,32 Increasing amounts of GA (2.5–7.5 wGA/wMC) reduce the water uptake of 1% w/v MC films (swelling ratio: 3.16 ± 0.29 vs. 7.07 ± 0.25 for 7.5 and 2.5 wGA/wMC, respectively) as a consequence of the chemical crosslinking among the –OH groups and the subsequent reduction in MC chain flexibility and mobility. GA has also been shown to affect the mechanical properties of MC samples. Increasing amounts of GA cause an increase in the tensile strength (
O bonds of GA, promoting the formation of acetal bridges (Fig. 4). Thus, HCl (0.1–1 M) is usually added as a catalyst in the polymer solution with GA.31,32 Increasing amounts of GA (2.5–7.5 wGA/wMC) reduce the water uptake of 1% w/v MC films (swelling ratio: 3.16 ± 0.29 vs. 7.07 ± 0.25 for 7.5 and 2.5 wGA/wMC, respectively) as a consequence of the chemical crosslinking among the –OH groups and the subsequent reduction in MC chain flexibility and mobility. GA has also been shown to affect the mechanical properties of MC samples. Increasing amounts of GA cause an increase in the tensile strength (![[small sigma, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d2.gif) = 67.3 vs. 74.7 MPa for 0 and 1.7 × 10−2 mol L−1 of GA, respectively) and a reduction in the strain at break (
= 67.3 vs. 74.7 MPa for 0 and 1.7 × 10−2 mol L−1 of GA, respectively) and a reduction in the strain at break (![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c6.gif) = 1.6 vs. 0.8% for 0 and 1.7 × 10−2 mol L−1 of GA, respectively) of 5% w/v MC films under dry conditions (T = 100 °C, 3 h).31
= 1.6 vs. 0.8% for 0 and 1.7 × 10−2 mol L−1 of GA, respectively) of 5% w/v MC films under dry conditions (T = 100 °C, 3 h).31
MC microspheres based on MC have been obtained using the water-in-oil emulsion method by adding GA and HCl in the oil phase to achieve crosslinking (crosslinking time range: 30 min to 3 h, room temperature). In this regard, MC has always been used in a blend with other polymers like alginate,33 chitosan,34–36 and polyvinyl alcohol (PVA)37 for the controlled release of drugs. Blending was selected to modify the blend's physicochemical properties (swelling and degradation) or provide additional functionalities (e.g., pH responsiveness and antibacterial activity for chitosan). GA-based crosslinking proved to be an effective way to achieve a sustained release of drugs for the microspheres (i.e., the slower drug release was observed at higher amounts of GA).
One major drawback of GA is the high cytotoxicity of the aldehyde groups, which have also been reported to cause severe inflammation.38 Although the studies on MC hydrogels crosslinked with GA do not assess the possible cytotoxic effects of the crosslinked gels, when GA was used to crosslink other biopolymers (e.g., collagen), the obtained specimens, when tested as prepared, showed poor biocompatibility, with poor cell attachment, poor spreading, and high levels of apoptosis.39 A possible strategy used in the literature to quench GA cytotoxicity is to achieve an adequate purification step of the samples after crosslinking, like using solutions containing free amine groups or amino acids like glycine.26 However, residual (not quenched) GA can still be present in the samples after purification, and even low amounts can lead to cell-growth inhibition.30 Thus, although GA displays high crosslinking efficacy, the fact that it can cause cell toxicity (and biohazard problems not covered in this review) has limited its extensive use in commercial products.26
In the studies mentioned above,40,41 the thermo-responsive character of the crosslinked samples was not considered. In this regard, it has been demonstrated how chemical crosslinking (even with crosslinking agents different from GA) of other thermo-responsive hydrogels, such as gelatin and poly-N-isopropylacrylamide (PNIPAAm), can lead to shifts in their transition temperature or the loss of their thermo-responsive character. However, only a few studies have been focused on assessing the preservation of the thermo-responsive character of MC hydrogels after chemical crosslinking. Indeed, the optimal tuning of the chemical crosslinking can be regarded as a successful strategy to preserve the thermo-responsive behavior of MC hydrogels.25
Cellulose derivatives (e.g., MC, hydroxypropyl methylcellulose (HPMC), hydroxyethyl cellulose (HEC), and carboxymethyl cellulose (CMC)) can be crosslinked with DVS.22,44,45 Such cellulose derivatives differ from MC in the substituent group at C2, C3, or C6 positions, which is a methyl group for MC. The reader is referred to ref. 3 for further information on the specific characteristics of other cellulose derivatives. In an alkaline environment (pH ∼ 12), DVS reacts with either the –OH groups on the backbone or the unsubstituted –OH groups (Fig. 5) of cellulose derivatives, saturating the DVS carbon–carbon double bond.44
Despite MC being crosslinked using DVS (pH = 12, crosslinking time 24 h, room temperature), no characterization other than quantifying the crosslinking density of the obtained gel has been carried out.22 However, HPMC, a thermo-responsive cellulose derivative similar to MC, has been crosslinked with DVS.22,45 As expected, the swelling degree of the HPMC gels has been reported to depend on the concentration of DVS (i.e., on the crosslinking degree of the obtained hydrogel). When DVS was used to crosslink HPMC gel microspheres (20 wt%), the swelling degree of the crosslinked HPMC samples decreased by increasing the amount of DVS (swelling degree (Q) at 25 °C): ∼60 vs. ∼20 for 0.012 and 0.023 gDVS/gHPMC, respectively), confirming the reduction in HPMC chain flexibility and mobility in the more crosslinked samples. Differences in the swelling degree were not detected at higher temperatures (T > Tt ∼ 65 °C) where the gels collapsed (i.e., gel state). Interestingly, detecting the Tt of the tested samples for the DVS ranges used in this work (0.012–0.023 gDVS/gHPMC) was also possible.45 Thus, despite a significant variation in the extent of swelling, the thermo-responsive character of the samples was preserved. A higher amount of DVS was not tested, but further crosslinking would have impaired the hydrogels’ thermo-responsiveness. Similar behavior was observed by Harsh et al.,22 whose work revealed that increasing amounts of DVS (0.006–0.05 gDVS/gHPMC) caused a slight increase in the Tt (54 to 62 °C) of crosslinked HPMC gels.
No studies describe the possible cytotoxic effects caused by MC gels crosslinked with DVS. However, the cytotoxicity of DVS has been assessed on DVS-crosslinked hyaluronic acid (HA) films.43 Cytotoxicity was found to be dependent on the concentration of DVS. Specifically, using a DVS bath to crosslink HA films was shown to cause noticeable cytotoxicity on the human epithelial cell line (ARPE-19 cells) when DVS concentrations higher than 100 mM were used.43 Unlike GA, no information about possible purification steps of crosslinked samples was found in the literature for DVS.
We have recently reported citric acid (CA) as an effective alternative to conventional crosslinkers for MC due to its low cost, low toxicity, and crosslinking efficacy.25,47,48 We successfully crosslinked MC hydrogels via an esterification-based reaction: at high temperatures (T > 165 °C), CA forms cyclic anhydrides, which esterify the reactive –OH groups of MC (Fig. 6). When di-esters arise between two different MC chains (i.e., inter-molecularly), this reaction leads to crosslinking.25 In this process, phosphorous-containing salts (e.g., NaH2PO4) increase the CA-based crosslinking efficiency of MC and other cellulose derivatives.25,49
 | ||
| Fig. 6 CA-based crosslinking of MC: at high temperatures (T > 165 °C), CA forms cyclic anhydrides which esterify MC's reactive –OH groups. R1 and R2 = AGUs of two different MC chains. | ||
MC crosslinking with CA is finely tunable by accurately selecting the crosslinker concentration (1–5% wCA/wMC), the crosslinking temperature (165–190 °C), and the crosslinking time (1–15 min). Consequently, the swelling rate of 8% w/v MC films changed from ∼5000% to less than 1000% under low and high crosslinking conditions, respectively. Accordingly, both the viscoelastic properties (G′ ∼ 10 Pa vs. 105 Pa, at T = 25 °C, for non-crosslinked and highly crosslinked MC specimens25) and the mechanical properties (E ∼ 5 kPa vs. 3.5 MPa for non-crosslinked and highly crosslinked MC specimens47) of MC hydrogels changed as a function of the crosslinking extent. Interestingly, we optimally crosslinked MC hydrogels, achieving a trade-off between increased mechanical properties and preserved thermo-responsive behavior.25 In this regard, optimally crosslinked MC gels displayed reduced swelling, extended stability in a water environment at a physiological temperature, and increased the rheological and mechanical properties compared with non-crosslinked control gels.25,47,50 Moreover, no significant shifts were detected in the Tt of the MC gels after crosslinking under optimized conditions.25 This can be considered a positive outcome, as no further optimization of the gel formulation was needed (e.g., to restore the Tt close to body temperature). Such an approach allowed the crosslinked responsive MC hydrogel platform design for cell sheet engineering47 and drug delivery.50 Lastly, unlike GA, CA was disclosed not to cause any cytotoxic effect, even at high (5% wCA/wMC) concentrations.47 These results align with other literature studies where CA amounts up to 20% (wCA/wpolymer) have been explored for crosslinking different polymers (e.g., PVA/starch51 and collagen52). Nevertheless, a possible disadvantage of using CA as the crosslinking agent is the need for high temperatures to achieve crosslinking. This limits the breadth of the potential application of this crosslinking approach. For instance, embedding cells or thermosensitive molecules (e.g., drugs or biomolecules) before crosslinking is prevented.
2.2. Crosslinking by high-energy radiation
A crosslinking method that uses no chemicals to achieve crosslinking involves applying high-energy radiation, i.e., gamma (γ) and electron beam (EB) radiations (Fig. 7). High-energy radiation has been used to crosslink water-soluble cellulose derivatives (e.g., MC, CMC, HPMC, and HEC) and other polysaccharide derivatives (e.g., carboxymethyl chitin and carboxymethyl chitosan).53 | ||
| Fig. 7 (A) Irradiation of MC results in the dehydrogenation of methylene groups. (B) Crosslinking between two MC chains occurs through the engagement of radicals on the substituent. | ||
MC has been crosslinked by exposing paste-like (i.e., highly concentrated: 5–40% w/w) MC solutions to either EB or gamma irradiation. During irradiation, water radiolysis creates free radicals interacting with MC side groups (Fig. 7A). Then, these radicals form covalent bonds (Fig. 7B) among MC chains, leading to crosslinking.54 In this regard, the properties of the crosslinked gels (e.g., swelling and degradation) have been demonstrated to depend on the concentration of MC and the radiation dose.24
Fig. 8A displays a representative gel fraction vs. the irradiation dose relationship for MC hydrogels crosslinked by EB and gamma rays. EB irradiation (open points) results in higher gel fractions than γ-rays (solid points), evidencing that the number of radicals formed by EB irradiation is higher than for γ irradiation. Moreover, independently from the irradiation source, the gel fraction results higher in MC with a higher molecular weight. This is caused by the fact that longer macromolecules are more prone to bond with each other.55 As expected, irradiation crosslinking has also been shown to affect the water uptake of crosslinked MC hydrogels. An increase in the crosslinking density leads to a decrease in the water absorption ability of MC hydrogels. Fig. 8B displays representative swelling vs. the irradiation dose relationship for MC and HEC hydrogels exposed to γ-irradiation. The swelling degree for MC samples decreases by increasing the dose of γ-irradiation. A different behavior can be observed for HEC hydrogels, where the swelling degree first decreases (γ-irradiation dose < 20–30 kGy) as a function of the irradiation dose and then increases (γ-irradiation dose > 30 kGy). This behavior can be explained by the scission that occurs in the HEC polymeric backbone during exposure to high irradiation doses, which reduces the degree of polymerization of the polymer itself.55 It should be noted that two main effects occur after exposure to high energy radiation: (i) scission of the main polymer chain (i.e., in the glycosidic bonds) with the consequent decrease of the molecular weight of the polymer, and (ii) crosslinking, which leads to the formation of an insoluble material.27,54
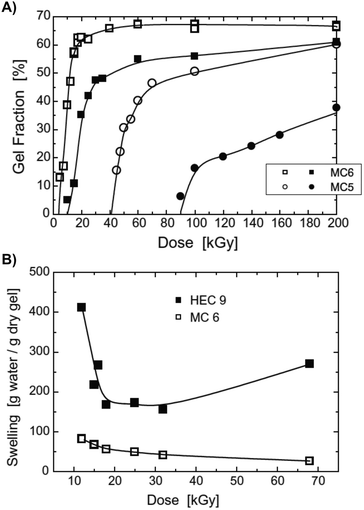 | ||
| Fig. 8 (A) Influence of irradiation (γ or EB) dose on the gel fraction of MC hydrogels. γ-rays (solid points) were applied to a 25% MC paste-like solution, while EB irradiation (open points) was applied to a 20% MC paste-like solution. MC6 (Mw = 14 × 105 Da) and MC5 (Mw = 1.2 × 105 Da). (B) Swelling of 20% MC and HEC hydrogels as a function of the γ-rays dose. Reproduced with permission from ref. 55. Copyright 2023 Elsevier. | ||
Scission is a significant limitation when dealing with high-energy radiation crosslinking since it competes against forming new covalent bonds in the crosslinked hydrogel. The trade-off between these two factors is the only way to obtain crosslinked hydrogels with the desired physical properties.55 Scission has been shown to dominate in low-concentrated MC aqueous solutions (i.e., <10% w/w).56 MC hydrogels generally used in the biomedical field are typically prepared at concentrations lower than 10% w/v (with few exceptions of concentrations up to 12–14% w/v3). Studies exploiting high-energy radiation crosslinking for MC hydrogels in this area are probably limited by the predominant scission process. Moreover, a significant limitation related to irradiation-based crosslinking is that high-energy radiations prevent any possible loading of cells before crosslinking. In such circumstances, high-energy irradiation should be avoided, and chemical crosslinking based on small molecules (Section 2.1) or photo-crosslinking (Section 2.3.1) should be preferred.
The studies mentioned above did not consider the crosslinked samples' thermo-responsive character. The main objective of these studies was to evaluate the crosslinking parameters (e.g., concentration and irradiation dose) for the EB and γ irradiation-based crosslinking of cellulose derivatives (MC and HEC).
2.3. Crosslinking by MC chemical modification
As other natural polymers (e.g., gelatin and hyaluronic acid), MC can be modified with functional groups that enable the covalent crosslinking of MC macromolecules. Three main MC modifications have been developed for crosslinking: (i) methacrylation, (ii) thiolation, and (iii) norbornene/methyl phenyl tetrazine functionalization.In the first case, MC was functionalized with methacrylate groups (MC-MA, Fig. 9A) via esterification of the non-substituted hydroxyl groups by reacting a MC solution with methacrylic anhydride in 20-fold excess (24 h, 4 °C, pH = 8).57 Crosslinking has been obtained using redox initiation systems (e.g., ammonium persulfate (APS)–ascorbic acid (AA)57 (crosslinking time 15–20 min) or APS – N,N,N′,N′-tetramethylethylenediamine (TEMED)58 (crosslinking time 15 min)).
Thiolated methylcellulose (MC-SH) was obtained in two steps: first, carboxylated methylcellulose (MC-CO2H) was obtained with the Williamson ether synthesis by reacting MC with sodium hydroxide and bromoacetic acid (3 h, 4 °C). Then, MC-CO2H was reacted with 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide (EDC) and 3,3′-dithiobis(propionic dihydrazide) (DTP) (2 h, RT, pH = 4.5). After neutralization, disulfide reduction with dithiothreitol (DTT) was achieved (24 h, room temperature, pH = 8.5), followed by acidification (pH = 3.5) to yield MC-SH (Fig. 9B).59
Adding poly(ethylene glycol)-bismaleimide (PEG-MI2) resulted in crosslinking by the Michael-type addition of MC-SH.60,61 In particular, Pakulska et al. developed an injectable dual-crosslinked MC (XMC) hydrogel for drug delivery to the injured spinal cord, exploiting MC-SH/PEG-MI2 crosslinking (5 wt% MC, 0.1 μmol thiol/100 μL, 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 maleimide:thiol ratio).60 The physically crosslinked MC hydrogel (5 wt% MC) gelled at a physiological temperature within 10 min (Fig. 10A). Conversely, the XMC hydrogel displayed a gel behavior (G′ > G′′) before exposure to the physiological temperature, i.e., at T < Tt, meaning that the chemical crosslinking was complete before injection. Interestingly, the XMC hydrogel remained injectable after chemical crosslinking, providing favorable properties (e.g., easy storage and simple injection timing). After exposure to physiological temperature, physical crosslinks cause a further strengthening of the XMC gel (G′ increases 60 min after exposure to 37 °C, Fig. 10A) due to the preserved thermo-responsive character of MC. Moreover, physically crosslinked MC gels, containing or not PEG-MI2 (MC and MC + PEG-MI2), displayed significant mass loss 35 days after swelling in artificial cerebrospinal fluid (aCSF). At the same time, the XMC hydrogel was stable for the entire duration of the test (Fig. 10B). Both the rheological and swelling data confirmed the effectiveness of MC-SH/PEG-MI2 crosslinking in controlling the physicochemical and mechanical properties of the gel. This study unveiled the possibility to achieve hybrid crosslinking (i.e., chemical and physical crosslinking) to create injectable long-lasting hydrogels. However, no data were reported on the Tt (and possible shifts induced by crosslinking) of the obtained hybrid crosslinked hydrogels.
1 maleimide:thiol ratio).60 The physically crosslinked MC hydrogel (5 wt% MC) gelled at a physiological temperature within 10 min (Fig. 10A). Conversely, the XMC hydrogel displayed a gel behavior (G′ > G′′) before exposure to the physiological temperature, i.e., at T < Tt, meaning that the chemical crosslinking was complete before injection. Interestingly, the XMC hydrogel remained injectable after chemical crosslinking, providing favorable properties (e.g., easy storage and simple injection timing). After exposure to physiological temperature, physical crosslinks cause a further strengthening of the XMC gel (G′ increases 60 min after exposure to 37 °C, Fig. 10A) due to the preserved thermo-responsive character of MC. Moreover, physically crosslinked MC gels, containing or not PEG-MI2 (MC and MC + PEG-MI2), displayed significant mass loss 35 days after swelling in artificial cerebrospinal fluid (aCSF). At the same time, the XMC hydrogel was stable for the entire duration of the test (Fig. 10B). Both the rheological and swelling data confirmed the effectiveness of MC-SH/PEG-MI2 crosslinking in controlling the physicochemical and mechanical properties of the gel. This study unveiled the possibility to achieve hybrid crosslinking (i.e., chemical and physical crosslinking) to create injectable long-lasting hydrogels. However, no data were reported on the Tt (and possible shifts induced by crosslinking) of the obtained hybrid crosslinked hydrogels.
 | ||
Fig. 10 (A) G′ and G′′ for XMC (5 wt% MC, 0.1 μmol thiol/100 μL, 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 maleimide–thiol molar ratio) and MC hydrogels. (B) Swelling ratios for XMC, MC, and PEGMI containing MC hydrogels in aCSF. Reproduced with permission from ref. 60. Copyright Wiley 2023. 1 maleimide–thiol molar ratio) and MC hydrogels. (B) Swelling ratios for XMC, MC, and PEGMI containing MC hydrogels in aCSF. Reproduced with permission from ref. 60. Copyright Wiley 2023. | ||
In the third approach, norbornene-functionalized MC (MC-NB) was obtained (Fig. 9C, left) in a two-step reaction: first, carboxylated methylcellulose (MC-CO2H) was obtained and then NB functionalization was obtained via amidation by reacting 5-norbornene-2-methylamine in the presence of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) (3 days, 4 °C). In addition, methylphenyltetrazine (Tz) was also reacted with a disulfide-containing linker (i.e., DTP-functionalized MC) via amidation to produce redox-cleavable MC-DTP-Tz (Fig. 9C, right). Lastly, MC-NB and MC-DTP-Tz were mixed, and gelation (<15 min) was induced via the inverse electron demand Diels–Alder reaction.62 Conversely, to the first two approaches, no other reactive species were added to achieve crosslinking (Fig. 3C, reactive species not present).
Compared to photo-crosslinking (Par. 2.3.1), no irradiation (UV or visible light) is required in the above-mentioned processes to achieve crosslinking. Still, crosslinking is achieved simply by mixing the modified polymer and the crosslinking agent. The crosslinking reaction occurs at physiological pH and temperature without catalysts. This represents an advantage over photo-crosslinkable MC hydrogels for specific applications, e.g., injectable formulations, since no further manipulation after in vivo injection is required.60 Nevertheless, chemical modification of MC is needed before proceeding with crosslinking, representing a possible limitation in terms of the ease of the production process (e.g., chemical crosslinking with small molecule crosslinkers). In addition, despite the favorable results, no information about possible Tt shifts (or alteration of the thermo-responsive character) induced by the chemical crosslinking of modified MC has been reported.
Photo-crosslinkable groups are non-native functionalities, requiring a chemical modification of a native polymer before crosslinking.64 Furthermore, photo-crosslinking is usually coordinated by the action of photo-initiators. Photo-initiators absorb light at a specific wavelength (e.g., UV, λ = 250–370 nm; visible blue light, λ = 405–550 nm; red light, λ = 750–810 nm) either decomposing (i.e., cleavage at C–C, C–Cl, C–O, or C–S bonds) or abstracting hydrogen from an H-donor molecule, lastly forming radicals.63,65
MC photo-crosslinking has been obtained by further (i.e., in addition to –OCH3) substitution of MC with methacrylate,66 allyl,67 tyramine,68 or norbornene69 groups. MC-MA was obtained as reported in section 2.3. Allyl-modified MC (MC-allyl, Fig. 9D) was obtained by dissolving MC in NaOH solution and then adding allyl bromide in solution (overnight and at room temperature).67 For methacrylate- and allyl-modified MC, photo-crosslinking is induced by exposure to UV light (λ = 365 nm, for 10–20 min), using Irgacure 2959 as the photo-initiator.66,67 Tyramine-modified MC (MC-Tyr, Fig. 9E) was obtained in a two-step reaction: first, carboxylated methylcellulose (MC-CO2H) was obtained and then the amino groups of tyramine were reacted with the carboxylic groups on MC-COOH via the conventional EDC/NHS coupling reaction (24 h, room temperature).68MC-Tyr was photo-crosslinked after exposure to visible light (λ = 440 nm, 120 s) in the presence of riboflavin (RF) or riboflavin 5′-monophosphate (RFp) as the photo-initiator and ammonium persulfate (APS) as the co-initiator.68 As reported in section 2.3, norbornene-functionalized MC (MC-NB, Fig. 9C, left) was obtained MC-NB readily reacting with a thiol group via light-mediated thiol-NB photoclick-chemistry (λ = 365 nm, 120 s) in the presence of the lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) photo-initiator.69
Fig. 11A shows the swelling curves for dual (i.e., physical + chemical) crosslinkable MC-Tyr hydrogels (8 wt%). The effect of physical (temperature-induced sol–gel transition), chemical (photo-crosslinking), and double (physical + chemical) crosslinking was studied in terms of the hydrogel water uptake. As expected, chemical crosslinking causes a reduction in the swelling rate of MC-Tyr hydrogels (600 vs. 200%) compared to physical crosslinking because of the reduced mobility of MC macromolecules. Double crosslinked samples display even lower swelling values due to an additional increase in the crosslinking extent of the hydrogels. Photo-crosslinking also significantly affects the mechanical performance of MC-Tyr gels (Fig. 11B). The compressive strength of MC-Tyr hydrogels increases in chemically crosslinked samples compared to physically crosslinked ones (50 vs. 5 kPa when RFp was used) and even more in dual crosslinked samples (>100 kPa). Interestingly, the dual crosslinked hydrogels retained their structural stability for up to 60 days in PBS, whereas the physically crosslinked ones steadily collapsed. Moreover, after chemical crosslinking, the described dual crosslinkable MC hydrogels preserved their thermo-responsive character, displaying a Tt ∼ 37 °C. This allows the customization of the properties (e.g., mechanical) of the chemically crosslinked gels based on temperature, specifically for 3D bioprinting purposes.68 It is worth mentioning that dual crosslinked systems allow further controlling and tuning over the properties of crosslinked MC gels, offering the possibility to decide in which order to achieve crosslinking (i.e., chemical or physical first) depending on the fabrication method and the final application of the MC structure.60,68
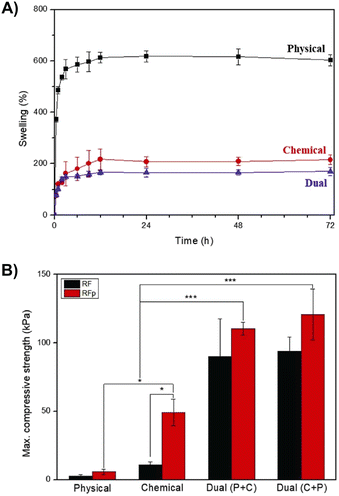 | ||
| Fig. 11 (A) Swelling and (B) compressive strength of MC-Tyr hydrogels with different crosslinking methods (physical, chemical, and dual crosslinking). RF = riboflavin and RFp = riboflavin 5′-monophosphate. Reproduced with permission from ref. 68. Copyright Elsevier 2023. | ||
In this study, visible light and RF or RFp allowed crosslinking without cytotoxic effects in the cells laden in the gel before irradiation.68 Conversely to this work, most studies in the literature (not limited to MC) exploit UV light to achieve crosslinking. However, a significant limitation of UV radiation is the possible DNA damage of light-exposed cells.63 For this reason, visible light photo-initiators should be preferred when designing the gels for biomedical purposes (e.g., 3D bioprinting and cell delivery).
Moreover, independently from the light source (UV or visible), MC modification before photo-crosslinking can represent a limitation, particularly in terms of ease and rapidity of the production of crosslinked samples.
3. Conclusions
3.1. MC crosslinking strategies: comparison and selection
This review provided an overview of the current approaches reported in the literature for MC chemical crosslinking (Table 1); however, it is important to underline that comparing the different crosslinking methods is non-trivial. In this regard, the swelling degree is primarily reported as a preliminary way to assess the effectiveness of hydrogels’ crosslinking.70 A quantitative evaluation of the extent of crosslinking can be carried out by measuring the parameters defining the network structure of the gels, i.e., the average molecular weight between crosslinks (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) C), the crosslinking density (ρc), and the mesh size (ξ).25 In this regard, two difficulties arise when comparing crosslinked MC hydrogels described in the literature: first, only a few works calculated these parameters. Second, the parameters were computed by applying the Flory–Rehner model, starting from the swelling data.25,57,71 However, these latter strictly depend on the hydrogel formulation, which displays high variability in the works considered here. To provide just a few examples, the main parameters involved are the MC molecular weight, MC concentration, the presence of additives (e.g., salts), and the blending with other polymers (e.g., chitosan, alginate, and PVA). As a result, such variability in the reviewed works makes it impossible to extrapolate the effect of the single parameter (e.g., crosslinking type) on the properties (e.g., swelling extent) of the considered gel formulation. The appropriate approach to compare different crosslinking agents would require keeping the MC hydrogel formulation fixed. This has been carried out on other biopolymers (e.g., hyaluronic acid,70 chitosan,72,73 and gelatin74), but nothing similar has been reported in the literature for MC. Such a study on MC could reveal the efficacy of different crosslinkers, disclosing optimal dosage ranges for each one and the effects of each crosslinker on the properties (e.g., physicochemical, mechanical, and biological) of the crosslinked MC gel.
C), the crosslinking density (ρc), and the mesh size (ξ).25 In this regard, two difficulties arise when comparing crosslinked MC hydrogels described in the literature: first, only a few works calculated these parameters. Second, the parameters were computed by applying the Flory–Rehner model, starting from the swelling data.25,57,71 However, these latter strictly depend on the hydrogel formulation, which displays high variability in the works considered here. To provide just a few examples, the main parameters involved are the MC molecular weight, MC concentration, the presence of additives (e.g., salts), and the blending with other polymers (e.g., chitosan, alginate, and PVA). As a result, such variability in the reviewed works makes it impossible to extrapolate the effect of the single parameter (e.g., crosslinking type) on the properties (e.g., swelling extent) of the considered gel formulation. The appropriate approach to compare different crosslinking agents would require keeping the MC hydrogel formulation fixed. This has been carried out on other biopolymers (e.g., hyaluronic acid,70 chitosan,72,73 and gelatin74), but nothing similar has been reported in the literature for MC. Such a study on MC could reveal the efficacy of different crosslinkers, disclosing optimal dosage ranges for each one and the effects of each crosslinker on the properties (e.g., physicochemical, mechanical, and biological) of the crosslinked MC gel.
Getting back to the studies mentioned in this review, it can be noted that no crosslinking approach is a priori better than the others. The choice depends on the final aim and application of the crosslinked MC gel (Table 2). In this regard, it is possible to draw some useful guidelines to assist the reader in choosing the best crosslinking approach.
A priori, toxic crosslinking agents must be avoided. Among small molecule crosslinkers, these non-toxic or easy to quench crosslinkers (e.g., CA, DVS, and STMP) should be selected. When dealing with cell-laden hydrogels (e.g., bioinks and injectable drug/cell carriers), mild crosslinking approaches should be considered: crosslinking by chemical modification (including photo-crosslinking) is the suggested choice, while high-energy radiation and high temperatures (e.g., to achieve crosslinking with CA or HDI) must be avoided.
As MC is used as a food ingredient or contact material in both cases, toxic crosslinking agents must be avoided.78 Moreover, mild crosslinking conditions (e.g., low temperature and γ irradiation) should be preferred when dealing with edible coating development to avoid food quality modifications.
3.2. Further design options
In our opinion, more studies in this direction would allow further tuning of the properties of crosslinked MC gels according to the intended application.
However, sterilization may cause changes to the chemical and physical properties of materials, affecting their performance and impairing their possible applications.82,83 In this regard, a systematic investigation on sterilization has been carried out by Hodder et al.,82 who assessed how autoclave treatment, supercritical CO2 (scCO2) treatment, and UV and γ irradiation affected MC properties, with a focus on 3D bioprinting. They observed that UV irradiation, autoclave treatment, and scCO2 treatment of MC powder (before hydrogel preparation) did not affect printability, indicating negligible effects on viscosity. Instead, γ irradiation caused a decrease in MC hydrogels’ viscosity, attributed to a decrease in the molecular mass of MC after irradiation. Overall, since UV treatment is not considered a fully effective sterilization method and scCO2 implies time-consuming optimization, autoclave treatment was suggested as the optimal (fast, effective, and cost-effective) method for MC sterilization.
It is worth mentioning that the choice of the sterilization technique is even more critical when it comes to crosslinked MC systems. The risk of over-crosslinking must be taken into consideration. As an example, sterilization under gamma irradiation could lead to further crosslinking of MC chains (Par. 2.2). Another example is the use of high temperatures (e.g., autoclave sterilization) in citric-acid crosslinked MC, where residual (i.e., non-reacted) CA could lead to over-crosslinking. Again, sterilization by UV light may lead to the premature crosslinking of photo-crosslinkable MC hydrogel formulations. Once again, the correct design of the MC-based system is therefore essential and requires considering all aspects, from synthesis/preparation to crosslinking and sterilization, foreseeing the final application.
3.3. Limitations and future directions
Chemical crosslinking allows increasing the physicochemical and mechanical properties of MC hydrogels, endowing new design and application strategies for MC hydrogels, particularly in the biomedical area. Nevertheless, great attention has been paid to preserving the thermo-responsive character of MC hydrogels after chemical crosslinking. As discussed in this work, only a few studies assess the influence of chemical crosslinking on the thermo-responsive behavior of the MC hydrogels and possible detrimental effects on their Tt. Most of the works focus more on the physicochemical and mechanical performance of the chemically crosslinked MC gels rather than assessing if the MC gels have retained their smart properties after crosslinking. Such foresight is instead fundamental for developing MC-based hydrogel systems with a defined smart response.Keeping in mind these considerations, two main hints arise when envisioning the future trends in the research on crosslinked MC hydrogels: (i) the need to use biocompatible, tunable, and easy-to-use crosslinking agents for MC; (ii) the maintenance of MC thermo-responsive behavior after chemical crosslinking. In this regard, optimal/dual crosslinking (i.e., a trade-off between physical and chemical crosslinking) can be regarded as the leading strategy to preserve MC thermo-responsiveness around the set value, providing the crosslinked samples with superior performance compared to non-crosslinked MC. In this direction, countless envisioned applications of crosslinked MC hydrogels in the biomedical area are expected. From in situ gelling, tough, and long-lasting smart hydrogels to responsive hydrogels for enhanced loading and controlled release of drugs to intelligent cell culture surfaces capable of recapitulating the mechanical and topographic properties of different native tissues.
Author contributions
Conceptualization, writing – original draft, writing – review and editing, and visualization: L. B.; conceptualization, writing – review and editing, and supervision: L. D. N.; conceptualization, writing – review and editing, and supervision: S. F. All the authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. D. N. thanks “ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods” funded by the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.3—D.D. 341 15/03/2022, PE00000003).Notes and references
- P. Nasatto, F. Pignon, J. Silveira, M. Duarte, M. Noseda and M. Rinaudo, Polymers, 2015, 7, 777–803 CrossRef CAS.
- N. Sarkar, J. Appl. Polym. Sci., 1979, 24, 1073–1087 CrossRef CAS.
- L. Bonetti, L. De Nardo and S. Farè, Tissue Eng., Part B, 2021, 27, 486–513 CrossRef CAS PubMed.
- M. L. Coughlin, L. Liberman, S. P. Ertem, J. Edmund, F. S. Bates and T. P. Lodge, Prog. Polym. Sci., 2021, 112, 101324 CrossRef CAS.
- T. Chatterjee, A. I. Nakatani, R. Adden, M. Brackhagen, D. Redwine, H. Shen, Y. Li, T. Wilson and R. L. Sammler, Biomacromolecules, 2012, 13, 3355–3369 CrossRef CAS PubMed.
- T. Heinze, O. A. El Seoud and A. Koschella, Cellulose Derivatives, Springer International Publishing, Cham, 2018 Search PubMed.
- K. Kobayashi, C. Huang and T. P. Lodge, Macromolecules, 1999, 32, 7070–7077 CrossRef CAS.
- F. Tanaka and M. Ishida, J. Chem. Soc., Faraday Trans., 1995, 91, 2663 RSC.
- B. Niemczyk-Soczynska, A. Gradys, D. Kolbuk, A. Krzton-Maziopa and P. Sajkiewicz, Polymers, 2019, 11, 1772 CrossRef CAS PubMed.
- A. Haque and E. R. Morris, Carbohydr. Polym., 1993, 22, 161–173 CrossRef CAS.
- N. Sarkar, Carbohydr. Polym., 1995, 26, 195–203 CrossRef CAS.
- S. Morozova, Polym. Int., 2020, 69, 125–130 CrossRef CAS.
- P. W. Schmidt, S. Morozova, S. P. Ertem, M. L. Coughlin, I. Davidovich, Y. Talmon, T. M. Reineke, F. S. Bates and T. P. Lodge, Macromolecules, 2020, 53, 398–405 CrossRef CAS.
- J. R. Lott, J. W. McAllister, S. A. Arvidson, F. S. Bates and T. P. Lodge, Biomacromolecules, 2013, 14, 2484–2488 CrossRef CAS PubMed.
- J. R. Lott, J. W. McAllister, M. Wasbrough, R. L. Sammler, F. S. Bates and T. P. Lodge, Macromolecules, 2013, 46, 9760–9771 CrossRef CAS.
- P. W. Schmidt, S. Morozova, P. M. Owens, R. Adden, Y. Li, F. S. Bates and T. P. Lodge, Macromolecules, 2018, 51, 7767–7775 CrossRef CAS.
- S. Thirumala, J. Gimble and R. Devireddy, Cells, 2013, 2, 460–475 CrossRef CAS PubMed.
- T. Ahlfeld, V. Guduric, S. Duin, A. R. Akkineni, K. Schütz, D. Kilian, J. Emmermacher, N. Cubo-Mateo, S. Dani, M. V. Witzleben, J. Spangenberg, R. Abdelgaber, R. F. Richter, A. Lode and M. Gelinsky, Biomater. Sci., 2020, 8, 2102–2110 RSC.
- H. Li, Y. J. Tan, K. F. Leong and L. Li, ACS Appl. Mater. Interfaces, 2017, 9, 20086–20097 CrossRef CAS PubMed.
- K. Schütz, A.-M. Placht, B. Paul, S. Brüggemeier, M. Gelinsky and A. Lode, J. Tissue Eng. Regener. Med., 2017, 11, 1574–1587 CrossRef PubMed.
- D. Ribezzi, R. Pinos, L. Bonetti, M. Cellani, F. Barbaglio, C. Scielzo and S. Farè, Front. Biomater. Sci., 2023, 2, 1081065 CrossRef.
- D. C. Harsh and S. H. Gehrke, J. Controlled Release, 1991, 17, 175–185 CrossRef CAS.
- A. Cochis, L. Bonetti, R. Sorrentino, N. Contessi Negrini, F. Grassi, M. Leigheb, L. Rimondini and S. Fare’, Materials, 2018, 11, 1–14 CrossRef PubMed.
- T. Fekete, J. Borsa, E. Takács and L. Wojnárovits, Cellulose, 2014, 21, 4157–4165 CrossRef CAS.
- L. Bonetti, L. De Nardo, F. Variola and S. Fare’, Soft Matter, 2020, 16, 5577–5587 RSC.
- A. Oryan, A. Kamali, A. Moshiri, H. Baharvand and H. Daemi, Int. J. Biol. Macromol., 2018, 107, 678–688 CrossRef CAS PubMed.
- S. Rimdusit, K. Somsaeng, P. Kewsuwan, C. Jubsilp and S. Tiptipakorn, Eng. J., 2012, 16, 15–28 CrossRef.
- I. Migneault, C. Dartiguenave, M. J. Bertrand and K. C. Waldron, Biotechniques, 2004, 37, 790–802 CrossRef CAS PubMed.
- A. Bigi, G. Cojazzi, S. Panzavolta, K. Rubini and N. Roveri, Biomaterials, 2001, 22, 763–768 CrossRef CAS PubMed.
- W. Hennink and C. van Nostrum, Adv. Drug Delivery Rev., 2002, 54, 13–36 CrossRef CAS PubMed.
- J.-S. Park and E. Ruckenstein, Carbohydr. Polym., 2001, 46, 373–381 CrossRef CAS.
- J.-S. Park, J.-W. Park and E. Ruckenstein, Polymer, 2001, 42, 4271–4280 CrossRef CAS.
- V. Ramesh Babu, M. Sairam, K. M. Hosamani and T. M. Aminabhavi, Carbohydr. Polym., 2007, 69, 241–250 CrossRef CAS.
- O. Şanlı, A. Kahraman, E. Kondolot Solak and M. Olukman, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 950–959 Search PubMed.
- E. Bulut, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 1098–1108 CAS.
- A. P. Rokhade, N. B. Shelke, S. A. Patil and T. M. Aminabhavi, Carbohydr. Polym., 2007, 69, 678–687 CrossRef CAS.
- A. G. Sullad, L. S. Manjeshwar and T. M. Aminabhavi, J. Appl. Polym. Sci., 2010, 116, 1226–1235 CAS.
- E. Zeiger, B. Gollapudi and P. Spencer, Mutat. Res., Rev. Mutat. Res., 2005, 589, 136–151 CrossRef CAS PubMed.
- J. E. Gough, C. A. Scotchford and S. Downes, J. Biomed. Mater. Res., 2002, 61, 121–130 CrossRef CAS PubMed.
- T. Chen, H. D. Embree, E. M. Brown, M. M. Taylor and G. F. Payne, Biomaterials, 2003, 24, 2831–2841 CrossRef CAS PubMed.
- G. Malucelli, J. Dore, D. Sanna, D. Nuvoli, M. Rassu, A. Mariani and V. Alzari, Front. Chem., 2018, 6, 585 CrossRef CAS PubMed.
- J. Morales-Sanfrutos, F. Lopez-Jaramillo, M. Elremaily, F. Hernández-Mateo and F. Santoyo-Gonzalez, Molecules, 2015, 20, 3565–3581 CrossRef PubMed.
- J.-Y. Lai, Carbohydr. Polym., 2014, 101, 203–212 CrossRef CAS PubMed.
- U. Anbergen and W. Oppermann, Polymer, 1990, 31, 1854–1858 CrossRef CAS.
- S. M. O’Connor and S. H. Gehrke, J. Appl. Polym. Sci., 1997, 66, 1279–1290 CrossRef.
- N. Reddy, R. Reddy and Q. Jiang, Trends Biotechnol., 2015, 33, 362–369 CrossRef CAS PubMed.
- L. Bonetti, L. De Nardo and S. Farè, Gels, 2021, 7, 141 CrossRef CAS PubMed.
- L. Bonetti, L. De Nardo, F. Variola and S. Farè, Mater. Lett., 2020, 274, 128011 CrossRef CAS.
- R. E. Wing, Starch – Starke, 1996, 48, 275–279 CrossRef CAS.
- L. Bonetti, A. Fiorati, A. D’Agostino, C. M. Pelacani, R. Chiesa, S. Farè and L. De Nardo, Gels, 2022, 8, 298 CrossRef CAS PubMed.
- R. Shi, J. Bi, Z. Zhang, A. Zhu, D. Chen, X. Zhou, L. Zhang and W. Tian, Carbohydr. Polym., 2008, 74, 763–770 CrossRef CAS.
- Q. Jiang, N. Reddy, S. Zhang, N. Roscioli and Y. Yang, J. Biomed. Mater. Res., Part A, 2013, 101A, 1237–1247 CrossRef CAS PubMed.
- J. Garner and K. Park, Polysaccharides, Springer International Publishing, Cham, 2015, pp. 1555–1582 Search PubMed.
- R. A. Wach, H. Mitomo and F. Yoshii, J. Radioanal. Nucl. Chem., 2004, 261, 113–118 CrossRef CAS.
- R. A. Wach, H. Mitomo, N. Nagasawa and F. Yoshii, Nucl. Instrum. Methods Phys. Res., Sect. B, 2003, 211, 533–544 CrossRef CAS.
- P. Petrov, E. Petrova, R. Stamenova, C. B. Tsvetanov and G. Riess, Polymer, 2006, 47, 6481–6484 CrossRef CAS.
- G. T. Gold, D. M. Varma, P. J. Taub and S. B. Nicoll, Carbohydr. Polym., 2015, 134, 497–507 CrossRef CAS PubMed.
- G. T. Gold, D. M. Varma, D. Harbottle, M. S. Gupta, S. S. Stalling, P. J. Taub and S. B. Nicoll, J. Biomed. Mater. Res., Part A, 2014, 102, 4536–4544 Search PubMed.
- K. Vulic and M. S. Shoichet, J. Am. Chem. Soc., 2012, 134, 882–885 CrossRef CAS PubMed.
- M. M. Pakulska, K. Vulic, R. Y. Tam and M. S. Shoichet, Adv. Mater., 2015, 27, 5002–5008 CrossRef CAS PubMed.
- M. M. Pakulska, C. H. Tator and M. S. Shoichet, Biomaterials, 2017, 134, 13–21 CrossRef CAS PubMed.
- V. Delplace, A. J. Pickering, M. H. Hettiaratchi, S. Zhao, T. Kivijärvi and M. S. Shoichet, Biomacromolecules, 2020, 21, 2421–2431 CrossRef CAS PubMed.
- W. Hu, Z. Wang, Y. Xiao, S. Zhang and J. Wang, Biomater. Sci., 2019, 7, 843–855 RSC.
- N. D. Pham, R. B. Parker and J. J. Kohler, Curr. Opin. Chem. Biol., 2013, 17, 90–101 CrossRef CAS PubMed.
- K. T. Nguyen and J. L. West, Biomaterials, 2002, 23, 4307–4314 CrossRef CAS PubMed.
- S. S. Stalling, S. O. Akintoye and S. B. Nicoll, Acta Biomater., 2009, 5, 1911–1918 CrossRef CAS PubMed.
- S. Morozova, M. L. Coughlin, J. T. Early, S. P. Ertem, T. M. Reineke, F. S. Bates and T. P. Lodge, Macromolecules, 2019, 52, 7740–7748 CrossRef CAS.
- J. Y. Shin, Y. H. Yeo, J. E. Jeong, S. A. Park and W. H. Park, Carbohydr. Polym., 2020, 238, 116192 CrossRef CAS PubMed.
- M. H. Kim and C.-C. Lin, Biofabrication, 2021, 13, 045023 CrossRef CAS PubMed.
- M. N. Collins and C. Birkinshaw, J. Appl. Polym. Sci., 2007, 104, 3183–3191 CrossRef CAS.
- P. J. Flory and J. Rehner, J. Chem. Phys., 1943, 11, 521–526 CrossRef CAS.
- S. N. Mikhailov, A. N. Zakharova, M. S. Drenichev, A. V. Ershov, M. A. Kasatkina, L. V. Vladimirov, V. V. Novikov and N. R. Kildeeva, Nucleosides, Nucleotides Nucleic Acids, 2016, 35, 114–129 CrossRef CAS PubMed.
- F. Ruini, C. Tonda-Turo, V. Chiono and G. Ciardelli, Biomed. Mater., 2015, 10, 065002 CrossRef CAS PubMed.
- G. Yang, Z. Xiao, H. Long, K. Ma, J. Zhang, X. Ren and J. Zhang, Sci. Rep., 2018, 8, 1616 CrossRef PubMed.
- L. Bonetti, A. Caprioglio, N. Bono, G. Candiani and L. Altomare, Biomater. Sci., 2023, 11, 2699–2710 RSC.
- T. Sanz, M. Falomir and A. Salvador, Food Hydrocolloids, 2015, 46, 19–27 CrossRef CAS.
- Y. Liu, S. Ahmed, D. E. Sameen, Y. Wang, R. Lu, J. Dai, S. Li and W. Qin, Trends Food Sci. Technol., 2021, 112, 532–546 CrossRef CAS.
- E. Ruggeri, S. Farè, L. De Nardo and B. Marelli, Sustainable Food Packaging Technology, Wiley, 2021, pp. 57–105 Search PubMed.
- Y. Kim, S.-P. Chang and Y. Song, ACS Appl. Electron. Mater., 2022, 4, 2227–2237 CrossRef CAS.
- F. Yu, H. Zhang, L. Zhao, Z. Sun, Y. Li, Y. Mo and Y. Chen, Carbohydr. Polym., 2020, 246, 116622 CrossRef CAS PubMed.
- Z. Dai, J. Ronholm, Y. Tian, B. Sethi and X. Cao, J. Tissue Eng., 2016, 7, 204173141664881 CrossRef PubMed.
- E. Hodder, S. Duin, D. Kilian, T. Ahlfeld, J. Seidel, C. Nachtigall, P. Bush, D. Covill, M. Gelinsky and A. Lode, J. Mater. Sci.: Mater. Med., 2019, 30, 10 CrossRef PubMed.
- A. Fatimi, J.-F. Tassin, R. Turczyn, M. A. V. Axelos and P. Weiss, Acta Biomater., 2009, 5, 3423–3432 CrossRef CAS PubMed.
- S. Rimdusit, S. Jingjid, S. Damrongsakkul, S. Tiptipakorn and T. Takeichi, Carbohydr. Polym., 2008, 72, 444–455 CrossRef CAS.
- C. López de Dicastillo, F. Rodríguez, A. Guarda and M. J. Galotto, Carbohydr. Polym., 2016, 136, 1052–1060 CrossRef PubMed.
- C. López de Dicastillo, F. Bustos, A. Guarda and M. J. Galotto, Food Hydrocolloids, 2016, 60, 335–344 CrossRef.
- H. Hatakeyama, T. Onishi, T. Endo and T. Hatakeyama, Carbohydr. Polym., 2007, 69, 792–798 CrossRef CAS.
- T. Fekete, J. Borsa, E. Takács and L. Wojnárovits, Radiat. Phys. Chem., 2016, 118, 114–119 CrossRef CAS.
- H. Shen, Y. Ma, Y. Luo, X. Liu, Z. Zhang and J. Dai, Colloids Surf., B, 2015, 135, 332–338 CrossRef CAS PubMed.
- H. Wang, Y. Liao, A. Wu, B. Li, J. Qian and F. Ding, Polymers, 2019, 11, 368 CrossRef CAS PubMed.
- F. Yoshii, L. Zhao, R. A. Wach, N. Nagasawa, H. Mitomo and T. Kume, Nucl. Instrum. Methods Phys. Res., Sect. B, 2003, 208, 320–324 CrossRef CAS.
- E. Bulut, J. Biomater. Sci., Polym. Ed., 2020, 31, 1671–1688 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |