Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
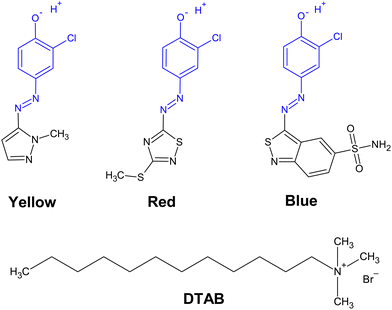
Comparative study of the co-assembly behaviour of 3-chloro-4-hydroxy-phenylazo dyes with DTAB†
Wenke
Müller
 *a,
Ralf
Schweins
*a,
Ralf
Schweins
 a,
Bernd
Nöcker
b,
Joachim
Kohlbrecher
a,
Bernd
Nöcker
b,
Joachim
Kohlbrecher
 c,
Glen J
Smales
c,
Glen J
Smales
 d and
Klaus
Huber
d and
Klaus
Huber
 e
e
aInstitut Laue-Langevin, DS/LSS, 71 Avenue des Martyrs, 38000 Grenoble, France. E-mail: we-mue@gmx.net
bKAO Germany GmbH, Pfungstädter Straße 98-100, 64297 Darmstadt, Germany
cPaul Scherrer Institut, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
dBundesanstalt für Materialforschung und –prüfung, Unter den Eichen 87, 12205 Berlin, Germany
eUniversität Paderborn, Warburger Straße 100, 33098 Paderborn, Germany
First published on 9th June 2023
Abstract
The co-assembly of three one-fold negatively charged 3-chloro-4-hydroxy-phenylazo dyes (Yellow, Blue and Red) with the cationic surfactant dodecyltrimethylammoniumbromide (DTAB) was studied to probe dye–DTAB binding stoichiometry and assembly morphology. For each dye, phase separation was observed above a given dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB ratio with the ratio depending on the dye. While Yellow and DTAB showed liquid/liquid phase separation above Yellow
DTAB ratio with the ratio depending on the dye. While Yellow and DTAB showed liquid/liquid phase separation above Yellow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.67, crystalline dye–DTAB complexes were observed for Blue–DTAB and Red–DTAB above Blue
1.67, crystalline dye–DTAB complexes were observed for Blue–DTAB and Red–DTAB above Blue![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.56 and Red
2.56 and Red![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.94 respecively. In homogeneous solution, UV/vis spectroscopic investigations suggest stochiometries of Yellow
2.94 respecively. In homogeneous solution, UV/vis spectroscopic investigations suggest stochiometries of Yellow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, Blue
2, Blue![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and Red
3 and Red![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. It was concluded, that Yellow exhibits the highest dye
4. It was concluded, that Yellow exhibits the highest dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB binding stoichiometry in both, dye–surfactant complexes in the 2-phase region and in solution, whereas the lowest dye
DTAB binding stoichiometry in both, dye–surfactant complexes in the 2-phase region and in solution, whereas the lowest dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB binding stoichiometry was observed for Red–DTAB in both cases. The observed stoichiometries are inversely correlated to the impact dye addition has on the morphology of DTAB micelles. Generally, addition of dye to DTAB micelles leads to a reduction in spontaneous curvature of these micelles and to the formation of triaxial ellipsoidal or cylindrical micelles from oblate ellipsoidal DTAB micelles. At a DTAB concentration of 30 mM and a dye concentration of 5 mM, this effect was most pronounced for Red and least pronounced for Yellow, whilst Blue showed an intermediate effect.
DTAB binding stoichiometry was observed for Red–DTAB in both cases. The observed stoichiometries are inversely correlated to the impact dye addition has on the morphology of DTAB micelles. Generally, addition of dye to DTAB micelles leads to a reduction in spontaneous curvature of these micelles and to the formation of triaxial ellipsoidal or cylindrical micelles from oblate ellipsoidal DTAB micelles. At a DTAB concentration of 30 mM and a dye concentration of 5 mM, this effect was most pronounced for Red and least pronounced for Yellow, whilst Blue showed an intermediate effect.
Introduction
Non-covalent intermolecular interactions, leading to self- and co-assembly, are pivotal to life. They enable structures and functions ranging from cell membranes over metabolic processes to the DNA double helix. Inspired by nature, scientists are trying to exploit the spontaneous assembly of one or more components for the creation of functional materials, which could be used in regenerative medicine,1,2 as carrier systems,3,4 for solar cells,5 as sensors6,7 or in photonics.8,9The complexity of intermolecular interactions exacerbates the prediction of assembly behaviour by simple models, as not only forces like van der Waals-, dipole–dipole-, electrostatic- or π–π-interactions play a role, but other phenomena such as entropic effects, repulsive interactions, cooperativity and external forces might need to be considered.10 This highlights the necessity of comprehensive investigations of co- and self-assembling systems to obtain a better understanding of their fundamental principles as well as the system specific features that govern assembly structures and properties.
Polyelectrolytes are a typical family of building blocks for self-assembly processes in aqueous systems, while also being a simple model system for biological building units.11–13 One reason for this is that assembly of linear polyelectrolytes or dendrimers can be triggered by oppositely charged counter ions. To give but an example, specific counter ion binding of Ca2+ was used to induce micellization of a block-copolyelectrolyte.14 Morphological changes of polyelectrolyte micelles in solution can also be triggered by the addition of charged amphiphilic molecules, which is interesting for the design of well-defined nanostructures.15 In a recent example it was shown, that the addition of ionic surfactants to an amphoteric diblock-copolyelectrolyte resulted in the formation of core–shell complexes, with an internal crystalline core structure and either positive or negative surface charge, dependent on the charge of the surfactant.16 Moreover, like the addition of inorganic counter ions or amphiphilic molecules, the introduction of charged dye molecules into polyelectrolyte systems yields supramolecular structures. These structures may be responsive to light as a trigger for morphological changes.17 This was, among others, shown in a recent example where assembly morphology between oppositely charged polyelectrolyte and azo dye was controlled by a change in N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-bond configuration, demonstrating the complexity of underlying intermolecular interactions.18 Due to the interest in light-switchability, the interaction between dye molecules and polyelectrolytes has been studied for a long time, revealing that such systems form well-defined supramolecular nanoparticles of adjustable size, based on electrostatic assembly with various azo dyes.19 Furthermore, the assembly morphology was shown to not only depend on structural features of the dye, but also on its self-assembly behaviour.20
N-bond configuration, demonstrating the complexity of underlying intermolecular interactions.18 Due to the interest in light-switchability, the interaction between dye molecules and polyelectrolytes has been studied for a long time, revealing that such systems form well-defined supramolecular nanoparticles of adjustable size, based on electrostatic assembly with various azo dyes.19 Furthermore, the assembly morphology was shown to not only depend on structural features of the dye, but also on its self-assembly behaviour.20
The use of polymeric building blocks does, however, come along with some drawbacks. Apart from challenges arising due to availability of some polymers in sufficient purity at the needed degree of polymerization and dispersity, pathways in polymer assembly are frequently subjected to kinetic limitations.21–23 Substituting polyelectrolytes with low molecular weight surfactants that assemble into micelles could be a pathway to circumvent such problems while obtaining assemblies with interesting and well-defined properties. To give an example, the Faul group observed the formation of highly ordered complexes between azo dye and oppositely charged surfactant molecules.24 Some of these complexes showed pleochroism.25 Considering the defined and regular shape of charged dye molecules and their potential to not only interact electrostatically, but also to form π–π-interactions makes them an interesting system for studying intermolecular interactions.26
Herein we report investigations on the stoichiometry of dye–surfactant binding between the positively charged surfactant dodecyltrimethylammoniumbromide (DTAB) and three negatively charged azo dyes (Fig. 1). The stoichiometry of dye–surfactant binding in solution is compared to the stoichiometry of dye–surfactant binding in solid complexes as obtained from the corresponding phase diagrams. Furthermore, differences in the morphology of dye–DTAB micelles were elucidated with small-angle neutron scattering (SANS).
Experimental
Chemicals and sample preparation
Three azo dyes Yellow (HC Yellow 16, ≥99%), Blue (HC Blue 18, ≥99.8%) and Red (HC Red 18, ≥99%) were provided by KAO GmbH, Germany. Completely hydrogenated dodecyltrimethylammoniumbromide (DTAB, 99%) was obtained from abcr GmbH, Germany. Completely deuterated dodecytrimethylammoniumbromide (d34-DTAB, 99% isotopic purity) was obtained from INNOVACHEM SAS, France. Tail-deuterated dodecyltrimethylammoniumbromide (d25-DTAB, 99.1% isotopic purity) was obtained from C/D/N Isotopes Inc., Canada. The buffer salts sodium carbonate Na2CO3 (≥99.8%) and sodium bicarbonate NaHCO3 (≥99.7%) were obtained from Sigma Aldrich Chemie GmbH, Germany. MilliQ water was used to prepare the NaHCO3/Na2CO3 buffer solutions (pH = 10.5, ionic strength) for UV/vis-spectroscopy and light scattering samples. D2O was used to prepare the NaHCO3/Na2CO3 buffer solutions (pD = 10.7, ionic strength I ≈ 0.25 M) for small-angle neutron scattering samples. D2O (99.90% D) was obtained from Eurisotop, France. Chemicals were used without further purification. Samples were prepared from stock solutions, followed by a minimum equilibration time of 20 h at room temperature prior to analysis.Phase diagrams
Phase diagrams were established by stepwise addition of a dye stock solution to a DTAB solution. NaHCO3/Na2CO3 buffer solution (pH = 10.5, ionic strength I ≈ 0.25 M) was prepared in H2O and used as a solvent in all cases. Dye stock solutions contained dye molar concentrations of 10 mM, and 15 mM for Yellow, Blue and Red respectively. The concentration of the employed DTAB stock solution was chosen according to the desired sample composition. After each addition of dye stock solution, the sample was vortexed for approximately 30 s and its visual appearance observed immediately. Phase diagrams were established at room temperature (≈22 °C). Longer timescales were not systematically investigated. It should, however, be noted that at low DTAB-concentrations (below its critical micelle concentration of 9 mM) some initially stable samples showed precipitation after 24 h.UV/vis spectroscopy
UV/vis spectra of solutions containing dye and DTAB were recorded with a Lambda-19 spectrometer from PerkinElmer. A Hellma quartz glass cuvette with an optical path length of 0.01 cm was used. The spectrometer was equipped with a thermostat to guarantee a constant measurement temperature of 25 °C. Samples showing precipitation were filtered prior to measurement (MACHEREY-NAGEL, CHROMAFIL Xtra H-PTFE syringe filters, pore size 0.2 μm).Small-angle neutron scattering
Samples for small-angle neutron scattering (SANS) measurements were obtained by mixing the solvent, a dye stock solution and a stock solution containing DTAB at appropriate ratios. The solvent was a NaHCO3/Na2CO3 buffer in D2O (pD = 10.7, ionic strength I ≈ 0.25 M). The sample containing [Blue] = 5 mM and [DTAB] = 30 mM represents an exception to this procedure, as it was initially prepared for another project. For this sample, the solvent, a dye stock solution and a stock solution containing d25-DTAB and d34-DTAB at a ratio of 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 (v/v) were mixed at appropriate ratios. The solvent was a NaHCO3/Na2CO3 buffer prepared in a mixture of H2O
54 (v/v) were mixed at appropriate ratios. The solvent was a NaHCO3/Na2CO3 buffer prepared in a mixture of H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O = 50
D2O = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) (pH/D = 10.5, ionic strength I ≈ 0.25 M). The resulting SANS curve of this sample and its fit were scaled by a factor of 3.6 in Fig. 5 to account for the difference in contrast.
50 (v/v) (pH/D = 10.5, ionic strength I ≈ 0.25 M). The resulting SANS curve of this sample and its fit were scaled by a factor of 3.6 in Fig. 5 to account for the difference in contrast.
After their preparation, sample solutions were filtered (MACHEREY-NAGEL, CHROMAFIL Xtra H-PTFE syringe filters, pore size 0.2 μm) into a dust-free sample vial and equilibrated for a minimum of 20 h at room temperature.
SANS measurements were performed at the instrument D11 at the Institut Laue-Langevin (ILL, Grenoble, France) and at the instrument SANS-I at the Paul Scherrer Institute (PSI, Villigen, Switzerland).
At the ILL, different setups were used: (1) the sample containing no dye and [DTAB]tot = 30 mM and the sample containing [Blue]tot = 5 mM and [DTAB]tot = 30 mM were measured at three sample-to-detector distances (28 m collimation 28 m), (8 m collimation 8 m), (1.7 m collimation 4.0 m) at a neutron wavelength of 6 Å to cover a q-range of 0.002 Å−1 to 0.5 Å−1. A circular neutron beam with a diameter of 15 mm was used. (2) All samples containing Red were measured at three sample-to-detector distances (38.0 m collimation 40.5 m), (10.5 m collimation 10.5 m), (2.5 m collimation 2.5 m) at a neutron wavelength of 6 Å to cover a q-range of 0.0014 Å−1 to 0.5 Å−1. A circular neutron beam with a diameter of 14 mm was used. Neutrons were detected with a 3He-detector (Reuter-Stokes multi-tube detector consisting of 256 tubes with a tube diameter of 8 mm and a pixel size of 8 mm × 4 mm), detector images azimuthally averaged, corrected to the transmission of the direct beam and scaled to absolute intensity using the Mantid software.27,28 Solvent scattering and empty cell scattering were subtracted from the scattering curves.29 SANS data were collected at a sample temperature of 25 °C.
The sample containing [Blue]tot = 5 mM and [DTAB]tot = 20 mM and all samples containing Yellow were measured at the PSI SANS-I instrument. The measurements were performed at 3 sample-to-detector distances (18.0 m collimation 18.0 m), (6.0 m collimation 6.0 m), (1.6 m collimation 6.0 m) at a wavelength of 6 Å to cover a q-range of 0.003 Å−1 to 0.4 Å−1. A circular neutron beam with a diameter of 14 mm was used. Neutrons were detected with a 2D MWPC CERCA 3He-detector with 128 × 128 elements of 7.5 × 7.5 mm2. Detector images were corrected for pixel efficiency using scattering from a water sample which also served as a secondary standard, corrected for transmission and azimuthally averaged using the BerSANS software.30 Solvent scattering and empty cell scattering were subtracted from the scattering curves.29 SANS data were collected at a sample temperature of 25 °C.
Data analysis was performed with the SasView small angle scattering analysis software. In all cases, the solvent scattering length density was fixed to the known value and the assembly volume fraction was calculated based on known sample composition and partial molar volumes of each component, assuming that all molecules take part in assembly formation. This yielded an estimation of the assembly volume fraction, which was fixed to the calculated value during fitting to avoid over parameterization. The scattering length density (SLD) of the assembly was fitted. Molar volumes and complete parameter sets can be found in the ESI.†
Discussion
Phase diagrams
Adding dye to a solution of the cationic surfactant DTAB leads to a concentration-dependency of solution stability. This is visualized by phase diagrams shown in Fig. 2. In all cases, a homogeneous solution was obtained for sufficient surfactant excess and denoted as 1-phase region in each phase diagram. Phase separation and concomitant entry into a 2-phase region was observed at different dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB ratios for each dye and will be discussed in the following. Within the selected concentration region, the correlation between the dye concentration [Dye]tot and DTAB concentration [DTAB]tot where phase separation occurs can be described by a linear relationship (eqn (1)).
DTAB ratios for each dye and will be discussed in the following. Within the selected concentration region, the correlation between the dye concentration [Dye]tot and DTAB concentration [DTAB]tot where phase separation occurs can be described by a linear relationship (eqn (1)).| [Dye]tot = A·[DTAB]tot + B | (1) |
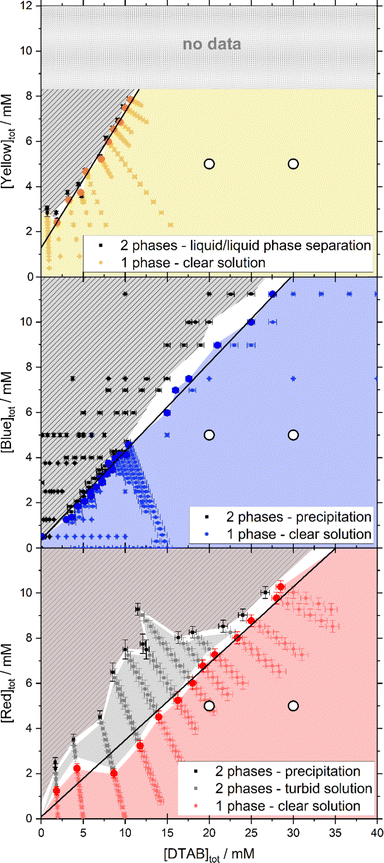 | ||
| Fig. 2 Concentration-dependent phase behaviour and linear fit of phase transition threshold line (eqn (1)) for Yellow (A = 0.60, B = 1.29 mM), Blue (A = 0.39, B = 0.38 mM) and Red (A = 0.34, B = 0.09 mM) in combination with DTAB. The phase transition threshold line was obtained from fitting to the sample compositions marked with bold points in the 1-phase region. All phase diagrams were observed 30 s after mixing and at room temperature. The solvent was an aqueous NaHCO3/Na2CO3-buffer in all cases. In the Yellow/DTAB diagram, dye concentrations higher than 8 mM were not accessible, as the Yellow stock solution used for sample preparation would have exceeded the solubility limit of Yellow. White circles (○) display the composition of samples which were investigated with SANS. | ||
In case of Yellow and DTAB a liquid/liquid phase separation is observed. Combining either Blue or Red with the cationic surfactant DTAB gives rise to the formation of solid complexes above a threshold ratio of dye and DTAB concentration. The formation of such solid dye–surfactant complexes, from oppositely charged species, was described previously for various systems.24,25,31,32
Determining the phase transition threshold line according to eqn (1) indicates the stoichiometry of dye–surfactant complex formation within the observed concentration range (2 mM ≤ [DTAB]tot ≤ 30 mM). This was done by a linear fit to the compositions of samples containing the highest concentration of dye still being within the 1-phase region. Data points used for fitting are highlighted in the corresponding phase diagrams.
For Yellow and DTAB, A = 0.60 and B = 1.29 mM suggests a stoichiometry of [Yellow]tot![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [DTAB]tot = 1:1.67 for an entry into the 2-phase region. At low DTAB concentration, a deviation from this linearity was observed and not included into the linear fit. This is not unexpected, as the 1-phase region should extend to [Yellow]tot = 11 mM, i.e. the solubility limit of Yellow in the absence of DTAB.33 As Blue and Red show a very high solubility (>25 mM) in the present buffer system and the absence of DTAB, a similar deviation at low DTAB concentration is expected for both of them.33 It was, however, not observed in the investigated DTAB concentration range and likely happens at even lower DTAB concentrations.
[DTAB]tot = 1:1.67 for an entry into the 2-phase region. At low DTAB concentration, a deviation from this linearity was observed and not included into the linear fit. This is not unexpected, as the 1-phase region should extend to [Yellow]tot = 11 mM, i.e. the solubility limit of Yellow in the absence of DTAB.33 As Blue and Red show a very high solubility (>25 mM) in the present buffer system and the absence of DTAB, a similar deviation at low DTAB concentration is expected for both of them.33 It was, however, not observed in the investigated DTAB concentration range and likely happens at even lower DTAB concentrations.
The phase diagram of Blue and DTAB shows a defined phase transition. In the 2-phase region precipitates with crystalline appearance are formed. This suggests high ordering of the complexes, which was confirmed by wide-angle X-ray scattering (WAXS) measurements shown in the ESI† (Fig. S3). From a linear fit based on eqn (1) a stoichiometry of [Blue]tot![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [DTAB]tot = 1
[DTAB]tot = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.56 was determined.
2.56 was determined.
The phase transition threshold between the 1- and 2-phase region is less defined for Red and DTAB. Within the reported observation time of 30 s, two different 2-phase-regions were observed, one appearing as a turbid solution, likely due to liquid/liquid phase separation, and the other one showing unambiguous precipitation of crystalline Red–DTAB-complexes. A linear fit based on eqn (1) was performed to obtain information on the transition from the 1-phase-region into the 2-phase-region, independent of the appearance of the 2-phase-region. It yielded a stoichiometry for Red–DTAB complex formation of [Red]tot![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [DTAB]tot = 1
[DTAB]tot = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.94. It should be noted that not all samples containing dye and DTAB in the 1-phase-region were long-term stable. Some samples close to the precipitation threshold were stable within the observation time of 30 s, but precipitated after 1 h to 1 day. This occurred more frequently at [DTAB]tot below the critical micelle concentration of DTAB.
2.94. It should be noted that not all samples containing dye and DTAB in the 1-phase-region were long-term stable. Some samples close to the precipitation threshold were stable within the observation time of 30 s, but precipitated after 1 h to 1 day. This occurred more frequently at [DTAB]tot below the critical micelle concentration of DTAB.
To conclude this section, the excess of DTAB molecules needed to enter the soluble 1-phase-region differs for all three dyes and increases from 1.67 for Yellow over 2.56 for Blue to 2.94 for Red. The formation of dye–surfactant complexes with a defined stoichiometry is observed in most of the investigated DTAB concentration range.
UV/vis-spectroscopy
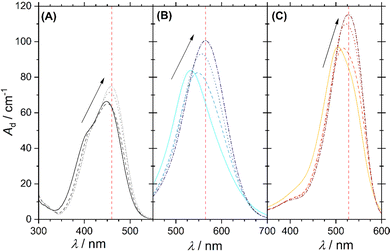
Dye–surfactant aggregation is commonly studied by means of UV/vis-spectroscopy and various models were developed to quantitatively evaluate dye–surfactant interaction.32,34–37 These models rely on a change in dye absorption upon addition of surfactant, which is explained by the interaction between dye and surfactant causing a polarity change in the microenvironment of the dye.34,38 For the dyes Yellow, Blue and Red a bathochromic shift of their absorption maximum and an increase in its extinction upon successive addition of DTAB in the 1-phase region was observed (Fig. 3). It should be pointed out, that for a given dye concentration the 1-phase region only exists at sufficiently high DTAB concentrations (Fig. 2). At low DTAB concentrations, the 2-phase region occurs due to liquid/liquid phase separation or precipitation of dye–DTAB complexes. Therefore, Fig. 3 only shows absorption spectra of dye–DTAB solutions in the 1-phase region in comparison to the absorption spectrum of the solution of pure dye. The total dye concentration was [Dye]tot = 2.5 mM in all cases. The value Ad designating the y-axis of all spectra corresponds to the absorbance A of the sample divided by the optical path length d. The optical path length was d = 0.01 cm in all cases. In the present work, Ad was used consistently in place of A in order to simplify calculations and mathematical expressions by not having to consider the path length. [Dye]tot = 2.5 mM was chosen to provide a dye concentration high enough to obtain a reasonable scattering signal during contrast variation SANS experiments, which will be reported more extensively in a follow-up publication. At the same time it is low enough to permit the recording of UV/vis spectra in a cuvette with 0.01 cm path length while not significantly exceeding a maximum absorbance of A = 1.As visible from Fig. 3, bathochromic shifts of absorption maxima of Δλmax = 11 nm, Δλmax = 34 nm and Δλmax = 24 nm were observed for Yellow, Blue and Red between a solution containing only dye and a solution containing dye and [DTAB]tot = 90 mM. These values signal a bathochromic shift significant enough to perform quantitative evaluation of recorded UV/vis-spectra with respect to an underlying dye–surfactant association equilibrium. For this purpose, dye absorbance was monitored as a function of DTAB concentration at a given wavelength. For Yellow, this wavelength was chosen to λ = 460 nm, for Blue to λ = 565 nm and for Red to λ = 528 nm. These wavelengths are indicated in Fig. 3 by a red, vertical line for each dye. They correspond to the wavelength of maximum absorbance in the sample with highest surfactant excess. [Dye]tot was kept constant at 2.5 mM while varying DTAB concentration between 0 mM < [DTAB]tot < 90 mM.
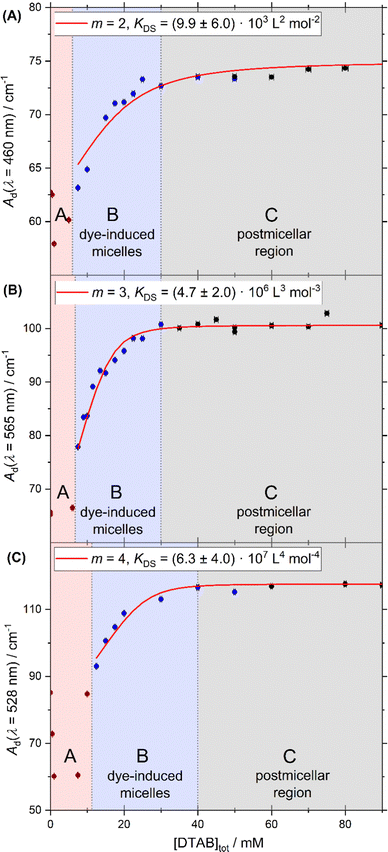
Fig. 4 shows the development of Ad as a function of [DTAB]tot at [Dye]tot = 2.5 mM for all three dyes at wavelengths indicated in Fig. 3 by the red lines. Fig. 4 permits the distinction of three surfactant concentration regions commonly referred to when observing the aggregation between oppositely charged dye and surfactant:32,36,39
Within the last 70 years, various models were developed for the quantitative evaluation of equilibrium constants from UV/vis-spectroscopic data.40–42 In the case of solute-surfactant association it is possible to either assume a thermodynamic equilibrium between a solute molecule and m surfactant molecules or between one solute molecule and one surfactant micelle. The latter gives rise to the well-known Benesi–Hildebrand equation frequently used in case of high surfactant excess.32,34,36,43 In the current system, however, the application of a dye-micelle equilibrium model is not suitable as (1) surfactant concentrations studied are too low to assume surfactant excess and (2) some of the studied surfactant concentrations lay below the cmc of DTAB without the dye. Therefore, an equilibrium between one dye molecule Dye and m free surfactant molecules S on one side and the complex DyeSm on the other side is considered, assuming that micelle formation does only occur due to the interaction between dye and surfactant.
| Dye + m S ⇌ DyeSm | (2) |
The assumption of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m rather than a 1
m rather than a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for dye–surfactant association in solution is reasonable considering observed stoichiometries for dye–surfactant binding determined in eqn (1) for the solid dye–surfactant complex. The equilibrium– or dye–surfactant association constant KDS for eqn (2) can be written as:
1 stoichiometry for dye–surfactant association in solution is reasonable considering observed stoichiometries for dye–surfactant binding determined in eqn (1) for the solid dye–surfactant complex. The equilibrium– or dye–surfactant association constant KDS for eqn (2) can be written as:
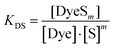 | (3) |
[DyeSm], [Dye] and [S] denote molar equilibrium concentrations of the dye–surfactant complex, dye monomer and surfactant monomer. Our model based on eqn (2) is fully compatible with the choice of [Dye]tot = 2.5 mM. The model formally describes the co-assembly of one dye molecule with m surfactant molecules in equilibrium with monomeric dye and monomeric surfactant. This feature well includes the possibility of mixed micelles with the same stoichiometry as DyeSm. In other words, if this co-assembly triggers micelle formation it is automatically included.
It is emphasized that preliminary self-assembly equilibria of dye molecules are neglected. Indeed, previous studies on the self-assembly of the three dyes showed, that Blue self-assembles to dimers and Red to fractal-like aggregates, whereas Yellow remains molecularly dissolved in their pure solution without surfactant.33 However, self-assembly was suggested to be mainly driven by π–π-stacking and hydrogen bonding interactions, which are generally weaker than the strong electrostatic attraction between oppositely charged dye- and surfactant molecules.26 Therefore, dye–dye complexes are considered to be easily broken apart within dye–DTAB mixed micelles due to strong electrostatic interaction between negatively charged dye molecules and cationic surfactant molecules. An observation confirming preferential binding between dye and DTAB molecules is the precipitation of solid dye–DTAB complexes at small surfactant concentrations (2-phase region, Fig. 2).
Taking total molar concentrations of the dye monomer [Dye]tot and the surfactant [S]tot = [DTAB]tot into account, eqn (3) can be written as:
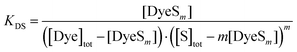 | (4) |
Assuming that only the dye and the dye–surfactant complex [DyeSm] absorb at the relevant wavelength (indicated by the red vertical lines of Fig. 3) and assuming the validity of Beer–Lambert Law for both species, the absorbance of a sample can be expressed as:
| Ad = εDye·[Dye] + εDSm·[DyeSm] | (5) |
With [Dye] = [Dye]tot − [DyeSm] and with Ad,0 = εDye [Dye]tot, eqn (6) as an expression for [DyeSm] is revealed:
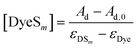 | (6) |
ε Dye and εDSm are the molar extinction coefficients of the dye and the dye–surfactant complex respectively. Assuming a stoichiometry m, a value KDS can therefore be calculated from each data pair Ad = f([S]tot) by substituting eqn (6) into eqn (4). Ad,0 and εDye are known from the absorption spectrum of pure dye, whereas εDS was obtained from the sample with maximum surfactant excess, located in the postmicellar region where dye absorption does not change upon further addition of surfactant.
Model fits were carried out with six stoichiometries. For a given stoichiometry m, KDS was determined from the Ad = f([S]tot) data pair of N samples mostly located in the region of dye-induced micelles and indicated as blue data points in Fig. 4. The average of all KDS obtained for this stoichiometry m was then taken and used to calculate the theoretical absorbance Ad,theo for each of the N samples. The deviation of Ad,theo from the recorded absorbance Ad of the respective sample yielded the χred2 value defined by eqn (7).
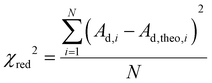 | (7) |
This procedure was performed for six stoichiometries. Corresponding χred2 values are reported in Table 1 for each dye. Due to the absence of specific spectral features signalling a build-up and disassembly or modification of soluble dye–surfactant complexes DyeSm at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m ratio, it was not possible to employ a more straightforward analysis technique to obtain information on the stoichiometry of dye–surfactant binding.44 Furthermore, as discussed earlier, the application of a previously reported analysis technique assuming 1
m ratio, it was not possible to employ a more straightforward analysis technique to obtain information on the stoichiometry of dye–surfactant binding.44 Furthermore, as discussed earlier, the application of a previously reported analysis technique assuming 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of dye–surfactant binding in the region of dye-induced micelles (B, Fig. 4) would have been unreasonable.45 Therefore, strategy to obtain information on stoichiometry m and association KDS of dye–surfactant binding, presented here, is considered to be most suitable for the system under consideration.
1 stoichiometry of dye–surfactant binding in the region of dye-induced micelles (B, Fig. 4) would have been unreasonable.45 Therefore, strategy to obtain information on stoichiometry m and association KDS of dye–surfactant binding, presented here, is considered to be most suitable for the system under consideration.
| Dye | Yellow | Blue | Red |
|---|---|---|---|
| Data interval | 7.5 mM ≤ [S]tot ≤ 50 mM | 7.5 mM ≤ [S]tot ≤ 25 mM | 12.5 mM ≤ [S]tot ≤ 55 mM |
| m | χ red 2 | χ red 2 | χ red 2 |
| 1 | 29.78 | 145.97 | 44.53 |
| 2 | 1.64 | 137.88 | 11.26 |
| 3 | 51.28 | 2.22 | 4.64 |
| 4 | 66.02 | 378.88 | 3.35 |
| 5 | 71.83 | 626.11 | 4.02 |
| 6 | 74.03 | 608.56 | 5.19 |
The data are best described by a stoichiometry m at which the lowest χred2 is obtained. This stoichiometry, together with the corresponding dye–surfactant association constant KDS is summarized in Table 2. Ad,theo = f([S]tot) based on these values is displayed in Fig. 4 as a red curve. Experimental data points are reasonably well described by these curves so that the model can be considered to be a relatively good approximation under the given conditions.
| Dye | Yellow | Blue | Red |
|---|---|---|---|
| m | 2 | 3 | 4 |
| K DS(m) [Lm mol−m] | (9.9 ± 6.0) × 103 | (4.7 ± 2.0) × 106 | (6.3 ± 4.0) × 107 |
| χ red 2 | 1.64 | 2.22 | 3.35 |
Association constants KDS obtained by this procedure suffer from considerable uncertainties. However, information about their order of magnitude is obtained. In addition to that, all KDS lay well above 1, which means that dye–surfactant complex formation is strongly favoured.
It is remarkable that the stoichiometry of dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB binding decreases from 1
DTAB binding decreases from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for Yellow over 1
2 for Yellow over 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for Blue to 1
3 for Blue to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for Red. The same trend was established for the stoichiometry of dye
4 for Red. The same trend was established for the stoichiometry of dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB binding from evaluation of the phase transition threshold line in the phase diagrams (Fig. 2). In addition to that, the number of DTAB molecules aggregating with one dye molecule in the homogeneous 1-phase region is always higher than that obtained from the analysis of the phase transition threshold in the phase diagram. This is consistent with the assumption that soluble DyeSm aggregates are only formed below a certain dye
DTAB binding from evaluation of the phase transition threshold line in the phase diagrams (Fig. 2). In addition to that, the number of DTAB molecules aggregating with one dye molecule in the homogeneous 1-phase region is always higher than that obtained from the analysis of the phase transition threshold in the phase diagram. This is consistent with the assumption that soluble DyeSm aggregates are only formed below a certain dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB threshold ratio.
DTAB threshold ratio.
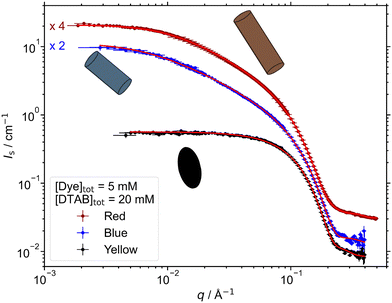
Small-angle neutron scattering
SANS studies were performed on samples which, according to their dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
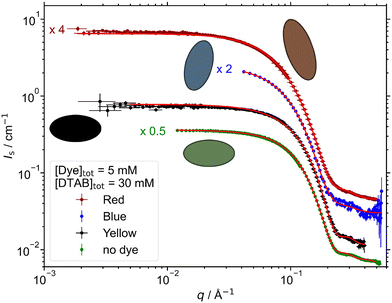
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB ratio, lay within the region of dye-induced micelles. The impact of adding different dye molecules at a concentration of [Dye]tot = 5 mM on the morphology of DTAB micelles was studied. Fig. 5 displays SANS curves from solutions containing [Dye]tot = 5 mM and [DTAB]tot = 30 mM in comparison to the SANS curve of pure DTAB at that concentration. The latter was described with the form factor model of an oblate ellipsoid including a structure factor derived by Hayter and Penfold to consider inter-micellar interaction.46,47 The choice of this model was based on work by Bergström and Pedersen whom investigated the morphology of DTAB micelles in brine solution.48 The use of such a structure factor significantly improved quality of the fit, even though no obvious correlation peak is visible in the dilute solution of DTAB (Fig. 5), which also contains a rather high concentration of buffer salt (ionic strength I ≈ 0.25 M).
DTAB ratio, lay within the region of dye-induced micelles. The impact of adding different dye molecules at a concentration of [Dye]tot = 5 mM on the morphology of DTAB micelles was studied. Fig. 5 displays SANS curves from solutions containing [Dye]tot = 5 mM and [DTAB]tot = 30 mM in comparison to the SANS curve of pure DTAB at that concentration. The latter was described with the form factor model of an oblate ellipsoid including a structure factor derived by Hayter and Penfold to consider inter-micellar interaction.46,47 The choice of this model was based on work by Bergström and Pedersen whom investigated the morphology of DTAB micelles in brine solution.48 The use of such a structure factor significantly improved quality of the fit, even though no obvious correlation peak is visible in the dilute solution of DTAB (Fig. 5), which also contains a rather high concentration of buffer salt (ionic strength I ≈ 0.25 M).
For the description of SANS curves originating from solutions containing both, dye and DTAB, no structure factor was used, as the anionic dye is assumed to interact with the positively charged DTAB head group, which leads to charge screening and reduces medium- to long-range ordering caused by electrostatic interactions.49 The SANS curve of Yellow was well described using the form factor of an oblate ellipsoid with dimensions similar to the pure DTAB micelle (Table 3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) as described in the experimental part. For fitting the SANS curve emerging from a pure DTAB solution, the product of an oblate ellipsoidal form factor and a structure factor by Hayter and Penfold was used.46,47 In the case of the form factor model of a triaxial ellipsoid, the length parameter is equivalent to the longest radius
50 v/v) as described in the experimental part. For fitting the SANS curve emerging from a pure DTAB solution, the product of an oblate ellipsoidal form factor and a structure factor by Hayter and Penfold was used.46,47 In the case of the form factor model of a triaxial ellipsoid, the length parameter is equivalent to the longest radius
| Dye | Model | Cross section | Length | |
|---|---|---|---|---|
| r minor/Å | r major/Å | L/Å | ||
| a Oblate ellipsoid × structure factor; symbols: rp – polar radius, req – equatorial radius, rminor – minor cross section radius, rmajor – major cross section radius, L – length. | ||||
| No dye | Oblate ellipsoida | r eq = 22.357 ± 0.009 | r p = 14.048 ± 0.008 | |
| Yellow | Oblate ellipsoid | r eq = 21.73 ± 0.04 | r p = 13.98 ± 0.06 | |
| Blue | Triaxial ellipsoid | 15.3 ± 0.2 | 22.0 ± 0.3 | 32.0 ± 0.3 |
| Red | Triaxial ellipsoid | 15.93 ± 0.02 | 22.32 ± 0.03 | 40.31 ± 0.04 |
For the description of SANS curves emerging from solutions of Blue with DTAB and Red with DTAB, the assumption of a more anisometric micellar morphology became necessary. This was realized by moving to a triaxial ellipsoid model to fit the data.50 Resulting geometrical dimensions are displayed in Table 3. Interestingly, the two smaller radii of each triaxial ellipsoid are similar to the polar and equatorial radius rp and req of oblate ellipsoidal DTAB micelles. This points towards an one-dimensional growth of DTAB micelles upon addition of dye, like it is frequently observed for cationic surfactant micelles upon addition of salt or hydrotrope.51–53
Moving on to a DTAB concentration of [DTAB]tot = 20 mM while remaining at [Dye]tot = 5 mM, micelle morphologies were found to become more anisometric compared to [DTAB]tot = 30 mM (Fig. 6). This is in agreement with literature. A decrease in the spontaneous curvature of surfactant micelles was frequently observed upon hydrotrope addition.53–55 This was also the case for the addition of dye molecules to surfactant systems, with the morphology of assemblies depending on the system in use.56–58
In the present work, the SANS curve from a solution of Yellow and DTAB was described with the form factor model of a triaxial ellipsoid, whereas SANS curves from solutions of Blue or Red and DTAB were described with the form factor model of a cylinder with elliptical cross section.46 The corresponding fit parameters can be found in Table 4. Micelles of Blue or Red and DTAB are very polydisperse in length, which makes sense considering the underlying dynamic equilibrium present in micellar surfactant solutions.59 To fit the data, the ratio between the root-mean-square deviation from the average length (σ) and the average length (Lavg) was set to σ/Lavg = 0.95 for Blue–DTAB micelles according to a strategy employed by Bergström and Pedersen who observed polydisperse mixed micelles formed from sodiumdodecylsulphate and DTAB.60 It was intended to use the same σ/Lavg for the SANS curve emerging from the corresponding Red–DTAB solution. However, the data was better described using σ/Lavg = 0.5. Strikingly, the dimensions of the elliptical cylinder cross section remain very similar to req and rp found for DTAB micelles at [DTAB]tot = 30 mM (Table 4). This confirms the assumption of an unidimensional growth of these micelles upon increase of the dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB ratio from 1
DTAB ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 to 1
6 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
| Dye | Model | Cross Section | Length | |
|---|---|---|---|---|
| r minor/Å | r major/Å | L/Å | ||
| Symbols: σ/Lavg – ratio of the root-mean-square deviation from the average length and the average length. | ||||
| Yellow | Triaxial ellipsoid | 13.8 ± 0.2 | 21.6 ± 0.6 | 24.4 ± 0.6 |
| Blue | Cylinder with elliptical cross section | 13.1 ± 0.2 | 19.6 ± 0.4 | L = 199 ± 3 |
| σ/Lavg = 0.95 | ||||
| Red | Cylinder with elliptical cross section | 14.19 ± 0.02 | 23.64 ± 0.05 | L = 233.5 ± 0.7 |
| σ/Lavg = 0.5 | ||||
The differences in the morphologies of dye–DTAB micelles observed for the different dyes correlate with the stoichiometries found for dye–DTAB binding from the phase transition threshold as well as in solution. This can easily be related to the SANS sample composition relative to the phase transition threshold in the concentration-dependent phase diagrams of Fig. 2. For the dye Red, the sample compositions investigated with SANS, i.e. [Dye]tot = 5 mM and [DTAB]tot = 20 mM or 30 mM lay much closer to its phase transition threshold than for Blue or Yellow. Conversely, the phase transition threshold in the phase diagram of Yellow and DTAB is the farthest away from these sample compositions. The micelles formed between [Yellow]tot = 5 mM and [DTAB]tot = 30 mM are about the same size as pure DTAB micelles, indicating that the composition of this sample presumably lays close to the post-micellar region, where assembly morphology is determined by DTAB micelles due to their excess presence. Furthermore, it is logical to assume a DTAB excess at a Yellow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB ratio of 1
DTAB ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, considering that Yellow and DTAB interact at a stoichiometry of 1
6, considering that Yellow and DTAB interact at a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Table 2).
2 (Table 2).
To conclude, the morphology of dye–surfactant micelles depends on the dye added to the solution. The magnitude of observed deviations from the morphology of pure DTAB micelles at a given sample composition inversely correlates with dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB binding stoichiometries found at the phase transition threshold as well as in solution. The one-dimensional growth of DTAB micelles upon dye addition was strongest for the addition of Red, which binds to DTAB at a stoichiometry of Red
DTAB binding stoichiometries found at the phase transition threshold as well as in solution. The one-dimensional growth of DTAB micelles upon dye addition was strongest for the addition of Red, which binds to DTAB at a stoichiometry of Red![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in solution, medium for the addition of Blue with a stoichiometry of Blue
4 in solution, medium for the addition of Blue with a stoichiometry of Blue![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and weakest for Yellow with a stoichiometry of Yellow
3 and weakest for Yellow with a stoichiometry of Yellow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. A general increase in micellar anisometry when increasing the dye
2. A general increase in micellar anisometry when increasing the dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB ratio from 1
DTAB ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 to 1
6 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, i.e. when moving closer to the phase transition threshold from the side of DTAB excess, was observed for all dyes.
4, i.e. when moving closer to the phase transition threshold from the side of DTAB excess, was observed for all dyes.
Conclusions
Three 3-chloro-4-hydroxy-phenylazo dyes, each of them carrying a negative charge due to deprotonation of the phenolic hydroxyl group at alkaline pH, were shown to differ in the stoichiometry of their co-assembly with the cationic surfactant DTAB.For all dyes, phase separation of dye–DTAB solutions was observed above a given dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB ratio with the ratio depending on the dye. It was observed to decrease from Yellow (1
DTAB ratio with the ratio depending on the dye. It was observed to decrease from Yellow (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.67) over Blue (1
1.67) over Blue (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.56) to Red (1
2.56) to Red (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.94) and gives an indication of the stoichiometry of dye–DTAB binding in the coacervate phase for Yellow or the solid precipitate for Blue and Red.
2.94) and gives an indication of the stoichiometry of dye–DTAB binding in the coacervate phase for Yellow or the solid precipitate for Blue and Red.
Furthermore, the stoichiometry of dye–DTAB binding in solution, i.e. the 1-phase region, was studied using UV/vis spectroscopy. Stoichiometries of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were found for Yellow, Blue and Red with DTAB respectively. The lower dye
4 were found for Yellow, Blue and Red with DTAB respectively. The lower dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB ratios obtained in homogeneous solution as compared to the ratios at the respective phase transition threshold can nicely be reconciled with a larger amount of DTAB molecules required for the solubilisation of dye compared to that of the formation of solid complexes.
DTAB ratios obtained in homogeneous solution as compared to the ratios at the respective phase transition threshold can nicely be reconciled with a larger amount of DTAB molecules required for the solubilisation of dye compared to that of the formation of solid complexes.
Furthermore, the morphology of dye–DTAB micelles was studied using SANS. DTAB micelles show an uniaxial growth upon dye addition with its extend being dependent upon the dye and being inversely correlated to dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB binding stoichiometry at a set total concentration of dye and DTAB. This was attributed to a sample composition being closer to the postmicellar region if the dye
DTAB binding stoichiometry at a set total concentration of dye and DTAB. This was attributed to a sample composition being closer to the postmicellar region if the dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB binding stoichiometry is high, e.g. Yellow
DTAB binding stoichiometry is high, e.g. Yellow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, or closer to the phase transition threshold or region of dye–surfactant ion pair formation for a low binding stoichiometry, e.g. Red
2, or closer to the phase transition threshold or region of dye–surfactant ion pair formation for a low binding stoichiometry, e.g. Red![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTAB = 1
DTAB = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. A sample being close to the postmicellar region indicates surfactant excess, which leads to micellar morphology being largely determined by the morphology of surfactant micelles.
4. A sample being close to the postmicellar region indicates surfactant excess, which leads to micellar morphology being largely determined by the morphology of surfactant micelles.
Following the presented investigations, differences in the co-assembly behaviour of three structurally similar azo dyes with DTAB were elucidated. Differences in co-assembly morphology were related to the stoichiometry of dye–DTAB binding. It becomes clear, that even structurally similar molecules can show significant differences in their interaction with a surfactant.
Author contributions
Conceptualization: WM, RS, BN, KH, experimental: WM, RS, GS, JK, analysis: WM with contributions from RS, GS, KH, BN, writing: WM, editing: RS, BN, KH, GS, funding acquisition: RS, BN, KHConflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Institut Laue-Langevin for the provision of internal beamtime (https://doi.ill.fr/10.5291/ILL-DATA.INTER-557). The authors thank the Paul Scherrer Institut for the provision of internal beamtime (Proposal 20221454). The authors gratefully acknowledge the use of the Partnership for Soft Condensed Matter (PSCM) facilities. The authors acknowledge the Bundesanstalt für Materialforschung und –prüfung (BAM) for measurements at the MOUSE SAXS-instrument. W. M. acknowledges funding for a PhD scholarship from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 847439 within the InnovaXN framework (Project number XN2019-ILL23). This work benefited from the use of the SasView application, originally developed under NSF award DMR-0520547. SasView contains code developed with funding from the European Union's Horizon 2020 research and innovation programme under the SINE2020 project, grant agreement No. 654000.Notes and references
- G. A. Silva, C. Czeisler, K. L. Niece, E. Beniash, D. A. Harrington, J. A. Kessler and S. I. Stupp, Science, 2004, 303, 1352–1355 CrossRef CAS PubMed.
- K. Rajangam, H. A. Behanna, M. J. Hui, X. Han, J. F. Hulvat, J. W. Lomasney and S. I. Stupp, Nano Lett., 2006, 6, 2086–2090 CrossRef CAS PubMed.
- S. Zhou, C. Burger, B. Chu, M. Sawamura, N. Nagahama, M. Toganoh, U. E. Hackler, H. Isobe and E. Nakamura, Science, 2001, 291, 1944–1947 CrossRef CAS PubMed.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967–973 CrossRef CAS PubMed.
- A. P. H. J. Schenning, J. v Herrikhuyzen, P. Jonkheijm, Z. Chen, F. Würthner and E. W. Meijer, J. Am. Chem. Soc., 2002, 124, 10252–10253 CrossRef CAS PubMed.
- A. N. Shipway, E. Katz and I. Willner, ChemPhysChem, 2000, 1, 18–52 CrossRef CAS PubMed.
- O. D. Velev and E. W. Kaler, Langmuir, 1999, 15, 3693–3698 CrossRef CAS.
- D. J. Norris and Yu. A. Vlasov, Adv. Mater., 2001, 13, 371–376 CrossRef CAS.
- O. D. Velev and E. W. Kaler, Adv. Mater., 2000, 12, 531–534 CrossRef CAS.
- L. C. Palmer, Y. S. Velichko, M. Olvera de la Cruz and S. I. Stupp, Philos. Trans. R. Soc., A, 2007, 365, 1417–1433 CrossRef CAS PubMed.
- M. Rubinstein and G. A. Papoian, Soft Matter, 2012, 8, 9265–9267 RSC.
- H. J. Kwon and J. P. Gong, Curr. Opin. Colloid Interface Sci., 2006, 11, 345–350 CrossRef CAS.
- G. Du, D. Belić, A. Del Giudice, V. Alfredsson, A. M. Carnerup, K. Zhu, B. Nyström, Y. Wang, L. Galantini and K. Schillén, Angew. Chem., 2022, 134, e202113279 Search PubMed.
- N. Carl, S. Prévost, R. Schweins and K. Huber, Soft Matter, 2019, 15, 8266–8271 RSC.
- M. Antonietti, J. Conrad and A. Thuenemann, Macromolecules, 1994, 27, 6007–6011 CrossRef CAS.
- S. G. Trindade, L. Piculell and W. Loh, Langmuir, 2022, 38, 2906–2918 CrossRef CAS PubMed.
- I. Willerich and F. Gröhn, Angew. Chem., Int. Ed., 2010, 49, 8104–8108 CrossRef CAS PubMed.
- N. Carl, W. Müller, R. Schweins and K. Huber, Langmuir, 2020, 36, 223–231 CrossRef CAS PubMed.
- A. Zika, M. Agarwal, R. Schweins and F. Gröhn, Chem. – Eur. J., 2022, 29, e202203373 Search PubMed.
- I. Willerich and F. Gröhn, J. Am. Chem. Soc., 2011, 133, 20341–20356 CrossRef CAS PubMed.
- S. Kanai and M. Muthukumar, J. Chem. Phys., 2007, 127, 244908 CrossRef PubMed.
- H. M. Fares and J. B. Schlenoff, J. Am. Chem. Soc., 2017, 139, 14656–14667 CrossRef CAS PubMed.
- K. Kolman, G. Poggi, M. Baglioni, D. Chelazzi, P. Baglioni, M. Persson, K. Holmberg and R. Bordes, J. Colloid Interface Sci., 2022, 615, 265–272 CrossRef CAS PubMed.
- Y. Guan, M. Antonietti and C. F. J. Faul, Langmuir, 2002, 18, 5939–5945 CrossRef CAS.
- C. F. J. Faul and M. Antonietti, Chem. – Eur. J., 2002, 8, 2764–2768 CrossRef CAS PubMed.
- C. F. J. Faul and M. Antonietti, Adv. Mater., 2003, 15, 673–683 CrossRef CAS.
- Mantid Project, Mantid (2013): Manipulation and Analysis Toolkit for Instrument Data DOI:10.5286/SOFTWARE/MANTID.
- O. Arnold, J. C. Bilheux, J. M. Borreguero, A. Buts, S. I. Campbell, L. Chapon, M. Doucet, N. Draper, R. Ferraz Leal, M. A. Gigg, V. E. Lynch, A. Markvardsen, D. J. Mikkelson, R. L. Mikkelson, R. Miller, K. Palmen, P. Parker, G. Passos, T. G. Perring, P. F. Peterson, S. Ren, M. A. Reuter, A. T. Savici, J. W. Taylor, R. J. Taylor, R. Tolchenov, W. Zhou and J. Zikovsky, Nucl. Instrum. Methods Phys. Res., Sect. A, 2014, 764, 156–166 CrossRef CAS.
- Neutrons, X-rays, and light: scattering methods applied to soft condensed matter, ed. P. Lindner and T. Zemb, Elsevier, Amsterdam, Boston, 1st edn, 2002 Search PubMed.
- U. Keiderling, Appl. Phys. A: Mater. Sci. Process., 2002, 74, s1455–s1457 CrossRef CAS.
- G. K. G. Carvalho Barros, L. J. N. Duarte, R. P. F. Melo, F. W. B. Lopes and E. L. Barros Neto, J. Polym. Environ., 2022, 30, 2534–2546 CrossRef CAS.
- O. Owoyomi, O. Z. Junaid and O. E. Olaoye, Phys. Chem. Liq., 2021, 60, 129–140 CrossRef.
- W. Müller, R. Schweins, B. Nöcker, H. Egold and K. Huber, Soft Matter, 2023 10.1039/d3sm00500c.
- R. Sabaté, M. Gallardo, A. de la Maza and J. Estelrich, Langmuir, 2001, 17, 6433–6437 CrossRef.
- S. S. Shah, S. W. H. Shah and K. Naeem, Encyclopedia of Surface and Colloid Science, Taylor & Francis, 3rd edn, 2015, pp. 7141–7149 Search PubMed.
- P. Shah, S. Kumari Jha and A. Bhattarai, J. Mol. Liq., 2021, 340, 117200 CrossRef CAS.
- R. K. Dutta and S. N. Bhat, Can. J. Chem., 1993, 71, 1785–1791 CrossRef CAS.
- A. Rehman, M. U. Nisa, M. Usman, Z. Ahmad, T. H. Bokhari, H. M. A. U. Rahman, A. Rasheed and L. Kiran, J. Mol. Liq., 2021, 115345 CrossRef CAS.
- D. C. Ghosh, P. K. Sen and B. Pal, Int. J. Chem. Kinet., 2021, 53, 1228–1238 CrossRef CAS.
- N. J. Rose and R. S. Drago, J. Am. Chem. Soc., 1959, 81, 6138–6141 CrossRef CAS.
- B. K. Seal, A. K. Mukherjee and D. C. Mukherjee, BCSJ, 1979, 52, 2088–2090 CrossRef CAS.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- J. R. Perussi, V. E. Yushmanov, S. C. Monte, H. Imasato and M. Tabak, Physiol. Chem. Phys. Med. NMR, 1995, 27, 1–15 CAS.
- K. K. Karukstis, D. A. Savin, C. T. Loftus and N. D. D’Angelo, J. Colloid Interface Sci., 1998, 203, 157–163 CrossRef CAS.
- M. Bielska, A. Sobczyńska and K. Prochaska, Dyes Pigm., 2009, 80, 201–205 CrossRef CAS.
- L. A. Feigin and D. I. Svergun, Structure Analysis by Small-Angle X-Ray and Neutron Scattering, Springer, US, 1987 Search PubMed.
- J. B. Hayter and J. Penfold, Mol. Phys., 1981, 42, 109–118 CrossRef CAS.
- M. Bergstrom and J. Pedersen, Phys. Chem. Chem. Phys., 1999, 1, 4437–4446 RSC.
- P. A. Hassan, G. Fritz and E. W. Kaler, J. Colloid Interface Sci., 2003, 257, 154–162 CrossRef CAS PubMed.
- J. A. Finnigan and D. J. Jacobs, J. Phys. D: Appl. Phys., 1971, 4, 72 CrossRef.
- C. A. Dreiss, Soft Matter, 2007, 3, 956–970 RSC.
- F. Quirion and L. J. Magid, J. Phys. Chem., 1986, 90, 5435–5441 CrossRef CAS.
- N. Onoda-Yamamuro, O. Yamamuro, N. Tanaka and H. Nomura, J. Mol. Liq., 2005, 117, 139–145 CrossRef CAS.
- C. A. Dreiss, Wormlike Micelles: Advances in Systems, Characterisation and Applications, Royal Society of Chemistry, 2017, pp. 1–8 Search PubMed.
- E. J. Creatto, F. B. Okasaki, M. B. Cardoso and E. Sabadini, J. Colloid Interface Sci., 2022, 627, 355–366 CrossRef CAS PubMed.
- D. Wang, P. Long, R. Dong and J. Hao, Langmuir, 2012, 28, 14155–14163 CrossRef CAS PubMed.
- L. Li, Y. Yang, J. Dong and X. Li, J. Colloid Interface Sci., 2010, 343, 504–509 CrossRef CAS PubMed.
- A. Kutz, G. Mariani and F. Gröhn, Colloid Polym. Sci., 2016, 294, 591–606 CrossRef CAS.
- A. Patist, S. G. Oh, R. Leung and D. O. Shah, Colloids Surf., A, 2001, 176, 3–16 CrossRef CAS.
- M. Bergström and J. S. Pedersen, Langmuir, 1999, 15, 2250–2253 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sm00501a |
| This journal is © The Royal Society of Chemistry 2023 |