Self-healing nanocomposites via N-doped GO promoted “click chemistry”†
R. V. Siva Prasanna
Sanka‡
 a,
Sravendra
Rana‡
a,
Sravendra
Rana‡
 *b,
Poonam
Singh
b,
Abhishek K.
Mishra
b,
Pankaj
Kumar
b,
Manjeet
Singh
c,
Nanda Gopal
Sahoo
*b,
Poonam
Singh
b,
Abhishek K.
Mishra
b,
Pankaj
Kumar
b,
Manjeet
Singh
c,
Nanda Gopal
Sahoo
 d,
Wolfgang H.
Binder
d,
Wolfgang H.
Binder
 *e,
Gun Jin
Yun
*f and
Chanwook
Park
*g
*e,
Gun Jin
Yun
*f and
Chanwook
Park
*g
aDepartment of Mechanical Engineering, University Institute of Engineering, Chandigarh University, Mohali, India
bSchool of Engineering, University of Petroleum & Energy Studies (UPES), Energy Acres, Bidholi, Dehradun, 248007, India. E-mail: srana@ddn.upes.ac.in
cDepartment of Chemistry, School of Physical Sciences, Mizoram University, Aizawl, 796004, Mizoram, India
dProf. Rajendra Singh Nanoscience and Nanotechnology Centre, Department of Chemistry, D. S. B. Campus, Kumaun University, Nainital, 263001, Uttarakhand, India
eChair of Macromolecular Chemistry, Institute of Chemistry, Faculty of Natural Science II, Martin Luther University Halle-Wittenberg, Von-Danckelmann-Platz 4, Halle, 06120, Germany. E-mail: wolfgang.binder@chemie.uni-halle.de
fDepartment of Aerospace Engineering, Seoul National University, Gwanak-gu Gwanak-ro 1, Seoul, 151-744, South Korea. E-mail: gunjin.yun@snu.ac.kr
gDepartment of Mechanical Engineering, Northwestern University, Evanston, 60208, IL, USA. E-mail: chanwookpark2024@u.northwestern.edu
First published on 24th November 2022
Abstract
N-doped graphene stabilized Cu(I)-catalyzed self-healing nanocomposites are developed. This study found the use of N-doped graphene as both a nanostructured material for enhancing mechanical and conductive properties and a catalyst promoter (a scaffold for catalytic copper(I) particles), helpful to trigger self-healing via “click chemistry”. Due to an increase in electron density on nitrogen atom doping, including the coordination of N-doped rGO with Cu+ ions, nitrogen-doped graphene-supported copper particles demonstrate a higher reaction yield at room temperature without adding any external ligand/base. In this study, only one component (an azide moiety containing a healing agent) was encapsulated, whereas another component (an alkyne moiety containing a healing agent) was as such (without encapsulation) homogeneously dispersed in a matrix. Triggered capsule rupture then induces the contact of the healing agents with the N-doped graphene-based catalyst and the alkyne molecules dispersed in the matrix, inducing a “click”-reaction, allowing onsite damage to be repaired as determined by mechanical measurements entirely. Tensile measurements were also performed using molecular dynamics (MD) simulations to support the findings. Given the enormous importance of autonomic repair of materials damage, this concept here reports a trustworthy and reliable chemical system with a high level of robustness.
1. Introduction
The whole idea of self-healing materials is inspired by nature in view of how natural healing of wounds and cuts occurs in the living species.1,2 Ultimately, materials undergo natural or artificial degradation and deterioration with time. In the case of structural materials, a long-time degradation gives rise to microcracks that promote materials failure, hence making repair indispensable to improve the reliability and lifetime of the materials. Different concepts to generate materials that can repair autonomically3,4 or via external stimuli such as heat,5 light,6 and pressure7 have been designed using various approaches such as capsule-based covalent systems,8 where the catalyst cost, stability, and processing still represent a challenge. Moreover, the addition of micro/-nanocapsules into matrices reduces the materials performance, especially the tensile strength.9 To achieve self-healing in thermosetting materials, non-covalent bonds such as hydrogen bonds,10,11 π–π stacking,12 and metal–ligand bonds were also introduced in the thermosetting matrix. However, such non-covalent adaptive bond interactions can lead to lower mechanical properties. Carbon derivatives such as carbon black, carbon nanotubes, and graphene demonstrate better mechanical properties when incorporated in the polymeric matrices.13–15 Graphene among the nano-fillers has a large effect on the performance of materials by enhancing its conductive and mechanical properties;16 furthermore, graphene serves as a catalyst to trigger organic reactions.17–19The objective of this study is to obtain self-healing at room temperature – a feature which often is not achieved in many reported self-healing materials, where healing often is accomplished at elevated temperatures, in turn impeding technical usability. We have used a highly stable and recyclable heterogeneous catalyst (nitrogen doped graphene oxide nanosheet immobilized copper(I) particles; NRGO/Cu(I)), which undergoes click reactions under both solvent and bulk conditions. Due to an increase in electron density on nitrogen atom doping, including the coordination of N-doped rGO with Cu+ ions, nitrogen-doped graphene-supported copper particles demonstrate a higher reaction yield at room temperature without adding any external ligand/base. Thus, Cu nanoparticle decorated graphene nanosheets can function as an effective catalyst in click chemistry and provide an application for achieving highly dispersed graphene composite materials with self-healing properties. In this study, the capsules contain trivalent azide as healing/crosslinking agents and alkyne molecules dispersed in the epoxy matrix (Scheme 1). Self-healing occurs after rupture formation on the surface of capsules, leading to the ‘click’ based crosslinking reaction in the presence of Cu(I) nanoparticles on the graphene surface. The healing agents were encapsulated using an in situ condensation process, and we observed the stability of healing agents inside the capsules for several months.
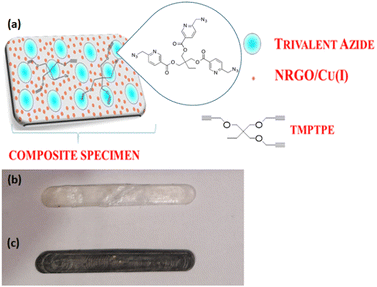 | ||
| Scheme 1 (a) Schematic diagram of the presence of healing agents and catalysts in an epoxy matrix; specimens (dimensions of 35 × 5 × 2 mm) – pure epoxy (b) and epoxy composites (c). | ||
In addition to the self-healing properties, mechanical reinforcement of graphene-based catalysts is studied using molecular dynamics (MD) simulations. MD is an atomic (or molecular) level computer simulation based on ab initio principles, enabling sophisticated studies for nanomaterials intractable in general experimental settings. MD has been widely adopted for characterizing the thermomechanical properties of nanomaterials and nanocomposites.20–23 It has also been used to study atomic-level mechanisms for various self-healing materials.24–26 In this study, mechanical tensile tests are performed via MD simulations to support the findings from the experimental tensile test.
This paper is organized as follows: Section 2 covers the synthesis and encapsulation of healing agents and their further utilization in developing self-healing composites. Section 3.1 includes the characterization of the developed healing agents and composite materials. The mechanical analysis, including the computation study, is covered in Section 3.2. Finally, Section 3.3 and, moreover, 3.4 cover the self-healing and conductive properties, respectively. Lastly, Section 4 concludes this study.
2. Experimental section
2.1 Materials and methods
Formaldehyde (30.031 g mol−1), trimethylamine (99% anhydrous), sodium dodecyl benzene sulphonate (SDBS) (348.5 g mol−1), diglycidylether of bisphenol A (DGEBA) (340.41 g mol−1) resin, and diethylenetriamine (DETA) (103.17 g mol−1) were purchased from Sigma-Aldrich. X-Ray photoelectron spectroscopy (XPS; XPS PHI Versa Probe 5000 spectrometer at a pressure of 1 × 10−9 torr) and high-resolution transmission electron microscopy (HRTEM; FEI Titan3 80–300 electron microscope with a cs-image aberration corrector (FEI Company) at an acceleration voltage of 300 kV) were performed to characterize the prepared catalyst. The synthesized nanocapsules and their dispersion in the epoxy matrix were analyzed using a field emission scanning electron microscope (FE-SEM; Phillips XL30 ESEM, with a beam diameter of 5 nm at 1 kV). The self-healing performance of the fabricated nanocomposite was evaluated via dynamic mechanical analysis (DMA; single-notch impact testing). The specimens were fabricated with dimensions of 35 × 5 × 2 mm and evaluated through a three-point bending test (ASTM D7264). Initially, a sharp “V” shaped notch of 1 mm depth was made at the center of the specimen (the specimen made of NRGO–Cu(I) (2 mol%) and 15 wt% capsules) was considered for the evaluation. The conductivity of the prepared specimens was investigated by the standard four-point probe method (electrometer model 6517A).2.2 Synthesis of healing agents
These reactions were studied to formulate materials characterized by high self-healing functionality based on inexpensive reactions. Thus, trivalent azide and alkyne were prepared using the earlier reported procedure27,28 (ESI;† Schemes S1, S2 and Fig. S1, S2).2.3 Fabrication of healing agents containing nanocapsules
The preparation of the encapsulation procedure was adopted as per the literature with slight modifications,5 where a trivalent azide-based healing agent was encapsulated. Firstly, poly(vinyl formal) (PVF) (100 mg) and trivalent azide (component 1, 100 mg) were dissolved in chloroform (2 mL) in order to form a dispersed phase. An aqueous surfactant solution (sodium dodecyl sulfate, 10 mL, 0.5 mg mL−1) was added to the above solution. The solution mixture was stirred at 1100 rpm for 1 h, followed by sonication for 2 min (30 s pulse and 15 s pause, Branson W450D Digital, 90% amplitude). CHCl3 was subsequently evaporated at 40 °C for overnight in an open beaker. Dynamic light scattering (DLS) (Viscotek 802) was performed to determine the hydrodynamic diameter and centrifuged at 5000 rpm for 30 min (Fig. S3, ESI†). The precipitate was redispersed in water, and the shape and the morphology of the core–shell nanocapsules were characterized via scanning electron microscopy. The dispersed nanocapsules were freeze-dried to obtain trivalent azide nanocapsules as a white powder.2.4 Composite specimen preparation
The preparation of epoxy-based self-healing nanocomposites was adopted according to the literature. Initially, diglycidylether of bisphenol A (DGEBA) known as the EPON 828 epoxy resin of 500 mg was added into a flask, and air was removed using the degassing technique. The calculated amount of nanocapsules containing the trivalent azide components of 5–20 wt% in total (azide + alkyne) was used for preparing the samples. The calculated amount of nano-capsules was then manually introduced into the mixture, followed by mixing using a vortex and a bath sonicator for 15 min. Subsequently, the alkyne component was introduced into the mixture and allowed to mix until it was finely dispersed in the matrix. Subsequently, the required amount of NRGO/Cu(I) catalysts (2 mol% per functional group of azide/alkyne) was mixed with the resin, followed by the addition of diethylenetriamine (DETA) (12 pph). The final mixture was poured into a silicone mold of standard size (30 × 5 × 2 mm) and the specimens were allowed to cure at room temperature for 24 h (Scheme 1). Furthermore, we have fabricated different specimens with different loadings of capsules (5 wt%, 10 wt%, 15 wt%, and 20 wt%) and catalysts ((CuPPh3)3F, thermally reduced graphene oxide nanosheet copper(I) particles [TRGO/Cu(I)] and NRGO/Cu(I)). The dispersion of capsules in the epoxy matrix was further investigated with the help of SEM.2.5 Molecular dynamics simulations
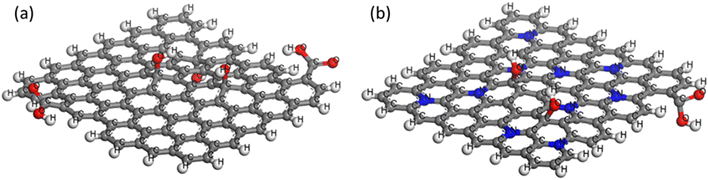
The mechanical properties of pristine epoxy, epoxy + TRGO/Cu(I), and epoxy + NRGO/Cu(I) are computed in MD simulations. The initial models are built-in Materials studio 2017 package. For epoxy resin, DGEBA (also known as EPON 828) and DETA were mixed at a molecular ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for consistency with experiments. TRGO/Cu(I) and NRGO/Cu(I) catalysts are modeled based on the atomic composition characterized in the previous paper of our group.29 Note that for MD tensile simulations, Cu particles are not modeled for several reasons: (1) Cu only constitutes ∼4 wt% of the catalyst (NRGO/Cu(I)); (2) in the atomic scale model, 4 wt% of Cu refers to one atom per graphene flake (Fig. 1); (3) this will have a negligible effect on the tensile properties of the nanocomposites. The generated TRGO and NRGO are described in Fig. 1 and Table S1 (ESI†). TRGO and NRGO are then mixed with epoxy resin (three GO flakes for each model) such that the wt% of the nanoparticles would be 4.16% and 4.15%, respectively. That is, three models are generated: (1) epoxy, (2) epoxy + TRGO (4.16 wt%), and (3) epoxy + NRGO (4.15 wt%). These models are crosslinked up to a crosslink density of 80% using the dynamic crosslinking algorithm reported in ref. 23. The large-scale atomic/molecular massively parallel simulator (LAMMPS)30 is used for MD simulations. Dynamic tensile tests are performed on each MD model with a strain rate of 2 × 108 s−1, which is a widely-accepted setting in MD studies.22 The detailed MD settings are found in the ESI.†
2 for consistency with experiments. TRGO/Cu(I) and NRGO/Cu(I) catalysts are modeled based on the atomic composition characterized in the previous paper of our group.29 Note that for MD tensile simulations, Cu particles are not modeled for several reasons: (1) Cu only constitutes ∼4 wt% of the catalyst (NRGO/Cu(I)); (2) in the atomic scale model, 4 wt% of Cu refers to one atom per graphene flake (Fig. 1); (3) this will have a negligible effect on the tensile properties of the nanocomposites. The generated TRGO and NRGO are described in Fig. 1 and Table S1 (ESI†). TRGO and NRGO are then mixed with epoxy resin (three GO flakes for each model) such that the wt% of the nanoparticles would be 4.16% and 4.15%, respectively. That is, three models are generated: (1) epoxy, (2) epoxy + TRGO (4.16 wt%), and (3) epoxy + NRGO (4.15 wt%). These models are crosslinked up to a crosslink density of 80% using the dynamic crosslinking algorithm reported in ref. 23. The large-scale atomic/molecular massively parallel simulator (LAMMPS)30 is used for MD simulations. Dynamic tensile tests are performed on each MD model with a strain rate of 2 × 108 s−1, which is a widely-accepted setting in MD studies.22 The detailed MD settings are found in the ESI.†
3. Results and discussion
3.1 Materials characterization
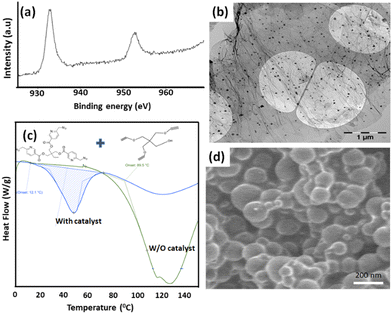
To enhance the catalytic activity of copper and the mechanical properties of the specimen, the nitrogen-doped reduced graphene oxide immobilized copper(I) nanoparticles (NRGO/Cu(I)) were synthesized.29 The XPS analysis of the prepared catalyst confirms the presence of nitrogen and copper in the form of Cu(I), required for the ‘click’ reaction (Fig. 2a), where the peak satellites at 932.3 eV and 952.3 eV confirm the presence of Cu(I), including a small associated peak at 932.7 eV corresponding to a stronger interaction of Cu(I) with N-doped RGO.29The TEM image confirms a uniform dispersion of the copper nanoparticles (Fig. 2b). Furthermore, ‘differential scanning calorimetry (DSC) was performed for the azide and alkyne components in the presence of a catalyst to analyze the ‘click’-based reaction. DSC thermograms at 5 °C min−1 with and without a catalyst are shown in Fig. 2c (2 mol% of the catalyst per functional group). The obtained results from DSC confirm that thermal ‘click’ crosslinking (W/O catalyst) takes place at a higher temperature with a Tonset at 60 °C and a Tp at 130 °C, whereas a lower Tonset (at around 15 °C) and Tp (at 42 °C) were observed for the NRGO/Cu(I) catalyst. Moreover, the azide and alkyne components in the epoxy matrix were stable even after several weeks, confirming the stability of the healing agents in the epoxy matrix. After confirming the stability of the healing agents, trivalent azide (healing agent) was encapsulated. FE-SEM analysis was performed to characterize the developed capsules, where the particle size of the capsules was measured to be about 100–150 nm (Fig. 2d).
After evaluating the stability of the catalyst and healing agents, self-healing nanocomposites were developed, where the azide component was encapsulated, whereas the alkyne component (TMPTE) was directly dispersed in an epoxy matrix (Scheme 1). The SEM images confirm a uniform dispersion of the capsules in the epoxy matrix (Fig. 3) and the stability of capsules during the fabrication of the composites. However, agglomeration of capsules was observed for the composites containing more than 15 wt% capsules. Thus, to investigate the mechanical and self-healing performances, a 15 wt% concentration of capsules containing healing agents and homogeneous and heterogeneous catalysts was taken into account, where capsules remain intact and are not aggregated. A core–shell structure comprising the healing component was observed by TEM analysis (after 2 weeks of fabrication), confirming the stability of capsules during the fabrication of composite materials (Fig. S4, ESI†).
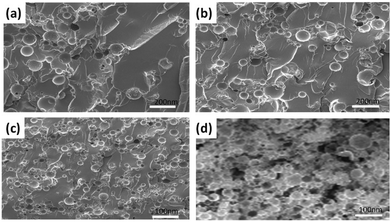 | ||
| Fig. 3 SEM images of capsules with (a) 5 wt%, (b) 10 wt%, (c) 15 wt% and (d) 20 wt% in the epoxy matrix. | ||
3.2 Mechanical properties
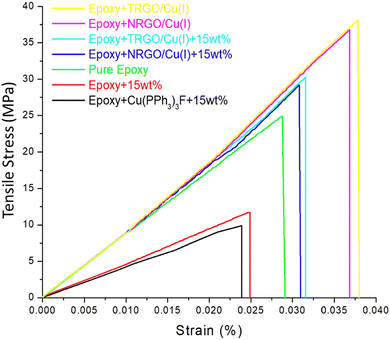
The prepared samples underwent tensile measurements for evaluating their mechanical properties (Table 1). Fig. 4 and Table 1 show a decrease in the Young's modulus and tensile strength for capsules containing specimens. The capsules consisting of healing agents are one of the essential components for developing the self-healing composites; however, the incorporation of capsules acts as inherent voids and thus interrupt the integral structure of materials, therefore decreases the tensile strength and Young's modulus of the composites. The results are in agreement with the empirical models proposed by Sudduth for particulate composites.9,31| Sample | Tensile strength (MPa) | Strain-at-break (%) | Young's modulus |
|---|---|---|---|
| Catalyst amount – 2 mol% per functional group. | |||
| Pure epoxy | 25.0 ± 1 | 2.8 ± 0.1 | 892 ± 10 |
| Epoxy + 15 wt% capsules | 11.7 ± 2 | 2.4 ± 0.3 | 487 ± 20 |
| Epoxy + 15 wt% capsules + Cu(PPh3)3F | 9.8 ± 1 | 2.3 ± 0.1 | 426 ± 20 |
| Epoxy + TRGO/Cu(I) | 38 ± 1 | 3.75 ± 0.2 | 1013 ± 10 |
| Epoxy + 15 wt% capsules + TRGO/Cu(I) | 30.2 ± 2 | 3.15 ± 0.3 | 958 ± 20 |
| Epoxy + NRGO/Cu(I) | 36.8 ± 2 | 3.6 ± 0.2 | 1022 ± 10 |
| Epoxy + 15 wt% capsules + NRGO/Cu(I) | 28.9 ± 2 | 3.0 ± 0.2 | 963 ± 20 |
Interestingly, there is an increase in Young's modulus and tensile strength for the NRGO/Cu(I) and TRGO/Cu(I) incorporated capsule-based composite specimens. This could be due to the effective load transfer between GO and epoxy via interfacial adhesion. However, there is a decrease in mechanical performance after adding capsules to the specimens containing TRGO/Cu(I) and NRGO/Cu(I), which states that an excess addition of capsules can contribute to a reduction of interfacial adhesion between the epoxy and the reinforcing agent.
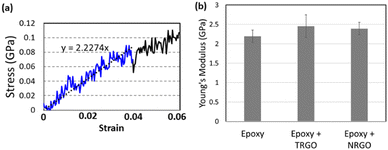
A similar trend was observed in molecular dynamics (MD) simulations. Three cases were studied: pure epoxy (DGEBA + DETA), epoxy + TRGO nanocomposite (4.16 wt%), and epoxy + NRGO nanocomposite (4.15 wt%). The details on the model generation are given in the ESI.† Note that, for each nanocomposite, three GO flakes (either TRGO or NRGO) were randomly dispersed in the epoxy matrix, followed by energy minimization simulations for mode equilibration. Uniaxial tensile tests were conducted on each MD model for three (x, y, and z) directions. The initial 4% of engineering strain of stress–strain curves were used to characterize Young's modulus (Fig. 5a). Assuming the isotropic mechanical properties, the isotropic Young's modulus is averaged over three directions. For each material case, six different models were studied to enhance the reliability of the results. The average Young's modulus is plotted in Fig. 5b, where both the nanocomposites have better Young's modulus than the pure epoxy. It is generally accepted that adding graphene-based nanoparticles enhances an elastic stiffness of nanocomposites unless there is a severe filler agglomeration.32 Since we generated well-dispersed nanocomposites, the increase of Young's modulus is acceptable. It is also important to note that epoxy + TRGO has higher Young's modulus than epoxy + NRGO, indicating that nitrogen doping has a negative effect on Young's modulus. These results are in consistent with previous MD studies on the N-doped CNT nanocomposites,22 where N-doped sites act as a defect to the sp2 hybridized bonds.
Motivated by these results, we further analysed the effect of mechanical performance based on the capsule loading. A series of test specimens with 5 wt%, 10 wt%, 15 wt% and 20 wt% were fabricated containing NRGO/Cu(I) as a catalyst. To investigate the influence of capsule loading on the mechanical performance, tensile testing was carried out. The stress–strain curves and the tensile strength of the prepared specimens are shown in Fig. 6 and Table 2. The results demonstrate a decrease in the tensile strength with an increase in capsule loading from 25 MPa of pure epoxy to 11.5 MPa for 20 wt% of capsules, and in a similar way, the strain at breakpoint decreased from 2.8 for pure epoxy to 2.3 for 20 wt% of capsules. Moreover, with the addition of NRGO/Cu(I), an increase in tensile strength and strain was observed.
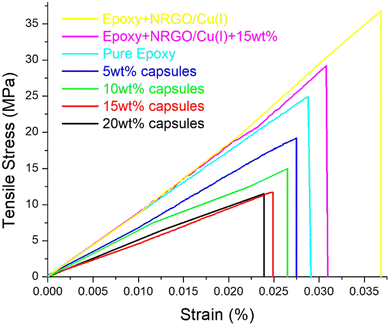 | ||
| Fig. 6 Plot of tensile stress vs. strain curve for the prepared pure epoxy and composite samples with different capsule loadings (strain rate: 0.1 mm min−1, 25 °C). | ||
| Sample | Tensile strength (Mpa) | Strain-at-break (%) | Young's modulus |
|---|---|---|---|
| Epoxy | 25.0 ± 1 | 2.8 ± 0.1 | 892 ± 10 |
| Epoxy + NRGO/Cu(I) | 36.8 ± 2 | 3.6 ± 0.2 | 1022 ± 10 |
| Epoxy + NRGO/Cu(I) + 15 wt% capsules | 28.9 ± 2 | 3.0 ± 0.2 | 963 ± 20 |
| 5 wt% Capsules | 19.1 ± 1 | 2.7 ± 0.2 | 707 ± 20 |
| 10 wt% Capsules | 14.8 ± 1 | 2.6 ± 0.2 | 569 ± 10 |
| 15 wt% Capsules | 11.7 ± 2 | 2.4 ± 0.3 | 487 ± 10 |
| 20 wt% Capsules | 11.5 ± 1 | 2.3 ± 0.1 | 500 ± 20 |
3.3 Self-healing performance
The self-healing performance of the fabricated nanocomposites was evaluated via dynamic mechanical analysis (DMA; single-notch impact testing) (Fig. 7). The healing efficiency was determined as the recovery of the storage modulus using the formula (ESH/EV × 100),33 where EV is the storage modulus of the virgin sample and ESH is the storage modulus of the healed sample. Three time tests were performed for each composition for obtaining similar results.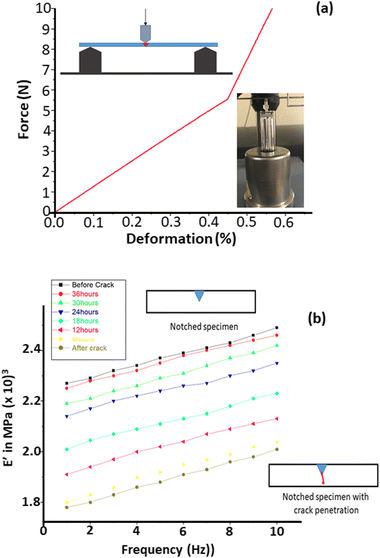 | ||
| Fig. 7 (a) Deformation curve for the applied load. (b) DMA analysis (before and after crack) for the prepared nanocomposites (contain 15 wt% azide capsules) at 25 °C. | ||
The specimens were fabricated with dimensions of 35 × 5 × 2 mm and evaluated through a three-point bending test (ASTM D7264). Initially, a sharp “V” shaped notch of 1 mm depth was made at the center of the specimen (the specimen made of NRGO–Cu(I) (2 mol%) and 15 wt% capsules) was considered for the evaluation. The E′ (2270 MPa) was obtained for the notched specimen of pure epoxy (Fig. 7). An impulsive load of 10 N was applied on the notched specimen via a tensile force in 3D bending mode (Fig. 7a) for allowing the crack to propagate.
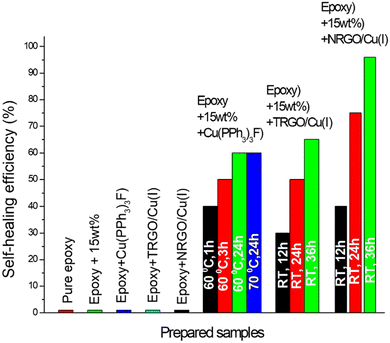
Fig. 7b clearly shows the deformation of the curve, indicating the occurrence of delamination in the specimen owing to crack propagation. During the delamination period, we observed a sudden decrease in E′ (1780 MPa) from DMA (Fig. 7b), confirming the propagation of a crack in the specimen. The recovery of the storage modulus (2250 MPa) was observed from DMA when the specimen was kept at 25 °C for 36 h, where almost 96% of recovery was observed at room temperature prevailing a triazole formation in the presence of NRGO/Cu(I). Specimens without healing agents, i.e., neat epoxy and epoxy with different homogeneous and heterogeneous catalysts (Fig. 8), did not demonstrate a change in E′. Furthermore, the epoxy with 15 wt% capsules also showed no progress in E′ in the absence of the copper catalyst. Interestingly, the specimen with the homogenous catalyst Cu(PPh3)3F and 15 wt% capsules showed a quick recovery of 40% at 60 °C within one h, but failed to achieve the full recovery even after 24 h at 70 °C (Fig. 8).
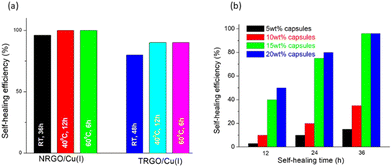
The specimen consisting of 15 wt% capsules and TRGO/Cu(I) demonstrates better results, where up to 85% recovery at 60 °C (36 h) was observed (Fig. 9a). However, the specimen consisting of 15 wt% capsules and the NRGO/Cu(I) catalyst displays 100% recovery at 60 °C even after 6 h and 95% recovery at room temperature after 36 h, which further showed the excellent efficiency of the NRGO/Cu(I) catalyst (Fig. 9a). With these exciting results displayed by NRGO/Cu(I), further investigation was carried out with different loading concentrations of capsules containing 5 wt%, 10 wt%, 15 wt%, and 20 wt% (Fig. 9b). The results state that self-healing at room temperature is even possible with loadings of 20 wt% and 15 wt%. There is no improvement (96%) in self-healing efficiency for both specimens even after 36 h, whereas 5 wt% and 10 wt% could not even demonstrate 50% of self-healing, presumably due to the unavailability of healing agents at the site.
The results demonstrate the higher catalytic activity of NRGO/Cu(I) compared to a commercially available catalyst (with a similar amount of catalyst loading in terms of per functional group, i.e. 2 mol%) and TRGO/Cu(I), due to the scaffold properties of graphene sheets. It promotes the prevention of agglomeration by copper particles due to the formation of the coordination of N-doped rGO with Cu+, including the high binding energy of Cu nanoparticles via a N-doped carbon support. Furthermore, the exclusive interactions of copper particles with N-doped reduced graphene sheets and the ability for electron transfer and capture were highly supportive to enhance the catalytic activity.
The arguments were further supported by TEM analysis (Fig. S5, ESI†), where agglomeration of the homogenous catalyst (Cu(PPh3)3F) in the epoxy matrix was observed, whereas a highly dispersed catalyst image was captured for the heterogeneous catalyst NRGO/Cu(I), required to achieve the self-healing behavior of the prepared composites. The agglomeration of Cu(PPh3)3F in the epoxy matrix leads to limited self-healing compared to the heterogeneous catalyst. Therefore, the results strongly support the significance of NRGO/Cu(I) for its high stability and uniform dispersion in the polymer matrix over the homogenous (Cu(PPh3)3F) and heterogeneous catalysts TRGO/Cu(I).
3.4 Conductivity
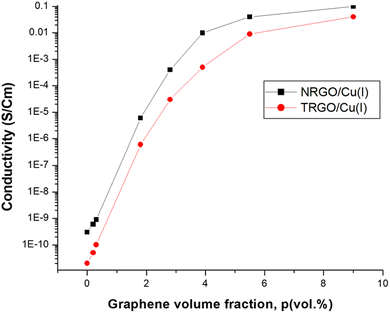
A standard four-point probe method was used to measure the conductivity of the prepared self-healing composites. Here, the direct current conductivity (σ) of the prepared composite materials as a function of the graphene volume fraction (p) was determined. From the obtained results, the NRGO/Cu(I) and TRGO/Cu(I) specimens displayed conductivities of 5 × 10−2 and 8 × 10−3 S cm−1, respectively, as shown in Fig. 10. The increase in the conductivity of NRGO/Cu(I) is due to the presence of nitrogen, strongly promoting the conductivity of a composite material.4. Conclusion
In summary, we have developed efficient graphene-based epoxy self-healing nanocomposites demonstrating self-healing at room temperature in the presence of synthesized catalysts NRGO/Cu(I) and TRGO/Cu(I). Self-healing is made possible by ‘click’ reactions between azides (present in the capsule) and alkynes (dispersed directly in the epoxy matrix) triggered by a Cu catalyst. The highly dispersible N-doped graphene-immobilized copper catalyst (NRGO–Cu2O) demonstrates an excellent combination of high catalytic performance for ‘click’-based network formation (crack healing) at room temperature and the required reinforcement inside the material, in turn allowing the compensation of tensile strength reduction exerted by the embedded capsules. Along with mechanical properties, the NRGO/Cu(I) based specimen also improves conductive properties. Tensile measurements were performed using MD simulations to support the findings. The results are quite promising in terms of reliability and robustness required to develop a low temperature self-healing material.Author contributions
Conceptualization: R. V. S. P. S., W. H. B. and S. R.; resources: W. H. B., G. J. Y., C. W. P. and S. R.; experimental: R. V. S. P. S., P. S., C. W. P. and M. S.; computational: C. W. P., G. J. Y. and A. K. M.; writing: R. V. S. P. S., P. S., A. K. M., P. K., M. S. and S. R.; editing: S. R., W. H. B., N. G. S., P. K., M. S. and G. J. Y.; funding acquisition: S. R., W. H. B. and G. J. Y.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the Science and Engineering Research Board (SERB-DST), Government of India (Grant No. CRG/2021/006957). We also acknowledge the support from the “Institute of Engineering Research at Seoul National University”. The authors also would like to acknowledge the financial support from the BAT4EVER project founded by European Union in the scope of H2020-LC-BAT-2020-3. A. K. M. acknowledges SEED Grant (Year 2021) UPES, Dehradun for computational facilities.Notes and references
- W. H. Binder, Self-healing polymers: from principles to applications, John Wiley & Sons, 2013 Search PubMed.
- Y. Yang and M. W. Urban, Chem. Soc. Rev., 2013, 42, 7446–7467 RSC.
- P. Michael, D. Döhler and W. H. Binder, Polymer, 2015, 69, 216–227 CrossRef CAS.
- S. Chen, N. Mahmood, M. Beiner and W. H. Binder, Angew. Chem., Int. Ed., 2015, 54, 10188–10192 CrossRef CAS.
- S. Rana, D. Döhler, A. S. Nia, M. Nasir, M. Beiner and W. H. Binder, Macromol. Rapid Commun., 2016, 37, 1715–1722 CrossRef CAS.
- F. Yavari, M. A. Rafiee, J. Rafiee, Z.-Z. Yu and N. Koratkar, ACS Appl. Mater. Interfaces, 2010, 2, 2738–2743 CrossRef CAS.
- B. C.-K. Tee, C. Wang, R. Allen and Z. Bao, Nat. Nanotechnol., 2012, 7, 825 CrossRef CAS PubMed.
- S. R. White, N. R. Sottos, P. H. Geubelle, J. S. Moore, M. R. Kessler, S. R. Sriram, E. N. Brown and S. Viswanathan, Nature, 2001, 409, 794–797 CrossRef CAS.
- B. J. Blaiszik, N. R. Sottos and S. R. White, Compos. Sci. Technol., 2008, 68, 978–986 CrossRef CAS.
- L. Guadagno, L. Vertuccio, C. Naddeo, E. Calabrese, G. Barra, M. Raimondo, A. Sorrentino, W. H. Binder, P. Michael and S. Rana, Polymers, 2019, 11, 903 CrossRef.
- S. Chen and W. H. Binder, Acc. Chem. Res., 2016, 49, 1409–1420 CrossRef CAS.
- M. Raimondo and L. Guadagno, Polym. Compos., 2013, 34, 1525–1532 CrossRef CAS.
- N. Kargarfard, N. Diedrich, H. Rupp, D. Döhler and H. W. Binder, Polymer, 2018, 10, 17 Search PubMed.
- N. Gopal, S. Rana, J. Whan, L. Li and S. Hwa, Prog. Polym. Sci., 2010, 35, 837–867 CrossRef.
- S. Rana, N. Karak, J. W. Cho and Y. H. Kim, Nanotechnology, 2008, 19, 495707 CrossRef PubMed.
- V. D. Punetha, S. Rana, H. J. Yoo, A. Chaurasia, J. T. McLeskey, M. S. Ramasamy, N. G. Sahoo and J. W. Cho, Prog. Polym. Sci., 2017, 67, 1–47 CrossRef CAS.
- A. S. Nia and W. H. Binder, Prog. Polym. Sci., 2017, 67, 48–76 CrossRef.
- B. Chen, D. Wang, B. Zhang, X. Zhong, Y. Liu, J. Sheng, Q. Zhang, X. Zou, G. Zhou and H.-M. Cheng, ACS Nano, 2021, 15, 9841–9850 CrossRef.
- A. S. Nia, S. Rana, D. Döhler, X. Noirfalise, A. Belfiore and W. H. Binder, Chem. Commun., 2014, 50, 15374–15377 RSC.
- B. Yu, S. Fu, Z. Wu, H. Bai, N. Ning and Q. Fu, Composites, Part A, 2015, 73, 155–165 CrossRef CAS.
- K. S. Khare, F. Khabaz and R. Khare, ACS Appl. Mater. Interfaces, 2014, 6, 6098–6110 CrossRef CAS.
- C. Park, J. Jung and G. J. Yun, Composites, Part B, 2019, 161, 639–650 CrossRef CAS.
- C. Park and G. J. Yun, J. Appl. Mech., 2018, 85, 091007 CrossRef.
- H. Yang, K. Yu, X. Mu, Y. Wei, Y. Guo and H. J. Qi, RSC Adv., 2016, 6, 22476–22487 RSC.
- Y. Sun, H. Yang and Y. Guo, Comput. Mater. Sci., 2021, 192, 110412 CrossRef CAS.
- C. Park, G. Kim, J. Jung, B. Krishnakumar, S. Rana and G. J. Yun, Polymer, 2020, 122862 CrossRef CAS.
- S. Neumann, D. Döhler, D. Ströhl and W. H. Binder, Polym. Chem., 2016, 7, 2342–2351 RSC.
- A. Shaygan Nia, S. Rana, D. Döhler, W. Osim and W. H. Binder, Polymer, 2015, 79, 21–28 CrossRef CAS.
- R. V. S. P. Sanka, K. Balaji, Y. Leterrier, S. Pandey, M. Srivastava, A. Srivastava, W. H. Binder, S. Rana and V. Michaud, Chem. Commun., 2019, 55, 6249–6252 RSC.
- A. P. Thompson, S. J. Plimpton and W. Mattson, J. Chem. Phys., 2009, 131, 154107 CrossRef PubMed.
- R. D. Sudduth, J. Compos. Mater., 2005, 40, 301–331 CrossRef.
- Z. Li, J. Chu, C. Yang, S. Hao, M. A. Bissett, I. A. Kinloch and R. J. Young, Compos. Sci. Technol., 2018, 163, 116–122 CrossRef CAS.
- L. Guadagno, M. Raimondo, M. Catauro, A. Sorrentino and E. Calabrese, J. Therm. Anal. Calorim., 2022, 147, 5463–5472 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sm01423h |
| ‡ These authors made equal contribution. |
| This journal is © The Royal Society of Chemistry 2023 |