Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fine comminution of torrefied wheat straw for energy applications: properties of the powder and energy balances of the production route
Rova-Karine
Rajaonarivony
a,
Xavier
Rouau
a,
Jean-Michel
Commandré
b,
Charlène
Fabre
a,
Jean-Eudes
Maigret
c,
Xavier
Falourd
cd,
Sophie
Le Gall
cd,
Bruno
Piriou
b,
Camille
Goudenhooft
e,
Sylvie
Durand
c,
Alain
Bourmaud
e,
Johnny
Beaugrand
c and
Claire
Mayer-Laigle
 a
a
aIATE, Université de Monpellier, INRAE, Montpellier Supagro, 34060 Montpellier, France. E-mail: claire.mayer@inrae.fr
bBIOWOOEB, Université de Montpellier, INRAE, Montpellier Supagro, 34060 Montpellier, France
cINRAE, UR BIA, 44316 Nantes, France
dINRAE, BIBS Facility, PROBE Infrastructure, 44316 Nantes, France
eUniv. Bretagne Sud, UMR CNRS 6027, IRDL, F-56100 Lorient, France
First published on 16th October 2023
Abstract
Lignocellulosic powders have a chemical composition that makes them explosive under certain conditions. In the form of ultrafine particles, they can be directly used as fuel in combustion devices to generate heat and mechanical power without the need to convert them into a liquid form. However, the production of such powder encounters two main challenges that affect engine efficiency: high energy consumption during grinding and poor flowability of the resulting powder. To tackle these challenges, one option is to modify the chemical and physical characteristics of the biomass before grinding. This can be effectively achieved through thermal pretreatment methods such as torrefaction, which alter the chemical structure of lignocellulosic biomass, making it more brittle, modifying its surface properties, and thereby improving its flowability. In this study, we investigated the impact of two torrefaction temperatures (220 °C and 280 °C) on the chemical and physical properties of wheat straw prior to fine milling. Our results indicate that the physical and chemical properties of the wheat straw were not significantly affected at 220 °C, whereas a significant change was observed at 280 °C. In particular, extensive chemical analysis and atomic force measurements show that the modifications of the properties are related to heat-induced degradation of cell wall polymers and an increase in cellulose crystallinity. For the wheat straw torrefied at 280 °C, both the grindability and flowability of the ground powder improved significantly. Meanwhile, the total energy consumption for the transformation remained constant, making the torrefaction process interesting for designing combustible powders.
1 Introduction
Annual cereal harvests produce huge amounts of renewable biomass in the form of non-edible by-products that can be used for the production of bio-energy in place of fossil feedstock. In particular, several studies demonstrated the potential of pulverized lignocellulosic materials for high-power applications such as gasification processes or even directly fuelling combustion engines.1–3 In this innovative technology, unlike current fuels derived from biomass, the powder is fed directly into the engine with no treatment other than ultra-fine milling.4 This invention relies on the fact that when used in sufficient quantity and concentration in a confined environment, finely milled biomass undergoes rapid combustion in the form of deflagration.5 The energy released can be recovered as a mechanical force and used for high-power generation. Previous studies by our team4,6–8 have demonstrated that an ultrafine lignocellulosic powder, with a median particle size of around 20 μm, is fully compatible with this application7 but the energy released depends on the physical and chemical properties of the powder. Thus, the production of such powdered fuel is entirely green, provided that the energy required for the milling remains lower than the heating values of the lignocellulosic biomass.However, obtaining powder of this order of magnitude requires the plant material to be fractionated below the cellular scale (<50 microns) which poses challenges in terms of energy requirement. Indeed, comminution results from fractures which are initiated and propagated mainly through defect zones9 naturally present in the structure of the plant. These zones are much more numerous at the plant tissue level than at the cellular scale. As a result, subcellular milling of plant materials usually consumes much more energy.
In addition, obtaining stable energy yields during engine combustion requires regular feeding which, in turn, means that the lignocellulosic powder must exhibit a good flowability. This is not a straightforward task when dealing with a powder of such size, primarily composed of cell fragments and possessing chemical groups that tend to strengthen interparticle cohesive forces.10
Among all the lignocellulosic biomasses, straws from cereal crops (such as wheat straw in Europe, rice straw in Asia…) appear to be suitable candidates for such applications, as their production and collection are spread over vast territories, rather than being limited to a few producer countries. However, previous work studying the grindability of various biomasses, including wheat straw11–15 has shown that such fibrous materials are more difficult to mill than more brittle materials such as pine bark, regardless of the mill used.14 Milling efficiency and powder quality differ with the dominant mechanical loading stress generated by the milling device. Milling devices that work by impact as the dominant mechanism tend to generate powders with a better flowability and lower potential agglomeration whereas milling devices that work by attrition as the dominant mechanism make better use of milling energy input and thus provide a more cost-effective comminution process.13,14,16Combining both modes of mechanical stress, such as in a stirred ball mill (SBM), appears to be the best compromise, especially for fibrous materials such as wheat straw. Nevertheless, for wheat straw, the milling energy required to reach a 20 μm average particle size (1.37 kW h kg−1) still represented ⅓ of the lower heating value of straw (4.1 kW h kg−1), i.e. the maximum energy that can be expected to be recovered.14,17,18
Improving the grindability of wheat straw and enhancing its energy content constitutes a challenge that needs to be overcome in order to improve the cost-effectiveness of powder production and the technical performances of the powders for different end-uses such as combustion or gasification.19
Torrefaction, a mild thermal treatment led under non-oxidizing conditions, is known to increase the energy density of the matter by removing oxygen chemical groups.20 Additionally, partial thermolysis in the bulk of the constituent polymer chains and modifications in cellulose crystallinity have also been reported, resulting in a weakening of the mechanical properties. This, in turn, facilitates the milling process and influences the properties of the resulting powders, such as surface chemistry, shape, and indeed, flowability.21–24
While previous studies have emphasized the impact of torrefaction prior to milling at the tissue scale, none, to the best of our knowledge, has explored its effects specifically for milling processes below the cellular level.
In this study, we first investigated the influence of torrefaction on the chemical and mechanical properties of wheat straw at the cellular level in order to determine the optimal operating conditions. In the second part, we examined how these conditions affect the processability of wheat straw and the properties of the resulting powder, taking into account the specifications required for combustion engine applications. Finally, in the last part, we conducted an energy balance analysis comparing the input processing energy costs and the potential heat release of control and torrefied powders, aiming to discuss the viability of these technical processing routes for high-power applications.
2 Materials and methods
Control wheat straw (WS) and torrefied wheat straw (TWS) were prepared according to two different process routes as summarized in Fig. 1. Each processing step is described in further detail below. | ||
| Fig. 1 Technological process routes for the production of control (WS) and torrefied wheat straw (TWS). | ||
2.1 Raw lignocellulosic biomass
Wheat straw (Triticum aestivum) was harvested in the south of France (Saint-Gilles, France) and stored dried in 25-kg bales. Moisture content was measured by weight loss after oven-drying for 2 h at 135 °C.16 The moisture content of the stored wheat straw was 11%.2.2 Processing step
Coarse milling of the raw materials: wheat straw (WS) was coarse-milled using a cutting mill (SM 300, Retsch®, Germany) with a 2 mm sieving grid, operated with manual feeding according to protocols previously developed.14The instantaneous power of all the milling devices was measured using a PX 120 wattmeter (METRIX, France) connected to WattCom (USA) data acquisition software. The measurements then served to compute the mean power demand, and milling energy consumption was determined by multiplying the mean power by the total milling time according to the procedure described by Rajaonarivony et al.14
The energy consumption of the torrefaction step was determined from the mean consumed power recorded directly by a Biogreen reactor multiplied by the residence time under stabilized temperature conditions (10 min). This measurement includes the energy consumed by all the components required for the process operation, such as the feeder unit, Spirajoule™ screw, cooled screw, refrigeration unit, regulation device, gas analyzer, and others.
2.3 Chemical characterization of the raw materials and powders
Supramolecular organization with solid-state NMR: 13C CP-MAS solid-state NMR spectra were acquired and processed as previously described.26Briefly, about 80 mg of each sample was rehydrated to 20–22% w/w with ultrapure water and then packed into a 4 mm-diameter rotor bed. The experiments were conducted under a contact time of 1.5 ms and an 8 s recycling delay. The 90° proton pulse was adjusted to 3.35 and 4 μs for control and torrefied wheat straw (prior to the milling), respectively.
The 13C chemical shifts were attributed as per Bardet et al.27 As the spectral resolution did not allow us to apply the peak associated with cellulose crystallinity Cr(Iα) (89.1 ppm), the approach developed by Larsson et al. (1997)28 was adapted to evaluate cellulose crystallinity from the deconvoluted C-4 peaks in the region 78–91 ppm.21
Chemical shift, full width at half height, peak area, and Gaussian–Lorentzian ratio were determined using a least-squares fitting method with Peakfit® software (Systat Software Inc., USA).
Cellulose crystallinity (CrI), lateral fibril dimension (LFD), lateral fibril aggregate dimension (LFAD) and proportion of β-O-4 bonds in lignin were estimated as previously described.26 Briefly, the area of the two broad signals was measured at 152.5 ppm, corresponding to the aromatic carbon atoms of lignin involved in β-O-4 bonds, such as the C-3 and C-5 of the S units, and at 147.5 ppm, corresponding to the same carbons but non-esterified. The abundance of β-O-4 bonds was considered representative of the entire lignin polymer.
The molar relative proportion of hemicelluloses was determined from the two peaks at 79 and 81 ppm after deconvolution of the 78–91 ppm region relating to both cellulose and hemicelluloses.
2.3.1.1 Polysaccharides. Total cell wall polysaccharide content was measured as previously described.29 Briefly, 5 mg of sample (control or torrefied at 280 °C) was acid-hydrolysed with sulfuric acid, and the resulting freed neutral sugars were derived into alditol acetates and analysed on a TG-225 GC column (30 × 0.32 mm ID) using a TRACE™ ultra gas chromatography system (Thermo Scientific™; temperature: 205 °C; carrier gas: H2). Uronic acids were quantified according to the MHDP method. All measurements were performed in triplicate using standard sugar solution and inositol as internal standards for calibration.
2.3.1.2Lignin. Lignin content was quantified by spectrophotometry using the acetyl bromide method (Hatfield & Fukushima, 2005) on samples of approximately 20 mg mass weight per assay.30 All chemicals were laboratory-grade from Sigma Aldrich, and the analyses were performed based on four independent assays. Lignin content was expressed as a percentage of dry weight.
2.3.1.3 Protein. Total C and N contents of the samples were quantified via the Dumas method using an elemental analyser (VarioMicro, ELEMENTAR, France). Protein content was determined from N content multiplied by a 5.7 coefficient routinely applied to non-storage proteins. Experiments were run in triplicate.
2.3.1.4 Ash. Measurements were carried out based on standard ISO 2171. Briefly 2 grams of the sample were dried in an oven at 130 °C for 90 minutes to obtain the dry mass (ms), measured through weighing. Subsequently, the sample was heated at 900 °C for 2 hours. After cooling down to room temperature inside a desiccator, the sample was weighed again to determine the residual mass (mr). The ash content was calculated as the ratio of the residual mass to the dry mass. The experiments were conducted in duplicate.
2.4 Physical characterization of raw materials and powders
| LHV = HHV − (212 × X) | (1) |
The span, which is an expression of the width of the distribution, was also calculated based on eqn (2), where d10, d50 and d90 are the 10th, 50th and 90th percentiles of the particle size distribution (PSD), respectively.
 | (2) |
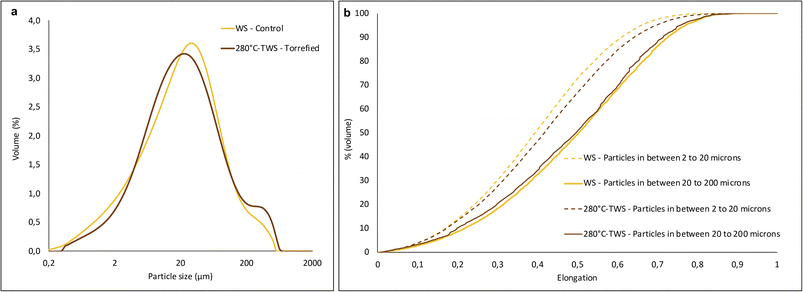
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles per sample, we defined 3 sampling zones per plate and then merged the results to determine the shape factors. As the median particle size of the powder was 20 μm, global distributions were constructed by post-processing, as described in previous work, by considering that particles below 20 μm and above 20 μm each accounted for 50% of the total.16
000 particles per sample, we defined 3 sampling zones per plate and then merged the results to determine the shape factors. As the median particle size of the powder was 20 μm, global distributions were constructed by post-processing, as described in previous work, by considering that particles below 20 μm and above 20 μm each accounted for 50% of the total.16
Particle elongation and convexity were determined from the images according to eqn 3
 | (3) |
Convexity was also calculated as the ratio of the convex hull (the smallest convex polygon that contains all the vertex of the particle) to the perimeter of the particle (eqn 4)
 | (4) |
The calculated convexity gives an indication of the particle surface condition: a perfectly smooth particle will have a convexity of 1.
| τ(σ) = Co + Fσ. | (5) |
 | (6) |
3 Results and discussion
3.1 Effects of the torrefaction step on the physical and chemical properties of wheat straw
The wheat straw (WS) to be torrefied was in the form of coarse-milled particles. The particle size distribution of the particles has a d10 = 182 μm, d50 = 600 μm, and d90 = 1254 μm. The torrefaction was carried out at 220 °C and 280 °C for 10 min under nitrogen flushing in the Biogreen reactor.| WS | 220 °C-TWS | 280°C-TWS | |
|---|---|---|---|
| Control | Torrified wheat straw at 220 °C | Torrified wheat straw at 280 °C | |
| Protein | 4.3 ± 0.1 | 4.5 ± 0.1 | 5.3 ± 0.1 |
| Lignin | 23.0 ± 0.1 | 27.9 ± 0.1 | 47.9 ± 0.1 |
| Ash | 5.39 ± 0.06 | 5.39 ± 0.36 | 7.44 ± 0.191 |
| Total CWP | 65.5 ± 1.25 | 62.7 ± 1.38 | 46.8 ± 0.61 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| CWP residues | |||
| Rha (rhamnose) | 0.29 ± 0.09 | 0.23 ± 0.05 | 0.14 ± 0.04 |
| Ara (arabinose) | 3.7 ± 0.23 | 4.0 ± 0.09 | 1.7 ± 0.2 |
| Xyl (xylose) | 20.8 ± 0.24 | 19.9 ± 0.71 | 7.4 ± 0.08 |
| Man (mannose) | 0.77 ± 0.11 | 0.95 ± 0.02 | 0.41 ± 0.1 |
| Gal (galactose) | 0.79 ± 0.2 | 0.73 ± 0.03 | 0.23 ± 0.1 |
| Glc (glucose) | 35.4 ± 0.49 | 33.6 ± 0.74 | 34.4 ± 0.39 |
| UA (uronic acid) | 3.7 ± 0.22 | 3.3 ± 0.07 | 2.6 ± 0.08 |
| Main CWPs | 54.3 ± 0.76 | 53.6 ± 1.32 | 39.1 ± 1.32 |
| Cellulose | 29.8 ± 0.47 | 29.7 ± 0.56 | 30 ± 1.1 |
| Arabinose (xylan) | 24.5 ± 0.29 | 23.9 ± 0.76 | 9.1 ± 0.22 |
| Cellulose crystallinity (%) | 35 ± 1 | 38% ± 1 | 38 ± 1 |
| Total crystallinity (including hemicellulose) | 25 ± 1 | 27 ± 1 | 32 ± 1 |
| LFD (nm) | 2.8 | 3.0 | 3.0 |
| LFAD (nm) | 26.4 | 25.1 | 30.9 |
| Estimated β–O–4 bonds (%) | 47 ± 1 | 40 ± 1 | 36 ± 1 |
| Proportion of hemicellulose in (CWP) | 29 ± 1 | 30 ± 1 | 16 ± 1 |
| W c (%) | 6.6 | 5.5 | 3.3 |
| T1 (°C) | 270° ± 1 | 278 °C ± 0 | 291 °C ± 0 |
| T2 (°C) | 448° ± 3 | 441° ± 0 | 456° ± 1 |
| Mass loss at 220 °C | 9.9% ± 0.5% | ||
| Mass loss at 280 °C | 22.0% ± 0.9% | ||
The 220 °C-torrefaction step affected the composition of wheat straw very slightly whereas the 280 °C-torrefaction step led to a 67% increase in protein, a 116% increase in lignin and a 28% decrease in cell wall polysaccharides.
Analysis of the monosaccharide composition of cell wall polysaccharides showed that non-cellulosic polysaccharides were more sensitive to thermolysis (cell wall polysaccharides, i.e. CWP residues, Table 1) than the cellulosic ones. In particular, the proportions of xylose, arabinose and galactose residues decreased by 65%, 54% and 71%, respectively, after the 280 °C-torrefaction.
The CrI (crystallinity index) by NMR was calculated according to Larsson et al. (1997),28 along with other methods that integrate the hemicellulose signal in the amorphous cellulose part. The spectra obtained for the different samples are shown in Fig. 2.
 | ||
| Fig. 2 NMR spectra for the control (N), 220 °C-torrified WS (T220) and 280 °C torrefied wheat straw (T280). | ||
Spectra show signals in the zone associated with polysachharides (60–110 ppm). Most of the signals correspond to cellulose (grey areas), but signals associated with hemicelluloses and pectins are also present (orange areas). Finally, signals characteristic of lignins are found in the 110–160 ppm zone. CrI values (Table 1) show a slight but significant38 increase for the torrefied samples regardless of the calculation method used. Comparison of the CrI values resulting from the different calculation methods highlights a greater degradation of the hemicelluloses than the celluloses, relating to the higher molar proportion of hemicelluloses.
The amorphous region of the cellulose, which is the major part, is known to be the cellulose fraction that is most sensitive to thermal degradation. Here, the higher final CrI indicates that the cellulose decrease observed in global biochemical analyses is mainly due to the thermal degradation of the amorphous region.
The LFD (lateral fibril dimension) remained constant during the torrefaction process, whereas the LFAD (lateral fibril aggregate dimension) increased. One hypothesis is that the cellulose aggregates collapse during the torrefaction process. Indeed, the degradation of amorphous parts could expose some crystalline regions on the surface of the aggregates, allowing them to interact and form aggregates with a larger cross-section.
The proportion of specific covalent linkages, such as β–O–4 bonds in the lignin fraction, which are representative of the entire lignin ultrastructure, decreases as a function of torrefaction temperature. This decrease can be attributed to a breakdown of the lignin network, as previously reported by Wen et al.39 In addition, the decrease in the proportion of hemicellulose (measured in moles in solid state NMR analysis) is well correlated with the decrease in arabinose, xylose, galactose and mannose measured by biochemical analyses in weight.
The mass losses observed at 220 °C and 280 °C were 9.9% ± 0.5% and 22.0% ± 0.9% respectively, corresponding to a net mass loss of 3.3% and 15.6% after deducting the water content. The weak mass loss at 220 °C is consistent with the low levels of chemical degradation observed at the same temperature. Interestingly, the mass loss at 280 °C is in the same range as the difference in the content of the main CWP(s) measured by the biochemical analyses (54.3 − 39.1 = 15.1%) reinforcing the reliability of both measurements.
| Cell wall region | Middle lamella | |
|---|---|---|
| Control | 14.9 ± 5.3 GPa | 12.3 ± 5.2 GPa |
| 220 °C torrefied sample | 15.5 ± 2.3 GPa | 10.9 ± 1.1 GPa |
| 280 °C torrefied sample | 10.7 ± 1.8 GPa | N.D. |
It should be noted that in contact mechanics, indentation properties are calculated from a theoretical contact area which is not always consistent with experimental reality. Here, the indentation modulus values found in the cell walls are around 15 GPa for the raw samples and those treated at 220 °C; these values are consistent with the values found in the literature for wood fibres that have similarities to the cells studied here, particularly in terms of xylose content.40 One can notice a significant drop in the mechanical properties after 280 °C exposure. This is well correlated with the behavior observed on other plant fibres such as flax cell walls.41
This drop can be attributed to the development of porosities within the cell wall due to the decrease in non-cellulosic polymers previously reported and the increase in the LFAD distance. This led to a decrease in cell wall density and consequently a loss in terms of mechanical properties. Interestingly, the AFM PF-QNM technique is resolutive enough for calculating stiffness values in local regions such as the middle lamellae. Here, values between 9 and 12 GPa are calculated in these areas. These values are fully in line with the CML indentation modulus obtained in CML regions of different xylan-type fibre bundles such as jute or kenaf.42 After being treated at 280 °C, morphological damage occurs on the cell wall, making it difficult to clearly identify the middle lamella. This explains why no value is reported for the 280 °C torrefied sample.
In general, morphological and mechanical assessments confirm the previous biochemical and structural results obtained through NMR and biochemistry, providing insights into the histological degradation of the straw. This also suggests a potential modification in the grindability of the materials. Cross-sectionally, this also demonstrates the high potential of AFM PF-QNM for a localised and highly resolving investigation of plant cell wall stiffness and microstructure.
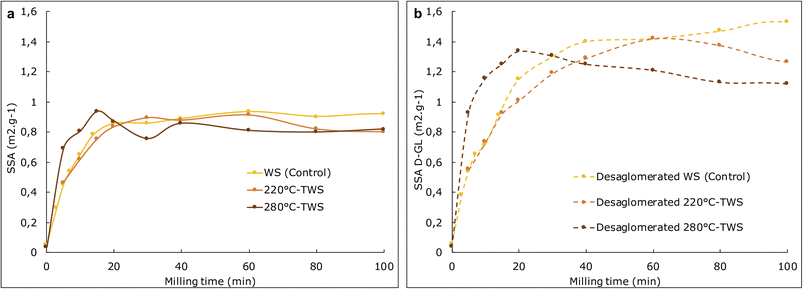 | ||
| Fig. 4 Evolution of specific surface area before (plain line, Fig. 2a)) and after (dashed line, Fig. 2b) de-agglomeration with ultrasonic treatment (UT) in control (WS) and torrefied samples (220 °C TWS and 280 °C-TWS) in a lab-scale MM400 ball-mill. | ||
| Grindability (m2 g−1 min−1) | R 2 | |
|---|---|---|
| WS (control) | 0.051 | 0.90 |
| 220 °C-TWS | 0.046 | 0.91 |
| 280 °C-TWS | 0.112 | 0.94 |
The grindability of the control and the 220 °C TWS is quite close (0.51 and 0.46 m2 g−1 min−1) and half those of the 280 °C TWS. This reveals an effect of fragilization of the material due to the thermal treatment at the highest temperature, as previously highlighted through AFM PF-QNM mapping. The degradation of some part of the lignocellulosic matrix, 15% in mass, in particular the hemicelluloses and amorphous cellulose creates microvoids in the cell wall and middle lamella structure that can serve as areas for initiation and propagation of fracture paths.
The evolution of the specific area over time can also be used to determine the agglomeration potential of the powders.44 Two types of agglomeration are generally distinguished.45
Reversible agglomeration, also known as soft agglomeration, can be assessed using the specific surface area that is released after applying ultrasonic treatment (UT), which allows the powder to de-agglomerate. Reversible agglomeration hampers the efficiency of the milling process but does not completely impede comminution. In contrast, irreversible agglomeration (hard agglomeration) occurs due to stronger interactions between biomass particles that cannot be broken by UT. Hard agglomeration is evident when the specific surface area decreases with increasing milling time after UT treatment (i.e., particle size increases).14
In Fig. 4a, we observe a plateau for the SSA, which can be attributed to the presence of agglomeration phenomena that compete with comminution. As shown in Fig. 4b, for longer milling times, the SSAD-LG value decreased for both the 280 °C torrefied wheat samples (starting from 20 minutes of milling) and the 220 °C torrefied wheat samples (starting from 60 minutes of milling), highlighting the presence of strong agglomeration. Interestingly, no irreversible agglomeration is observed in the control sample. While we do not know, currently, the exact origin for these interactions responsible for hard agglomeration, we can assume that the biochemical changes generated by torrefaction make certain chemical groups more accessible. These groups are then more likely to form stronger interactions with others.
Agglomeration is mainly governed by the ratio of interparticle forces (van der Waals forces, hydrogen bonds, and possibly covalent bonds due to the effects of mechanochemistry on gravity). These are related to the chemical surface composition of the particles, which is modified during the torrefaction process. Additionally, as the torrefaction process increases grindability, it also leads to an increase in the proportion of the smallest particles and stronger agglomeration, particularly in the case of the 280 °C temperature.
Therefore, the modification in the cellular structure observed with AFM at the 280 °C torrefaction temperature effectively leads to higher grindability. On the other hand, torrefaction also increases the total agglomeration regardless of the temperature considered.
3.2 Milling in a pilot-scale stirred ball mill and powder properties
The coarsely milled and torrefied powders were finely milled in a pilot-scale stirred ball mill (SBM) to reach a target mean particle size of 20 μm, which is the target particle size for feeding an internal combustion engine.42 The 280 °C-torrefied wheat straw powders were compared in terms of milling behaviour to the control samples that had been prepared with intermediate impact grinding in the UPZ mill instead of the torrefaction step.
Table 4 reports the main particle size characteristics of the sample prior to milling. As the control was submitted to an additional milling step, the d10, d50, and d90 values of torrefied straw powders were logically 35–40% higher than those of the controls. The span reflecting the spread of the distribution was similar between control and torrefied samples.
| SSA, m2 g−1 | d 10, μm | d 50, μm | d 90, μm | Span | ||
|---|---|---|---|---|---|---|
| Before fine milling | WS | 0.055 | 64 | 286 | 691 | 2.2 |
| 280 °C-TWS | 0.037 | 98 | 478 | 1102 | 2.1 | |
| After fine milling | WS | 0.831 | 2.6 | 20.5 | 97.3 | 5.9 |
| 280 °C-TWS | 1.017 | 3.3 | 20.1 | 122.8 | 4.6 | |
In the SBM, the milling time to reach a median particle size of d50 = 20 μm was 96 min and 16.8 min for the control and 280 °C-TWS respectively, i.e. a time reduction of more than 80% for the torrefied sample. This reduction is especially significant given that TWS had particles that were coarser (around 40%, see Table 4) than the control.
| Elongation | Convexity | ||||||
|---|---|---|---|---|---|---|---|
| El10 | El50 | El90 | Con10 | Con50 | Con90 | ||
| Control (WS) | Particles < 20 μm | 0.17 | 0.39 | 0.61 | 0.64 | 0.75 | 0.87 |
| Particles > 20 μm | 0.22 | 0.50 | 0.72 | 0.63 | 0.78 | 0.91 | |
| 280 °C-TWS | Particles < 20 μm | 0.17 | 0.42 | 0.61 | 0.75 | 0.89 | 0.98 |
| Particles above 20 μm | 0.20 | 0.50 | 0.71 | 0.56 | 0.72 | 0.88 | |
For the coarser particles (above 20 microns), the elongation distributions of the WS and the 208 °C-TWS powders were very close, with a median elongation of around 0.5 which corresponds to a length twice as large as the width. For the smaller particles, the WS powder showed lower median elongation (0.39) than 280 °C-TWS (0.42). This difference can be related to the shorter milling time of the 280 °C-TWS. Due to its higher grindability, the 280 °C-TWS powder had a lower residence time than control powder. The 280 °C-TWS powder was thus less rounded by the mill ball and kept its shape factor.
Convexity values showed little difference between finer (below 20 μm) and coarser particles (above 20 μm) for the control sample. The coarser particles appeared to be a little smoother than the smaller particles. However, the torrefied sample showed the opposite pattern, and the gap between the values for particles below and above 20 microns was much greater. Thus, in this case, the smaller particles were smoother and the coarser ones exhibited more shape irregularity. Many factors may explain this difference. First, similar observations were previously reported for powders obtained using a milling device that worked predominantly with attrition as the main mechanical force.16 As the input torrefied particles had skipped the intermediate milling step, the ratio between size of the milling media and size of the particles was lower for 280 °C TWS than for the control. This would lead to a better efficiency of the attrition mode in comparison to the impact loading mode generated by the device, which could explain these observations. In addition, due to the shorter residence time needed in the mill to reach the target particle size, the coarser particles are less polished by the grinding media. The torrefaction process also degrades some components (hemicellulose and amorphous cellulose) and could generate microvoids inside the torrefied particles. The preferential propagation of fracture paths through these microvoids could lead to the formation of irregular coarse particles.
We also demonstrated that the 280 °C-TWS powder has a greater tendency to agglomerate than the control, and agglomerates generally exhibit smoother and more regular shapes.
| Comp. (%) | Co (kPa) | |
|---|---|---|
| Control (WS) | 36.1 ± 0.5% | 0.42 ± 0.1 |
| 280 °C -TWS | 32.1 ± 0.7% | 0.22 ± 0.6 |
Compressibility is an indicator that quantifies the changes in density of a powder when a normal stress is applied. Compressibility is often compared to the Carr index, which expresses the compressibility of a powder when self-arranged after tapping down (i.e. under its own weight).47
The control WS had a compressibility of about 36% whereas the ground 280 °C TWS was less compressible with a value of 32%. If we compare the value obtained to the Carr index reference table, the torrefaction step moved the ground straw from the range of a very-poor-flowability powder (35–38%) to the range of a poor-flowability powder (28–35% compression).47 In practice, this index was defined to quantify the flow of a powder under quasi-static conditions, which refers to the flow of a powder under its own weight. In the context of high-power energy applications, achieving good flowability isn't strictly required, as forces are employed to set the powder in motion. However, even slight improvements, like this one, will facilitate the handling of the powder since less significant mechanical forces are needed to initiate the movement of the particles.
Compressibility is affected by the deformability of the particles. Under a normal stress, a deformable particle can fill any voids in the powder bed.48 The torrefaction process makes the straw particles more brittle (as shown by the increase in grindability), which decreases their deformability and thus the compressibility of the powder.
Cohesion was halved by the torrefaction process, shifting from 0.42 to 0.22 kPa. Cohesion can be seen as a measure of the intensity of inter-particle forces, which depends on the interactions between chemical groups and the physical defects at the surface of the particles and is also related to the distance between particles. All the powders here had the same d50 (∼20 μm), and we showed in Table 4 that the 280 °C torrefied powder had a higher d10 than the control (WS), which can be assimilated to a lower presence of smaller particles in the torrefied sample, probably due to the formation of agglomerates and a smoothing effect of the particle surface (see convexity values), thus decreasing the cohesion in the powder which flowed as it was made up of larger particles.
In lignocellulosic materials, it is widely agreed that hemicelluloses are hydrogen-bonded to cellulose and covalently bonded to lignins.49,50 The non-cellulosic components are strongly correlated with the cohesive forces within the cell wall of lignocellulosic materials. Studies have shown that the branches of xylan hemicelluloses such as arabinose, glucuronic acid or acetyl are responsible for these interactions by coordinating to hydrogen bond donor sites on the cellulose surface.51,52 Here, we found that the torrefaction process preferentially degraded the arabinoxylans. Again, we can assume that this decreases such interactions in the torrefied particles and leads to a decrease in inter-particle forces. The hydrophilizing effect of the powders conferred by the torrefaction treatment due to the elimination of such hydrophilic compounds would most likely further drive this decrease. Finally, torrefaction also leads to a densification of the matter (i.e., particles of the same size are heavier after the torrefaction step) and the chemical modifications of the lignocellulosic biomass due to the torrefaction process may significantly decrease the inter-particle force/weight decrease ratio, leading to a decrease in cohesion.
3.3 Energy balance and overall advantages of the torrefaction process
| Yields | |
| Solid matter (280 °-TWS) | 70% |
| Oil | 23.33% |
| Syngas | 6.67% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Properties of torrefaction's products | |
| Lower heating value (LHV) of raw WS | 15.2 MJ kg−1 of dry matter |
| Lower heating value (LHV) of 280 °C-TWS | 19.5 MJ kg−1 of dry matter |
| Main compound present in the syngas | CO2: 30.83% |
| CO: 16.71% | |
| Alkane: 5.37% | |
| Lower heating value (LHV) of syngas | 4.63 MJ m−3 |
The lower heating value of the 280 °C_TWS shows an increase of 22% compared to that of the control (before the torrefaction). This reflects the concentration of energy in the torrefied powder. The energy yield (Ey) in the torrefied biomass was defined as the ratio of the energy content in the torrefied samples to the energy content of the raw biomass:
| Energy consumption | WS | 280 °C-TWS |
|---|---|---|
| Coarse milling (kW h t−1) | 49.2 | 49.2 |
| Torrefaction (kW h t−1) | — | 1360 |
| Intermediate milling (kW h t−1) | 181.5 | — |
| Fine milling (kW h t−1) | 1368 | 217 |
| Total energy consumption (kW h t−1) | 1598.7 | 1626.6 |
| Residual energy (kW h t−1) | 4222.2 | 3802.4 |
| EROI | 2.64 | 2.33 |
The energy consumption reported here for fine milling takes into account the yield of biochar after the torrefaction step (70%). In a similar way, residual energy was calculated from the LHV multiplied by the yield of the torrefaction step. Table 8 also reports the residual energy and the energy return on investment (EROI) for control and torrefied samples which is defined as the amount of energy in the products divided by the amount of energy provided to the process.55 In this case, the EROI was calculated as the ratio of residual energy to total energy consumption for 1 ton of input biomass feedstock. The first point to note is that the total energy consumption was relatively similar (less than 2% difference) between control and torrefied samples (1598.7 vs. 1626.6 kW h t−1). Indeed, the significant energy gain obtained during the grinding step is offset by the energy cost of torrefaction.
During our experimentation, we measured an energy consumption of 1360 kW h t−1 (dry basis) for the torrefaction step, with a residence time of 10 minutes inside the reactor. This value appears to be high, around 5 to 7 times higher than other values reported in the literature (∼200 kW h t−1) for torrefaction of lignocellulosic biomass.53–56 The difference can be attributed to the low density of wheat straw and the fact that the energy consumption measurements in this study take into account the total consumption of the pilot reactor, including its peripherals. Additionally, it should be noted that this experimental torrefaction operation was not optimized for industrial process practice.
In an industrial process, we can expect to significantly reduce the total energy consumption needed for production of the 280 °C sample by optimizing the torrefaction step, which was not the purpose of this study. Currently the values measured for this step are 5 to 7 times higher than those in the literature. A simple 2-fold reduction of the torrefaction energy leads to an EROI of 4.1 (i.e. 1.5 times higher than the EROI of the WS).
In addition, this yield can be further improved by re-using the energy content in the syngas and oil to decrease the energy consumption of the torrefaction step. To give an order-of-magnitude idea, syngas yield was 22% with a LHV of 4.2 × 103 kW h m−3. Assuming that the volumetric mass density of the syngas is 1–1.5 kg m−3, the use of the energy contained in the syngas and oil could increase the EROI to between 2.97 and 3.28 kg m−3 with the current energy consumption measured for the torrefaction step, which would fully justify practical consideration of torrefaction as a pre-processing step.
In addition, torrefaction also increased the density of the materials and decreased the water uptake of the lignocellulosic biomass, thus facilitating its subsequent storage. Finally, the improvement of the flowability properties of the combustible powder facilitates its implementation in the combustion process, limiting potential blocking points during conveying and enabling better regulation of the feedstock feed, leading to better-quality energy recovery.
4. Conclusions
In this study, we explored the potential benefits of torrefaction as a pre-treatment before fine-milling wheat straw, with a specific focus on its applicability to produce powdered fuel intended for high-power energy applications. Our research indicates that a torrefaction temperature of 220 °C for 10 minutes is insufficient to bring about significant structural changes in the physical and chemical properties of wheat straw. In contrast, at a higher temperature of 280 °C, notable structural transformations occur, which have a substantial impact on both the grindability of the raw material and the rheological properties of the resulting powder. While the energy return on investment (EROI) is relatively consistent regardless of whether the wheat straw is raw or torrefied, there is untapped potential for enhancing the EROI by optimizing the energy efficiency of the torrefaction process and by capitalizing on the energy contained in the by-products of torrefaction. From an industrial perspective, torrefied powders offer distinct advantages, such as increased energy density and improved flow properties. These findings suggest that torrefaction could offer significant benefits in the biomass-to-energy value chain, especially for high-power energy applications.Abbreviations
| BFE | Basic flow energy |
| Co | Cohesion |
| Con | Convexity |
| Cp | Compressibility |
| CrI | Crystallinity index |
| CWPs | Cell wall polysaccharides |
| d 10, d50, and d90 | 10th, 50th and 90th percentiles of particle size distribution |
| E y | Energy yield |
| El | Elongation |
| EROI | Energy return on investment |
| LFAD | Lateral fibril aggregate dimension |
| LFD | Lateral fibril dimension |
| LHV | Lower heating value |
| NER | Net energy ratio |
| PSD | Particle size distribution |
| SBM | Stirred ball mill |
| SSA | Specific surface area |
| SSAD-GL | Specific surface area after an ultrasonic-treatment deagglomeration step |
| TWS | Torrefied wheat straw |
| WS | Wheat straw |
Author contributions
Rova-Karine Rajaonarivony: conceptualization, investigation, formal analysis, writing – original draft, writing – review & editing. Xavier Rouau: conceptualization, funding acquisition, investigation, formal analysis, writing – original draft, writing – review & editing. Jean-Michel Commandré: methodology, investigation, writing – review & editing. Charlène Fabre: methodology, investigation, writing – review & editing. Jean-Eudes Maigret: methodology, investigation. Xavier Falourd: methodology, investigation, formal analysis, writing – review & editing. Bruno Piriou: methodology, investigation, funding acquisition, review & editing. Alain Bourmaud: methodology, investigation, writing – review & editing. Camille Goudenhooft: methodology, investigation, review & editing. Sylvie Durand: methodology, investigation, review & editing. Johnny Beaugrand: methodology, investigation, writing – review & editing. Sophie Le Gall: methodology, investigation, writing – review & editing. Claire Mayer-Laigle: conceptualization, funding acquisition, investigation, formal analysis, writing – original draft, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors thank Huda Ashfaq and Bryan Hughes for their help with the biomass characterization phase. The milling and powder characterization were performed at the PLANET facilities hosted by the JRU 1208 IATE (https://eng-planet.isc.inrae.fr/). The cell wall polysaccharide analyses and NMR investigations were performed at the BIBS facility hosted by the RU1268 BIA. (http://www.bibs.inrae.fr/bibs_eng/, UR1268 BIA, IBiSA, Phenome-Emphasis-FR ANR-11-INBS- 0012, PROBE and CALIS infrastructures, Biogenouest).Notes and references
- J. Deng, et al., Pretreatment of agricultural residues for co-gasification via torrefaction, J. Anal. Appl. Pyrolysis, 2009, 86(2), 331–337 CrossRef CAS.
- M. Mandø, et al., Pulverized straw combustion in a low-NOx multifuel burner: Modeling the transition from coal to straw, Fuel, 2010, 89(10), 3051–3062 CrossRef.
- C. Mayer-Laigle, et al., Comminution of Dry Lignocellulosic Biomass, a Review: Part I. From Fundamental Mechanisms to Milling Behaviour, Bioengineering, 2018, 5(2), 41 CrossRef PubMed.
- B. Piriou, G. Vaitilingom, X. Rouau, Solid Fuel in the Form of a Powder, Including a Lignocellulosic Component, WO2013108177A1, France, 2013.
- T. Abbasi and S. A. Abbasi, Dust explosions–Cases, causes, consequences, and control, J. Hazard. Mater., 2007, 140(1), 7–44 CrossRef CAS PubMed.
- L. Stover, et al., A phenomenological description of biomass powder combustion in internal combustion engines, Energy, 2023, 274, 127287 CrossRef CAS.
- L. Stover, et al., The Biomass Dust-Fueled Engine, in 26th European Biomass Conference & Exhibition, Copenhagen, 2018 Search PubMed.
- B. Piriou, et al., Potential direct use of solid biomass in internal combustion engines, Prog. Energy Combust. Sci., 2013, 39(1), 169–188 CrossRef.
- N. Blanc, et al., Breakage of Flawed Particles by Peridynamic Simulations, Comput. Part. Mech., 2021, 8, 1019–1031 CrossRef.
- J. Cai, et al., Review of physicochemical properties and analytical characterization of lignocellulosic biomass, Renewable and Sustainable Energy Reviews, 2017, 76, 309–322 CrossRef CAS.
- G. G. D. Silva and S. G. X. Rouau, Successive centrifugal grinding and sieving of wheat straw, Powder Technol., 2011, 208(2), 266–270 CrossRef CAS.
- C. Gao, et al., Regularity and mechanism of wheat straw properties change in ball milling process at cellular scale, Bioresour. Technol., 2017, 241, 214–219 CrossRef CAS PubMed.
- C. Mayer-Laigle, et al., Properties of Biomass Powders Resulting from the Fine Comminution of Straw and Bark Feedstocks by Three Types of Ball-Mill Set-Up (RBM, SBM, VBM), Portail Data INRAE, 2021 Search PubMed.
- K. R. Rajaonarivony, et al., Comparative comminution efficiencies of rotary, stirred and vibrating ball-mills for the production of ultrafine biomass powders, Energy, 2021, 227, 120508 CrossRef.
- G. G. D. Silva, et al., Effects of grinding processes on enzymatic degradation of wheat straw, Bioresour. Technol., 2012, 103(1), 192–200 CrossRef CAS PubMed.
- R. Rajaonarivony, et al., Properties of biomass powders resulting from the fine comminution of lignocellulosic feedstocks by three types of ball-mill set-up [version 2; peer review: 2 approved], Open Research Europe, 2022, 1(125) Search PubMed.
- M. J. Prins, K. J. Ptasinski and F. J. J. G. Janssen, More efficient biomass gasification via torrefaction, Energy, 2006, 31(15), 3458–3470 CrossRef CAS.
- M. J. C. van der Stelt, et al., Biomass upgrading by torrefaction for the production of biofuels: A review, Biomass and Bioenergy, 2011, 35(9), 3748–3762 CAS.
- D. D. Hu, J. B. Zhuang and M. L. Ding, A Review of Studies on the Granular Agglomeration Mechanisms and Anti-Agglomeration Methods, Key Eng. Mater., 2012, 501, 515–519 Search PubMed.
- A. Álvarez, et al., Non-oxidative torrefaction of biomass to enhance its fuel properties, Energy, 2018, 158, 1–8 CrossRef.
- C. Couhert, S. Salvador and J. M. Commandré, Impact of torrefaction on syngas production from wood, Fuel, 2009, 88(11), 2286–2290 CrossRef CAS.
- F. Weiland, et al., Entrained flow gasification of torrefied wood residues, Fuel Process. Technol., 2014, 125, 51–58 CrossRef CAS.
- J. Pachón-Morales, et al., Flowability characterization of torrefied biomass powders: Static and dynamic testing, Biomass Bioenergy, 2020, 138, 105608 CrossRef.
- J. Pachón-Morales, et al., Effect of torrefaction intensity on the flow properties of lignocellulosic biomass powders, Biomass Bioenergy, 2019, 120, 301–312 CrossRef.
- B. Etia-group and S. A. S. Etia, Thermal Processing of Powders: Introducing the Spirajoule, Heating Screw Conveyor Powered by Electricity, 2019 Search PubMed.
- A. Leroy, et al., Evaluating polymer interplay after hot water pretreatment to investigate maize stem internode recalcitrance, Biotechnol. Biofuels, 2021, 14(1), 164 CrossRef CAS PubMed.
- M. Bardet, et al., Dynamics property recovery of archaeological-wood fibers treated with polyethylene glycol demonstrated by high-resolution solid-state NMR, Cellulose, 2012, 19(5), 1537–1545 CrossRef CAS.
- P. T. Larsson, K. Wickholm and T. Iversen, A CP/MAS13C NMR investigation of molecular ordering in celluloses, Carbohydr. Res., 1997, 302(1), 19–25 CrossRef CAS.
- M. Lahaye, et al., Cellulose, pectin and water in cell walls determine apple flesh viscoelastic mechanical properties, Carbohydr. Polym., 2020, 232, 115768 CrossRef CAS PubMed.
- R. Hatfield and R. S. Fukushima, Can Lignin Be Accurately Measured?, Crop Science, 2005, 45(3), 832–839 CrossRef CAS.
- Sader, Available from: https://sadermethod.org/ Search PubMed.
- Gwyddion, Available from: http://gwyddion.net Search PubMed.
- Afnor, Biocombustibles solides - Méthode pour la détermination du pouvoir calorifique, 2005, p. 66 Search PubMed.
- G. B. J. de Boer, et al., Laser Diffraction Spectrometry: Fraunhofer Diffraction Versus Mie Scattering, Part. Part. Syst. Charact., 1987, 4(1-4), 14–19 CrossRef CAS.
- K. Rajaonarivony, et al., Fine Comminution of Pine Bark: How Does Mechanical Loading Influence Particles Properties and Milling Efficiency?, Bioengineering, 2019, 6(4), 102 CrossRef CAS PubMed.
- R. Freeman, Measuring the flow properties of consolidated, conditioned and aerated powders — A comparative study using a powder rheometer and a rotational shear cell, Powder Technol., 2007, 174(1), 25–33 CrossRef CAS.
- A. Alonso-Simón, et al., High-throughput microarray profiling of cell wall polymers during hydrothermal pre-treatment of wheat straw, Biotechnol. Bioeng., 2010, 105(3), 509–514 CrossRef PubMed.
- A. Villares, et al., Lytic polysaccharide monooxygenases disrupt the cellulose fibers structure, Sci. Rep., 2017, 7(1), 40262 CrossRef CAS PubMed.
- J.-L. Wen, et al., Understanding the chemical and structural transformations of lignin macromolecule during torrefaction, Appl. Energy, 2014, 121, 1–9 CrossRef CAS.
- M. Eder, et al., Experimental micromechanical characterisation of wood cell walls, Wood Sci. Technol., 2013, 47(1), 163–182 CrossRef CAS.
- D. Siniscalco, et al., Monitoring temperature effects on flax cell-wall mechanical properties within a composite material using AFM, Polym. Test., 2018, 69, 91–99 CrossRef CAS.
- A. Melelli, et al., The Middle Lamella of Plant Fibers Used as Composite Reinforcement: Investigation by Atomic Force Microscopy, Molecules, 2020, 25 DOI:10.3390/molecules25030632.
- P. Rittinger, Lehrbuch Der Aufbereitungskunde in Ihrer Neuesten Entwicklung Und Ausbildung Systematisch Dargestellt, Ernst & Sohn, Berlin, Germany, 1867 Search PubMed.
- N. Blanc, et al., Evolution of grinding energy and particle size during dry ball-milling of silica sand, Powder Technol., 2020, 376, 661–667 CrossRef CAS.
- L. Opoczky, Fine grinding and agglomeration of silicates, Powder Technol., 1977, 17(1), 1–7 CrossRef CAS.
- M. Almendros, et al., Changes on wood powder morphology and flowability due to thermal pretreatment, in Récents Progrès en Génie des Procédés, SFGP, Paris, France, 2011 Search PubMed.
- R. L. Carr, Evaluating Flow Properties of Solids, Chem. Eng., 1965, 72, 69 CAS.
- H. F. Fischmeister and E. Arzt, Densification of Powders by Particle Deformation, Powder Metall., 1983, 26(2), 82–88 CrossRef.
- R. M. Rowell, Handbook of Wood Chemistry and Wood Composites, Taylor & Francis, 2nd edn, 2012 Search PubMed.
- M. C. Jarvis, Hydrogen Bonding and Other Non-covalent Interactions at the Surfaces of Cellulose Microfibrils, Cellulose, 2023, 30, 667–687 CrossRef CAS.
- R. L. Silveira, et al., Plant Biomass Recalcitrance: Effect of Hemicellulose Composition on Nanoscale Forces that Control Cell Wall Strength, J. Am. Chem. Soc., 2013, 135(51), 19048–19051 CrossRef CAS PubMed.
- J. C. Mortimer, et al., Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass, Proceedings of the National Academy of Sciences, 2010, 107(40), 17409–17414 CrossRef CAS PubMed.
- P. Basu, Chapter 4 - Torrefaction, in Biomass Gasification, Pyrolysis and Torrefaction, ed. P. Basu, Academic Press, Boston, 2nd edn, 2013, pp. 87–145 Search PubMed.
- R. Bass, et al., Carbon Implications of Converting a Coal-Fired Power Plant to Combustion of Torrefied Arundo Donax, Applied Bioenergy, 2014, 1 Search PubMed.
- L. J. R. Nunes, J. C. De Oliveira Matias, and J. P. Da Silva Catalão, Chapter 3 - Biomass Torrefaction Process, in Torrefaction of Biomass for Energy Applications, ed. L. J. R. Nunes, J. C. de Oliveira Matias, and J. P. Da Silva Catalão, Academic Press, 2018, pp. 89–124 Search PubMed.
- B. Colin, Modelling of Wood Chips Torrefaction in a Rotary Kiln and Experimental Validation in a Continuous Pilot-Scale Rotary Kiln, Ecole des Mines d'Albi-Carmaux, 2014 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |