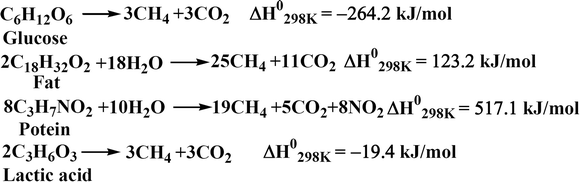Open Access Article
Open Access ArticleLactic acid and biomethane production from bread waste: a techno-economic and profitability analysis using pinch technology†
Swarnalatha
Mailaram
a,
Vivek
Narisetty
b,
Sunil K.
Maity
 *a,
Siddharth
Gadkari
*a,
Siddharth
Gadkari
 c,
Vijay Kumar
Thakur
c,
Vijay Kumar
Thakur
 d,
Stephen
Russell
e and
Vinod
Kumar
d,
Stephen
Russell
e and
Vinod
Kumar
 *bf
*bf
aDepartment of Chemical Engineering, Indian Institute of Technology Hyderabad, Kandi, Sangareddy-502285, Telangana, India. E-mail: sunil_maity@che.iith.ac.in; Tel: +91-40-23016202
bSchool of Water, Energy and Environment, Cranfield University, Cranfield MK43 0AL, UK. E-mail: Vinod.Kumar@cranfield.ac.uk; Tel: +44 (0)1234754786
cDepartment of Chemical and Process Engineering, University of Surrey, Guildford GU2 7XH, UK
dBiorefining and Advanced Materials Research Centre, Scotland's Rural College (SRUC), Edinburgh, UK
eClothworkers' Centre for Textile Materials Innovation for Healthcare, School of Design, University of Leeds, Leeds LS2 9JT, UK
fDepartment of Bioscience and Bioengineering, Indian Institute of Technology Roorkee, Uttarakhand 247667, India
First published on 7th June 2023
Abstract
Lactic acid (LA) is a vital platform chemical with diverse applications, especially for biodegradable polylactic acid. Bread waste (BW) is sugar-rich waste biomass generated in large quantities in residential and commercial operations. Recently, we evaluated the potential of BW for LA production by Bacillus coagulans under non-sterile conditions. This work presents a techno-economic and profitability analysis for valorizing 100 metric tons of BW per day to alleviate environmental pollution with concurrent production of LA and biomethane. We compared two fermentation approaches: acid-neutral (Scenario I) and low pH (Scenario II). Traditional esterification with methanol, followed by hydrolysis of methyl lactate, was employed for downstream separation to obtain polymer-grade LA. High-pressure steam was generated from solid debris via anaerobic digestion to complement energy demands partly. Energy consumption was further attenuated by process integration using pinch technology, with around 15% and 11% utility cost savings for Scenario I and II, respectively. These processes were capital-intensive, with 42–46% of LA production cost stemming from direct and indirect costs. Utilities were the major cost-contributing factor (19–21%) due to energy-intensive water evaporation from dilute fermentation broth. Due to additional processing steps, capital investment and operating costs were slightly higher in Scenario I than in Scenario II. LA manufacturing cost was thus more for Scenario I ($2.07 per kg) than Scenario II ($1.82 per kg). The minimum LA selling price for Scenario I and II were $3.52 and $3.22 per kg, respectively, with five-year payback periods and 8.5% internal rates of return. LA was slightly more expensive for decentralized BW processing than the market price.
1. Introduction
Today, most of the world's energy and industrially relevant organic chemicals are produced from fossil-based resources. The petrochemical route supply more than 80% and 90% of fuels and chemicals, respectively.1,2 However, continued dependency on these non-renewable resources to meet growing energy and commodity demands is unsustainable and inconsistent with achieving net-zero CO2 targets.2,3 Meanwhile, enormous quantities of organic waste generated by global human activities, such as food production, are not harnessed to produce industrially important organic chemicals, especially those utilised by the plastics and textile industries.4 The power of microbes to turn waste into value-added chemicals has attracted substantial interest among researchers to find ways of replacing fossil-based production routes with green technologies for manufacturing chemical building block with a concurrent reduction in environmental impacts. Transitioning to a sustainable decarbonized chemical industry requires a rapid shift from petroleum to renewable carbon-based resources and energy supplies.5Fossil-based resources originated from biomass. Therefore, biomass could be a sustainable resource for production of energy and chemical building blocks via a biorefinery approach. Lignocellulosic biomass (LCB) is the most abundant bioresource in the biosphere, and the carbohydrate fraction is the most researched for this purpose. Despite all efforts in the past few decades, LCB-based biorefineries are still under development and not yet industrialized. One of the major obstacles is the tedious and cumbersome process of extracting fermentable sugars from LCB due to its recalcitrant nature, making the overall manufacturing process complex, as well as capital and energy intensive. A possible alternative to LCB is the waste biomass, where the release of sugars is easier and economical and does not compete with food production.6
Food waste is a global problem resulting in enormous quantities of unused and wasted biomass across the supply chain (∼1.3 billion tons).7–9 Food waste is rich in starch, proteins, and lipids, which can be recovered, concentrated, and transformed into high-value commodity products. The use of food waste can also be more profitable than conventional processing methods.10 One example is bread waste (BW) which is rich in high-quality fermentable sugars (50–70%) and generated in large quantities in residential and commercial operations across Europe and the USA. BW is a major food waste across the globe. For example, the food waste in Finland, Netherlands, New Zealand, Norway, Portugal, Saudi Arabia, South Korea, and Sweden contains 13, 22, 23, 27, 7.9, 18.7, 2.2, and 12–17% of BW, respectively.11 Bread is the second most wasted food in the UK, and as per the WRAP report, 20 million slices of bread go to bin every day,12 and BW constitutes 10% of all the food waste generated in the UK.13 The annual availability of BW in the UK alone is approximately 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons (MT).7 The situation is similar in other European countries. For example, 27% of the edible household food waste in Norway comprises bakery products.14 In Sweden, 29
000 metric tons (MT).7 The situation is similar in other European countries. For example, 27% of the edible household food waste in Norway comprises bakery products.14 In Sweden, 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 tons of BW are generated domestically and 80
870 tons of BW are generated domestically and 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 tons across the supply chain.13,15 Bread has also been identified as a major waste in Austria, Sweden, Netherlands, and Finland.16–21 The valorisation of such food waste is important to mitigate environmental impacts, as well as to reduce dependency on fossil-based resources for the production of value-added products.7 There are several examples where BW has been used for fermentative production of high-value chemical products, such as succinic acid, ethanol, 2,3-butanediol, etc.6,22,23
410 tons across the supply chain.13,15 Bread has also been identified as a major waste in Austria, Sweden, Netherlands, and Finland.16–21 The valorisation of such food waste is important to mitigate environmental impacts, as well as to reduce dependency on fossil-based resources for the production of value-added products.7 There are several examples where BW has been used for fermentative production of high-value chemical products, such as succinic acid, ethanol, 2,3-butanediol, etc.6,22,23
Lactic acid (LA) is a vital commodity chemical that is included in the revised list of platform chemicals by the US Department of Energy.24–26 LA is one of the top ten biochemicals in the UK based on its commercial viability, functionality, and sustainability.27 As a platform chemical, LA can be transformed into a wide range of high-value products, such as propylene glycol, propylene oxide, lactic and acrylic acid esters, and others. LA finds applications in the plastics, textile, food, agriculture, cosmetic, pharmaceutical, and chemical industries. The global LA market was $2.6 billion in 2018 and is anticipated to reach $8.7 billion by 2025, with a compound annual growth rate of 18.7%.28 Biodegradable polymers such as polylactic acid (PLA) derived from LA are amongst the most well-established bio-based synthetic materials used by the plastics and fibre industry. The properties of PLA as a material compete to differing extents with several commodity fossil-based plastics, including polyethylene terephthalate (PET), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), and low-density polyethylene (LDPE). Further, unlike ethanol, the substrate carbon is conserved in product and there is no release of CO2 (C6H12O6 → 2C3H6O3) during LA fermentation. Thus, products like LA produced indirectly from CO2 (biomass) can make significant contribution towards net removal of CO2 from atmosphere which is highly needed in current time to curb carbon emission. Currently, 90% of LA is obtained through the microbiological fermentation route, mainly from corn starch, sugarcane, and sugar beet. However, these feedstocks are expensive and compete with food products. To address this challenge, an alternative low-cost LA production route that does not compete with food production and harnesses inexpensive unavoidable wastes from the food supply chain and agro-industrial sectors, could be developed to enable wider applications of LA.16,29
In our previous work, we evaluated the potential of BW as feedstock for LA production by Bacillus coagulans.30 BW, for this purpose, was saccharified via acid and enzymatic hydrolysis. We achieved a high-level LA production from BW with a titer of 155.4 g L−1 during fed-batch cultivation under non-sterile conditions, and the conversion yield was 0.42 g LA per g BW. All the biogenic residues obtained during saccharification and fermentation were collected and subjected to anaerobic digestion. On the other hand, techno-economic and feasibility studies are important to gauge the technological challenges of non-conventional feedstock and to demonstrate their commercial viability. However, techno-economic viability studies related to the production of LA from waste biomass, especially food waste, have been limited. Kwan et al. reported LA production from 10 MT per h of food waste and estimated the 80% LA production cost to be $1.066 per kg,31 and Li et al. reported techno-economic and life cycle analyses based on the production of LA from 2000 MT LCB per day for various scenarios.25
The conversion of various food wastes and LCB to LA has been previously reported in the literature,32,33 but these feedstocks are generally heterogeneous in composition. An attractive feature of BW is the comparative homogeneity of the composition. To our knowledge, a techno-economic analysis of the production of LA from BW has not been reported in the literature. Further, previous studies mainly focused on economic and life cycle assessment without in-depth process design, energy balance, and economic evaluation. A detailed process design is important to evaluate the complexity and estimate the capital investment, operating and production costs, as well as associated energy consumption precisely. It further highlights the critical economic barriers in the way of commercialization. This work thus delineated a comprehensive process design using Aspen Plus and an evaluation of the economic feasibility for LA production from BW.
Accordingly, the purpose of this work was to address these requirements based on a comprehensive process design using Aspen Plus and an evaluation of the economic feasibility of LA production from BW. Generally, energy is one of the main operating costs involved in industrial processes, and reducing energy consumption is a primary goal in improving thermal efficiency and hence, the economics of the process. In this work, we use appropriate thermodynamic models for separation units and validated kinetic models for the reactors for precise estimation of energy consumption. Pinch technology relies on a systematic approach to minimize utility consumption by designing a suitable heat exchanger network (HEN). Herein, integrated processes were developed using pinch technology to reduce energy consumption. Sensitivity and profitability analyses were conducted to identify the key cost-contributing parameters, estimate the minimum LA selling price and the internal rate of return (IRR). Traditionally, LA fermentation is carried out under neutral pH using lime to avoid microorganism inhibition by the acidic product. This approach involves subsequent hydrolysis of lactates by acid after fermentation to regenerate LA. Fermentation using acid-resistant microorganisms is a possible alternative to circumvent process complexity and cost.34 This approach also avoids the need for expensive chemicals and the disposal of calcium salt. The economic and profitability performance of traditional acid-neutral fermentation was further compared with low-pH equivalents for producing LA from BW.
2. Methodology
2.1 Feedstock
The BW composition used as a feedstock for LA production comprised 46% carbohydrates, 2.5% fiber, 7.9% protein, 2% saturated fats, and 1% salt, with the balance amount as water.30 The carbohydrate was represented as starch, with 3000 monomers units in the polymer chain. The fat and protein were expressed by linoleic acid and alanine, respectively, and the fiber was considered a non-conventional solid. The processes were developed using Aspen Plus software for the BW, with a feeding rate of 100 MT per day, which represented the decentralized processing of BW.2.2 Saccharification, fermentation, and anaerobic digestion
The saccharification of BW was carried out enzymatically at 60 °C with a solid loading of 20% (w/v) and 48 h residence time. The conversion of starch in the saccharification reactor was taken as 95%, which resulted in a glucose titer of ∼111 g L−1 and a yield of 0.48 g g−1 BW. The starch hydrolysis reaction is slightly exothermic with a standard heat of reaction of −65.5 kJ mol−1 glucose. The LA accumulating cell factories are sensitive to pH fluctuations. The fermentation is generally carried out around neutral pH of 7.0, which is maintained using neutralizing agents. In this work, the process was developed using acid-neutral microorganisms in fermentation (Scenario I). The pH in the fermenter was controlled using calcium hydroxide, which converted LA to calcium lactate. The calcium lactate was later hydrolyzed by sulfuric acid to regenerate LA, with the co-generation of calcium sulfate. The fermentation was carried out at 50 °C with a residence time of 120 h. B. coagulans used in the current work is a homofermentative LA bacteria, and the reaction stoichiometry for the fermentation is shown in Scheme 1. The conversion of glucose to LA in fermentation was taken as 95% with a LA concentration of around 106 g L−1 in the fermentation broth. The fermentation reaction is also exothermic with a standard heat of reaction of around −225.3 kJ mol−1 glucose. However, Scenario I involved complex neutralization of LA and hydrolysis of lactate, with added costs and created waste disposal problems. Therefore, one alternative is fermentation using acid-resistant microorganisms (Scenario II).34 Engineered microorganisms are reported to tolerate low pH, and Cargill (for example) has produced LA commercially using acid-tolerant (pH ≤ 3.0) microorganisms.34 Therefore, the economic performance of Scenario I was compared with low-pH fermentation using acid-resistant microorganisms (Scenario II). The RStoic reactor model was used for both the saccharification reactor and fermenter. The solid residues from the saccharification and fermentation reactions were pooled and used for anaerobic digestion to generate biogas (Scheme 2). The standard heat of reactions presented in Schemes 1 and 2 were calculated using Aspen Plus. The anaerobic digestion of glucose and LA is exothermic, while it is endothermic for protein and fat. The anaerobic digestion was carried out at 41 °C for 16 days.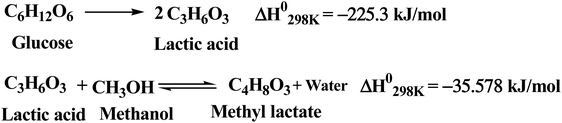 | ||
| Scheme 1 Reaction stoichiometry for fermentation of glucose to lactic acid (LA) and esterification of LA to methyl lactate. | ||
2.3 Lactic acid separation process
LA is a high boiling (122 °C at 15 mmHg) and thermally sensitive liquid. Therefore, unlike ethanol, LA cannot be distilled from the fermentation broth. However, if LA is recovered as the bottom product from the distillation column, it remains contaminated with heavy fermenter residues. These factors make the recovery of LA quite challenging from the fermentation broth. Various LA separation processes, such as liquid–liquid extraction and membrane separation, have been employed in the past, but they are not cost-effective.25 In contrast, the traditional microbial LA production process (Scenario I) uses the reactive separation method to recover LA from the fermentation broth. This method comprises (i) fermentative production of LA with the concurrent neutralization of LA by lime, (ii) the hydrolysis of calcium lactate by sulfuric acid to regenerate LA with the co-production of calcium sulfate, (iii) evaporation of water to obtain concentrated LA, (iv) esterification of LA with methanol in the presence of cation exchange resin (Amberlyst 15), (v) the recovery of methyl lactate as a distillate from heavy fermenter residues by distillation, (vi) hydrolysis of methyl lactate to LA over cation exchange resin catalyst, and (vii) purification of LA by vacuum distillation.35 The calcium lactate is highly miscible in water.36 The calcium lactate concentration in the fermenter was well below its solubility limit at a fermentation temperature of 50 °C and remained completely miscible in the fermentation broth. Methanol is the most volatile alcohol and easy to distill after the esterification and hydrolysis reactions. Methanol was thus chosen for the esterification reaction. However, esterification is an equilibrium-limited reaction, and the equilibrium conversion of LA is restricted by the product water (Scheme 1). The removal of water from the fermentation broth is thus necessary before the esterification reaction to maximize LA recovery at a moderate methanol/LA mole ratio. For Scenario II, the neutralization of LA and subsequent hydrolysis of the calcium lactate are redundant; but the remaining steps are the same as in Scenario I.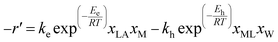 | (1) |
2.4 Kinetics of lactic acid (LA) esterification and methyl lactate hydrolysis
The heterogeneous kinetics of esterification of LA and hydrolysis of methyl lactate was taken from the literature, as shown in eqn (1).37 The esterification of LA was carried out in a fixed-bed reactor using Amberlyst 15 catalyst at 80 °C. The liquid phase of the reaction was ensured by maintaining an elevated pressure of 3 bar in the reactor. The esterification of LA with methanol is an equilibrium-limited reaction, and the equilibrium constant was calculated using Aspen Plus as 8.56 × 104 at 80 °C. Further, the esterification of LA with methanol is slightly exothermic (Scheme 1). The equilibrium of this reaction is thus favored at lower temperatures and higher methanol/LA mole ratios. However, using excessive methanol involves the separation of excess methanol after the reaction with added cost. The esterification reaction was thus carried out using a modest 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/LA mole ratio. Similarly, hydrolysis of methyl lactate was performed in the fixed-bed reactor using Amberlyst 15 catalyst at 80 °C and 3 bar pressure with 3
1 methanol/LA mole ratio. Similarly, hydrolysis of methyl lactate was performed in the fixed-bed reactor using Amberlyst 15 catalyst at 80 °C and 3 bar pressure with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water/methyl lactate mole ratio. The conversion of LA was about 85% in the esterification reaction under the reaction conditions. The per pass conversion of methyl lactate was around 55% during the hydrolysis of methyl lactate.
1 water/methyl lactate mole ratio. The conversion of LA was about 85% in the esterification reaction under the reaction conditions. The per pass conversion of methyl lactate was around 55% during the hydrolysis of methyl lactate.
2.5 Pinch analysis
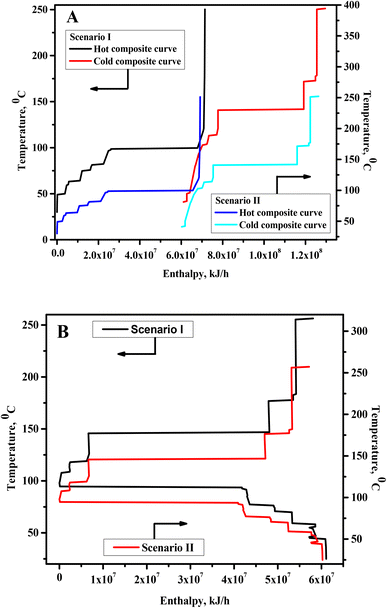
Pinch technology is a practical method for the optimal design of HEN with maximum process heat energy utilization.38 In this work, integrated processes were developed using pinch technology. For this purpose, inlet and outlet temperatures, enthalpy changes involved in hot and cold process streams, and mass flow rate were acquired from the developed processes. Heat duty was also involved in several isothermal unit operations. These unit operations were also included in the pinch analysis, considering their inlet to the outlet temperature difference of 1 °C. Temperature–enthalpy diagrams, i.e., composite and grand composite curves, were used to obtain maximum process heat energy exchange potentials, minimum utility consumption, and pinch point temperatures. The integrated process with in-built HEN was then developed for the highest possible process heat energy recovery considering ΔTmin as 10 °C. Cooling water and steam (100 psi and 400 psi) were the utilities in these processes. The cost of these utilities is shown in Table 1.| Bread waste (BW) | 100$ per MT |
| Enzymes | 3.08$ per kg (ref. 41) |
| Lime | 0.109$ per kg (ref. 41) |
| H2SO4 | 0.25$ per kg (ref. 42) |
| Cooling water | 0.032$/1000 L |
| Electricity | 0.077$ per kW h |
| Inoculum | 0.007$ per kg (ref. 43) |
| Nutrients | 0.238$ per kg (ref. 43) |
| Steam@100 psi | 0.018$ per kg |
| Steam@400 psi | 0.027$ per kg |
| Catalyst | 1.18$ per kg LA (ref. 44) |
2.6 Economic and profitability analysis
Process economics involves two cost components: fixed and operating costs. The fixed capital expenditure comprises equipment, direct, and indirect costs. The equipment cost was calculated based on the 6/10th rule (eqn (2)). The recommended values of exponent n were obtained from the literature.39 The fermenters, enzymatic hydrolysis reactors, and anaerobic digestor costs were estimated based on integer numbers of 500 m3 stirred reactors, with 75% of the total capacity as the working volume. The equipment cost includes the storage of chemicals for twelve days and products for twenty-five days. The capital investment for distillation columns included the cost of the tower, condenser and its accessories, reflux pump, and reboiler and was calculated using the Aspen Plus economic analyzer, considering sieve trays with 0.61 m tray spacing. Similarly, the design of the heat exchangers and their capital costs were estimated using Aspen Plus. However, reactors for the esterification of LA and hydrolysis of methyl lactate were considered equivalent to the vertical packed column for estimating their capital investment. The direct fixed cost consists of installation, instrumentation, controls, piping, electrical, building, yard improvements, service facilities, and land. The indirect fixed cost involves construction expenses, engineering, supervision, legal expenses, contingency, contractor fees, and working capital. The chemical engineering plant cost index of 708.0 for 2021 was used to estimate equipment cost. | (2) |
The direct operating costs include labor, maintenance, overhead charges, raw materials, chemicals, utilities, and electricity. The labor cost was estimated considering six operators and one supervisor with individual salaries of $20 and $35 per h, respectively. The BW feedstock is generated as municipal and commercial waste, and ideally, it should be available free of cost or marginal cost. However, we considered a nominal BW price of $100 per MT to reflect collection costs and transportation to the biorefinery facility. The economic analysis includes the costs of enzymes, nutrients, and inoculum, which were taken as 0.052 kg, 0.273 kg, and 1.43 kg per kg of LA, respectively.40 The costs of chemicals and utilities used in the economic analysis are listed in Table 1. The indirect operating costs were insurance, taxes, depreciation, and interest. Process economics was evaluated assuming twenty years of plant life and straight-line depreciation of equipment costs, with 20% of the initial equipment costs as salvage value at the end of their life. In this work, the total capital investment was considered financed by a bank with 5.5% interest rate per annum. The profitability study was performed considering the cost-escalation factor of 3.5% for raw materials, 3% for utilities, labor, and maintenance, and 5% for products. The minimum LA selling price was calculated assuming 34% tax on income, but it is recognized that sales tax varies widely depending on the policies of different countries. Therefore, sales tax was excluded in the estimation of the minimum LA selling price.
3. Results and discussion
3.1 Process for fermentative production of lactic acid (LA) from bread waste (BW)
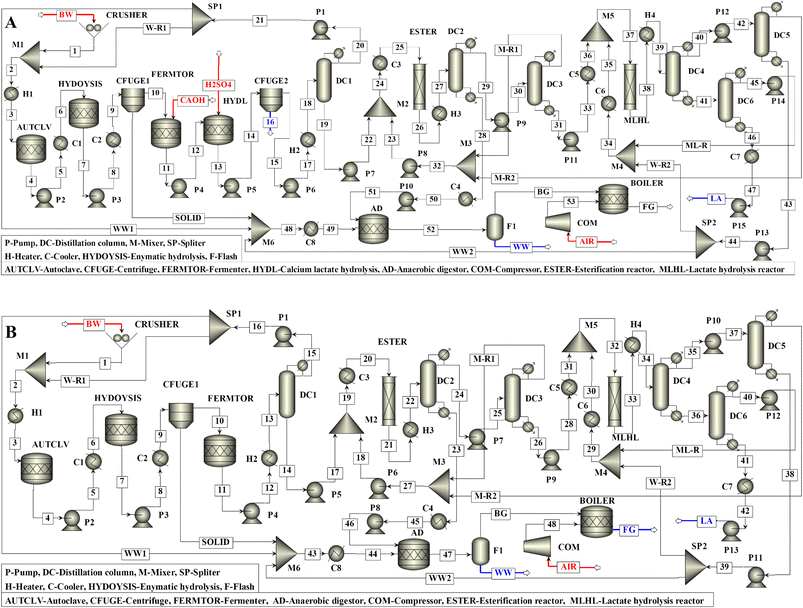
The basic flowsheet without process integration for fermentative production of LA from BW using acid-neutral microorganisms (Scenario I) is shown in Fig. 1A. The process started with shredding BW into small pieces. A suitable quantity of water was then added to the shredded BW to obtain the BW slurry with a solid loading of 20% (w/v). The BW slurry was heated to 121 °C and sterilized for 15 min in an autoclave. The mixture was then cooled to 60 °C and sent to the carbohydrate hydrolysis reactor, where the BW slurry was treated with an enzyme (Dextrozyme). The reaction mixture from the saccharification reactor was then cooled to 50 °C, and unconverted solids were separated by centrifuge. The clear sugar solution was then directed to the fermenter, and calcium hydroxide was added continuously to maintain a neutral pH. In the fermenter, LA was neutralized to calcium lactate, maintaining pH. Both fermentation and LA neutralization reactions are exothermic (Scheme 1), generating excessive heat energy in the fermenter. The calcium lactate was subsequently hydrolyzed to LA by sulfuric acid. In this reaction, calcium sulfate was formed, which was separated by a centrifuge. The liquid stream from the centrifuge containing LA, unconverted sugars, protein, fats, and salts were then sent to the distillation column (DC1) to evaporate around 99% water as distillate. Water evaporation from fermentation broth is essential to favor subsequent equilibrium-limited esterification reaction at a modest methanol/LA mole ratio (Scheme 1). Since BW was comprised of 40.6% water, the amount of water recovered from DC1 was more than required for saccharification. The requisite amount of water was thus recycled to the saccharification processes after purging the excess amount.The concentrated LA obtained from the bottom of DC1 had a temperature of around 140 °C. This stream was mixed with a suitable quantity of methanol equivalent to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/LA mole ratio, cooled to 80 °C, and sent to the LA esterification reactor. The excess methanol and methyl lactate were separated from unconverted LA and heavy fermenter residues by two distillation columns (DC2 and DC3). These distillation columns were operated at atmospheric pressure. The heavy fermenter residues and unconverted LA were obtained from the bottom of DC2. In comparison, methanol and methyl lactate were obtained as distillate and bottom products of DC3, respectively. The methanol recovered from DC3 was then recycled into the LA esterification reactor. The heavy fermenter residue obtained from the bottom of DC2 and solid residues separated after saccharification were sent for anaerobic digestion for biogas generation. About 2913.7 metric tons of methane was produced per annum, and a fraction of it was used to generate high-pressure steam to complement the energy demand in the process.
1 methanol/LA mole ratio, cooled to 80 °C, and sent to the LA esterification reactor. The excess methanol and methyl lactate were separated from unconverted LA and heavy fermenter residues by two distillation columns (DC2 and DC3). These distillation columns were operated at atmospheric pressure. The heavy fermenter residues and unconverted LA were obtained from the bottom of DC2. In comparison, methanol and methyl lactate were obtained as distillate and bottom products of DC3, respectively. The methanol recovered from DC3 was then recycled into the LA esterification reactor. The heavy fermenter residue obtained from the bottom of DC2 and solid residues separated after saccharification were sent for anaerobic digestion for biogas generation. About 2913.7 metric tons of methane was produced per annum, and a fraction of it was used to generate high-pressure steam to complement the energy demand in the process.
The methyl lactate stream obtained from the bottom of DC3 contained water formed during the esterification reaction and had a temperature of around 113 °C. This stream was cooled to 80 °C, mixed with an additional quantity of water corresponding to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water/methyl lactate mole ratio, and sent to the methyl lactate hydrolysis reactor. The hydrolysis reaction is slightly endothermic (Scheme 1), and the per pass conversion of methyl lactate was around 55% under the reactor conditions. The methanol, water, LA, and unconverted methyl lactate were separated by three distillation columns (DC4–DC6). In DC4, methanol and water were recovered together as distillate, with methyl lactate and LA together as the bottom product. The methanol was then separated from water in DC5 and recycled to the LA esterification reactor. The water was recycled to the methyl lactate hydrolysis reactor after purging the excess amount. The unconverted methyl lactate was separated from LA in DC6 and returned to the methyl lactate hydrolysis reactor. The DC4 and DC5 were designed at 1 bar pressure; however, DC6 was operated at 0.2 bar pressure to maintain the reboiler temperature below 180 °C to avoid the thermal decomposition of LA.35 LA obtained from this process had more than 99.9% purity, suitable for polymer manufacturing processes. RadFrac model was used for the distillation columns, with NRTL as the property method. They were designed considering 99.9% recovery of products. The optimum reflux ratio, number of stages, feed stage location, and column diameter for both scenarios are shown in Table 2.
1 water/methyl lactate mole ratio, and sent to the methyl lactate hydrolysis reactor. The hydrolysis reaction is slightly endothermic (Scheme 1), and the per pass conversion of methyl lactate was around 55% under the reactor conditions. The methanol, water, LA, and unconverted methyl lactate were separated by three distillation columns (DC4–DC6). In DC4, methanol and water were recovered together as distillate, with methyl lactate and LA together as the bottom product. The methanol was then separated from water in DC5 and recycled to the LA esterification reactor. The water was recycled to the methyl lactate hydrolysis reactor after purging the excess amount. The unconverted methyl lactate was separated from LA in DC6 and returned to the methyl lactate hydrolysis reactor. The DC4 and DC5 were designed at 1 bar pressure; however, DC6 was operated at 0.2 bar pressure to maintain the reboiler temperature below 180 °C to avoid the thermal decomposition of LA.35 LA obtained from this process had more than 99.9% purity, suitable for polymer manufacturing processes. RadFrac model was used for the distillation columns, with NRTL as the property method. They were designed considering 99.9% recovery of products. The optimum reflux ratio, number of stages, feed stage location, and column diameter for both scenarios are shown in Table 2.
| DC1 | DC2 | DC3 | DC4 | DC5 | DC6 | |
|---|---|---|---|---|---|---|
| Number of stages | 10 | 10 | 23 | 18 | 24 | 11 |
| Reflux ratio (mole) | 0.0028 | 0.0071 | 1.179 | 0.336 | 1.68 | 0.3515 |
| Feed stage | 6 | 4 | 17 | 11 | 16 | 5 |
| Column diameter, m | 4.27 | 1.22 | 1.07 | 1.68 | 0.61 | 0.76 |
The basic flowsheet without process integration for fermentative production of LA from BW using acid-tolerant microorganisms (Scenario II) is shown in Fig. 1B. In this scenario, the fermentation broth containing LA was directly sent to the DC1, and the remaining process was the same as in Scenario I. However, heat energy generated in the fermenter was less than in Scenario I due to the absence of the exothermic LA neutralization reaction. The theoretical LA yield was about 0.46 kg kg−1 BW. However, the overall yield of LA in this process was 0.39 kg kg−1 BW. The yield loss in this process was due to incomplete carbohydrate hydrolysis, sugars conversion in the fermenter, and LA conversion in the esterification reactor.
3.2 Process integration by pinch technology
| Scenario I | Scenario II | |
|---|---|---|
| Cooling water, MT per annum | 2.66 × 107 | 2.62 × 107 |
| Steam, MT per annum | 1.78 × 105 | 1.74 × 105 |
| Electricity, kW h h−1 | 377.3 | 342.1 |
| Ca(OH)2, MT per annum | 7.06 × 103 | — |
| H2SO4, MT per annum | 9.35 × 103 | — |
| LA, MT per annum | 1.43 × 104 | |
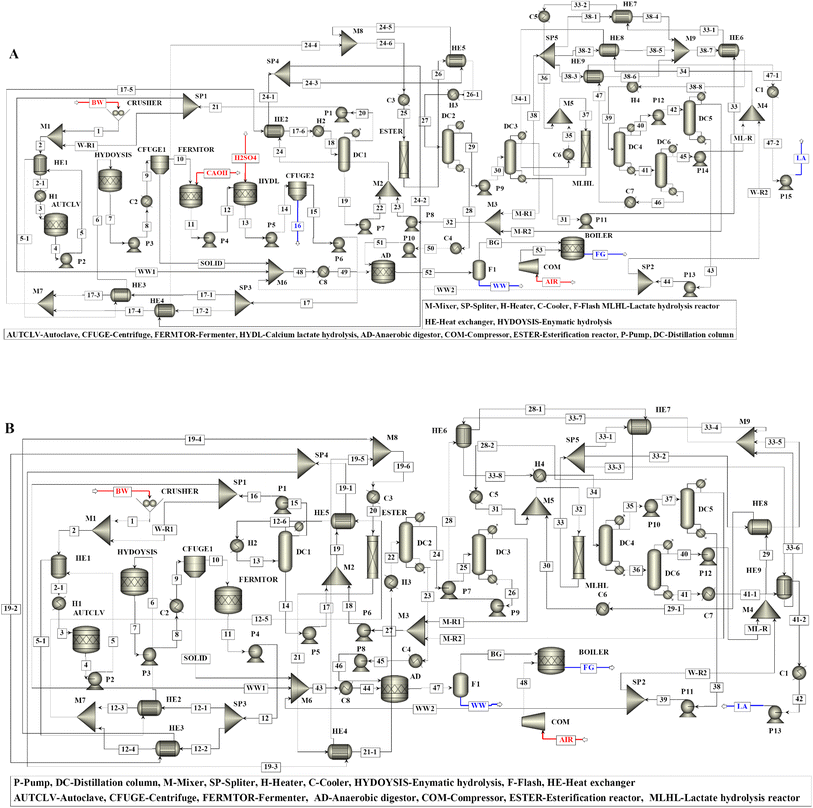
The HEN and grid diagram for microbial LA production from BW under neutral pH (Scenario I) is shown in Fig. 3. Below pinch point temperature, the calcium lactate and methyl lactate hydrolysis reactors and anaerobic digestor were heated using the heat duty of the DC1 condenser. Both C3 and H2 were separately split into two streams. The heat duty of H3 was met by C3 with a split fraction of 0.7, while C3 with a split fraction of 0.3 was used to heat the H2 stream with a split fraction of 0.0574. The H2 stream with a split fraction of 0.9426 was heated to pinch pint temperature using C1. The H4 was divided into three streams with split fractions of 0.35, 0.40, and 0.25, and they were heated to pinch point temperature by C5, C6, and C7, respectively. The remaining heat duty of the C3 and C5–C7 and all other hot streams below pinch point temperature were met by cooling water. Above the pinch point temperature, the C1, C3, and C5 were cooed to pinch point temperature by heat exchange with H1, H2, and H4, respectively, and the remaining heat duty of the H1, H2, and H4 was met by steam. The heat duty of C4 and C7 was partially utilized (around 90%) for heating the reboiler of DC5. Similarly, all other cold streams above pinch point temperature were heated by steam. The integrated process with the HEN is shown in Fig. 4A.
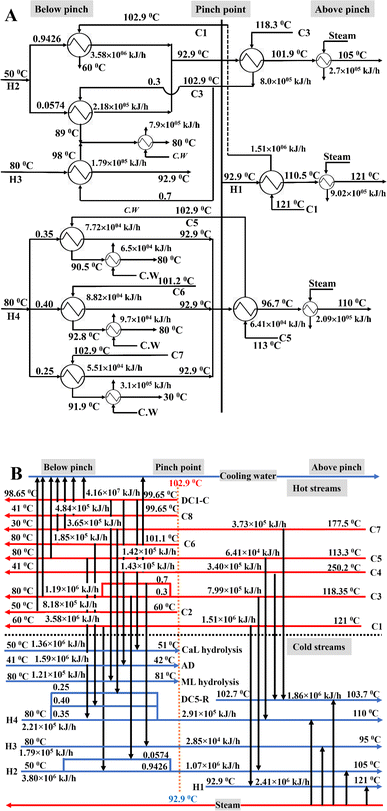 | ||
| Fig. 3 (A) Heat exchanger network (HEN) and (B) grid diagram for fermentative production of lactic acid (LA) from bread waste (BW) using acid-neutral microorganisms (Scenario I). | ||
3.3 Economic analysis
| Equipment | Scenario I | Scenario II |
|---|---|---|
| Crusher | 0.195 | 0.195 |
| Sterilizer | 0.089 | 0.089 |
| Hydrolysis | 1.920 | 1.920 |
| Fermenters | 4.481 | 4.481 |
| Calcium lactate hydrolysis | 0.103 | 0.000 |
| Reactors | 0.101 | 0.101 |
| Anaerobic digesters | 1.920 | 1.920 |
| Distillation columns | 1.279 | 1.274 |
| Heat exchangers | 0.238 | 0.237 |
| Pumps and compressors | 0.546 | 0.534 |
| Centrifuges | 0.104 | 0.022 |
| Boiler | 0.287 | 0.287 |
| Storage tanks | 0.303 | 0.126 |
| Total equipment cost | 11.568 | 11.186 |
| Installation | 6.015 | 5.817 |
| Instrumentation and controls | 3.470 | 3.356 |
| Piping | 8.676 | 8.390 |
| Electricals | 1.388 | 1.342 |
| Building | 2.314 | 2.237 |
| Yard improvements | 1.272 | 1.230 |
| Service facilities | 8.907 | 8.613 |
| Land | 0.578 | 0.559 |
| Direct fixed cost (A) | 44.188 | 42.731 |
| Engineering and supervision | 3.977 | 3.846 |
| Construction expenses | 4.861 | 4.700 |
| Legal expenses | 0.442 | 0.427 |
| Contractor fees | 2.651 | 2.564 |
| Contingency | 5.303 | 5.128 |
| Indirect fixed cost (B) | 17.233 | 16.665 |
| Fixed capital cost (C = A + B) | 61.421 | 59.396 |
| Working capital (D) | 3.071 | 2.970 |
| Total capital expenditure (C + D) | 64.492 | 62.366 |
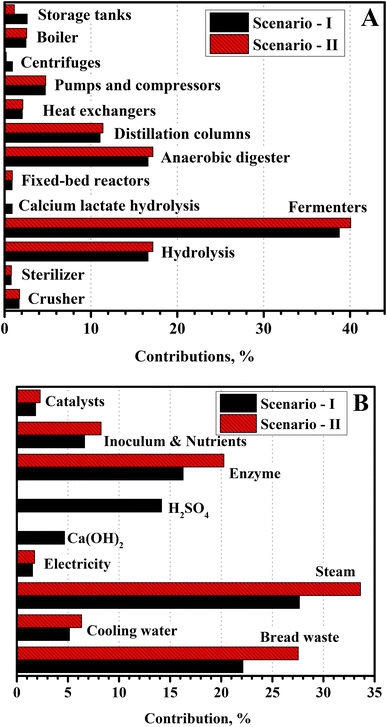 | ||
| Fig. 5 Contribution of the individual (A) equipment and (B) raw materials and utilities on their respective total costs. | ||
The fermenters have been reported to contribute 27% of the equipment cost for LA production from food waste.31 Distillation columns were another major unit operation involved in this process, accounting for about 11% of the total equipment costs (Table S1†). However, DC1 alone contributed approximately 34% of the total cost of the distillation columns due to the voluminous feed flow rate with a large diameter column (Table 2). The share of all other equipment was less than 5% of the total equipment cost. Scenario I involved the additional calcium lactate hydrolysis reactor, centrifuge to separate calcium sulfate, and storage vessel for sulfuric acid and calcium hydroxide (Table 4). In Scenario I, water was additionally generated due to the neutralization of LA in the fermenter, with the slightly higher cost of the distillation columns, heat exchangers, and pumps. Total equipment costs in Scenario I was thus marginally higher than in Scenario II.
| Scenario I | Scenario II | |
|---|---|---|
| Direct cost | ||
| Operating labor | 1.358 | 1.358 |
| Maintenance | 3.870 | 3.742 |
| Operating charges | 0.339 | 0.339 |
| Plant overhead charges | 2.614 | 2.550 |
| Bread waste (BW) | 3.650 | 3.650 |
| Utilities (cooling water and steam) | 5.415 | 5.298 |
| Electricity | 0.254 | 0.231 |
| Chemicals | 6.886 | 3.778 |
| Catalysts | 0.303 | 0.303 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Indirect costs | ||
| Insurance & taxes | 0.174 | 0.168 |
| Depreciation | 0.463 | 0.447 |
| Interest | 3.547 | 3.430 |
| General & administration expenses | 0.654 | 0.639 |
| Total operating cost (A) | 29.526 | 25.934 |
| LA produced, MT per annum (B) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 273.0 273.0 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 273.0 273.0 |
| Production cost, $ per kg (A/B) | 2.069 | 1.817 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Without pinch analysis | ||
| Additional utility (A′) | 0.969 | 0.670 |
| Production cost, $ per kg [(A + A′)/B] | 2.14 | 1.86 |
3.4 Cost-controlling operating parameters and sensitivity analysis
The direct operating costs, associated with labor, maintenance, operating charges, and overhead, were the governing factors for the LA production cost, with around 28–31% share (Fig. 6). On the other hand, indirect operating expenses, which included depreciation, interest, insurance, and taxes, contributed around 14–16% of the LA production cost. These two factors were directly related to the capital investment, and their cumulative contribution was 42–46% of the LA production costs. The considerable contribution of these factors implied a relatively large capital investment associated with these processes, mainly due to the capital-intensive reactive separation of LA from the fermentation broth. Besides, utilities were another prominent operating expense, with 19–21% contribution to the LA production cost. The large external utility consumption was due to the evaporation of large volumes of water from the fermentation broth. The contribution from chemicals and BW was 23–15% and 12–14% of the LA production cost, respectively. Developing novel separation processes, such as reactive distillation, solvent extraction, membrane-based separation, etc., could markedly improve the economic performance of the process. Further research in this direction should focus on reducing utility consumption and capital investments by simplifying the separation process. These process improvements are likely to improve the economics of microbial LA production based on BW feedstock in decentralized processing.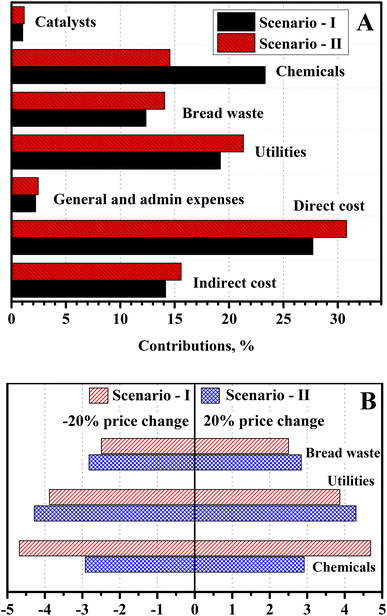 | ||
| Fig. 6 (A) Cost-controlling operating parameters on the LA manufacturing cost. (B) Effect of ±20% price deviation of BW, utilities, and chemicals on the LA manufacturing cost. | ||
The retail prices of feedstock, chemicals, and utilities are sensitive and vary with time and geographical region. For example, electricity is highly expensive in the UK (∼0.1585 $ per kW h), which will inflate the LA production cost to $2.09 and $1.83 per kg for Scenario I and II, respectively.45Fig. 6B thus shows the effect of a ±20% variation of base prices on the LA production cost. The results reveal that utility price is the most sensitive factor, followed by chemicals and feedstock. The LA production cost varied between ±3.8–4.3%, ±4.7–2.9%, and ±2.5–2.8% for ±20% change in the price of utilities, chemicals, and feedstock, respectively. Lower-cost BW feedstock would also reduce LA manufacturing costs based on the processing scenarios outlined herein. For example, BW can be free of cost when utilized at the generation site, especially commercial operation, or subsidized by the government for achieving the clean environmental goal. In this case, the LA can be produced at $1.81 and $1.56 per kg for Scenario I and II, respectively. Under this consideration, LA production costs could be reduced by around 12–14%.
3.5 Profitability analysis
Profitability analysis enables the economic sustainability of a process to be evaluated from an investment perspective. It is measured by various parameters, such as payback period and IRR. A short payback period and high IRR imply a profitable investment for a fixed product selling price. It also estimates the minimum product selling price for a desired IRR and payback period. The minimum selling price was considered as the product's cost, giving a net zero present value of all future cash flows. In this work, the minimum LA selling price was estimated for payback periods of 5–10 years and IRR of 8.5–15%. LA was cheaper for a higher payback period and a lower IRR (Fig. 7). For Scenario I, the minimum LA selling price per kg was estimated as $3.52 and $2.71 for a payback period of five- and ten-years, respectively, with 8.5% IRR. For a higher IRR of 15%, the minimum LA selling price was escalated to $3.82 and $3.02, respectively. However, the minimum LA selling price was lower in Scenario II than in Scenario I due to lower capital investment and operating (chemicals and utility) costs. For a payback period of five years, the minimum unitary LA selling price was $3.22 and $3.52 for IRR of 8.5% and 15%, respectively. The minimum unitary LA selling price was reduced to $2.44 and $2.74 for IRR of 8.5% and 15%, respectively, when the payback period was increased to ten years. The results exhibited that the minimum LA selling price was much higher than the production costs (Table 5). The significant difference between the selling price and production costs reflected the large capital investment involved in these processes, with a high share of direct and indirect operating costs.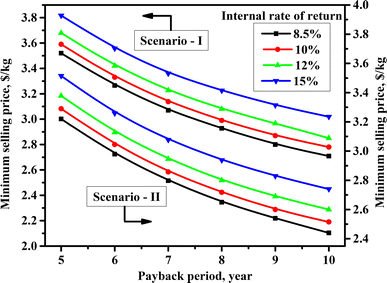 | ||
| Fig. 7 Minimum selling price of fermentative lactic acid (LA) from bread waste (BW) with 100 MT feeding rate per day for various payback periods and internal rate of return. | ||
The minimum LA selling price based on LCB feedstock has been reported as $1.38–1.91 per kg for 2000 MT per day plant capacity.25 Marchesan et al. reported an economic analysis to produce LA from 212 MT sugarcane per h. They evaluated economic performance at different pH in the fermenter with a 24 h fermentation time, 130 g L−1 sugar, and LA yield of 0.97 g g−1 glucose.34 The minimum LA selling price was estimated as $1.134 and $1.057 per kg for acid-neutral and low pH fermentation, respectively, considering thirty years of plant life and 10% IRR. The minimum 80% LA selling price was reported to be $0.943 per kg from 10 MT food waste per hour.31 However, the minimum LA selling price estimated in this work was much higher than that previously reported in the literature. This is principally due to the assumption of decentralized BW processing, combined with low plant capacity and long fermentation periods. Further, the market price of 80% LA has been reported as $1.874 per kg,31 although the selling price of polymer-grade LA is much higher and varies from country to country, for example, $2.793 per kg in Germany and $2.858 per kg in the USA.46 The minimum selling price of LA produced from BW in a decentralized facility in this study was also slightly higher than the reported market prices for conventionally produced LA. In the future, a cost-effective downstream LA purification process could potentially reduce the price differential. Developing a LA production process with higher productivity and a low-cost downstream LA purification process will possibly reduce this gap in the future. For example, LA production cost was reduced to $1.91 and $1.65 per kg by decreasing the fermentation period to 48 h alone. In this case, the minimum LA selling price was dropped to $3.07 per kg for Scenario I and $2.77 per kg for Scenario II for five years payback period and 8.5% IRR. The selling price could be further reduced by assuming cost-free BW. Under this consideration, the minimum LA selling price was found to be $2.82 per kg for Scenario I and $2.52 per kg for Scenario II for the same payback period and IRR.
4. Conclusions
LA is an increasingly important platform chemical to produce biodegradable polylactic acid for the manufacturing of plastics, textile fibres, and many other consumer and industrial products. However, alternative methods of producing LA are required that do not compete with food production. This study elucidated the techno-economic feasibility of decentralized processing of BW (100 MT per day) for microbial production of polymer-grade LA. The integrated processes were developed using pinch technology for acid-neutral and low-pH scenarios. The utility costs were saved by around 15% and 11% using pinch analysis for Scenario I and II. Scenario I involved extra processing steps with added equipment and chemicals. Further, water was formed during the neutralization of LA, with slightly higher utility consumption and equipment costs for distillation columns, heat exchangers, and pumps. These factors were responsible for marginally higher unitary LA manufacturing cost for Scenario I ($2.07) than for Scenario II ($1.82). The direct and indirect cost was the major cost-contributing factor (42–46%) of the total production cost, implying massive capital investment. Utility costs were the governing factor driving LA manufacturing costs (accounting for 19–21% of the total). For a five-year payback period and 8.5% IRR, the minimum LA selling price per kg was $3.52 for Scenario I and $3.22 for Scenario II. The decentralized production of polymer-grade LA from BW was slightly more expensive than the present market price. Though acid-tolerant microorganisms improved economic performance marginally, the primary economic barriers were the excessive capital investment and utility consumption associated with the complex reactive separation of LA. Therefore, future research should be directed toward developing LA fermentation with higher productivity and cheaper downstream LA recovery processes. All these should reduce capital investment and utility consumption for improving the economic viability of decentralized LA manufacturing using BW as a feedstock.Abbreviations
| BW | Bread waste |
| IRR | Internal rate of return |
| LA | Lactic acid |
| HEN | Heat exchanger network |
| MT | Metric tons |
Conflicts of interest
There are no conflicts to declare.References
- S. K. Maity, Renewable Sustainable Energy Rev., 2015, 43, 1427–1445 CrossRef CAS.
- S. K. Maity, Renewable Sustainable Energy Rev., 2015, 43, 1446–1466 CrossRef CAS.
- S. Takkellapati, T. Li and M. A. Gonzalez, Clean Technol. Environ. Policy, 2018, 20, 1615–1630 CrossRef CAS PubMed.
- A. V. Shah, A. Singh, S. Sabyasachi Mohanty, V. Kumar Srivastava and S. Varjani, Bioresour. Technol., 2022, 349, 126835 CrossRef CAS PubMed.
- V. Narisetty, R. Cox, R. Bommareddy, D. Agrawal, E. Ahmad, K. Kumar, A. K. Chandel, S. Kant Bhatia, D. Kumar, P. Binod, V. K. Gupta and V. Kumar, Sustainable Energy Fuels, 2022, 6, 29–65 RSC.
- V. Narisetty, S. Nagarajan, S. Gadkari, V. v. Ranade, J. Zhang, K. Patchigolla, A. Bhatnagar, M. Kumar Awasthi, A. Pandey and V. Kumar, Energy Convers. Manage., 2022, 266, 115784 CrossRef CAS.
- V. Narisetty, R. Cox, N. Willoughby, E. Aktas, B. Tiwari, A. Singh Matharu, K. Salonitis and V. Kumar, Sustainable Energy Fuels, 2021, 5, 4842–4849 RSC.
- T. H. Kwan, K. L. Ong, M. A. Haque, S. Kulkarni and C. S. K. Lin, Process Saf. Environ. Prot., 2019, 121, 194–208 CrossRef CAS.
- P. Sharma, A. Vimal, R. Vishvakarma, P. Kumar, L. porto de Souza Vandenberghe, V. Kumar Gaur and S. Varjani, Int. J. Food Microbiol., 2022, 372, 109691 CrossRef CAS PubMed.
- V. Kumar and P. Longhurst, Curr. Opin. Green Sustainable Chem., 2018, 13, 118–122 CrossRef.
- J.-M. Jung, J. Y. Kim, J.-H. Kim, S. M. Kim, S. Jung, H. Song, E. E. Kwon and Y.-E. Choi, J. Cleaner Prod., 2022, 365, 132795 CrossRef CAS.
- WRAP, Food Surplus and Waste in the UK – Key Facts, WRAP, 2021, accessed 3.24.22, https://wrap.org.uk/resources/report/food-surplus-and-waste-ukkey-fact Search PubMed.
- P. Brancoli, K. Bolton and M. Eriksson, Waste Manage., 2020, 117, 136–145 CrossRef PubMed.
- O. J. Hanssen, F. Syversen and E. Stø, Resour., Conserv. Recycl., 2016, 109, 146–154 CrossRef.
- P. Brancoli, M. Lundin, K. Bolton and M. Eriksson, Resour., Conserv. Recycl., 2019, 147, 128–136 CrossRef.
- V. Kumar, P. Brancoli, V. Narisetty, S. Wallace, D. Charalampopoulos, B. Kumar Dubey, G. Kumar, A. Bhatnagar, S. Kant Bhatia and M. J. Taherzadeh, Bioresour. Technol., 2023, 369, 128449 CrossRef CAS PubMed.
- A. E. Stensgård and O. J. Hanssen, Food Waste in Norway 2010–2015, Norway, 2016 Search PubMed.
- S. Lebersorger and F. Schneider, Waste Manage., 2014, 34, 1911–1919 CrossRef CAS PubMed.
- P. Brancoli, K. Rousta and K. Bolton, Resour., Conserv. Recycl., 2017, 118, 39–46 CrossRef.
- C. van Dooren, O. Janmaat, J. Snoek and M. Schrijnen, Waste Manage., 2019, 94, 153–164 CrossRef PubMed.
- J.-M. Katajajuuri, K. Silvennoinen, H. Hartikainen, L. Heikkilä and A. Reinikainen, J. Cleaner Prod., 2014, 73, 322–329 CrossRef.
- C. C. J. Leung, A. S. Y. Cheung, A. Y.-Z. Zhang, K. F. Lam and C. S. K. Lin, Biochem. Eng. J., 2012, 65, 10–15 CrossRef CAS.
- V. Narisetty, L. Zhang, J. Zhang, C. Sze Ki Lin, Y. Wah Tong, P. Loke Show, S. Kant Bhatia, A. Misra and V. Kumar, Bioresour. Technol., 2022, 358, 127381 CrossRef CAS PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC.
- Y. Li, S. S. Bhagwat, Y. R. Cortés-Peña, D. Ki, C. V. Rao, Y.-S. Jin and J. S. Guest, ACS Sustainable Chem. Eng., 2021, 9, 1341–1351 CrossRef CAS.
- E. Cubas-Cano, J. P. López-Gómez, C. González-Fernández, I. Ballesteros and E. Tomás-Pejó, Renewable Energy, 2020, 153, 759–765 CrossRef CAS.
- LBNet and BBSRC-NIBB, The ten green chemicals which can create growth, jobs and trade for the UK: Highlights from a report commissioned by The Lignocellulosic Biorefinery Network (LBNet), a Biotechnology and Biological Sciences Research Council Network in Industrial Biotechnology and Bioenergy (BBSRC-NIBB), 2018 Search PubMed.
- E. Augustiniene, E. Valanciene, P. Matulis, M. Syrpas, I. Jonuskiene and N. Malys, Crit. Rev. Biotechnol., 2022, 42, 342–360 CrossRef CAS PubMed.
- M. Alexandri, R. Schneider, K. Mehlmann and J. Venus, Food Technol. Biotechnol., 2019, 57, 293–304 CrossRef CAS PubMed.
- R. Cox, V. Narisetty, S. Nagarajan, D. Agrawal, V. V. Ranade, K. Salonitis, J. Venus and V. Kumar, Fuel, 2022, 313, 122976 CrossRef CAS.
- T. H. Kwan, Y. Hu and C. S. K. Lin, J. Cleaner Prod., 2018, 181, 72–87 CrossRef CAS.
- J. Lee, W.-H. Chen and Y.-K. Park, Bioresour. Technol., 2022, 366, 128204 CrossRef CAS PubMed.
- L. Song, D. Yang, R. Liu, S. Liu, L. Dai and X. Dai, Bioresour. Technol., 2022, 345, 126052 CrossRef CAS PubMed.
- A. N. Marchesan, J. Felipe, L. Silva, R. M. Filho, M. Regina and W. Maciel, ACS Sustainable Chem. Eng., 2021, 9, 12120–12131 CrossRef CAS.
- M. Morales, P. Y. Dapsens, I. Giovinazzo, J. Witte, C. Mondelli, S. Papadokonstantakis, K. Hungerbühler and J. Pérez-Ramírez, Energy Environ. Sci., 2015, 8, 558–567 RSC.
- N. Kubantseva and R. W. Hartel, Food Rev. Int., 2002, 18, 135–149 CrossRef CAS.
- M. T. Sanz, R. Murga, S. Beltrán, J. L. Cabezas and J. Coca, Ind. Eng. Chem. Res., 2004, 43, 2049–2053 CrossRef CAS.
- S. Mailaram and S. K. Maity, J. Renewable Sustainable Energy, 2019, 11, 025906 CrossRef.
- M. S. Peters and K. D. Timmerhaus, Plant Design and Economics for Chemical Engineers, McGraw-Hill, Inc., 5th edn, 2003 Search PubMed.
- S. Mailaram and S. K. Maity, Fuel, 2022, 312, 122932 CrossRef CAS.
- L. Tao and A. Aden, In Vitro Cell. Dev. Biol.: Plant, 2009, 45, 199–217 CrossRef.
- https://www.chemanalyst.com/Pricing-data/sulphuric-acid-70 .
- S. Mailaram, V. Narisetty, V. V. Ranade, V. Kumar and S. K. Maity, Ind. Eng. Chem. Res., 2022, 61, 2195–2205 CrossRef CAS.
- G. W. Diederichs, M. Ali Mandegari, S. Farzad and J. F. Görgens, Bioresour. Technol., 2016, 216, 331–339 CrossRef CAS PubMed.
- https://www.gov.uk/government/statistical-data-sets/gas-and-electricity-prices-in-the-non-domestic-sector .
- E. T. Grasa, Ó. Ögmundarson, H. N. Gavala and S. Sukumara, Biofuels, Bioprod. Biorefin., 2021, 15, 257–281 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se00119a |
| This journal is © The Royal Society of Chemistry 2023 |