Open Access Article
Open Access ArticleFunctional nanostructures in analytical chemistry: new insights into the optical and electrochemical sensing of animal hormones in food, environmental and biological samples
Juhi Bhadresh
Raval
a,
Vaibhavkumar N.
Mehta
b,
Sanjay
Jha
b,
Rakesh Kumar
Singhal
 c,
Hirakendu
Basu
c,
Hirakendu
Basu
 c and
Suresh Kumar
Kailasa
c and
Suresh Kumar
Kailasa
 *a
*a
aDepartment of Chemistry, Sardar Vallabhbhai National Institute of Technology, Surat, 395007, Gujarat, India. E-mail: sureshkumarchem@gmail.com; skk@chem.svnit.ac.in
bASPEE SHAKILAM Biotechnology Institute, Navsari Agricultural University, Surat, 395007, Gujarat, India
cAnalytical Chemistry Division, Bhabha Atomic Research Center, Trombay, Mumbai, 400085, India
First published on 6th June 2023
Abstract
Hormones, which are complex biomolecules, play a vital role in various biochemical pathways and the growth of animals. Hormones, both natural and synthetic, are widely used in agriculture and dairy farms; however, they pose serious health risks. They are responsible for major changes in living organisms related to reproductive health, stress control, pigmentation, glucose metabolism, etc. The quantity of hormones in living bodies is quite low, and thus efficient techniques for their detection are required. However, conventional analytical strategies for the detection of animal hormones exhibit some limitations, such as being time-consuming, expensive, frequently inaccurate, and challenging to use. In this review, we present a detailed classification of animal hormones and a brief description of different sample preparations from food matrices for hormone assays. As summarized in this review, nanostructured material-based optical (colorimetric and fluorescence) and electrochemical sensors have become promising miniaturized analytical tools for the selective and sensitive detection of animal hormones in food samples. Furthermore, we provide an overview on the integration of various nanomaterials (carbon nanostructures, metal nanoclusters, metal nanoparticles, metal–organic frameworks, and quantum dots) with UV-visible, fluorescence, and electrochemical analytical methods for the identification of various animal hormones in food samples with minimized sample volume and preparation.
1. Introduction
In multi-cellular organisms, hormones are signalling molecules that are delivered to distal organs to control a variety of physiological and behavioural processes. Animal hormones are present in the form of steroids and catecholamine.1 However, the detection of hormones is difficult due to their ultra-trace levels in sample extracts, which also have a wide range of interfering substances during their assay. Thus, appropriate analytical techniques need to be developed for assaying these trace compounds in tissues and identifying hormones in real samples. Specifically, steroid and corticoid hormones are excreted in the environment by animals. In this case, the sensitive and selective detection of hormones is necessary given that unusual concentrations of these biomolecules present various health issues, such as reduced sperm count in males, hyper-pigmentation, and feminization of fish in the aquatic environment due to the elevated level of estrogen hormones.2 According to literature, approximately about 1.6–2.5 μg of 17β estradiol (17β-E) is released in the environment via the faecal route. In this case, once hormones are excreted from the living entity, they cannot be degraded in soil or water. Consequently, animals are exposed to these organic molecules, posing serious threats to the normal functioning of their organs.3 The utilization of biosensors for hormone monitoring is becoming increasingly popular given that they are quick, practical, affordable, and have good selectivity and sensitivity. The progress in the determination of animal hormones using nanostructured materials as sensors has been promoted by the improvement in the analytical features of conventional techniques, thereby enabling their application for the analysis of hormones in food, clinical, environmental, and industrial samples.4 The presence of hormones at the parts per million (ppm) level in the environment and edible matrices may affect the health of living organisms. Therefore, it is necessary to develop sensors that are highly sensitive for ultra-trace-level detection.Various traditional analytical techniques have been used for the detection of target hormones such as enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), and antibody-based assay.5 The highly sensitive mass-spectrometry and chromatographic techniques have also been used for the detection of animal hormones. In an interesting work using liquid chromatography-mass spectrometry (LC-MS), steroid hormones were detected with a good low limit of detection (LOD).6 A similar procedure was followed by another group of authors, employing LC-MS for the detection of steroids in environmental samples.7 The magnetic solid-phase extraction (SPE) method was used to prepare samples for the extraction and pre-concentration of hormones. For example, various steroid hormones such as progesterone, testosterone and cortisol were quantified using SPE-LC-MS.8 It was observed that this method is highly sensitive compared to RIA and other available bio-assays; however, it exhibits several limitations such as the use of organic solvents for extraction and highly sophisticated instrumentation, and inability for real-time analysis. Various single-molecule-detection (SMD) techniques (e.g., UV-visible and fluorescence spectrometric techniques) have also been employed for the ultrasensitive detection of the hormones. In a recent review related to the recent progress in food safety, the SMD techniques and recent applications based on the detection of contaminants in edible matrices were discussed.9 SMD exhibits several limitations including the need for a highly pure sample, inability to differentiate between analogous molecules, and the problem of cross-contamination in complex samples.10 ELISA, which is an antibody-based immunoassay, has also been widely employed for the detection of various target molecules including hormones;11–14 however, this tool has several limitations such as the use of high-cost antibodies, cross-reaction of the antibodies, and use of organic solvents.
Thus, to overcome the limitations of the above-mentioned techniques, nanomaterial-based analytical techniques have been the recent choice of researchers for trace-level molecular assays. Nanomaterials have been widely employed for the detection of hormones due to their diverse optical and chemical properties.15 Among them, carbon-based nanomaterials, quantum dots, and metal nanoclusters have been effectively employed in the detection of hormones. Recent studies demonstrated that electrochemical, colorimetric and fluorescence methods are the current state of the art for the quantitative and qualitative detection of hormones.16 The objective of this review is to discuss in detail the developments in the field of animal hormone analysis using nanostructured materials as probes. Also, the advantages and limitations of the available traditional methods are briefly summarized. The analytical features of nanostructured material-integrated analytical techniques (colorimetric, electrochemical and fluorescence) for the assay of animal hormones are highlighted and discussed. In addition, we also briefly illustrate the available sample preparation tools for the pre-concentration of animal hormones from real samples. Finally, the promising applications and future perspectives of nanomaterial-based probes for the assay of hormones in real samples are presented.
2. Brief outline on hormones
Hormones can be scientifically defined as organic molecules synthesized and secreted by specialized cells in living bodies.1 In animals, hormones are secreted from specialized glands called endocrine glands into the bloodstream of mammals or the tissue fluid-like lymph in invertebrates. Hormones are analogous to other chemical mediators such as neurotransmitters and cytokines.17 Also, they can function locally as neurotransmitters and interact with cytokines and neurotransmitters to play a major role in physiological functions. Rapid clinical diagnosis and monitoring approaches combining quick performance and selective and sensitive sample determination are urgently needed for the detection and quantification of hormones given that they play a vital role in important catabolic and anabolic processes in plants and animals. In this case, nanomaterial-mediated biosensors have paved a new way for the detection of biomolecules such as hormones in real time without complicated sample treatment.18 In this section, we provide a brief summary on the classification of animal hormones and their functions in living organisms.2.1 Classification of hormones
Hormones are classified as plant hormones and animal hormones, which is based on their structure, function and source. Animal hormones are a combination of organic molecules with complex structures. The response of animal hormones is faster compared to plant hormones. Animal hormones are secreted in the blood, and then transported to the target organ. In this review, we focus on animal hormones and techniques for their assays.i Steroids. The first class of animal hormones steroids is divided into corticoids and sex hormones.20 The corticoids consist of adrenal corticol hormones and are responsible for the release of aldosterone, which is a mineralocorticoid, and cortisol is a glucocorticoid. Aldosterone aids in controlling the concentration of salt in tissues and blood, and glucocorticoid controls the sugar balance in the body. The sex hormones in the category of steroids consist of male and female sex hormones. The female sex hormones consist of estrogen and progesterone. Estrogen is released by the ovaries and is responsible for the female reproductive characters, while progesterone is released after ovulation. The main role of these hormones is to regulate the menstrual cycle in females and prepare the uterus for pregnancy. Accordingly, changes in the concentration of these hormones can lead to malformations in the reproductive system and infertility. The male sex hormone androgen is a steroid responsible for the release of the hormone testosterone. It is secreted by the testicles in men. This hormone is responsible for masculine characters in males, and also the healthy development of sperm.
ii Peptides or proteins. This class of animal hormones consists of insulin and glucagon. This hormone is responsible for the maintenance of sugar in the blood and urine. These hormones are released from the islet cells present in the pancreatic duct. Insulin is secreted to control the sugar level in the blood and prevent its concentration from becoming low, a condition called hypoglycemia, whereas glucagon is secreted to prevent the blood sugar level from becoming too high (hyperglycemia).
iii Monoamines. This class of animal hormones consists of adrenaline, triiodothyronine (T3) and thyroxine (T4). Epinephrine, commonly known as adrenaline, is a drug and a vital hormone that plays a role in visceral function regulation (e.g., respiration). Biological investigations demonstrated that adrenaline is produced by the adrenal glands and a few neurons in the medulla oblongata. It helps in fight or flight responses by increasing blood flow to the muscles, pupil dilation response, cardiac output via the SA node, and blood sugar level. The T3 and T4 hormones are controlled by thyroid stimulating hormones, which are produced in the pituitary gland.21 T3 helps to promote brain activity, stomach function, and heart functioning. It plays a major role in regulating the metabolic rate and bone health. T4 hormone is also responsible for regulating metabolism in the human body, and in addition it affects the mood and body temperature in humans.
iv Lipid derivatives. They consist of hormones such as luteinizing hormone (LH), thyroid stimulating hormone (TSH) and follicle stimulating hormone (FSH). LH is released by a tiny gland located behind the brain known as the pituitary gland. LH is responsible for regulating sexual function and sexual development. During the menstrual cycle, the release of an egg from the ovary is also regulated by the secretion of LH. In men, LH surge causes Leydig cells to generate more testosterone. In women, LH promotes steroid production from the ovaries, ovulation, and the release of progesterone from the corpus luteum after ovulation. FSH is also released by the pituitary gland, and LH and FSH work simultaneously, playing an important role in sexual development, which also involved in regulating the menstrual cycle and helps in the growth of eggs in the ovaries in females. TSH is also secreted by the pituitary gland directly into the bloodstream. It helps in the release of the thyroid hormones triiodothyronine and thyroxine by binding to receptors on thyroid gland cells.
3. Sample preparation for the detection of animal hormones in various matrices
The sample preparation for the analysis of animal hormones is very complex due to the presence of several fats, lipids and other interfering molecules. The presence of potential contaminants make the pre-concentration of the matrix challenging.3 In solid matrixes, solvent extraction is the main method for the extraction of hormones. The recent literature demonstrated that SPE has been widely employed for the extraction and pre-concentration of animal hormones from various samples matrices (hair, manure, dairy products, urine, faecal matter, and agriculture soil) prior to their identification by various analytical strategies.22 The extraction of target animal hormones is performed using organic solvents. Firstly, the samples are finely chopped, freeze dried, crushed, homogenized, and then used for the further extraction of the cell lysates. For the purification of the lysates, liquid–liquid extraction (LLE) and solid–liquid extraction can also be employed.11 Alternatively, sophisticated methods such as supercritical fluid extraction and accelerated solvent extraction have also been commonly used for the isolation of animal hormones in food samples.22 Similarly, enzyme-mediated hydrolysis has been adopted for the extraction of hormones, but it has poor accuracy due to the cleavage of the targeted hormone prior to its analysis. Another research group reported microwave-assisted extraction, in which the sample is heated using microwave radiation, aiding in the extraction of solid samples without sample distortion.23 The combination of molecular imprinted polymers and SPE is a widely used technique for the extraction of specific hormones from various matrices (urine, beef-liver, blood sera, faecal matter and meat).24 These investigations demonstrated that sample preparation plays an important role in extracting and pre-concentrating trace-level animal hormones prior to their analysis. A recent investigation demonstrated that nanostructured material-integrated optical approaches minimize the use of sample preparation tools, offering animal hormone assays with high selectivity and sensitivity.4. Analysis of animal hormones by conventional analytical techniques
Significant work has been devoted to studying hormones and obtaining reference ranges to distinguish between healthy and unwell people. Analytical methods play an important role in the quantitative and qualitative analysis of animal hormones. The traditional analytical techniques such as high performance liquid chromatography (HPLC), gas chromatography and mass spectrometry have been widely employed for the detection of animal hormones, but only certain hormones have been standardized using these methods. Also, these methods exhibit poor sensitivity, are labour intensive, and require highly skilled laboratory personnel and high-tech instruments, limiting their wider use in the assaying of animal hormones.25Noticeably, nanomaterial-based modern analytical techniques such as electroanalytical, colorimetric and fluorescence methods have shown several features compared to traditional analytical techniques. Generally, electrochemical sensors are used as disposable sensors, which can be used once for a single target molecule, and then discarded.26 The ease of use and real-time analysis by electrochemical sensors have allowed the commercialization of these sensors for the analysis of various animal hormones such as insulin, human chorionic gonadotrophin (HCG) and LH. However, although these biosensors have been widely utilized for hormone sensing, they are associated with several drawbacks in terms of reproducibility, replicability, sample pre-treatment, immobilization of the target analyte and their regeneration. Another method employed for the detection of animal hormones is colorimetry.27 This method is widely accepted due to its naked-eye detection and real-time monitoring. Colorimetric sensors contain chromogenic substrates or color labels, showing excellent sensitivity and selectivity towards the target hormones. Dye-based colorimetric sensors have been widely applied to assay trace-level target hormones because of their low cost and good stability. However, despite these advantages, dye-based sensors exhibit some limitations such as poor reproducibility, toxicity and sensitivity towards the target hormones. Therefore, dye-based colorimetric sensors have been replaced with nanomaterial-based colorimetric devices, which show high selectivity and sensitivity towards the target animal hormones. Due to the feasibility of these sensors, reduced number of sample pre-treatment steps, small sample volume, minimal instrumentation, integration with smart phones, and portability, colorimetric sensors have been widely applied in various fields of bio-medical science and pharmacology. Fluorescence sensors have been used for the analysis of various target molecules due to their biocompatibility and optoelectronics properties.28 Also, they possess various advantages such as being portable, rapid, and user friendly. However, traditional fluorescent sensors exhibit several limitations including multi-step reactions for the synthesis of fluorophores and toxicity, which are toxic to several bioactive compounds and organs. In this case, nanomaterial-based fluorescent sensors offer significant advantages such as selectivity, simplicity, eco-friendly nature and sensitivity. Consequently, nanomaterial-based optical sensors have been successfully employed in a broad range of applications including hormone analysis.
5. Nanomaterial-based analytical techniques for hormone detection
Due to the increasing interest in the determination of hormones in various complex matrices, the requirement for highly sensitive analytical techniques has increased. Traditional techniques such as mass spectrometry, chromatographic techniques, immuno-based techniques, and bio-assays have been widely employed for the detection of animal hormones. Furthermore, different spectroscopic techniques such as nuclear magnetic resonance spectroscopy, infrared spectroscopy, UV-visible spectroscopy and fluorescence spectroscopy have also been reported for the detection of animal hormones. For hormone analysis, immunological assays offer extraordinarily high theoretical sensitivity and specificity for the purification and detection of animal hormones; however, their cross-reactions pose a number of problems. Similarly, traditional methods for hormones assay exhibit several limitations such as huge sample-pre-treatment, high cost, and use of costly precursors. Thus, to overcome these limitations, various nanostructures coupled with colorimetric, fluorescence and electrochemical tools have been employed for the analysis of animal hormones.29 The application of nanostructures in analytical techniques has potential to improve the sensitivity and selectivity of the method, which make them convenient and suitable tools for the real-time analysis of target molecules. Among the various nanomaterials, nanoclusters (NCs), nanorods, nanodiamonds, metal–organic frameworks (MOFs), carbon-based nanomaterials (carbon nanotubes,30,31 carbon dots (CDs), and carbon nanohorns) have attracted wide attention due to their high conductivity, catalytic activity, biocompatibility and unusual optical properties. Two-dimensional nanomaterials such as graphene sheets and MXenes have also gained popularity due to their exceptional physio-chemical properties. For example, graphene electrodes functionalized with nanomaterials have been widely utilized for electroanalytical applications for the detection of biomolecules and stress biomarkers.32,33 Another advantage of using nanomaterials is that they eliminate the use of toxic dyes and organic solvents, making them highly compatible for biological matrices. Fig. 2 depicts the detection of melamine using a colorimetric assay, employing gold nanoparticles (AuNPs) decorated with an aptamer.34 The color change can be visualized through the naked eye, which helps to quantify the target molecules based on the color intensity. Among the various analytical techniques, nanomaterial-integrated electroanalytical, colorimetric and fluorometric techniques have gained increasing popularity due to their feasibility, good lower detection limits, and selectivity.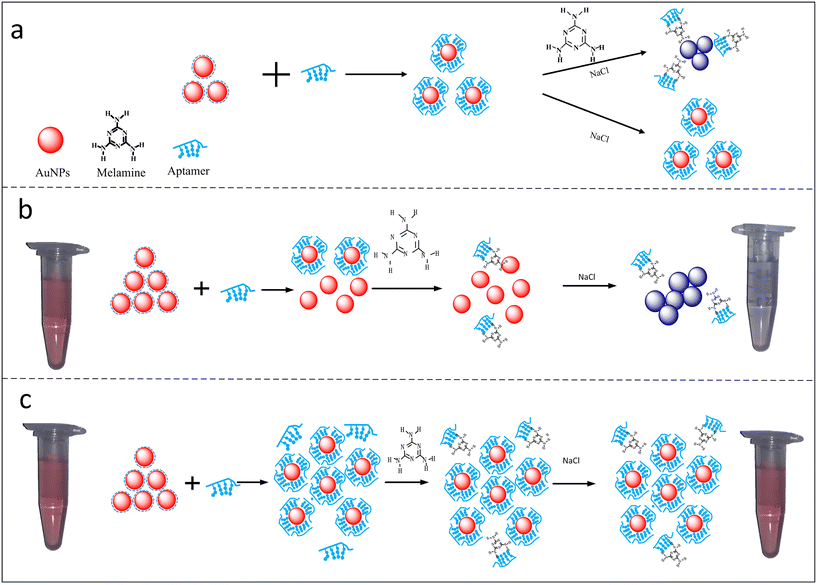 | ||
| Fig. 2 Development of colorimetric assay for melamine using aptamer–Au NPs as a colorimetric sensor. a) It shows coordination bonding between AuNPs and aptamers, and a significant blue shift in presence of NaCl. b) Aggregation of aptamer–AuNPs in presence of melanine molecules showing a visible blue colour. c) It represent competitive binding, large number of aptamers present in the solution will not allow efficient binding of melamine molecules resulting in no visible colour change. Reprinted from ref. 34 with permission from [Plos One], Copyright [2018]. | ||
5.1 Electroanalytical methods for animal hormone detection
Electrochemical biosensors function based on the fundamental idea that the chemical reaction between an immobilized biomolecule and the target analyte produces or consumes ions or electrons, which changes the quantifiable electrical properties (electric current or potential) of the solution. In electroanalytical chemistry, sensors can be further divided into amperometric, potentiometric, conductometric and impedimetric sensors.35 Amperometric sensors are widely employed for assaying various target molecules. They work on the basic principle of the generation of a current due to the electrochemical reduction or oxidation of the target analytes. The constant amplitude voltage is maintained using electrodes made from gold, silver and platinum (working electrodes) and a reference electrode. When the measurement of the current is performed at a constant potential, it is called amperometry, while the change in the potential is referred to as cyclic voltammetry (CV). Potentiometric sensors work based on the difference in potential, which occurs between the reference and working electrode due to redox reactions occurring in the sample. Conductometric biosensors are based on a bio-recognition event, causing a change in conductance due to the presence of the target analyte. Changes in the concentration of ionic species can lead to a change in conductance, which can be measured using an ohmmeter. Impedimetric sensors have also been widely utilized in the field of analytical chemistry. They works on the principle of electrical changes, which occur due to the target analyte at the surface of the functionalized electrodes. These sensors are largely used to quantify the biomolecules and to find the charge transfer resistance.29 Electrochemical-based biosensors are widely employed for the analysis of animal hormones due to their miniaturization, which can be used as lab on chip or point of detection devices, enabling the assay various target molecules including glucose, LH, and HCG. Table 2 summarizes the nanostructure materials used in the development of electrochemical sensors for the analysis of animal hormones in real samples.| Name of hormones | Nanomaterial | Functionalization | Linear range | LOD | Real sample | Ref. |
|---|---|---|---|---|---|---|
| Dopamine | CDs and graphene | Ionic liquid | 0.1–600 μM | 30 nM | Fetal bovine serum | 72 |
| HCG | Nanoporous gold foil and graphene sheets | — | 0.0136–1.0899 nM | 0.00092 nM | — | 41 |
| 17β-Estradiol | CDs | Citric acid and ethylene diamine | 0.1 μM–0.001 nM | 0.0005 nM | River water | 54 |
| Estriol | Carbon black nanoballs | Ag NPs | 0.2–3.0 μM | 0.16 μM | Creek water | 59 |
| 17β-Estradiol | Pt NPs | rGO | 1.5–22 M | 0.48 M | Water and urine | 56 |
| HCG | Chitosan–Au NPs | — | 0.0027–0.6811 nM | 0.00043 nM | Pregnant lady urine | 43 |
| — | ||||||
| HCG | Graphene–IL–Chit | Pt NPs | 34.79–6.9 × 10−5 nM and 6.9 × 10−5−0.0114 nM | 1.149 × 10−8 nM | — | 44 |
| — | ||||||
| HCG | Au NPs | — | 0.0027 nM to 0.027 nM | 0.00272 nM | — | 46 |
| HCG | Au NPs | Primary and secondary antibody | 3.28 × 10−5−0.00328 nM | 1.18 × 10−5 nM | Human serum | 47 |
| 17β-Estradiol | Au NPs | — | 0.0019–49.93 nM | 0.00308 nM | Bovine serum | 52 |
| Progesterone | Au NPs | — | 0.254−22.253 nM | 0.254 nM | Bovine serum | 64 |
| Estrogens | Carbon film | Poly(L-proline) | 0.01–2.0 μM | 5.0 nM | Female blood serums | 57 |
| Estradiol | Antimony NPs | — | 0.20–1.4 μM | 0.5 nM | Natural water | 61 |
| Estradiol | rGO-Au NPs | Potato starch | 1.5–22 μM | 0.48 μM | Water and human urine | 63 |
| Estradiol and progesterone | GQDs | Poly(sulfosalicylic acid) | Estrogen: −0.001–6.0 μM | Estrogen: 0.23 nM | Blood serum | 67 |
| Progesterone: −0.001–6.0 μM | Progesterone: 0.31 nM | |||||
| Estradiol | GO films | Di-hexadecyl phosphate film | 0.4–10 μM | 0.077 μM | Synthetic human urine | 55 |
| Testosterone | Nanoclusters | CuO–CeO2 | 0.1–0.01 nM | 9.30 ± 0.47 pM | Human serum | 71 |
| HCG | Au NPs | Monoclonal antibody | 0.001362–0.272 nM | 0.00215 nM | — | 42 |
| Follicle stimulating hormone | Nanocomposites | r-GO-MWCNTs/thionine/Au NPs | 4.0 × 10−5–0.01 nM | 2.0 × 10−6 nM | Human serum | 69 |
| Progesterone | Nanocomposites | Graphene oxide | 0.01 pM–1000 nM | 0.17 pM | Water | 65 |
| Estriol | Graphene sheets | Sb2O5 | 25 nM–1.03 mM | 11 nM | Urine | 60 |
| Progesterone | Tin nanorods | — | 40–600 μM | 0.12 μM | Pharmaceutical commercial tablets | 66 |
| Estriol | Carbon paste electrode | Ferrimagnetic NPs | 2.98–110.96 μM | 2.7 μM | Tablets and human urine | 62 |
HCG hormone is a glycoprotein produced by syncytiotrophoblastic cells.36 This glycoprotein of approx. 37 kDa normally consists of α- and β-subunits.37 The α-subunit is designated for all the pituitary glycoprotein hormones, whereas the β-subunit is responsible for all the biological activities of the hormone. The occurrence of HCG in serum or urine at the start of conception and its quick upsurge in concentration are good parameters for the identification and confirmation of pregnancy.38 However, raised HCG concentrations are also often associated with non-trophoblastic and trophoblastic neoplasms, which lead to certain deformities before embryological development.39 This is the main reason why the quick detection and low LOD of HCG are necessary, enabling the control some of life-threatening diseases. In comparison with the available immune-based sensors, electrochemical sensors are highly selective, sensitive and accurate. A unique approach based on the antibody-free electrochemical detection of HCG was developed using peptide-functionalized Ag NPs as redox reporters.40 The HCG peptide present on the electrode and in solution together with AgNPs forms an AgNP-peptide based network. This network generates an augmented electrochemical signal via the solid-state Ag/AgCl reaction with the AgNPs. When the electrode binds to HCG molecules, it loses its ability to form the AgNP-peptide-based network, which results in a decrease in the electrochemical signal, hence aiding the detection of HCG molecules (Fig. 3).
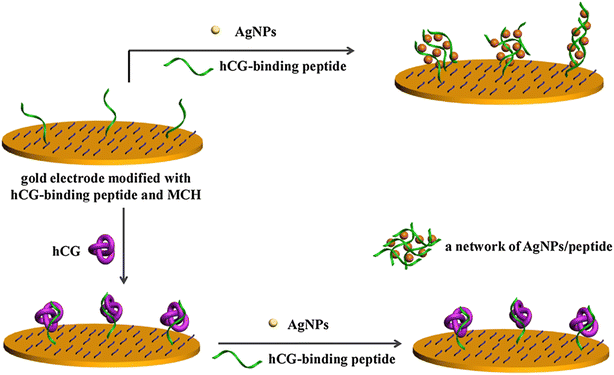 | ||
| Fig. 3 Schematic illustration of the principle of electrochemical-based detection of HCG using functional Ag NPs. Reproduced from ref. 40 with permission from [Elsevier], Copyright [2017]. | ||
An electrochemical-based immunosensor with high precision and accuracy was constructed using hydroquinone (HQ) redox species as the indicator and nanoporous gold (NPG) foils.41 The electrode was constructed by polishing a glassy carbon electrode (GCE) with α-Al2O3, and a graphene sheet solution was added to the electrode to modify its surface. The fabricated composite was dried and functionalized with NPG foil to form the NPG/graphene sheet-modified GCE electrode. The antibody anti-HCG was coated on the NPG/graphene sheet-modified GCE electrode. A diagrammatic representation of the fabrication of the sensor is shown in Fig. 4. NPG has a high active surface area, enabling the feasible immobilization of anti-HCG. The fabricated sensor showed good biocompatibility and high sensitivity towards various antigens and analytes, making it a promising candidate for bio-sensing and clinical applications.
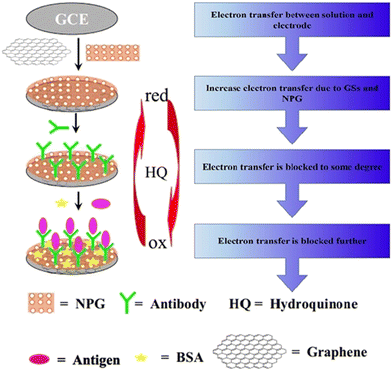 | ||
| Fig. 4 Schematic illustration of the synthesis of an immunosensor for the detection of HCG. Reproduced from ref. 41 with permission from [Elsevier], Copyright [2011]. | ||
A similar approach using AuNP-labelled HCG antibodies was studied for the detection of HCG.42 It was based on a sandwich-type electrochemical immune-based biosensor using an open circuit potential (OCP). The synthesized conjugate showed great potential for developing miniaturized analytical devices for hormone analysis. An antibody-free novel approach using nanomaterials based on electrochemical impedance spectroscopy (EIS) was described for hormone assay using chitosan films and AuNPs as substrates.43 The sensor showed a proportional increase in resistance to electron transfer with an increase in the concentration of HCG (Fig. 5). The biosensor exhibited a linear response to the logarithm-HCG concentration (0.0027 nM to 0.6811) with a lower limit of detection (LOD) of 0.00043 nM.
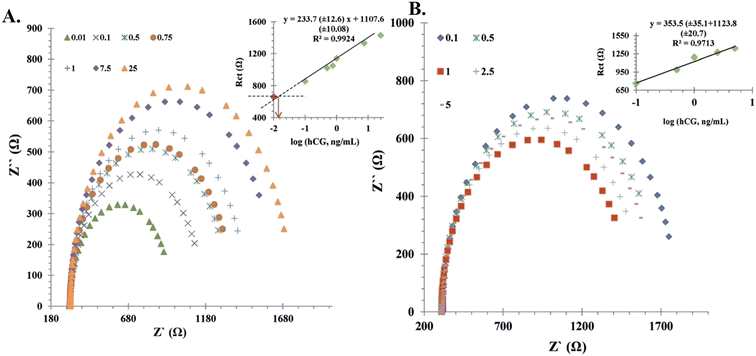 | ||
| Fig. 5 Calibration curve of the immunosensor. (A) Nyquist plots of the fabricated sensor at pH 7.4 and (B) Nyquist plots of immunosensor at pH 6.5 incubated in various concentrations of HCG. Reproduced from ref. 43 with permission from [Wiley], Copyright [2014]. | ||
Further, other nanomaterials have also been employed for the electrochemical sensing of HCG. In one study, PtNPs were covalently immobilised on a strong nanocomposite combining chitosan (Chit), graphene (Gr), and 1-methyl-3-octyl imidazolium tetrafluoroborate as an ionic liquid (IL). It was observed that the Gr–IL–Chit nanocomposite accelerated the kinetics of electron transfer, and also increased the electrode surface area in the biosensor (Fig. 6).44 When HCG recognizes its antibody, the peak current of rutin (RU) is lowered as a result of the formation of an HCG–anti HCG complex, which is depicted as curves in the CV. For the detection of HCG, the immunosensor was shown to be stable, repeatable, and highly specific in the presence of other molecules.
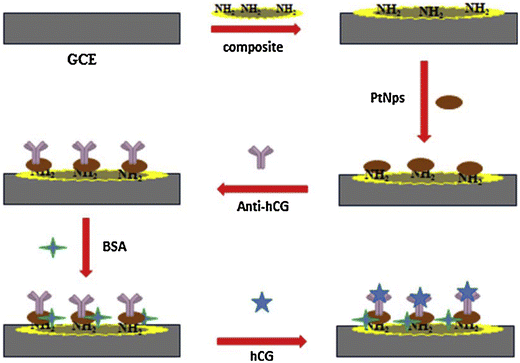 | ||
| Fig. 6 Fabrication of electrochemical-based sensor for the detection of HCG. Reproduced from ref. 44 with permission from [Elsevier], Copyright [2016]. | ||
Voltammetric detection was employed for the detection of HCG using an AgNP-based electrode.45 AgNPs were covalently immobilised on a nanocomposite including Chit, 1-methyl-3-octylimidazolium tetrafluoroborate as an IL and graphene (g-IL) to create the immunosensor. The modified electrode was immobilised with HCG antibody using AgNPs as a linker. The AgNP/g-IL chitosan nanocomposite was used to covalently link the amino groups in the antibody. Another approach was based on disposable EIS based on the AuNP-mediated detection of HCG.46 EIS and differential pulse voltammetry (DPV) were used to analyse the conjugate, enabling the detection of HCG in the range of 0.0027–0.027 nM of HCG with an LOD of 0.00272 nM. This adaptable platform can be used for a variety of purposes such as lab on chip and point of care diagnostics or the sensing of other therapeutically useful hormones. Given that there has been a rapid surge in point of care diagnostics and lab on-chip devices, a novel method was developed using a paper-based microfluidic electrochemical immunosensor (μ-PEI), which formulated based on the principle of screen-printing technology and photolithography.47 DPV was used to measure the electrochemical signal. The fabricated sensor exhibited numerous advantages such as low cost, quick and real-time response, convenient use and disposability. The proposed μ-PEI exhibited an LOD of 1.18 × 10−5 nM. Another important mammalian hormone is 17β-estradiol (17β-E), followed by estriol and estrone, which are synthesized naturally and show maximum estrogen-based activity in mammals.48 The importance of these hormones stems from the fact that their concentration levels have an impact on mammalian health.10 Estrogen is a natural hormone that has a variety of physiological effects. Its actions are related to neuroendocrine mechanisms, developmental difficulties, ovulation control, implantation during reproduction and fertilisation preparation as its major impacts in women.49 The metabolic effects of estrogen on carbohydrates, proteins, and lipids are the most significant. After menstruation, the concentration of 17β-E is normally <50 pg mL−1, which increases with follicular growth (max. 200 pg mL−1), declines to some degree at the time of ovulation, and once more increases in the luteal phase.50 Thus, monitoring the 17β-E levels in females is a good predictor of ovarian activity. This monitoring enables the diagnosis of menstrual disorders and the detection of hypoestrogenism and menopause.51 In this case, electrochemical-based biosensors are the dominant analytical tools for the detection of various steroids hormones.
Recently, a simple analytical approach was established for the identification of 17β-E in bovine serum. In this approach, AuNP disk electrodes were used to incubate bovine serum samples, and then a given concentration of 17β-E was added.52 Then, the sensor was transferred to citrate buffer solution with a pH of 5.00 and various concentrations of pyrocatechol (H2Q), H2O2 and horseradish peroxidase (HRP) were added and their electrochemical signals measured. HRP reacts with the enzyme 17β-E and co-substrate H2Q. In the presence of H2O2, HRP does not affect 17β-E and catalyses the oxidation of H2Q to o-benzoquinone. The modified gold electrode surface encourages the reverse electrochemical reduction of quinone to H2Q, which was identified using square wave voltammetry (SWV). The amount of H2Q interacting with the enzyme is proportional to the electrochemical signal, which is inversely related to the concentration of 17β-E in serum samples. The developed biosensor offered an impressive LOD of 0.00308 nM. The recovery percentages were excellent (99.7%, 106%, and 105%), demonstrating its practical application for the analysis of H2Q in real samples. A double template approach, employing a dendritic gold layered microstructure fabricated on a boron-doped diamond (BDD) electrode surface was employed for the detection of 17β-E.53 The entry of 17β-E in the detection system mediates its reaction with aptamers, causing a rise in the interfacial electron transfer resistance and allowing 17β-E to be quantified. A similar electrochemical biosensor was fabricated for the detection of estradiol using CDs. In this case, the authors functionalized CDs with a 76-mer aptamer probe.54 The comparative impedance changes before and after estradiol addition was the identification parameter for the detection of the hormone. A linear relationship of estradiol concentration in the range of 0.1 μM to 0.001 nM, with and LOD of 0.0005 nM was observed. Furthermore, the proposed sensor had good selectivity for estradiol and no interfering molecules such as bisphenol A (BPA), estriol, and progesterone were detected, making it highly specific and sensitive for the estradiol hormone. Another biosensor working on the principle of an electrochemical device was fabricated, where a graphene oxide (GO) film was produced via chemical exfoliation, and then coated on the electrode surface for electrochemical sensing of the hormone.55 In this method, besides sodium dodecyl sulfate (SDS), another surfactant, dihexadecyl phosphate (DHP), was employed as a dispersant for the development of a graphene oxide film for electroanalysis. Using stripping voltammetry, the changes in electrochemical signals were used for the identification of the hormone. Further, a 17β-E electrochemical assay was performed by constructing a molecular imprinting electrochemical sensor (MIES) with Pt NPs and reduced GO (rGO), thereby favouring a sandwich immunoassay with improved sensitivity.56 An analytical curve was obtained under ideal conditions, which showed that the concentration of 17β-E was in the linear range, exhibiting good selectivity and sensitivity, favouring the assay of 17β-E in biological and environmental samples. The proposed electrode was employed to define the concentration of 17β-E in urine and water samples, with good recovery rates (92.1% to 106%), demonstrating that the biosensor is a feasible option for the detection of 17β-E and can attract interest for various electrochemical-based detection applications. For the detection of estrogen hormone in human samples, many nanomaterial-based electrochemical strategies have been successfully demonstrated for the detection of animal hormones in the literature. Briefly, a poly(L-proline)-ordered mesoporous carbon film was used for the electrochemical sensing of 17β-E.57 The fabricated electrodes showed good electrocatalytic activity, and hence could be employed for detection in human samples. Using the SWV technique, a broad linear range (0.01 to 2.0 μM) was observed for the detection of 17β-E with an LOD of 5.0 nM. Similarly, a nanoporous polymeric film was functionalized with monomers of 1-butyl-3-[3-(N-pyrrole) propyl] imidazolium tetrafluoroborate ionic liquid and decorated on the electrode surface for the accurate detection of 17β-E in human serum.58
Estriol, as an estrogen present in environmental samples, could also be detected using nanostructure-based electrochemical sensors. For example, carbon black nanoballs were decorated on the surface of AgNPs for the electrochemical sensing of estriol in a creek water environmental sample.59 The electrode had a diameter of 20–25 nm for carbon nanoballs and 5–6 nm for AgNPs. The EIS and DPV experiments showed a significant increase in the current signal when estriol was present in the sample. Consequently, this approach showed a quantification limit of 0.5 μM and a good detection limit of 0.16 μM for estriol. Another study demonstrated the use of an rGO-Sb2O5 hybrid nanostructure for the functionalization of laccase enzyme on the electrode surface for the amperometric detection of estriol.60 The combined effect of rGO, Sb and laccase enzyme enabled the electrocatalytic transformation of the estriol hormone, making it highly sensitive and selective for estriol hormone assay within 4 s. Similarly, a similar sensor was developed for estriol assay in natural water using an rGO-Sb NP-modified electrode, which showed good sensitivity and selectivity toward estriol hormone.61 A simple and eco-friendly electrochemical detector was constructed for the detection of estriol employing ferrimagnetic NPs.62 An upsurge in estriol concentration in the range of 2.98 μM to 110.96 μM caused a linear enhancement in the anodic current with an increase in hormone concentration, which exhibited an LOD of 2.7 μM. Over a period of at least 90 days, the modified electrode demonstrated outstanding stability and good sensing ability toward hormone analysis in real samples (pharmaceutical and urine) samples. Another novel approach for estriol determination was demonstrated using rGO-AuNPs and the starch granules present in potato.63 The CV graphs demonstrated that strong anodic and cathodic currents were produced by the conjugation of the electrode with nanostructure-starch. Under the given physiological conditions, the sensor had the ability to detect the target hormone with a wide linear range of 1.5–22 μM, which offered a low LOD of 0.48 μM. The application of the sensor showed a good recovery range from 92.1% to 106% for the hormone in urine and water samples.
Another important steroid animal sex hormone is progesterone, which is a biologically active steroid hormone synthesized by the testes, ovaries, placenta and adrenal cortex. It plays a vital role in maintaining pregnancy, regulating the menstrual cycle, sexual development, oral contraceptives, menopausal hormone therapy, and reproductive-related activities of mammals. It is secreted by both male and female sexes. The accepted range of progesterone in human blood serum is ∼0.48 to ∼79.5 nM, which can increase to ∼731 nM in pregnant women. Inadequate progesterone levels are linked to negative physiological effects such as reproductive system malformations, menstrual disorders, urinary tract infections, anxiety, infertility, body pain and mood swings. Progesterone is also responsible for changes in the prostate, accessory glands, seminal vesicles, secretion, spermatogenesis and breast enlargement in men. Thus, to avoid these threatening conditions, it is necessary to maintain adequate concentrations of progesterone. The sensitive and facile detection of progesterone was performed based on the principle of antibodies using voltammetry.64 A modified gold disk electrode was fabricated with AuNPs and decorated with anti-progesterone monoclonal antibody. The calibration curve revealed a good LOD of 0.254 nM and a broad linear range of 0.254 nM to 22.253 nM. For a sample concentration of 1 ng mL−1, the recovery value was 109.6 and for 10 ng mL−1, the recovery value was 110.1%. This electrochemical assay is quite useful for determining progesterone in biofluids given that it does not need any sample preparation or sample pre-treatment. A similar approach was employed using magnetic nanocomposites of graphene oxide (MGO).65 The MGO electrodes were combined with anti-progesterone antibodies as a bio-recognition element. EIS and CV electrochemical methods were applied to measure the change in current in the presence of progesterone, thereby allowing to assay progesterone in water with remarkable selectivity and excellent sensitivity. Further, progesterone was successfully detected by integrating tin nanorods in an electrochemical device.66 The detection mechanism is based on the use of a surfactant, cetyltrimethylammonium bromide (CTAB), which increased the electrocatalytic efficiency in the presence of progesterone and helped in the electrochemical-based detection of the hormone. The inclusion of CTAB in the presence of progesterone molecules resulted in a shift in the positive cathodic peak potentials. Other surfactants did not give a vital change in the peak currents. This phenomenon can be ascribed to the synergistic adsorption of progesterone and CTAB molecules on the electrode surface, which resulted in a shift in the peak potential and enhancement in the reduction peak current. Commercial pharmaceutical formulations were used for the analysis of progesterone in real samples, which showed good sensitivity and selectivity irrespective of the interference. The selection of the electrode plays a key role in developing sensitive electrochemical sensors, for example, graphene quantum dots (GQDs) were inserted in a poly(sulfosalicylic acid) (GQD-PSSA)-based glass electrode and used as an electrochemical device for the simultaneous detection of 17-β-E and progesterone.67 The as-modified electrode with GQD-PSSA exhibited different active sites, thereby improving the analytical features of the biosensor for 17-β-E and progesterone assays in real samples. The DPV studies using the biosensor were clearly able to identify the different oxidation peaks of estradiol and progesterone hormones. Under the optimal condition, the developed biosensor exhibited two independent linear ranges of 0.001 to 6.0 μM and 0.001 to 6.0 μM for estradiol and progesterone, respectively. The LODs were 0.23 and 0.31 nM for estradiol and progesterone, respectively. The practical application of this biosensor was demonstrated for the analysis of both hormones in pharmaceuticals and human serum samples.
Follicle stimulating hormone (FSH) is a gonadotropin hormone that can help with developmental processes and human reproduction. Importantly, FSH levels that are too high or too low can cause endocrine problems and infertility.68 Thus, the detection of FSH is important for the medical diagnosis of hormone-related diseases. Nanocomposites containing MWCNTs, thionine (Thi), AuNPs, and rGO were decorated on the surface of the electrode to increase the specific surface area for the adsorption of molecules and signal amplification.69 The FSH assay was based on the formation of an immunocomplex, which decreased the electron transfer to Thi, eventually leading to a decrease in the DPV currents, which signifies the presence of FSH in the sample. The rGO/MWCNT/Thi/AuNP-modified electrochemical sensor showed great accuracy and sensitivity for the identification of FSH in a broad range of 4.0 × 10−5 nM to 0.01 nM, with a detection limit of 2.0 × 10−6 nM. The biosensor was effectively used to determine FSH in serum samples with a considerable recovery rate of 94.0–109.8%. Another sex hormone produced by males is testosterone, which is also a steroid hormone of the androgen group. This hormone is formed in the testes of males, the ovaries of females, and the adrenal glands of both sexes. In males, testosterone plays a vital role in their reproductive organs such as the testis and prostate for its maturation and development of secondary sexual characteristics.70 An enzyme-free electrochemical approach was used for the detection of testosterone hormone. For the testosterone assay, CuO–CeO2 NC hybrid nanostructures were deposited on a glassy carbon electrode and applied for the analysis of testosterone.71 At the physiological pH, the designed electrode effectively detected testosterone in the biofluid of a mouse, rabbit and human. Dopamine is an animal hormone that regulates several physiological activities, which also acts as a potent neurotransmitter. A variation in the concentration of dopamine leads to several neurological disorders such as Parkinsonism, epilepsy and schizophrenia. Therefore, it is important to detect and to quantify dopamine in vivo and in vitro. A novel nanocomposite, CD–graphene–IL, was used for the modification of the electrode for dopamine assay.72 The authors observed that the –COOH group of CDs showed high affinity toward the –NH2 group of dopamine, resulting strong electrostatic interaction and their stabilizing in the IL. Consequently, this sensor showed good linearity in the dopamine concentration range of 0.1 to 600 μM, with an LOD of 30 nM. These studies illustrate that the modification of the electrodes with nanostructures greatly improves several analytical features of electrochemical devices for the analysis of animal hormones in real samples with high rapidity, selectivity and sensitivity.
5.2 Colorimetric methods for the detection of animal hormones
Colorimetric approaches have gained tremendous interest in recent years due to their feasibility and real-time analysis.73 Noticeably, the analytical features of UV-visible spectrometry have been greatly improved by integrating it with various functional nanostructures, improving its sensitivity as with sophisticated instruments. Furthermore, the cost-effective and quick detection of biomaterials using colorimetric sensors has opened the doors for its use in environmental sensing, diagnosis, bio-recognition element detection, etc. The colorimetric detection is based on the principle of a change in the surface plasmon resonance (SPR) band of nanostructures due to the introduction of an analyte.74 However, due to the low extinction coefficients of dyes, difficulty in recognizing minor colour changes, their toxicity, and tedious synthetic steps, the use of dyes in colorimetric sensors has been reduced. Thus, to overcome these issues, several research groups have focused their research on the fabrication of nanostructures as colorimetric probes for the detection of various target molecules, offering several advantages such as real-time detection, low toxicity, cost-effectiveness and recognizable differences in the color change, which signify that nanomaterial-based colorimetric probes are prime choices for the detection of trace-level target molecules.75Due to their unique physicochemical and optical features, Au NPs are highly flexible substances for use in sensing components. Glutathione disulfide-linked AuNPs (AuNP-GSSG) were developed to evaluate their colorimetric sensing toward HCG.76 Glutathione increases the flexibility for surface conjugation, causing the typical red-shift only with HCG due to the aggregation of the AuNPs induced by HCG. The authors noticed a good linearity between the absorbance ratio (A518/A715) and concentration of HCG, offering an impressive LOD of 0.00436 μM. The developed probe was successfully demonstrated for HCG assay in biofluids. A similar approach was employed for the determination of HCG using Au NPs as a probe.77 The detection mechanism was based on the prevention of the aggregation of NPs due to the positively charged HCG peptide by adding the HCG molecule. The peptide will engage with the HCG molecules to form a high-affinity hCG/peptide complex, which inhibits the AuNPs from aggregating, allowing the solution to remain red. The as-prepared Au NPs acted as a rapid, facile and user friendly probe for the analysis of HCG.
Another study also utilized Ag NPs as a probe for HCG assay.78 In this work, AgNPs were functionalized with CTAB and the release of Ag+ ions from CTAB-Ag NPs studied, which increased the catalytic activity of CTAB-AGNPs. Colorimetric signals were observed when the substrate (3,3′,5,5′-tetramethylbenzidine [TMB] + H2O2) underwent catalytic oxidation. Fig. 7 presents an overview of the development of a colorimetric approach for the detection of HCG using CTAB-AgNPs as a nanozyme.
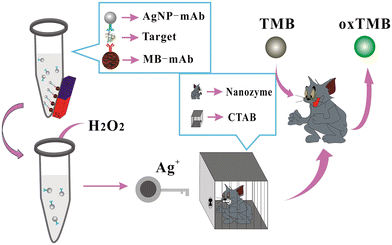 | ||
| Fig. 7 Schematic illustration of the colorimetric assay of HCG using AgNPs as labels, magnetic beads as carriers, H2O2/TMB/AgNPs@CTAB, for signal transduction. Reproduced from ref. 78 with permission from [Elsevier], Copyright [2020]. | ||
The happy hormone dopamine is also an important hormone released in the animal body and is responsible for various actions of the nervous system such as emotions, learning, and control of locomotion. A novel colorimetric technique was employed for the detection of dopamine using an IL functionalized with AgNPs (IL-AgNPs).79 This conjugate was synthesized using the etching method, which in the presence of dopamine changed the shape of the NPs from hexagonal to round. This change in the shape was responsible for the maximum absorption band shift from 585 nm to 500 nm owning to the color change from green to red. The described method was used to detect the dopamine levels in a human serum sample. This approach proved to be a good sensing platform for detecting dopamine in biological fluids with minimal sample preparation. To decrease the use of harmful chemicals, a green approach was employed for the synthesis of Ag NPs using pine (Pinus densiflora) as a reducing agent, and also used for the detection of dopamine.80 In this case, rGO@AgNPs were synthesized via a one-step procedure (10 min) and the as-prepared rGO@AgNPs interacted efficiently with dopamine, causing a significant color change from greenish-yellow to brown, which demonstrated that the aggregation of rGO@AgNPs was induced by dopamine. When Cu2+ is present, the development of dopamine and Cu2+ complexes is suppressed, it initiates dopamine-induced aggregation, and consequently a color change is noticed in the sample. Thus, a simple dual-sensing colorimetric probe for determining dopamine and Cu2+ was developed, which is highly efficient and can be applied in real samples. Another approach for the detection of dopamine was employed using S-doped CDs-AuNPs as a probe.81 This approach is based on the development of a complex between the -NH2 group of dopamine and the carboxyl side of S-CDs@AuNPs. The aggregation is caused in the presence of Fe3+ ions, indicating that the Fe3+ ion acts as a linker to form nanoaggregates, which causes a red shift in the LSPR peak of S-CDs@AuNPs from 520 to 670 nm. Under identical conditions, this method exhibited a wider linear range of dopamine concentration (0.81–16.80 μM) with an LOD of 0.23 μM, allowing dopamine assay in biofluids (urine and serum) of humans. Another novel colorimetric technique was developed for the detection of dopamine using Cu3(OH)2(MoO4)2 cuboidal nanostructures (CMCNs) and their rGO/CMCN nanocomposites.82 At slightly acidic pH, these nanostructures exhibited good enzyme-mimicking activity. The colorimetric detection of dopamine is based on the oxidation of colourless TMB peroxidase substrate to a bluish-colored product in the presence of H2O2. It was noticed that dopamine binds to ˙OH free radicals and prevents TMB from oxidising, allowing it to be detected and giving a visible color change. Progesterone is a steroid hormone that is required for mammary tissue development and pregnancy maintenance. It also aids females in controlling their menstrual cycle and is used in menopausal hormone therapy and oral contraception.83 To explore Au NPs as a promising colorimetric probe for hormone assay, a specific aptamer was functionalized on the surface of Au NPs and used as a visual reader for progesterone detection.84 This assay was based on the aggregation of the aptamer–Au NPs induced by the progesterone hormone, leading to a noticeable color change from red to blue, which could be identified by the naked eyes. Consequently, the ratio at A650/A520 exhibited good linearity with the hormone concentration of 0.89–500 nM and LOD of 2.62 nM. This method has high ability to assay progesterone in biofluids without the aid of high-end analytical techniques. Another female sex hormone, estriol, was also detected using AuNPs.85 The working principle of the sensor was based on antibody-mediated aggregation. The AuNPs were functionalized with polyclonal antibody, showing high affinity toward estriol. Fig. 8a illustrates the antibody-based colorimetric detection approach for 17-β-estriol assay based on AuNPs in a saline environment. The antibody of 17-β-estriol (anti-17-β-estriol) was conjugated with AuNPs. In the presence of 17-β-estriol, the antibody gets detached from the AuNPs and binds to the 17-β-estriol molecule, leading to a visible color change from red to blue under high salt condition. Fig. 8b summarizes the assembly and disassembly of AuNPs in saline condition. It was observed that AuNPs in the presence of antibody gets dispersed in a high NaCl concentration, while in absence of antibody the AuNPs aggregated. This approach showed a good sensitivity of 10 pM and a detection limit of 100 pM for the target hormone. The biosensor was applied for the detection of estriol in serum samples, which proves its efficiency in treating reproductive-related issues. Table 3 presents a summary of the nanomaterials employed as colorimetric readers for the detection of hormones from various biological samples.
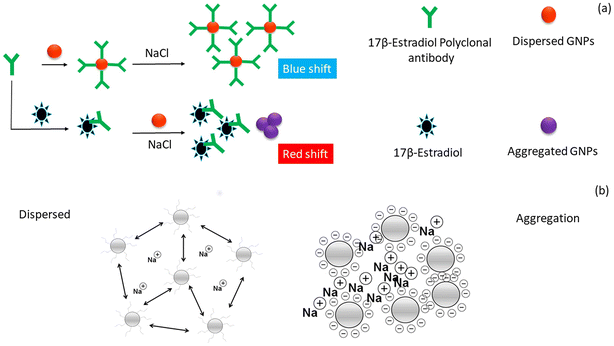 | ||
| Fig. 8 Development of colorimetric approach for the detection of estriol. a) Antibody-based colorimetric detection of 17-β-estriol using AuNPs under appropriate salt condition and b) nature of AuNPs in the presence of a high salt concentration. Reproduced from ref. 85 with permission from [Elsevier], Copyright [2020]. | ||
| Hormone | Nanomaterials | Functionalization | Linear range | LOD | Absorbance (nm) | Real sample | Ref. |
|---|---|---|---|---|---|---|---|
| HCG | Au NPs | Glutathione-disulphide linked | — | 0.00436 μM | (A518/A715) | — | 76 |
| Dopamine | Ag NPs | Pyridium-based TSIL | 0.1–7.5 μM | 0.031 μM | (A500/A585) | Human serum | 79 |
| Dopamine | Ag NPs | Graphene nanocomposites | 0.1–4.5 μM | 16 nM | (A405/A515) | Human urine | 80 |
| HCG | Au NPs | — | 0.00164 to 0.03282 nM | 0.000817 nM | (A600/A525) | Human serum | 77 |
| HCG | Au NPs | CTAB | 0.041 to 0.328 nM | 0.00025 nM | — | Human serum | 78 |
| Dopamine | S-doped CDs | Au NPs | 0.81–16.80 μM | 0.23 μM | (A520/A670) | Human urine and human serum | 81 |
| Progesterone | Au NPs | Hexadecyltrimethylammonium bromide | 0.89–500 nM | 2.62 nM | A650/A520 | Human urine and human serum | 84 |
| Dopamine | GO-nanocomposites | Copper molybdates | 1–10 μM | 0.17 μM | — | Human urine and human serum | 82 |
| 17-β-Estriol | Au NPs | Polyclonal antibody | — | 100 pM | — | Human serum | 85 |
5.3 Fluorescence-based methods for the detection of animal hormones
Biosensors utilizing fluorescence-based approaches have gained popularity for a wide range of applications in several scientific frontiers because of their ultra-sensitivity, decreased detection limits, and optical sensing characteristics. The fundamental working principle of fluorescent sensors depends on the addition of analytes, which results in a decrease in emission intensity, serving as the sensing signal.86 Fluorophores are the best option for a fluorescence-based optical sensing system because they provide a high fluorescence signal, are not photo-bleached, and can be effectively quenched by a designated quencher. This method is used to assess the lowest concentrations in complex matrices. Considering this, several materials, such as fluorescent dyes, fluorescent materials (nanomaterials), and fluorescence biomolecules have been introduced as fluorescence readers for the recognition of trace-level target molecules. Therefore, there is an unlimited number of possibilities to combine them for the detection of bio-recognition elements. For multiple detection, the phenomenon of fluorescence turn ON and turn OFF is also employed, which works by quenching the fluorescence in the presence of one analyte, while restoring it in the presence of another analyte.87 Importantly, various nanostructure materials have been used as promising nanoprobes for the detection of animal hormones due to their selectivity, simplicity and sensitivity. Table 4 demonstrates the applications of functional nanomaterials for the fluorescence analysis of animal hormones in various samples.| Name of hormones | Nanomaterial | Functionalization | Linear range | LOD | Emission and excitation (nm) | Real sample | Ref. |
|---|---|---|---|---|---|---|---|
| Melatonin | Zr-based MOF | — | 4.310–431.03 nM | 0.775 nM | 397 and 270 | Grape and cherry juice | 88 |
| Dopamine | GQDs | — | 0.25–50 μM | 0.09 μM | 453 and 390 | Human serum | 95 |
| Progesterone | CDs | Curcumin | — | 9.3 nM | 560 and 410 | Human serum | 90 |
| HCG | GO | FITC-labelled hCG-specific binding peptide aptamer | 0.0016–0.6565 nM | 0.00065 nM | 518 and 470 | Human urine | 92 |
| — | |||||||
| Progesterone | CDs | GO | 0.1–120 nM | 0.033 nM | 450 and 355 | Milk | 89 |
| Dopamine | Ag NPs | DA-quinone | 0.5 nM–1 μM | 0.1 nM | 444 and 343 | Human serum | 96 |
| Dopamine | NaYF4:Yb3+,Tm3+ UCNPs | Polyethylenimine | 0.25–150 μM | 124 nM | 473 and 980 | Fresh urine and human serum | 99 |
| Dopamine | CDs | Methyl coumarin | 0–33.6 μM | 5.67 nM | 455, 505 and 300 | Human serum | 97 |
| Dopamine | CDs | Au NCs | 5–180 nM | 2.9 nM | 420, 610, and 380 | Human serum | 98 |
| Dopamine | Polydopamine NPs | Ficin | 10 nM-5.0 μM | 5.5 nM | 476 and 400 | Human serum | 100 |
| Progesterone | Carbon nanotubes | Methacrylic polymers and chitosan layer | — | 0.00953 nM | 453 and 367 | Industrial wastewater | 91 |
| Testosterone | N, S co-doped CDs | β-Cyclodextrin | 0–280 μM | 0.51 μM | 425 and 355 | Groundwater | 93 |
| Epinephrine | Au NCs | BSA | — | 910 pM | 650 and 450 | Human serum | 94 |
Melatonin is a hormone that plays key roles in brain functioning and the regulation of sleeping activities. To detect this hormone, a fluorescence-based biosensor was developed using Zr-based MOFs.88 The detection mechanism is based on the “turn-on” fluorescence signal. An intense emission appeared at 397 nm when it was excited at 270 nm. The results demonstrated that trace levels of melatonin could be detected in actual samples such as sour cherry juice and grapes. For trace quantities of melatonin, the linear range and LOD were determined to be 4.310–431.03 nM and 0.775 nM, respectively. Another animal hormone, progesterone, which plays a major part in the maintenance of pregnancy health in females was detected using this method. A fluorescence approach was developed for progesterone assay using GO-CDs as a probe.89 A conjugate of progesterone and CDs with graphene oxide formed an aptamer-based sensor for the detection of hormone. The as-prepared CDs showed the highest excitation at 355 nm with an intense emission at 450 nm. The fluorescence intensity was recovered by the CDs, exhibiting a linear relationship with the progesterone concentration. Furthermore, the developed approach showed good recoveries for progesterone analysis in milk, indicating that this approach has a lot of promise for food safety. Similarly, progesterone was effectively detected by using yellow-green emissive CDs as a fluorescent probe.90 The CDs were synthesized using curcumin via a green synthetic approach. Fig. 9 illustrates the fluorescence detection of progesterone hormone using CDs as a fluorescent nanoprobe. The as-synthesized CDs showed good selectivity and sensitivity for progesterone under ideal conditions, as well as a fast-responding time (within 1 min). The fluorescence sensing device was expanded to monitor progesterone in human serum samples with acceptable recoveries.
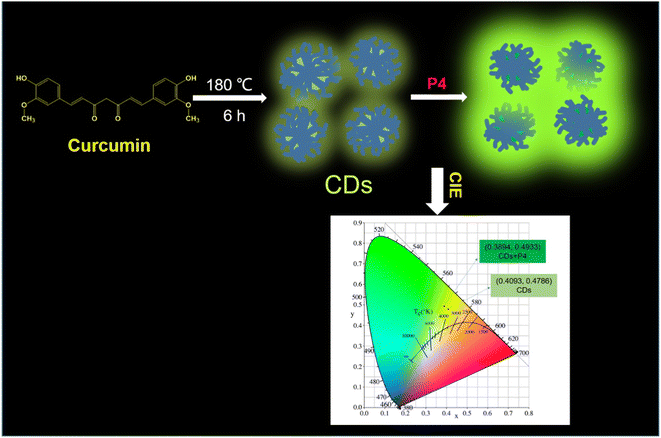 | ||
| Fig. 9 Schematic representation of the fluorescence-based detection of progesterone hormone using CDs as a nanoprobe. Reproduced from ref. 90 with permission from [Elsevier], Copyright [2021]. | ||
Another fluorescence-based strategy employed for the analysis of progesterone used a carbon nanomaterial conjugated with methacrylic.91 A good linearity was observed under ideal conditions, with a correlation coefficient of 0.9998 and LOD of 0.00953 nM. Consequently, this method could be utilised to identify progesterone in industrial effluent, as well as providing a new way for detecting progesterone in other environmental fluids. HCG hormone is also an important animal hormone, which is responsible for the maintenance of the progesterone hormone levels during the pregnancy phase. A sensitive and selective fluorescence study was reported for the analysis of HCG using GO as a fluorescent platform.92 A fluorescein isothiocyanate (FITC)-labelled HCG-specific binding peptide aptamer (denoted as FITC-PPLRINRHILTR) was used as the probe. The peptide was released from the GO surface, which resulted in the specific interaction of the HCG molecule with the aptamer. Consequently, there was an increase in the fluorescence signal (Fig. 10a), exhibiting good linearity in the range of 0.0016 nM to 0.6565 nM, where the emission peak intensity was directly proportional to the HCG concentration (Fig. 10b). Fig. 10c shows the graph of HCG as a function of time due to the accumulation of the peptide, which is detached from the GO surface. Fig. 10d illustrates the sensitivity and selectivity of the method. Compared with the blank and other interfering molecules, a change in fluorescence intensity was only observed in the presence of HCG. The LOD of the sensor was reported to be 0.00065 nM. This strategy is suitable for HCG analysis in human body fluids, which was proven by testing the HCG levels in urine samples (Fig. 10e).
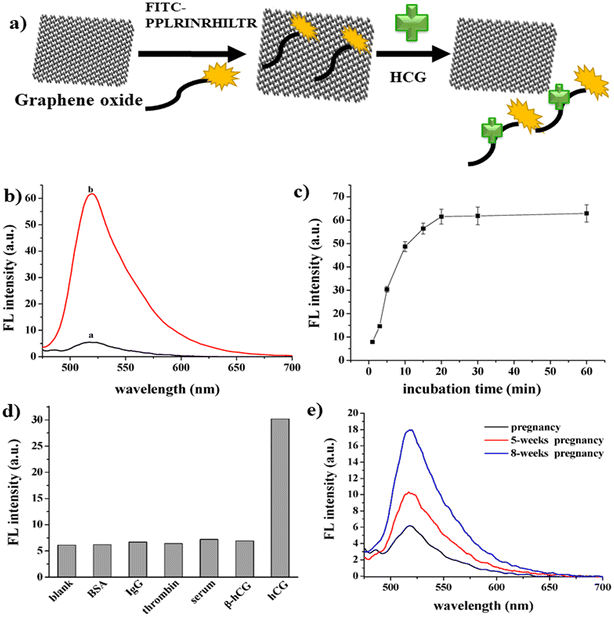 | ||
| Fig. 10 Development of fluorescence strategy for HCG assay. a) Schematic representation of the mechanism for the detection of HCG. b) Fluorescence quenching in the presence (a) and absence (b) of HCG. (c) Fluorescence restoration spectra of the synthesized material by HCG as a function of time, (d) selectivity and sensitivity of the proposed method and (e) fluorescence analysis of real sample. Reproduced from ref. 92 with permission from [MDPI], Copyright [2016]. | ||
Another sex hormone in males known as testosterone was also detected using fluorescent analysis. In this method, nitrogen and sulphur-doped carbon was functionalized with β-cyclodextrin.93 The fluorescence intensity exhibited a linear response with an increase in testosterone concentration (0–280 μM) with an LOD of 0.51 μM. The sensor was tested for the detection of testosterone in ground water. These results confirm the efficiency of the synthesized biosensor. Another novel fluorescence strategy introduced for the detection of epinephrine hormone used BSA–Au NCs.94 The as-fabricated BSA–Au NCs exhibited a strong red emission at 650 nm when excited at 450 nm. The as-synthesized BSA–Au NCs acted as a probe for the fluorescence detection of epinephrine in human serum, showing high specificity toward epinephrine. Also, the well-known neurotransmitter and hormone dopamine was detected using fluorescence methods. A simple fluorescence method was adopted for the detection of dopamine using GQDs.95 The quenching process entailed electron transfer from the photoexcited GQDs to dopamine–quinine, which was generated by oxidising dopamine in basic solution with ambient O2. In the range of 0.25–50 μM, the quenching efficiency was directly proportional to the concentration of dopamine, with an LOD of 0.09 μM. The biosensor was highly reliable, sensitive, cost effective and handheld. Likewise, an AgNP-based approach was used for the detection of dopamine. In this method, the AgNPs and dopamine-quinone quenched the emission intensity of SiNPs effectively via synergistic electron transfer effect.96Fig. 11a illustrates the mechanism for the detection of dopamine, where under the optimal alkaline condition, dopamine is responsible for the reduction of Ag+ to AgNPs and dopamine gets oxidized to dopamine-quinone, which act as an electron acceptor. The fluorescence of SiNPs was quenched due to the synergistic electron transfer between AgNPs and dopamine-quinone. Fig. 11b shows the fluorescence spectra of SiNPs in combination with dopamine, dopamine+ + Ag+ and Ag+, where the maximum quenching was observed in the presence of dopamine + Ag+. The dopamine concentration was determined by the decrease in the fluorescence intensity. Fig. 11c shows the decrease in the fluorescence intensity with an increase in the concentration of dopamine hormone. The sensor showed a typical emission at 444 nm when excited at 343 nm. The proposed biosensor showed an LOD of 0.1 nM and successfully applied to detect dopamine in human serum.
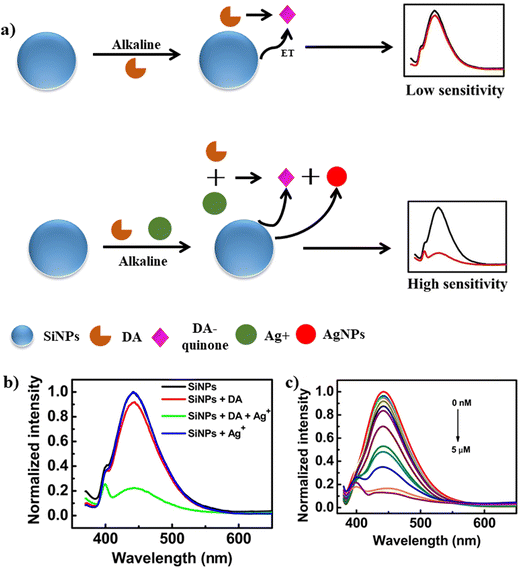 | ||
| Fig. 11 a) Schematic representation for the detection of DA using SiNPs, b) fluorescence spectra of SiNPs and SiNPs in the presence of DA, DA+ and Ag+ and c) change in fluorescence intensity with an increase in the concentration of DA (0 nM to 5 μM). Reproduced from ref. 96 with permission from [Elsevier], Copyright [2018]. | ||
Further, CDs were functionalized with 7-amino-4-methylcoumarin (AMC) and used as an eco-friendly probe for the determination of dopamine.97 CDs and AMC, which were linked by amide bonds, produced dual-emissions peaks at 455 and 505 nm, respectively, when excited at a wavelength of 300 nm. This sensor was also tested against a human serum sample for its specificity. Similarly, the combination of CDs and Au NCs was explored for the detection of dopamine via the Forster resonance energy transfer (FRET) mechanism.98 The as-fabricated probe exhibited two emission peaks at 420 and 610 nm upon excitation at 380 nm. This FRET probe is made up of a two-fluorophore with CD as the energy donor and AuNCs as the acceptor. When dopamine was added to this probe solution, the emission peak at 610 nm was quenched, but the emission peak at 420 nm was recovered, showing good recoveries in the range of 95–105%. Rare-earth-doped upconversion NPs (UCNPs) were also used as a probe for the fluorescence detection of dopamine.99 Different surface additives such as NaYF4:Yb3+,Tm3+ UCNPs were used to prepare the conjugate. The synthesized UCNPs showed a strong emission at 473 nm under continuous excitation at 980 nm. The developed sensor exhibited a linear range of 0.25–150 μM with an LOD of 124 nM for dopamine. This biosensor was applied in real samples such as fresh urine and human serum samples, which proved the efficiency and reliability of the method for dopamine assay. Moreover, a simple fluorescence strategy was developed for the detection of dopamine using polydopamine NPs.100 In this method, ficin was used for mimicking the activity of peroxidase, which induces intrinsic fluorescence. This fluorescence developed due to the oxidation of dopamine into its quinine derivatives. Eventually with an increase in the concentration of dopamine, ficin converts it into its quinine derivatives, which enhances the fluorescence intensity. Notably, the proposed sensing method was used for dopamine assay in human serum samples, showing great sensitivity and selectivity, which can be used as a promising platform for the analysis of dopamine in clinical samples.
6. Conclusions
Functional nanostructures exhibit outstanding analytical features for the analysis of animal hormones. Their unique surface chemistry, optical properties and tunable structures/properties make them good candidates for the analysis of animal hormones in various samples via electrochemical, colorimetric and fluorescence techniques, respectively. In this review, we presented in detail the use of functional nanomaterials (metal NPs, NCs, CDs, GQDs, and hybrid nanostructures) for the development of electrochemical and optical (fluorescence and colorimetry) analytical strategies for the analysis of various animal hormones. Further, a detailed analysis of different animal hormones was presented, considering the diseases related to an imbalance of these hormones. The analytical characteristics of functional nanomaterial-based electrochemical, colorimetric and fluorometric sensors for the detection of various animal hormones were summarized in tables, providing a quick reference about analytical data for the analysis of animal hormones. Importantly, the integration of nanostructure materials with unique surface chemistry and optical and electrochemical properties with electrochemical, fluorescence and colorimetric techniques not only improved the analytical features (higher sensitivity and selectivity) but also reduced the sample volume and minimized the sample preparation. However, despite the remarkable progress in functional nanostructures for the analysis of animal hormones, these developed analytical strategies still face some issues, which need to be addressed for their effective use in the assaying of animal hormones. For instance, to tune the selectivity of nanostructured materials, specific binding agents (aptamer or proteins or antibody) are required for the modification of the nanomaterial surface, which hinder their wider use in the real-time analysis of animal hormones. To realize the use of surface-modified nanostructured materials as probes, efforts need to be devoted to the ligand chemistry of nanostructured materials with good reproducibility, thereby offering their extended use in validating devices for the analysis of animal hormones for real-world applications. Finally, we anticipate that functionalized nanostructured materials will show significant improvements in the development of miniaturized analytical devices for the detection of multiple animal hormones in the near future.Ethics declarations
The authors declare that there are no biological studies.Conflicts of interest
The authors declare no competing interests.Acknowledgements
Authors thank Gujarat State Biotechnology Mission, Government of Gujarat for providing financial support (Ref. No: Project No: GSBTM/JD(R&D)/662/2022-23/00292169, dated 10.03.2023) to this work. SKK thanks the Director, Sardar Vallabhbhai National Institute of Technology, Surat, for providing infrastructural facilities for this work.References
- R. J. Nelson, Encyclopedia of Animal Behavior, 2009, pp. 97–105 Search PubMed.
- A. J. Tilbrook and C. R. Ralph, Anim. Prod. Sci., 2018, 58, 408–415 CrossRef CAS.
- K. L. Edwards, H. M. McArthur, T. Liddicoat and S. L. Walker, Conserv. Physiol., 2014, 2, 1–8 CrossRef PubMed.
- L. E. Christian, D. O. Everson and S. L. Davis, J. Anim. Sci., 1978, 46, 699–706 CrossRef CAS PubMed.
- S. A. Thayer, Vitam. Horm., 1946, 4, 311–361 CrossRef.
- G. D. Luche, S. B. Nash, J. R. Kucklick, F. M. J. Mingramm and A. S. P. Boggs, Conserv. Physiol., 2019, 7, 1–13 Search PubMed.
- Y. Zhao, X. Bai, T. Song, G. Zhang, Y. Yuan and Y. Liu, Microchem. J., 2019, 151, 104212 CrossRef CAS.
- M. Nouri, K. Kroll, M. Webb and N. Denslow, J. Endocrinol., 2020, 296, 113543 CAS.
- Z. Su, T. Li, D. Wu, Y. Wu and G. Li, J. Agric. Food Chem., 2022, 70, 458–469 CrossRef CAS PubMed.
- J. Bai, Z. Gao, W. Wang, Y. Peng, J. Wu, M. Zhang, Q. Li, Z. Zhao, M. Liu, J. Wang and G. Cao, Anal. Chem., 2021, 93, 4488–4496 CrossRef PubMed.
- B. B. Hirpessa, B. H. Ulusoy and C. Hecer, J. Food Qual., 2020, 1–12 CrossRef.
- S. Wang, H. Zhang, W. Li, Z. Birech, L. Ma, D. Li, S. Li, L. Wang, J. Shang and J. Hu, Microchim. Acta, 2020, 187, 1–9 CrossRef PubMed.
- M. Van Tieu, A. Go, Y. J. Park, H. V. Nguyen, S. Y. Hwang and M. H. Lee, IEEE Sens. J., 2019, 19, 9826–9831 Search PubMed.
- G. Jaria, V. Calisto, M. Otero and V. I. Esteves, Anal. Bioanal. Chem., 2020, 412, 3983–4008 CrossRef CAS PubMed.
- O. S. Kwon, H. S. Song, T. H. Park and J. Jang, Chem. Rev., 2019, 119, 36–93 CrossRef CAS PubMed.
- H. Noppe, B. Le Bizec, K. Verheyden and H. F. De Brabander, Anal. Chim. Acta, 2008, 611, 1–16 CrossRef CAS PubMed.
- M. C. Y. Ang, N. Dhar, D. T. Khong, T. T. S. Lew, M. Park, S. Sarangapani, J. Cui, A. Dehadrai, G. P. Singh, M. B. Chan-Park, R. Sarojam and M. Strano, ACS Sens., 2021, 6, 3032–3046 CrossRef CAS PubMed.
- A. El-Ansary and L. M. Faddah, Nanotechnol., Sci. Appl., 2010, 3, 65–76 CrossRef CAS PubMed.
- S. D. McCormick, Integr. Comp. Biol., 2009, 49, 408–422 CrossRef PubMed.
- K. Sláma, Biochem. Physiol. Pflanz., 1980, 175, 177–193 CrossRef.
- J. F. Silva, N. M. Ocarino and R. Serakides, Biol. Reprod., 2018, 99, 907–921 Search PubMed.
- A. Mpupa, S. K. Selahle, B. Mizaikoff and P. N. Nomngongo, Chemosensors, 2021, 9, 151 CrossRef CAS.
- H. Wang, X. Zhou, Y. Zhang, H. Chen, G. Li, Y. Xu, Q. Zhao, W. Song, H. Jin and L. Ding, J. Agric. Food Chem., 2012, 60, 10343–10351 CrossRef CAS PubMed.
- A. Rúbies, A. Cabrera and F. Centrich, J. AOAC Int., 2007, 90, 626–632 CrossRef.
- S. A. Wudy, G. Schuler, A. Sánchez-Guijo and M. F. Hartmann, J. Steroid Biochem. Mol. Biol., 2018, 179, 88–103 CrossRef CAS PubMed.
- A. Nezami, R. Nosrati, B. Golichenari, R. Rezaee, G. I. Chatzidakis, A. M. Tsatsakis and G. Karimi, TrAC, Trends Anal. Chem., 2017, 94, 95–105 CrossRef CAS.
- S. Patel, R. Jamunkar, D. Sinha, T. Kumar, T. Kant, K. Dewangan and K. Shrivas, Trends Environ. Anal. Chem., 2021, 31, e00136 CrossRef CAS.
- H. S. Hwang, J. W. Jeong and Y. A. Kim, Micromachines, 2020, 11, 814 CrossRef PubMed.
- E. B. Bahadir and M. K. Sezginturk, Biosens. Bioelectron., 2015, 68, 62–71 CrossRef CAS PubMed.
- X. Ji, R. Kadara, J. Krussma, Q. Chen and C. Banks, Electroanalysis, 2009, 22, 7–19 CrossRef.
- S. Rathinavel, K. Priyadharshini and D. Panda, Mater. Sci. Eng., B, 2021, 268, 115095 CrossRef CAS.
- M. Allen, V. Tung and R. Kaner, Chem. Rev., 2010, 110, 132–145 CrossRef CAS PubMed.
- D. Brownson, C. Foster and C. Banks, Analyst, 2012, 137, 1815–1823 RSC.
- X. Hu, K. Chang, S. Wang, X. Sun, J. Hu and M. Jiang, PLoS One, 2018, 13, 1–15 Search PubMed.
- L. Sun, Q. Feng, Y. Yan, Z. Pan, X. Li, F. Song, H. Yang, J. Xu, N. Bao and H. Gu, Biosens. Bioelectron., 2014, 60, 154–160 CrossRef CAS PubMed.
- F. J. Morgan and R. E. Canfield, Endocrinology, 1971, 88, 1045–1053 CrossRef CAS PubMed.
- T. Kosasa, L. Levesque, D. P. Goldstein and M. L. Taymor, J. Clin. Endocrinol. Metab., 1973, 36, 622–624 CrossRef CAS PubMed.
- J. Lu, S. Liu, S. Ge, M. Yan, J. Yu and X. Hu, Biosens. Bioelectron., 2012, 33, 29–35 CrossRef CAS PubMed.
- J. Y. Liao, Appl. Microbiol. Biotechnol., 2007, 74, 1385–1391 CrossRef CAS PubMed.
- N. Xia, Z. Chen, Y. Liu, H. Ren and L. Liu, Sens. Actuators, B, 2017, 243, 784–791 CrossRef CAS.
- R. Li, D. Wu, H. Li, C. Xu, H. Wang, Y. Zhao, Y. Cai, Q. Wei and B. Du, Anal. Biochem., 2011, 414, 196–201 CrossRef CAS PubMed.
- K. Charoenkitamorn, P. Trong Tue, M. Chikae, O. Chailapakul and Y. Takamura, Electroanalysis, 2018, 30, 1766–1772 CrossRef CAS.
- S. Teixeira, N. S. Ferreira, R. S. Conlan, O. J. Guy and M. G. F. Sales, Electroanalysis, 2014, 26, 2591–2598 CrossRef CAS.
- M. Roushani and A. Valipour, Sens. Actuators, B, 2016, 222, 1103–1111 CrossRef CAS.
- M. Roushani and A. Valipour, Microchim. Acta, 2016, 183, 845–853 CrossRef CAS.
- N. Li, Q. Li, X. R. Chen, A. J. Veloso, V. W. S. Hung, D. Dhar and K. Kerman, ECS Trans., 2013, 50, 15–21 CrossRef.
- L. Cao, C. Fang, R. Zeng, X. Zhao, Y. Jiang and Z. Chen, Biosens. Bioelectron., 2017, 92, 87–94 CrossRef CAS PubMed.
- X. Lu, J. Sun and X. Sun, TrAC, Trends Anal. Chem., 2020, 127, 115882 CrossRef CAS.
- Z. Yan, Y. Liu, H. Sun and G. Lu, Environ. Sci. Pollut. Res., 2018, 25, 7566–7574 CrossRef CAS PubMed.
- N. H. Torres, G. D. O. S. Santos, L. F. R. Ferreira, J. H. P. Américo-Pinheiro, K. I. B. Eguiluz and G. R. Salazar-Banda, Chemosphere, 2021, 267, 128888 CrossRef CAS PubMed.
- E. Hampson, Horm. Behav., 2020, 119, 104655 CrossRef CAS PubMed.
- M. J. Monerris, F. J. Arévalo, H. Fernández, M. A. Zon and P. G. Molina, Sens. Actuators, B, 2015, 208, 525–531 CrossRef CAS.
- H. Ke, M. Liu, L. Zhuang, Z. Li, L. Fan and G. Zhao, Electrochim. Acta, 2014, 137, 146–153 CrossRef CAS.
- M. H. M. Zaid, J. Abdullah, N. Rozi, A. A. M. Rozlan and S. A. Hanifah, Nanomaterials, 2020, 10, 1–14 Search PubMed.
- B. C. Janegitz, F. A. Dos Santos, R. C. Faria and V. Zucolotto, Mater. Sci. Eng., C, 2014, 37, 14–19 CrossRef CAS PubMed.
- T. Wen, C. Xue, Y. Li, Y. Wang, R. Wang, J. Hong, X. Zhou and H. Jiang, J. Electroanal. Chem., 2012, 682, 121–127 CrossRef CAS.
- L. Luo, F. Li, L. Zhu, Y. Ding and D. Deng, Sens. Actuators, B, 2013, 187, 78–83 CrossRef CAS.
- Z. Wang, P. Wang, X. Tu, Y. Wu, G. Zhan and C. Li, Sens. Actuators, B, 2014, 193, 190–197 CrossRef CAS.
- P. A. Raymundo-Pereira, A. M. Campos, F. C. Vicentini, B. C. Janegitz, C. D. Mendonça, L. N. Furini, N. V. Boas, M. L. Calegaro, C. J. L. Constantino, S. A. S. Machado and O. N. Oliveira, Talanta, 2017, 174, 652–659 CrossRef CAS PubMed.
- F. H. Cincotto, T. C. Canevari, S. A. S. Machado, A. Sánchez, M. A. R. Barrio, R. Villalonga and J. M. Pingarron, Electrochim. Acta, 2015, 174, 332–339 CrossRef CAS.
- I. Cesarino, F. H. Cincotto and S. A. S. Machado, Sens. Actuators, B, 2015, 210, 453–459 CrossRef CAS.
- J. P. da Silveira, J. V. Piovesan and A. Spinelli, Microchem. J., 2017, 133, 22–30 CrossRef.
- L. V. Jodar, F. A. Santos, V. Zucolotto and B. C. Janegitz, J. Solid State Electrochem., 2018, 22, 1431–1438 CrossRef CAS.
- M. J. Monerris, F. J. Arévalo, H. Fernández, M. A. Zon and P. G. Molina, Sens. Actuators, B, 2012, 166–167, 586–592 CrossRef CAS.
- P. Kumari, M. K. Nayak and P. Kumar, J. Mater. Chem. B, 2021, 9, 5264–5271 RSC.
- A. Das and M. V. Sangaranarayanan, Sens. Actuators, B, 2018, 256, 775–789 CrossRef CAS.
- M. Arvand and S. Hemmati, Talanta, 2017, 174, 243–255 CrossRef CAS PubMed.
- A. Al Matari, A. Combès, J. Camperi, T. Fournier, V. Pichon and N. Delaunay, Anal. Bioanal. Chem., 2020, 412, 5729–5741 CrossRef CAS PubMed.
- Y. Fan, Y. Guo, S. Shi and J. Ma, Anal. Methods, 2021, 13, 3821–3828 RSC.
- A. Levent, A. Altun, Y. Yardim and Z. Senturk, Electrochim. Acta, 2014, 128, 54–60 CrossRef CAS.
- M. M. Alam, M. M. Rahman, A. M. Asiri and M. A. Fazal, J. Mater. Sci.: Mater. Electron., 2021, 32, 5259–5273 CrossRef CAS.
- X. Zhuang, H. Wang, T. He and L. Chen, Microchim. Acta, 2016, 183, 3177–3182 CrossRef CAS.
- S. K. Biswas, S. Chatterjee, S. Bandyopadhyay, S. Kar, N. K. Som, S. Saha and S. Chakraborty, ACS Sens., 2021, 6, 1077–1085 CrossRef CAS PubMed.
- L. Yu and N. Li, Chemosensors, 2019, 7, 53 CrossRef CAS.
- J. F. Chen, Q. Lin, Y. M. Zhang, H. Yao and T. B. Wei, Chem. Commun., 2017, 53, 13296–13311 RSC.
- M. Anas, Y. Mohd, M. Hafiz, A. Hassan and S. Azhari, Malays. J. Sci., 2020, 5, 1–5 Search PubMed.
- C. Chang, C. Chen, C. Y. Chen and C. W. Lin, Anal. Methods, 2015, 7, 29–33 RSC.
- Y. Guo, Y. Zhou, S. Xiong, L. Zeng, X. Huang and Y. Leng, Sens. Actuators, B, 2020, 305, 127439 CrossRef CAS.
- S. Rostami, A. Mehdinia, A. Jabbari, E. Kowsari, R. Niroumand and T. J. Booth, Sens. Actuators, B, 2018, 271, 64–72 CrossRef CAS.
- S. Basiri, A. Mehdinia and A. Jabbari, Sens. Actuators, B, 2018, 262, 499–507 CrossRef CAS.
- M. Amiri, S. Dadfarnia, A. M. Shabani and S. Sadjadi, J. Pharm. Biomed. Anal., 2019, 172, 223–229 CrossRef CAS PubMed.
- S. Gajendar, K. Amisha and S. Manu, CrystEngComm, 2021, 23, 599–616 RSC.
- P. Kumari, M. K. Nayak and P. Kumar, Mater. Today: Proc., 2019, 48, 583–586 Search PubMed.
- G. Du, L. Wang, D. Zhang, X. Ni, X. Zhou, H. Xu, L. Xu, S. Wu, T. Zhang and W. Wang, Anal. Biochem., 2016, 514, 2–7 CrossRef CAS PubMed.
- X. Zhang, Z. Shen, W. Su, H. Wu, S. C. B. Gopinath and R. Chen, Process Biochem., 2020, 99, 21–26 CrossRef CAS.
- M. Liu, NANO, 2020, 1, 1–12 Search PubMed.
- C. Li, Z. Qin, M. Wang, W. Liu, H. Jiang and X. Wang, Anal. Chim. Acta, 2020, 1104, 125–131 CrossRef CAS PubMed.
- E. A. Afshar, M. A. Taher, F. Karimi, C. Karaman and O. Moradi, Chemosphere, 2022, 295, 133869 CrossRef CAS PubMed.
- H. Cui, H. Lu, J. Yang, Y. Fu, Y. Huang, L. Li and Y. Ding, J. Fluoresc., 2021, 32(3), 927–936 CrossRef PubMed.
- M. Zan, S. An, L. Cao, Y. Liu, L. Li, M. Ge, P. Liu, Z. Wu, W. F. Dong and Q. Mei, Appl. Surf. Sci., 2021, 566, 150686 CrossRef CAS.
- X. Cui, H. Shu, L. Wang, G. Chen, J. Han, Q. Hu, K. Bashir, Z. Luo, C. Chang, J. Zhang and Q. Fu, Environ. Sci. Pollut. Res., 2021, 28, 62306–62320 CrossRef CAS PubMed.
- N. Xia, X. Wang and L. Liu, Sensors, 2016, 16, 1–10 Search PubMed.
- M. Luo, Y. Hua, Y. Liang, J. Han, D. Liu, W. Zhao and P. Wang, Biosens. Bioelectron., 2017, 98, 195–201 CrossRef CAS PubMed.
- S. Govindaraju, A. S. Reddy, J. Kim and K. Yun, Appl. Surf. Sci., 2019, 498, 143837 CrossRef CAS.
- J. Zhao, L. Zhao, C. Lan and S. Zhao, Sens. Actuators, B, 2016, 223, 246–251 CrossRef CAS.
- Q. Lu, X. Chen, D. Liu, C. Wu, M. Liu, H. Li, Y. Zhang and S. Yao, Talanta, 2018, 182, 428–432 CrossRef CAS PubMed.
- J. An, M. Chen, N. Hu, Y. Hu, R. Chen, Y. Lyu, W. Guo, L. Li and Y. Liu, Spectrochim. Acta, Part A, 2020, 243, 118804 CrossRef CAS PubMed.
- Y. S. He, C. G. Pan, H. X. Cao, M. Z. Yue, L. Wang and G. X. Liang, Sens. Actuators, B, 2018, 265, 371–377 CrossRef CAS.
- B. Zhao and Y. Li, Talanta, 2018, 179, 478–484 CrossRef CAS PubMed.
- Y. Pang, Y. Shi, Y. Pan, Y. Yang, Y. Long and H. Zheng, Sens. Actuators, B, 2018, 263, 177–182 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |







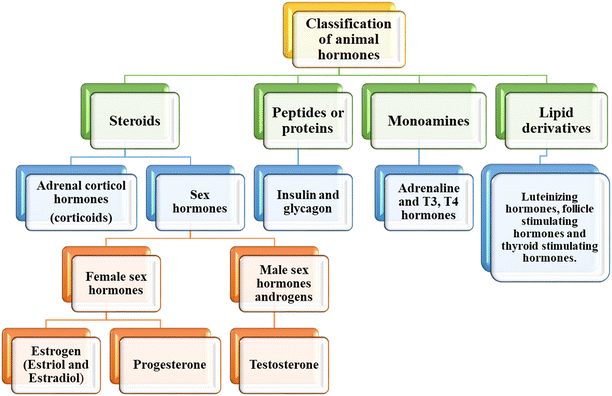
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da)
000 Da)