Open Access Article
Open Access ArticlePreparation of Z-scheme 3D ZnO/Au/g-C3N4 heterostructures for the photoelectrochemical sensing of glutathione†
Weixin
Li
a,
Xinyang
Wang
a,
Jiaji
Huang
b,
Min
Zhao
*cd,
Jiao
Yang
a,
Fang
Luo
a,
Bin
Qiu
 a,
Jian
Wang
a,
Jian
Wang
 *a and
Zhenyu
Lin
*a and
Zhenyu
Lin
 *a
*a
aMinistry of Education Key Laboratory for Analytical Science of Food Safety and Biology, Fujian Provincial Key Laboratory of Analysis and Detection for Food Safety, College of Chemistry, Department of Chemistry, Fuzhou University, Fuzhou, Fujian 350116, China. E-mail: jwang@fzu.edu.cn; zylin@fzu.edu.cn
bClinical Medicine Major, School of Medicine, Xiamen University, Xiamen, Fujian 361005, China
cDepartment of Gynecology and Obstetrics, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, Fujian 361005, China. E-mail: xmzmdyyy@xmu.edu.cn
dComputer Management Center, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, Fujian 361005, China
First published on 28th March 2023
Abstract
Three-dimensional (3D) ZnO nanostructures were used as the support for gold nanoparticles (AuNPs) and graphite carbon nitride (g-C3N4) to form a Z-scheme heterostructure photoelectrode (3D ZnO/Au/g-C3N4) in this work. The 3D structure of the ZnO nanorods provided a directional channel for fast carrier transport and abundant reaction sites. ZnO and g-C3N4 formed the Z-scheme heterojunction, and the AuNPs sandwiched between ZnO and g-C3N4 acted as the mediators and greatly reinforced vector electron transfer in the Z-scheme heterojunction. Then, the 3D ZnO/Au/g-C3N4 photoelectrode was applied to develop a sensitive photoelectrochemical (PEC) biosensor for reduced glutathione (GSH) detection. GSH can act as a hole scavenger and reduce the recombination of photogenerated electron–hole pairs, which results in an increase in the photocurrent of the system. The enhanced current showed good linearity with GSH concentration in the range of 7–2000 μM, and the system had a limit of detection of 2.3 μM. The proposed biosensor exhibited good selectivity and reproducibility, obtaining satisfactory results in the analysis of the target in human serum.
Introduction
The photoelectrochemical (PEC) detection technique has the characteristics of high sensitivity, low background and simple equipment.1 Photoactive substances that act as transducers to convert light into electrical signal form an essential component in the development of PEC biosensors.1,2 Zinc oxide (ZnO) is a kind of n-type semiconductor with a wide bandgap, and the advantages of high electron mobility and morphological plasticity, as well as low cost, low toxicity and ease of preparation.3 Low-dimensional ZnO nanostructures can provide fast charge transport through micron-scale axial lengths, enabling effective charge separation across nanoscale diameters.4,5 Compared with simple low-dimensional structures, three-dimensional (3D) ZnO nanostructures not only inherit excellent properties but also exhibit a large surface-area-to-volume ratio and better permeability, which facilitate enhanced light capture, more reactive sites, and faster electron and ion transport capability, due to which they have been applied in the development of PEC biosensors frequently.6 For instance, Ren's group prepared different structures of ZnO, such as nanotrees, nanorod clusters and parent nanorods, by electrodeposition and hydrothermal growth.7 In terms of PEC water-splitting performance, they found that the strongest material was the nanotree, while the weakest was the parent nanorod. The key reason for improved performance is the very large surface-to-volume ratios in the branched 3D nanostructures.Single photoactive materials always exhibit low photoelectric response signals due to the rapid recombination of photogenerated electron–hole pairs, which is easily disturbed by the environment or the substrate, subsequently affecting the accuracy of the detection results. The construction of heterojunctions can address this concern easily.8,9 Graphite carbon nitride (g-C3N4) is a kind of photocatalyst with a unique structure, small interlayer distance and a bandgap of 2.7 eV, which can be driven by visible light.10–12 Due to its well-matched band edge position with ZnO, g-C3N4 has been applied to prepare ZnO/g-C3N4 heterojunction systems previously.13 For example, Bian's group prepared a ZnO/g-C3N4 heterojunction photocatalyst, and it was applied for H2O2 production under sunlight irradiation.14 Its H2O2 productivity was approximately 2.7 times greater than that on bare g-C3N4, which benefited from the construction of the heterojunction.
Z-Scheme heterojunctions consist mostly of two semiconductors and an electron dielectric made of a noble metal. Under sunlight irradiation, light-induced electrons on the conduction band (CB) of one semiconductor migrate to the electron dielectric and subsequently recombine with the light-induced holes on the valence band (VB) of the other semiconductor, which has a relatively strong redox and efficient charge transfer abilities.8,15,16 Gold nanoparticles (AuNPs) with localized surface plasmon resonance (LSPR) can form Schottky junctions with semiconductor materials. The presence of AuNPs not only broadens the light-absorption range to the visible region but also suppresses the complexation of electron–hole pairs, thereby enhancing the intensity of the photoelectronic signal.17 Earlier studies have shown that AuNPs sandwiched in ternary heterojunctions can act as mediators to increase the vector mobility of the photogenerated electron–hole pairs.18 For instance, Li's group facilitated charge transfer from ZnO-based photoanodes and improved the PEC cell conversion efficiency by immobilizing Au-core CdS-shell nanostructures (Au@CdS) on ZnO nanowires, wherein the Au core formed unique nano-junctions with CdS and ZnO.19 Zou's group20 constructed a ternary BiVO4–Au–Cu2O heterostructure by anchoring the Au–Cu2O composite structure on the face of the truncated BiVO4 octahedron. The synthesized BiVO4–Au–Cu2O could activate inert BiVO4 and Cu2O and exhibited increased CO2 reduction activity, which was 5 times greater than that of BiVO4–Cu2O. However, to the best of our knowledge, AuNPs have never been sandwiched in 3D ZnO and g-C3N4 to enhance the performance of the 3D ZnO/Au/g-C3N4 Z-scheme heterostructure photoelectrode.
Reduced glutathione (GSH) is one of the antioxidants that are widely present in the body. Abnormal GSH levels are closely associated with a variety of diseases, such as Alzheimer's disease and cardiovascular diseases.21,22 GSH can act as a hole scavenger, producing electrons that effectively prevent the rapid compounding of photogenerated electron–hole pairs.23 In this study, 3D ZnO nanostructures have been used as the body to support AuNPs and g-C3N4 to form a Z-scheme heterostructure photoelectrode (3D ZnO/Au/g-C3N4). This structure combines the advantage of each component and allows efficient electron–hole separation, resulting in a strong PEC photocurrent, as detected. The presence of GSH causes a change in the photocurrent intensity of the system, and the relationship between GSH and the photocurrent is established in this work. Based on this, a sensitive PEC biosensor for GSH can be developed.
Experimental sections
Reagents and instruments
Complete information on the reagents and instrumentations used in this experiment is shown in the ESI.†Preparation of the photoelectrodes
The procedures employed for the preparation of materials to fabricate 3D ZnO/Au/g-C3N4 have been shown in ESI.† The indium tin oxide (ITO) electrodes were cleaned with acetone, ethanol, and deionized water, respectively, by ultrasonication and blown dry with N2. 20 μL of a 2.0 mg mL−1 3D ZnO/Au/g-C3N4 solution was applied dropwise to the ITO electrode by drop-casting and dried in the air. Then, it was used as the photoelectrode for PEC testing.The procedure of GSH detection
A pre-configured GSH solution was added to the electrolyte solution and mixed thoroughly. The photocurrent signal was detected using a xenon lamp as the excitation light source with a switching frequency of 10 s and an applied voltage of 0.6 V. The PEC test results were quantified by calculating the average of three independent parallel tests.Results and discussion
Characterization of the synthesized materials
Fig. 1a shows the TEM of 3D ZnO, which has a sea urchin-like microstructure consisting of a large number of one-dimensional (1D) nanorods. These nanorods were reoriented toward the same point at the center, with dimensions of about 5 μm and diameters in the range of 300–500 nm. As shown in Fig. 1b, the SEM images further demonstrated that the sea urchin-like structure of 3D ZnO is composed of many 1D nanorods with a remarkably smooth surface. The TEM image in Fig. 1c illustrates the uniform reduction of AuNPs on the 3D ZnO surface. Fig. 1d shows the excellent dispersion of AuNPs with a size of 30–60 nm on ZnO. Fig. 1f shows the successful attachment of the g-C3N4 nanospheres. The 3D ZnO/Au surface is quite rough due to the attachment of the g-C3N4 nanospheres, but they do not change the 3D ZnO morphology significantly (Fig. 1e). The high-resolution transmission electron microscopy (HRTEM) image of 3D ZnO (Fig. 1g) clearly showed an interplanar distance of 0.26 nm, which belongs to the (002) plane of ZnO.24,25Fig. 1h shows the HRTEM image of 3D ZnO/Au/g-C3N4 captured near the edges of the nanorods; the AuNP (111) crystalline surfaces were tightly attached to the surface of the ZnO nanorods.26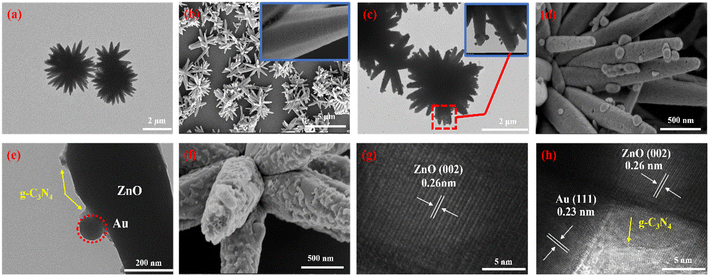 | ||
| Fig. 1 The TEM and SEM images of (a and b) 3D ZnO, (c and d) 3D ZnO/Au and (e and f) 3D ZnO/Au/g-C3N4, respectively. The HRTEM images of (g) 3D ZnO and (h) 3D ZnO/Au/g-C3N4. | ||
Energy-dispersive X-ray spectroscopy (EDX) (Fig. S2†) revealed the content of each element in the composite. The characteristic peak intensity was the strongest for Zn, second highest for O, and weaker for Au, C, and N. The reason is that ZnO is the main component in the composite. As shown in Fig. S3,† the crystalline phases and crystallinity of 3D ZnO, 3D ZnO/Au, g-C3N4, 3D ZnO/g-C3N4 and 3D ZnO/Au/g-C3N4 were determined by X-ray diffraction (XRD). In comparison with the other materials (Fig. S3a†), 3D ZnO/Au/g-C3N4 (purple curve) not only presented the characteristic diffraction peak of ZnO but also exhibited the characteristic peak of Au. After the vapour phase deposition of g-C3N4, the characteristic peak belonging to g-C3N4 (27.5°) also appeared in the pattern (Fig. S3b†). This further supports the highly crystalline nature of the prepared material. The states of the elements in the composite were studied by X-ray photoelectron spectroscopy (XPS) measurements. As shown in Fig. S4a,† the two main peaks in the C1s spectrum belonged to C–C (285.1 eV) and the sp2 hybridized carbon in N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (289.2 eV). Similarly, Fig. S4b† shows three characteristic peaks in the N1s spectrum corresponding to C–N
N (289.2 eV). Similarly, Fig. S4b† shows three characteristic peaks in the N1s spectrum corresponding to C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (398.5 eV), C–N–H (402.6 eV) and C
C (398.5 eV), C–N–H (402.6 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (405.3 eV), respectively. The UV-vis absorption spectra confirmed the improved light absorption of 3D ZnO/g-C3N4 in both the UV and visible regions at 455 nm compared with pristine ZnO due to the g-C3N4 modification (Fig. S5†).
N (405.3 eV), respectively. The UV-vis absorption spectra confirmed the improved light absorption of 3D ZnO/g-C3N4 in both the UV and visible regions at 455 nm compared with pristine ZnO due to the g-C3N4 modification (Fig. S5†).
Performance of the PEC electrode
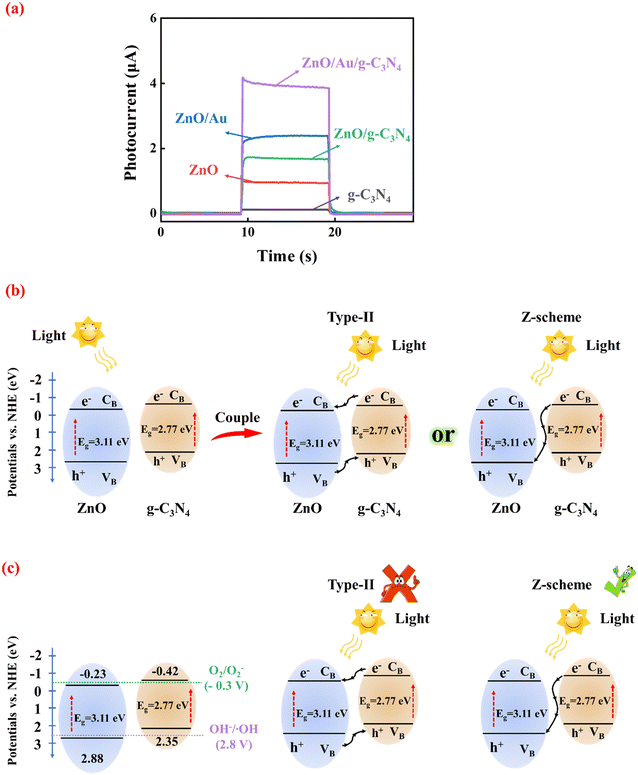
As shown in Fig. 2a, both 3D ZnO and g-C3N4 exhibited low photocurrents due to the recombination of photoinduced carriers. However, the combination of 3D ZnO and g-C3N4 led to significantly enhanced photocurrent due to the construction of heterojunctions. When AuNPs were introduced to form 3D ZnO/Au/g-C3N4, the photoelectrode showed the highest photocurrent response, approximately four times that of pristine 3D ZnO and twice that of 3D ZnO/g-C3N4.To investigate the possible mechanism underlying the changing photocurrent of the system, the corresponding reflectance of ZnO and g-C3N4 was obtained through UV-vis DRS measurements (as detailed in Fig. S6†). The calculated bandgap was 3.11 eV for ZnO (Fig. S6a†) and 2.77 eV (versus vacuum energy (Evac)) for g-C3N4 (Fig. S6b†). Obviously, ZnO has a wider band gap. Thus, the formation of heterojunction between ZnO and g-C3N4 is possible. As displayed in Fig. S7 in ESI,† the values of the Fermi level (Ef) of ZnO and g-C3N4 were 1.94 eV and 1.76 eV Evac, which were calculated using the ultraviolet photoelectron spectroscopy (UPS) work function derived from the excitation energy (HeI, 21.2 eV), and the VB edge (Evb) values for ZnO and g-C3N4 were 7.32 eV and 6.80 eV versus Evac, respectively. Furthermore, the CB energies (Ecb) of ZnO and g-C3N4 were determined to be 4.21 eV and 4.03 eV versus Evac, respectively (based on the equation: Ecb = Evb − Eg). The values of Evb and Ecb of ZnO and g-C3N4 were corresponding to 2.88 eV, 2.35 eV, −0.23 eV and −0.42 eV versus RHE, respectively. Fig. 2b shows the energy band arrangement of ZnO and g-C3N4 before and after the formation of the composite. Due to their complexation, the g-C3N4 energy band edge bends upward, and the ZnO energy band edge bends downward.27 From the bandgap positions of ZnO and g-C3N4, it can be inferred that only a type-II heterojunction or Z-scheme heterojunction can be formed between them.
The mechanism of the proposed Z-scheme heterojunction was verified using photoluminescence (PL). Typically, the photocatalyst (2 mg) was dispersed in an aqueous solution (5 mL) of 0.5 mM terephthalic acid and 2 mM NaOH. It was irradiated with a 300 W xenon lamp for 1 h and sampled and analyzed on an EI FLS980 spectroscopy test system with an excitation wavelength of 320 nm. The use of terephthalic acid as a probe molecule is attributed to the fact that hydroxyl radicals (˙OH) produced on the surfaces of 3D ZnO, pure g-C3N4, ZnO/g-C3N4 and ZnO/Au/g-C3N4 can be detected by terephthalic acid. Therefore, PL detection was carried out under the light. Fig. S8 in ESI† shows the comparative PL results of 3D ZnO, g-C3N4, ZnO/g-C3N4 and ZnO/Au/g-C3N4 after 1 h of illumination. All samples had a strong PL signal at 425 nM, indicating that ˙OH were produced on their surfaces. This is attributed to the energy band positions of ZnO and g-C3N4 with the OH−/˙OH (2.8 V vs. NHE) and O2/O2− (−0.3 V vs. NHE) standard redox potentials, which suggests that the photogenerated holes from the VB of ZnO produce ˙OH directly and the photogenerated electrons from the CB of g-C3N4 also produce ˙OH but indirectly by deriving it from superoxide radicals (O2−).28 As shown in Fig. 2c, ˙OH and O2− cannot be detected if a type-II heterojunction is formed. ZnO/g-C3N4 exhibited a stronger PL intensity than ZnO and g-C3N4, which indicates that more ˙OH was produced by ZnO/g-C3N4. It is well-known that Z-scheme heterojunctions have stronger redox ability. Therefore, ZnO/g-C3N4 supported sufficient oxidation to generate ˙OH and sufficient reduction to generate O2−. In addition, ZnO/Au/g-C3N4 showed the strongest PL intensity signal because the LSPR effect of the AuNP intermediates effectively promotes carrier transfer between ZnO and g-C3N4. According to the experimental results of PL, the charge carrier transfer in the ZnO/Au/g-C3N4 composite photocatalysis system matches the direct Z-scheme type than type-II semiconductors, which is consistent with our inference. Under xenon lamp irradiation, the photogenerated carriers are accelerated by transport across the Z-scheme heterojunction. The charge transfer is greatly enhanced, which is responsible for the significant increase in photocurrent intensity.
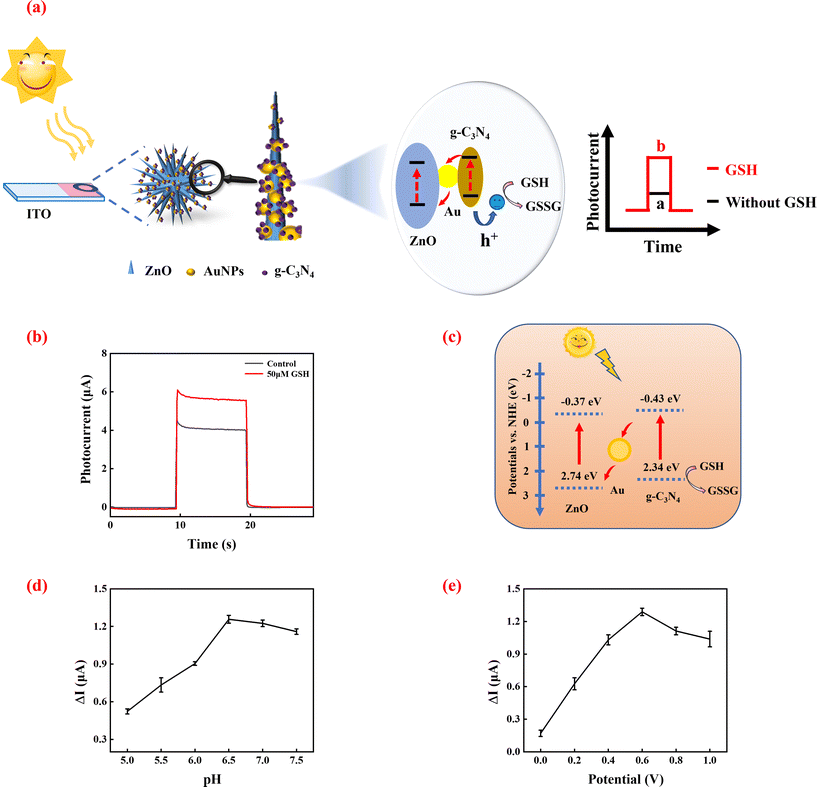
The principle of GSH detection by the PEC biosensor
GSH, which is a reducing substance, has the potential to serve as a hole scavenger. The principle of GSH sensing based on the 3D ZnO/Au/g-C3N4 heterostructure is shown in Fig. 3a. The electrons of GSH eliminate the photogenerated holes in the VB of g-C3N4, which prevent the rapid recombination of photogenerated electron–hole pairs in 3D ZnO/Au/g-C3N4. This results in the enhancement of the photocurrent detected. As the GSH concentration increases, more photogenerated holes are consumed, and the photocurrent rises accordingly. Based on this, it is possible to develop a sensitive PEC biosensor for GSH detection.23To verify the feasibility of the above sensing strategy, a simple PEC test was first performed. As shown in Fig. 3b, 3D ZnO/Au/g-C3N4 exhibited a high photocurrent intensity response. The system photocurrent was enhanced obviously after the addition of GSH. The reason is that GSH can be oxidized by holes as it is an electron donor, which effectively reduces the recombination of photogenerated electron–hole pairs, leading to enhanced photocurrent. These results demonstrate the feasibility of the sensor built using the 3D ZnO/Au/g-C3N4 heterojunction for GSH detection.
Fig. 3c shows a schematic of the charge transfer at the 3D ZnO/Au/g-C3N4 heterojunction when GSH is added to the system. When 3D ZnO/Au/g-C3N4 is exposed to the xenon lamp, ZnO and g-C3N4 are excited simultaneously, producing electron–hole pairs, respectively.
Under illumination,
| ZnO/Au/g-C3N4 + hv → h+ + e− | (1-1) |
| H2O + 2h+ → 1/2O2 + 2H+ | (1-2) |
| 2H+ + 2e− → H2 | (1-3) |
The Z-scheme heterojunction promotes fast transport of the photogenerated carriers. AuNPs act as mediators and also facilitate photoelectron transfer from ZnO to g-C3N4. When GSH is present in the system, GSH acts as a hole scavenger and effectively slows down the complexation of the electron–hole pairs so that a stronger photocurrent can be detected.
Under illumination,
| ZnO/Au/g-C3N4 + hv → h+ + e− | (2-1) |
| GSH + 2h+ → GSSG + 2H+ | (2-2) |
| 2H+ + 2e− → H2 | (2-3) |
Optimization of the experimental conditions
To achieve the best GSH sensing performance, some parameters of the system were optimized. GSH solutions are susceptible to oxidation, which is influenced by temperature, pH, light and other conditions, producing oxidized glutathione. pH is a crucial factor, which affected the performance of the GSH assay greatly. As shown in Fig. 3d, the enhanced photocurrent (ΔI = I − I0, where I0 is the photocurrent intensity value for the blank group (without GSH), and I is the photocurrent intensity value in the presence of 50 μM GSH) gradually increased with the pH from 5.0 to 6.5. When the pH was higher than 6.5, the enhanced photocurrent began to decrease gradually, which is attributed to the fact that GSH can be easily oxidized to form oxidized glutathione (GSSG), and GSSG cannot provide electrons that neutralize the holes in the VB of g-C3N4. Therefore, the pH of the assay was determined to be 6.5.The effect of bias potential was also optimized. As shown in Fig. 3e, ΔI increased with increasing bias potential and reached a maximum value at 0.6 V. Then, ΔI gradually decreased when the bias potential was further increased to 1.0 V. This is because, under certain conditions of the incident light, the bias increases the kinetic energy of the photoelectrons and results in the enhancement of the photocurrent. However, the photocurrent is limited by the number of photoelectrons and will not increase further. Therefore, 0.6 V was chosen as the optimum potential.
The performance of the biosensor
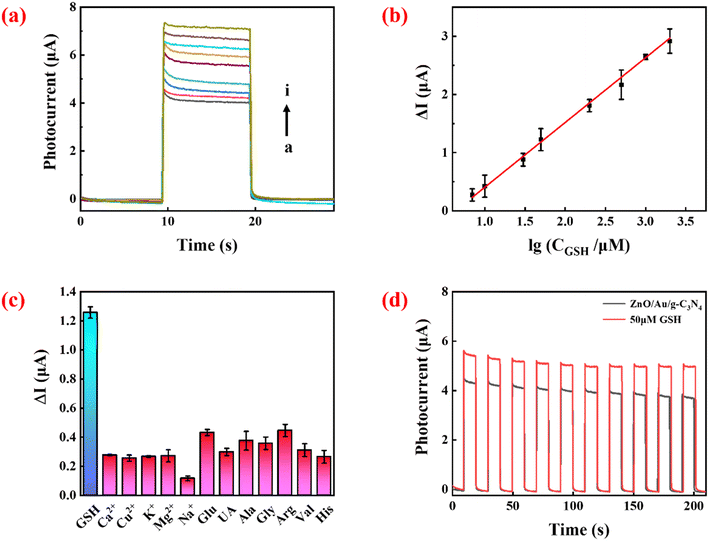
As shown in Fig. 4a, under optimized conditions, the measured photocurrent signal increased with increasing GSH concentration. Fig. 4b shows that there was a linear relationship between ΔI and the logarithm of target concentration between 7 and 2000 μM. The linear equation (as in eqn (3)) wasΔI = 1/11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CGSH − 0.71 R2 = 0.9984 CGSH − 0.71 R2 = 0.9984 | (3) |
Photocurrents in the presence of various interfering substances (e.g. metal ions, common chemical and biological samples) were measured to verify the selectivity of the sensor. The concentration of GSH was 50 μM, while the concentration of the interfering agent was 500 μM. As shown in Fig. 4c, compared with GSH, much smaller responses were detected in the presence of the interfering molecules. Although high concentrations of Glu and Arg could also produce a PEC response in the system, their levels (μM levels) in biological systems are much lower than that of GSH (mM levels).29,30 Therefore, these compounds will not cause a great interference to GSH detection in real samples.
The stability of the sensor is shown in Fig. 4d. There was no significant change in photocurrent intensity after 10 cycles of on/off irradiation in the absence and presence of 50 μM GSH, signifying that the sensor has excellent stability. To explore the reproducibility of the sensor, five independent tests were performed at 50 μM GSH, and the relative standard deviation (RSD) of the measured photocurrent intensity was 4.9%, showing that the constructed sensing platform has excellent reproducibility.
The performance of the proposed PEC sensor was compared with other GSH detection methods (Table S1†). The results showed the superior performance of this method with a wider linear range and a relatively lower LOD. This can be attributed to the development of a multi-component high-sensitivity PEC platform by the modification of the 3D ZnO surface using AuNPs and g-C3N4. Using the sea urchin-like, surface plasmon resonance effect and the synergistic effect of the Z-scheme heterojunction, the carrier separation and transfer are greatly enhanced, and more holes are trapped by GSH as a hole scavenger.
Real samples analysis
To assess the practical application of the proposed biosensor, GSH in three real serum samples (donated by volunteers from the Affiliated Hospital of Putian University, China) was detected, and spiked recovery experiments were carried out. This study was approved by the local ethics committee of the First Affiliated Hospital of Putian University and compliant with the Declaration of Helsinki (2008). Written informed consent was obtained from all participants. The results are shown in Table 1. The spiked recoveries ranged from 98.9% to 110.5%, and the relative standard deviations of the three parallel independent experiments ranged from 1.7% to 4.8%. This demonstrates the potential of the sensor in practical sample detection.| Samples | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0 | 28.5 | — | 3.1 |
| 10 | 38.8 | 102.4 | 2.3 | |
| 100 | 139.0 | 110.5 | 2.6 | |
| 1000 | 1064.5 | 103.6 | 2.2 | |
| 2 | 0 | 24.4 | — | 1.9 |
| 10 | 34.5 | 101.0 | 2.6 | |
| 100 | 131.7 | 107.3 | 3.2 | |
| 1000 | 1093.4 | 106.9 | 4.1 | |
| 3 | 0 | 22.9 | — | 1.7 |
| 10 | 33.4 | 105.0 | 2.9 | |
| 100 | 121.8 | 98.9 | 4.8 | |
| 1000 | 1022.1 | 99.9 | 3.6 |
Conclusion
In this study, a Z-scheme 3D ZnO/Au/g-C3N4 heterojunction photoelectrode was designed, and it exhibited significantly enhanced photocurrent because of the following unique advantages: (1) the 3D sea urchin-like structure composed of nanorods provided a directional channel for fast carrier transport and abundant reaction sites; (2) the Z-scheme has a stronger redox ability along with more efficient charge transfer and separation; (3) the AuNPs sandwiched between 3D ZnO and g-C3N4 act as mediators and greatly reinforce vector electron transfer in the Z-scheme heterojunction. GSH was chosen as the model target to test the potential application of the designed photoelectrode. The proposed system shows satisfactory results in the specific detection of GSH in serum samples.Author contributions
Weixin Li: investigation, data curation, writing – original draft. Xinyang Wang: software, methodology. Jiaji Huang: resources, software. Min Zhao: resources, software. Jiao Yang: data curation. Fang Luo: resources, software. Bin Qiu: resources. Jian Wang: resources, software. Zhenyu Lin: writing-review & editing, project administration, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence thee work reported in this paper.Acknowledgements
The authors gratefully acknowledge the financial support of the National Sciences Foundation of China (21974020).References
- Y.-T. Xu, S.-Y. Yu, Y.-C. Zhu, G.-C. Fan, D.-M. Han, P. Qu and W.-W. Zhao, TrAC, Trends Anal. Chem., 2019, 114, 81–88 CrossRef CAS.
- W. W. Zhao, J. J. Xu and H. Y. Chen, Anal. Chem., 2018, 90, 615–627 CrossRef CAS PubMed.
- R. M. Hewlett and M. A. McLachlan, Adv. Mater., 2016, 28, 3893–3921 CrossRef CAS PubMed.
- Z. Wang, M. Cao, L. Yang, D. Liu and D. Wei, Analyst, 2017, 142, 2805–2811 RSC.
- J. Tang, B. Kong, Y. Wang, M. Xu, Y. Wang, H. Wu and G. Zheng, Nano Lett., 2013, 13, 5350–5354 CrossRef CAS PubMed.
- J. Elias, C. Levy-Clement, M. Bechelany, J. Michler, G. Y. Wang, Z. Wang and L. Philippe, Adv. Mater., 2010, 22, 1607–1612 CrossRef CAS PubMed.
- X. Ren, A. Sangle, S. Zhang, S. Yuan, Y. Zhao, L. Shi, R. L. Hoye, S. Cho, D. Li and J. L. MacManus-Driscoll, J. Mater. Chem. A, 2016, 4, 10203–10211 RSC.
- J. Low, J. Yu, M. Jaroniec, S. Wageh and A. A. Al-Ghamdi, Adv. Mater., 2017, 29, 1601694 CrossRef PubMed.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- Y. Zhou, H. Yin and S. Ai, Coord. Chem. Rev., 2021, 447, 214156 CrossRef CAS.
- S. Patnaik, D. P. Sahoo and K. Parida, Carbon, 2021, 172, 682–711 CrossRef CAS.
- S. S. Low, Z. Chen, Y. Li, Y. Lu and Q. Liu, TrAC, Trends Anal. Chem., 2021, 145, 116454 CrossRef CAS.
- Š. Hajduk, S. P. Berglund, M. Podlogar, G. Dražić, F. F. Abdi, Z. C. Orel and M. Shalom, Adv. Mater. Interfaces, 2017, 4, 1700924 CrossRef.
- X. Geng, L. Wang, L. Zhang, H. Wang, Y. Peng and Z. Bian, Chem. Eng. J., 2021, 420, 129722 CrossRef CAS.
- X. Liang, J. Zhao, T. Wang, Z. Zhang, M. Qu and C. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 33034–33044 CrossRef CAS PubMed.
- Y. Fu, Z. Ren, J. Wu, Y. Li, W. Liu, P. Li, L. Xing, J. Ma, H. Wang and X. Xue, Appl. Catal., B, 2021, 285, 119785 CrossRef CAS.
- K. Leng, W. Mai, X. Zhang, R. Liu, X. Lin, J. Huang, H. Lou, Y. Xie, R. Fu and D. Wu, Chem. Commun., 2018, 54, 7159–7162 RSC.
- N. Zhang, S. Xie, B. Weng and Y.-J. Xu, J. Mater. Chem. A, 2016, 4, 18804–18814 RSC.
- C. X. Guo, J. Xie, H. Yang and C. M. Li, Adv. Sci., 2015, 2, 1500135 CrossRef PubMed.
- C. Zhou, S. Wang, Z. Zhao, Z. Shi, S. Yan and Z. Zou, Adv. Funct. Mater., 2018, 28, 1801214 CrossRef.
- X. L. Fu, F. Hou, F. R. Liu, S. W. Ren, J. T. Cao and Y. M. Liu, Biosens. Bioelectron., 2019, 129, 72–78 CrossRef CAS PubMed.
- Q.-X. Sun, S.-Q. Zhang, X. Wei, T. Yang, J.-H. Wang and M.-L. Chen, Anal. Chim. Acta, 2022, 1221, 340100 CrossRef CAS PubMed.
- D. Liu, X. Bai, J. Sun, D. Zhao, C. Hong and N. Jia, Sens. Actuators, B, 2022, 359, 131542 CrossRef CAS.
- R. Lv, T. Wang, F. Su, P. Zhang, C. Li and J. Gong, Nano Energy, 2014, 7, 143–150 CrossRef CAS.
- S. S. Patil, M. A. Johar, M. A. Hassan, D. R. Patil and S.-W. Ryu, Appl. Catal., B, 2018, 237, 791–801 CrossRef CAS.
- Z. Yin, B. Chen, M. Bosman, X. Cao, J. Chen, B. Zheng and H. Zhang, Small, 2014, 10, 3537–3543 CrossRef CAS PubMed.
- Z.-F. Huang, J. Song, X. Wang, L. Pan, K. Li, X. Zhang, L. Wang and J.-J. Zou, Nano Energy, 2017, 40, 308–316 CrossRef CAS.
- J. W. Jang, S. Cho, G. Magesh, Y. J. Jang, J. Y. Kim, W. Y. Kim, J. K. Seo, S. Kim, K. H. Lee and J. S. Lee, Angew. Chem., Int. Ed., 2014, 53, 5852–5857 CrossRef CAS PubMed.
- Q. Y. Cai, J. Li, J. Ge, L. Zhang, Y. L. Hu, Z. H. Li and L. B. Qu, Biosens. Bioelectron., 2015, 72, 31–36 CrossRef CAS PubMed.
- X. Dong, C. Xu, C. Yang, F. Chen, A. G. Manohari, Z. Zhu, W. Zhang, R. Wang, D. You and J. Chen, J. Mater. Chem., 2019, 7, 5624–5629 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental details on the characterization of the prepared materials, such as EDX testing of 3D ZnO/Au/g-C3N4, XRD testing of various components, XPS testing of 3D ZnO/Au/g-C3N4, UV-vis absorption testing of various components, UV-vis diffuse reflectance and UPS measurement of 3D ZnO and g-C3N4. See DOI: https://doi.org/10.1039/d3sd00040k |
| This journal is © The Royal Society of Chemistry 2023 |