DOI:
10.1039/D2SD00165A
(Critical Review)
Sens. Diagn., 2023,
2, 36-45
Research progress of electrode materials for non-enzymatic glucose electrochemical sensors
Received
15th September 2022
, Accepted 4th October 2022
First published on 13th December 2022
Abstract
Glucose biosensors are mainly divided into two types. Traditional enzymatic glucose sensors with high sensitivity and selectivity were developed and used early; however, they are sensitive to temperature and pH and come at a high cost. In contrast, non-enzymatic glucose sensors have been widely studied because of their stability and economy. Their research directions mainly focus on improving the detection ability and immediacy. Under these research directions, different electrode sensitive materials have been continuously developed and studied. This article reviewed the research progress of non-enzymatic glucose electrochemical sensor sensitive materials in the past five years. We also introduced the basic principles of electrochemical detection, various electrode sensitive materials and the development direction of non-enzymatic glucose sensors.
1 Introduction
The commonly used methods of glucose detection are high performance liquid chromatography,1 spectrophotometry2 and a glucose biosensor method.3 Most of the commercially available glucose detection devices are based on biological enzymes, and they are the most researched and commercialized biosensors in the field of biosensing.4,5 In 1964, Lyons et al.6 proposed the use of biological enzymes as an electrode to detect glucose, which laid a theoretical foundation for the development of bio-enzyme glucose detection. The enzymatic glucose sensor needs the participation of oxygen in the process of catalyzing glucose, and the detection result is greatly affected by oxygen concentration. The electrode active material of the non enzymatic glucose electrochemical sensor is an inorganic material, which gets rid of some limitations of biological enzymes. Therefore, compared with the enzymatic glucose electrochemical sensor, the non-enzymatic glucose electrochemical sensor has the following advantages: firstly, as active substances, inorganic materials have better chemical stability and thermal stability than biological enzymes. Secondly, the synthesis method for inorganic materials is relatively simple, and the cost is low. Finally, inorganic materials have a relatively long storage time and better reproducibility.7
2 Detection mechanisms of non-enzymatic glucose sensors
The most important factor in the response of non-enzymatic glucose sensors to glucose is the electrode-sensitive material on the electrode surface. The reaction mechanism between electrode-sensitive materials and glucose can be explained by the following two models. The first is the activated chemisorption model proposed by Pletcher8 in 1984, and the second is the initial hydrous oxide/atomic medium model (IHOAM) proposed by Burke9 in 1994.
As shown in Fig. 1, Pletcher assumes that glucose molecules are first adsorbed on the electrode surface during the catalytic oxidation of glucose. Due to the unpaired d-electrons and unfilled d-orbitals of the transition metal atoms on the surface of the electrode, they are susceptible to redox reactions with the adsorbed glucose molecules. The C–H bond on the hemiacetal carbon of the glucose molecule breaks and combines rapidly with the electrode surface to form a chemical bond.
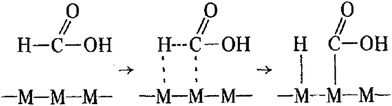 |
| | Fig. 1 Mechanism of the activated chemisorption model8 (M: metal atom, C: hemiacetal carbon atom). | |
The activated chemisorption model only explains the adsorption process on the electrode surface but does not consider the oxidation of hydroxyl radicals. Burke discovered that the active atoms on the electrode surface have low lattice coordination values and lattice stability, and the polycrystalline surface of the discontinuous region is directly exposed to the solution, which has higher reactivity and is prone to pre-monolayer oxidation. Therefore, he proposed an initial aqueous oxide atomic medium model in 1994, as shown in Fig. 2, the active metal atom on the surface of the electrode undergoes pre-monolayer oxidation to form an initial hydrated oxide layer. The activation layer acts as a medium and an inhibitor in the oxidation and reduction processes to oxidize glucose to gluconolactone.
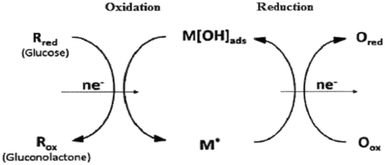 |
| | Fig. 2 Mechanism of IHOAM.9 | |
3 Structure of nanomaterials for glucose detection
There are many structures of nanomaterials, such as hollow sphere,10 slice type,11 rod shape,12 flower shape13etc. The catalytic effect of different nanostructured electrode materials on glucose is mainly reflected in two aspects: the number of exposed active sites and the size of a surface area. Glucose oxidation involves surface adsorption sites and hydroxyl radicals, both of which can promote the electrochemical oxidation of glucose. Nanoparticles, nanowires and other structures can expose more active sites, which can enhance the electrocatalysis of the electrode surface; in addition, the rough surface can increase the electrochemically active area, participate in more electrochemical reactions, and generate a larger response current. Wei14 believed that electrode materials with complex hollow architectures, large specific surface area, and porosity, as well as more inner cavities, are widely employed for high-performance supercapacitors. They prepared a complex nickel cobalt manganese sulfide yolk–shell hollow sphere and proved their potential application in high-performance electrochemical energy storage (Fig. 3).
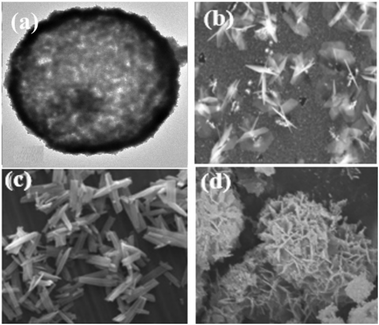 |
| | Fig. 3 (a) SEM image of the CuC2O4 hollow sphere.10 (b) SEM image of the Cu2O/AuCu/Cu sheet.11 (c) SEM image of the NiCo2S4 nanorods.12 (d) SEM image of flower-like NiO.13 | |
The roughness of the electrode material can be used to increase the Faraday current of slow reaction, Park et al.15 used this principle to construct an electrode material with a mesoporous structure on the surface of a platinum electrode and used it for the detection of glucose. Park et al.16 studied the electrochemical behavior of a 3D-npPt membrane in the electrocatalytic oxidation, O2 reduction and H2O2 reduction of glucose. By comparing the glucose oxidation process on 3D-npPt and 1D nanoporous Pt (1D-npPt), they found that the overall electrode activity of 3D-npPt was significantly higher than that of 1D-npPt, and the high catalytic activity is attributed to the 3D structure of 3D-npPt, which increases the active area of the electrode and reduces the pore resistance.
4 Electrode sensitive material for non-enzymatic glucose sensors
4.1 Precious metal-based non-enzymatic glucose sensors
Pt has high catalytic activity for small molecular substances, it will not dissolve even in a strong acid, strong alkali and high concentration salt medium. The surface of Pt easily adsorbs small molecules due to insufficient d orbital loading and further redox reaction with adsorbate at a certain potential. Therefore, it is considered to be a highly efficient electrocatalyst and is widely used in fuel cells, water decomposition and glucose detection. Au can also react with glucose to generate redox current under neutral or basic conditions, but the effect is not as good as Pt.17
As shown in Fig. 4, Shim et al.18 incorporated Au into Au@Pt NPs to obtain Au@Pt/Au NPs by an acousto chemical method and a potential step method. The Au core and the Pt shell can increase the selectivity and sensitivity to glucose by synergistic catalytic effects, and the bimetallic core–shell structure prevents metal passivation. Electrochemical performance tests showed that the electrode has a good linear relationship to glucose in the wide linear range of 0.5–10 μM and 100–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM, and the detection limit is 445 nM. Table 1 summarizes the glucose sensors based on Au and Pt in recent years and shows the key performance parameters such as sensitivity, detection range and minimum detection limit.
000 μM, and the detection limit is 445 nM. Table 1 summarizes the glucose sensors based on Au and Pt in recent years and shows the key performance parameters such as sensitivity, detection range and minimum detection limit.
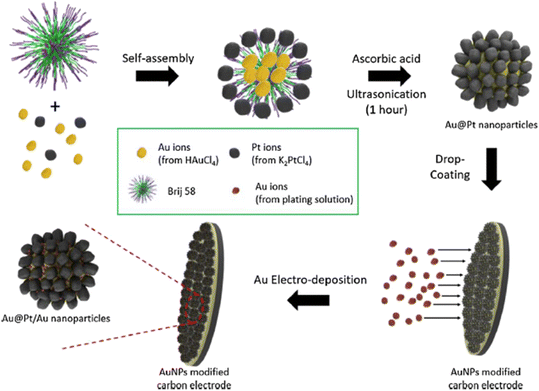 |
| | Fig. 4 Schematic illustration of the synthesis of Au@Pt/Au NPs.18 | |
Table 1 List of Au-based and Pt-based electrochemical non-enzymatic glucose sensors
| Electrode materials |
Sensitivity (μA mM−1 cm−2) |
Linear range (μM) |
LOD (μM) |
Operation potential (V) |
Chemical environment |
Ref. |
| Au foam |
— |
0.5–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.14 |
0.10 |
0.3 M NaOH |
19
|
| Au/ZnO |
4416 |
∼15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.12 |
0.80 |
0.1 M PBS (pH = 7.0) |
20
|
| Pt/Au |
— |
0.5–10, 10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.45 |
0.35 |
0.1 M PBS (pH = 7.4) |
18
|
| Ag@Ni-MOF |
160.08 |
5–500 |
5 |
0.50 |
0.1 M NaOH |
21
|
4.2 Non-enzymatic glucose sensors based on transition metals and their compounds
Transition metal sensing materials have the characteristics of rapid response, high sensitivity, good stability and low cost, which showed unique advantages in the field of glucose enzyme free electrochemical sensors.22 In the process of catalytic oxidation of glucose, transition metal-based electrode sensitive materials mainly involve redox reactions between transition metal compounds with different valence states.23 The hemiacetal carbon atom of the glucose molecule is dehydrogenated on the surface of the electrode and then combined with the active site adjacent to the surface of the electrode. The dehydrogenated free radical intermediate is further oxidized to gluconolactone and finally oxidized to gluconolactone by hydrolysis. During the reaction, the transition peak of the transition metal compound coincides with the oxidation peak of glucose, which proves that the transition metal acts as a reactive center to participate in the catalytic oxidation process of glucose.24 Metal and metal compound electrodes based on Cu, Co, and Ni have become research hotspots due to their good catalytic properties among many transition metal materials.
4.2.1 Non-enzymatic glucose sensors based on copper and its compounds.
Copper is a very promising metal material with good electrical conductivity and abundant reserves on earth. Various forms of Cu-based glucose sensors have been developed, such as Cu,25 Cu2O,26 CuO,27 and CuS.28 The oxidation peak range of Cu based materials is wider than that of many catalysts, about 0.3 to 0.5 V (relative to Ag/AgCl). However, the oxidation peak of Cu(II) contains a high background current, and it is difficult to distinguish when the intensity of the oxidation peak is weak.
Liu et al.29 synthesized a copper oxide nano-array (CuO NWA) on copper foam (CF) (Fig. 5); the nano-array on the CF increases the specific surface area and the number of active sites, and it also increases the electron transport rate and enhances the electrocatalytic performance. The CuO NWA electrode exhibits an extremely high sensitivity (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 μA mM−1 cm−2) to glucose in the concentration range of 10 to 500 μM and an ultra-low detection limit (20 nM). It has a lower relative standard deviation when the sensor is used to detect glucose in iced black tea. Table 2 summarizes the Cu-based glucose sensors in the past three years and shows the key performance parameters in detail.
330 μA mM−1 cm−2) to glucose in the concentration range of 10 to 500 μM and an ultra-low detection limit (20 nM). It has a lower relative standard deviation when the sensor is used to detect glucose in iced black tea. Table 2 summarizes the Cu-based glucose sensors in the past three years and shows the key performance parameters in detail.
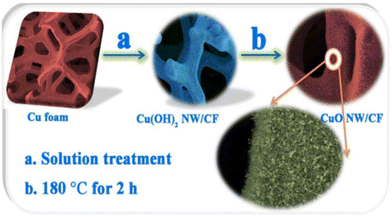 |
| | Fig. 5 Fabrication procedure of CuO NWA/CF.29 | |
Table 2 List of Cu-based electrochemical non-enzymatic glucose sensors
| Electrode materials |
Sensitivity (μA mM−1 cm−2) |
Linear range (μM) |
LOD (μM) |
Operation potential (V) |
Chemical environment |
Ref. |
| CuS |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 620 |
160–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
2.72 |
0.50 |
0.1 M NaOH |
28
|
| Cu2O |
1470.85 |
1–700 |
— |
0.50 |
0.1 M NaOH |
30
|
| Cu/CuO |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
1–5000 |
0.33 |
0.65 |
1.0 M NaOH |
31
|
| CuxO/CuO |
1212.02, 852.80 |
10–200, 500–1600 |
— |
0.40 |
0.5 M NaOH |
32
|
| Cu/CuS |
5020 |
0.1–500 |
0.33 |
0.55 |
0.1 M NaOH |
33
|
4.2.2 Non-enzymatic glucose sensors based on cobalt and its compounds.
Cobalt-based metal compounds have been widely studied as catalysts in the field of glucose sensor sensitive materials.34 Cobalt oxide has three polymorphic substances: CoO, Co2O3 and Co3O4. Compared with the other two polymorphic materials, Co3O4 is cheap and environmentally friendly and has high electrical conductivity and electrocatalytic properties.35 Cobalt exists in various forms in an alkaline solution, and there are many redox peaks in the cyclic voltammogram of cobalt involving Co (0)/Co(II), Co(II)/Co(III), and Co(III)/Co(IV) transitions. Studies have found that most catalytic oxidation reactions for glucose are carried out by Co(III)/Co(IV) redox couples.36 Co3O4 has a special spinel structure in which one of the three Co atoms is in the Co(II) state, and the other two are in the Co(III) state. Under strong alkaline conditions, Co3O4 may be oxidized to CoOOH to catalytically oxidize glucose.
Li et al.37 used CuCl2 and CoCl2 as raw materials to synthesize Co-MOF nanosheets on foamed nickel by a hydrothermal method. As can be seen in Fig. 6, the synthesized Co-MOF is an ultra-thin layered structure.38 In an alkaline medium, the coordination interaction between Co2+ and organic molecules becomes weak, and the released Co2+ reacts with OH− in solution to form CoOOH.39 The reaction mechanism can be expressed by eqn (1)–(3):40–42
| | | Co2+ + nOH− + H2O ↔ CoOOH + ne− | (1) |
| | | CoOOH + H2O ↔ Co4+ + 3OH− + e− | (2) |
| | | Co-MOF + glucose → CoOOH + glucolactone + H2O | (3) |
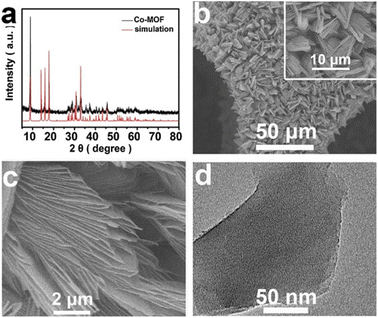 |
| | Fig. 6 (a) XRD pattern of CoMOF and the synthesized CoMOF sample. (b and c) SEM images. (d) HRTEM images.37 | |
Co-MOF/NiF is a glucose-sensitive material with excellent performance. The response range for glucose is 1–3000 μM, the sensitivity is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 886 μA mM−1 cm−2, and the detection limit is 1.3 nM. Table 3 summarizes the Co-based glucose sensors in the past three years and shows the key performance parameters in detail.
886 μA mM−1 cm−2, and the detection limit is 1.3 nM. Table 3 summarizes the Co-based glucose sensors in the past three years and shows the key performance parameters in detail.
Table 3 List of Co-based electrochemical non-enzymatic glucose sensors
| Electrode materials |
Sensitivity (μA mM−1 cm−2) |
Linear range (μM) |
LOD (μM) |
Operation potential (V) |
Chemical environment |
Ref. |
| Co |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 886 886 |
1–3000 |
0.0013 |
0.50 |
0.1 M NaOH |
37
|
| Co3O4 |
2550 |
1–122 |
0.28 |
0.50 |
0.1 M NaOH |
43
|
| CoTe2 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
10–5000 |
0.59 |
0.60 |
0.1 M NaOH |
44
|
| Co(OH)2 |
1646 |
0–3000 |
0.5 |
0.55 |
0.1 M KOH |
45
|
| Cu/Co(OH)2 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1–250 |
0.073 |
0.50 |
0.1 M KOH |
46
|
4.2.3 Non-enzymatic glucose sensors based on nickel and its compounds.
Similar to the above two metal elements, nickel and its compounds are also widely studied by scientists. Nickel based compounds are widely used in energy storage and electrocatalysis due to their low natural abundance, low cost and excellent electrochemical activity.47 Their active centers mainly come from the redox pairs of Ni(II)/Ni(III) (Fig. 7).48
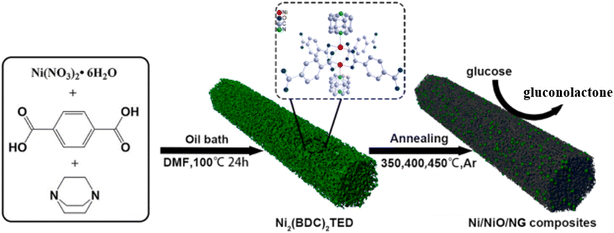 |
| | Fig. 7 The construction schematic of the Ni/NiO/NG nanocomposite electrode as well as the schematic of its glucose sensing mechanism.49 | |
Wang et al.49 prepared composites (Ni/NiO/NG) in doped graphene by isolated air calcination. Ni/NiO/NG has a mixed valence state, and the special microporous scaffold structure provides a strong support force and responds well to glucose. The sensitivity of the Ni/NiO/NG electrode is 3251.8 μA mM−1 cm−2 with a detection limit of 32 nM; in addition, the electrode also shows excellent selectivity, repeatability, long-term stability and high tolerance to chloride ions. Therefore, the Ni/NiO/NG non-enzymatic glucose sensor is expected to be a candidate for practical applications. Table 4 summarizes the Ni-based glucose sensors in the past three years and shows the key performance parameters in detail.
Table 4 List of Ni-based electrochemical non-enzymatic glucose sensors
| Electrode materials |
Sensitivity (μA mM−1 cm−2) |
Linear range (μM) |
LOD (μM) |
Operation potential (V) |
Chemical environment |
Ref. |
| NiO |
206.9 |
0.1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.16 |
0.55 |
0.1 M KCl + 0.5 M NaOH |
50
|
| Ni(OH)2 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 171 171 |
1–400 |
— |
0.60 |
0.1 M KOH |
51
|
| Ni–N–C |
1181 |
1–1200 |
0.15 |
0.50 |
0.1 M NaOH |
52
|
| Ni (OH)2/3DGF |
2366 |
0.32–2200 |
0.32 |
0.40 |
0.2 M NaOH |
53
|
| Ni/Ni(OH)2 |
1078 |
200–6000 |
200 |
0.60 |
2.0 M NaOH |
54
|
4.2.4 Non-enzymatic glucose sensor based on other transition metals and their compounds.
As glucose sensitive material, the three transition metals mentioned above and their compounds are the most studied by researchers. In addition, some materials are also used in the field of glucose sensors, but there are few related reports, such as Zn,55 Fe,56 Mn,57etc. Their elemental or single oxides are not very effective in the catalytic oxidation of glucose, but they can be combined with those with high catalytic properties to improve the performance by utilizing the synergistic relationship between different metal elements. In addition, transition metal based nitride,58 phosphide,59 sulfide28 and selenide60 are also commonly used catalyst materials for non-enzymatic glucose oxidation. Table 5 summarizes the non-enzymatic glucose sensors based on other transition metals and their compounds in recent years and shows the key performance parameters in detail.
Table 5 List of other transition metals-based electrochemical non-enzymatic glucose sensors
| Electrode materials |
Sensitivity (μA mM−1 cm−2) |
Linear range (μM) |
LOD (μM) |
Operation potential (V) |
Chemical environment |
Ref. |
| Cu–Ag–Zn |
3572 |
0–6000 |
0.37 |
0.10 |
0.1 M NaOH |
61
|
| Ni–Al–Mn |
2253 |
15–8000 |
1.49 |
0.80 |
0.1 M NaOH |
62
|
| Co–P/Cu |
1818 |
500–2500 |
0.378 |
0.60 |
0.1 M KOH |
63
|
| SnO2 |
121.2 |
0.12–7800 |
0.04 |
0.90 |
0.1 M PBS (pH = 6) |
64
|
| Fe3O4/rGO |
81.81 |
0–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.1 |
0.40 |
0.02 M PBS |
65
|
| 21.54 |
1000–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.2 |
4.3 Non-enzymatic glucose sensors with specific binding for glucose
In addition to the several sensitive materials described above, we can find a functional material that has a specific response to glucose, such as boric acid and its derivatives, and a conductive polymer based on molecular imprinting technology (MIP), which has excellent selectivity for glucose molecules.
Kuivila et al.66 first reported that phenylboronic acid and its derivatives could bind to sugars and form cyclic lactones in 1954, opening the door to the use of boric acid as a sugar recognition molecule. Lorand et al.67 further studied the principle of binding between phenylboronic acid and sugar and pointed out that in an alkaline environment, the phenylboronic acid neutral molecule is converted to an anionic state, the boron atom is changed from sp2 hybridization to sp3 hybridization, and the boric acid configuration is changed from a planar triangle to a tetrahedral configuration. They also pointed out that boric acid could combine with 1,2- or 1,3-dihydroxy compounds to form a five- or six-membered cyclic lactone. The cis-ortho-dihydroxy group in the sugar ring structure has a stronger binding ability to boric acid than the simple chain-like ortho-dihydroxy compound (such as ethylene glycol). Therefore, boric acid can be used as a recognition site for saccharide sensors in the field of glucose detection. Liu et al.68 presented a continuous glucose monitoring platform consisting of a quartz crystal microbalance (QCM) quartz and a flexible hydrogel recognition system. A stretchable hydrogel is employed as the support material and antifouling membrane, and glucose-sensitive monomer phenylboronic acid (PBA) is employed as the recognition part. The hydrogel membrane with PBA firmly adhered onto the electrode surface of the quartz crystal, and the lower limit of glucose detection of the platform was 0.15 mg L−1.
MIT refers to the process of preparing a polymer with specific recognition for a specific target molecule,69 it is a powerful technology for synthesizing high molecular type artificial receptors,70 MIT has been widely used in the fields of chromatographic separation,71 chiral separation72 and sensing probe.73 MIT-based polymers (MIP) have a selective response to target molecules of natural receptors,74 and in MIP-based glucose sensors, a monomer having a functional moiety is polymerized with glucose to produce a specific polymer template for identifying glucose, which can be detected by reading an identification signal. MIP has high selectivity to glucose, good stability and low cost; however, MIP has a longer response time to glucose and is susceptible to external environments such as temperature and pH. There are currently few papers on the detection of glucose molecules. Table 6 summarizes the non-enzymatic glucose sensors based on boric acid and MIP in recent years and shows the key performance parameters in detail.
Table 6 List of boric acid and MIP-based electrochemical non-enzymatic glucose sensors
| Electrode materials |
Sensitivity |
Linear range (μM) |
LOD (μM) |
Chemical environment |
Ref. |
| MIP/CD |
— |
0.5–40, 50–600 |
0.09 |
0.05 M PBS |
74
|
| MIP |
— |
0.32–1000 |
0.19 |
0.1 M PBS (pH = 7.4) |
75
|
| GO-MIP |
— |
10–6000 |
0.02 |
PBS (pH = 5.0) |
76
|
5 Summary
In this paper, we review the electrode materials available in non-enzymatic glucose sensors in recent years and their research status, including precious metals, transition metals, and substances that specifically respond to glucose. Precious metals have high electrocatalytic activity for glucose, but their sensitivity and stability are poor. Transition metal, represented by Cu, Co and Ni, have good electrocatalytic activity and stability for glucose, but the selectivity is poor, and the catalytic activity is greatly affected by nanomaterials. Electrode materials with a specific selectivity for glucose are too dependent on the balance between glucose and functional groups, the reaction conditions are harsh, and the linear range and stability need to be improved. We also need to conduct deeper research to combine the advantages of materials to make up for their problems and shortcomings in sensing performance (Fig. 8).
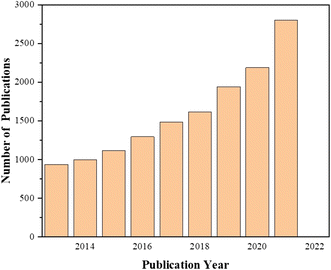 |
| | Fig. 8 Chart of the latest publications for enzyme-free glucose sensors as a function of year (results collected using the ScienceDirect database with the “non-enzymatic glucose sensors” as the search term). | |
6 Outlook
Since 2000, non-enzymatic glucose sensors have achieved tremendous development, and the number of articles on glucose sensors has been increasing year by year. In the past three years, the number of articles on glucose sensors on ScienceDirect is 1910 (2019), 2190 (2020), and 2804 (2021), indicating that there is still room and potential for the development of non-enzymatic glucose sensors. The development of nanomaterials and nanotechnology can provide more options and possibilities for the further development of glucose sensors.
Previously, the detection of glucose basically requires blood, which is a non-continuous invasive test. If we develop a non-invasive detection method to detect the concentration of glucose in the body, such as saliva,77 tears,78 sweat,79etc., this will be a huge advancement in glucose detection technology and a huge boon for patients. At present, some research teams have tried in this direction. Garcia-Carmona et al.80 designed a pacifier-type wireless device that can detect blood sugar levels by detecting the baby's saliva. Liu et al.81 reported a new device that can detect the amount of glucose in sweat. The team integrated highly sensitive In2O3 nanostrip field effect transistors (FETs) into substrates and devices for glucose detection. Non-intrusive sensors have too many interference factors in the detection process, and the accuracy and repeatability are yet to be confirmed; besides, the correlation between saliva, tears, other fluids and blood glucose concentrations remains to be verified. Researchers need to conduct deeper research on the sensing mechanisms and influencing factors to provide more effective solutions for the development of commercial non-enzyme sensors.82
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
There are no conflicts to declare.
References
- G. Lamprecht, F. Pichlmayer and E. R. Schmid, Determination of the authenticity of vanilla extracts by stable isotope ratio analysis and component analysis by HPLC, J. Agric. Food Chem., 1994, 42, 1722–1727 CrossRef CAS.
- Y. Ni, G. Zhang and S. Kokot, Simultaneous spectrophotometric determination of maltol, ethyl maltol, vanillin and ethyl vanillin in foods by multivariate calibration and artificial neural networks, Food Chem., 2005, 89, 465–473 CrossRef CAS.
- H. Kawakami, Y. Ito, Y.-A. Chien, C.-Y. Chen, W.-T. Chiu, P. Chakraborty, T. Nakamoto, M. Sone and T.-F. M. Chang, Development of polypyrrole/nano-gold composite for non-enzymatic glucose sensors, Micro Nano Eng., 2022, 14, 100109 CrossRef CAS.
- E.-H. Yoo and S.-Y. Lee,
et al., Glucose Biosensors: An Overview of Use in Clinical Practice, Sensors, 2010, 10, 4558–4576 CrossRef PubMed; E.-H. Yoo and S.-Y. Lee, Sensors, 2010, 10, 1–2 CrossRef PubMed.
- A. Heller and B. Feldman, Electrochemistry in Diabetes Management, Acc. Chem. Res., 2010, 43, 963–973 CrossRef CAS PubMed.
- C. Lyons, L. Clark, H. McDowell and K. McArthur, Cerebral Venous Oxygen Content During Carotid Thrombintimectomy, Ann. Surg., 1964, 160, 561–567 CrossRef CAS PubMed.
- E. Ding, J. Hai, T. Li, J. Wu, F. Chen, Y. Wen, B. Wang and X. Lu, Efficient Hydrogen-Generation CuO/Co3O4 Heterojunction Nanofibers for Sensitive Detection of Cancer Cells by Portable Pressure Meter, Anal. Chem., 2017, 89, 8140–8147 CrossRef CAS PubMed.
- D. Pletcher, Electrocatalysis, 1984, 14, 403–415 CAS.
- L. D. Burke, Premonolayer oxidation and its role in electrocatalysis, Electrochim. Acta, 1994, 39, 1841–1848 CrossRef CAS.
- M. Sun, J. Liu, S. Wang, Z. Wang and X. Song, Solvothermal synthesis of hollow spherical CuCo2O4 as high-performance non-enzymatic glucose sensors, Ionics, 2021, 27, 2257–2266 CrossRef CAS.
- X. Liang, W. Li, H. Han and Z. Ma, Non-enzymatic electrochemical sensor based on Cu2O/AuCu/Cu composite for hydrogen peroxide detection, J. Electroanal. Chem., 2019, 834, 43–48 CrossRef CAS.
- B. Li, B. Luo, J. Zhao, Y. Pan, H. Zhou, Y. Xiao, S. Lei and B. Cheng, High electrical conductivity-induced enhancement effect of electrochemical performance in mesoporous NiCo2S4 nanorod-based supercapacitor, J. Energy Storage, 2019, 26, 100955 CrossRef.
- Z. Cui, H. Yin, Q. Nie, D. Qin, W. Wu and X. He, Hierarchical flower-like NiO hollow microspheres for non-enzymatic glucose sensors, J. Electroanal. Chem., 2015, 757, 51–57 CrossRef CAS.
- C. Wei, Q. Chen, C. Cheng, R. Liu, Q. Zhang and L. Zhang, Mesoporous nickel cobalt manganese sulfide yolk–shell hollow spheres for high-performance electrochemical energy storage, Inorg. Chem. Front., 2019, 6, 1851–1860 CAS.
- S. Park, T. D. Chung and H. C. Kim, Nonenzymatic Glucose Detection Using Mesoporous Platinum, Anal. Chem., 2003, 75, 3046–3049 CrossRef CAS PubMed.
- S. Park, Y. J. Song, J.-H. Han, H. Boo and T. D. Chung, Structural and electrochemical features of 3D nanoporous platinum electrodes, Electrochim. Acta, 2010, 55, 2029–2035 CrossRef CAS.
- S. Biella, L. Prati and M. Rossi, Selective Oxidation of D-Glucose on Gold Catalyst, J. Catal., 2002, 206, 242–247 CrossRef CAS.
- K. Shim, W.-C. Lee, M.-S. Park, M. Shahabuddin, Y. Yamauchi, M. S. A. Hossain, Y.-B. Shim and J. H. Kim, Au decorated
core-shell structured Au@Pt for the glucose oxidation reaction, Sens. Actuators, B, 2019, 278, 88–96 CrossRef CAS.
- N. N. Shen, H. J. Xu, W. C. Zhao, Y. M. Zhao and X. Zhang, Highly Responsive and Ultrasensitive Non-Enzymatic Electrochemical Glucose Sensor Based on Au Foam, Sensors, 2019, 19(5), 1203 CrossRef CAS PubMed.
- A. Awais, M. Arsalan, X. Qiao, W. Yahui, Q. Sheng, T. Yue and Y. He, Facial synthesis of highly efficient non-enzymatic glucose sensor based on vertically aligned Au-ZnO NRs, J. Electroanal. Chem., 2021, 895, 115424 CrossRef CAS.
- J. Cao, J. Yun, N. Zhang, Y. Wei, H. Yang and Z. Xu, Bifunctional Ag@Ni-MOF for high performance supercapacitor and glucose sensor, Synth. Met., 2021, 282, 116931 CrossRef CAS.
- D.-W. Hwang, S. Lee, M. Seo and T. D. Chung, Recent advances in electrochemical non-enzymatic glucose sensors – A review, Anal. Chim. Acta, 2018, 1033, 1–34 CrossRef CAS.
- S. Berchmans, H. Gomathi and G. P. Rao, Electrooxidation of alcohols and sugars catalysed on a nickel oxide modified glassy carbon electrode, J. Electroanal. Chem., 1995, 394, 267–270 CrossRef.
- J. M. Marioli and T. Kuwana, Electrochemical characterization of carbohydrate oxidation at copper electrodes, Electrochim. Acta, 1992, 37, 1187–1197 CrossRef CAS.
- A. El Golli, M. Echabaane and C. Dridi, Development of an electrochemical nanoplatform for non-enzymatic glucose sensing based on Cu/ZnO nanocomposite, Mater. Chem. Phys., 2022, 280, 125844 CrossRef CAS.
- W. Liu, X. Zhao, Y. Dai and Y. Qi, Preparation of three dimensional Cu2O/Au/GO hybrid electrodes and its application as a non-enzymatic glucose sensor, Microchem. J., 2022, 179, 107451 CrossRef CAS.
- L. Yang, J. Yang, Q. Dong, F. Zhou, Q. Wang, Z. Wang, K. Huang, H. Yu and X. Xiong, One-step synthesis of CuO nanoparticles based on flame synthesis: As a highly effective non-enzymatic sensor for glucose, hydrogen peroxide and formaldehyde, J. Electroanal. Chem., 2021, 881, 114965 CrossRef CAS.
- K. P. Sharma, M. Shin, G. P. Awasthi, M. B. Poudel, H. J. Kim and C. Yu, Chitosan polymer matrix-derived nanocomposite (CuS/NSC) for non-enzymatic electrochemical glucose sensor, Int. J. Biol. Macromol., 2022, 206, 708–717 CrossRef CAS PubMed.
- X. Liu, W. Yang, L. Chen and J. Jia, Three-Dimensional Copper Foam Supported CuO Nanowire Arrays: An Efficient Non-enzymatic Glucose Sensor, Electrochim. Acta, 2017, 235, 519–526 CrossRef CAS.
- F. Sun and Z. Wang, Highly-branched dendrite cuprous oxide films for non-enzymatic amperometric glucose sensor, Mater. Lett., 2019, 234, 62–65 CrossRef CAS.
- L. Zhang, H. Liang, X. Ma, C. Ye and G. Zhao, A vertically aligned CuO nanosheet film prepared by electrochemical conversion on Cu-based metal-organic framework for non-enzymatic glucose sensors, Microchem. J., 2019, 146, 479–485 CrossRef CAS.
- S. Wang, L. Jiang, J. Hu, Q. Wang, S. Zhan and Y. Lu, Dual-functional CuxO/Cu electrodes for supercapacitors and non-enzymatic glucose sensors fabricated by femtosecond laser enhanced thermal oxidation, J. Alloys Compd., 2020, 815, 152105 CrossRef CAS.
- G.-R. Xu, C. Ge, D. Liu, L. Jin, Y.-C. Li, T.-H. Zhang, M. M. Rahman, X.-B. Li and W. Kim, In-situ electrochemical deposition of dendritic Cu-Cu2S nanocomposites onto glassy carbon electrode for sensitive and non-enzymatic detection of glucose, J. Electroanal. Chem., 2019, 847, 113177 CrossRef CAS.
- V. R. Shinde, S. B. Mahadik, T. P. Gujar and C. D. Lokhande, Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis, Appl. Surf. Sci., 2006, 252, 7487–7492 CrossRef CAS.
- Q. Balouch, Z. H. Ibupoto, G. Q. Khaskheli, R. A. Soomro, S. S. Sirajuddin, M. K. Samoon and V. K. Deewani, Cobalt Oxide Nanoflowers for Electrochemical Determination of Glucose, J. Electron. Mater., 2015, 44, 3724–3732 CrossRef CAS.
- T. R. I. Cataldi, A. Guerrieri, I. G. Casella and E. Desimoni, Study of a cobalt-based surface modified glassy carbon electrode: Electrocatalytic oxidation of sugars and alditols, Electroanalysis, 1995, 7, 305–311 CrossRef CAS.
- Y. Li, M. Xie, X. Zhang, Q. Liu, D. Lin, C. Xu, F. Xie and X. Sun, Co-MOF nanosheet array: A high-performance electrochemical sensor for non-enzymatic glucose detection, Sens. Actuators, B, 2019, 278, 126–132 CrossRef CAS.
- J. Zhao, P. Su, Y. Zhao, M. Li, Y. Yang, Q. Yang and C. Li, Systematic morphology and phase control of Mg-ptcda coordination polymers by Ostwald ripening and self-templating, J. Mater. Chem., 2012, 22, 8470–8475 RSC.
- P. Su, S. Liao, F. Rong, F. Wang, J. Chen, C. Li and Q. Yang, Enhanced lithium storage capacity of Co3O4 hexagonal nanorings derived from Co-based metal organic frameworks, J. Mater. Chem. A, 2014, 2, 17408–17414 RSC.
- C. Barbero, G. A. Planes and M. C. Miras, Redox coupled ion exchange in cobalt oxide films, Electrochem. Commun., 2001, 3, 113–116 CrossRef CAS.
- Q.-Q. Sun, M. Wang, S.-J. Bao, Y. C. Wang and S. Gu, Analysis of cobalt phosphide (CoP) nanorods designed for non-enzyme glucose detection, Analyst, 2016, 141, 256–260 RSC.
- Y. Ding, Y. Wang, L. Su, M. Bellagamba, H. Zhang and Y. Lei, Electrospun Co3O4 nanofibers for sensitive and selective glucose detection, Biosens. Bioelectron., 2010, 26, 542–548 CrossRef CAS PubMed.
- X. Lin, Y. Wang, M. Zou, T. Lan and Y. Ni, Electrochemical non-enzymatic glucose sensors based on nano-composite of Co3O4 and multiwalled carbon nanotube, Chin. Chem. Lett., 2019, 30, 1157–1160 CrossRef CAS.
- A. Farid, L. Pan, M. Usman, I. A. Khan, A. S. Khan, A. U. Ahmad and M. Javid, In-situ growth of porous CoTe2 nanosheets array on 3D nickel foam for highly sensitive binder-free non-enzymatic glucose sensor, J. Alloys Compd., 2021, 861, 158642 CrossRef CAS.
- Y. Tao, Q. Liu, Q. Chang, J. Duan, Z. Tao, H. Guan, G. Chen, Y. Mao, J. Xie and C. Dong, In situ fabrication of Co(OH)2 by hydrothermal treating Co foil in MOH (M = H, Li, Na, K) for non-enzymatic glucose detection, J. Alloys Compd., 2019, 781, 1033–1039 CrossRef CAS.
- L. Wang, S. Zhuang, L. Wang, N. Wang, H. Mo, Y. Tang, Y. Chen, Y. Sun and P. Wan, One step synthesis of hierarchical Cu nanoparticles-Co(OH)2 nanoflakes/Nifoam electrode for ultrasensitive detection of glucose, Appl. Surf. Sci., 2019, 467–468, 773–781 CrossRef CAS.
- C. Wei, J. Sun, Y. Zhang, Y. Liu, Z. Guo, W. Du, L. Liu and Y. Zhang, Hierarchical Ni(OH)2–MnO2 hollow spheres as an electrode material for high-performance supercapacitors, Inorg. Chem. Front., 2022, 9, 3542–3551 RSC.
- N. Salarizadeh, M. Habibi-Rezaei and S. J. Zargar, NiO–MoO3 nanocomposite: A sensitive non-enzymatic sensor for glucose and urea monitoring, Mater. Chem. Phys., 2022, 281, 125870 CrossRef CAS.
- Q. Wang, S. Zheng, T. Li and Z. Wang, Ni/NiO multivalent system encapsulated in nitrogen-doped graphene realizing efficient activation for non-enzymatic glucose sensing, Ceram. Int., 2021, 47, 22869–22880 CrossRef CAS.
- C. Heyser, R. Schrebler and P. Grez, New route for the synthesis of nickel (II) oxide nanostructures and its application as non-enzymatic glucose sensor, J. Electroanal. Chem., 2019, 832, 189–195 CrossRef CAS.
- X. Zhong, Z. He, H. Chen and Y. Chen, Enhancing the non-enzymatic glucose detection performance of Ni(OH)2 nanosheets via defect engineering, Surf. Interfaces, 2021, 25, 101234 CrossRef CAS.
- X. Chen, X. He, J. Gao, J. Jiang, X. Jiang and C. Wu, Three-dimensional porous Ni, N-codoped C networks for highly sensitive and selective non-enzymatic glucose sensing, Sens. Actuators, B, 2019, 299, 126945 CrossRef CAS.
- W. Mao, H. He, Z. Ye and J. Huang, Three-dimensional graphene foam integrated with Ni(OH)2 nanosheets as a hierarchical structure for non-enzymatic glucose sensing, J. Electroanal. Chem., 2019, 832, 275–283 CrossRef CAS.
- E. M. Almutairi, M. A. Ghanem, A. Al-Warthan, M. R. Shaik, S. F. Adil and A. M. Almutairi, Chemical deposition and exfoliation from liquid crystal template: Nickel/nickel (II) hydroxide nanoflakes electrocatalyst for a non-enzymatic glucose oxidation reaction, Arabian J. Chem., 2022, 15, 103467 CrossRef CAS.
- F. Zhou, Y. Li, Y. Tang, F. Gao, W. Jing, Y. Du and F. Han, A novel flexible non-enzymatic electrochemical glucose sensor of excellent performance with ZnO nanorods modified on stainless steel wire sieve and stimulated via UV irradiation, Ceram. Int., 2022, 48, 14395–14405 CrossRef CAS.
- H. Shu, S. Peng, T. Lai, X. Cui, J. Ren, T. Chen, X. Xiao and Y. Wang, Nickel foam electrode decorated with Fe-CdIn2O4 nanoparticles as an effective electrochemical sensor for non-enzymatic glucose detection, J. Electroanal. Chem., 2022, 919, 116524 CrossRef CAS.
- Q. Dong, Z. He, X. Tang, Y. Zhang, L. Yang, K. Huang, X. Jiang and X. Xiong, One-step synthesis of Mn3O4@ZIF-67 on carbon cloth: As an effective non-enzymatic glucose sensor, Microchem. J., 2022, 175, 107203 CrossRef CAS.
- B. Zhang, L. Huang, X. Zhang, Y. Du, H. Sun, C. Jin, T. Zuo, L. He and W. Fa, Tantalum nitride nanotube structured electrode for non-enzymatic hydrogen peroxide sensing via photoelectrochemical route, J. Electroanal. Chem., 2022, 912, 116262 CrossRef CAS.
- L. He, J. Li, J. Cao, X. Li, X. Feng, J. Zhang and Y. Yang, High performance of non-enzymatic glucose biosensors based on the design of microstructure of Ni2P/Cu3P nanocomposites, Appl. Surf. Sci., 2022, 593, 153395 CrossRef CAS.
- Z. Rahmati, M. Roushani and H. Hosseini, Hierarchical hollow sea-urchin-like Ni–Co diselenide encapsulated in N-doped carbon networks as an advanced core-shell bifunctional electrocatalyst for fabrication of nonenzymatic glucose and hydrogen peroxide sensors, Sens. Actuators, B, 2020, 324, 128730 CrossRef CAS.
- S. Liu, B. Liu, C. Gong and Z. Li, A nanoporous Cu-Ag thin film at the Cu-Ag-Zn alloy surface by spontaneous dissolution of Zn and Cu in different degrees as a highly sensitive non-enzymatic glucose sensor, Electrochim. Acta, 2019, 320, 134599 CrossRef CAS.
- N. I. Chandrasekaran and M. Manickam, A sensitive and selective non-enzymatic glucose sensor with hollow Ni-Al-Mn layered triple hydroxide nanocomposites modified Ni foam, Sens. Actuators, B, 2019, 288, 188–194 CrossRef CAS.
- H. Chen, P. Sun, M. Qiu, M. Jiang, J. Zhao, D. Han, L. Niu and G. Cui, Co-P decorated nanoporous copper framework for high performance flexible non-enzymatic glucose sensors, J. Electroanal. Chem., 2019, 841, 119–128 CrossRef CAS.
- C. Duan and J. Zheng, Porous coralloid Polyaniline/SnO2-based enzyme-free sensor for sensitive and selective detection of nitrite, Colloids Surf., A, 2019, 567, 271–277 CrossRef CAS.
- A. Ghosh, D. Ganguly and R. Sundara, A new approach to in-situ uniform growth of Fe3O4 nanoparticles over thermally exfoliated rGO sheet for
the non-enzymatic and enzymatic detection of glucose, J. Electroanal. Chem., 2021, 895, 115386 CrossRef CAS.
- H. Kuivila, A. Keough and E. Soboczenski, Areneborates from diols and polyols, J. Org. Chem., 1954, 19, 780–783 CrossRef CAS.
- J. Lorand and J. Edwards, Polyol Complexes and Structure of the Benzeneboronate Ion, J. Org. Chem., 1959, 24(6), 769–774 CrossRef CAS.
- N. Liu, X. Xiang, M. Sun, P. Li, H. Qin, H. Liu, Y. Zhou, L. Wang, L. Wu and J. Zhu, Flexible hydrogel non-enzymatic QCM sensor for continuous glucose monitoring, Biosens. Bioelectron.: X, 2022, 10, 100110 CAS.
- S. Azad, M. Khosravi, A. Nikzad and S. K. Mishra, A novel contemporary molecular imprinting technique for non-enzymatic selective glucose detection, Opt. Laser Technol., 2022, 148, 107786 CrossRef CAS.
- T. Chen, A. Zhang, Y. Cheng, Y. Zhang, D. Fu, M. Liu, A. Li and J. Liu, A molecularly imprinted nanoreactor with spatially confined effect fabricated with nano-caged cascaded enzymatic system for specific detection of monosaccharides, Biosens. Bioelectron., 2021, 188, 113355 CrossRef CAS PubMed.
- T. Kubo and K. Otsuka, Recent progress in molecularly imprinted media by new preparation concepts and methodological approaches for selective separation of targeting compounds, TrAC, Trends Anal. Chem., 2016, 81, 102–109 CrossRef CAS.
- T. J. Ward and K. D. Ward, Chiral Separations: A Review of Current Topics and Trends, Anal. Chem., 2012, 84, 626–635 CrossRef CAS PubMed.
- L. Uzun and A. P. F. Turner, Molecularly-imprinted polymer sensors: realising their potential, Biosens. Bioelectron., 2016, 76, 131–144 CrossRef CAS PubMed.
- W. Zheng, H. Wu, Y. Jiang, J. Xu, X. Li, W. Zhang and F. Qiu, A molecularly-imprinted-electrochemical-sensor modified with nano-carbon-dots with high sensitivity and selectivity for rapid determination of glucose, Anal. Biochem., 2018, 555, 42–49 CrossRef CAS PubMed.
- D.-M. Kim, J.-M. Moon, W.-C. Lee, J.-H. Yoon, C. S. Choi and Y.-B. Shim, A potentiometric non-enzymatic glucose sensor using a molecularly imprinted layer bonded on a conducting polymer, Biosens. Bioelectron., 2017, 91, 276–283 CrossRef CAS PubMed.
- S. Alexander, P. Baraneedharan, S. Balasubrahmanyan and S. Ramaprabhu, Highly sensitive and selective non enzymatic electrochemical glucose sensors based on Graphene Oxide-Molecular Imprinted Polymer, Mater. Sci. Eng., C, 2017, 78, 124–129 CrossRef CAS PubMed.
- D. Wang, C. Zheng, Y. Li, C. Han, H. Fang, X. Fang and H. Zhao, Sensitive non-invasive electrochemical sensing of glucose in saliva using amorphous SnOx decorated one-dimensional CuO nanorods rich in oxygen vacancy defects, Appl. Surf. Sci., 2022, 592, 153349 CrossRef CAS.
- J. Zhu, S. Liu, Z. Hu, X. Zhang, N. Yi, K. Tang, M. G. Dexheimer, X. Lian, Q. Wang, J. Yang, J. Gray and H. Cheng, Laser-induced graphene non-enzymatic glucose sensors for on-body measurements, Biosens. Bioelectron., 2021, 193, 113606 CrossRef CAS PubMed.
- F. Vasiliou, A. K. Plessas, A. Economou, N. Thomaidis, G. S. Papaefstathiou and C. Kokkinos, Graphite paste sensor modified with a Cu(II)-complex for the enzyme-free simultaneous voltammetric determination of glucose and uric acid in sweat, J. Electroanal. Chem., 2022, 116393 CrossRef CAS.
- L. Garcia-Carmona, A. Martin, J. R. Sempionatto, J. R. Moreto, M. C. Gonzalez, J. Wang and A. Escarpa, Pacifier Biosensor: Toward Noninvasive Saliva Biomarker Monitoring, Anal. Chem., 2019, 91(21), 13883–13891 CrossRef CAS PubMed.
- Q. Liu, Y. Liu, F. Wu, X. Cao, Z. Li, M. Alharbi, A. N. Abbas, M. R. Amer and C. Zhou, Highly Sensitive and Wearable In2O3 Nanoribbon Transistor Biosensors with Integrated On-Chip Gate for Glucose Monitoring in Body Fluids, ACS Nano, 2018, 12, 1170–1178 CrossRef CAS PubMed.
- J. Zhang, Z. Kuang, H. Li, S. Li and F. Xia, Electrode surface roughness greatly enhances the sensitivity of electrochemical non-enzymatic glucose sensors, J. Electroanal. Chem., 2022, 919, 116541 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM, and the detection limit is 445 nM. Table 1 summarizes the glucose sensors based on Au and Pt in recent years and shows the key performance parameters such as sensitivity, detection range and minimum detection limit.
000 μM, and the detection limit is 445 nM. Table 1 summarizes the glucose sensors based on Au and Pt in recent years and shows the key performance parameters such as sensitivity, detection range and minimum detection limit.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 μA mM−1 cm−2) to glucose in the concentration range of 10 to 500 μM and an ultra-low detection limit (20 nM). It has a lower relative standard deviation when the sensor is used to detect glucose in iced black tea. Table 2 summarizes the Cu-based glucose sensors in the past three years and shows the key performance parameters in detail.
330 μA mM−1 cm−2) to glucose in the concentration range of 10 to 500 μM and an ultra-low detection limit (20 nM). It has a lower relative standard deviation when the sensor is used to detect glucose in iced black tea. Table 2 summarizes the Cu-based glucose sensors in the past three years and shows the key performance parameters in detail.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620
620![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760
760![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950
950
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 886 μA mM−1 cm−2, and the detection limit is 1.3 nM. Table 3 summarizes the Co-based glucose sensors in the past three years and shows the key performance parameters in detail.
886 μA mM−1 cm−2, and the detection limit is 1.3 nM. Table 3 summarizes the Co-based glucose sensors in the past three years and shows the key performance parameters in detail.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 886
886![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 171
171![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000
