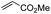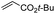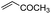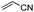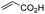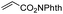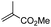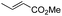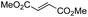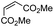Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Red-light-mediated Barton decarboxylation reaction and one-pot wavelength-selective transformations†
Hiroki
Yamamoto‡
,
Kohei
Yamaoka‡
,
Ann
Shinohara
,
Kouhei
Shibata
,
Ken-ichi
Takao
 * and
Akihiro
Ogura
* and
Akihiro
Ogura
 *
*
Department of Applied Chemistry, Keio University, Hiyoshi, Kohoku-ku, Yokohama, 223-8522, Japan. E-mail: takao@applc.keio.ac.jp; ogura@applc.keio.ac.jp
First published on 28th September 2023
Abstract
In organic chemistry, selecting mild conditions for transformations and saving energy are increasingly important for achieving sustainable development goals. Herein, we describe a red-light-mediated Barton decarboxylation using readily available red-light-emitting diodes as the energy source and zinc tetraphenylporphyrin as the catalyst, avoiding explosive or hazardous reagents or external heating. Mechanistic studies suggest that the reaction probably proceeds via Dexter energy transfer between the activated catalyst and the Barton ester. Furthermore, a one-pot wavelength-selective reaction within the visible light range is developed in combination with a blue-light-mediated photoredox reaction, demonstrating the compatibility of two photochemical transformations based on mechanistic differences. This one-pot process expands the limits of the decarboxylative Giese reaction beyond polarity matching.
Introduction
Visible-light-mediated organic transformations have progressed enormously in the past decade. In particular, photoredox chemistry has attracted widespread attention and various reactions have been developed.1–9 Photoredox chemistry depends on the electrochemical potential of the catalyst activated by light energy. Blue light or white light containing short wavelengths has generally been used for activation because the high energy of blue light (450 nm: 266 kJ mol−1) can easily meet the electron potential required for reactions to occur. However, blue light-emitting diode (LED) light sources need higher electric voltages compared with those with longer wavelengths, and there are also concerns about adverse health effects, for example, on circadian rhythms10 and the retina.11 Furthermore, the low penetration rate of short wavelength-light can cause problems, especially in scaling up reactions.In this context, red light has started to gather attention from organic chemists as a source of energy for organic reactions because of its safety. In addition to widely used singlet oxygen generation,12–16 red-light-mediated organic transformation involving the photoredox mechanism or intramolecular charge transfer have been reported.17–33 Very recently, we reported the red-light-mediated Barton–McCombie reaction.34 In the presence of chlorophyll a as the catalyst35 and tris(trimethylsilyl)silane or a Hantzsch ester as the hydrogen source, the methyl xanthate moiety was removed under red-light irradiation. The reaction mechanism was probably via the formation of a complex between the substrate and the photocatalyst, followed by charge transfer.36 The new conditions were safer and milder than the original conditions.37
Based on this previous work, we wondered whether wavelength-selective activation of a molecule could be achieved through sequential, preferably one-pot, visible-light irradiation. The narrow emission wavelength band of LEDs would be beneficial for activating the catalyst selectively, and thus we expected new chemical selectivity would be possible. Although this type of reaction selectivity has been achieved in polymer synthesis,38–41 its use in fine organic synthesis has been rare. Despite the recent achievement of one-pot white/blue sequential transformation,42 sequential chemical transformation by a specific wavelength of visible light in a one-pot reaction has not been reported. With numerous examples of blue-light-mediated redox reactions,1–9 we considered that to achieve selectivity, namely, that the red-light-mediated reaction should not affect the redox-active moiety, a red-light-triggered reaction not involving a redox mechanism would be preferable. Thus, energy transfer catalysis would be an attractive choice.43,44 Among the candidate reactions for exploring this idea, we decided to focus on the decarboxylative functionalization reaction under red-light irradiation because its blue light-mediated counterpart has been investigated extensively with many examples of redox reactions.1–9 Herein, we describe a red-light-mediated Barton decarboxylation reaction via an energy transfer mechanism and one-pot wavelength-selective decarboxylative functionalization reactions.
Results and discussion
Red-light-mediated decarboxylative reactions
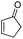
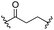
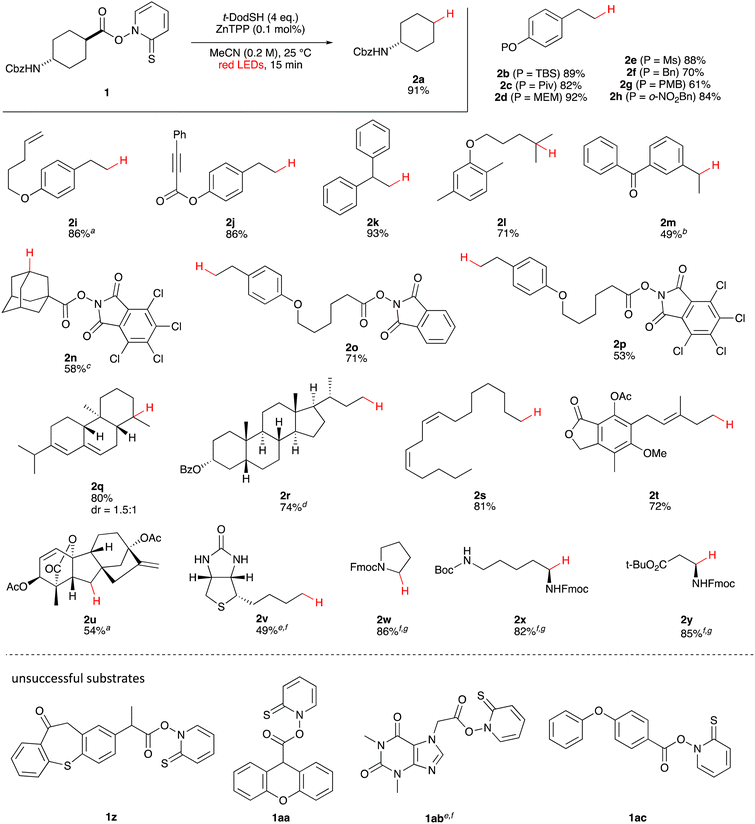
First, we established the simple red-light-mediated Barton decarboxylation reaction.45,46 Although high-energy UV light directly excites the substrate, the red-light-mediated reaction has not been reported. Thus, model compound 1a was irradiated with 4 W red LEDs in the presence of photocatalysts and a hydrogen source (see Table S1† for summary of optimization). The optimized conditions used 4 equiv of t-dodecanethiol as the hydrogen source, 0.1 mol% zinc tetraphenylporphyrin (ZnTPP)47,48 as the catalyst, 0.2 M acetonitrile and 15 min of red-light irradiation to provide decarboxylated product 2a. These conditions do not require an explosive azo reagent, a toxic organotin reagent, or energy-consuming heat or UV sources.49,50 Use of t-dodecanethiol is preferable, because unlike low molecular weight thiols, t-dodecanethiol is regarded as a low-odour reagent. Also, reaction time was much shorter than the direct excitation in the original study.45,46 Shorter irradiation times (15 min) and lower voltages than the conventional blue-light-mediated reaction (several hours to days) also save energy.Having optimized the reaction conditions, we investigated the substrate scope. Various Barton esters were irradiated with red LEDs in the presence of ZnTPP (Table 1). A range of hydroxy protecting groups were tolerated (2b to 2g), including a UV-labile o-nitrobenzyl group (2h).51,52 Terminal olefin (2i) and phenylpropiolate (2j) moieties were also compatible, and products via tertiary (2l) or benzyl radicals (2m) were obtained in moderate yields. Notably redox-active phthalimide esters53–55 were stable under the conditions (2n to 2p). A wide variety of biology-related carboxylic acids were also smoothly converted to the corresponding decarboxylated products with an occasional slight modification (2q to 2u). Barton ester derivatives from biotin and amino acids were unstable to silica gel purification,56 but the one-pot conversion from the carboxylic acid afforded decarboxybiotin and amines (2v to 2y). In contrast, substrates that generated certain benzyl or phenyl radicals gave unsatisfactory results (1z to 1ac); the high electronic stability of benzyl radicals with an electron-donating group lead to low hydrogen atom transfer reactivity against thiol, which resulted in side reactions, such as homodimerization or ketone formation.57 For benzoate, hydrogenation with thiol occurred before decarboxylation at ambient temperature.58
We then investigated decarboxylative functionalization reactions.59 Model Barton ester 3 was irradiated with red LEDs in the presence of ZnTPP and various reagents (Table 2, see Tables S2 to S10† for optimization). Halogenation (4a–4c),60 oxygenation (4d),61 nitrogenation (4e62 and 4f63,64), sulfidation (4g),65,66 selenidation (4h),65,66 and borylation (4i)67,68 all worked well, and afforded the corresponding functionalized products in good yields. However, decarboxylative fluorination69 was not possible due to the incompatibility of Barton esters with fluorine sources.
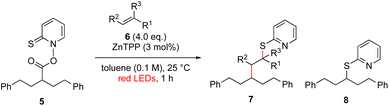
Another important reaction using a decarboxylated radical intermediate is the Giese reaction, which is a radical-mediated conjugate addition.70–72 The decarboxylative Giese reaction has been a focus of research64,73–77 because it enables convenient chemical bond recombination beyond the classical synthon approach. Thus, Barton ester 5 was treated with various unsaturated carbonyl compounds (6) in the presence of ZnTPP and red-light irradiation (Table 3, see Table S11† for optimization). Acrylates (entries 1 and 2), vinyl ketone (entry 3), and acrylonitrile (entry 4) all afforded corresponding conjugate addition products 7 in moderate to good yields, with pyridyl sulfide 8 as a minor product. The highest yield was obtained with a simple acrylic acid as the radical acceptor (entry 5), whereas the redox-active phthalimidyl ester only gave a small amount of 8 (entry 6). Substitution generally decreased the yield.70 Methyl methacrylate afforded the Giese product in low yield (entry 7), and crotonate did not provide 7 (entry 8). Extra addition of various Lewis acids78 did not improve the yields. Whereas fumarate and maleate with a lower LUMO afforded 7 in moderate yield (entries 9 and 10), cyclic ketones gave 8 as the major product (entries 11 and 12).
One-pot wavelength-selective transformations
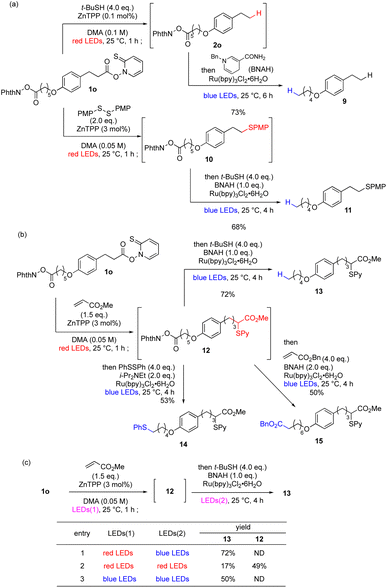
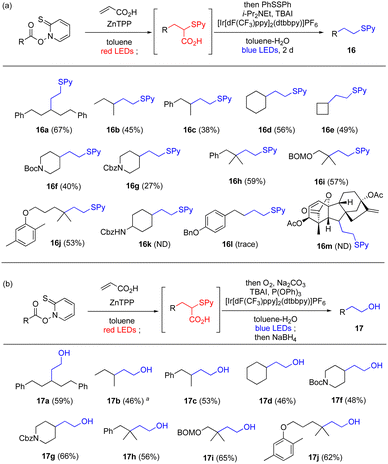
Having established red-light-mediated transformations, we moved on to wavelength-selective sequential transformations (Scheme 1a). First, we performed a parallel reaction, in which one molecule with two photoactivated functional groups was transformed sequentially in a wavelength-selective one-pot reaction. To differentiate the two photoreactive functional groups, 1o was selected as the substrate, which carries a red-light-reactive Barton ester moiety and a blue-light-reactive phthalimidyl ester moiety. Treatment with t-butyl mercaptan and ZnTPP under red-light irradiation afforded 2o as the intermediary product, as in Table 1. Subsequently, 1-benzyl-1,4-dihydronicotinamide (BNAH) and a ruthenium complex were added to the reaction mixture and it was irradiated by blue light.79 Photoredox decarboxylation proceeded smoothly to provide 9 in good overall yield. Similarly, red-light-mediated decarboxylative sulfidation provided intermediate 10, which upon blue-light-mediated decarboxylation afforded 11 in good overall yield. Importantly, the presence of ZnTPP did not affect the reactivity of the ruthenium catalyst, and the bisulfide was not observed. Next, 1o was subjected to red-light-mediated Giese reaction with methyl acrylate to afford intermediary product 12 (Scheme 1b). One-pot photoredox decarboxylation with blue light gave expected product 13 in 72% yield. Furthermore, blue-light-mediated decarboxylative sulfidation80 or another Giese reaction81 of 12 proceeded to give 14 and 15, respectively, thus achieving chemoselective functionalization based on one-pot wavelength-selective photoactivation (more examples in Scheme S1†). To prove the wavelength dependence of these transformations, 1o was converted to 13 with different combinations of light wavelengths (Scheme 1c). Optimized red-blue irradiation afforded 13 in 72% yield (entry 1). However, red–red (entry 2) or blue–blue irradiation (entry 3) decreased the yield greatly and increased the amount of byproducts, which may be explained by insufficient activation or uncontrolled attack by the catalyst, respectively, indicating that activation of photocatalyst with appropriate wavelength is necessary for optimum performance.In addition to the parallel reactions, a sequential series reaction was investigated (Table 4a). We define the series reaction as a one-pot reaction82 in which the photoactivated functional group is converted into another photoactivated functional group, which is then subjected to another photoreaction. Because 5 could be converted to carboxylic acid 7e by our red-light-mediated Giese reaction with acrylic acid (Table 3), optimization of the subsequent decarboxylation was first performed based on the conditions developed by MacMillan group83 and Nicewicz group84 (see Table S12† for detail). A biphasic system with the addition of tetrabutylammonium iodide as a phase transfer catalyst greatly improved the yield of this single reaction, and gave 16a in 95% yield. When the two reactions were performed as a one-pot reaction, desired product 16a was obtained in satisfactory overall yield of 67% (see Table S13† for optimization).
| a GC yield. |
|---|
|
The Giese reaction and decarboxylative oxygenation were chosen for a further example of a series reaction. First, the decarboxylative oxygenation was optimized independently (see Table S14† for optimization). Thus, 7e was subjected to conditions established by MacMillan group,85 and reductive treatment afforded alcohol 17a. The reaction likely involved the decarboxylative formation of the thioester,85 followed by additional reduction (detailed proposed mechanism in Scheme S2†).86 The addition of phosphine increased the yield, which suggested that phosphine-mediated heterolytic cleavage of the hydroperoxide moiety converted the peroxide to the corresponding aldehyde by expelling pyridine thiol (see Scheme S3†). This reaction can be conducted as a wavelength-selective one-pot reaction with the red-light-mediated Barton decarboxylative Giese reaction without major modification (Table 4b).
Under the conditions to give 16a and 17a described above, the substrate scope for this one-pot wavelength-selective transformation was studied (Table 4). For the Giese reaction/decarboxylation reaction, a wide variety of Barton esters that give secondary or tertiary radicals afforded the corresponding thioethers generally in medium to good yields (Table 4a, 16a–16k). However, in some cases, the complexation of the reaction system (16g) or insufficient substrate solubility (16k) led to unsatisfactory results. Substrates without α-carbon substitution (16l) or with high steric congestion (16m) did not afford the desired products because of an unproductive Giese reaction. The Giese reaction/decarboxylative oxygenation also proceeded, affording the corresponding alcohols in satisfactory yields (Table 4b, 17a–17j). It should be emphasized that the products obtained by these one-pot sequences are hard to construct via normal Giese reactions using vinyl sulfides or enol equivalents because they are polarity mismatched. This one-pot procedure enables polarity switching,87 broadening the utility of the Giese reaction.
Mechanistic analysis
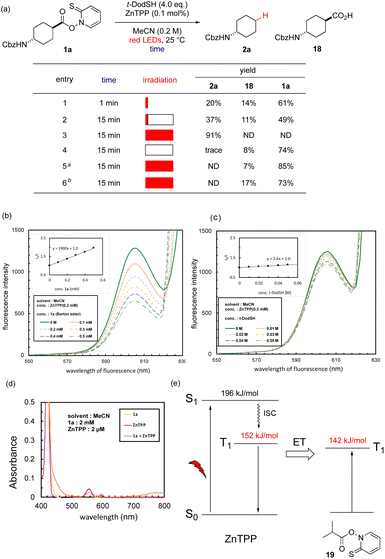
We performed several experiments to elucidate the mechanism of the red-light-mediated reaction. First, the reaction with 1a was run for only 1 min and immediately quenched, which gave 20% of 2a (entry 1, Fig. 1a). Irradiating the reaction flask for 1 min, and then stirring for another 14 min increased the yield to 37% (entry 2). This observation shows that the reaction involved a chain mechanism, which proceeded autonomously after initiation. However, comparison with entry 3 (irradiation time: 15 min) suggests that the chain length was not long enough for the reaction to be completed. The irradiation, catalyst, and thiol were all essential for the reaction because the removal of any of these factors resulted in a total loss of yield (entries 4–6).Stern–Volmer quenching experiments were performed for ZnTPP in the presence of 1a (Fig. 1b) or t-dodecanethiol (Fig. 1c). Although t-dodecanethiol was not involved in fluorescence quenching of ZnTPP, 1a showed efficient quenching. Photon flow was calculated according to the procedure for the red-light range,88 and the quantum yield was calculated to be 62, which strongly supports the chain mechanism. The chain length was estimated to be 63 (see ESI† for details).89
The following five mechanisms for radical generation could be proposed: (i) a redox pathway, where photoactivated ZnTPP in the S1 or T1 state reduces the Barton ester; (ii) triplet–triplet annihilation of ZnTPP to form S2 state species, which reduce or sensitize the Barton ester; (iii) Förster energy transfer, where S1 state ZnTPP passes energy to the Barton ester and activates it to the S1 state; or (iv) Dexter energy transfer, where T1 state ZnTPP exchanges electrons with the Barton ester to generate the T1 state; (v) excitation of complex between Barton ester and ZnTPP followed by charge transfer.
First, a single-electron transfer pathway was considered. The reduction potential of 1a was measured as −1.76 V vs. saturated calomel electrode (SCE) in acetonitrile by differential pulse voltammetry. The redox potentials of singlet activated state and triplet state ZnTPP are −1.36 and −0.88 V (vs. SCE),90,91 respectively, according to the Rehm–Weller equation. These data suggest that a simple photoredox pathway is not feasible.
Castellano and coworkers recently showed that triplet–triplet annihilation of ZnTPP produces the high-energy S2 state of ZnTPP, which triggers polymerization.91 In their experiment, ZnTPP was excited to the S1 state, and S2 Soret-band fluorescence generated through triplet–triplet annihilation was quenched by an acrylate, whereas the S1 Q-band emission remained intact. However, in our system, the Q-band emission was quenched by the Barton ester, which suggests that a completely different mechanism occurred. Furthermore, redox-active esters remained intact in the red-light-mediated reaction (e.g., 2n, 2o, 2p in Table 1); these functional groups are expected to be more easily reduced by a photoredox catalyst (E1/2 = −1.20 V vs. SCE for phthalimidyl92 and E1/2 = −0.79 V vs. SCE for tetrachlorophthalimidyl93) than the Barton ester. The chemoselective reaction of the Barton ester moiety suggests that there is a different pathway from the redox mechanism. In addition, ZnTPP remained intact in the reaction mixture (see ESI†), which suggests that an irreversible redox pathway is unlikely. Thus, although we cannot completely rule out a limited occurrence of the triplet–triplet annihilation pathway, there was sufficient evidence to suggest another mechanism occurred.
Förster energy transfer is not feasible due to absence of large absorption peak in the red or near-infrared region (Fig. 1d). Thus, we focused on Dexter energy transfer.94–97 In this mechanism, electron exchange occurs between an excited sensitizer and the substrate. This type of reaction is known to be solvent-independent, which we observed during our optimization (Table S1†). For this reaction to occur, the T1 energy of the sensitizer (152 kJ mol−1 for ZnTPP)90 should be larger than that of the acceptor. We performed a DFT calculation94 for model Barton ester 19, and the T1 state was above the S0 state by 142 kJ mol−1, which was ideal for the Dexter energy transfer mechanism to happen (Fig. 1e). Although a red-light-triggered energy transfer mechanism has been proposed between pheophorbide a and dithiocarbonate, the thermodynamic validity has not been discussed.39 Usually, Dexter energy transfer occurs from triplet state, and the singlet excited state is not involved. This should result in no change in fluorescence, whereas we observed quenching of fluorescence by the Barton ester (Fig. 1b). This phenomenon can be interpreted as an external heavy-atom effect98 from the sulfur atom of the Barton ester that facilitates intersystem crossing to the T1 state, which appears as formal quenching of Soret-band fluorescence. This mechanism is supported by the partial formation of a complex between ZnTPP and Barton ester 19 because a slight change in the absorption spectrum (460–500 nm, >720 nm) is observed when ZnTPP and 19 coexist (Fig. 1d). On the other hand, direct excitation of this complex, the fifth possibility, is unlikely: we performed calculation on complex between ZnTPP and 19, which revealed that HOMO–LUMO energy gap was 260 kJ mol−1, corresponding to 459 nm of light, which cannot be covered by red-light range.
Conclusions
We have developed a red-light-mediated Barton decarboxylation reaction via Dexter energy transfer. The reaction was characterized by remarkably mild conditions, absence of hazardous materials, and low energy consumption. A number of related decarboxylative conversions, including new carbon–carbon bond formation, were also achieved in good yield. Furthermore, one-pot wavelength-selective transformation within the visible light region was achieved by using blue and red light, which contributed to polarity switching and broadens the scope of the Giese reaction. Further research on red light as a reaction energy source is currently underway in our laboratory.Data availability
All supporting data is provided in the ESI.†Author contributions
A. O. conceived the project. H. Y., K. Y., A. S., and K. S. performed the experimental studies. A. O. performed computational study. K. T. and A. O. prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by JSPS KAKENHI Grant Number 19K15571 and 22K05100, Tobe Maki Scholarship Foundation, and Keio Gijuku Academic Development Funds. We acknowledge Prof. Yasushi Katayama, Dr Nobuyuki Serizawa, and Mr Shodai Kato at Keio University for electrochemical measurements. We thank Prof. Fumitoshi Kakiuchi, Prof. Takuya Kochi, and Mr Kazuma Muto at Keio University for gas chromatography.Notes and references
- J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC.
- J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed.
- M. A. Ischay and T. P. Yoon, Eur. J. Org. Chem., 2012, 3359 CrossRef CAS.
- M. Reckenthäler and A. G. Griesbeck, Adv. Synth. Catal., 2013, 355, 2727 CrossRef.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed.
- Y. Xi, H. Yi and A. Lei, Org. Biomol. Chem., 2013, 11, 2387 RSC.
- K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035 CrossRef CAS PubMed.
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef CAS PubMed.
- M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898 CrossRef CAS PubMed.
- T. A. LeGates, D. C. Fernandez and S. Hattar, Nat. Rev. Neurosci., 2014, 15, 443 CrossRef CAS PubMed.
- F. Behar-Cohen, C. Martinsons, F. Viénot, G. Zissis, A. Barlier-Salsi, J. P. Cesarini, O. Enouf, M. Garcia, S. Picaud and D. Attia, Prog. Retinal Eye Res., 2011, 30, 239 CrossRef CAS PubMed.
- M. Prein and W. Adam, Angew Chem. Int. Ed. Engl., 1996, 35, 477 CrossRef CAS.
- A. Greer, Acc. Chem. Res., 2006, 39, 797 CrossRef CAS PubMed.
- T. Montagnon, M. Tofi and G. Vassilikogiannakis, Acc. Chem. Res., 2008, 41, 1001 CrossRef CAS PubMed.
- A. A. Ghogare and A. Greer, Chem. Rev., 2016, 116, 9994 CrossRef CAS PubMed.
- I. Pibiri, S. Buscemi, A. P. Piccionello and A. Pace, ChemPhotoChem, 2018, 2, 535 CrossRef CAS.
- J. Lee, J. W. Papatzimas, A. D. Bromby, E. Gorobets and D. J. Derksen, RSC Adv., 2016, 6, 59269 RSC.
- S. Kim, G. Park, E. J. Cho and Y. You, J. Org. Chem., 2016, 81, 7072 CrossRef CAS PubMed.
- K. Matsuzaki, T. Hiromura, E. Tokunaga and N. Shibata, ChemistryOpen, 2017, 6, 226 CrossRef CAS PubMed.
- X. Wang and J. A. Kalow, Org. Lett., 2018, 20, 1716 CrossRef CAS PubMed.
- D. E. Yerien, M. V. Cooke, M. C. García Vior, S. Barata-Vallejo and A. Postigo, Org. Biomol. Chem., 2019, 17, 3741 RSC.
- D. E. Yerien, S. Barata-Vallejo, E. W. Mora Flores and A. Postigo, Catal. Sci. Technol., 2020, 10, 5113 RSC.
- L. Mei, J. M. Veleta and T. L. Gianetti, J. Am. Chem. Soc., 2020, 142, 12056 CrossRef CAS PubMed.
- Z. Shen, S. Zheng, S. Xiao, R. Shen, S. Liu and J. Hu, Angew. Chem., Int. Ed., 2021, 60, 20452 CrossRef CAS PubMed.
- Y. Ishikawa, T. Kameyama, T. Torimoto, H. Maeda, M. Segi and T. Furuyama, Chem. Commun., 2021, 57, 13594 RSC.
- N. Toriumi, T. Inoue and N. Iwasawa, J. Am. Chem. Soc., 2022, 144, 19592 CrossRef CAS PubMed.
- S. L. Goldschmid, N. E. S. Tay, C. L. Joe, B. C. Lainhart, T. C. Sherwood, E. M. Simmons, M. Sezen-Edmonds and T. Rovis, J. Am. Chem. Soc., 2022, 144, 22409 CrossRef CAS PubMed.
- F. Glaser and O. S. Wenger, JACS Au, 2022, 2, 1488 CrossRef CAS PubMed.
- P. Yan, R. Zeng, B. Bao, X.-M. Yang, L. Zhu, B. Pan, S.-L. Niu, X.-W. Qi, Y.-L. Li and Q. Ouyang, Green Chem., 2022, 24, 9263 RSC.
- B. F. Buksh, S. D. Knutson, J. V. Oakley, N. B. Bissonnette, D. G. Oblinsky, M. P. Schwoerer, C. P. Seath, J. B. Geri, F. P. Rodriguez-Rivera, D. L. Parker, G. D. Scholes, A. Ploss and D. W. C. MacMillan, J. Am. Chem. Soc., 2022, 144, 6154 CrossRef CAS PubMed.
- K. Rybicka-Jasińska, T. Wdowik, K. Łuczak, A. J. Wierzba, O. Drapała and D. Gryko, ACS Org. Inorg. Au, 2022, 2, 422 CrossRef PubMed.
- F. Glaser and O. S. Wenger, Chem. Sci., 2023, 14, 149 RSC.
- L. Zeng, L. Huang, W. Lin, L.-H. Jiang and G. Han, Nat. Commun., 2023, 14, 1102 CrossRef CAS PubMed.
- A. Ogura, N. Ichii, K. Shibata and K. Takao, Bull. Chem. Soc. Jpn., 2020, 93, 936 CrossRef CAS.
- S. Shanmugam, J. Xu and C. Boyer, Chem. Sci., 2015, 6, 1341 RSC.
- P. Seal, J. Xu, S. De Luca, C. Boyer and S. C. Smith, Adv. Theory Simul., 2019, 2, 1900038 CrossRef.
- D. H. R. Barton and S. W. McCombie, J. Chem. Soc., Perkin Trans. 1, 1975, 1574 RSC.
- A. Ohtsuki, L. Lei, M. Tanishima, A. Goto and H. Kaji, J. Am. Chem. Soc., 2015, 137, 5610 CrossRef CAS PubMed.
- J. Xu, S. Shanmugam, C. Fu, K.-F. Aguey-Zinsou and C. Boyer, J. Am. Chem. Soc., 2016, 138, 3094 CrossRef CAS PubMed.
- L. Delafresnaye, K. Jung, C. Boyer and C. Barner-Kowollik, Polym. Chem., 2020, 11, 6453 RSC.
- A. Bagheri, C. M. Fellows and C. Boyer, Adv. Sci., 2021, 8, 2003701 CrossRef CAS PubMed.
- D. Kalaitzakis, A. Bosveli, K. Sfakianaki, T. Montagnon and G. Vassilikogiannakis, Angew. Chem., Int. Ed., 2021, 60, 4335 CrossRef CAS PubMed.
- F. Strieth-Kalthoff, M. J. James, M. Teders, L. Pitzer and F. Glorius, Chem. Soc. Rev., 2018, 47, 7190 RSC.
- T. Patra, S. Mukherjee, J. Ma, F. Strieth-Kalthoff and F. Glorius, Angew. Chem., Int. Ed., 2019, 58, 10514 CrossRef CAS PubMed.
- D. H. R. Barton, D. Crich and W. B. Motherwell, J. Chem. Soc., Chem. Commun., 1983, 939 RSC.
- D. H. R. Barton and G. Kretzschmar, Tetrahedron Lett., 1983, 24, 5889 CrossRef CAS.
- H. Cao, G. Wang, Y. Xue, G. Yang, J. Tian, F. Liu and W. Zhang, ACS Macro Lett., 2019, 8, 616 CrossRef CAS PubMed.
- R. Costa e Silva, L. O. da Silva, A. de Andrade Bartolomeu, T. J. Brocksom and K. T. de Oliveira, Beilstein J. Org. Chem., 2020, 16, 917 CrossRef PubMed.
- T. Constantin, M. Zanini, A. Regni, N. S. Sheikh, F. Juliá and D. Leonori, Science, 2020, 367, 1021 CrossRef CAS PubMed.
- T. Constantin, B. Górski, M. J. Tilby, S. Chelli, F. Juliá, J. Llaveria, K. J. Gillen, H. Zipse, S. Lakhdar and D. Leonori, Science, 2022, 377, 1323 CrossRef CAS PubMed.
- U. Zehavi, B. Amit and A. Patchornik, J. Org. Chem., 1972, 37, 2281 CrossRef CAS.
- U. Zehavi and A. Patchornik, J. Org. Chem., 1972, 37, 2285 CrossRef CAS.
- K. Okada, K. Okamoto and M. Oda, J. Am. Chem. Soc., 1988, 110, 8736 CrossRef CAS.
- K. Okada, K. Okamoto, N. Morita, K. Okubo and M. Oda, J. Am. Chem. Soc., 1991, 113, 9401 CrossRef CAS.
- T. Qin, J. Cornella, C. Li, L. R. Malins, J. T. Edwards, S. Kawamura, B. D. Maxwell, M. D. Eastgate and P. S. Baran, Science, 2016, 352, 801 CrossRef CAS PubMed.
- C. Commandeur, C. Chalumeau, J. Dessolin and M. Laguerre, Eur. J. Org. Chem., 2007, 3045 CrossRef CAS.
- E. J. Ko, G. P. Savage, C. M. Williams and J. Tsanaktsidis, Org. Lett., 2011, 13, 1944 CrossRef CAS PubMed.
- D. H. R. Barton, B. Lacher and S. Z. Zard, Tetrahedron, 1987, 43, 4321 CrossRef CAS.
- Z. Zeng, A. Feceu, N. Sivendran and L. J. Gooßen, Adv. Synth. Catal., 2021, 363, 2678 CrossRef CAS.
- D. H. R. Barton, D. Crich and W. B. Motherwell, Tetrahedron Lett., 1983, 24, 4979 CrossRef CAS.
- D. H. R. Barton, D. Crich and W. B. Motherwell, J. Chem. Soc., Chem. Commun., 1984, 242 RSC.
- P. Girard, N. Guillot, W. B. Motherwell, R. S. Hay-Motherwell and P. Potier, Tetrahedron, 1999, 55, 3573 CrossRef CAS.
- L. Chen, C. S. Chao, Y. Pan, S. Dong, Y. C. Teo, J. Wang and C.-H. Tan, Org. Biomol. Chem., 2013, 11, 5922 RSC.
- Y. Miyake, K. Nakajima and Y. Nishibayashi, Chem. Commun., 2013, 49, 7854 RSC.
- D. H. R. Barton, D. Bridon and S. Z. Zard, Tetrahedron Lett., 1984, 25, 5777 CrossRef CAS.
- D. H. R. Barton, D. Bridon and S. Z. Zard, Heterocycles, 1987, 25, 449 CrossRef CAS.
- C. Li, J. Wang, L. M. Barton, S. Yu, M. Tian, D. S. Peters, M. Kumar, A. W. Yu, K. A. Johnson, A. K. Chatterjee, M. Yan and P. S. Baran, Science, 2017, 356, eaam7355 CrossRef PubMed.
- A. Fawcett, J. Pradeilles, Y. Wang, T. Mutsuga, E. L. Myers and V. K. Aggarwal, Science, 2017, 357, 283 CrossRef CAS PubMed.
- S. Ventre, F. R. Petronijevic and D. W. C. MacMillan, J. Am. Chem. Soc., 2015, 137, 5654 CrossRef CAS PubMed.
- B. Giese and J. Meister, Chem. Ber., 1977, 110, 2588 CrossRef CAS.
- B. Giese and J. Meister, Angew Chem. Int. Ed. Engl., 1977, 16, 178 CrossRef.
- G. S. C. Srikanth and S. L. Castle, Tetrahedron, 2005, 61, 10377 CrossRef CAS.
- Y. Yoshimi, S. Washida, Y. Okita, K. Nishikawa, K. Maeda, S. Hayashi and T. Morita, Tetrahedron Lett., 2013, 54, 4324 CrossRef CAS.
- L. Chu, C. Ohta, Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 10886 CrossRef CAS PubMed.
- T. Chinzei, K. Miyazawa, Y. Yasu, T. Koike and M. Akita, RSC Adv., 2015, 5, 21297 RSC.
- T. Patra, P. Bellotti, F. Strieth-Kalthoff and F. Glorius, Angew. Chem., Int. Ed., 2020, 59, 3172 CrossRef CAS PubMed.
- A. L. Gant Kanegusuku and J. L. Roizen, Angew. Chem., Int. Ed., 2021, 60, 21116 CrossRef CAS PubMed.
- M. P. Sibi and J. Ji, J. Am. Chem. Soc., 1996, 118, 3063 CrossRef CAS.
- K. Okada, K. Okubo, N. Morita and M. Oda, Tetrahedron Lett., 1992, 33, 7377 CrossRef CAS.
- Z. Xiao, L. Wang, J. Wei, C. Ran, S. H. Liang, J. Shang, G.-Y. Chen and C. Zheng, Chem. Commun., 2020, 56, 4164 RSC.
- J. Schwarz and B. König, Green Chem., 2016, 18, 4743 RSC.
- Z. Wu, M. Wu, K. Zhu, J. Wu and Y. Lu, Chem, 2023, 9, 978 CAS.
- Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle and D. W. C. MacMillan, Science, 2014, 345, 437 CrossRef CAS PubMed.
- J. D. Griffin, M. A. Zeller and D. A. Nicewicz, J. Am. Chem. Soc., 2015, 137, 11340 CrossRef CAS PubMed.
- T. M. Faraggi, W. Li and D. W. C. MacMillan, Isr. J. Chem., 2020, 60, 410 CrossRef CAS.
- B. Kang and S. H. Hong, Chem. Sci., 2017, 8, 6613 RSC.
- S. Paul, D. Filippini and M. Silvi, J. Am. Chem. Soc., 2023, 145, 2773 CrossRef CAS PubMed.
- H.-J. Adick, R. Schmidt and H.-D. Brauer, J. Photochem. Photobiol., A, 1989, 49, 311 CrossRef CAS.
- M. A. Cismesia and T. P. Yoon, Chem. Sci., 2015, 6, 5426 RSC.
- M. Otake, M. Itou, Y. Araki, O. Ito and H. Kido, Inorg. Chem., 2005, 44, 8581 CrossRef CAS PubMed.
- N. Awwad, A. T. Bui, E. O. Danilov and F. N. Castellano, Chem, 2020, 6, 3071 CAS.
- K. N. Tripathi, M. Belal and R. P. Singh, J. Org. Chem., 2020, 85, 1193 CrossRef CAS PubMed.
- Y. Zhang and D. Zhang, Org. Biomol. Chem., 2020, 18, 4479 RSC.
- F. Strieth-Kalthoff, M. J. James, M. Teders, L. Pitzer and F. Glorius, Chem. Soc. Rev., 2018, 47, 7190 RSC.
- Q.-Q. Zhou, Y.-Q. Zou, L.-Q. Lu and W.-J. Xiao, Angew. Chem., Int. Ed., 2019, 58, 1586 CrossRef CAS PubMed.
- N. Corrigan, J. Xu, C. Boyer and X. Allonas, ChemPhotoChem, 2019, 3, 1193 CrossRef CAS.
- J. Christmann, A. Ibrahim, V. Charlot, C. Croutxé-Barghorn, C. Ley and X. Allonas, ChemPhysChem, 2016, 2309 CrossRef CAS PubMed.
- G. G. Giachino and D. R. Kearns, J. Chem. Phys., 1970, 52, 2964 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc03643j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: