Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tellurolate: an effective Te-atom transfer reagent to prepare the triad of group 5 metal bis(tellurides)†
Shuruthi
Senthil
a,
Seongyeon
Kwon
bc,
Richard Y.
Kong
 d,
Samantha N.
MacMillan
d,
Samantha N.
MacMillan
 d,
Pavel
Zatsepin
d,
Pavel
Zatsepin
 a,
Michael R.
Gau
a,
Patrick J.
Carroll
a,
Mu-Hyun
Baik
a,
Michael R.
Gau
a,
Patrick J.
Carroll
a,
Mu-Hyun
Baik
 *cb,
Kyle M.
Lancaster
*cb,
Kyle M.
Lancaster
 *d and
Daniel J.
Mindiola
*d and
Daniel J.
Mindiola
 *a
*a
aDepartment of Chemistry, University of Pennsylvania, Philadelphia, PA 19104, USA. E-mail: mindiola@sas.upenn.edu
bDepartment of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea. E-mail: mbaik2805@kaist.ac.kr
cCenter for Catalytic Hydrocarbon Functionalizations, Institute for Basic Science (IBS), Daejeon 34141, Republic of Korea
dDepartment of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853, USA. E-mail: kml236@cornell.edu
First published on 5th October 2023
Abstract
We show in this work how lithium tellurolate Li(X)nTeCH2SiMe3 (X = THF, n = 1, 1; X = 12-crown-4, n = 2, 2), can serve as an effective Te-atom transfer reagent to all group 5 transition metal halide precursors irrespective of the oxidation state. Mononuclear and bis(telluride) complexes, namely (PNP)M(Te)2 (M = V; Nb, 3; Ta, 4; PNP− = N[2-PiPr2-4-methylphenyl]2), are reported herein including structural and spectroscopic data. Whereas the known complex (PNP)V(Te)2 can be readily prepared from the trivalent precursor (PNP)VCl2, two equiv. of tellurolate, and elemental Te partially solubilized with PMe3, complex 3 can also be similarly obtained following the same procedure but with or without a reductant, Na/NaCl. Complex 4 on the other hand is formed from the addition of four equiv. of tellurolate to (PNP)TaF4. Having access to a triad of (PNP)M(Te)2 systems for group 5 metals has allowed us to compare them using a combination of theory and spectroscopy including Te-L1 edge XANES data.
Introduction
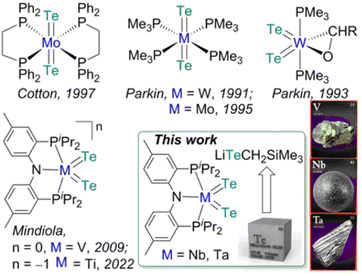
In comparison to the development of transition metal complexes with multiple bonds to the lighter congeners of group 16 elements such as oxides and sulfides, analogous examples of the heavier elements, selenides and tellurides, remain far more underdeveloped.1 The tendency of these heavy elements to bridge or catenate,1b their large difference in σ- and π-bond energies (which results in their weak π overlap to transition metals),1a and the lack of soluble and convenient synthetic routes to deliver these atoms render such moieties LnM![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X (X = Se, Te) difficult to access in well-defined complexes.1a,b Furthermore, metal complexes with two terminal telluride ligands (bis(tellurides)) are even rarer with the few structurally characterized examples mostly being confined to group 6 metals: Mo(L)2(Te)2 (L = dppee, dppe),2 M(PMe3)4(Te)2 (M = W,3 Mo4) and W(PMe3)2(Te)2(η2-OCHR) (R = H, Ph)5 (top, Fig. 1). Previously, we reported the synthesis and reactivity of the first 3d transition metal bis(telluride), (PNP)V(Te)2 (PNP− = N[2-PiPr2-4-methylphenyl]2, bottom, Fig. 1), from (PNP)V(CH2tBu)2 6 and Te, and showed how the bis(telluride) complex was a masked form of “(PNP)V” fragment in the presence of various oxidants.7 More recently, we synthesized a bis(telluride) titanate,
X (X = Se, Te) difficult to access in well-defined complexes.1a,b Furthermore, metal complexes with two terminal telluride ligands (bis(tellurides)) are even rarer with the few structurally characterized examples mostly being confined to group 6 metals: Mo(L)2(Te)2 (L = dppee, dppe),2 M(PMe3)4(Te)2 (M = W,3 Mo4) and W(PMe3)2(Te)2(η2-OCHR) (R = H, Ph)5 (top, Fig. 1). Previously, we reported the synthesis and reactivity of the first 3d transition metal bis(telluride), (PNP)V(Te)2 (PNP− = N[2-PiPr2-4-methylphenyl]2, bottom, Fig. 1), from (PNP)V(CH2tBu)2 6 and Te, and showed how the bis(telluride) complex was a masked form of “(PNP)V” fragment in the presence of various oxidants.7 More recently, we synthesized a bis(telluride) titanate, 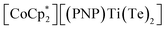 (bottom left, Fig. 1) starting from the trivalent bis(alkyl) complex,8 and probed its ability to act as a metallo bis(telluride) ligand.9
(bottom left, Fig. 1) starting from the trivalent bis(alkyl) complex,8 and probed its ability to act as a metallo bis(telluride) ligand.9
Since transition metal bis(telluride) motifs remain quite scant and given our breakthrough with 3d metals, vanadium and titanium,7,9 we inquired if such functionality can be extended down the group to niobium and tantalum by using a similar protocol. Unfortunately, the respective low-valent niobium or tantalum hypothetical bis or trisalkyl complexes are inherently unstable, and in the case of Nb, these ligands readily undergo disproportionation and/or β-hydrogen abstraction/hydrogen elimination reactions10 resulting in the bis(alkylidene) complex, (PNP)Nb(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHtBu)2.10b Therefore, we decided to explore a systematic approach to delivering the Te atom that would work irrespective of the oxidation state of the metal since the stability of group 5 transition metal halide precursors tend to differ depending on their periodic trends. For instance, VIII chloride (e.g., VCl3(THF)3) is a common starting material, whereas NbCl4(THF)2/NbCl5 and TaCl5 are routinely used for heavier congeners. Inspired by the decomposition of M(TeR)n to [MTe]n from solid state thermolysis,11 we hypothesized that terminal metal bis(telluride) ligands could likewise be assembled via the elimination of telluroethers,11a,b,12 akin to how Arnold and co-workers prepared a Ta
CHtBu)2.10b Therefore, we decided to explore a systematic approach to delivering the Te atom that would work irrespective of the oxidation state of the metal since the stability of group 5 transition metal halide precursors tend to differ depending on their periodic trends. For instance, VIII chloride (e.g., VCl3(THF)3) is a common starting material, whereas NbCl4(THF)2/NbCl5 and TaCl5 are routinely used for heavier congeners. Inspired by the decomposition of M(TeR)n to [MTe]n from solid state thermolysis,11 we hypothesized that terminal metal bis(telluride) ligands could likewise be assembled via the elimination of telluroethers,11a,b,12 akin to how Arnold and co-workers prepared a Ta![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te mono(telluride) complex using silyltelluroether as the leaving group.12e
Te mono(telluride) complex using silyltelluroether as the leaving group.12e
Herein we demonstrate how an easily prepared tellurolate salt [Li(THF)TeCH2SiMe3]x, can be an effective Te-atom transfer reagent not only to VIII, but also to NbIV and TaV halide precursors. Our approach demonstrates that low or high-valent precursors, which are generally more common for the lighter or heavier group 5 metals, respectively, can all be adequate reagents for the assembly of the bis(telluride) motif when using [Li(THF)TeCH2SiMe3]x and if needed, an appropriate oxidant or reductant.
Results and discussion
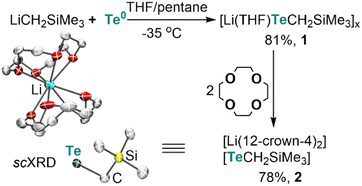
Lithium alkyltellurolate [Li(THF)TeCH2SiMe3]x (1) could be readily prepared by slow and dropwise addition of elemental tellurium as a suspension in THF to a cold pentane solution of recrystallized and commercially available LiCH2SiMe3 (−35 °C). Despite no single-crystal X-ray diffraction (sc-XRD) data for 1, NMR spectroscopic analysis of the isolated product corroborates its formation in 81% yield13 as a pale yellow residue (Scheme 1).To obtain solid-state structural information by sc-XRD, we prepared the discrete salt, [Li(12-crown-4)2][TeCH2SiMe3] (2), in 78% yield via the addition of two equiv. of 12-crown-4 to the tellurolate solution at −35 °C (Scheme 1). Although sterically hindered aryl and silyl tellurolates are known,14 structurally characterized alkyl tellurolate is limited to just one example: dimeric [Li(TMEDA)TenBu]2,15 thus making [TeCH2SiMe3]− a rare example of a one coordinate alkyl tellurolate anion. The Te–C bond distance in 2 (2.196(4) Å) is similar to the dimeric [Li(TMEDA)TenBu]2 (2.191(4) Å).15 A sharp 125Te{1H} resonance was located for 1 at −1790 ppm (Fig. S6†)14c whereas a broad resonance at −569 ppm (Δν1/2 = 282 Hz) was found for 2 (Fig. S14†).16 However, no 7Li–125Te coupling was resolved in 1 (Fig. S7†), which can be attributed to its fluxional behavior in solution.16
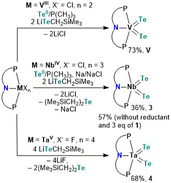
Having the tellurolate 1 in hand, we turned our attention to independently making the bis(telluride) complex (PNP)V(Te)2 using the VIII dichloride precursor, (PNP)VCl2.17 Accessing (PNP)V(Te)2 directly from the dichloride precursor offers two advantages: (1) this circumvents the need to prepare the thermally unstable bisalkyl complex [(PNP)V(CH2tBu)2], which is known to not only activate N2,10a but also convert to the transient alkylidene [(PNP)V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHtBu] that has been shown to intermolecularly activate C–H bonds in C6H6,18 and (2) avoids the cumbersome synthetic route involving the reaction of [(PNP)V(CH2tBu)2] with elemental Te under a static vacuum at 90 °C for four days.7 However, in order to convert VIII to VV, Te (solubilized with a catalytic amount of PMe3) is required as a two-electron oxidant. Treatment of (PNP)VCl2 with two equiv. of 1 in the presence of Te/PMe3 for 16 hours resulted in the gradual formation of (PNP)V(Te)2 which could be isolated from the mixture in 73% yield as a dark green, and highly insoluble material in most common organic solvents.
CHtBu] that has been shown to intermolecularly activate C–H bonds in C6H6,18 and (2) avoids the cumbersome synthetic route involving the reaction of [(PNP)V(CH2tBu)2] with elemental Te under a static vacuum at 90 °C for four days.7 However, in order to convert VIII to VV, Te (solubilized with a catalytic amount of PMe3) is required as a two-electron oxidant. Treatment of (PNP)VCl2 with two equiv. of 1 in the presence of Te/PMe3 for 16 hours resulted in the gradual formation of (PNP)V(Te)2 which could be isolated from the mixture in 73% yield as a dark green, and highly insoluble material in most common organic solvents.
Unlike V, Nb poses a challenge since the putative trivalent precursor “(PNP)NbCl2” is unstable.10b As a result we used instead the NbIV precursor (PNP)NbCl310c under similar conditions in combination with tellurolate 1, but in the presence of a reductant. Accordingly, treatment of (PNP)NbCl3 with two equiv. of 1,19 in the presence of Te/PMe3 and one equiv. of Na/NaCl in THF resulted in the formation of (PNP)Nb(Te)2 (3) as a dark green colored crystalline material isolated in 36% yield after work-up of the reaction mixture (Scheme 2). We found later that complex 3 could also be prepared using instead 3 equiv. of 1, and in the presence of Te/PMe3 in 57% yield (Scheme 2). Additionally, formation of Te(CH2SiMe3)2 20 is confirmed in both reactions by 1H and 125Te NMR spectroscopy (Fig. S24 and S25†).13
Analogous to Arnold's construction of a terminal mono(telluride) ligand onto TaV dihalide,12e one should intuitively conceive the assembly of a bis(telluride) motif using the appropriate TaV tetrahalide precursor. Conveniently, Ozerov and co-workers have reported a TaV starting material, namely (PNP)TaF4.21 Treating (PNP)TaF4 with 4 equiv. of 1 in THF over 16 hours at room temperature resulted in the formation of (PNP)Ta(Te)2 (4) in 68% yield as a red, crystalline material (Scheme 2). Examination of the side-product formed from this reaction via1H and 125Te NMR spectroscopy again verified the formation of Te(CH2SiMe3)2 along with other intractable products (Fig. S33 and S34†).13 Interestingly, the loss of Te(CH2SiMe3)2 was not observed in the formation of V. It is noteworthy that in all three cases, the reduction of metal halides (PNP)MXn (M = V, Nb, Ta; X = Cl, F; n = 2, 3, 4) in the presence of Te(0) did not provide species (PNP)M(Te)2, highlighting the utility of 1 as a Te-atom delivery reagent.
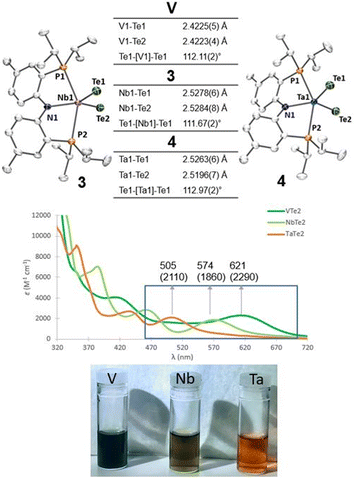
Single crystals of compounds 3 and 4 were obtained from a THF/pentane mixture at −35 °C as green and red needles respectively, and sc-XRD confirmed the formation of isomorphous five-coordinate bis(telluride) structures (top, Fig. 2). The M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te bond lengths in 3 and 4 are similar to the anionic Ti bis(telluride) complex, [(PNP)Ti(Te)2]− (2.5185(6) Å),9 but longer than that of (PNP)V(Te)27 owing to the increase in ionic radii of the metal as one descends the group. To date, the only documented example of a terminally bound telluride ligand to Nb and Ta include the mono(telluride) complexes, [(C5Me5)Nb(NAr)(Te)(PMe3)] (Ar = 2,6-C6H3iPr2; not structurally characterized),22 [((Me3SiNCH2CH2)3N)Ta(Te)] (Ta
Te bond lengths in 3 and 4 are similar to the anionic Ti bis(telluride) complex, [(PNP)Ti(Te)2]− (2.5185(6) Å),9 but longer than that of (PNP)V(Te)27 owing to the increase in ionic radii of the metal as one descends the group. To date, the only documented example of a terminally bound telluride ligand to Nb and Ta include the mono(telluride) complexes, [(C5Me5)Nb(NAr)(Te)(PMe3)] (Ar = 2,6-C6H3iPr2; not structurally characterized),22 [((Me3SiNCH2CH2)3N)Ta(Te)] (Ta![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te, 2.568(1) Å),12e and
Te, 2.568(1) Å),12e and 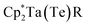 (R = H, Ta
(R = H, Ta![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te, 2.588(2) Å),23 reported by Gibson, Arnold, and Parkin respectively. Notably, the Ta
Te, 2.588(2) Å),23 reported by Gibson, Arnold, and Parkin respectively. Notably, the Ta![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te bond distance in 4 is shorter than Arnold's and Parkin's high-valent mono(telluride) complexes. The 1H and 31P NMR spectroscopic features for 3 and 4 are indicative of a C2-symmetric complex in solution with one 31P singlet resonance for the symmetrically equivalent phosphorus atoms (V: 142 ppm, 3: 91 ppm and 4: 111 ppm). Markedly, the 125Te{1H} NMR spectrum shows a broad resonance at 3896 ppm for 3 (Δν1/2 = 404 Hz) and 3041 ppm for 4 (Δν1/2 = 370 Hz) whereas the titanium bis(telluride) anion, [(PNP)Ti(Te)2]− (3260 ppm, Δν1/2 = 560 Hz) is in between 3 and 4. All bis(telluride) complexes in this work are air-sensitive, highly colored (V: dark green, Nb: green in solution, Ta: red; bottom, Fig. 2), stable as a solid up to 180 °C,13 and show a modest increase in solubility down the group. The latter feature is counterintuitive as one would expect the Ta
Te bond distance in 4 is shorter than Arnold's and Parkin's high-valent mono(telluride) complexes. The 1H and 31P NMR spectroscopic features for 3 and 4 are indicative of a C2-symmetric complex in solution with one 31P singlet resonance for the symmetrically equivalent phosphorus atoms (V: 142 ppm, 3: 91 ppm and 4: 111 ppm). Markedly, the 125Te{1H} NMR spectrum shows a broad resonance at 3896 ppm for 3 (Δν1/2 = 404 Hz) and 3041 ppm for 4 (Δν1/2 = 370 Hz) whereas the titanium bis(telluride) anion, [(PNP)Ti(Te)2]− (3260 ppm, Δν1/2 = 560 Hz) is in between 3 and 4. All bis(telluride) complexes in this work are air-sensitive, highly colored (V: dark green, Nb: green in solution, Ta: red; bottom, Fig. 2), stable as a solid up to 180 °C,13 and show a modest increase in solubility down the group. The latter feature is counterintuitive as one would expect the Ta![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te bond in 4 to be more ionic and thus less soluble relative to Nb
Te bond in 4 to be more ionic and thus less soluble relative to Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te in 3 and V
Te in 3 and V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te in (PNP)V(Te)2 based on periodic trends. To probe the electronic features in these complexes, we collected UV-vis absorption spectra in THF at the same concentrations (0.056 mM). In addition to the high energy ligand-to-ligand transitions, the spectra show the expected hypsochromic shift in absorptions down the group: (PNP)V(Te)2 (λ = 621 nm, ε = 2290 M−1 cm−1); 3 (574 nm, 1860 M−1 cm−1); 4 (505 nm, 2110 M−1 cm−1), that can be ascribed to ligand-to-metal charge transfer (LMCT) transitions (middle, Fig. 2, and S36–S39†).
Te in (PNP)V(Te)2 based on periodic trends. To probe the electronic features in these complexes, we collected UV-vis absorption spectra in THF at the same concentrations (0.056 mM). In addition to the high energy ligand-to-ligand transitions, the spectra show the expected hypsochromic shift in absorptions down the group: (PNP)V(Te)2 (λ = 621 nm, ε = 2290 M−1 cm−1); 3 (574 nm, 1860 M−1 cm−1); 4 (505 nm, 2110 M−1 cm−1), that can be ascribed to ligand-to-metal charge transfer (LMCT) transitions (middle, Fig. 2, and S36–S39†).
We conducted density functional theory (DFT) calculations to better understand the nature of M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te bonds in the series. After an extensive DFT functional benchmark study (Fig. S40–S42†), we used the MN15/TZ2P//DZP level of theory24 implemented in the Amsterdam Density Functional (ADF) software25 package to compute and analyze charge-transfer characters. Fig. 3 illustrates the frontier orbitals of the three complexes and the orbital transitions involved in the excitation. The HOMO–LUMO gap increases down the group in accord with the periodic trends: 3.652 eV (V), 3.942 eV (Nb) and 4.201 eV (Ta). Two frontier orbital transitions corresponding to the experimentally observed absorptions13 were identified where the T1 transitions involve the pincer-to-metal charge transfer from HOMO to LUMO (M = Nb and Ta) or LUMO+2 (M = V). In contrast, the T2 transitions are mostly localized on the metal center (M = V) or the telluride moiety (M = Nb and Ta) depending on the charge transfer character (Fig. S43†). The table inset in Fig. 3 clearly illustrates that the contribution of T1 follows the expected trend based on the average hole–electron distance, Δr,26 obtained from natural transition orbital (NTO) analysis. We performed a Hirshfeld charge analysis to probe the bond polarity whereby the Nb center (+0.35e) in 3 (Te: −0.19e) is more electron-deficient relative to Ta (+0.23e) in 4 (Te: −0.17e) which results in an increased polarization in the binding of the ligands to the transition metal. This difference supports the increased solubility observed in the Ta complex relative to Nb. Furthermore, we hypothesize that the slight decrease in the positive charge of Ta in 4 can be attributed to relativistic contractions of the p orbitals, making the 5p orbitals of Ta more electronegative compared to the 4p orbitals of Nb.
Te bonds in the series. After an extensive DFT functional benchmark study (Fig. S40–S42†), we used the MN15/TZ2P//DZP level of theory24 implemented in the Amsterdam Density Functional (ADF) software25 package to compute and analyze charge-transfer characters. Fig. 3 illustrates the frontier orbitals of the three complexes and the orbital transitions involved in the excitation. The HOMO–LUMO gap increases down the group in accord with the periodic trends: 3.652 eV (V), 3.942 eV (Nb) and 4.201 eV (Ta). Two frontier orbital transitions corresponding to the experimentally observed absorptions13 were identified where the T1 transitions involve the pincer-to-metal charge transfer from HOMO to LUMO (M = Nb and Ta) or LUMO+2 (M = V). In contrast, the T2 transitions are mostly localized on the metal center (M = V) or the telluride moiety (M = Nb and Ta) depending on the charge transfer character (Fig. S43†). The table inset in Fig. 3 clearly illustrates that the contribution of T1 follows the expected trend based on the average hole–electron distance, Δr,26 obtained from natural transition orbital (NTO) analysis. We performed a Hirshfeld charge analysis to probe the bond polarity whereby the Nb center (+0.35e) in 3 (Te: −0.19e) is more electron-deficient relative to Ta (+0.23e) in 4 (Te: −0.17e) which results in an increased polarization in the binding of the ligands to the transition metal. This difference supports the increased solubility observed in the Ta complex relative to Nb. Furthermore, we hypothesize that the slight decrease in the positive charge of Ta in 4 can be attributed to relativistic contractions of the p orbitals, making the 5p orbitals of Ta more electronegative compared to the 4p orbitals of Nb.
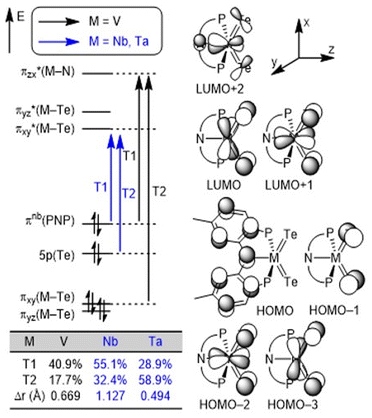 | ||
| Fig. 3 Conceptual MO diagrams of (PNP)M(Te)2 (M = V, Nb, and Ta) complexes and the most contributing orbital transitions denoted as T1 and T2, respectively. | ||
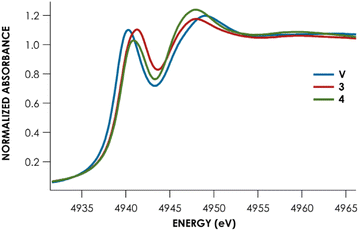
Lastly, Te L1-edge X-ray absorption spectra (XAS) were obtained for compounds V, 3, and 4 (Fig. 4 and Table 1). TDDFT calculations13 of the Te L1-edge XAS spectrum were performed using geometries derived from the respective sc-XRD-derived structures. Good agreement is observed between the experimental Te L1 spectra and the calculated spectra (Fig. S50–S52†).13 In all three cases, the L1 peak corresponds to a Te 2s → Te 5p transition with varying M d and ligand-based orbital contributions. In both the calculated and measured spectra, the L1 edge peak for V is red-shifted relative to 3 and 4 which are measured to be essentially coincident with each other. This likely reflects the more contracted V 3d orbitals, compared to Nb and Ta 4d and 5d orbitals respectively. This results in poor M d/Te p overlap and ultimately in greater charge density localized on the Te atoms. This is supported by inspection of the Löwdin calculated charges: Te is calculated to have an average charge of −0.21e in V, whereas Te is calculated to have an average charge of +0.09e and +0.07e in 3 and 4, respectively. However, we found that irrespective of the charge analysis method used (Hirshfeld vs. Löwdin), the metal center is more electropositive than the Te atom. Inspection of the empty frontier orbitals of V, 3, and 4 (Table S9†) shows that there is more TM d orbital character than Te indicative of overall nucleophilic character on Te and positive character on the metal center.
| Complex | Measured L1 peak (eV) | Measured L1 peak area | Calculated L1 peaka (eV) |
|---|---|---|---|
| a Calculated as the intensity weighted average position: Epeak = (∑(Eabs × Iabs)/∑(Iabs)), where Eabs is the calculated energy of the state contributing to the peak, and Iabs is the calculated intensity. | |||
| V | 4940.3 ± 0.01 | 5.9 ± 0.5 | 4940.6 |
| 3 (Nb) | 4941.1 ± 0.02 | 6.6 ± 0.5 | 4940.9 |
| 4 (Ta) | 4941.1 ± 0.03 | 6.0 ± 0.6 | 4941.0 |
Remarkably, little variance in the Te 5p character of the acceptor orbitals is observed across the measured series (24–27%). This is reflected in the fact that no discernible trend can be observed in the measured Te L1 peak area – all areas are within error of each other (Table 1). The transition metal M d character is observed to decrease down the group (V > Nb > Ta). Concomitantly, the acceptor orbitals appear to have increasing ligand character (Ta > Nb > V). Taken together, we can observe that the bonding of Te to group 5 transition metal centers is substantively similar down the group and that varying the metal identity primarily changes the binding of the transition metal to the PNP scaffold, rather than inducing noteworthy changes to the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te bonds.
Te bonds.
Conclusions
In summary, we have established a convenient one-step synthetic route to bis(telluride) complexes of group 5 transition metals. In contrast to the protocol of using elemental Te or TePR3 to access bis(telluride) motifs, this work presents the groundwork that a lithium alkyl tellurolate can be used to access the rare bis(telluride) species regardless of the starting metal halide complexes and their oxidation state. By combining spectroscopic and theoretical investigation, we identified the electronic structures of the series of complexes and their excitation profiles in the UV-vis region. Outside of the expected periodic trends, Hirshfeld charge analysis indicates increased polarity between Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te bonds relative to Ta
Te bonds relative to Ta![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te, and the Te-L1 XAS peak areas are indifferent between these complexes indicating similarity in the bonding between Te and the respective metal center. We are now investigating this strategy for the synthesis of other terminal bis(chalogenides) (e.g. Se and/or S) and of transition metals outside of group 5.
Te, and the Te-L1 XAS peak areas are indifferent between these complexes indicating similarity in the bonding between Te and the respective metal center. We are now investigating this strategy for the synthesis of other terminal bis(chalogenides) (e.g. Se and/or S) and of transition metals outside of group 5.
Data availability
All the experimental and computational data are available in the ESI: experimental details, characterization and analytical data. CCDC data available for 2262094 (complex 2), 2262095 (complex 3), and 2262098 (complex 4).†Author contributions
SS: conceptualization, formal analysis, data curation, writing – original draft preparation, writing – review & editing; SK: methodology, formal analysis, data curation, writing – original draft preparation, writing – review & editing; RYK: methodology, formal analysis, funding acquisition, data curation, writing – original draft preparation, writing – review & editing; SNM: methodology, writing – review & editing; PZ: conceptualization, writing – review & editing; MRG: data curation; PJC: data curation; MHB: funding acquisition, writing – review & editing, supervision; KML: funding acquisition, writing – review & editing, supervision; DJM: conceptualization, funding acquisition, writing – original draft preparation, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the University of Pennsylvania, the U.S. National Science Foundation (NSF; CHE-2154620 and NSF-MRI CHE-2117783 to D. J. M., CHE-2247818 to K. M. L.), and the Institute for Basic Science in Korea for financial support (IBS-R010-A1 to M.-H. B.). R. Y. K. thanks Cornell University for a Klarman Postdoctoral Fellowship. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (P30GM133894).Notes and references
- (a) G. Parkin, in Prog. Inorg. Chem., ed. K. D. Karlin, John Wiley & Sons, Inc., 1997, ch. 1, pp. 1–165 Search PubMed; (b) D. Rabinovich and G. Parkin, Inorg. Chem., 1995, 34, 6341–6361 CrossRef CAS; (c) L. C. Roof and J. W. Kolis, Chem. Rev., 1993, 93, 1037–1080 CrossRef CAS.
- F. A. Cotton and G. Schmid, Inorg. Chem., 1997, 36, 2267–2278 CrossRef CAS PubMed.
- D. Rabinovich and G. Parkin, J. Am. Chem. Soc., 1991, 113, 9421–9422 CrossRef CAS.
- V. J. Murphy and G. Parkin, J. Am. Chem. Soc., 1995, 117, 3522–3528 CrossRef CAS.
- D. Rabinovich and G. Parkin, J. Am. Chem. Soc., 1993, 115, 9822–9823 CrossRef CAS.
- U. J. Kilgore, C. A. Sengelaub, H. Fan, J. Tomaszewski, J. A. Karty, M.-H. Baik and D. J. Mindiola, Organometallics, 2009, 28, 843–852 CrossRef CAS.
- U. J. Kilgore, J. A. Karty, M. Pink, X. Gao and D. J. Mindiola, Angew Chem. Int. Ed. Engl., 2009, 48, 2394–2397 CrossRef CAS PubMed.
- B. C. Bailey, J. C. Huffman, D. J. Mindiola, W. Weng and O. V. Ozerov, Organometallics, 2005, 24, 1390–1393 CrossRef CAS.
- P. Zatsepin, J. H. Kim, M. R. Gau, P. J. Carroll, B. Pudasaini, M. H. Baik and D. J. Mindiola, J. Am. Chem. Soc., 2022, 144, 13066–13070 CrossRef CAS PubMed.
- (a) U. J. Kilgore, C. A. Sengelaub, M. Pink, A. R. Fout and D. J. Mindiola, Angew Chem. Int. Ed. Engl., 2008, 47, 3769–3772 CrossRef CAS PubMed; (b) U. J. Kilgore, J. Tomaszewski, H. Fan, J. C. Huffman and D. J. Mindiola, Organometallics, 2007, 26, 6132–6138 CrossRef CAS; (c) U. J. Kilgore, X. Yang, J. Tomaszewski, J. C. Huffman and D. J. Mindiola, Inorg. Chem., 2006, 45, 10712–10721 CrossRef CAS.
- (a) D. E. Gindelberger and J. Arnold, Organometallics, 1994, 13, 4462–4468 CrossRef CAS; (b) V. Christou and J. Arnold, J. Am. Chem. Soc., 1992, 114, 6240–6242 CrossRef CAS; (c) D. R. Cary and J. Arnold, J. Am. Chem. Soc., 1993, 115, 2520–2521 CrossRef CAS.
- (a) L. K. Knight, W. E. Piers and R. McDonald, Chem. - Eur. J., 2000, 6, 4322–4326 CrossRef CAS PubMed; (b) W. E. Piers, J. Chem. Soc., Chem. Commun., 1994, 309–310 RSC; (c) W. E. Piers, L. R. MacGillivray and M. Zaworotko, Organometallics, 1993, 12, 4723–4725 CrossRef CAS; (d) V. Christou, S. P. Wuller and J. Arnold, J. Am. Chem. Soc., 1993, 115, 10545–10552 CrossRef CAS; (e) V. Christou and J. Arnold, Angew Chem. Int. Ed. Engl., 1993, 32, 1450–1452 CrossRef.
- See ESI.†.
- (a) P. J. Bonasia and J. Arnold, J. Organomet. Chem., 1993, 449, 147–157 CrossRef CAS; (b) H. Gornitzka, S. Besser, R. Herbst-Irmer, U. Kilimann and F. T. Edelmann, Angew Chem. Int. Ed. Engl., 1992, 31, 1260–1261 CrossRef; (c) P. J. Bonasia, D. E. Gindelberger, B. O. Dabbousi and J. Arnold, J. Am. Chem. Soc., 1992, 114, 5209–5214 CrossRef CAS; (d) B. O. Dabbousi, P. J. Bonasia and J. Arnold, J. Am. Chem. Soc., 1991, 113, 3186–3188 CrossRef CAS; (e) P. J. Bonasia and J. Arnold, J. Chem. Soc., Chem. Commun., 1990, 1299–1301 RSC.
- W. Clegg, R. P. Davies, R. Snaith and A. E. H. Wheatley, Eur. J. Inorg. Chem., 2001, 2001, 1411–1413 CrossRef.
- The broad resonance of 2 could be attributed to its rigid structure enforced by the [Li(12-crown-4)2]+ in solution relative to 1 which is possibly an oligomer in solution. This causes a slower relaxation time, T2, for 2 and results in a broader peak width.
- U. J. Kilgore, C. A. Sengelaub, H. Fan, J. Tomaszewski, J. A. Karty, M.-H. Baik and D. J. Mindiola, Organometallics, 2008, 28, 843–852 CrossRef.
- J. G. Andino, U. J. Kilgore, M. Pink, A. Ozarowski, J. Krzystek, J. Telser, M.-H. Baik and D. J. Mindiola, Chem. Sci., 2010, 1, 351–356 RSC.
- Complex 1 could be an oligomer in solution.
- R. W. Gedridge, K. T. Higa and R. A. Nissan, Magn. Reson. Chem., 1995, 33, 441–448 CrossRef CAS.
- L. C. H. Gerber, L. A. Watson, S. Parkin, W. Weng, B. M. Foxman and O. V. Ozerov, Organometallics, 2007, 26, 4866–4868 CrossRef CAS.
- U. Siemeling and V. C. Gibson, J. Chem. Soc., Chem. Commun., 1992, 1670–1671 RSC.
- J. H. Shin and G. Parkin, Organometallics, 1994, 13, 2147–2149 CrossRef CAS.
- H. S. Yu, X. He, S. L. Li and D. G. Truhlar, Chem. Sci., 2016, 7, 5032–5051 RSC.
- (a) G. te Velde and E. J. Baerends, J. Comput. Phys., 1992, 99, 84–98 CrossRef CAS; (b) L. Versluis and T. Ziegler, J. Chem. Phys., 1988, 88, 322–328 CrossRef CAS; (c) E. J. Baerends and P. Ros, Chem. Phys., 1973, 2, 52–59 CrossRef CAS; (d) E. J. Baerends, D. E. Ellis and P. Ros, Chem. Phys., 1973, 2, 41–51 CrossRef CAS; (e) H. J. J. te Riele, T. J. Dekker and H. A. van der Vorst, Algorithms and Applications on Vector and Parallel Computers, Elsevier, Amsterdam, 1987 Search PubMed.
- C. A. Guido, P. Cortona, B. Mennucci and C. Adamo, J. Chem. Theory Comput., 2013, 9, 3118–3126 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2262094, 2262095 and 2262098. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc03470d |
| This journal is © The Royal Society of Chemistry 2023 |